Summary
In the past couple of decades, evidence from prospective observational studies and clinical trials has converged to support the importance of individual nutrients, foods, and dietary patterns in the prevention and management of type 2 diabetes. The quality of dietary fats and carbohydrates consumed is more crucial than the quantity of these macronutrients. Diets rich in whole grains, fruits, vegetables, legumes, nuts, moderate in alcohol consumption, and lower in refined grains, red/processed meats, and sugar-sweetened beverages have demonstrated to reduce diabetes risk and improve glycemic control and blood lipids in patients with diabetes. Several healthful dietary patterns emphasizing the overall diet quality can be adapted to appropriate personal and cultural food preferences and calorie needs for weight control and diabetes prevention and management. Although considerable progress has been made in developing and implementing evidence-based nutrition recommendations in developed countries, concerted global efforts and policies are warranted to alleviate regional disparities.
Introduction
Globally, 382 million adults (8.3%) are living with diabetes, and the estimate is projected to rise to over 592 million by 2035.1 At least 147 billion USD was spent on diabetes healthcare in Europe, while North America and the Caribbean spent 263 billion USD in 2013.1 Diabetes has become a major cause of death in people under the age of 60.1 Investment in effective diabetes prevention and management has become necessary to battle this global epidemic. Along with urbanization and economic growth, many countries have experienced dietary changes favoring increased caloric consumption.2 Although an unhealthful diet has been considered a major contributor to diabetes development for a long time, only in the past two decades has the evidence vastly accumulated from both prospective observational studies and randomized controlled trials (RCTs). In this review, we examine the role of diet in prevention and management of diabetes.
Search strategy and selection criteria
We searched PubMed and Google Scholar, mainly for original research articles, meta-analysis/systematic reviews, and organization recommendations published up to January, 2014. We used the main search terms “type 2 diabetes”, “nutrition”, “diet”, “prevention”, and “management” in combination with specific terms on nutrient or dietary pattern. We largely selected publications in the past 5 years, but did not exclude commonly referenced and highly regarded older publications. We also searched the reference lists of articles identified by this search strategy and selected those we judged relevant. Review articles and book chapters are cited to provide readers with more details and references.
Nutrition transition and global dietary trends
At a macro-level, the type 2 diabetes epidemic has been attributed to urbanization and environmental transitions, including work pattern changes from heavy labor to sedentary occupations, increased computerization and mechanization, and improved transportation. Economic growth and environmental transitions have led to drastic changes in food production, processing, and distribution systems and increased the accessibility of unhealthful foods.3 Fast food restaurant establishments have experienced exponential global expansion in recent decades. This increased availability of fast foods has contributed to unhealthful diets with high calorie content; large portion sizes; and large amounts of processed meat, highly refined carbohydrates, sugary beverages, and unhealthy fats. Another key component in the food system transition has been the saturation of large chain supermarkets, which displace fresh local food and farm shops and serve as a source of highly processed foods, high-energy snacks, and sugary beverages.3
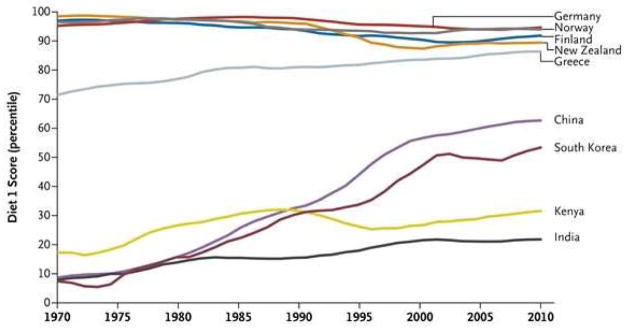
Parts of the world undergoing epidemiological transition have experienced a livestock revolution, which leads to increased production of beef, pork, dairy products, eggs, and poultry.3,4 Based on the United Nations Food and Agriculture Organization data, this change has been especially drastic in Asian countries (Figure 1).4 Another characteristic of nutrition transition is increased refinement of grain products. Milling and processing whole grains to produce refined grains such as polished white rice and refined wheat flour reduce the nutritional content of grains, including their fiber, micronutrients, and phytochemicals.
Figure 1. Global dietary trend changes over time.
A high diet 1 score indicates a high availability of sugars; meat, animal products, animal fats, milk, and eggs; and total calories, in addition to low availability of pulses and cereals based on the United Nations Food and Agriculture Organization food balance sheets.4
Dietary factors for the prevention of diabetes
Positive energy balance and excess adiposity
In recent decades, men and women around the globe have gained weight, largely due to changes in dietary patterns and decreased physical activity levels.4 Excess adiposity reflected by higher body mass index (BMI) is the strongest risk factor for diabetes, and Asians tend to develop diabetes at a much lower BMI than those of European ancestry.5 The risk of diabetes rises as excessive body fat increases, starting from the lower end of a healthful BMI or waist circumference.6 A meta-analysis of prospective cohort studies suggests that the risk associated with a higher waist circumference is slightly stronger than the risk associated with a higher BMI.7 In clinical practice, it is important to monitor both BMI and waist circumference. Weight gain since young adulthood is another independent predictor of diabetes risk even after adjusting for current BMI.5
Lifestyle intervention involving calorie-restriction and exercise to promote weight-loss, as demonstrated in the Diabetes Prevention Program, significantly reduced conversion to diabetes among high risk patients with impaired glucose tolerance by 58%.8 The beneficial effect of lifestyle modification was documented in various populations including multiethnic American,8 Finnish,9 Chinese,10 and Indian.11
Quantity and quality of dietary fat
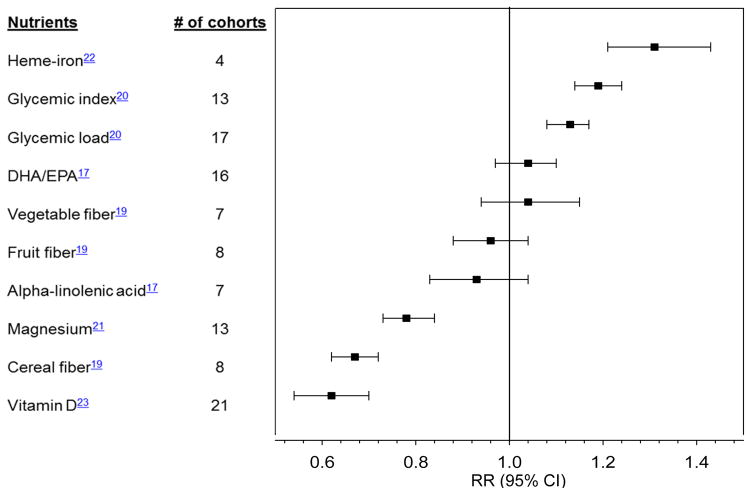
While it has been hypothesized that higher total fat intake contributes to diabetes directly by inducing insulin resistance and indirectly by promoting weight gain, results from metabolic studies in humans do not support that high-fat diets per se have a detrimental effect on insulin sensitivity.12 In several observational studies, total fat intake was not associated with diabetes risk.13, 14 In the Women’s Health Initiative, the incidence of diabetes was not reduced among women who consumed a low-fat diet compared to the control group.15 The quality of fat is more important than total fat intake, and diets that favor plant-based fats over animal fats are more advantageous.13 In particular, greater intake of omega-6 polyunsaturated fatty acids (PUFA) was associated with lower diabetes risk in the Nurses’ Health Study.16 Replacing saturated fat with omega-6 PUFA was related to lower risk of developing diabetes.13 However, the relationship between omega-3 PUFA and diabetes risk has been inconsistent (Figure 2; Supplemental Table 1).17
Figure 2. Summary of meta-analyses of prospective cohort studies on nutrient intake and type 2 diabetes.
DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid. Relative risks (RR) are comparison of extreme categories, except for DHA/EPA (per 250 mg/d increase) and alpha-linolenic acid (per 0.5 g/d). All nutrients were assessed from dietary intake, except vitamin D for which blood 25-hydroxyvitamin D was used.
Quantity and quality of carbohydrates
Prospective observational evidence suggests that the relative carbohydrate proportion of a diet does not appreciably influence diabetes risk.18 However, a diet rich in fiber, especially cereal fiber, may reduce diabetes risk. A meta-analysis of prospective cohort studies demonstrated an inverse association between fiber from cereal products and type 2 diabetes risk (Figure 2).19 Compared to cereal fiber, fiber from fruits had a weaker inverse association with diabetes risk.19
Carbohydrate quality can be determined by evaluating the glycemic response to carbohydrate-rich foods such as the glycemic index (GI) and the glycemic load (GL). In meta-analyses of prospective studies, low GI and GL diets were associated with lower risk for diabetes compared with diets with higher GI and GL (Figure 2),20 independent of the amount of cereal fiber in the diet.
Vitamins and minerals
Emerging evidence has supported the associations of specific minerals with type 2 diabetes using assessments of dietary intake and/or biomarkers (Figure 2). In a meta-analysis of prospective studies, magnesium intake was inversely associated with diabetes risk.21 This association was more pronounced among overweight than normal weight participants.21 Conversely, higher heme-iron intake was associated with higher diabetes risk.22 Similarly, higher iron stores reflected by elevated ferritin concentrations were associated with higher diabetes risk.22
An inverse association was shown between circulating 25-hydroxyvitamin D concentrations and diabetes risk in a meta-analysis of prospective studies from diverse populations.23 However, plasma vitamin D may be a marker of an overall healthful lifestyle such as frequent outdoor physical activities exposing to sunlight. Further, vitamin D supplementations did not improve hemoglobin A1C (HbA1C), fasting plasma glucose, or insulin sensitivity in small RCTs.24 Ongoing large RCTs will provide more conclusive evidence on the role of vitamin D in preventing type 2 diabetes.
Individual foods and food groups
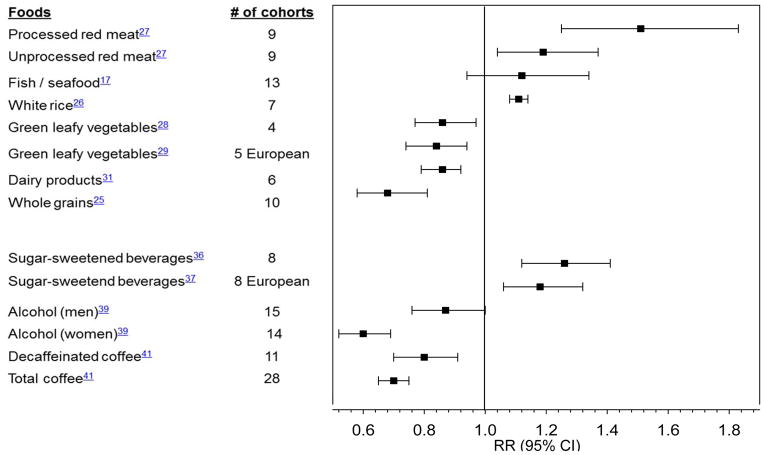
Prospective studies have provided evidence that intake of several individual food items or food groups may play a role in diabetes prevention (Figure 3; Supplemental Table 2). Whole grain intake has been consistently associated with lower diabetes risk.25 Conversely, greater intake of white rice, a processed grain, was associated with increased diabetes risk,26 especially among Asian populations with white rice as a staple food and a main source of calories. Frequent consumption of red meats, especially processed red meats such as bacon, sausages, and hot dogs, was strongly associated with higher diabetes risk.27
Figure 3. Summary of meta-analyses of prospective cohort studies on food and beverage intake and type 2 diabetes.
Relative risks (RR) are comparison of extreme categories, except for processed meat (per 50 g/d increase), unprocessed red meat and fish/sea food (per 100g/d), white rice (per each serving/d), whole grains (per 3 servings/d), sugar-sweetened beverages in European cohorts (per 336g/d), alcohol (abstainers with 22 g/d for men and with 24 g/d for women)
In a meta-analysis of prospective cohort studies, fish and/or seafood consumption was not significantly associated with diabetes risk.17 Interestingly, a difference in the direction of the association between fish/seafood consumption and diabetes risk was observed between geographical regions.17 Higher fish/seafood consumption was associated with higher diabetes risk in North America and Europe, but it was associated with lower risk in Asia.17 The reason for this regional variation is unclear, but may be explained by the differences in types of fish consumed, cooking methods used, and/or levels of exposure to pollutants in different geographical locations.
Total intake of fruits and vegetables was not associated with diabetes risk, but greater green leafy vegetable intake was associated with lower risk.28, 29 Further, consumption of specific whole fruits such as blueberries, grapes, and apples was significantly associated with lower diabetes risk based on three large prospective cohort studies.30
Consuming a greater amount of dairy products has been associated with moderately lower diabetes risk,31 and the benefits of yogurt appear to be more consistent than other types of dairy products. Consumption of nuts, which are high in PUFA and monounsaturated fatty acids (MUFA), may have beneficial effects on diabetes prevention. Greater nut consumption, especially walnuts, was associated with lower diabetes risk.32, 33 In the PREvención con DIeta MEDiterránea (PREDIMED) trial, supplementation of mixed nuts significantly reduced incident diabetes in a preliminary analysis from one center34 and a non-significant 18% reduction in the entire cohort.35 However, the nuts were supplemented in the context of a Mediterranean diet in this trial and, therefore, the beneficial results might not be solely attributed to nut consumption. Despite their high fat and energy contents, regular consumption of nuts was not associated with increased obesity, but instead conferred benefits in weight control.32
Beverages
Greater intake of sugar-sweetened beverages (SSBs) has been associated with higher type 2 diabetes risk in a meta-analysis36 and a recent pooled analysis of European cohorts37 (Figure 3). This association remains significant even after adjusting for BMI, suggesting that the deleterious effects of SSBs on diabetes are not entirely mediated by body weight. Substituting plain water, coffee, or tea for SSBs was associated with lower diabetes risk.38
Alcohol consumption is associated with diabetes in a U-shaped fashion (Figure 3).39 Based on a meta-analysis, the amounts of alcohol consumption most protective of diabetes were 24 g/d in women and 22 g/d in men, but alcohol became harmful at a consumption level above 50 g/d in women and 60 g/d in men.39 In a randomized trial,40 moderate alcohol consumption improved insulin sensitivity.
Coffee consumption has been consistently associated with lower risk (Figure 3). In a meta-analysis of 28 prospective cohort studies, coffee consumption was inversely associated with diabetes risk in a dose-response manner.41 Furthermore, both caffeinated and decaffeinated coffee intakes were associated with lower diabetes risk, suggesting that bioactive compounds other than caffeine may be responsible for the benefits.41
Dietary patterns and overall diet quality
Instead of considering individual food items in isolation, the application of food pattern techniques has led to a variety of different food patterns related to diabetes risk (Table 1). Mediterranean-style diets have been associated with lower incident type 2 diabetes in prospective cohort studies.34, 35, 42, 43 In the PREDIMED trial after a 4.1-year follow-up, participants assigned to a Mediterranean diet without calorie-restriction had a significant 40% diabetes risk reduction with extra-virgin olive oil supplementation and a non-significant 18% risk reduction with mixed nut supplementation compared to a low-fat control diet.35
Table 1.
Summary of observational and intervention studies on dietary patterns for diabetes prevention and management
| Main components | Diabetes prevention | Diabetes management | |
|---|---|---|---|
| Mediterranean diet | high consumption of minimally processed plant based foods; olive oil as the principal source of fat; low- to-moderate consumption of dairy products, fish, and poultry; low consumption of red meat; and low-to- moderate consumption of wine with meals | Mediterranean dietary patterns were associated with lower risk of type 2 diabetes in prospective cohort studies and RCTs.34, 35, 42, 43 | Mediterranean diets compared to a conventional diet for diabetes management improved glycemic control and insulin sensitivity, and reduced risk of CVD.43, 74, 80, 81 |
| Dietary Approaches to Stop Hypertension (DASH) | rich in vegetables, fruits, and low-fat dairy products, including whole grains, poultry, fish, and nuts; lower in saturated fat, red meat, sweets, and sugar containing beverages; and often reduced in sodium | Adherence to the DASH diet was associated with lower risk of diabetes.46, 47 | The DASH diet with 2 400mg/d sodium restriction had beneficial effects on glycemic control and CVD risk factors.86, 87 |
| Vegetarian and vegan | vegan, diets devoid all animal-derived products; vegetarian diets, diets devoid of some animal products including lacto- ovo (consuming dairy and/or eggs), pesco (consuming fish, eggs, and/or dairy), semi (consuming all but no red meat and poultry) | Vegan, lacto-ovo and semi-vegetarian diets were associated with lower risk of type 2 diabetes.47 | Improved glycemic control or CVD risk was not consistently reported,87, 88 and the effect of vegetarian diets was difficult to isolate because calorie-restriction was often implemented. |
| Dietary guidelines - Alternative Healthy Eating Index (AHEI) | indices of the diet quality created based on foods and nutrients predictive of chronic disease risk, including greater intake of vegetables and fruits, whole grains, nuts and legumes, long-chain omega-3 fatty acids, PUFAs; lower intake of sugar-sweetened beverages and fruit juice, red/processed meat, trans fat, sodium; and moderate alcohol consumption | Adherence to high quality diet assessed by AHEI was strongly associated with lower risk of diabetes.44 | NA |
| Prudent pattern | dietary patterns higher in fruits, vegetables, whole grains, and vegetable fats and lower in red meats, refined grains, and sugared soft drinks | Prudent dietary patterns over Western dietary patterns were associated with lower type 2 diabetes risk.48–53 | NA |
PUFA, polyunsaturated fatty acid; NA, not available
Adherence to a high quality diet assessed by the Alternative Healthy Eating Index (AHEI) was strongly associated with lower diabetes risk.44 Further, adherence to the Dietary Approaches to Stop Hypertension (DASH) diet, which is a dietary plan rich in vegetables, fruits, and low-fat dairy products, was also associated with lower diabetes risk.45, 46 Vegetarian diets devoid in animal products were demonstrated to reduce diabetes risk.47
Prospective studies using exploratory methods to define dietary patterns further supported that these dietary patterns favoring fruits, vegetables, whole grains, and legumes at the expense of red meats, refined grains, and SSBs are beneficial for diabetes prevention.48–53
Major knowledge gaps in the dietary prevention of diabetes
Although much has been learned about the role of various dietary factors in the development of diabetes, further studies are warranted to examine synergistic effects of individual components of various dietary patterns and to understand the biological mechanisms underlying the observed associations. Additional high quality, large prospective studies are needed to examine the role of different food choices and dietary habits for diabetes prevention in non-Western populations.
Dietary factors for the management of diabetes
Body weight-loss intervention trials and surgeries
The current nutritional therapy recommendations from various organizations for diabetes management support intensive lifestyle interventions to achieve modest weight-loss and weight-maintenance.54–57 In the Action for Health in Diabetes (Look AHEAD) trial, an intensive lifestyle intervention for weight-loss among overweight or obese adults with type 2 diabetes, weight-loss was greater in the intervention group than the control group (8.6% and 0.7% at 1-year; 6.0% and 3.5% at 9.6-year follow-up).58, 59 The participants randomized to the intervention experienced health benefits including reduced sleep apnea, depression, and urinary incontinence, in addition to improved health-related quality of life including requiring less medication for glycemic control and management of cardiovascular disease (CVD) risk factors.60–63 However, the Look AHEAD trial did not show a reduction in the rate of cardiovascular events in the intensive lifestyle intervention arm compared with the diabetes support and education arm.58, 59 This might be explained by several factors, including an unbalanced use of cardioprotective medications between the groups and very low event rates, which led to inadequate power for the hard endpoints.64 The intervention was focused on lowering caloric and fat intake, which potentially compromised the long-term compliance, and thus overall nutritional quality should have been a higher priority.65
If diabetes and associated comorbidities are difficult to control with lifestyle and pharmacological therapy, bariatric and metabolic surgeries may be considered in diabetes patients with BMI ≥35kg/m2.66 A meta-analysis showed that bariatric surgery, when compared to non-surgical treatments, led to greater body weight-loss (mean difference -26kg) and higher partial or complete remission rates of diabetes. 67 Further, participants in the Swedish Obese Subjects study who underwent bariatric surgery had lower cardiovascular events than those who received a conventional treatment.68
Macronutrients
Organizations vary in their recommendations for optimal macronutrient distributions for diabetes management. In more recent guidelines, a transition to favoring individualized goals and focusing on the quality of macronutrient intake was provided (Table 2).54–56 The latest 2014 American Diabetes Association (ADA) position statement recommends individualizing macronutrient distribution needs based on current eating patterns, preferences, and metabolic goals.54 Although the 2013 Canadian Diabetes Association (CDA) provides ranges of ideal macronutrient distribution for the management of diabetes, the guidelines also emphasize the importance of individualized dietary goals and quality of specific macronutrients.55
Table 2.
A comparison of nutrition therapy main recommendations for patients with type 2 diabetes from various organizations
| ADA 201454 | CDA 201355 | DNSG-EASD 200456 | |
|---|---|---|---|
| Energy balance | reducing energy intake while maintaining a healthful eating pattern to promote weight loss for overweight or obese adults | a nutritionally balanced calorie-reduced diet to achieve and maintain a lower, healthier body weight in overweight or obese people | reduced caloric intake to loose or maintain body weight among those BMI >25kg/m2 |
| Macronutrient distribution | use of individualized assessment because evidence suggests no one ideal distribution for all people | individualization within ranges of 45–60% carbohydrate, 15–20% protein, 20–35% fat of total energy | ranges of 45–60% carbohydrate, 10–20% protein, <35% fat of energy |
| Dietary eating patterns | a variety of eating patterns are acceptable with consideration for personal preferences and metabolic goals | a variety of dietary patterns are acceptable with consideration for personal preferences, values, and abilities | no specific recommendations |
| Glycemic index and glycemic load | substitute low glycemic load foods for higher glycemic load foods may be beneficial | choose food sources from a low glycemic index | low glycemic index foods are suitable as carbohydrate-rich choices |
| Dietary fiber and whole grains | consume at least the amount recommended for the general public (14 g/1000kcal or 25 g/d for women and 38g/d for men) | consume higher intake than those for the general public (25–50g/d or 15–25g/1000kcal) | consume fiber intake >40g/d (or 20g/1000kcal/d) with half as soluble; choose cereal-based foods high in fiber and whole grains |
| Sucrose and fructose | limit or avoid intake of sugar sweetened beverages | added sucrose or fructose can be substituted for other carbohydrate as a mixed meal up to a maximum of 10% total daily energy intake | moderate intake of free sugars (up to 50g/d) recommended without exceeding 10% total energy |
| Protein | reducing the amount of dietary protein below usual intake is not recommended for people with diabetes and kidney disease | usual intake recommended for those without kidney disese, but consider restricting protein to 0.8g/kg bodyweight for people with diabetes and chronic kidney disease | insufficient evidence to recommend protein restriction for those with type 2 diabetes and incipient nephropathy |
| MUFAs and PUFAs | MUFA-rich eating pattern may be beneficial | MUFAs up to 20% of energy and PUFAs up to 10% | 10–20% of energy from MUFAs and below 10% from PUFAs |
| Omega-3 fatty acids | no support for omega-3 fatty acid supplements | no support for omega-3 fatty acid supplements | no support for omega-3 fatty acid supplements |
| Saturated fat, dietary cholesterol, and trans fat | same as recommended for general public (<10% of energy, aiming for 300mg dietary cholesterol/d, limiting trans fat as much as possible) | no more than 7% of energy from saturated fats; limit intake of trans fatty acids to a minimum | under 10% of energy from saturated and trans fatty acids (<8% if LDL cholesterol is elevated); below 300mg/d cholesterol |
| Micronutrient supplements | no support for vitamin or mineral supplements | routine vitamin and mineral supplementation is generally not recommended | no recommendation for vitamin and mineral supplements |
| Alcohol | advised to drink in moderation with consideration for managing delayed hypoglycemia | same precautions as in the general public with additional consideration for risk of hypoglycemia and weight gain | moderate use of alcohol is acceptable with consideration for prolonged hypoglycemia and weight control |
| Sodium | reduce sodium intake less than 2 300 mg/d in general, and further reduction in sodium is to be individualized | no clear limit recommended for people with type 2 diabetes | restrict salt intake under 6g/d |
ADA, American Diabetes Association; CDA, Canadian Diabetes Association; DNSG-EASD, Diabetes and Nutrition Study Group of the. European Association for the Study of Diabetes; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid
Quality of carbohydrates
In a meta-analysis of RCTs with interventions >4 weeks among people with diabetes, participants on a low-GI diet had a significant reduction in HbA1C than those on a high-GI diet.69 Educating a person with diabetes to use GI and GL as tools is generally supported by various organizations to improve glycemic control.54–57 However, the literature regarding GI and GL is difficult to isolate from the benefits of dietary fiber because studies often investigate a combination of high-fiber and low-GI foods.54
Soluble fiber interventions have been shown to reduce HbA1C and plasma fasting glucose in people with diabetes.70 Several organizations recommended increasing fiber intake for diabetes management (Table 2).55, 56 However, the latest ADA guidelines did not recommend increasing above the level for the general public because the amount of fiber needed was unrealistically high (>50g/d) for modest lowering of HbA1C and preprandial glucose.54
Limiting intake of added sugars or SSBs has been recommended for diabetes management by various organizations (Table 2).54–56 Overconsumption of high fructose-sweetened beverages has adverse effects on selective deposition of visceral fat, lipid metabolism, blood pressure, insulin sensitivity, and de novo lipogenesis in overweight and obese individuals.71 However, naturally occurring fructose from whole fruits is unlikely to be deleterious because of its relatively slow digestion and absorption unless consumed in an excess amount (>10% of energy).55 Nonnutritive sweeteners may have potential to reduce overall calorie and carbohydrate intake.54 Short-term studies have shown that replacing added sugar with nonnutritive sweeteners reduces body weight and improves glycemic control, but the long-term effects need to be investigated.72
Protein
The current nutrition recommendations for adults with type 2 diabetes do not indicate prescribing a protein restriction.54, 55 For people on energy-reduced diets for weight-loss, however, it is important to maintain or increase protein intake because using a fixed percentage of total calories to estimate a protein requirement may result in inadequate protein intake and lean muscle loss.55
For individuals with diabetic kidney disease, either micro- or macroalbuminuria, the current recommendations for protein intake vary among organizations (Table 2).54–56 The European Association for the Study of Diabetes (EASD) states that there is insufficient evidence to make a firm recommendation.56 The CDA recommends to consider prescribing a protein restriction.55 The ADA, meanwhile, recommends against a protein restriction.54 A meta-analysis of RCTs did not show beneficial renal effects from low-protein diets in patients with diabetes.73
Fats
Evidence indicates that the type of fat consumed is more important than total fat intake in supporting metabolic goals.54, 74 Although the specific distributions of fat composition recommendations vary, organizations generally support limiting intake of saturated fat and trans fat from industrial hydrogenation to reduce CVD risk (Table 2).54–56 In a cohort study of women with diabetes, greater intake of saturated fat and cholesterol was associated with higher CVD risk,75 and greater intake of fish and long-chain omega-3 PUFA from food was associated with lower coronary heart disease incidence.76 However, omega-3 PUFA supplementation did not reduce risk of all-cause mortality, CVD mortality, or CVD events in a meta-analysis of RCTs.77 In the Outcome Reduction with an Initial Glargine Intervention (ORIGIN) trial involving 12,536 people with or at risk for diabetes, supplementation of omega-3 PUFA did not show a CVD or mortality benefit.78 Omega-3 PUFA supplementation is not recommended for people with diabetes,54–56 but an increase in foods containing omega-3 PUFA is recommended as it is for the general public.
In a meta-analysis of RCTs, a high MUFA diet (>12% of energy) was associated with improved fat mass, in addition to systolic and diastolic blood pressure.79 Substituting MUFA for carbohydrates or saturated fats among individuals with diabetes, those who consumed high MUFA diets had favorable glycemic control after 2-y intervention.80
Dietary patterns
Several dietary patterns consisting of combinations of different foods or food groups are beneficial for diabetes management (Table 3). Organizations have recommended using these dietary patterns with consideration for personal preferences and metabolic goals.54, 55 In a systematic review of 5 RCTs among people with type 2 diabetes,43 improvement in glycemic control and insulin sensitivity was greater in participants on a Mediterranean diet than other commonly used diets, although the magnitude of results needs to be interpreted with a caution because energy restriction was also included in few RCTs80, 81 A Mediterranean diet reduced anti-diabetes medications in overweight patients with newly diagnosed diabetes compared to those on a low-fat diet.81 In a subgroup of moderately obese participants with diabetes from the Dietary Intervention Randomized Controlled Trial (DIRECT), a calorie-restricted Mediterranean diet resulted in more favorable fasting plasma glucose and insulin concentrations at 2 years than a low-fat diet.80 In a subgroup of the PREDIMED trial participants with diabetes, Mediterranean diet interventions supplemented with extra-virgin olive oil or nuts, without calorie-restriction, significantly reduced incidence of major CVD events after a median 4.8-year follow-up.74
The DASH diet has been shown to lower blood pressure in people without (or controlled) diabetes.82, 83 In a small 8-week RCT among people with diabetes, the DASH diet, including the 2,400mg/d sodium restriction, had favorable effects on glycemic control, high-density lipoprotein and low-density lipoprotein (LDL) cholesterol, blood pressure, and inflammatory biomarkers.84, 85 In one observational study, low sodium intake was associated with increased mortality in people with diabetes,86 but reverse causation might explain this result. The current sodium intake recommendation for diabetes management from the ADA is ≤2,300mg/d,54 and other organizations’ recommendations are summarized in Table 2.
Several low-fat vegetarian or vegan diet trials have been conducted in people with diabetes,87 but improved glycemic control or CVD risk was not consistently reported in these studies.87 The effect of vegetarian diets might have been difficult to isolate because many trials implemented calorie-restriction. In a 74-week intervention trial, a low-fat vegan diet, without energy restriction, resulted in weight-loss and improved fasting glucose, triglyceride, and LDL cholesterol, and the low-fat vegan diet was more beneficial than a conventional diet after controlling for medication changes.88
A meta-analysis of RCTs suggested that various dietary patterns such as low-carbohydrate, low-GI, Mediterranean, and high-protein diets were effective in improving glycemic control and CVD risk factors compared to diets in diabetic patients.87 These results provide a range of dietary options for diabetes management, paying attention to overall diet quality, treatment goals, and personal and cultural food preferences.
Vitamin and mineral supplementation
The current nutrition therapy recommendations do not support vitamin or mineral supplementation in people with diabetes who do not have underlying deficiencies (Table 2).54–56 However, people with diabetes should be informed about the importance of acquiring daily vitamin and mineral requirements through a well-balanced diet because people with poorly controlled diabetes often experience micronutrient deficiencies.54 Select populations with diabetes, including the elderly, pregnant and lactating women, vegetarians, and those on calorie-restricted diets, should be aware of additional supplemental needs specific to that individual population.
Alcohol
Organizations from North America and Europe recommend moderate alcohol consumption for people with diabetes as in the general public with consideration for risk of weight gain and hypoglycemia, especially if taking insulin or insulin secretgogues.54–56 Similar to the general public, moderate alcohol consumption has been associated with a lower risk of mortality and coronary heart disease in people with diabetes.89 In a metabolic study, people with type 2 diabetes did not experience delayed hypoglycemia when alcohol was consumed with food.90 However, the recommendations need to be delivered in a culturally appropriate context because excess alcohol drinking is one of the top leading causes of disease burden in Eastern Europe and Latin America, and alcohol consumption is increasing steadily in many Asian countries.4
Major knowledge gaps in the dietary management of diabetes
Larger and longer term RCTs are needed to compare relative efficacy and effectiveness of various dietary approaches in the diabetes management. Personalized nutrition therapy, a promising concept, is yet to be investigated in the context of diabetes management. High quality, large sample size intervention and observational studies and region-specific recommendations are lacking from diverse populations and cultures.
Summary and global perspectives
Economic growth and environmental transitions have led to drastic changes in food production, processing, and distribution systems and have increased the accessibility of unhealthful foods.3 With nutritional transitions, men and women around the globe have experienced excess body weight gain accompanied by increased diabetes incidence and mortality.4
In the past two decades, evidence from prospective cohort studies and RCTs has surged to highlight the importance of individual nutrients, foods, and dietary patterns in type 2 diabetes prevention and management. The convergence of dietary factors for prevention and management of diabetes was observed, and healthful dietary patterns for diabetes prevention and management were typically rich in whole grains, fruits and vegetables, nuts, legumes, moderate in alcohol consumption, and lower in refined grains, red/processed meats, and SSBs. To achieve long-term adherence, individuals can have flexibility in food choices without compromising overall diet quality.
The vast majority of present knowledge on dietary prevention and management of diabetes has been derived from Western populations. It is critically important to conduct original investigations in other populations with different disease susceptibility and eating habits. Evidence-based nutrition therapy recommendations have been developed and implemented in many developed countries.54–56 However, further development of region specific guidelines is needed to provide practical educational instruments considering variation in dietary patterns, accessibility to foods, and agricultural in different regions and cultures
Global public health policies are warranted across multiple sectors to create healthful food environment and promote corporate social responsibility. Potential strategies include nutrition and agricultural policies that favor the production and distribution of healthful food, for example, taxing highly processed food and instituting agricultural subsidies that increase accessibility and affordability of whole foods. Global efforts, such as homogenizing standardization of front-of-package nutrition labels and nutrition facts in conjunction with public education campaigns, will reshape the trajectory of nutritional transition and the global food supply helping to curb the type 2 diabetes epidemic.
Supplementary Material
Supplemental Table 1. Summary of meta-analyses of prospective cohort studies on nutrient intake and type 2 diabetes
Supplemental Table 2. Summary of meta-analyses of prospective cohort studies on food and beverage intake and type 2 diabetes
References
- 1.International Diabetes Federation. [accessed January 30, 2014];IDF Diabetes Atlas. (6). 2013 http://www.idf.org/diabetesatlas. [PubMed]
- 2.Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care. 2011;34(6):1249–57. doi: 10.2337/dc11-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70(1):3–21. doi: 10.1111/j.1753-4887.2011.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezzati M, Riboli E. Behavioral and dietary risk factors for noncommunicable diseases. N Engl J Med. 2013;369(10):954–64. doi: 10.1056/NEJMra1203528. [DOI] [PubMed] [Google Scholar]
- 5.Hu FB. Obesity epidemiology. New York, New York: Oxford University Press; 2008. Metabolic consequences of obesity; pp. 149–73. [Google Scholar]
- 6.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 7.Vazquez G, Duval S, Jacobs DR, Jr, Silventoinen K. Comparison of body mass index, waist circumference, and waist/hip ratio in predicting incident diabetes: a meta-analysis. Epidemiol Rev. 2007;29:115–28. doi: 10.1093/epirev/mxm008. [DOI] [PubMed] [Google Scholar]
- 8.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tuomilehto J, Lindström J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 10.Pan XR, Li GW, Hu YH, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 11.Ramachandran A, Snehalatha C, Mary S, Mukesh B, Bhaskar AD, Vijay V. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49(2):289–97. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 12.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res. 2009;48(1):44–51. doi: 10.1016/j.plipres.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44(7):805–17. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- 14.Halton TL, Liu S, Manson JE, Hu FB. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am J Clin Nutr. 2008;87(2):339–46. doi: 10.1093/ajcn/87.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tinker LF, Bonds DE, Margolis KL, et al. Low-fat dietary pattern and risk of treated diabetes mellitus in postmenopausal women: the Women’s Health Initiative randomized controlled dietary modification trial. Arch Intern Med. 2008;168(14):1500–11. doi: 10.1001/archinte.168.14.1500. [DOI] [PubMed] [Google Scholar]
- 16.Salmerón J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr. 2001;73(6):1019–26. doi: 10.1093/ajcn/73.6.1019. [DOI] [PubMed] [Google Scholar]
- 17.Wu JH, Micha R, Imamura F, et al. Omega-3 fatty acids and incident type 2 diabetes: a systematic review and meta-analysis. Br J Nutr. 2012;107(Suppl 2):S214–S27. doi: 10.1017/S0007114512001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauner H, Bechthold A, Boeing H, et al. Evidence-based guideline of the German Nutrition Society: carbohydrate intake and prevention of nutrition-related diseases. Ann Nutr Metab. 2012;60(Suppl 1):1–58. doi: 10.1159/000335326. [DOI] [PubMed] [Google Scholar]
- 19.Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and magnesium intake and incidence of type 2 diabetes: a prospective study and meta-analysis. Arch Intern Med. 2007;167(9):956–65. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- 20.Bhupathiraju SN, Tobias DK, Malik VS, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014 doi: 10.3945/ajcn.113.079533. Under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong J-Y, Xun P, He K, Qin L-Q. Magnesium intake and risk of type 2 diabetes: meta-analysis of prospective cohort studies. Diabetes Care. 2011;34(9):2116–22. doi: 10.2337/dc11-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Z, Li S, Liu G, et al. Body iron stores and heme-iron intake in relation to risk of type 2 diabetes: a systematic review and meta-analysis. PLoS ONE. 2012;7(7):e41641. doi: 10.1371/journal.pone.0041641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song Y, Wang L, Pittas AG, et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of prospective studies. Diabetes Care. 2013;36(5):1422–8. doi: 10.2337/dc12-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitri J, Muraru MD, Pittas AG. Vitamin D and type 2 diabetes: a systematic review. Eur J Clin Nutr. 2011;65(9):1005–15. doi: 10.1038/ejcn.2011.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28(11):845–58. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- 26.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ. 2012:344. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan A, Sun Q, Bernstein AM, et al. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am J Clin Nutr. 2011;94(4):1088–96. doi: 10.3945/ajcn.111.018978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ. 2010;341:c4229. doi: 10.1136/bmj.c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper AJ, Forouhi NG, Ye Z, et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr. 2012;66(10):1082–92. doi: 10.1038/ejcn.2012.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ. 2013:347. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tong X, Dong JY, Wu ZW, Li W, Qin LQ. Dairy consumption and risk of type 2 diabetes mellitus: a meta-analysis of cohort studies. Eur J Clin Nutr. 2011;65(9):1027–31. doi: 10.1038/ejcn.2011.62. [DOI] [PubMed] [Google Scholar]
- 32.Jiang R, Manson JE, Stampfer MJ, Liu S, Willett WC, Hu FB. Nut and peanut butter consumption and risk of type 2 diabetes in women. JAMA. 2002;288(20):2554–60. doi: 10.1001/jama.288.20.2554. [DOI] [PubMed] [Google Scholar]
- 33.Pan A, Sun Q, Manson JE, Willett WC, Hu FB. Walnut consumption is associated with lower risk of type 2 diabetes in women. J Nutr. 2013;143(4):512–8. doi: 10.3945/jn.112.172171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salas-Salvadó J, Bulló M, Babio N, et al. Reduction in the incidence of type 2 diabetes with the Mediterranean diet: results of the PREDIMED-Reus nutrition intervention randomized trial. Diabetes Care. 2011;34(1):14–9. doi: 10.2337/dc10-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Salas-Salvadó J, Bulló M, Estruch R, et al. Prevention of diabetes With Mediterranean diets: A subgroup analysis of a randomized trial. Ann Intern Med. 2014;160(1):1–10. doi: 10.7326/M13-1725. [DOI] [PubMed] [Google Scholar]
- 36.Malik VS, Popkin BM, Bray GA, Després J-P, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33(11):2477–83. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The Inter Act Consortium. Consumption of sweet beverages and type 2 diabetes incidence in European adults: results from EPIC-InterAct. Diabetologia. 2013;56(7):1520–30. doi: 10.1007/s00125-013-2899-8. [DOI] [PubMed] [Google Scholar]
- 38.Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. Am J Clin Nutr. 2012;95(6):1454–60. doi: 10.3945/ajcn.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baliunas DO, Taylor BJ, Irving H, et al. Alcohol as a risk factor for type 2 diabetes. Diabetes Care. 2009;32(11):2123–32. doi: 10.2337/dc09-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joosten MM, Beulens JWJ, Kersten S, Hendriks HFJ. Moderate alcohol consumption increases insulin sensitivity and ADIPOQ expression in postmenopausal women: a randomised, crossover trial. Diabetologia. 2008;51(8):1375–81. doi: 10.1007/s00125-008-1031-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding M, Bhupathiraju SN, Chen M, van Dam R, Hu FB. Caffeinated and decaffeinated coffee consumption and riskof type 2 diabetes: a systematic review and a dose-response meta-analysis. Diabetes Care. 2014;37(2):569–86. doi: 10.2337/dc13-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.The Inter Act Consortium. Mediterranean diet and type 2 diabetes risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) study: the InterAct project. Diabetes Care. 2011;34(9):1913–8. doi: 10.2337/dc11-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esposito K, Maiorino MI, Ceriello A, Giugliano D. Prevention and control of type 2 diabetes by Mediterranean diet: a systematic review. Diabetes Res Clin Pract. 2010;89(2):97–102. doi: 10.1016/j.diabres.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 44.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liese AD, Nichols M, Sun X, D’Agostino RB, Haffner SM. Adherence to the DASH Diet is inversely associated with incidence of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes Care. 2009;32(8):1434–6. doi: 10.2337/dc09-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Koning L, Chiuve SE, Fung TT, Willett WC, Rimm EB, Hu FB. Diet-quality scores and the risk of type 2 diabetes in men. Diabetes Care. 2011;34(5):1150–6. doi: 10.2337/dc10-2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tonstad S, Stewart K, Oda K, Batech M, Herring RP, Fraser GE. Vegetarian diets and incidence of diabetes in the Adventist Health Study-2. Nutr Metab Cardiovasc Dis. 2013;23(4):292–9. doi: 10.1016/j.numecd.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heidemann C, Hoffmann K, Spranger J, et al. A dietary pattern protective against type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam Study cohort. Diabetologia. 2005;48(6):1126–34. doi: 10.1007/s00125-005-1743-1. [DOI] [PubMed] [Google Scholar]
- 49.Imamura F, Lichtenstein AH, Dallal GE, Meigs JB, Jacques PF. Generalizability of dietary patterns associated with incidence of type 2 diabetes mellitus. Am J Clin Nutr. 2009;90(4):1075–83. doi: 10.3945/ajcn.2009.28009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liese AD, Weis KE, Schulz M, Tooze JA. Food intake patterns associated with incident type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2009;32(2):263–8. doi: 10.2337/dc08-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McNaughton SA, Mishra GD, Brunner EJ. Dietary patterns, insulin resistance, and incidence of type 2 diabetes in the Whitehall II Study. Diabetes Care. 2008;31(7):1343–8. doi: 10.2337/dc07-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82(3):675–84. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fung TT, Schulze M, Manson JE, Willett WC, Hu FB. DIetary patterns, meat intake, and the risk of type 2 diabetes in women. Arch Intern Med. 2004;164(20):2235–40. doi: 10.1001/archinte.164.20.2235. [DOI] [PubMed] [Google Scholar]
- 54.Evert AB, Boucher JL, Cypress M, et al. Nutrition therapy recommendations for the management of adults with diabetes. Diabetes Care. 2014;37(Supplement 1):S120–S43. doi: 10.2337/dc14-S120. [DOI] [PubMed] [Google Scholar]
- 55.Canadian Diabetes Assocaition Clinical Practice Guidelines Expert Committee. Clinical Practice Guidelines: Nutrition Therapy. Can J Diabetes. 2013;37:S45–S55. doi: 10.1016/j.jcjd.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 56.Mann JI, De Leeuw I, Hermansen K, et al. Evidence-based nutritional approaches to the treatment and prevention of diabetes mellitus. Nutr Metab Cardiovasc Dis. 2004;14(6):373–94. doi: 10.1016/s0939-4753(04)80028-0. [DOI] [PubMed] [Google Scholar]
- 57.Dyson PA, Kelly T, Deakin T, et al. Diabetes UK evidence-based nutrition guidelines for the prevention and management of diabetes. Diabet Med. 2011;28(11):1282–8. doi: 10.1111/j.1464-5491.2011.03371.x. [DOI] [PubMed] [Google Scholar]
- 58.The Look AHEAD Research Group. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30(6):1374–83. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.The Look AHEAD Research Group. Cardiovascular Effects of Intensive Lifestyle Intervention in Type 2 Diabetes. N Engl J Med. 2013;369(2):145–54. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Faulconbridge LF, Wadden TA, Rubin RR, et al. One-year changes in symptoms of depression and weight in overweight/obese individuals with type 2 diabetes in the Look AHEAD study. Obesity. 2012;20(4):783–93. doi: 10.1038/oby.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster GD, Borradaile KE, Sanders MH, et al. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: The sleep ahead study. Arch Intern Med. 2009;169(17):1619–26. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Phelan S, Kanaya AM, Subak LL, et al. Weight loss prevents urinary incontinence in women with type 2 diabetes: results from the Look AHEAD trial. J Urol. 2012;187(3):939–44. doi: 10.1016/j.juro.2011.10.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williamson DA, Rejeski J, Lang W, et al. Impact of a weight management program on health-related quality of life in overweight adults with type 2 diabetes. Arch Intern Med. 2009;169(2):163–71. doi: 10.1001/archinternmed.2008.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gerstein HC. Do lifestyle changes reduce serious outcomes in diabetes? N Engl J Med. 2013;369(2):189–90. doi: 10.1056/NEJMe1306987. [DOI] [PubMed] [Google Scholar]
- 65.Martínez-González MA, Salas-Salvadó J, Estruch R. Intensive Lifestyle Intervention in Type 2 Diabetes. N Engl J Med. 2013;369(24):2357. doi: 10.1056/NEJMc1312802. [DOI] [PubMed] [Google Scholar]
- 66.American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37(Supplement 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 67.Gloy VL, Briel M, Bhatt DL, et al. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347 doi: 10.1136/bmj.f5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307(1):56–65. doi: 10.1001/jama.2011.1914. [DOI] [PubMed] [Google Scholar]
- 69.Thomas DE, Elliott EJ. The use of low-glycaemic index diets in diabetes control. Br J Nutr. 2010;104(06):797–802. doi: 10.1017/S0007114510001534. [DOI] [PubMed] [Google Scholar]
- 70.Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Gross JL, Azevedo MJ. Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev. 2013;71(12):790–801. doi: 10.1111/nure.12076. [DOI] [PubMed] [Google Scholar]
- 71.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gardner C, Wylie-Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2012;126(4):509–19. doi: 10.1161/CIR.0b013e31825c42ee. [DOI] [PubMed] [Google Scholar]
- 73.Pan Y, Guo LL, Jin HM. Low-protein diet for diabetic nephropathy: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2008;88(3):660–6. doi: 10.1093/ajcn/88.3.660. [DOI] [PubMed] [Google Scholar]
- 74.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368(14):1279–90. doi: 10.1056/NEJMoa1200303. [DOI] [PubMed] [Google Scholar]
- 75.Tanasescu M, Cho E, Manson JE, Hu FB. Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am J Clin Nutr. 2004;79(6):999–1005. doi: 10.1093/ajcn/79.6.999. [DOI] [PubMed] [Google Scholar]
- 76.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107(14):1852–7. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 77.Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: A systematic review and meta-analysis. JAMA. 2012;308(10):1024–33. doi: 10.1001/2012.jama.11374. [DOI] [PubMed] [Google Scholar]
- 78.ORIGIN Trial Investigators. Bosch J, Gerstein HC, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 79.Schwingshackl L, Strasser B, Hoffmann G. Effects of monounsaturated fatty acids on cardiovascular risk factors: a systematic review and meta-analysis. Ann Nutr Metab. 2011;59(2–4):176–86. doi: 10.1159/000334071. [DOI] [PubMed] [Google Scholar]
- 80.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229–41. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 81.Esposito K, Maiorino MI, Ciotola M, et al. Effects of a Mediterranean-style diet on the need for antihyperglycemic drug therapy in patients with newly diagnosed type 2 diabetes: a randomized trial. Ann Intern Med. 2009;151(5):306–14. doi: 10.7326/0003-4819-151-5-200909010-00004. [DOI] [PubMed] [Google Scholar]
- 82.Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med. 1997;336(16):1117–24. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 83.Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med. 2001;344(1):3–10. doi: 10.1056/NEJM200101043440101. [DOI] [PubMed] [Google Scholar]
- 84.Azadbakht L, Fard NRP, Karimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care. 2011;34(1):55–7. doi: 10.2337/dc10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Azadbakht L, Surkan PJ, Esmaillzadeh A, Willett WC. The Dietary Approaches to Stop Hypertension eating plan affects C-reactive protein, coagulation abnormalities, and hepatic function tests among type 2 diabetic patients. J Nutr. 2011;141(6):1083–8. doi: 10.3945/jn.110.136739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ekinci EI, Clarke S, Thomas MC, et al. Dietary salt intake and mortality in patients with type 2 diabetes. Diabetes Care. 2011;34(3):703–9. doi: 10.2337/dc10-1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr. 2013;97(3):505–16. doi: 10.3945/ajcn.112.042457. [DOI] [PubMed] [Google Scholar]
- 88.Barnard ND, Cohen J, Jenkins DJ, et al. A low-fat vegan diet and a conventional diabetes diet in the treatment of type 2 diabetes: a randomized, controlled, 74-wk clinical trial. Am J Clin Nutr. 2009;89(5):1588S–96S. doi: 10.3945/ajcn.2009.26736H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Koppes LLJ, Dekker JM, Hendriks HFJ, Bouter LM, Heine RJ. Meta-analysis of the relationship between alcohol consumption and coronary heart disease and mortality in type 2 diabetic patients. Diabetologia. 2006;49(4):648–52. doi: 10.1007/s00125-005-0127-x. [DOI] [PubMed] [Google Scholar]
- 90.Bantle AE, Thomas W, Bantle JP. Metabolic effects of alcohol in the form of wine in persons with type 2 diabetes mellitus. Metabolism. 2008;57(2):241–5. doi: 10.1016/j.metabol.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Summary of meta-analyses of prospective cohort studies on nutrient intake and type 2 diabetes
Supplemental Table 2. Summary of meta-analyses of prospective cohort studies on food and beverage intake and type 2 diabetes