Abstract
Since 2009, the HIV vaccine field has worked to define correlates of risk associated with HIV-1 acquisition based upon the partial efficacy found in the RV144 trial. Both immunological and genetic pressure on the virus has been demonstrated by Fc antiviral antibodies largely directed at conserved regions of the V1V2 loop including antibody dependent cellular cytotoxicity (ADCC) to HIV envelope in the absence of inhibiting serum IgA antibodies. CD4+ T-cell responses to HIV envelope also correlate with reduced acquisition. Recently, NHP studies using vaccine regimens that differ from that used in RV144 also indicate that non-neutralizing antibodies are associated with protection from experimental lentivirus challenge. These immunological correlates have provided the basis for the design of a next generation of vaccine regimens to improve upon the qualitative and quantitative degree of magnitude of these immune responses on HIV acquisition.
Introduction
Over 30 years into the HIV-1 pandemic, the need for a globally effective HIV-1 vaccine is more compelling than ever. Biomedical interventions to reduce HIV-1 acquisition, such as population-based antiretroviral therapy, large scale circumcision programs, pre- and post-exposure prophylaxis with tenofovir-based regimens for high risk individuals, and the use of antiretroviral drugs to prevent mother to child transmission of HIV-1 have favorably influenced the trajectory of HIV-1 infections in several populations throughout the world (1). However, micro-epidemics of HIV-1 persist throughout all affected countries, and overall population prevalence of HIV-1 remains relatively stable, including in the US and Europe (2, 3), and UNAIDS estimates that there were 2.1 million new infections in 2013 (4). Vaccines are historically the primary public health intervention for prevention of a wide range of infectious diseases, and thus would provide the most cost effective, durable, and accepted approach to reduce HIV-1 infection. However, developing a safe and effective HIV-1 vaccine has proven to be a considerable scientific challenge (5, 6). A milestone in the field of HIV-1 vaccine development was achieved in September 2009 with the report of the RV144 trial, which evaluated a regimen consisting of a replication-defective canarypox vector (ALVAC) in combination with a recombinant gp120 protein (AIDSVAX), administered intramuscularly to over 16,000 heterosexual men and women at risk of HIV-1 infection in Thailand (7). This regimen demonstrated a statistically significant, albeit modest, reduction in HIV-1 acquisitions. While the first year vaccine efficacy (VE) approached 60%, efficacy waned over time and overall VE over the 3.5 years of the trial was 31.2%.
The results of this trial were, in many quarters, quite unexpected. Preclinical testing had indicated that the replication-defective pox virus vector (ALVAC) was less immunogenic than many other candidate prototypes in human testing, such as recombinant adenovirus vectors (8, 9), and the AIDSVAX® clade B/E protein vaccine, used as a boost to the ALVAC vector, had when used alone proven ineffective in preventing HIV-1 among IV drug users in Bangkok (8–10). Moreover, a bivalent clade B gp120 recombinant protein vaccine was ineffective in reducing acquisition of HIV-1 in men who have sex with men (MSM) in North America, Australia and the Netherlands (11). That the combination of these 2 immunogens would achieve a statistically significant reduction in HIV-1 acquisition among heterosexual Thai men and women was met with surprise and, by some, skepticism, especially since the number of infections was relatively small as compared to the size of the large trial participant population studied (125 infections in 16,395 participants, of which 51 infections occurred in 8,197 vaccinees). Shortly after announcing the results, the organizers of the RV144 trial (the US Military HIV Research Program [USMHRP] and the Thai government) in collaboration with the National Institute of Allergy and Infectious Diseases (NIAID), the primary funder of the trial, mobilized a major scientific effort to evaluate whether one could define immune responses among vaccinated individuals that were associated with protection, with the goal of developing biological underpinnings and/or hypotheses that could lead to a better understanding of the trial findings. More importantly, could these studies provide a roadmap to construct future iterations of HIV-1 vaccine regimens that could improve upon the RV144 outcome? Defining such responses would markedly enhance the ability to construct vaccine regimens that could be tested in areas of the world with markedly higher prevalence and higher exposure rates than those in Thailand.
Over the last 4 years, an international collaboration has produced a significant number of provocative scientific findings that raise the hypothesis that antibody-mediated protection played the predominant role in the efficacy observed in the RV144 trial. This article will outline these findings, describe how follow-up vaccine trials, planned to be conducted predominantly in southern Africa, are being designed to build upon these results, and hopefully provide both benchmarks and additional insights into how to develop the globally effective vaccines that are needed to successfully reduce HIV-1 acquisition worldwide.
Defining Correlates of Immunity
The process of defining correlates of immunity associated with vaccination in the RV144 trial was driven by both laboratory scientists and statisticians, and followed an analysis plan that included examining a large variety of laboratory assays so that statistical validity could be defined from the available samples. The overall strategy utilized a case control format in which specimens at the week 26 visit (2 weeks post last vaccination) from all 41 vaccine recipients who acquired HIV-1 infection after week 26 were included (12). For comparison, week 26 specimens from a random sample of 205 controls (vaccine recipients who were HIV-1 negative at the end of the study follow-up period at month 42) were also included. In addition, specimens from 40 placebo recipients were included.
Prior to beginning the case control study, a pilot study, which was conducted between November 2009 and July 2011, evaluated 32 assays from 20 immunology laboratories of innate immunity, T-cell, and antibody responses on 4 criteria: low false positive rate (comparing vaccine responses to baseline and to placebo responses), broad variability of responses across vaccinees, low correlation of responses (non-redundant responses), and high reproducibility of within-vaccinee replicate samples. The pilot study assessed samples from both vaccine (n=80 or n=40) and placebo (n=20 or n=10) recipients who were HIV-1 negative at month 42. Seventeen assays measuring a wide range of both antibody and T-cell responses were chosen for this initial case control evaluation, from which six immune response variables were pre-specified for the primary correlates analysis which then provided evidence for their association with vaccine efficacy (VE) 1) the binding of IgG antibodies to the V1V2 region of gp120; 2) the binding of plasma IgA antibodies to Env; 3) the avidity of IgG antibodies for Env; 4) antibody dependent cellular cytotoxicity (ADCC); 5) neutralizing antibodies; and 6) the magnitude of CD4+ T cells specific for HIV-1 Env. Immune responses 1) and 2) were significantly associated with vaccine efficacy after multiplicity correction. In secondary analyses without multiplicity correction, immune responses 3) through 6) were also significantly associated with vaccine efficacy in vaccinees with low plasma IgA responses (12).
Non-neutralizing Antibodies and Correlates of Efficacy
The observed protective efficacy in RV144 was surprising given the low titer and narrow scope of neutralizing antibodies elicited by the RV144 vaccine regimen (8, 12, 13). None of the sera from RV144 recipients, even at peak levels of antibody response, neutralized a panel of 20 contemporaneous isolates of HIV-1 circulating in Thailand during the course of the trial. While neutralizing antibodies were uncommon, essentially all RV144 vaccine recipients developed binding antibodies to gp120 (12). Titers of these antibodies peaked shortly after the 6 month boost, and as seen in Figure 1 rapidly waned over time, a pattern that tracked the degree of vaccine efficacy. To pursue whether unique regions of gp120 were more closely associated with protection, several linear and conformational peptides were evaluated both in the pilot and case control studies. Postvaccination sera tested against a linear peptide array derived from the A244 CF01_AE strain of HIV-1 (used in both the ALVAC vector and gp120 protein) exhibited a high binding pattern to peptides in the V1V2 and V3 regions of the HIV-1 envelope, a pattern of reactivity that differed considerably from HIV-1 infected patients (14, 15). The secondary case control analysis indicated that IgG Ab responses to a series of relatively conserved amino acids on the crown of the V1V2 loop (residues 163–178) were associated with reduced HIV-1 acquisition (relative risk [RR], 0.57 for reactive vs. non-reactive vaccinees, vaccine efficacy [VE] for reactive vaccinees 43% over the 3.5 year study). Due to insertion of an HSV gD sequence utilized for purification of the gp120, the A244 CF01_AE gp120 Env in RV144 contained an N-terminal deletion of 11 amino acids in the HIV-1 sequence that enhanced exposure of these V1V2 epitopes (16).
Figure 1. Kinetics of vaccine induced antibody response and vaccine protection in RV144.
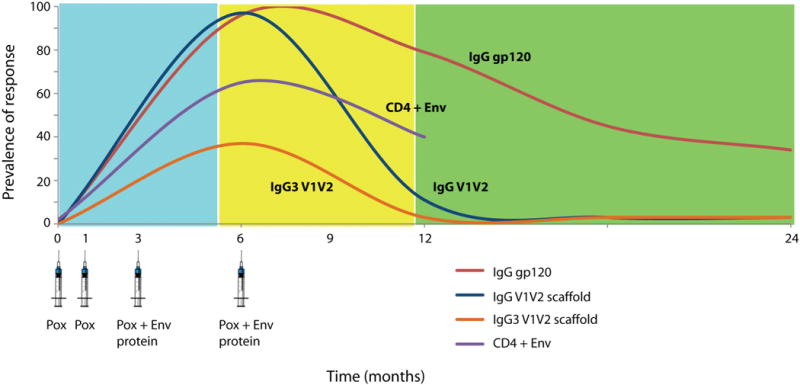
Schematic representation of selected immune responses to vaccination with RV144 regimen ALVAC (pox) followed by gp120 (protein).
Vaccinees with the highest binding antibody titers were more likely to be protected than those with lower titers, with a reasonably linear association between the peak concentration to the V1V2 scaffold and VE (Figure 2a). Follow-up studies using a wide variety of V1V2 antigens including antigens derived from a variety of clade C isolates indicated similar associations between high binding to V1V2 and reduced HIV-1 acquisition (17). Overall, about 84% of vaccine recipients developed antibodies to the V1V2 loop (14). Those with the highest magnitude exhibited greater protection from infection; those with the highest titers to the original gp70-V1V2 protein in the upper third having VE of 60% (12), compared to no protection for those with negative or lowest third titers (Figure 2b), a result that was recapitulated for titer responses to eight other gp70-V1V2 proteins (average VE=47% for the upper third of responders) (17). The concentration of IgG to the V1V2 correlates in RV144 was a median of 1.63 mcg/mL, with those in the lower tertile below 0.98 mcg/mL and those in the upper tertile, associated with a decrease in HIV infection, having a titer of at least 2.98 mcg/mL (Figure 2c). Of note, the V1V2 antibody response rate and magnitude waned over time in temporal association with the waning efficacy.
Figure 2. Estimated vaccine efficacy in RV144.
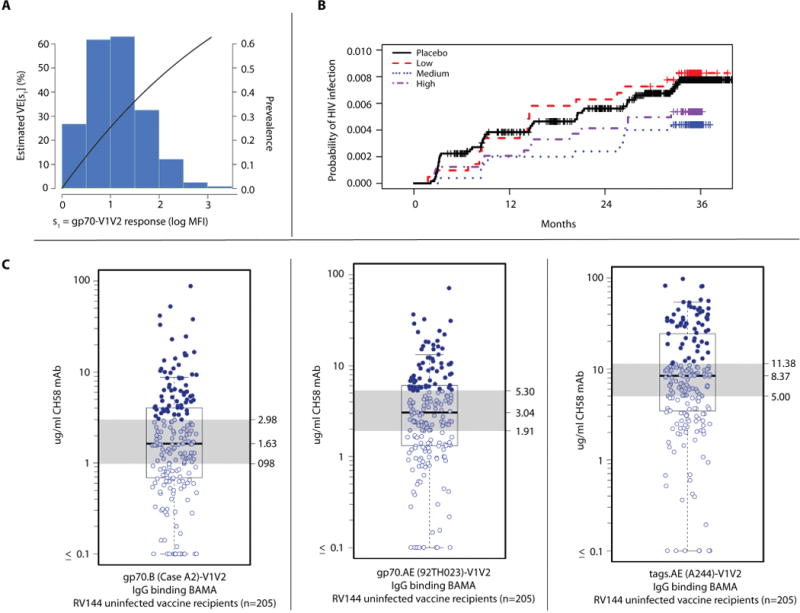
Estimated vaccine efficacy in RV144 as a function of the level of IgG binding antibody to gp70-scaffolded V1V2 in a model assuming VE of 0% in vaccinees with no V1V2 antibodies (black line) and the distribution of IgG levels among vaccinees (histogram) (Panel A). Kaplan-Meir curve of the probability of acquiring HIV infection in vaccine recipients with Low, Medium and High V1V2-scaffold IgG Ab responses, measured by BAMA at Week 26 (Panel B). The median, upper, and lower bounds of antibody concentrations to 3 of the V1V2 antigens that were correlated to vaccine efficacy: the left panel shows the cross-clade clade B gp70 levels; the middle panel the V1V2 response to the AE isolate in the ALVAC vector used in RV144; and the third panel shows the V1V2 responses to the AE gp120 used in the protein boost in RV144 (Panel C).
Epitope mapping of the antibodies to V1V2 indicated that much of the immune response was directed at a linear epitope interval including a lysine residue at amino acid 169 in the Env V2 region. Antibodies from RV144 vaccinees that bound to the K169 V2 region did not neutralize nor capture hard to neutralize (tier 2) AE viruses but did bind to Env on tier 2 AE virus-infected CD4 T cells and mediate ADCC. While antibodies to the C1 region themselves did not correlate with decreased transmission risk (15), addition of conformational C1-specific antibodies from RV144 induced memory B cells to A244 CF01_AE -infected CD4 T cells synergized with RV144 V2 antibodies for enhanced ADCC activity (18). These data give rise to the hypothesis that one candidate of the protective anti-HIV-1 antibody effector function induced by the RV144 vaccine was a non-broadly neutralizing and most likely FcR-mediated action that included ADCC activity (see below) (19). Isolation of antibodies that bind to the V2 region around K169 demonstrated that all K169-directed antibodies from humans (20) and rhesus macaques (21) after the RV144 vaccine regimen use a lambda light chain CDR2 aspartic acid, glutamic acid (ED) motif to bind to K169. Moreover, this lambda light chain motif is conserved throughout primate phylogeny, suggesting an evolutionary advantage to this dominant recognition mode. The response to Env induced by the RV144 vaccine regimen was remarkably dominant to this light chain motif. It is of interest that V1V2 responses were not substantially elicited by the DNA/Ad5 regimen utilized in the HIV Vaccine Trials Network’s (HVTN) HVTN 505 vaccine trial, even though these sequences were present in 2 of the 3 envelope vaccine constructs (22). The low level of V1V2 scaffold responses in association with a vaccine regimen in which no protection was seen suggests the potential importance of responses to the V1V2 scaffold in protection against HIV acquisition. Finally, in the secondary analysis strong but transient linear V2 IgG responses, especially against V2 in the A244 CF01_AE were associated with a lower risk of HIV-1 infection in RV144 vaccinees.
Linking of the Immune Response with Immune Pressure on the Virus
Genetic sieve analysis of the viral isolates from subjects who became HIV-1 infected in RV144 revealed that isolates from vaccinated subjects were less likely to possess a lysine at K169 of the Env V2 region than placebo recipients, and vaccine efficacy was significantly higher against HIV-1 manifesting a lysine at K169 than against HIV-1 with a different residue at position 169 (23). This lysine is found in over 85% of circulating HIV-1 strains in Thailand, yet was present in only 66% of RV144 HIV-1 infected vaccine recipients, suggesting vaccine immune pressure at this region of the virus. Thus, both the immunological and virological data point to the V2 region of HIV-1 as a vulnerability point for the virus, and a target site of protective antibodies associated with vaccine efficacy of the RV144 regimen.
A similar correlation between vaccine induced antibody responses to the third variable (V3) loop region of the circulating A244 CF01_AE isolates in Thailand and genetic pressure on circulating strains of HIV-1 in vaccinees was also detected (24). The RV144 regimen elicited V3 specific antibodies at week 26 (peak point of antibody response, 2 weeks post 4th dose) in 88%–95% of recipients, depending upon the antigen used. Similar to the V2 antibody response, the V3 antibody response was largely cross-reactive among strains, and similar to the V2 antibody response, the V3 antibody levels markedly declined over time (reduction in prevalence at week 52 of 50%–70% depending on the antigen). Past studies indicated such antibodies may demonstrate some cross clade in vitro neutralizing activity (25). Analogs of the viral isolates of the A244 CF01_AE breakthrough viruses from 43 vaccine and 66 placebo recipients demonstrated an 85% VE against viruses with amino acids mismatching the vaccine at V3 site 317 (P=.004 for higher VE than against F317 matched HIV-1), and 52% VE against viruses matching the vaccine at V3 site 307 (P=.004 for higher VE than against I307 mismatched HIV-1). When point mutations similar to those found in breakthrough isolates were made in the V3 crown at isoleucine site 307, the titers of antibodies in RV144 vaccinees to the V3 crown were markedly reduced, demonstrating the specificity of the RV144 regimen to this region of V3 (24). Taken together, these data indicate that the RV144 regimen induced both V2 and V3 specific antibodies that imposed immune pressure on infectious viruses, and provide strong support for antibody mediated protection. These observations also provide both a qualitative and quantitative basis for evaluating future vaccine regimens that should be considered for advanced clinical development.
Isotype-specific Antibodies and Protection
Since IgG subclasses have been implicated in both viral induced and vaccine induced protection (26, 27), a detailed evaluation was initiated of IgG subclass specific antibodies comparing the RV144 regimen with that of the ineffective AIDSVAX B/E® gp120 regimen used in VAX003 and VAX004. ALVAC priming with gp120 boosting was associated with both a significantly higher and broader IgG3 responses to the HIV-1 envelope glycoprotein, and significantly lower IgG2 and IgG4 response rates than gp120 vaccination alone (27, 28). Geometric mean titers of IgG3 antibodies to V1V2 were 10 times higher in RV144 than in VAX003 recipients. Vaccinees with high IgG3 responses were shown to have higher ADCC antibodies, and IgG3 antibodies to the V1V2 region were associated with VE (RR 0.32 for vaccinees positively vs. negatively responding to a gp70-V1V2 scaffold protein with a lysine placed at position 169; P=.003), especially to viruses in which V169K was present (27). Interestingly, IgG3 responses to HIV-1 Env declined rapidly over time from 79% prevalence at peak immunity (week 26) to 0% at year 1, correlating with the rapid loss of VE during this time period. Of note, there was no significant correlation between individuals with high V1V2 IgG3 responses and those with high IgG2 and IgG4 responses, suggesting there are unique determinants to IgG isotypes. These associations between IgG3 antibodies to HIV-1 envelope and protection offer a potential discrimination between ALVAC priming/gp120 vs. gp120 alone. Subclass specific antibody responses differ between Rhesus macaques and humans; hence evaluation of this potential correlate of risk must continue to be done within the context of human vaccine trials.
IgA Inhibitory Antibodies
One puzzling observation from the primary correlates of protection studies associated with RV144 was the strong direct correlation between high serum IgA antibodies to specific HIV-1 envelopes, and an increased risk of infection. This response was one of the strongest statistical associations influencing VE (estimated VE = 0% for the highest third IgA responding vaccinees compared to estimated VE = 43% for the lower two-thirds of IgA responding vaccinees) (12). Subjects with IgA binding levels to HIV-1 envelope >3,000 MFI had a VE of 0%. Why certain serum IgA antibodies were associated with lack of protection was at first perplexing. Epitope mapping indicated that some of the serum IgA antibodies seen post vaccination bound to the same conformational C1 region of gp120 that ADCC antibodies were shown to bind (29, 30). From 75% to 90% of RV144 vaccinees exhibited ADCC activity after vaccination. Much of this activity was blocked by C1 region specific antibodies to the A244 CF01_AE isolates, suggesting that the IgA antibodies inhibited functional ADCC activity, providing a direct hypothesis for the inverse correlation between high serum levels of IgA and increased HIV-1 acquisition. Moreover, natural IgA antibodies to the C1 region isolated from RV144 vaccinees also blocked the mediation of ADCC by RV144 IgG antibodies to the same epitope (30). Additional data supporting the presence of inhibitory antibodies are that individuals with low levels of IgA antibodies to Env also had high levels of antibodies to V3 peptides. VE approached 70% among the subgroup of vaccinees who had low to no serum IgA responses to gp120 and high V3 antibodies (15, 24). Interestingly, high serum IgA responses to HIV-1 gp120 were also seen with the DNA/Ad5 regimen utilized in the HVTN 505 vaccine trial, a regimen that showed no vaccine efficacy in MSM in the US (22). Development of regimens that do not induce inhibitory antibodies provides a potential strategy for improving vaccine efficacy, especially if such alterations resulted in enhanced induction of functional antibodies that mediate ADCC or neutralization. One of the impediments to a more detailed evaluation of ADCC responses and HIV vaccine protection is the lack of information on the correlation of ADCC assays between NHPs and humans. Greater attention to such issues may allow the field to pursue the observed connection between ADCC and VE more completely.
Fc Receptor Functions and their Role in HIV-1 Acquisition
In 2007 Hessel et al. demonstrated that the Fc-gamma receptor binding function of a monoclonal antibody to HIV-1 was critical for the ability of this antibody to protect against experimental challenge with SHIV in a monkey model (31). More recently, in a paper evaluating the mechanisms by which live, attenuated SIV vaccine protects against experimental SIV challenge, gp41 antibodies were concentrated by neonatal FcR in cervical and vaginal epithelium, and such local antibodies transported to epithelial surfaces through the neonatal FcR were an important correlate of protection (32). Moreover, a second correlate in this model was the pre-existence of virus specific immune complexes that correlated with the FcγRIIB inhibition in the epithelium lining the cervix, and these complexes blocked CD4+ T cell recruitment and likely inhibited virus expansion (33). In RV144, individuals with a CT or TT at a SNP in the FCRGR2C gene had an estimated 91% VE against HIV-1 with lysine at position 169 in the V2 loop, compared to 15% VE for individuals with a CC (19). This suggests that RV144 responses selectively blocked infection of HIV-1 isolates with lysine at position 169 only among CT or TT subjects (19). The prevalence of these polymorphisms is seen in only 28% of Thais and in an estimated 49% of South Africans (34), perhaps predicting higher efficacy for RV144-like vaccines in Africa. These data support the assertion that both vaccine induced and host factors are important determinants of the non-neutralizing antibody effector function induced by RV144 vaccination. However, the mechanistic link between such a polymorphism and antibody mediated immune response remains undefined.
Passive Protection Studies Using Non-neutralizing Monoclonal Antibodies after RV144 Vaccination
While studies employing neutralizing antibodies to HIV-1 have shown protection in NHP models, studies defining the efficacy of non-neutralizing antibodies in protection against experimental challenge in NHP models are incomplete (35). In established SIV infections in macaques, polyclonal non-neutralizing anti-SIV IgG, administered by passive infusion induced ADCC and an associated decline in SIV viremia (36). Since the passively administered sera possessed low levels of neutralizing activity, the protective nature of ADCC-only-mediating antibodies was not apparent. To explore whether non-broadly-neutralizing antibodies can protect against rapid disease progression, Binley et al (37) infused polyclonal anti-SIV IgG purified from macaques infected with mac251 SIV, at a concentration of 170 mg/kg, into 2 rhesus macaques who were rapid progressors infected with the mac251 SIV strain. Following the infusion, the anti envelope antibody levels in these animals were temporarily restored to levels typically found in non-rapid progressors, and these antibodies killed SIV-infected cells, presumably through an effector function such as ADCC.
While non-neutralizing ADCC mediating monoclonal antibodies reactive with the A244 CF01_AE -infected tier 2 CD4+ T cells are available for testing in passive protection trials, these studies have been hindered by lack of availability of R5 tier 2 SHIV-1s with the K169 and full V2 epitope of putatively protective V2 antibodies from RV144. SHIV-1s are under development (38, 39).
T-cell Responses
Recent studies have shown that CD4+ T-cell immune responses to HIV-1 envelope independently influenced VE in the RV144 trial (12, 40). Sixty percent of RV144 recipients who had exhibited CD4+ T-cell responses to envelope recognized the V2 peptides, and intracellular cytokine staining (ICS) confirmed these responses were mediated by polyfunctional effector memory CD4+ T cells, which produced more than 1 cytokine in 58% of the samples, predominantly IL-2 and TNF-α. The predominant IFN-γ response (25%) was to the Env V2 region at amino acid positions 145–208. The main peptide recognized in the vaccine group contained the integrin α4β7 binding motif, a region of the envelope involved in the initial encounter of HIV-1 and CD4+ T cells (12). Using more sophisticated single cell analyses, vaccinees with CD4+ T cells that secreted IL-2, TNF-α, IFN-γ, IL-4, and CD154 to HIV-1 envelope peptides had a reduced rate of infection (RR = 0.57, P=.006) vs. those who did not make such a polyfunctional response. Moreover, this response was an independent correlate of infection after accounting for the primary correlates of IgG binding to V2 and IgA binding to Env (41). These data indicate the potential importance of initiating a strong helper T-cell response with vaccination, and study of the CD4+ T cell – B cell interaction may provide insights into enhanced immunogenicity.
Building Upon the Correlates for the Construction of Next Generation Vaccines
With only one vaccine approach demonstrating efficacy against HIV acquisition, there is at present uncertainty as to whether the above described correlates will be effective guideposts for a globally effective HIV vaccine (42). The differences between the immune responses in RV144 and those seen in HVTN 505 and VAX003, two vaccine efficacy trials with no observed efficacy against HIV-1 acquisition, provide indirect evidence of the potential importance of the types of non-neutralizing antibodies important in reducing HIV-1 acquisition. Thus, while the possibility of a false positive result always exists in any given study, the confidence that RV144 demonstrated real efficacy has increased substantially based upon the viral sieve and immune correlates analyses. Importantly, a series of recent studies in NHP with different vaccine prototypes such as a replication deficient Ad26 alone or in combination with MVA have shown a correlation between non-neutralizing antibodies and protection from experimental challenges with both SIV and SHIV (Table 2) (43). Addition of a trimeric gp120 protein boost increases several antibody effector functions and is associated with enhanced protection from experimental challenge (43–45). These NHP studies support the notion that enhancing antibody effector functions and CD4+ T-cell responses offer the potential for providing HIV-1 vaccine efficacy. It was recognized that a series of more rapid iterative trials were needed to address the multitude of scientific questions to be answered from the immune correlates analyses (46). This was best done by conducting Test of Concept efficacy trials in populations with higher acquisition rates than those in Thailand, such as in sub-Saharan Africa, where clade C viruses predominate (47). Moreover, as the antibody responses associated with protection in RV144 were predominantly clade specific, the use of regimens that would provide clade C coverage should be utilized. Moreover, improving the durability of the envelope specific immune responses was essential (Figure 1). Studies with HIV envelope and other recombinant proteins have suggested that alternative adjuvants such as MF59 and MPL-like adjuvants such as AS01B can enhance the durability of immune responses over those seen with alum adjuvanted proteins (48, 49). The degree of somatic hypermutation, breadth of immune response and functional activities of antibodies induced by protein immunogenicity can be influenced by adjuvants (50). As such, comparative studies of recombinant HIV clade C envelope proteins (gp120 and trimeric gp140) adjuvanted with ASO1B, MF59, and alum adjuvants (Table 1), are underway. In addition, more frequent boosting of immune responses by dosing the vaccine regimen more frequently are under evaluation. Another approach for improving upon RV 144 is to utilize more immunogenic vector platforms to improve B- and T-cell priming, especially to envelope proteins. Second generation regimens, including a bivalent ALVAC (env and gag-pol on separate constructs), NYVAC/DNA constructs and combinations of replication incompetent adenovirus such as Ad26 in combination with MVA are in clinical development (51–53). One hope from the ALVAC and Ad26/MVA programs is that these different vaccine approaches will elicit immune responses that correlate with each other and with protection, and hence provide a critical surrogate marker for future vaccine development. The finding that non neutralizing antibody responses such as binding antibodies to HIV envelope, ADCC and other non neutralizing functional antibodies are correlates of protection in these NHP experimental challenge studies provide some optimism for this approach (54, 55). Validation of such a marker would allow the field to do “bridging immunogenicity studies” as a way of initiating a comprehensive global vaccine strategy.
Table 2.
Comparison of Immune responses that influence VE in RV 144 versus past and ongoing HIV vaccine trials.
| Immune response identified as a correlate in RV144 | Responses in non-RV144 efficacy trials | Responses in vaccines in current development |
|---|---|---|
| Total IgG to V1V2 scaffold | Lower in HVTN 505 compared with RV144 | Higher titers in DNA / NYVAC / gp120 than RV144. Correlates of protection in heterologous NHP challenge studies with Ad26/Ad35 and Ad26/MVA studies. |
| Serum IgA to gp120 (higher IgA = lower VE) | Higher IgA (including IgA to A1ConEnv) in HVTN 505, compared to RV144 |
|
| IgG3 to V1V2 | Lower in HVTN 505 and VAX003 than RV144 | Under evaluation |
| ADCC activity | Minimal ADCC in HVTN 505 | ADCC correlates with protection in NHP using Ad26 +/− trimeric gp120 High ADCC in DNA/MVA regimen |
| Tier 1 nAbs | Higher frequency in RV144 compared with HVTN 505 | Clade C regions under study |
| High avidity to gp120 | Not measured in HVTN 505 program Env IgG avidity with low IgA correlated with decreased risk of infection |
DNA / MVA containing regimens have high avidity. Other products under study. |
| CD4+ T cells with polyfunctional response | Different cytokine profile in HVTN 505 vs. RV144 | DNA / NYVAC and Ad26/MVA increase prevalence and magnitude of Env specific CD4+ T cells |
Table 1.
Post RV144 vaccine combinations
| Improve Frequency, Magnitude, and Polyfunctionality of CD4+ T-cell response to HIV-1 envelope products | Improve Clade-specific Antibody Responses to gp120 | Improve Durability of Antibody Response through Adjuvants |
|---|---|---|
| ALVAC (ZM96) +/−DNA NYVAC (ZM96) +/− DNA |
Bivalent clade C recombinant gp120 protein (strains 1086 and TV-1) construct | Alum, MF59, AS01B |
| Ad26+/− MVA | gp140 clade C trimeric protein +/− gp140 mosaic trimeric protein | Alum, AS01B |
While these developments occur in defining non-neutralizing antibody approaches to HIV-1 protection, there is also a concerted international effort toward developing immunogens that will elicit broader neutralizing antibodies than currently demonstrated by immunization with any of the current vaccine prototypes (56). The lack of broadly neutralizing antibodies elicited by the RV144 like regimens is an acknowledged deficiency in the immune profile of these approaches. Enhancing basic and translational scientific programs in attempting to elicit such broadly neutralizing antibodies is an important component of the research agenda of the HIV vaccine effort. Recent developments in developing stable HIV-1 envelope trimers provides evidence for progress in this area of research (55, 57). The continued elucidation of novel targets of binding neutralizing is also providing new insights on how to develop immunogens that can elicit such antibodies. Importantly, this agenda includes the use of passively administered broadly neutralizing antibodies on a 1–3 monthly basis (antibody mediated protection [AMP]) to reduce HIV acquisition (58, 59). A test of concept study to demonstrate how effective such antibodies would be in reducing HIV acquisition, and what level of neutralizing activity is required to reduce HIV-1 acquisition, is an important milestone for development of an effective neutralizing based vaccine.
In summary, the past 3 years have seen an unprecedented scientific effort that has provided a wealth of new information on the immune responses that are potentially associated with and responsible for protection from HIV acquisition. New understanding of regions of the HIV envelope heretofore largely ignored (such as the V2 loop) and non-neutralizing antibody responses, as well as the role that CD4 T cells play in B cell maturation, are important concepts that have emerged from these studies. These data have provided hypotheses and new benchmarks for the development of new vaccine strategies (60). Whether increased VE will be achieved by these efforts remains to be determined; however, this collaborative effort has produced a momentum and series of immune targets that will hopefully lead to an effective global vaccine effort.
Acknowledgments
The authors would like to thank Allan deCamp, Shannon Grant, Ying Huang, Yunda Huang, and Holly Janes for statistical analysis for Figure 2; The HVTN Laboratory led by Dr. Juliana McElrath at Fred Hutchinson Cancer Research Center; neutralizing antibody assays by Dr. David Montefiori at Duke University; and Dr. Nelson Michael who directed the USMHRP RV144 study and its correlates program. We thank Ms. Adi Ferrara and Dr. Mindy Miner for help in the preparation of this manuscript. Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under award numbers UM1AI068618, U01AI 068614-05, and UM1AI068635. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pharris A, Spiteri G, Noori T, Amato-Gauci AJ. Ten years after Dublin: principal trends in HIV surveillance in the EU/EEA, 2004 to 2013. Euro Surveill. 2014;19:20968. doi: 10.2807/1560-7917.es2014.19.47.20968. [DOI] [PubMed] [Google Scholar]
- 3.Prevention CfDCa. Today’s HIV/AIDS epidemic. 2014 Accessed Februrary, 11, 2015. [Google Scholar]
- 4.UNAIDS. The GAP Report 2014 [Google Scholar]
- 5.Johnston MI, Fauci AS. HIV vaccine development–improving on natural immunity. N Engl J Med. 2011;365:873–875. doi: 10.1056/NEJMp1107621. [DOI] [PubMed] [Google Scholar]
- 6.Fauci AS, Marston HD. Ending AIDS–is an HIV vaccine necessary? N Engl J Med. 2014;370:495–498. doi: 10.1056/NEJMp1313771. [DOI] [PubMed] [Google Scholar]
- 7.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 8.Nitayaphan S, Pitisuttithum P, Karnasuta C, Eamsila C, de Souza M, Morgan P, Polonis V, Benenson M, VanCott T, Ratto-Kim S, Kim J, Thapinta D, Garner R, Bussaratid V, Singharaj P, El Habib R, Gurunathan S, Heyward W, Birx D, McNeil J, Brown AE. Safety and immunogenicity of an HIV subtype B and E prime-boost vaccine combination in HIV-negative Thai adults. J Infect Dis. 2004;190:702–706. doi: 10.1086/422258. [DOI] [PubMed] [Google Scholar]
- 9.McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–1905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, Hu D, Tappero JW, Choopanya K. Randomized, Double-Blind, Placebo-Controlled Efficacy Trial of a Bivalent Recombinant Glycoprotein 120 HIV-1 Vaccine among Injection Drug Users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 11.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–665. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 12.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. Magnitude and Breadth of the Neutralizing Antibody Response in the RV144 and Vax003 HIV-1 Vaccine Efficacy Trials. J Infect Dis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karasavvas N, Billings E, Rao M, Williams C, Zolla-Pazner S, Bailer RT, Koup RA, Madnote S, Arworn D, Shen X, Tomaras GD, Currier JR, Jiang M, Magaret C, Andrews C, Gottardo R, Gilbert P, Cardozo TJ, Rerks-Ngarm S, Nitayaphan S, Pitisuttithum P, Kaewkungwal J, Paris R, Greene K, Gao H, Gurunathan S, Tartaglia J, Sinangil F, Korber BT, Montefiori DC, Mascola JR, Robb ML, Haynes BF, Ngauy V, Michael NL, Kim JH, de Souza MS. The Thai Phase III HIV Type 1 Vaccine trial (RV144) regimen induces antibodies that target conserved regions within the V2 loop of gp120. AIDS Res Hum Retroviruses. 2012;28:1444–1457. doi: 10.1089/aid.2012.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottardo R, Bailer RT, Korber BT, Gnanakaran S, Phillips J, Shen X, Tomaras GD, Turk E, Imholte G, Eckler L, Wenschuh H, Zerweck J, Greene K, Gao H, Berman PW, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Robb ML, Michael NL, Kim JH, Zolla-Pazner S, Haynes BF, Mascola JR, Self S, Gilbert P, Montefiori DC. Plasma IgG to linear epitopes in the V2 and V3 regions of HIV-1 gp120 correlate with a reduced risk of infection in the RV144 vaccine efficacy trial. PLo SONE. 2013;8:e75665. doi: 10.1371/journal.pone.0075665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alam SM, Liao HX, Tomaras GD, Bonsignori M, Tsao CY, Hwang KK, Chen H, Lloyd KE, Bowman C, Sutherland L, Jeffries TL, Jr, Kozink DM, Stewart S, Anasti K, Jaeger FH, Parks R, Yates NL, Overman RG, Sinangil F, Berman PW, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Karasavva N, Rerks-Ngarm S, Kim JH, Michael NL, Zolla-Pazner S, Santra S, Letvin NL, Harrison SC, Haynes BF. Antigenicity and immunogenicity of RV144 vaccine AIDSVAX clade E envelope immunogen is enhanced by a gp120 N-terminal deletion. J Virol. 2013;87:1554–1568. doi: 10.1128/JVI.00718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zolla-Pazner S, DeCamp A, Gilbert PB, Williams C, Yates NL, Williams WT, Howington R, Fong Y, Morris DE, Soderberg KA, Irene C, Reichman C, Pinter A, Parks R, Pitisuttithum P, Kaewkungwal J, Rerks-Ngarm S, Nitayaphan S, Andrews C, O’Connell RJ, Yang ZY, Nabel GJ, Kim JH, Michael NL, Montefiori DC, Liao HX, Haynes BF, Tomaras GD. Vaccine-Induced IgG Antibodies to V1V2 Regions of Multiple HIV-1 Subtypes Correlate with Decreased Risk of HIV-1 Infection. PLo SONE. 2014;9:e87572. doi: 10.1371/journal.pone.0087572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang KK, Gurley TC, Kozink DM, Armand LC, Marshall DJ, Whitesides JF, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, O’Connell RJ, Kim JH, Michael NL, Montefiori DC, Tomaras GD, Liao HX, Haynes BF, Ferrari G. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol. 2014;88:7715–7726. doi: 10.1128/JVI.00156-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li SS, Gilbert PB, Tomaras GD, Kijak G, Ferrari G, Thomas R, Pyo CW, Zolla-Pazner S, Montefiori D, Liao HX, Nabel G, Pinter A, Evans DT, Gottardo R, Dai JY, Janes H, Morris D, Fong Y, Edlefsen PT, Li F, Frahm N, Alpert MD, Prentice H, Rerks-Ngarm S, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Robb ML, O’Connell RJ, Haynes BF, Michael NL, Kim JH, McElrath MJ, Geraghty DE. FCGR2C polymorphisms associate with HIV-1 vaccine protection in RV144 trial. JClinInvest. 2014 doi: 10.1172/JCI75539. doi:75539 [pii];10.1172/JCI75539 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity. 2013;38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiehe K, Easterhoff D, Luo K, Nicely NI, Bradley T, Jaeger FH, Dennison SM, Zhang R, Lloyd KE, Stolarchuk C, Parks R, Sutherland LL, Scearce RM, Morris L, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Sinangil F, Phogat S, Michael NL, Kim JH, Kelsoe G, Montefiori DC, Tomaras GD, Bonsignori M, Santra S, Kepler TB, Alam SM, Moody MA, Liao HX, Haynes BF. Antibody Light-Chain-Restricted Recognition of the Site of Immune Pressure in the RV144 HIV-1 Vaccine Trial Is Phylogenetically Conserved. Immunity. 2014;41:909–918. doi: 10.1016/j.immuni.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammer SM, Sobieszczyk ME, Janes H, Karuna ST, Mulligan MJ, Grove D, Koblin BA, Buchbinder SP, Keefer MC, Tomaras GD, Frahm N, Hural J, Anude C, Graham BS, Enama ME, Adams E, DeJesus E, Novak RM, Frank I, Bentley C, Ramirez S, Fu R, Koup RA, Mascola JR, Nabel GJ, Montefiori DC, Kublin J, McElrath MJ, Corey L, Gilbert PB. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. NEnglJ Med. 2013;369:2083–2092. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rolland M, Edlefsen PT, Larsen BB, Tovanabutra S, Sanders-Buell E, Hertz T, Decamp AC, Carrico C, Menis S, Magaret CA, Ahmed H, Juraska M, Chen L, Konopa P, Nariya S, Stoddard JN, Wong K, Zhao H, Deng W, Maust BS, Bose M, Howell S, Bates A, Lazzaro M, O’Sullivan A, Lei E, Bradfield A, Ibitamuno G, Assawadarachai V, O’Connell RJ, DeSouza MS, Nitayaphan S, Rerks-Ngarm S, Robb ML, McLellan JS, Georgiev I, Kwong PD, Carlson JM, Michael NL, Schief WR, Gilbert PB, Mullins JI, Kim JH. Increased HIV-1 vaccine efficacy against viruses with genetic signatures in Env V2. Nature. 2012;490:417–420. doi: 10.1038/nature11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zolla-Pazner S, Edlefsen PT, Rolland M, Kong XP, DeCamp A, Gottardo R, Williams C, Tovanabutra S, Sharpe-Cohen S, Mullins JI, DeSouza MS, Karasavvas N, Nitayaphan S, Rerks-Ngarm S, Pitisuttihum P, Kaewkungwal J, O’Connell RJ, Robb ML, Michael NL, Kim JH, Gilbert P. Vaccine-induced Human Antibodies Specific for the Third Variable Region of HIV-1 gp120 Impose Immune Pressure on Infecting Viruses. E Bio Medicine. 2014;1:37–45. doi: 10.1016/j.ebiom.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zolla-Pazner S, Kong XP, Jiang X, Cardozo T, Nadas A, Cohen S, Totrov M, Seaman MS, Wang S, Lu S. Cross-clade HIV-1 neutralizing antibodies induced with V3-scaffold protein immunogens following priming with gp120 DNA. J Virol. 2011;85:9887–9898. doi: 10.1128/JVI.05086-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomaras GD, Haynes BF. HIV-1-specific antibody responses during acute and chronic HIV-1 infection. Curr Opin HIV AIDS. 2009;4:373–379. doi: 10.1097/COH.0b013e32832f00c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yates NL, Liao HX, Fong Y, DeCamp A, Vandergrift NA, Williams WT, Alam SM, Ferrari G, Yang ZY, Seaton KE, Berman PW, Alpert MD, Evans DT, O’Connell RJ, Francis D, Sinangil F, Lee C, Nitayaphan S, Rerks-Ngarm S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Pinter A, Zolla-Pazner S, Gilbert PB, Nabel GJ, Michael NL, Kim JH, Montefiori DC, Haynes BF, Tomaras GD. Vaccine-Induced Env V1-V2 IgG3 Correlates with Lower HIV-1 Infection Risk and Declines Soon After Vaccination. Sci Transl Med. 2014;6:228ra239. doi: 10.1126/scitranslmed.3007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast AS, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. Polyfunctional Fc-Effector Profiles Mediated by IgG Subclass Selection Distinguish RV144 and VAX003 Vaccines. Sci Transl Med. 2014;6:228ra238. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 29.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang KK, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao CY, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao HX, Haynes BF. ADCC-Mediating Antibodies from an HIV-1 Vaccine Efficacy Trial Target Multiple Epitopes and Preferentially Use the VH1 Gene Family. J Virol. 2012 doi: 10.1128/JVI.01023-12. doi:JVI.01023-12 [pii];10.1128/JVI.01023-12 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci USA. 2013;110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hessell AJ, Hangartner L, Hunter M, Havenith CE, Beurskens FJ, Bakker JM, Lanigan CM, Landucci G, Forthal DN, Parren PW, Marx PA, Burton DR. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 32.Smith AJ, Wietgrefe SW, Shang L, Reilly CS, Southern PJ, Perkey KE, Duan L, Kohler H, Muller S, Robinson J, Carlis JV, Li Q, Johnson RP, Haase AT. Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J Immunol. 2014;193:3126–3133. doi: 10.4049/jimmunol.1400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko SY, Pegu A, Rudicell RS, Yang ZY, Joyce MG, Chen X, Wang K, Bao S, Kraemer TD, Rath T, Zeng M, Schmidt SD, Todd JP, Penzak SR, Saunders KO, Nason MC, Haase AT, Rao SS, Blumberg RS, Mascola JR, Nabel GJ. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. doi: 10.1038/nature13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.R L, CT T. Fc-gamma receptor variability in the South African population-Will this impact on HVTN097 and vaccine efficacy? AIDS Res Hum Retroviruses. 2014;30:A219. [Google Scholar]
- 35.Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X, Todd JP, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243ra288. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Florese RH, Van Rompay KK, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, Haigwood N, Venzon D, Kalyanaraman VS, Marthas ML, Robert-Guroff M. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol. 2006;177:4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 37.Binley JM, Clas B, Gettie A, Vesanen M, Montefiori DC, Sawyer L, Booth J, Lewis M, Marx PA, Bonhoeffer S, Moore JP. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology. 2000;270:237–249. doi: 10.1006/viro.2000.0254. [DOI] [PubMed] [Google Scholar]
- 38.Asmal M, Luedemann C, Lavine CL, Mach LV, Balachandran H, Brinkley C, Denny TN, Lewis MG, Anderson H, Pal R, Sok D, Le K, Pauthner M, Hahn BH, Shaw GM, Seaman MS, Letvin NL, Burton DR, Sodroski JG, Haynes BF, Santra S. Infection of monkeys by simian-human immunodeficiency viruses with transmitted/founder clade C HIV-1 envelopes. Virology. 2015;475:37–45. doi: 10.1016/j.virol.2014.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang HW, Tartaglia LJ, Whitney JB, Lim SY, Sanisetty S, Lavine CL, Seaman MS, Rademeyer C, Williamson C, Ellingson-Strouss K, Stamatatos L, Kublin J, Barouch DH. Generation and Evaluation of Clade C Simian-Human Immunodeficiency Virus Challenge Stocks. J Virol. 2014 doi: 10.1128/JVI.03279-14. doi:JVI.03279-14 [pii];10.1128/JVI.03279-14 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin L, Finak G, Ushey K, Seshadri C, Hawn TR, Frahm N, Scriba TJ, Mahomed H, Hanekom W, Bart PA, Pantaleo G, Tomaras GD, Rerks-Ngarm S, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Michael NL, Kim JH, Robb ML, O’Connell RJ, Karasavvas N, Gilbert P, S CDR, McElrath MJ, Gottardo R. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol. 2015;33:610–616. doi: 10.1038/nbt.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin L, Finak G, Ushey K, Seshadri C, Hawn T, Frshm N, Scriba T, Mahomed H, Hanekom E, Bart P, Pantaleo G, Tomaras G, Rerks-Ngarm S, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Michael N, Kim J, Robb M, O’Connell R, Karasavvas N, Gilbert P, DeRosa S, McElrath M, Gottardo R. Combinatorial polyfunctionality analysis of antigen-specific T-cell subsets identified novel cellular subsets correlated with clinical outcomes. Nature Biotechnology. 2015 doi: 10.1038/nbt.3187. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enterpris GHV, HO/UNAIDS Program UMHR, Health TMoP. Recommendations for the Future Utility of the RV144 Vaccines to the Thai Ministry of Health 2010 [Google Scholar]
- 43.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, Borducchi EN, Smith KM, Nkolola JP, Liu J, Shields J, Parenteau L, Whitney JB, Abbink P, Ng’ang’a DM, Seaman MS, Lavine CL, Perry JR, Li W, Colantonio AD, Lewis MG, Chen B, Wenschuh H, Reimer U, Piatak M, Lifson JD, Handley SA, Virgin HW, Koutsoukos M, Lorin C, Voss G, Weijtens M, Pau MG, Schuitemaker H. HIV-1 vaccines. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science. 2015;349:320–324. doi: 10.1126/science.aab3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Barouch DH, Stephenson KE, Borducchi EN, Smith K, Stanley K, McNally AG, Liu J, Abbink P, Maxfield LF, Seaman MS, Dugast AS, Alter G, Ferguson M, Li W, Earl PL, Moss B, Giorgi EE, Szinger JJ, Eller LA, Billings EA, Rao M, Tovanabutra S, Sanders-Buell E, Weijtens M, Pau MG, Schuitemaker H, Robb ML, Kim JH, Korber BT, Michael NL. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–539. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corey L, Nabel GJ, Dieffenbach C, Gilbert P, Haynes BF, Johnston M, Kublin J, Lane HC, Pantaleo G, Picker LJ, Fauci AS. HIV-1 vaccines and adaptive trial designs. Sci Transl Med. 2011;3:79ps13. doi: 10.1126/scitranslmed.3001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert P, Grove D. A Sequential Two-stage Trial Design for Evaluating Efficacy and Immune Correlates for Multiple Vaccine Regimens. HVT News. 2011;3:2–4. doi: 10.2202/1948-4690.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh M, Ugozzoli M, Kazzaz J, Chesko J, Soenawan E, Mannucci D, Titta F, Contorni M, Volpini G, Del GG, O’Hagan DT. A preliminary evaluation of alternative adjuvants to alum using a range of established and new generation vaccine antigens. Vaccine. 2006;24:1680–1686. doi: 10.1016/j.vaccine.2005.09.046. [DOI] [PubMed] [Google Scholar]
- 49.Lal H, Cunningham AL, Godeaux O, Chlibek R, Diez-Domingo J, Hwang SJ, Levin MJ, McElhaney JE, Poder A, Puig-Barbera J, Vesikari T, Watanabe D, Weckx L, Zahaf T, Heineman TC, Group ZOES. Efficacy of an Adjuvanted Herpes Zoster Subunit Vaccine in Older Adults. N Engl J Med. 2015 doi: 10.1056/NEJMoa1501184. [DOI] [PubMed] [Google Scholar]
- 50.Jardine JG, Ota T, Sok D, Pauthner M, Kulp DW, Kalyuzhniy O, Skog PD, Thinnes TC, Bhullar D, Briney B, Menis S, Jones M, Kubitz M, Spencer S, Adachi Y, Burton DR, Schief WR, Nemazee D. HIV-1 VACCINES. Priming a broadly neutralizing antibody response to HIV-1 using a germline-targeting immunogen. Science. 2015;349:156–161. doi: 10.1126/science.aac5894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bart PA, Goodall R, Barber T, Harari A, Guimaraes-Walker A, Khonkarly M, Sheppard NC, Bangala Y, Frachette MJ, Wagner R, Liljestrom P, Kraehenbuhl JP, Girard M, Goudsmit J, Esteban M, Heeney J, Sattentau Q, McCormack S, Babiker A, Pantaleo G, Weber J. EV01: a phase I trial in healthy HIV negative volunteers to evaluate a clade C HIV vaccine, NYVAC-C undertaken by the EuroVacc Consortium. Vaccine. 2008;26:3153–3161. doi: 10.1016/j.vaccine.2008.03.083. [DOI] [PubMed] [Google Scholar]
- 52.Pantaleo G, Esteban M, Jacobs B, Tartaglia J. Poxvirus vector-based HIV vaccines. Curr Opin HIV AIDS. 2010;5:391–396. doi: 10.1097/COH.0b013e32833d1e87. [DOI] [PubMed] [Google Scholar]
- 53.Johnson JA, Barouch DH, Baden LR. Nonreplicating vectors in HIV vaccines. Curr Opin HIV AIDS. 2013;8:412–420. doi: 10.1097/COH.0b013e328363d3b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dosenovic P, von Boehmer L, Escolano A, Jardine J, Freund NT, Gitlin AD, McGuire AT, Kulp DW, Oliveira T, Scharf L, Pietzsch J, Gray MD, Cupo A, van Gils MJ, Yao KH, Liu C, Gazumyan A, Seaman MS, Bjorkman PJ, Sanders RW, Moore JP, Stamatatos L, Schief WR, Nussenzweig MC. Immunization for HIV-1 Broadly Neutralizing Antibodies in Human Ig Knockin Mice. Cell. 2015;161:1505–1515. doi: 10.1016/j.cell.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanders RW, van Gils MJ, Derking R, Sok D, Ketas TJ, Burger JA, Ozorowski G, Cupo A, Simonich C, Goo L, Arendt H, Kim HJ, Lee JH, Pugach P, Williams M, Debnath G, Moldt B, van Breemen MJ, Isik G, Medina-Ramirez M, Back JW, Koff WC, Julien JP, Rakasz EG, Seaman MS, Guttman M, Lee KK, Klasse PJ, LaBranche C, Schief WR, Wilson IA, Overbaugh J, Burton DR, Ward AB, Montefiori DC, Dean H, Moore JP. HIV-1 VACCINES. HIV-1 neutralizing antibodies induced by native-like envelope trimers. Science. 2015;349:aac4223. doi: 10.1126/science.aac4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haynes BF, Verkoczy L. AIDS/HIV. Host controls of HIV neutralizing antibodies. Science. 2014;344:588–589. doi: 10.1126/science.1254990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen J, Kovacs JM, Peng H, Rits-Volloch S, Lu J, Park D, Zablowsky E, Seaman MS, Chen B. HIV-1 ENVELOPE. Effect of the cytoplasmic domain on antigenic characteristics of HIV-1 envelope glycoprotein. Science. 2015;349:191–195. doi: 10.1126/science.aaa9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li Y, O’Dell S, Walker LM, Wu X, Guenaga J, Feng Y, Schmidt SD, McKee K, Louder MK, Ledgerwood JE, Graham BS, Haynes BF, Burton DR, Wyatt RT, Mascola JR. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J Virol. 2011;85:8954–8967. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lewis GK, DeVico AL, Gallo RC. Antibody persistence and T-cell balance: two key factors confronting HIV vaccine development. Proc Natl Acad Sci USA. 2014;111:15614–15621. doi: 10.1073/pnas.1413550111. [DOI] [PMC free article] [PubMed] [Google Scholar]


