Abstract
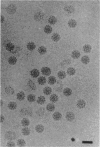
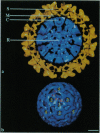
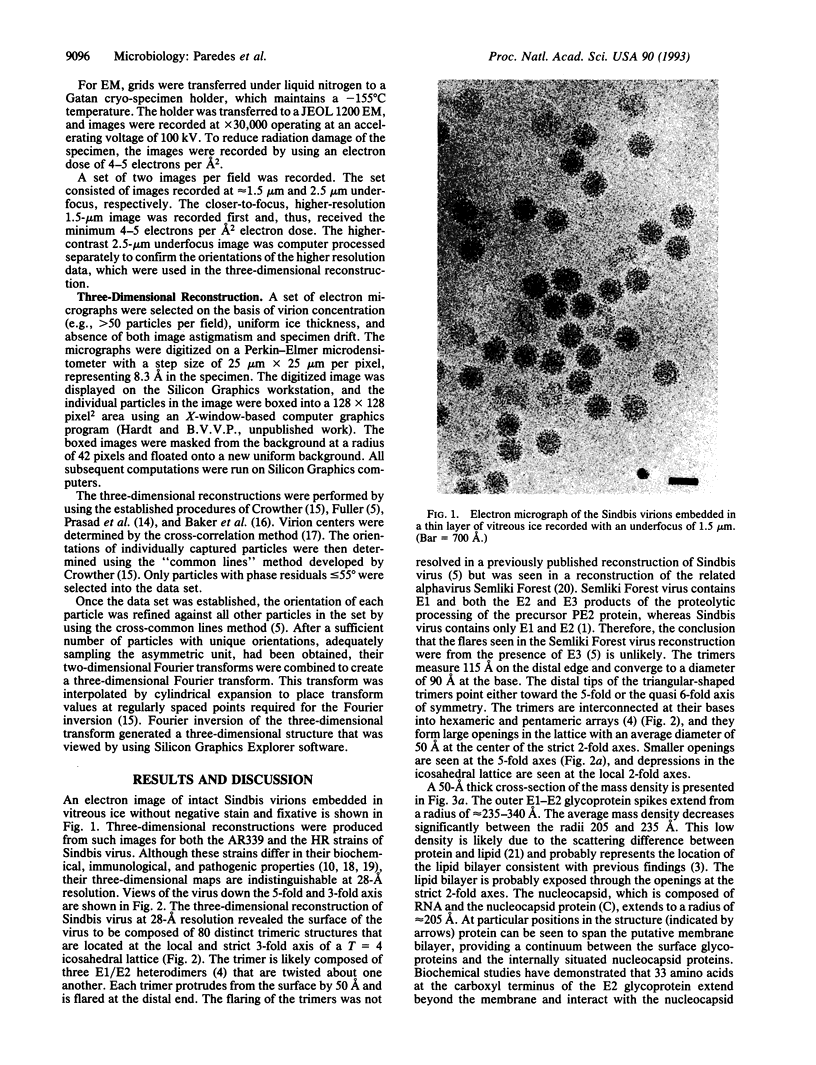
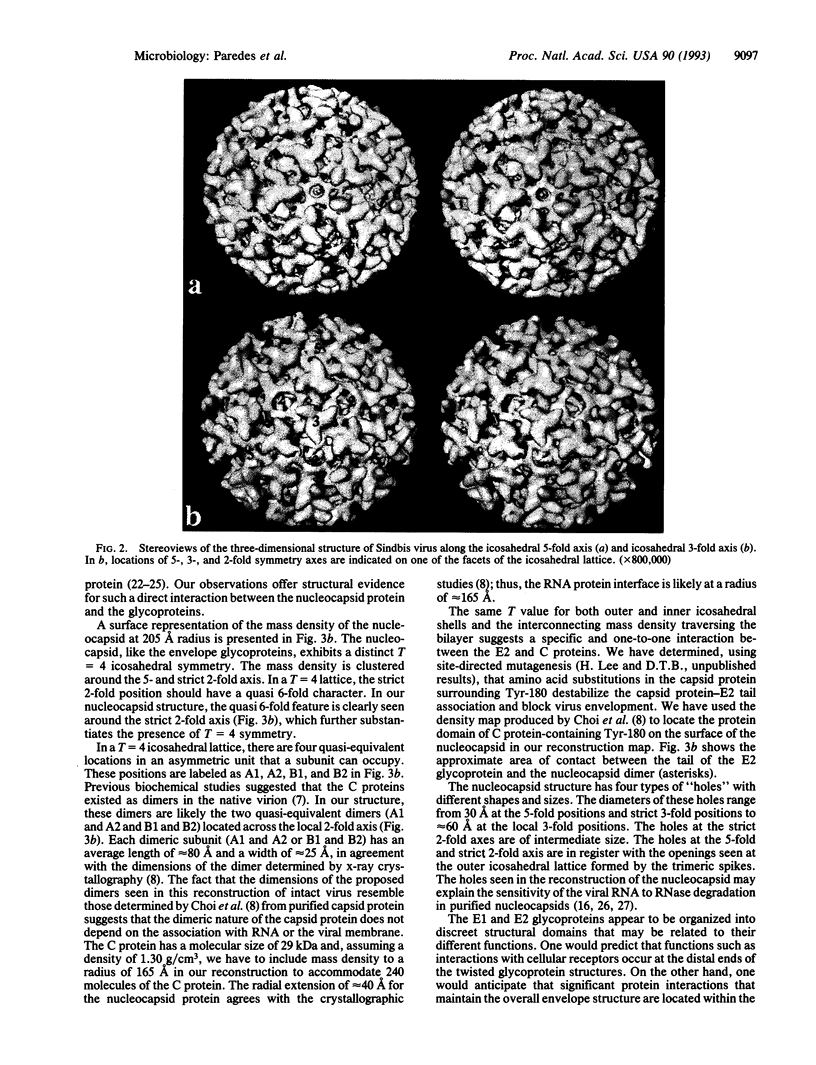
The structure of Sindbis virus was determined by electron cryomicroscopy. The virion contains two icosahedral shells of viral-encoded proteins separated by a membrane bilayer of cellular origin. The three-dimensional structure of the ice-embedded intact Sindbis virus, reconstructed from electron images, unambiguously shows that proteins in both shells are arranged with the same icosahedral lattice of triangulation number T = 4. These studies also provide structural evidence of contact between the glycoprotein and the nucleocapsid protein across the membrane bilayer. The structural organization of Sindbis virus has profound implications for the morphogenesis of the alphaviruses. The observed interactions confirm stoichiometric and specific protein associations that may be crucial for virion stability and predict a mechanism for assembly.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abell B. A., Brown D. T. Sindbis virus membrane fusion is mediated by reduction of glycoprotein disulfide bridges at the cell surface. J Virol. 1993 Sep;67(9):5496–5501. doi: 10.1128/jvi.67.9.5496-5501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian M., Dubochet J., Lepault J., McDowall A. W. Cryo-electron microscopy of viruses. Nature. 1984 Mar 1;308(5954):32–36. doi: 10.1038/308032a0. [DOI] [PubMed] [Google Scholar]
- Anthony R. P., Brown D. T. Protein-protein interactions in an alphavirus membrane. J Virol. 1991 Mar;65(3):1187–1194. doi: 10.1128/jvi.65.3.1187-1194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. P., Paredes A. M., Brown D. T. Disulfide bonds are essential for the stability of the Sindbis virus envelope. Virology. 1992 Sep;190(1):330–336. doi: 10.1016/0042-6822(92)91219-k. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggs W. M., Hahn C. S., Strauss E. G., Strauss J. H., Griffin D. E. Low pH-dependent Sindbis virus-induced fusion of BHK cells: differences between strains correlate with amino acid changes in the E1 glycoprotein. Virology. 1989 Apr;169(2):485–488. doi: 10.1016/0042-6822(89)90178-5. [DOI] [PubMed] [Google Scholar]
- Choi H. K., Tong L., Minor W., Dumas P., Boege U., Rossmann M. G., Wengler G. Structure of Sindbis virus core protein reveals a chymotrypsin-like serine proteinase and the organization of the virion. Nature. 1991 Nov 7;354(6348):37–43. doi: 10.1038/354037a0. [DOI] [PubMed] [Google Scholar]
- Coombs K. M., Brown D. T. Form-determining functions in Sindbis virus nucleocapsids: nucleosomelike organization of the nucleocapsid. J Virol. 1989 Feb;63(2):883–891. doi: 10.1128/jvi.63.2.883-891.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs K., Brown B., Brown D. T. Evidence for a change in capsid morphology during Sindbis virus envelopment. Virus Res. 1984;1(4):297–302. doi: 10.1016/0168-1702(84)90018-2. [DOI] [PubMed] [Google Scholar]
- Coombs K., Brown D. T. Organization of the Sindbis virus nucleocapsid as revealed by bifunctional cross-linking agents. J Mol Biol. 1987 May 20;195(2):359–371. doi: 10.1016/0022-2836(87)90657-7. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Adrian M., Chang J. J., Homo J. C., Lepault J., McDowall A. W., Schultz P. Cryo-electron microscopy of vitrified specimens. Q Rev Biophys. 1988 May;21(2):129–228. doi: 10.1017/s0033583500004297. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Hahn C. S., Strauss J. H. Site-directed mutagenesis of the proposed catalytic amino acids of the Sindbis virus capsid protein autoprotease. J Virol. 1990 Jun;64(6):3069–3073. doi: 10.1128/jvi.64.6.3069-3073.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Brown D. T. Transient translocation of the cytoplasmic (endo) domain of a type I membrane glycoprotein into cellular membranes. J Cell Biol. 1993 Feb;120(4):877–883. doi: 10.1083/jcb.120.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Garoff H. Oligomers of the cytoplasmic domain of the p62/E2 membrane protein of Semliki Forest virus bind to the nucleocapsid in vitro. J Virol. 1990 Oct;64(10):4678–4683. doi: 10.1128/jvi.64.10.4678-4683.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer W. J., Gidwitz S., Ayers V. K., Schoepp R. J., Johnston R. E. Conformational alteration of Sindbis virion glycoproteins induced by heat, reducing agents, or low pH. J Virol. 1992 Jun;66(6):3504–3513. doi: 10.1128/jvi.66.6.3504-3513.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paredes A. M., Simon M. N., Brown D. T. The mass of the Sindbis virus nucleocapsid suggests it has T = 4 icosahedral symmetry. Virology. 1992 Mar;187(1):329–332. doi: 10.1016/0042-6822(92)90322-g. [DOI] [PubMed] [Google Scholar]
- Polo J. M., Davis N. L., Rice C. M., Huang H. V., Johnston R. E. Molecular analysis of Sindbis virus pathogenesis in neonatal mice by using virus recombinants constructed in vitro. J Virol. 1988 Jun;62(6):2124–2133. doi: 10.1128/jvi.62.6.2124-2133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B. V., Prevelige P. E., Marietta E., Chen R. O., Thomas D., King J., Chiu W. Three-dimensional transformation of capsids associated with genome packaging in a bacterial virus. J Mol Biol. 1993 May 5;231(1):65–74. doi: 10.1006/jmbi.1993.1257. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheefers H., Scheefers-Borchel U., Edwards J., Brown D. T. Distribution of virus structural proteins and protein-protein interactions in plasma membrane of baby hamster kidney cells infected with Sindbis or vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7277–7281. doi: 10.1073/pnas.77.12.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrag J. D., Prasad B. V., Rixon F. J., Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989 Feb 24;56(4):651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Unwin N. Nicotinic acetylcholine receptor at 9 A resolution. J Mol Biol. 1993 Feb 20;229(4):1101–1124. doi: 10.1006/jmbi.1993.1107. [DOI] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]
- Wahlberg J. M., Boere W. A., Garoff H. The heterodimeric association between the membrane proteins of Semliki Forest virus changes its sensitivity to low pH during virus maturation. J Virol. 1989 Dec;63(12):4991–4997. doi: 10.1128/jvi.63.12.4991-4997.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Sindbis virus glycoproteins form a regular icosahedral surface lattice. J Virol. 1975 Jul;16(1):141–145. doi: 10.1128/jvi.16.1.141-145.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]