Abstract
Background
The accurate evaluation of favorable response to neoadjuvant chemotherapy (NCT) is critical to determine the extent of surgery. We investigated independent clinicopathological and radiological predictors to discriminate no residual carcinoma (ypT0) from residual ductal carcinoma in situ (ypTis) in breast cancer patients who received NCT.
Patients and Methods
Parameters of 117 patients attaining pathological complete response (CR) in the breast after NCT between January 2010 and December 2013 were retrospectively evaluated by univariate and multivariate analyses. All patients underwent mammography, ultrasound, and magnetic resonance imaging (MRI) before and after NCT.
Results
There were 67 (57.3%) patients with ypT0. These patients were associated with hormone receptor-negative status, human epidermal growth factor receptor-2 (HER2)-negative tumors, and a higher likelihood of breast-conservation surgery. Baseline mammographic and MRI presentation of the main lesion, absence of associated microcalcifications, shape, posterior features, and absence of calcifications on ultrasound were significantly associated with ypT0. CR in mammography, ultrasound, or MRI after NCT was also related to ypT0. By multivariate analysis, independent predictors of ypT0 were the triple-negative subtype [Odds ratio (OR), 4.23; 95% confidence interval (CI), 1.11–16.09] and CR in MRI after NCT (OR, 5.23; 95% CI, 1.53–17.85). Stratified analysis by breast cancer subtype demonstrated that MRI well predicted ypT0 in all subtypes except the HER2-positive subtype. In particular, of 40 triple-negative subtypes, 22 showed CR in MRI and 21 (95.5%) were ypT0 after NCT.
Conclusion
Among imaging modalities, breast MRI can potentially distinguish between ypT0 and ypTis after NCT, especially in patients with triple-negative breast cancer. This information can help clinicians evaluate tumor response to NCT and plan surgery for breast cancer patients of all subtypes except for those with HER2-enriched tumors after NCT.
Introduction
Neoadjuvant chemotherapy (NCT) is now commonly considered for breast cancer patients who are potential candidates for adjuvant chemotherapy and it has been reported to have similar oncologic outcomes to adjuvant chemotherapy [1]. In addition, NCT increases the chances of successful breast-conservation surgery, facilitates tumor biology research, and most importantly, provides information about prognosis [1–3]. For these advantages to be of use in real clinical practice, accurate evaluation of response during NCT and preoperative assessment of residual tumor burden through imaging modalities are critical for planning the extent of surgery and for predicting prognosis. Recently, a meta-analysis suggested that breast magnetic resonance imaging (MRI) showed good performance in predicting pathologic complete response (pCR) after NCT [4].
Residual ductal carcinoma in situ (DCIS) components of breast cancer after NCT are considered as pCR; however, surgery is differently planned if these components are of no residual invasive and in situ carcinoma (ypT0). Obtaining clear resection margins with accurate preoperative evaluation helps decrease operation time and reduces the chances of repeating surgery or early local recurrence. Chen et al. [5] demonstrated that positive cavity margin was the only independent predictor for local-regional failure in patients treated with NCT before breast-conservation surgery according to univariate and multivariate analysis. Most clinicians usually plan the extent of surgery to achieve negative resections based on radiological examinations and clinicopathological parameters. However, it has not been established which parameters should have higher priority in daily practice.
In our review of previous literatures, there was only one article that dealt with discriminating ypT0 from residual DCIS in the breast after NCT [6]. In that study, the dynamic contrast-enhanced MRI was reported to show good performance for distinguishing between lesions with or without residual DCIS in breast cancer patients who demonstrated no residual invasive cancer after NCT [6]. However, the study sample was limited, including only 15 cases of residual in situ carcinoma. It is therefore difficult to generalize their results to other samples, or to analyze clinicopathological factors such as breast cancer phenotype, Ki-67 levels, or the use of human epidermal growth factor receptor-2 (HER2) targeted therapy [7,8]. Thus, more comprehensive studies are necessary to determine the potential of MRI alongside future analyses of clinicopathological findings of breast cancer patients who receive NCT.
The aim of this study was to investigate independent clinicopathological and radiological characteristics, including breast cancer subtypes, in order to discriminate between ypT0 and residual DCIS alone (ypTis) on final pathology in breast cancer patients who responded well to NCT.
Patients and Methods
Patient selection
A total of 163 patients who achieved pCR in the breast after receiving NCT and who subsequently underwent definitive surgery of the breast and axilla from January 2010 to December 2013 at the Severance Hospital of Yonsei University College of Medicine, Seoul, Republic of Korea were retrospectively selected. All patients in the study cohort were histologically confirmed to have primary invasive breast carcinoma at initial presentation. After therapeutic surgery, permanent pathologic findings of the breast for all patients were reported as no residual invasive and in situ carcinoma (ypT0) or residual in situ carcinoma alone (ypTis), irrespective of pathologic nodal stage (ypNany). Forty-six (28.2%) patients who did not undergo mammography, ultrasound, and breast MRI both prior to and after NCT were excluded from analysis. Therefore, 117 patients were finally included in our study.
NCT regimens were mainly composed of 4 cycles of anthracycline plus cyclophosphamide (AC) followed by 4 cycles of docetaxel (T) every 3 weeks in 91 (77.8%) patients. Twelve (10.3%) patients received AC followed by T plus TS-1. Of the remaining 14 (12.0%) patients, 8 were treated with 6 cycles of T plus carboplatinum with bevacizumab and 2 received paclitaxel plus carboplatinum. Each patient went through one of four regimens: four cycles of AC, 6 cycles of TAC, T plus carboplatinum with trastzumab, or paclitaxel plus trastzumab. This study was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System, Seoul, Republic of Korea (IRB No. 4-2015-0247). The requirement for written informed consent was waived and patient information was anonymized and de-identified prior to analysis.
Clinicopathological characteristics
Clinicopathological information, including expression of the estrogen receptor (ER), progesterone receptor (PR), HER2, and Ki-67, was obtained through reviews of medical records and pathology reports. Tumors with ≥1% nuclear-stained cells by immunohistochemistry of core needle biopsy specimens prior to NCT were considered positive for hormone receptors (HRs) according to the American Society of Clinical Oncology/College of American Pathologists (ASCO/CAP) guidelines [9]. HER2 staining was scored as 0, 1+, 2+, or 3+ according to ASCO/CAP guidelines [10]. In cases with HER2 2+ results, fluorescence in situ hybridization (FISH) was performed using a PathVysion HER2 DNA Probe Kit (Vysis, Downers Grove, IL, USA) and HER2 gene amplification was defined with a HER2 gene/chromosome 17 copy number ratio ≥2.0 according to ASCO/CAP guidelines [10]. HER2 was considered positive with immunohistochemistry scores of 3+ or gene amplification by FISH. Ki-67 levels were scored by counting the number of positively stained nuclei and were expressed as a percentage of total tumor cells.
Breast cancer subtypes were categorized by HRs and HER2 expression as follows: HRs+/HER2-, ER-positive or PR-positive, and HER2-negative; HRs+/HER2+, ER-positive or PR-positive, and HER2-positive; HRs-/HER2+, ER-negative, PR-negative, and HER2-positive; HRs-/HER2-, ER-negative, PR-negative, and HER2-negative, a subtype also known as triple-negative breast cancer.
Interpretation and analysis of imaging study
A radiologist (MJK) with more than 10 years of experience specializing in breast imaging interpreted mammography, ultrasound, and MRI images before and after NCT while blinded to clinicopathological information. Data on mammographic factors were collected by reviewing mammography before and after NCT and the mammographic factors reviewed were as follows. For mammography before NCT, tumor size (the largest diameter on mammography), the extent of the tumor [single and multiple: the presence of two of more tumor foci within a single quadrant of the breast (multifocal) or within different quadrants of the same breast (multicentric)], the presentation pattern of the main lesion (mass alone, the presence of microcalcifications regardless of mass, and non-visualization on mammography), the presence of associated microcalcifications for the main lesion, and other imaging characteristics of the main lesion (shape, margin, and density) and breast parenchymal patterns classified with the Breast Imaging Reporting and Data System (BI-RADS) by the American College of Radiology were studied [11]. For mammography after NCT, the mammographic factors studied for the presentation of the main lesion were as follows; complete response including cases undetected on mammography before NCT and residual mass or microcalcifications.
Ultrasonographic factors were reviewed and categorized as follows: For ultrasound before NCT, the tumor size was defined as the largest diameter on ultrasound and imaging findings of the main lesion were classified with BI-RADS [11]. Complete response or residual disease after NCT was also determined for ultrasound.
MRIs were reviewed before and after NCT and tumor size was defined as the largest diameter on the second post-contrast subtracted image. Background parenchymal enhancement was categorized into one of four levels (1. minimal, 2. mild, 3. moderate, and 4. marked). The type of lesion presented, the shape of the main lesion, the margin, the internal enhancement pattern, and the time-intensity curve (washout, plateau, and persistent) were assessed [11]. The time-intensity curve was evaluated using an automated software program (CADstream, Merge Healthcare, Chicago, IL, USA). The presence of intratumoral necrosis, fibrosis, perilesional edema, and the signal intensity of the lesion were evaluated with T2-weighted images (T2WI). Residual tumors were assessed on MRI after NCT. An enhancing area distinct from the background parenchymal enhancement was considered to indicate the presence of residual tumors. The absence of a distinct enhancing area was considered to indicate complete response to chemotherapy.
Statistical analyses
Differences between the groups according to clinicopathological parameters were evaluated using the chi-square test. Fisher’s exact test was used when appropriate. The independent two sample t-test was used to compare the means of continuous numerical data. The predictive value of imaging modality for the detection of residual DCIS at the time before surgery was analyzed by receiver operating characteristic (ROC) curve analysis with calculated area under the ROC curve (AUC). A logistic regression analysis was used to investigate independent parameters including breast cancer subtype associated with ypT0 after completion of NCT. The Cochran-Mantel-Haenszel test was used in stratified analyses according to breast cancer subtype to explore the relationships between MRI findings after NCT and ypT0. All statistical tests were two-sided, and p-values <0.05 were considered statistically significant. The SPSS software version 20.0 (IBM Inc., Armonk, NY, USA) was used for all analyses.
Results
Of 117 patients, 67 (57.3%) were ypT0 and 50 (42.7%) were ypTis after breast surgery. Mean age at diagnosis was 49.4 ± 9.7 years for the entire study sample. Table 1 presents clinicopathological characteristics according to presence of residual disease. There were no differences in clinical features, tumor burden at presentation, pathologic nodal status after NCT, histologic grade, Ki-67 proliferative index at diagnosis, or regimens of NCT between the two groups. Patients with ypT0 were more likely to have ER-negative, PR-negative, and HER2-negative tumors. Therefore, triple-negative breast cancer was significantly more common in the ypT0 group. Breast-conservation surgeries were more frequently performed in patients with ypT0.
Table 1. Clinicopathological findings of patients with ypT0 and ypTis in the breast after neoadjuvant chemotherapy.
| Parameters | ypT0 (n = 67, %) | ypTis (n = 50, %) | P-value |
|---|---|---|---|
| Age (year) | |||
| Mean ± SD | 49.6 ± 10.4 | 49.1 ± 8.7 | 0.772a |
| ≤40 | 13 (68.4) | 6 (31.6) | 0.283 |
| >40 | 54 (55.1) | 44 (44.9) | |
| Menopause | |||
| Premenopause | 35 (56.5) | 27 (43.5) | 0.850 |
| Postmenopause | 32 (58.2) | 23 (41.8) | |
| BMI (kg/m2) | |||
| <25 | 46 (57.5) | 34 (42.5) | 0.940 |
| ≥25 | 21 (56.8) | 16 (43.2) | |
| Clinical tumor stage at presentation | |||
| T1 | 26 (66.7) | 13 (33.3) | 0.340 |
| T2 | 34 (53.1) | 30 (46.9) | |
| T3-4 | 7 (50.0) | 7 (50.0) | |
| Node status at presentation | |||
| Negative | 9 (64.3) | 5 (35.7) | 0.571 |
| Positive | 58 (56.3) | 45 (43.7) | |
| Regimens of NCT | |||
| AC followed by T | 49 (73.1) | 42 (84.0) | 0.070 |
| AC followed by T+TS1 | 6 (9.0) | 6 (12.0) | |
| Others | 12 (17.9) | 2 (4.0) | |
| Pathologic node status after NCT | |||
| ypN0 | 58 (60.4) | 38 (39.6) | 0.141 |
| ypN1-3 | 9 (42.9) | 12 (57.1) | |
| Histologic grade | |||
| I/II | 36 (56.2) | 28 (43.8) | 0.807 |
| III | 31 (58.5) | 22 (41.5) | |
| ER | |||
| Negative | 43 (65.2) | 23 (34.8) | 0.050 |
| Positive | 24 (47.1) | 27 (52.9) | |
| PR | |||
| Negative | 56 (62.2) | 34 (37.8) | 0.048 |
| Positive | 11 (40.7) | 16 (59.3) | |
| HER2 | |||
| Negative | 50 (68.5) | 23 (31.5) | 0.002 |
| Positive | 17 (38.6) | 27 (61.4) | |
| Breast cancer subtype | |||
| HRs+/HER2- | 17 (51.5) | 16 (48.5) | 0.001 |
| HRs+/HER2+ | 7 (36.8) | 12 (63.2) | |
| HRs-/HER2+ | 10 (40.0) | 15 (60.0) | |
| HRs-/HER2- | 33 (82.5) | 7 (17.5) | |
| Ki-67 before NCT (n = 105) | |||
| ≤15% | 17 (45.9) | 20 (54.1) | 0.119 |
| >15% | 42 (61.8) | 26 (38.2) | |
| Surgery | |||
| Breast-conservation | 46 (68.7) | 21 (31.3) | 0.004 |
| Total mastectomy | 21 (42.0) | 29 (58.0) |
SD, standard deviation; BMI, body mass index; NCT, neoadjuvant chemotherapy; AC, anthracycline plus cyclophosphamide; T, docetaxel; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; HRs, hormone receptors.
aIndependent samples t-test.
Mammographic findings for patients with ypT0 and ypTis are compared in Table 2 and S1 Appendix. At initial presentation, the size, extent, shape, and margin of the main lesion, mammographic parenchymal pattern, and density did not differ between patients with ypT0 and ypTis. The baseline main tumor frequently presented as microcalcifications with or without mass in the ypTis group. Associated microcalcifications were more frequent in patients with ypTis. After completion of NCT, mammographic findings of patients with ypT0 were significantly noted as either complete response or undetected.
Table 2. Mammographic findings of patients with ypT0 and ypTis.
| Parameters | ypT0 (%) | ypTis (%) | P-value |
|---|---|---|---|
| Before NCT | |||
| Mean Size ± SD | 29.4 ± 21.8 | 33.7 ± 21.0 | 0.287a |
| Extent | |||
| Single | 46 (63.0) | 27 (37.0) | 0.105 |
| Multiple | 21 (47.7) | 23 (52.3) | |
| Parenchymal pattern | |||
| b | 19 (65.5) | 10 (34.5) | 0.078b |
| c | 41 (51.2) | 39 (48.8) | |
| d | 7 (87.5) | 1 (12.5) | |
| Presentation of main lesion | |||
| Mass alone | 48 (70.6) | 20 (29.4) | <0.001 |
| Microcalcifications ± mass | 11 (28.9) | 27 (71.1) | |
| Undetected | 8 (72.7) | 3 (27.3) | |
| Associated microcalcifications | |||
| Present | 15 (31.2) | 33 (68.8) | <0.001 |
| Absent | 52 (75.4) | 17 (24.6) | |
| Shape | |||
| Round or oval | 38 (63.3) | 22 (36.7) | 0.252 |
| Irregular | 15 (57.7) | 11 (42.3) | |
| Undetected | 14 (45.2) | 17 (54.8) | |
| Margin | |||
| Circumscribed | 4 (100.0) | 0 (0.0) | 0.302b |
| Microlobulated | 7 (58.3) | 5 (41.7) | |
| Spiculated | 12 (57.1) | 9 (42.9) | |
| Indistinct | 29 (63.0) | 17 (37.0) | |
| Obscured | 1 (33.3) | 2 (66.7) | |
| Undetected | 14 (45.2) | 17 (54.8) | |
| Density | |||
| High density | 23 (65.7) | 12 (34.3) | 0.295 |
| Equal density | 29 (58.0) | 21 (42.0) | |
| Low density or undetected | 15 (46.9) | 17 (53.1) | |
| After NCT | |||
| Complete response or undetected | 26 (81.2) | 6 (18.8) | 0.003b |
| Residual microcalcifications alone | 3 (50.0) | 3 (50.0) | |
| Residual mass ± microcalcifications | 38 (48.1) | 41 (51.9) |
aIndependent samples t-test.
bFisher’s exact test.
Table 3 presents the ultrasound results for the ypT0 and ypTis group. Baseline sonographic size, margin, orientation, and echogenicity did not differ between the ypT0 and ypTis group. Round shape of the main lesion, posterior enhancement, and sonographic absence of calcifications were more frequently observed in patients with ypT0. After NCT, ultrasound findings of patients attaining ypT0 showed a higher proportion of complete response.
Table 3. Ultrasound findings of patients with ypT0 and ypTis.
| Parameters | ypT0 (%) | ypTis (%) | P-value |
|---|---|---|---|
| Before NCT | |||
| Mean Size ± SD | 29.9 ± 20.0 | 31.6 ± 18.5 | 0.641a |
| Shape | |||
| Oval | 37 (58.7) | 26 (41.3) | 0.015 |
| Round | 11 (91.7) | 1 (8.3) | |
| Irregular | 19 (45.2) | 23 (54.8) | |
| Margin | |||
| Circumscribed | 5 (100.0) | 0 (0.0) | 0.166 |
| Indistinct | 23 (60.5) | 15 (39.5) | |
| Angular | 9 (69.2) | 4 (30.8) | |
| Microlobulated | 20 (51.3) | 19 (48.7) | |
| Spiculated | 10 (45.5) | 12 (54.5) | |
| Orientation | |||
| Parallel | 38 (50.7) | 37 (49.3) | 0.054 |
| Non-parallel | 29 (69.0) | 13 (31.0) | |
| Echogenicity | |||
| Hyper-echo | 1 (33.3) | 2 (66.7) | 0.466b |
| Iso-echo | 11 (68.8) | 5 (31.2) | |
| Hypo-echo | 55 (56.1) | 43 (43.9) | |
| Posterior features | |||
| Enhancement | 28 (71.8) | 11 (28.2) | 0.038 |
| Shadowing | 10 (40.0) | 15 (60.0) | |
| No posterior features | 29 (54.7) | 24 (45.3) | |
| Calcification | |||
| Present | 9 (28.1) | 23 (71.9) | <0.001 |
| Absent | 58 (68.2) | 27 (31.8) | |
| After NCT | |||
| Complete response | 27 (75.0) | 9 (25.0) | 0.010 |
| Residual disease | 40 (49.4) | 41 (50.6) |
aIndependent samples t-test.
bFisher’s exact test.
MRI findings are shown in Table 4. Before NCT, the size, shape, and margin of the main lesion, background parenchymal enhancement, internal enhancement, T2WI, presence of necrosis, and peritumoral edema did not differ between the two groups. The main lesion of the ypT0 group was more likely to present as a mass, but non-mass enhancement was more frequent in the ypTis group. MRI findings for patients with ypT0 after NCT mostly indicated complete response.
Table 4. Magnetic resonance findings of patients with ypT0 and ypTis.
| Parameters | ypT0 (%) | ypTis (%) | P-value |
|---|---|---|---|
| Before NCT | |||
| Mean Size ± SD | 30.5 ± 19.9 | 32.1 ± 17.4 | 0.656a |
| BPE | |||
| 1 | 56 (60.2) | 37 (39.8) | 0.452b |
| 2 | 8 (44.4) | 10 (55.6) | |
| 3 | 3 (50.0) | 3 (50.0) | |
| Presentation of main lesion | |||
| Mass | 59 (62.1) | 36 (37.9) | 0.028 |
| Non-mass enhancement | 8 (36.4) | 14 (63.6) | |
| Shape | |||
| Round | 14 (60.9) | 9 (39.1) | 0.282 |
| Oval | 39 (61.9) | 24 (38.1) | |
| Irregular | 14 (45.2) | 17 (54.8) | |
| Margin | |||
| Circumscribed | 13 (65.0) | 7 (35.0) | 0.403b |
| Irregular | 48 (53.9) | 41 (46.1) | |
| Spiculated | 6 (75.0) | 2 (25.0) | |
| Internal enhancement | |||
| Heterogeneous | 34 (50.0) | 34 (50.0) | 0.133 |
| Homogeneous | 26 (70.3) | 11 (29.7) | |
| Rim enhancement | 7 (58.3) | 5 (41.7) | |
| Time-intensity curve | |||
| Washout | 64 (95.5) | 49 (98.0) | 0.830 |
| Plateau or persistent | 3 (4.5) | 1 (2.0) | |
| T2WI | |||
| High | 42 (58.3) | 30 (41.7) | 0.768 |
| Iso or low | 25 (55.6) | 20 (44.4) | |
| Presence of necrosis | |||
| No | 58 (56.9) | 44 (43.1) | 0.819 |
| Yes | 9 (60.0) | 6 (40.0) | |
| Peritumoral edema | |||
| No | 48 (57.1) | 36 (42.9) | 0.966 |
| Yes | 19 (57.6) | 14 (42.4) | |
| After NCT | |||
| Complete response | 47 (74.6) | 16 (25.4) | <0.001 |
| Residual disease | 20 (37.0) | 34 (63.0) |
BPE, Background parenchymal enhancement; T2WI, T2-weighted image.
aIndependent samples t-test.
bFisher’s exact test.
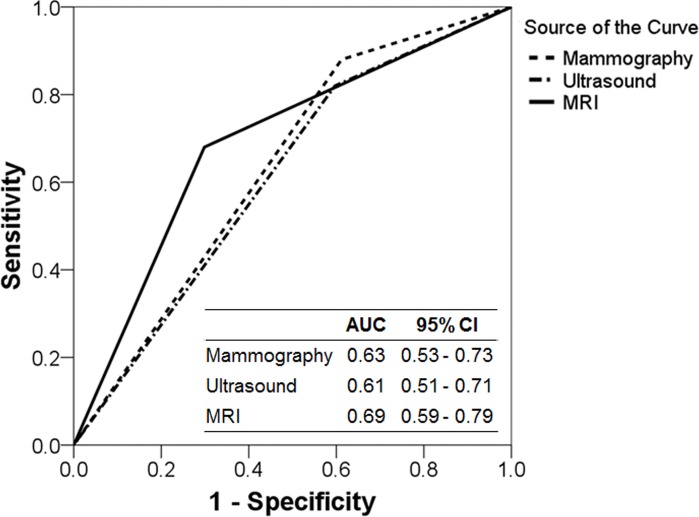
The sensitivity, specificity, and accuracy for the detection of residual DCIS at the time before surgery was 88.0%, 38.8%, and 59.8% for mammography, respectively, 82.0%, 40.3%, and 58.1% for ultrasound, respectively, and 68.0%, 70.1%, and 62.9% for MRI, respectively. Fig 1 shows ROC curve analysis for detecting ypTis. AUC of mammography, ultrasound, and MRI was 0.63 (95% confidence interval [CI], 0.53–0.73), 0.61 (95% CI, 0.51–0.71), and 0.69 (95% CI, 0.59–0.79), respectively.
Fig 1. Receiver operating characteristics (ROC) curve analysis for detecting ypTis.
AUC, area under the ROC curve; CI, confidence interval; MRI, magnetic resonance imaging.
Multivariate logistic regression analyses were conducted to identify independent predictors of ypT0 after completion of NCT (Table 5). The triple-negative subtype and complete response in MRI after NCT were significant predictors of ypT0. There was no significant interaction between breast cancer subtypes and MRI results in the multivariate model. Since breast cancer subtypes and MRI findings after NCT were the most important predictors, we conducted stratified analyses according to breast cancer subtype to explore the relationship between MRI findings and residual tumor burden after NCT (Table 6). In all breast cancer subtypes except the HER2-positive subtype, breast MRI well predicted ypT0 with statistical significance. In HRs+/HER2- and HRs+/HER2+ tumors, approximately two-thirds of the patients with complete response observed on MRI after NCT were determined to be ypT0 after surgery, with significant difference. In particular, 22 of 40 patients with HRs-/HER2- tumors showed complete response according to MRI after NCT and among these patients, 21 (95.5%) were ypT0. However, there were no significant differences in patients with HRs-/HER2+ tumors. During the study period, only 2 patients were treated with trastuzumab in combination with chemotherapy since anti-HER2 targeted therapy for neoadjuvant treatment is not covered by the Korean National Health Insurance. When these 2 cases were excluded, MRI findings after NCT were not associated with residual disease in 23 HRs-/HER2+ tumors (p = 0.193, Fisher’s exact test).
Table 5. Predictors of ypT0 in the breast after completion of NCT.
| Parameters | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Breast cancer subtype | 0.098 | ||
| HRs+/HER2- | Ref | ||
| HRs+/HER2+ | 0.70 | 0.16–3.00 | 0.633 |
| HRs-/HER2+ | 0.97 | 0.26–3.54 | 0.958 |
| HRs-/HER2- | 4.23 | 1.11–16.09 | 0.034 |
| MMG presentation of the main lesion | |||
| Mass or undetected | Ref | ||
| Microcalcifications ± mass | 0.29 | 0.03–2.51 | 0.263 |
| MMG associated microcalcifications | |||
| Absent | Ref | ||
| Present | 0.84 | 0.13–5.60 | 0.855 |
| US shape | 0.370 | ||
| Irregular | Ref | ||
| Oval | 1.11 | 0.39–3.17 | 0.846 |
| Round | 5.83 | 0.49–69.80 | 0.164 |
| US posterior features | 0.543 | ||
| No posterior features | Ref | ||
| Shadowing | 1.40 | 0.38–3.17 | 0.846 |
| Enhancement | 1.92 | 0.60–6.21 | 0.275 |
| US calcification | |||
| No | Ref | ||
| Yes | 1.34 | 0.22–8.27 | 0.751 |
| MRI presentation of the main lesion | |||
| Mass | Ref | ||
| Non-mass enhancement | 1.18 | 0.32–4.31 | 0.807 |
| MMG after NCT | |||
| Residual disease | Ref | ||
| Complete response | 0.90 | 0.20–4.06 | 0.890 |
| US after NCT | |||
| Residual disease | Ref | ||
| Complete response | 2.69 | 0.79–9.19 | 0.114 |
| MRI after NCT | |||
| Residual disease | Ref | ||
| Complete response | 5.23 | 1.53–17.85 | 0.008 |
CI, confidence interval; Ref, reference; MMG, mammography; US, ultrasound; MRI, magnetic resonance imaging.
Table 6. MRI findings after NCT according to postoperative pathologic results stratified by breast cancer subtype.
| MRI after NCT | ypT0 | ypTis | P-value | P-valuea |
|---|---|---|---|---|
| HRs+/HER2- (n = 33) | <0.001 | |||
| Complete response | 13 (68.4) | 6 (31.6) | 0.024 | |
| Residual disease | 4 (28.6) | 10 (71.4) | ||
| HRs+/HER2+ (n = 19) | ||||
| Complete response | 6 (66.7) | 3 (33.3) | 0.020b | |
| Residual disease | 1 (10.0) | 9 (90.0) | ||
| HRs-/HER2+ (n = 25) | ||||
| Complete response | 7 (53.8) | 6 (46.2) | 0.226b | |
| Residual disease | 3 (25.0) | 9 (75.0) | ||
| HRs-/HER2- (n = 40) | ||||
| Complete response | 21 (95.5) | 1 (4.5) | 0.033b | |
| Residual disease | 12 (66.7) | 6 (33.3) |
aCochran-Mantel-Haenszel test.
bFisher’s exact test.
Discussion
Recent pooled analyses of clinical trials of NCT indicate that achievement of pCR is associated with improved survival among breast cancer patients [3]. However, the implications of these findings are thought to vary among breast cancer subtypes [12]. Although several classifications have been suggested for pathologic response to NCT in the breast, pCR of the breast in practice is defined as no residual carcinoma (ypT0) or no residual invasive tumor with DCIS present (ypTis) [13,14]. The prognostic implications of pCR are somewhat controversial but in general, there are no differences in survival between patients with ypT0 and patients with ypT0/is when ypN0 is attained [3,12]. However, ypT0 and ypTis cannot be accurately distinguished before definitive surgery, and some tumors do not respond in uniform patterns to NCT [15,16]. The major clinical advantage of NCT is an increased success rate of breast-conservation surgery, which can be applied to patients with favorable response to NCT who fulfill the criteria for breast-conservation surgery. Therefore, monitoring response to NCT and evaluating residual tumor extent before surgery are clinically important practices.
Mammography is clinically useful for evaluating the extent of malignant-appearing calcifications. Lesions with residual DCIS frequently show calcifications on pre-chemotherapy mammograms [6], as observed in this study. When main lesions initially presented as microcalcifications with or without masses or when associated microcalcifications were detected, ypTis was more likely to be observed after NCT. However, calcifications are also indicative of necrotic tumor cells in patients who have received NCT. Our results showed that approximately half of all cases with residual masses or microcalcifications after completion of NCT were identified as ypT0. This result is similar to previous studies which indicated that remnant calcifications after NCT are not correlated with residual tumor burden [6,17,18]. Moreover, 33.3% of stable microcalcifications and 27.7% of newly developed or additional calcifications after NCT turned out to be pCR at the time of surgery, while 100% of calcifications in cases with increased mass showed residual malignancy [19]. In HER2-positive breast cancers, adjacent DCIS could be completely eradicated by NCT combined with trastuzumab [20]. Therefore, remnant calcifications on mammography after NCT should not be considered to constitute evidence of residual DCIS. While practical guidelines indicate that findings of diffuse suspicious or malignant-appearing microcalcifications absolutely contraindicate breast-conserving therapy [21], a comprehensive clinical and imaging analysis which considers breast cancer subtypes and therapeutic regimens is necessary to plan surgery after completion of NCT.
Recently, Lee et al. [22] summarized inaccuracies among current practical tools used to evaluate residual tumor volumes in response to NCT and demonstrated that two-dimensional and three-dimensional ultrasound and breast MRI show similar performances for the estimation of residual breast cancer volume and prediction of pCR. In a retrospective analysis of patients enrolled in the GeparTrio trial, ultrasound showed a high sensitivity for predicting ypT0 and ypN0 and modestly improved the prediction of pCR by patient characteristics, which was concluded to be a potentially useful modality for early prediction of pCR, despite breast MRI not being included in the study [23]. In addition, ultrasound provides clinical advantages over MRI including lower complexity, easier accessibility, shorter procedure time, easier interpretation, cheaper costs, and lack of the hazards associated with contrast agents [22,23]. In the present study, univariate analyses demonstrated that round shape on baseline sonographic analyses, posterior features, sonographic absence of calcification, and complete response after NCT were associated with a higher possibility of ypT0. However, there was no significant effect observed in multivariate analysis, and more studies are required to confirm the role of ultrasound in the prediction of residual tumor burden after NCT. Some potential explanations could lie in the fact that all cases included in our study showed pCR at the time of surgery, which means that residual disease was determined by in situ components of permanent pathology. No imaging modality other than mammography is currently accepted in the evaluation of DCIS. For example, ultrasound has limited ability to detect microcalcifications due to technical issues [24]. Although there are several circumstances in which ultrasound may be beneficial in the evaluation of patients with DCIS, sonographic findings of DCIS are very subtle. Therefore, even though ultrasound might help predict pCR based [23], its ability to differentiate ypT0 from ypTis needs to be investigated. In addition, interobserver variability is a well-known limitation of ultrasound [24]. Examining the exact primary site can be difficult in cases with markedly decreased tumor burden size due to the limited number of landmarks available for ultrasound.
A meta-analysis of MRI in the prediction of pCR after NCT revealed a high specificity of 0.91 and a relatively low sensitivity of 0.63 [4]. However, the performance of MRI can be influenced by pCR rates, Ki-67 index, and breast cancer subtype [4,8,25,26]. The accuracy of MRI for predicting residual tumor size was greatest in patients with the triple-negative phenotype or HER2-positive breast cancers, and a better correlation was noted in the triple-negative subtype with higher Ki-67 levels [8,25,26]. In this study, the triple-negative breast cancer subtype and complete response on MRI after NCT were independent predictors for discriminating ypT0 from ypTis. This is supported by previous study results which have shown that mass enhancement is an imaging characteristic of triple-negative breast cancer and that associated DCIS is rare in cases without non-mass enhancement [27]. However, Moon et al. [7] reported that the use of HER2-targeted agents resulted in less accurate MRI in patients with HER2-positive tumors. In the present study, although most patients with HER2-positive tumors did not receive HER2-directed therapy, MRI after NCT showed poor performance for the prediction of ypT0 in HRs-/HER2+ tumors. Breast cancer subtypes are associated with pCR rates after NCT, and the incorporation of HER2-targeted agents into NCT significantly improved pCR rates in HER2-positive breast cancers [2,12,28]. The relationship between biologic mechanisms and MRI used to discriminate ypT0 from ypTis in HER2-positive tumors has yet to be determined.
Potential limitations of the present study are that it was a retrospective analysis using a single institution database, and that the interpretations of imaging modalities were performed by a single radiologist (although the radiologist was blinded to clinicopathological information). In addition, patients with non-pCR after NCT were not analyzed, and confirmative parameters for the discrimination of ypT0 from ypTis or residual invasive carcinoma were not evaluated. Nevertheless, our study has two major strengths. One is that more than 100 cases attaining pCR in the breast after NCT were investigated and the other is that all patients underwent mammography, ultrasound, and breast MRI prior to and after NCT. Therefore, we were able to comprehensively analyze the impact of all three imaging modalities before and after NCT on the prediction of pCR while considering clinicopathological factors including breast cancer subtype. Our multivariate analyses suggest that MRI after NCT affects discrimination between ypT0 and ypTis differently according to breast tumor phenotype. Of note, since the high false-positive rate and the subsequently frequent overcall rate are weaknesses of MRI, further study with a larger multicenter cohort is necessary to validate our results and to evaluate the clinical benefits and risks of MRI.
In conclusion, we demonstrated that the triple-negative breast cancer subtype and complete response in MRI after NCT are independent predictors of ypT0. Among imaging modalities, breast MRI could be suggested as a modality that accurately discriminates between ypT0 and ypTis after NCT, especially in patients with triple-negative breast cancer. However, statistically low AUC value and relatively high false-positive rate of MRI given in the present study suggest that our findings are not definitive and additional study should be conducted. Until finding out more clinically relevant imaging modalities and appropriate patient selection criteria, this information can be useful in the evaluation of tumor response to NCT and in the planning of surgery for breast cancer patients of all subtypes except for HER2-positive tumors after NCT.
Supporting Information
(PDF)
Data Availability
All relevant data are within the paper.
Funding Statement
The work was supported by the Bumsuk Academic Research Fund in 2014 (Grant No. 2014-31-0768), SP. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Berruti A, Generali D, Kaufmann M, Puztai L, Curigliano G, Aglietta M, et al. International expert consensus on primary systemic therapy in the management of early breast cancer: highlights of the Fourth Symposium on Primary Systemic Therapy in the Management of Operable Breast Cancer, Cremona, Italy (2010). J Natl Cancer Inst Monogr. 2011;2011: 147–151. 10.1093/jncimonographs/lgr037 . [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann M, von Minckwitz G, Mamounas EP, Cameron D, Carey LA, Cristofanilli M, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19: 1508–1516. 10.1245/s10434-011-2108-2 . [DOI] [PubMed] [Google Scholar]
- 3.Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384: 164–172. 10.1016/s0140-6736(13)62422-8 . [DOI] [PubMed] [Google Scholar]
- 4.Yuan Y, Chen XS, Liu SY, Shen KW. Accuracy of MRI in prediction of pathologic complete remission in breast cancer after preoperative therapy: a meta-analysis. AJR Am J Roentgenol. 2010;195: 260–268. 10.2214/ajr.09.3908 . [DOI] [PubMed] [Google Scholar]
- 5.Chen K, Jia W, Li S, He J, Zeng Y, Yang H, et al. Cavity margin status is an independent risk factor for local-regional recurrence in breast cancer patients treated with neoadjuvant chemotherapy before breast-conserving surgery. Am Surg. 2011;77: 1700–1706. . [PubMed] [Google Scholar]
- 6.Choi HK, Cho N, Moon WK, Im SA, Han W, Noh DY. Magnetic resonance imaging evaluation of residual ductal carcinoma in situ following preoperative chemotherapy in breast cancer patients. Eur J Radiol. 2012;81: 737–743. 10.1016/j.ejrad.2011.01.013 . [DOI] [PubMed] [Google Scholar]
- 7.Moon HG, Han W, Ahn SK, Cho N, Moon WK, Im SA, et al. Breast cancer molecular phenotype and the use of HER2-targeted agents influence the accuracy of breast MRI after neoadjuvant chemotherapy. Ann Surg. 2013;257: 133–137. 10.1097/SLA.0b013e3182686bd9 . [DOI] [PubMed] [Google Scholar]
- 8.Kim MJ, Kim EK, Park S, Moon HJ, Kim SI, Park BW. Evaluation with 3.0-T MR imaging: predicting the pathological response of triple-negative breast cancer treated with anthracycline and taxane neoadjuvant chemotherapy. Acta Radiol. 2015;56: 1069–77. 10.1177/0284185114548507 . [DOI] [PubMed] [Google Scholar]
- 9.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28: 2784–2795. 10.1200/jco.2009.25.6529 ; PubMed Central PMCID: PMCPmc2881855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31: 3997–4013. 10.1200/jco.2013.50.9984 . [DOI] [PubMed] [Google Scholar]
- 11.Mercado CL. BI-RADS update. Radiol Clin North Am. 2014;52: 481–487. 10.1016/j.rcl.2014.02.008 . [DOI] [PubMed] [Google Scholar]
- 12.von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30: 1796–1804. 10.1200/jco.2011.38.8595 . [DOI] [PubMed] [Google Scholar]
- 13.Marchio C, Sapino A. The pathologic complete response open question in primary therapy. J Natl Cancer Inst Monogr. 2011;2011: 86–90. 10.1093/jncimonographs/lgr025 . [DOI] [PubMed] [Google Scholar]
- 14.Gebreamlak EP, Tse GM, Niu Y. Progress in evaluation of pathologic response to neoadjuvant chemotherapy of breast cancer. Anticancer Agents Med Chem. 2013;13: 222–226. . [DOI] [PubMed] [Google Scholar]
- 15.Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008;26: 791–797. 10.1200/jco.2007.15.0326 . [DOI] [PubMed] [Google Scholar]
- 16.Wang S, Zhang Y, Yang X, Fan L, Qi X, Chen Q, et al. Shrink pattern of breast cancer after neoadjuvant chemotherapy and its correlation with clinical pathological factors. World J Surg Oncol. 2013;11: 166 10.1186/1477-7819-11-166 ; PubMed Central PMCID: PMCPmc3728037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiss A, Lee KC, Romero Y, Ward E, Kim Y, Ojeda-Fournier H, et al. Calcifications on mammogram do not correlate with tumor size after neoadjuvant chemotherapy. Ann Surg Oncol. 2014;21: 3310–3316. 10.1245/s10434-014-3914-0 . [DOI] [PubMed] [Google Scholar]
- 18.Li JJ, Chen C, Gu Y, Di G, Wu J, Liu G, et al. The role of mammographic calcification in the neoadjuvant therapy of breast cancer imaging evaluation. PLoS One. 2014;9: e88853 10.1371/journal.pone.0088853 ; PubMed Central PMCID: PMCPmc3921249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adrada BE, Huo L, Lane DL, Arribas EM, Resetkova E, Yang W. Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2015;22: 1111–1117. 10.1245/s10434-014-4113-8 . [DOI] [PubMed] [Google Scholar]
- 20.von Minckwitz G, Darb-Esfahani S, Loibl S, Huober J, Tesch H, Solbach C, et al. Responsiveness of adjacent ductal carcinoma in situ and changes in HER2 status after neoadjuvant chemotherapy/trastuzumab treatment in early breast cancer-results from the GeparQuattro study (GBG 40). Breast Cancer Res Treat. 2012;132: 863–870. 10.1007/s10549-011-1621-0 . [DOI] [PubMed] [Google Scholar]
- 21.Gradishar WJ, Anderson BO, Blair SL, Burstein HJ, Cyr A, Elias AD, et al. Breast cancer version 3.2014. J Natl Compr Canc Netw. 2014;12: 542–590. . [DOI] [PubMed] [Google Scholar]
- 22.Lee MC, Gonzalez SJ, Lin H, Zhao X, Kiluk JV, Laronga C, et al. Prospective trial of breast MRI versus 2D and 3D ultrasound for evaluation of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2015;22: 2888–2894. 10.1245/s10434-014-4357-3 . [DOI] [PubMed] [Google Scholar]
- 23.Marinovich ML, Houssami N, Macaskill P, von Minckwitz G, Blohmer JU, Irwig L. Accuracy of ultrasound for predicting pathologic response during neoadjuvant therapy for breast cancer. Int J Cancer. 2015;136: 2730–2737. 10.1002/ijc.29323 . [DOI] [PubMed] [Google Scholar]
- 24.Hooley RJ, Scoutt LM, Philpotts LE. Breast ultrasonography: state of the art. Radiology. 2013;268: 642–659. 10.1148/radiol.13121606 . [DOI] [PubMed] [Google Scholar]
- 25.De Los Santos JF, Cantor A, Amos KD, Forero A, Golshan M, Horton JK, et al. Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017. Cancer. 2013;119: 1776–1783. 10.1002/cncr.27995 ; PubMed Central PMCID: PMCPmc3939707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mukhtar RA, Yau C, Rosen M, Tandon VJ, Hylton N, Esserman LJ. Clinically meaningful tumor reduction rates vary by prechemotherapy MRI phenotype and tumor subtype in the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). Ann Surg Oncol. 2013;20: 3823–3830. 10.1245/s10434-013-3038-y ; PubMed Central PMCID: PMCPmc3824937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trop I, LeBlanc SM, David J, Lalonde L, Tran-Thanh D, Labelle M, et al. Molecular classification of infiltrating breast cancer: toward personalized therapy. Radiographics. 2014;34: 1178–1195. 10.1148/rg.345130049 . [DOI] [PubMed] [Google Scholar]
- 28.Brown-Glaberman U, Dayao Z, Royce M. HER2-targeted therapy for early-stage breast cancer: a comprehensive review. Oncology (Williston Park). 2014;28: 281–289. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All relevant data are within the paper.