Abstract
In the midst of the current antimicrobial pipeline void, alternative approaches are needed to reduce the incidence of infection and decrease reliance on last-resort antibiotics for the therapeutic intervention of bacterial pathogens. In that regard, mupirocin ointment-based decolonization and wound maintenance practices have proven effective in reducing Staphylococcus aureus transmission and mitigating invasive disease. However, the emergence of mupirocin-resistant strains has compromised the agent's efficacy, necessitating new strategies for the prevention of staphylococcal infections. Herein, we set out to improve the performance of mupirocin-based ointments. A screen of a Food and Drug Administration (FDA)-approved drug library revealed that the antibiotic neomycin sulfate potentiates the antimicrobial activity of mupirocin, whereas other library antibiotics did not. Preliminary mechanism of action studies indicate that neomycin's potentiating activity may be mediated by inhibition of the organism's RNase P function, an enzyme that is believed to participate in the tRNA processing pathway immediately upstream of the primary target of mupirocin. The improved antimicrobial activity of neomycin and mupirocin was maintained in ointment formulations and reduced S. aureus bacterial burden in murine models of nasal colonization and wound site infections. Combination therapy improved upon the effects of either agent alone and was effective in the treatment of contemporary methicillin-susceptible, methicillin-resistant, and high-level mupirocin-resistant S. aureus strains. From these perspectives, combination mupirocin-and-neomycin ointments appear to be superior to that of mupirocin alone and warrant further development.
INTRODUCTION
Staphylococcus aureus has been designated one of the six ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) bacterial pathogens of greatest concern to health care in the United States (1). The organism is a predominant cause of nosocomial and community-associated bacterial infections and has developed resistance to all currently available antibiotics (2). The S. aureus annual U.S. mortality rate has already surpassed that of HIV/AIDS and is predicted to worsen given the downsizing of most pharmaceutical antimicrobial programs (3, 4). Consequently, new strategies are needed for the prevention and treatment of staphylococcal infections.
The anterior nares of humans is a principal ecological niche for S. aureus, and nasal carriage is a recognized risk factor for staphylococcal disease, particularly among patient populations undergoing surgical procedures or hemodialysis or requiring long-term intensive care unit stays (reviewed in reference 5). S. aureus nasal decolonization reduces colonization of other body sites, the risk of transmission, and subsequent infection (5). Consequently, infection control practices routinely include nasal decolonization procedures as a means to prevent staphylococcal disease.
Mupirocin is an antimicrobial agent that inhibits bacterial isoleucyl-tRNA synthetase-mediated Ile-tRNA aminoacylation and protein translation (6–8). This agent displays excellent antibacterial activity toward most Gram-positive species and lacks cross-resistance to current antibiotics, but it is also unstable in vivo and thus not well-suited for systemic use in humans (9). However, mupirocin-based ointments have proven effective for the treatment of S. aureus skin and wound infections (9–13) and have also recently emerged as the standard of care for presurgical nasal decolonization (reviewed in reference 14). Indeed, mupirocin-mediated nasal decolonization has been shown to be effective in reducing infections in burn wound, dialysis, and surgical patient populations, as well as S. aureus transmission among health care workers and intensive care unit patients (15–21). In addition to nasal decolonization, topical mupirocin has been used to successfully treat hemodialysis central venous catheter exit sites, impetigo, eczema, surgical wound sites, skin and soft tissue wounds, the breasts of breast-feeding mothers, and tympanic membrane lesions (22–27). However, the emergence of S. aureus mupirocin resistance has reduced the agent's efficacy both as a nasal decolonization agent and as a treatment option for skin and wound infections.
Low-level mupirocin-resistant (LL-MR) S. aureus strains are defined as exhibiting an MIC of 8 to 256 μg ml−1 due to point mutations in the organism's native isoleucyl-tRNA synthetase gene (ileRS) and develop rapidly in both the laboratory and clinical settings (28). High-level mupirocin resistance (HL-MR) (MIC of >256 μg ml−1) occurs less frequently and is attributable to the acquisition of a mobile genetic element harboring either mupA, which codes for an alternate isoleucyl-tRNA synthetase, or the less-characterized mupB gene (29, 30). Both LL-MR and HL-MR lead to mupirocin treatment failure (31). Indeed, while low-level resistant strains initially respond to therapy, they frequently reemerge quickly; relapse is hypothesized to be due to latent LL-MR subpopulations that are not eradicated by mupirocin dosing (31, 32). Conversely, HL-MR S. aureus bacteria are recalcitrant to mupirocin ointments (31). Thus, the emergence of mupirocin resistance has prompted renewed interest in developing alternative decolonization and wound infection treatment strategies.
S. aureus RNase P is an essential riboprotein complex consisting of RnpA and ribozyme rnpB that acts upstream of tRNA synthetases in the tRNA maturation pathway (33, 34). More specifically, RNase P is hypothesized to catalyze removal of the 5′ leader sequences from precursor tRNA species, thereby creating mature tRNA substrates for tRNA synthetases, including isoleucyl-tRNA synthetase (the cellular target for mupirocin) (33–39). Recognizing that two antimicrobials targeting independent steps in the same metabolic pathway can have combined antibacterial effects, it has been hypothesized that combination therapies involving mixtures of RNase P inhibitors together with mupirocin would display increased antimicrobial efficacy and the potential to overcome mupirocin resistance (33, 40).
Herein, we report the results of a screen of a Food and Drug Administration (FDA)-approved drug library for agents that potentiate the antimicrobial properties of mupirocin toward S. aureus. The antibiotic neomycin sulfate, which is approved for topical use and previously shown to inhibit Escherichia coli RNase P, was among the hits identified (41). Assays revealed that neomycin also inhibits S. aureus in vitro RNase P function, that it confers an additive antimicrobial advantage to mupirocin, and that the combination could be effectively formulated in ointment format. Topical application of the combination displayed significantly improved murine nasal decolonization toward a panel of S. aureus strains compared to either agent alone. Likewise, the combination led to the near eradication of contemporary methicillin-susceptible, methicillin-resistant, and high-level mupirocin-resistant strains in a murine wound model of colonization.
MATERIALS AND METHODS
Bacterial strains and animals.
All bacterial studies were performed with S. aureus strain UAMS-1, a well-characterized antibiotic-susceptible clinical isolate commonly used to study the organism's biofilm formation and colonization properties (42), USA300, a neomycin- and methicillin-resistant community-acquired clinical isolate (43), or BAA-1708, a high-level mupirocin-resistant strain containing mupA obtained from the American Type Culture Collection (Manassas, VA). Unless indicated otherwise, strains were grown overnight in tryptic soy broth (TSB) and were then used to inoculate fresh (1:100 dilution) medium, grown to early exponential phase (1 × 108 CFU/ml), and processed as described below. Female BALB/c mice 4 to 6 weeks of age were obtained from Charles River (Wilmington MA) and housed according to approved University of Rochester Medical Center Council on Animal Research (UCAR) protocol UCAR-2013-024.
Preparation of test articles.
The polyethylene glycol (PEG) ointment base was prepared by mixing PEG 400 (70%, wt/vol) with PEG 3350 (30%, wt/vol) as described by the U.S. Pharmacopeia and The National Formulary (USP 24-NF 19). Mupirocin (AppliChem, Chicago, IL) and neomycin (Sigma, St. Louis, MO) were suspended in 250 μl of dimethyl sulfoxide (DMSO) to create working concentrations of 100 mg and 50 mg, respectively. The mixtures were then added directly to 5 g of PEG ointment preliquefied by heating at 60°C for 30 min to create suspensions containing both 2% mupirocin and 1% neomycin and then cooled to room temperature to solidify the suspension. The same procedure was used to create DMSO vehicle control and 2% mupirocin–1% neomycin PEG mixtures by adding a combination of 100 mg mupirocin and 50 mg neomycin in a total 250-μl volume of DMSO.
Screen of Selleck library.
Members of the Selleck library of Food and Drug Administration-approved drugs (catalog no. L1300; Selleck Chemicals, Houston, TX) were screened for agents that potentiate the antimicrobial activity of mupirocin toward S. aureus strain UAMS-1. To do so, 1 × 105 CFU of S. aureus UAMS-1 was added to individual wells of a 96-well microtiter plate, mixed with 0.03 μg ml−1 mupirocin (0.5× MIC) and 50 μM test agent in Mueller-Hinton broth (MHB) (100-μl total well volume). Microtiter plates were incubated at 37°C for 16 h, and individual wells were inspected for growth. Wells lacking growth were considered to represent agents that either potentiated the antimicrobial properties of mupirocin or mupirocin-independent antimicrobial microbial properties. All drugs that resulted in no growth were confirmed in duplicate and were plated without mupirocin to measure their inherent antimicrobial activity.
RNase P ptRNA processing assay.
S. aureus RNase P activity assays were performed as previously described (33). Briefly, RNase P was first reconstituted by mixing an equimolar ratio of denatured rnpB and RnpA for 15 min at 37°C, and 2.5 pmol of this mixture was then added to 5 pmol of precursor tRNATyr (ptRNATyr) and increasing concentrations of the indicated concentration of neomycin or the known RNase P inhibitor RNPA2000 (33) in a total volume of 20 μl. Mixtures were incubated for 5 min at 37°C, stopped by adding 20 μl of 2× RNA loading dye (95% formamide, 0.025% SDS, 0.025% bromophenol blue, 0.025% xylene cyanol FF, 0.5 mM EDTA), and 30 μl of each sample was electrophoresed in a 7 M urea–8% polyacrylamide gel and stained with ethidium bromide (0.5 μg ml−1). A FluorChem 5500 imaging system was used to visualize RNA products and quantified using ImageJ software (National Institutes of Health, Bethesda, MD). The percent RNase P activity was then calculated using the following equation: test compound tRNATyr signal/mock tRNATyr signal.
Antimicrobial susceptibility testing.
MIC was tested in accordance with the Clinical and Laboratory Standards Institute guidelines. Briefly, 1 × 105 CFU of the indicated S. aureus strain was added to individual wells of a microtiter plate containing 88 μl of MHB medium and 2-fold increasing concentrations of mupirocin or test agent (0 to 128 μg ml−1). The plates were incubated for 16 h at 37°C, and the wells were visually inspected for growth. The lowest concentration of mupirocin or test agent that inhibited S. aureus growth was considered to be the MIC. Fractional inhibitory concentration index (FIC) testing was performed to measure interactions between mupirocin and neomycin, as previously described (44). Briefly, in checkerboard format, each row of the plate contained increasing concentrations of mupirocin (2-fold increments; 0 to 32 μg ml−1), whereas each column contained increasing concentrations of neomycin (2-fold increments; 0 to 32 μg ml−1). To every well (100-μl total volume), MHB containing 3 × 105 CFU of S. aureus strain UAMS-1 was added, and the plate was incubated at 37°C for 16 h. The FIC was determined using the following formula: (MIC of drug A in combination/MIC of drug A alone) + (MIC of drug B in combination/MIC of drug B alone) = FIC. A synergistic interaction was defined as an FIC value of ≤0.5, an additive interaction was defined as an FIC value of 0.5 to 1.0, no interaction was defined as an FIC of 1 to 4, and an antagonistic interaction was defined as an FIC of >4.
In vitro ointment antimicrobial testing.
Antimicrobial zones of inhibition were measured for PEG ointment compilations using the indicated S. aureus strains. To do so, 100 μl of 1 × 108 CFU ml−1 of S. aureus was spread on tryptic soy agar (TSA) plates. The plates were dried for 10 min, and 40 μl of ointment was pipetted onto the center of the plate. The plates were incubated at 37°C for 16 h, and zones of bacterial clearance were measured using ImageJ software (NIH).
Nasal colonization and treatment of mice.
Ointments were evaluated for in vivo antimicrobial activity using an S. aureus nasal colonization model as previously described (45) but with modifications. The nostrils of awake mice were inoculated with 1 × 107 CFU of the indicated S. aureus strain by pipetting 10 μl of culture directly into the nostrils and confirmed by the visualization of air bubbles appearing as the mouse breathed in and out. The nostrils of the mice were then treated with 10 μl PEG ointment (brought to 55°C in a heat block to liquefy) containing either vehicle alone or the indicated antibiotic 45 min postinoculation, and treatments were repeated every 8 h for 3 days. Mice were then euthanized via CO2 asphyxiation and cervical dislocation. The full nares from the back of the soft palate to the tip of the nostrils was collected by gross dissection and placed in microcentrifuge tubes containing 1 ml of freshly made phosphate-buffered saline (PBS). Samples were homogenized for 5 min, serially diluted, and plated on mannitol salt agar (MSA) (Thermo Scientific, Waltham MA). The plates were incubated for 16 h, and the number of S. aureus was determined.
Dermal wound model of infection and treatment of mice.
The effects of ointment compilations were evaluated for in vivo antimicrobial activity using an S. aureus dermal wound model (46) but with modifications. Mice were anesthetized by intraperitoneal injection with a mixture of 100 mg ml−1 ketamine (Hospira Inc., Lake Forest, IL) and 20 mg ml−1 Xylazine (Lloyd Laboratories, Shenandoah, IA) in 0.9% NaCl at 5 μl per g of body weight. Pain relief in the form of 20 μl of 0.5% Sensorcaine (APP Pharmaceuticals, Schaumburg, IL) was administered prior to dermal wounding. The dorsal midsection of the mouse was shaved and cleaned with a series of betadine scrub (Fisher Scientific), povidone-iodine pads (Professional Disposables International Inc.; Orangeburg, NY), and isopropyl alcohol pads (Fisher Scientific) for a total contact time of 2 min. A single wound was created in this sterile field on the mouse with a 6-mm biopsy punch (Fisher Scientific) to remove only the dermal layer and not disrupt the underlying musculature. The wounds of the mice were inoculated with 1 × 107 CFU of the indicated S. aureus strain by pipetting 10 μl of culture directly onto the wound. Mice were then treated with ointment formulations (50 μl) containing either vehicle alone, or indicated antibiotics 45 min postinoculation; treatments were repeated every 12 h for 3 days. Mice were then euthanized via CO2 asphyxiation and cervical dislocation, per UCAR-approved methodology, the wound and underlying muscle were excised with an 8-mm biopsy punch and placed in microcentrifuge tubes containing 1 ml of freshly made PBS. Samples were homogenized for 5 min, serially diluted, and plated on MSA. The plates were incubated for 16 h, and the number of S. aureus was enumerated.
In vivo toxicity testing.
Ointment toxicity was tested in a modified dermal wound model. Mice in groups of three per indicated treatment group were wounded as described above but were not inoculated with S. aureus. The wound was treated with ointments containing vehicle, 2% mupirocin, 1% neomycin, or 2% mupirocin plus 1% neomycin combination twice daily for 14 days. Mice were weighed and assessed for grooming and alertness, and images of the wound were obtained daily to measure wound contraction using ImageJ (NIH). Wound contraction was calculated as a percentage of wound area reduction using the following formula: WCd = (1 − WAd/WA0) × 100, where WCd is the wound contraction on day d, WAd is the wound area on day d, and WA0 is the wound area on the initial day, as previously described (47).
Statistical analyses.
Statistical analyses were performed using Graphpad Prism software version 6.0. For zone of inhibition assays, a Student t test was used to determine the statistical power between each treatment group. For murine studies, measures were log transformed and subjected to an one-way analysis of variance (ANOVA) analysis to determine the statistical power.
RESULTS
Agents that potentiate the antimicrobial activity of mupirocin.
Members of the Selleck library of 853 FDA-approved drugs were screened for agents that potentiate the activity of mupirocin. To do so, the antibiotic-susceptible S. aureus strain UAMS-1 was inoculated into individual wells of a microtiter plate containing 0.5× the strain's mupirocin MIC (0.0625 μg ml−1) and 50 μM library material. A total of 101 library members (11.8%), including 61 antibiotics, inhibited bacterial growth, suggesting that they may represent agents that (i) potentiate the antimicrobial activity of mupirocin, (ii) exhibit mupirocin-independent antimicrobial activity, or (iii) both (see Table S1 in the supplemental material).
To distinguish between these possibilities, the MIC of each compound was determined in medium lacking or containing 0.5× the strain's mupirocin MIC. Of the 101 compounds, 98 (97%) evaluated displayed similar antimicrobial activities regardless of whether mupirocin was present, indicating that they do not potentiate the antibacterial effects of mupirocin. Conversely, the antimicrobial activity of nitazoxanide, nitrofurazone, and neomycin sulfate increased in the presence of mupirocin. Fractional inhibitory concentration index (FIC) measures confirmed that each agent displayed an additive effect (FICs = 0.75) when combined with mupirocin indicating that they have the capacity to potentiate the activity of mupirocin (Table 1). More specifically, nitazoxanide and nitrofurazone reproducibly displayed modest antimicrobial activities of 16 μg ml−1 and 8 μg ml−1 in the absence and presence of 0.5× MIC mupirocin, respectively. The aminoglycoside antibiotic neomycin sulfate exhibited the most potent activity against the test strain in the absence (0.5 μg ml−1) and presence (0.125 to 0.25 μg ml−1) of 0.5× MIC mupirocin (0.0625 μg ml−1). Given that no other antibiotics within the Selleck library, including other aminoglycosides, displayed improved antimicrobial properties in the presence of mupirocin, and expanded FIC testing revealed that neomycin did not improve the antimicrobial activity of rifampin, vancomycin, sulfamethoxazole, meropenem, minocycline, ciprofloxacin, ceftriaxone, or erythromycin (data not shown), the additive effects between neomycin and mupirocin appeared to be specific to this combination of antibiotics.
TABLE 1.
Selleck library members with mupirocin-associated improved activitya
| Drug | MIC (μg ml−1) of drugb |
|
|---|---|---|
| (−) Mup | (+) Mup | |
| Nitazoxanide | 16 | 8 |
| Nitrofurazone | 16 | 8 |
| Neomycin sulfate | 0.5 | 0.25 |
The fractional inhibitory concentration index (FIC) was 0.75 for all three drugs.
The MIC of the drug was determined in the presence (+) or absence (−) of 0.5× mupirocin (Mup) MIC (0.0625 μg ml−1).
Neomycin inhibits S. aureus RNase P in vitro activity.
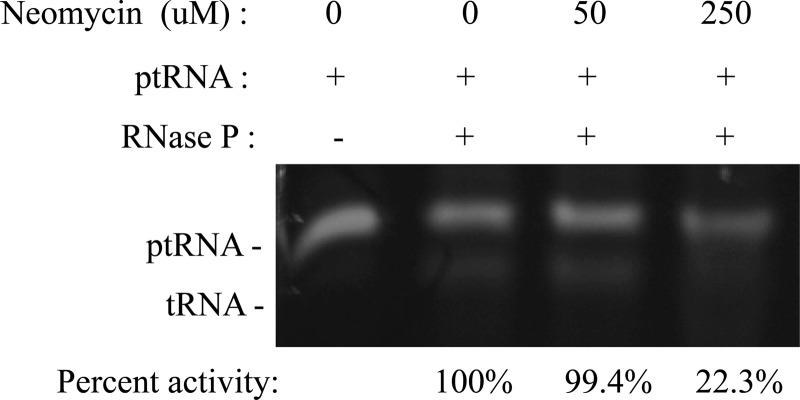
As noted above, it has been hypothesized that inhibitors of RNase P function would display improved antimicrobial effects when combined with mupirocin. In that regard, aminoglycoside antibiotics bind the major groove of the 16S rRNA to disrupt the fidelity of tRNA selection and block protein translation, but recent studies have revealed that they can also bind and affect the function of mRNAs, tRNAs, and catalytic RNAs (41, 48–50). Indeed, neomycin B and/or derivatives have been shown to bind to the rnpB component of RNase P and/or precursor tRNA molecules in a manner that inhibits Escherichia coli, Neisseria gonorrhoeae, Porphyromonas gingivalis, Streptococcus pneumoniae, and Bacillus subtilis RNase P function (41, 51, 52). Accordingly, we evaluated whether neomycin also inhibits S. aureus RNase P activity using an in vitro precursor tRNA processing assay (33). As shown in Fig. 1, results revealed that high concentrations (250 μM) of neomycin inhibit S. aureus RNase P's ability to catalyze the maturation of precursor tRNATyr, suggesting that the agent's ability to potentiate mupirocin may, in part, be mediated by its ability to inhibit the organism's RNase P activity.
FIG 1.
Effects of neomycin on S. aureus RNase P-mediated ptRNATyr processing. The mobility of precursor tRNATyr in the presence (+) and absence (−) of S. aureus RNase P enzyme and the indicated concentration of neomycin (in micromolar) is shown. Densitometry measured percent activity shown (tRNA product formed) normalized to the value for DMSO-treated enzyme alone.
Antimicrobial effects of mupirocin and neomycin combination in ointment formation.
Because neomycin improves the antimicrobial potency of mupirocin and the two antibiotics have differing mechanisms of action, we reasoned that combination ointments containing both agents would overcome mupirocin resistance. As a first test of this hypothesis, antimicrobial plate assays were used to monitor the antimicrobial effects of PEG-based ointments containing either DMSO (vehicle), 2% mupirocin, 1% neomycin, or the combination of 2% mupirocin plus 1% neomycin toward a neomycin- and mupirocin-susceptible clinical isolate (UAMS-1), a neomycin-resistant clinical isolate (USA300; MIC > 128 μg ml−1; data not shown), and a strain containing the mupA gene that confers high-level mupirocin resistance (BAA-1708; MIC > 256 μg ml−1; data not shown).
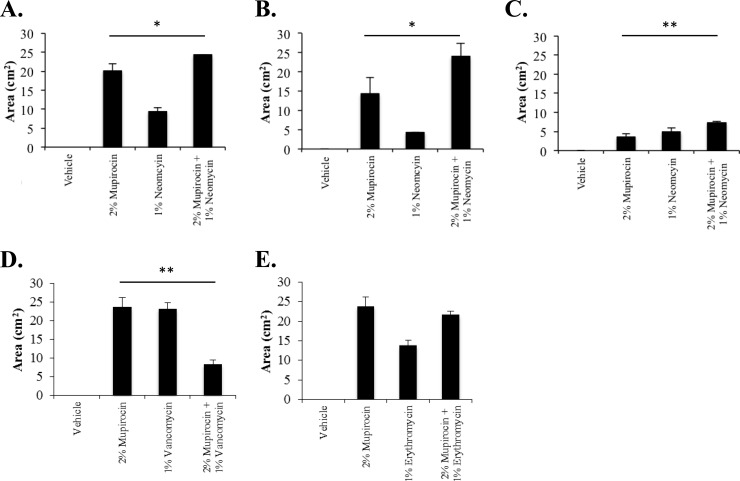
As shown in Fig. 2A, measures of each treatment's zone of inhibition revealed that while vehicle alone did not affect S. aureus UAMS-1 growth, both antibiotics, alone and in combination, produced zones of growth inhibition, suggesting that the ointment formulation did not antagonize the antimicrobial properties of either agent. More specifically, 2% mupirocin generated a zone of inhibition of 20 (±2) cm2, whereas 1% neomycin exhibited an average zone of clearance of 9.4 (±1.1) cm2. The combination of 2% mupirocin and 1% neomycin displayed the greatest zone of inhibition (24.3 ± 1 cm2), which was statistically improved over the zone of inhibition caused by mupirocin or neomycin alone. We considered that the improved activity of the combination could be attributed to either the additive effects of the specific antibiotic combination or merely reflect an overall increase in active antimicrobial ingredients. However, similar improvements in antimicrobial clearance were not observed in tests of 2% mupirocin in combination with 1% concentration of kanamycin, vancomycin, erythromycin, or oxacillin. Representative results for vancomycin and erythromycin, which exhibited an antagonistic interaction and no improvement in combination, respectively, toward the strain are shown in Fig. 2D and E. These results indicate that the additive effects of the mupirocin-plus-neomycin combination observed in liquid culture conditions also occur in ointment format.
FIG 2.
Antimicrobial zone of growth inhibition measures. (A to E) The average zones of inhibition (y axis; in square centimeters) of PEG-based ointments containing the indicated antibiotic or antibiotic mixture (x axis) toward S. aureus strain UAMS-1 (A, D, and E), USA300 (B) or BAA-1708 (C) are plotted. Significant increases in growth inhibition zones by Student's t test (n = 4), compared to the growth inhibition zones observed with 2% mupirocin, are indicated by bars and asterisks as follows: *, P ≤ 0.1; **, P ≤ 0.05.
As shown in Fig. 2B, tests of the neomycin-resistant strain USA300 revealed that mupirocin elicited a zone of growth inhibition of 14.0 (± 4) cm2. Interestingly, 1% neomycin ointment produced a small halo-like zone of inhibition (4.3 [± 0.01] cm2) despite the strain's resistance to the agent, indicating that the concentration tested is able to overcome the organism's resistance phenotype to a certain extent. Moreover, the combination treatment showed a significantly increased inhibition zone (24.0 [± 3.4] cm2) compared to the inhibition zone for either agent alone. Testing of the high-level mupirocin-resistant strain BAA-1708 (Fig. 2C) demonstrated that the strain was resistant to 2% mupirocin ointment compared to both UAMS-1 and USA300 but did generate a small zone of growth inhibition (3.6 [± 0.86] cm2). Conversely, 1% neomycin ointment elicited a clear zone of inhibition (4.9 [± 1.1] cm2), which was significantly increased by combination treatment (7.3 [± 0.4] cm2).
Taken together, these results indicate that mupirocin and neomycin are compatible in the ointment format tested here. Further, the combination of 2% mupirocin plus 1% neomycin exhibited increased antimicrobial activity in comparison to either agent alone and displayed activity against all strains irrespective of their resistance profile. From these perspectives, we hypothesized that the combination would be similarly therapeutically beneficial in host environments that mupirocin alone is typically used for the prevention and/or therapeutic intervention of staphylococcal infections.
Effects of mupirocin and neomycin on S. aureus nasal decolonization.
A murine model of S. aureus nasal colonization was used to compare the antimicrobial efficacy of mupirocin, neomycin, and the two agents when applied in combination. To do so, the nasal passages of BALB/c mice were inoculated with ∼1 × 107 CFU of S. aureus and then treated three times a day for a total of 3 days, at which point the bacterial burden was measured and the antibiotic susceptibility of 10 isolates from each animal was measured by MIC testing.
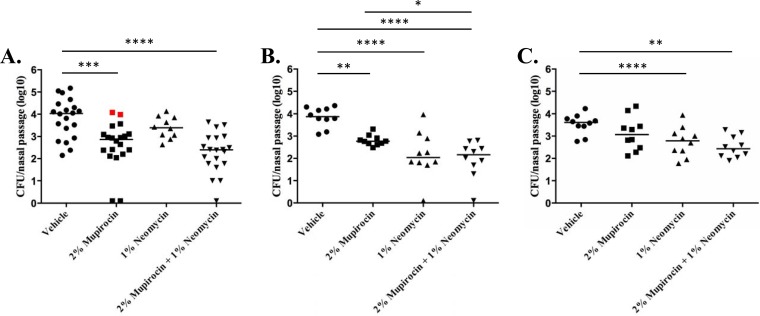
Consistent with previous reports, 2% mupirocin treatment resulted in a 1.1-log-unit reduction in S. aureus strain UAMS-1 nasal colonization (Fig. 3A) (53). However, two mice displayed uncharacteristically high burdens; upon testing, these isolates were found to exhibit an 4-fold increase in mupirocin resistance (MIC of 0.5 μg ml−1) compared to the inoculating strain as well as isolates from the other animals within the treatment group (MIC of 0.125 μg ml−1), suggesting that mupirocin alone selected for low-level resistant derivatives. One percent neomycin treatment displayed a slight, although not statistically significant, 0.5-log-unit reduction in bacterial burden compared to the bacterial burden in animals treated with vehicle alone, whereas combination treatment with 2% mupirocin plus 1% neomycin resulted in the greatest reduction in S. aureus colonization (1.7 log units) and did not appear to select for low-level mupirocin resistance. Similar results were observed for USA300 nasal decolonization (Fig. 3B). More specifically, 2% mupirocin treatment resulted in a 1-log-unit decrease in bacterial burden, whereas treatment with 1% neomycin alone resulted in nearly a 1.8-log-unit reduction in USA300 burden. The combination of mupirocin and neomycin appeared to consistently reduce bacterial burden to the greatest extent (1.7-log-unit reduction). Likewise, combination treatment exhibited increased efficacy toward S. aureus strain BAA-1708, in comparison to each agent alone (Fig. 3C). Despite displaying a high-level mupirocin-resistant phenotype, the strain exhibited a moderate reduction in burden (0.54-log-unit reduction) following mupirocin (alone) treatment, a 0.9-log-unit reduction in 1% neomycin-treated animals and a 1.2-log-unit reduction following combination treatment. The observed improved activity of the combination toward each strain, combined with the notoriously low resolution of the nasal models available (45, 53, 54), prompted us to evaluate the combination's ability to reduce S. aureus wound site colonization.
FIG 3.
Murine nasal decolonization measures. (A to C) The numbers of CFU per mouse nasal passage (y axis) after 3 days of dosing with PEG-based ointment containing the indicated antibiotic or antibiotic mixture (x axis) are plotted. Results for S. aureus strain UAMS-1 (A), USA300 (B), and BAA-1708 (C) are shown; red data points indicate low-level mupirocin-resistant isolates. Each symbol represents the value for an individual mouse. Each horizontal bar is the mean for the group of mice. Significant reductions in bacterial burden compared to the bacterial burden for mice treated with vehicle by one-way ANOVA are indicated by bars and asterisks as follows: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001.
Effects of mupirocin and neomycin on S. aureus wound clearance.
A murine dermal wound model was used to evaluate the decolonization properties of 2% mupirocin, 1% neomycin, and 2% mupirocin plus 1% neomycin. To do so, dermal wounds were created on the backs of BALB/c mice, inoculated with either S. aureus strain UAMS-1, USA300, or BAA-1708, and then treated with test agent suspended in PEG-based ointment twice a day for a total of 3 days, at which point the bacterial burden was determined.
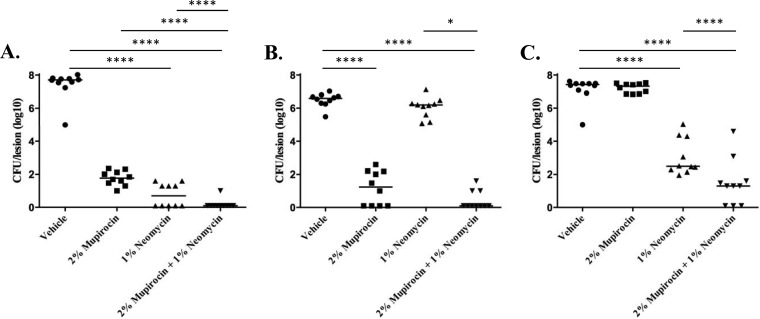
As shown in Fig. 4A, 3-day treatment with 2% mupirocin resulted in an approximately 6-log-unit reduction in S. aureus UAMS-1 colonization (8.7 × 101 CFU per lesion) of the wound site compared to animals that were treated with vehicle alone (4.8 × 107 CFU per lesion). One percent neomycin treatment exhibited improved clearance compared to mupirocin (alone), resulting in 1.4 × 101 CFU per lesion with no bacteria recovered from 5 of the 10 (50%) of the animals within the treatment group. Combination treatment displayed the greatest efficacy. No bacteria were recovered from 9 of the 10 animals (90%) treated with 2% mupirocin plus 1% neomycin, whereas a single UAMS-1 colony was recovered from the remaining animal.
FIG 4.
Murine wound decolonization measures. Shown are the numbers of CFU per lesion (y axis) following 3 days of dosing with PEG-based ointment containing the indicated antibiotic or antibiotic mixture (x axis). Results for S. aureus strains UAMS-1 (A), USA300 (B), and BAA-1708 (C) are shown. Significant reductions in bacterial burden between treatment groups by one-way ANOVA are indicated by bars and asterisks as follows: *, P ≤ 0.05; ****, P ≤ 0.0001.
Testing of the neomycin-resistant strain, USA300, showed that 2% mupirocin was effective, resulting in a 5-log-unit reduction in bacterial wound site burden, with no bacteria recovered from 4 of the 10 (40%) animals in the treatment group (Fig. 4B). As expected, neomycin treatment alone had minimal effects on decolonization, presumably due to the strain's neomycin resistance phenotype, while the greatest efficacy was observed for the group treated with the combination, in which no USA300 cells were recovered from 7 of 10 (70%) of the animals tested. Similarly, the combination of mupirocin and neomycin displayed the greatest efficacy in tests of the mupirocin-resistant strain BAA-1708 (Fig. 4C). More specifically, as expected, 2% mupirocin treatment alone did not reduce wound site colonization compared to vehicle-treated cells, whereas neomycin treatment alone resulted in a 4.9-log-unit decrease in recoverable bacteria. The combination of mupirocin plus neomycin produced the greatest reduction in colonization, resulting in a 6.1-log-unit decrease in wound site bacteria and no recoverable bacteria in 3 of the 10 (30%) animals tested. These results indicate that mupirocin-plus-neomycin ointments are more effective in reducing wound site S. aureus burden than either agent alone and that the combination is capable of overcoming resistance to either agent.
Antimicrobial potential of mupirocin-and-neomycin combination ointment toward other bacterial species.
Mupirocin and neomycin are predominantly active toward Gram-positive and Gram-negative species, respectively. Consequently, we predicted that the combination would display increased spectrum of activity, compared to either agent alone, and could improve treatment options for polyclonal wound site infections composed of mixtures of both Gram-positive and -negative organisms.
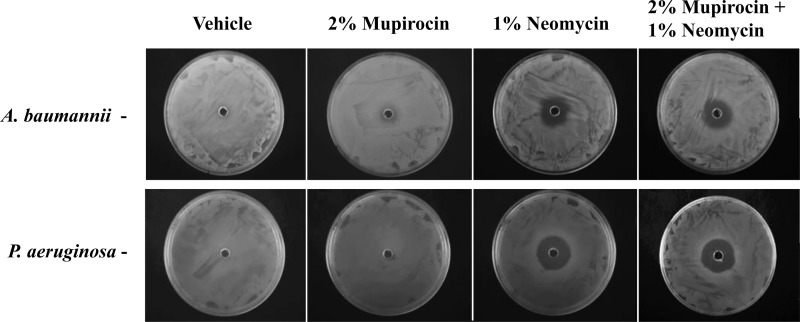
As a preliminary test of that hypothesis, zone of inhibition assays were performed for 2% mupirocin, 1% neomycin, and 2% mupirocin plus 1% neomycin using Acinetobacter baumannii and Pseudomonas aeruginosa, two Gram-negative organisms that are frequent causes of wound site infections. As shown in Fig. 5, 2% mupirocin ointment did not appear to restrict growth of A. baumannii strain 98-37-09 or P. aeruginosa strain PAO1. Conversely, neomycin, both alone and in combination with mupirocin, restricted growth of both organisms, indicating that the combination of 2% mupirocin plus 1% neomycin may be useful in the prevention and/or treatment of complicated wound infections. Both agents, independently and in combination, also limited growth of Staphylococcus epidermidis, Escherichia coli, and Streptococcus pyogenes strains tested (data not shown).
FIG 5.
Antimicrobial effects of PEG-based ointments toward A. baumannii and P. aeruginosa. The antimicrobial effects of PEG ointments containing vehicle, 2% mupirocin, 1% neomycin, and the combination of 2% mupirocin plus 1% neomycin toward A. baumannii strain 98-37-09 or P. aeruginosa strain PAO1 are shown.
Effects of mupirocin and neomycin on wound healing.
The above results indicate that combination ointments comprised of mupirocin and neomycin display improved antimicrobial efficacy, overcome mupirocin resistance, and are likely to exhibit increased spectrum of activity toward other bacterial species compared to mupirocin (alone). Such a combination therapeutic would most likely be of value in the context of the wound setting. In that regard, although both mupirocin and neomycin are FDA-approved antibiotics for topical use, we evaluated whether the mixture of both agents exhibited overt detrimental side effects at the wound site. To do so, dermal wounds were created, and animals were treated with either vehicle, 2% mupirocin, 1% neomycin, or the combination twice daily for a total of 14 days. Each day, animals were assessed for alertness and grooming, weight, and wound size.
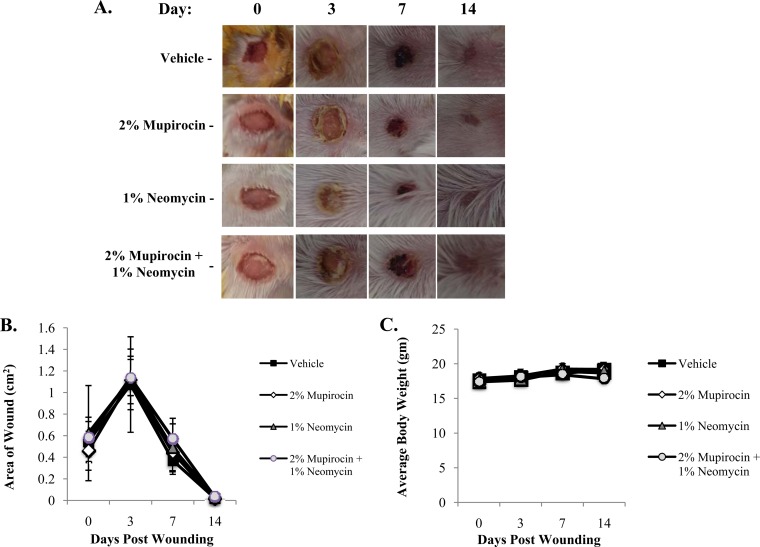
No significant differences in wound contraction were observed for any of the treatment groups (n = 3 for each treatment) compared to vehicle-containing ointment (Fig. 6A and B). Regardless of the ointment used, the size of the wound increased 3 days after lesion formation and was followed by a linear increase in wound contraction, such that the wound healing was completed and hair growth had been restored at 14 days of treatment. Likewise, no significant differences in weight were recorded for any animals in any of the treatment groups (Fig. 6C).
FIG 6.
Effects of PEG-based ointments on wound healing and animal health. (A) Representative wound healing images following 0, 3, 7, and 14 days of treatment with PEG-based ointments containing vehicle, 2% mupirocin, 1% neomycin, or the combination of 2% mupirocin plus 1% neomycin. (B) Average measures (n = 3) of wound contraction under the same conditions as in panel A. (C) Average body weight of animals (y axis) at the indicated day (x axis) after lesion formation and treatment with PEG-based ointment supplemented with the indicated agent.
DISCUSSION
More than 30 million patients undergo surgery in the United States annually, and up to 20% of those patients acquire a postoperative nosocomial infection, resulting in increased rates of morbidity and mortality, systemic antibiotic use, and health care costs of $5 to $10 billion (55, 56). Mupirocin-based ointments (2% mupirocin) have proven successful in the prevention and/or treatment of staphylococcal disease. Indeed, in the United Kingdom, it is recommended that methicillin-resistant S. aureus (MRSA) carriers undergo nasal decolonization with mupirocin as a prophylactic measure prior to surgical intervention (57). However, mupirocin use has predictably selected for resistance that has, in turn, mitigated the agent's efficacy.
The incidence of S. aureus low- and high-level mupirocin resistance within individual health care institutions is highly variable and is presumably influenced by differences in corresponding infection control practices and between the strains circulating at local and regional levels. One retrospective survey of methicillin-resistant S. aureus nasal and blood isolates collected from 23 U.S. hospitals revealed that 3% and 5% of the strains tested displayed high-level mupirocin resistance, respectively (58). However, single-center studies have recorded higher prevalences both in the United States and abroad. For instance, one New York hospital recently reported that 31% of pediatric isolates tested exhibited high-level resistance (59), and in one extreme case, 47% and 79% of community- and hospital-associated MRSA isolates collected from a Korean neonatal intensive care unit exhibited high-level mupirocin resistance (60). Single-center low-level mupirocin resistance rates of 0 to 80% have been recorded in the United States (58). From these perspectives, it is not surprising that recent studies have called into question the advantageous effects of mupirocin ointments, highlighting the need for new approaches for S. aureus decolonization and wound care management.
Drug combinations are a mainstay therapeutic strategy in the treatment of cancer, HIV, asthma, hypercholesterolemia, malaria, and tuberculosis (61). Several current antibiotics represent combination therapeutics, such as sulfonamides and trimethoprim and β-lactam antibiotics in conjunction with β-lactamase inhibitors (62, 63). A central tenet of the combination approach is that the sum of the ingredients is greater than the individual components themselves, and a highly successful strategy for development of multicomponent drugs has been to combine single-compound drugs that already exist; early examples include Advair (fluticasone plus salmeterol), Advicor (niacin plus lovastatin), Combivir (azidothymidine plus lamivudine), and Trizivir (azidothymidine plus lamivudine plus abacavir) (64–66). In that regard, we set out to improve the performance of mupirocin ointment via the addition of an FDA-approved agent with the goal of creating an improved antimicrobial ointment with increased antimicrobial efficacy and capable of overcoming high-level mupirocin resistance.
Numerous studies have made it apparent that the simple addition of two agents does not reliably correlate with improved combined activity. Indeed, that has also been our experience. Screening of an 853-member FDA-approved drug library identified 101 agents that displayed antimicrobial activity against the antibiotic-susceptible test strain UAMS-1. However, only three of those agents, nitazoxanide, nitrofurazone, and the antibiotic neomycin sulfate, were found to exhibit increased antistaphylococcal activity when combined with mupirocin. Of these, neomycin displayed the greatest potency, both alone and in the presence of mupirocin, and is currently available as 0.25% to 4% (wt/vol) ointment for topical antimicrobial use. Thus, we chose to focus effort on characterizing the effects of combinations of mupirocin and neomycin, with the anticipation that they may have the greatest likelihood of having a clinical impact and ease of advancement.
While neomycin is known to bind 16S rRNA and inhibit bacterial protein translation, more-recent studies indicate that it also has off-target effects that may contribute to its antimicrobial activity. In that regard, while other translational inhibitors, including several aminoglycosides, exhibited antimicrobial activity toward the test strain, they did not potentiate the activity of mupirocin. Thus, it seemed reasonable to predict that neomycin's off-target effects contribute to its potentiation of mupirocin. Neomycin binding to the rnpB component of the RNase P holoenzyme interferes with the enzyme's ability to catabolize precursor tRNA processing and consequently generation of mature tRNA substrates for tRNA synthetases, including the primary cellular target of mupirocin, isoleucyl-tRNA synthetase. Consequently, neomycin may limit S. aureus cellular RNase P activity resulting in a limited supply of mature tRNAIle species, thereby requiring less mupirocin to generate an antimicrobial phenotype. As a first test of that prediction, it was found that S. aureus RNase P activity is inhibited by neomycin (250 μM) during in vitro conditions that admittedly may be vastly different than are expected of the enzyme within bacterial cells (buffer conditions and cofactors). Even so, neomycin's RNase P inhibitory activity approximates the concentration required to potentiate mupirocin in liquid format (50 μM) and is well below its potentiating activity in topical format (16 mM), suggesting that the agent's ability to improve mupirocin's antimicrobial effects may be, in part, mediated by the cellular inhibition of RNase P. Further, neomycin did not increase the antimicrobial properties of other antibiotics tested in combination, supporting the notion that the agent's off-target effects may account for its ability to potentiate the antimicrobial activity of mupirocin and that these results are specific to mupirocin.
Combinations of 2% mupirocin and ≥1% neomycin proved to display improved antimicrobial activity in zone of inhibition assays designed to measure the combination's performance in topical format, compared to either agent when tested alone. For that reason, all studies were conducted with 2% mupirocin and/or 1% neomycin. As noted earlier, combinations of 2% mupirocin and 1% of other antibiotics evaluated did not exhibit improved antimicrobial effects or cause an antagonistic effect, suggesting that the improved performance of the combination was specific to neomycin and mimicked their performance in liquid format.
Using a murine nasal colonization model that has admittedly proven highly variable in terms of establishing S. aureus colonization and measuring the performance of antimicrobial agents, such as mupirocin, in the past, we found that the combination of mupirocin plus neomycin displayed greater efficacy than either agent alone. In initial studies designed to measure the model's performance using S. aureus strain UAMS-1, it was found that optimal colonization was achieved using 1 × 107 CFU and when animals were allowed to breathe in and out the inoculum, whereas colonization occurred in ∼70% of the animals challenged with fewer cells and/or animals that were anesthetized at the time of inoculation. Moreover, testing of various dosing regimens showed that optimal mupirocin decolonization was observed following three nasal treatments per day (data not shown), which consequently served as the standard dosing for nasal dosing studies. Complete antimicrobial-associated decolonization was rarely observed and may reflect poor distribution of the test agents throughout the nasal passage. In the model, mupirocin treatment displayed efficacy to various degrees for the three strains evaluated, with greatest decolonization observed for strains UAMS-1 and USA300 and less activity measured for the high-level mupirocin-resistant strain BAA-1708. Presumably, the dosing regimen used may partially override the resistance phenotype of the strain and/or the mupirocin resistance determinant may be only partially expressed during nasal colonization. Similar effects were observed for neomycin (alone) treatment for all strains, including neomycin-resistant USA300. In all cases, the combination of mupirocin and neomycin resulted in the greatest extent of nasal decolonization, and this occurred regardless of the strain used, suggesting that the combination may have greater promise in decolonizing at-risk patient populations than mupirocin (alone) ointments.
Similarly, the combination exhibited pronounced improvement in a murine wound model of S. aureus decolonization compared to either mupirocin or neomycin alone. In this model, twice-a-day mupirocin dosing consistently exhibited efficacy toward the mupirocin-susceptible strains evaluated, thus, each topical formulation was tested twice daily (as opposed to three times daily for nasal decolonization studies). Indeed, while twice-a-day 2% mupirocin treatment dramatically reduced S. aureus strain UAMS-1 and USA300 wound site colonization, the agent lacked efficacy toward the high-level mupirocin-resistant strain tested, mimicking what occurs in the clinical setting. One percent neomycin alone exhibited excellent decolonization activity toward UAMS-1 and BAA 1708 but no significant activity toward the neomycin-resistant strain USA300. The combination nearly eradicated each S. aureus strain tested, with either no measurable viable CFU or a single colony detected in 100% of UAMS-1-inoculated wounds, 90% of USA300-inoculated wounds, and 60% of BAA-1708-inoculated wounds.
Interestingly, as noted above, we observed differing antimicrobial effects of neomycin alone and mupirocin alone toward strains USA300 and BAA-1708, respectively, in the two animal model systems. The application of neomycin three times a day exhibited mild antimicrobial activity toward the neomycin-resistant strain USA300 in the nasal decolonization model but no activity toward the strain in the wound model when applied twice daily. Similarly, mupirocin dosing three times a day was associated with reduction in BAA-1708 nasal colonization but had no effect on wound decolonization. While there are likely to be vast differences between the bacterial physiology and host-pathogen dynamics in these two settings that may account for the observed differences in antibiotic susceptibility, these results could also suggest that more-frequent antibiotic application may allow drug accumulation to an extent that overrides each strain's resistance phenotype and may have corresponding clinical implications. Likewise, it is possible that extended time course or more-frequent dosing may further improve the combination's effects. Wound contraction and overt cytotoxic measures indicate that each agent, when used alone or in combination, is well tolerated over the course of 14 days when applied either twice or three times (not shown) a day to wound sites.
Taken together, the results presented indicate that the topical combinations of mupirocin and neomycin are likely to be superior to currently available mupirocin ointments in terms of promoting S. aureus nasal and wound site decolonization and may be particularly valuable in areas where high-level mupirocin resistance has emerged. Such combination therapies may offer a much needed option for improving S. aureus infection prevention, limiting disease progression and, consequently, systemic antibiotic usage. Further, by virtue of the increased spectrum of activity toward problematic Gram-negative organisms, such as A. baumannii and P. aeruginosa, neomycin-and-mupirocin combinations may provide the option to develop similar strategies for reducing the incidence of these organisms as well as additional options for treatment of polymicrobial infections.
In considering the development of any clinical candidate, including a combination ointment, one must also take into account that resistant isolates can and will emerge (if they do not already exist). In that regard, neither mupirocin or neomycin sulfate is routinely used for systemic treatment purposes, thus corresponding resistance surveillance data are sparse. However, a comprehensive assessment of gentamicin-resistant isolates collected between 1997 and 2002 in the United States revealed that all high-level mupirocin-resistant isolates collected were susceptible to neomycin, indicating that they would be responsive to mupirocin-and-neomycin combination therapeutics (67). The study also indicated that while neomycin resistance was observed frequently (31%) within S. aureus isolates collected, less than 1% of those strains were capable of tolerating 1:100th the level of neomycin present in topical formulations and would thus ostensibly be treatable by mupirocin-plus-neomycin ointments. Moreover, as noted above, neither agent is routinely used for systemic purposes or is associated with cross-resistance to currently used systemic antibiotics. From these perspectives, it is anticipated that combination neomycin-and-mupirocin ointments may hold great promise in the prevention and treatment of currently circulating S. aureus strains and that resistance to the multicomponent mixture will be slow to develop and unlikely to compromise the current antistaphylococcal armament. We also recognize that there will be limitations in the use of such a combination ointment. Indeed, one widely referenced study reported that neomycin-related contact allergy developed in 34% of patients with chronic dermatoses, who were patch tested with 20% neomycin (68). Thus, others have noted that the use of neomycin-containing topical preparations should be avoided or closely monitored for multiallergic individuals but advocated the use of neomycin in the majority of the general population, in whom the incidence of neomycin sensitivity is estimated to be 0.9% (69, 70).
Supplementary Material
ACKNOWLEDGMENTS
We thank Connie Winters and Kun Hyoe Rhoo for their technical support.
Funding Statement
This work was supported, in part, by a University of Rochester Technology Development Award and by National Institute of Allergy and Infectious Diseases (NIAID) grant number AI103507 (P.M.D.). S.D. and L.B. were supported by the University of Rochester Center for AIDS Research grant P30AI078498 and NIH/NIAID grant AI094511. Additionally, this publication was made possible by grant number GM068411-11 from the Institutional Ruth L. Kirschstein National Research Service Award (J.M.C.), the Phileoever Foundation (A.B.), and the Training Program in Oral Sciences T90FR021985 (C.B.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.02083-15.
REFERENCES
- 1.Rice LB. 2008. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J Infect Dis 197:1079–1081. doi: 10.1086/533452. [DOI] [PubMed] [Google Scholar]
- 2.Pendleton JN, Gorman SP, Gilmore BF. 2013. Clinical relevance of the ESKAPE pathogens. Expert Rev Anti Infect Ther 11:297–308. doi: 10.1586/eri.13.12. [DOI] [PubMed] [Google Scholar]
- 3.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. 2007. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA 298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 4.Projan SJ, Shlaes DM. 2004. Antibacterial drug discovery: is it all downhill from here? Clin Microbiol Infect 10(Suppl 4):S18–S22. [DOI] [PubMed] [Google Scholar]
- 5.Kluytmans J, van Belkum A, Verbrugh H. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin Microbiol Rev 10:505–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes J, Mellows G. 1978. On the mode of action of pseudomonic acid: inhibition of protein synthesis in Staphylococcus aureus. J Antibiot 31:330–335. [DOI] [PubMed] [Google Scholar]
- 7.Hughes J, Mellows G. 1978. Inhibition of isoleucyl-transfer ribonucleic acid synthetase in Escherichia coli by pseudomonic acid. Biochem J 176:305–318. doi: 10.1042/bj1760305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes J, Mellows G. 1980. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem J 191:209–219. doi: 10.1042/bj1910209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. 1985. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother 27:495–498. doi: 10.1128/AAC.27.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beale AS, Gisby J, Sutherland R. 1989. Efficacy of mupirocin calcium ointment in the treatment of experimental wound infections caused by methicillin-resistant strains of Staphylococcus aureus. J Chemother 1:397–398. [PubMed] [Google Scholar]
- 11.Moy JA, Caldwell-Brown D, Lin AN, Pappa KA, Carter DM. 1990. Mupirocin-resistant Staphylococcus aureus after long-term treatment of patients with epidermolysis bullosa. J Am Acad Dermatol 22:893–895. doi: 10.1016/0190-9622(90)70120-7. [DOI] [PubMed] [Google Scholar]
- 12.Rode H, de Wet PM, Millar AJ, Cywes S. 1988. Bactericidal efficacy of mupirocin in multi-antibiotic resistant Staphylococcus aureus burn wound infection. J Antimicrob Chemother 21:589–595. doi: 10.1093/jac/21.5.589. [DOI] [PubMed] [Google Scholar]
- 13.Rode H, Hanslo D, de Wet PM, Millar AJ, Cywes S. 1989. Efficacy of mupirocin in methicillin-resistant Staphylococcus aureus burn wound infection. Antimicrob Agents Chemother 33:1358–1361. doi: 10.1128/AAC.33.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coates T, Bax R, Coates A. 2009. Nasal decolonization of Staphylococcus aureus with mupirocin: strengths, weaknesses and future prospects. J Antimicrob Chemother 64:9–15. doi: 10.1093/jac/dkp159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mupirocin Study Group. 1996. Nasal mupirocin prevents Staphylococcus aureus exit-site infection during peritoneal dialysis. J Am Soc Nephrol 7:2403–2408. [DOI] [PubMed] [Google Scholar]
- 16.Gaspar MC, Uribe P, Sanchez P, Coello R, Cruzet F. 1992. Hospital personnel who are nasal carriers of methicillin-resistant Staphylococcus aureus. Usefulness of treatment with mupirocin. Enferm Infecc Microbiol Clin 10:107–110. (In Spanish.) [PubMed] [Google Scholar]
- 17.Gernaat-van der Sluis AJ, Hoogenboom-Verdegaal AM, Edixhoven PJ, Spies-van Rooijen NH. 1998. Prophylactic mupirocin could reduce orthopedic wound infections. 1,044 patients treated with mupirocin compared with 1,260 historical controls. Acta Orthop Scand 69:412–414. doi: 10.3109/17453679808999058. [DOI] [PubMed] [Google Scholar]
- 18.Kluytmans JA, Mouton JW, VandenBergh MF, Manders MJ, Maat AP, Wagenvoort JH, Michel MF, Verbrugh HA. 1996. Reduction of surgical-site infections in cardiothoracic surgery by elimination of nasal carriage of Staphylococcus aureus. Infect Control Hosp Epidemiol 17:780–785. doi: 10.2307/30141170. [DOI] [PubMed] [Google Scholar]
- 19.Mackie DP, van Hertum WA, Schumburg TH, Kuijper EC, Knape P, Massaro F. 1994. Reduction in Staphylococcus aureus wound colonization using nasal mupirocin and selective decontamination of the digestive tract in extensive burns. Burns 20(Suppl 1):S14–S18. doi: 10.1016/0305-4179(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 20.Talon D, Rouget C, Cailleaux V, Bailly P, Thouverez M, Barale F, Michel-Briand Y. 1995. Nasal carriage of Staphylococcus aureus and cross-contamination in a surgical intensive care unit: efficacy of mupirocin ointment. J Hosp Infect 30:39–49. [DOI] [PubMed] [Google Scholar]
- 21.Wenisch C, Laferl H, Szell M, Smolle KH, Grisold A, Bertha G, Krause R. 2006. A holistic approach to MRSA eradication in critically ill patients with MRSA pneumonia. Infection 34:148–154. doi: 10.1007/s15010-006-5107-7. [DOI] [PubMed] [Google Scholar]
- 22.McCann M, Moore ZE. 2010. Interventions for preventing infectious complications in haemodialysis patients with central venous catheters. Cochrane Database Syst Rev 2010:CD006894. doi: 10.1002/14651858.CD006894.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Walsh EE, Greene L, Kirshner R. 2011. Sustained reduction in methicillin-resistant Staphylococcus aureus wound infections after cardiothoracic surgery. Arch Intern Med 171:68–73. doi: 10.1001/archinternmed.2010.326. [DOI] [PubMed] [Google Scholar]
- 24.Hood R, Shermock KM, Emerman C. 2004. A prospective, randomized pilot evaluation of topical triple antibiotic versus mupirocin for the prevention of uncomplicated soft tissue wound infection. Am J Emerg Med 22:1–3. [DOI] [PubMed] [Google Scholar]
- 25.Furukawa M, Minekawa A, Haruyama T, Narui Y, Sugita G, Sugita R, Kusunoki T, Ikeda K. 2008. Clinical effectiveness of ototopical application of mupirocin ointment in methicillin-resistant Staphylococcus aureus otorrhea. Otol Neurotol 29:676–678. doi: 10.1097/MAO.0b013e31817ef4b7. [DOI] [PubMed] [Google Scholar]
- 26.Bass JW, Chan DS, Creamer KM, Thompson MW, Malone FJ, Becker TM, Marks SN. 1997. Comparison of oral cephalexin, topical mupirocin and topical bacitracin for treatment of impetigo. Pediatr Infect Dis J 16:708–710. doi: 10.1097/00006454-199707000-00013. [DOI] [PubMed] [Google Scholar]
- 27.Gong JQ, Lin L, Lin T, Hao F, Zeng FQ, Bi ZG, Yi D, Zhao B. 2006. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol 155:680–687. [DOI] [PubMed] [Google Scholar]
- 28.Lee AS, Gizard Y, Empel J, Bonetti EJ, Harbarth S, Francois P. 2014. Mupirocin-induced mutations in ileS in various genetic backgrounds of methicillin-resistant Staphylococcus aureus. J Clin Microbiol 52:3749–3754. doi: 10.1128/JCM.01010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fierobe L, Decre D, Muller C, Lucet JC, Marmuse JP, Mantz J, Desmonts JM. 1999. Methicillin-resistant Staphylococcus aureus as a causative agent of postoperative intra-abdominal infection: relation to nasal colonization. Clin Infect Dis 29:1231–1238. doi: 10.1086/313454. [DOI] [PubMed] [Google Scholar]
- 30.Seah C, Alexander DC, Louie L, Simor A, Low DE, Longtin J, Melano RG. 2012. MupB, a new high-level mupirocin resistance mechanism in Staphylococcus aureus. Antimicrob Agents Chemother 56:1916–1920. doi: 10.1128/AAC.05325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker ES, Vasquez JE, Dula R, Bullock H, Sarubbi FA. 2003. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus: does mupirocin remain effective? Infect Control Hosp Epidemiol 24:342–346. doi: 10.1086/502218. [DOI] [PubMed] [Google Scholar]
- 32.Coates A, Hu Y, Bax R, Page C. 2002. The future challenges facing the development of new antimicrobial drugs. Nat Rev Drug Discov 1:895–910. doi: 10.1038/nrd940. [DOI] [PubMed] [Google Scholar]
- 33.Eidem TM, Lounsbury N, Emery JF, Bulger J, Smith A, Abou-Gharbia M, Childers W, Dunman PM. 2015. Small-molecule inhibitors of Staphylococcus aureus RnpA-mediated RNA turnover and tRNA processing. Antimicrob Agents Chemother 59:2016–2028. doi: 10.1128/AAC.04352-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spitzfaden C, Nicholson N, Jones JJ, Guth S, Lehr R, Prescott CD, Hegg LA, Eggleston DS. 2000. The structure of ribonuclease P protein from Staphylococcus aureus reveals a unique binding site for single-stranded RNA. J Mol Biol 295:105–115. doi: 10.1006/jmbi.1999.3341. [DOI] [PubMed] [Google Scholar]
- 35.Buck AH, Dalby AB, Poole AW, Kazantsev AV, Pace NR. 2005. Protein activation of a ribozyme: the role of bacterial RNase P protein. EMBO J 24:3360–3368. doi: 10.1038/sj.emboj.7600805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guerrier-Takada C, Altman S. 1984. Catalytic activity of an RNA molecule prepared by transcription in vitro. Science 223:285–286. doi: 10.1126/science.6199841. [DOI] [PubMed] [Google Scholar]
- 37.Guerrier-Takada C, Gardiner K, Marsh T, Pace N, Altman S. 1983. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- 38.Kazantsev AV, Pace NR. 2006. Bacterial RNase P: a new view of an ancient enzyme. Nat Rev Microbiol 4:729–740. doi: 10.1038/nrmicro1491. [DOI] [PubMed] [Google Scholar]
- 39.Niranjanakumari S, Kurz JC, Fierke CA. 1998. Expression, purification and characterization of the recombinant ribonuclease P protein component from Bacillus subtilis. Nucleic Acids Res 26:3090–3096. doi: 10.1093/nar/26.13.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter VR, Simonson H. 1951. Sequential blocking of metabolic pathways in vivo. Proc Soc Exp Biol Med 76:41–46. doi: 10.3181/00379727-76-18383. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen NE, Brannvall M, Virtanen A, Kirsebom LA. 1999. Inhibition of RNase P RNA cleavage by aminoglycosides. Proc Natl Acad Sci U S A 96:6155–6160. doi: 10.1073/pnas.96.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63:3373–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J Clin Microbiol 41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 45.Kiser KB, Cantey-Kiser JM, Lee JC. 1999. Development and characterization of a Staphylococcus aureus nasal colonization model in mice. Infect Immun 67:5001–5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guthrie KM, Agarwal A, Tackes DS, Johnson KW, Abbott NL, Murphy CJ, Czuprynski CJ, Kierski PR, Schurr MJ, McAnulty JF. 2012. Antibacterial efficacy of silver-impregnated polyelectrolyte multilayers immobilized on a biological dressing in a murine wound infection model. Ann Surg 256:371–377. doi: 10.1097/SLA.0b013e318256ff99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amegbor K, Metowogo K, Eklu-Gadegbeku K, Agbonon A, Aklikokou KA, Napo-Koura G, Gbeassor M. 2012. Preliminary evaluation of the wound healing effect of Vitex doniana sweet (Verbenaceae) in mice. Afr J Tradit Complement Altern Med 9:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mikkelsen NE, Johansson K, Virtanen A, Kirsebom LA. 2001. Aminoglycoside binding displaces a divalent metal ion in a tRNA-neomycin B complex. Nat Struct Biol 8:510–514. doi: 10.1038/88569. [DOI] [PubMed] [Google Scholar]
- 49.Tok JB, Cho J, Rando RR. 1999. Aminoglycoside antibiotics are able to specifically bind the 5′-untranslated region of thymidylate synthase messenger RNA. Biochemistry 38:199–206. doi: 10.1021/bi9819428. [DOI] [PubMed] [Google Scholar]
- 50.von Ahsen U, Davies J, Schroeder R. 1992. Non-competitive inhibition of group I intron RNA self-splicing by aminoglycoside antibiotics. J Mol Biol 226:935–941. doi: 10.1016/0022-2836(92)91043-O. [DOI] [PubMed] [Google Scholar]
- 51.Eubank TD, Biswas R, Jovanovic M, Litovchick A, Lapidot A, Gopalan V. 2002. Inhibition of bacterial RNase P by aminoglycoside-arginine conjugates. FEBS Lett 511:107–112. doi: 10.1016/S0014-5793(01)03322-1. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Chen Y, Fierke CA. 2014. A real-time fluorescence polarization activity assay to screen for inhibitors of bacterial ribonuclease P. Nucleic Acids Res 42:e159. doi: 10.1093/nar/gku850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rouse MS, Rotger M, Piper KE, Steckelberg JM, Scholz M, Andrews J, Patel R. 2005. In vitro and in vivo evaluations of the activities of lauric acid monoester formulations against Staphylococcus aureus. Antimicrob Agents Chemother 49:3187–3191. doi: 10.1128/AAC.49.8.3187-3191.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kokai-Kun JF, Walsh SM, Chanturiya T, Mond JJ. 2003. Lysostaphin cream eradicates Staphylococcus aureus nasal colonization in a cotton rat model. Antimicrob Agents Chemother 47:1589–1597. doi: 10.1128/AAC.47.5.1589-1597.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E. 2005. The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 165:1756–1761. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 56.van Rijen MM, Bonten M, Wenzel RP, Kluytmans JA. 2008. Intranasal mupirocin for reduction of Staphylococcus aureus infections in surgical patients with nasal carriage: a systematic review. J Antimicrob Chemother 61:254–261. [DOI] [PubMed] [Google Scholar]
- 57.Coia JE, Duckworth GJ, Edwards DI, Farrington M, Fry C, Humphreys H, Mallaghan C, Tucker DR, Joint Working Party of the British Society of Antimicrobial Chemotherapy, the Hospital Infection Society, and the Infection Control Nurses Association . 2006. Guidelines for the control and prevention of meticillin-resistant Staphylococcus aureus (MRSA) in healthcare facilities. J Hosp Infect 63(Suppl 1):S1–S44. [DOI] [PubMed] [Google Scholar]
- 58.Hetem DJ, Bonten MJ. 2013. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect 85:249–256. doi: 10.1016/j.jhin.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 59.Antonov NK, Garzon MC, Morel KD, Whittier S, Planet PJ, Lauren CT. 2015. High prevalence of mupirocin resistance in Staphylococcus aureus isolates from a pediatric population. Antimicrob Agents Chemother 59:3350–3356. doi: 10.1128/AAC.00079-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park SH, Kim SY, Lee JH, Park C, Lee DG. 2013. Community-genotype strains of methicillin-resistant Staphylococcus aureus with high-level mupirocin resistance in a neonatal intensive care unit. Early Hum Dev 89:661–665. doi: 10.1016/j.earlhumdev.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 61.Keith CT, Borisy AA, Stockwell BR. 2005. Multicomponent therapeutics for networked systems. Nat Rev Drug Discov 4:71–78. doi: 10.1038/nrd1609. [DOI] [PubMed] [Google Scholar]
- 62.Stein GE, Gurwith MJ. 1984. Amoxicillin-potassium clavulanate, a beta-lactamase-resistant antibiotic combination. Clin Pharm 3:591–599. [PubMed] [Google Scholar]
- 63.Csonka GW, Knight GJ. 1967. Therapeutic trial of trimethoprim as a potentiator of sulphonamides in gonorrhoea. Br J Vener Dis 43:161–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kavuru M, Melamed J, Gross G, Laforce C, House K, Prillaman B, Baitinger L, Woodring A, Shah T. 2000. Salmeterol and fluticasone propionate combined in a new powder inhalation device for the treatment of asthma: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 105:1108–1116. [DOI] [PubMed] [Google Scholar]
- 65.Bays HE, Dujovne CA, McGovern ME, White TE, Kashyap ML, Hutcheson AG, Crouse JR. 2003. Comparison of once-daily, niacin extended-release/lovastatin with standard doses of atorvastatin and simvastatin (the ADvicor Versus Other Cholesterol-Modulating Agents Trial Evaluation [ADVOCATE]). Am J Cardiol 91:667–672. [DOI] [PubMed] [Google Scholar]
- 66.Larder BA, Kemp SD, Harrigan PR. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696–699. doi: 10.1126/science.7542804. [DOI] [PubMed] [Google Scholar]
- 67.Jones RN, Li Q, Kohut B, Biedenbach DJ, Bell J, Turnidge JD. 2006. Contemporary antimicrobial activity of triple antibiotic ointment: a multiphased study of recent clinical isolates in the United States and Australia. Diagn Microbiol Infect Dis 54:63–71. doi: 10.1016/j.diagmicrobio.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 68.Fraki JE, Peltonen L, Hopsu-Havu VK. 1979. Allergy to various components of topical preparations in stasis dermatitis and leg ulcer. Contact Dermatitis 5:97–100. doi: 10.1111/j.1600-0536.1979.tb04806.x. [DOI] [PubMed] [Google Scholar]
- 69.Bonomo RA, Van Zile PS, Li Q, Shermock KM, McCormick WG, Kohut B. 2007. Topical triple-antibiotic ointment as a novel therapeutic choice in wound management and infection prevention: a practical perspective. Expert Rev Anti Infect Ther 5:773–782. doi: 10.1586/14787210.5.5.773. [DOI] [PubMed] [Google Scholar]
- 70.Epstein E. 1966. Allergy to dermatologic agents. JAMA 198:517–520. doi: 10.1001/jama.1966.03110180061019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.