Abstract
Apoptosis is one of the mechanisms used by host cells to remove unwanted intracellular organisms, and often found to be subverted by pathogens through use of host anti-apoptotic proteins. In the present study, with the help of in vitro and in vivo approaches, we documented that the macrophage anti-apoptotic protein myeloid cell leukemia 1 (MCL-1) is exploited by the intra-macrophage parasite Leishmania donovani to protect their “home” from actinomycin D-induced mitochondria-dependent apoptosis. Among all the anti-apoptotic BCL-2 family members, infection preferentially up-regulated expression of MCL-1 at both the mRNA and protein levels and compared with infected control, MCL-1-silenced infected macrophages documented enhanced caspase activity and increased apoptosis when subjected to actinomycin D treatment. Phosphorylation kinetics and ChIP assay demonstrated that infection-induced MCL-1 expression was regulated by transcription factor CREB (cAMP-response element-binding protein) and silencing of CREB resulted in reduced expression of MCL-1 and increased apoptosis. During infection, MCL-1 was found to be localized in mitochondria and this was significantly reduced in Tom70-silenced macrophages, suggesting the active role of TOM70 in MCL-1 transport. In the mitochondria, MCL-1 interacts with the major pro-apoptotic protein BAK and prevents BAK-BAK homo-oligomer formation thereby preventing cytochrome c release-mediated mitochondrial dysfunction. Silencing of MCL-1 in the spleen of infected mice showed decreased parasite burden and increased induction of splenocyte apoptosis. Collectively our results showed that L. donovani exploited the macrophage anti-apoptotic protein MCL-1 to prevent BAK-mediated mitochondria-dependent apoptosis thereby protecting its niche, which is essential for disease progression.
Keywords: apoptosis, B-cell lymphoma 2 (Bcl-2) family, cytochrome c, Leishmania, macrophage
Introduction
Leishmania donovani, an obligate intracellular parasite, is the causative agent of the fatal visceral leishmaniasis (1). After entry into the macrophages, it survives and replicates inside the acidified phagolysosomes of macrophages (2). Its persistence to resist the host immune system determines the extent of pathogenicity and requires extensive manipulation of macrophage defense (3). One of the vital mechanisms by which host cells defend themselves against intracellular pathogens is the induction of apoptosis (4, 5). On the contrary, pathogens relentlessly try to defeat the host defense systems and evolved a number of ways to inhibit host cell apoptosis, which allows them more time to replicate (6). Various studies have documented the molecular mechanisms in virus (7), bacteria (8), and protozoan parasites (9) that tamper with the host defensive apoptotic machinery. Leishmania, being an obligate intracellular parasite, makes host cells resistant to a variety of pro-apoptotic signals in murine and human cell lines. L. donovani infection protects bone marrow-derived macrophages (BMDM)2 from growth factor withdrawal-induced apoptosis (10). Infection with Leishmania infantum reduces actinomycin D-induced cell death of human monocytic cell U-937 (11). Leishmania major successfully blocks the release of cytochrome c from mitochondria in BMDM isolated from BALB/c and C57BL/6 mice (12). A recent report showed the involvement of PI3K/AKT in the prevention of host cell apoptosis during Leishmania amazonensis and L. major infection (13) and another study documented that L. donovani-induced SOCS (suppressors of cytokine signaling) proteins inhibited reactive oxygen species-dependent macrophage apoptosis (14). All the evidences collectively indicate the ability of the Leishmania parasite in resisting host cell apoptosis. However, very little is known about the molecular mechanisms underlying this phenomenon.
The proteins primarily involved in controlling mitochondria-dependent apoptotic cell death are the BCL-2 (B cell lymphoma-2) family of proteins. The family is comprised of three major protein subgroups: pro-apoptotic proteins (BAK and BAX), anti-apoptotic proteins (BCL-2, BCL-XL, BCL-W, A1, and MCL-1), and BH3 only proteins (BAD, BIK, and NOXA), which play as apoptotic sensors (15, 16). Fine tuning among these proteins regulate the cell death machinery. During apoptosis, the activation of the two major pro-apoptotic proteins BAX and BAK play a key role in mitochondrial dysfunction and their activation is counter-regulated by the anti-apoptotic proteins BCL-2, BCL-XL, BCL-W, A1, and MCL-1 (17, 18). In various intracellular infections, the extremely labile and inducible protein of the BCL-2 family, MCL-1, acts as a key regulator in inhibition of cell death (19). Under normal physiological conditions, MCL-1 expression is rigidly controlled at different levels, involving transcriptional, post-transcriptional, and post-translational regulations (20). Experiments with conditional knock-out mice documented the indispensability of MCL-1 in the survival of many cell types including lymphocytes (21), hematopoietic stem cells (22), and neutrophils (23), whereas other anti-apoptotic BCL-2 family members were found to be more dispensable (21–23). MCL-1 played a crucial role in Staphylococcus aureus-induced prevention of host cell apoptosis (24) and it was observed that MCL-1-overexpressed cells delayed apoptosis when exposed to several apoptosis inducing stimuli (19). In Chlamydia trachomatis-infected cells, MCL-1 up-regulation led to prevention of host cell apoptosis and disease propagation (25) and MCL-1 induction was found to be essential for the survival of virulent Mycobacterium tuberculosis (26).
All these observations led us to speculate that MCL-1 could be a major target to be exploited by Leishmania for its intracellular survival and using the RAW 264.7 cell line, BMDM, and BALB/c mouse model, we documented the vital role played by MCL-1 in inhibition of macrophage apoptosis during Leishmania infection. Furthermore, our study revealed that L. donovani up-regulated MCL-1 expression through signaling pathways that had the active involvement of the transcription factor CREB. Infection-induced MCL-1 served to maintain the mitochondrial membrane integrity by sequestering BAK, thereby preventing its oligomerization and subsequent release of apoptogenic proteins.
Experimental Procedures
Cells and Parasites
L. donovani (MHOM/IN/1983/AG83) promastigotes were grown in medium M199 (Invitrogen) supplemented with Hanks' salt containing HEPES (12 mm), l-glutamine (20 mm), 10% heat-inactivated fetal bovine serum (FBS), 50 units/ml of penicillin, and 50 μg/ml of streptomycin (Invitrogen). BMDM were isolated from the femurs and tibiae of euthanized BALB/c mice (6 to 8 weeks old) as described earlier (27). Splenic macrophages from BALB/c mice were isolated as described earlier (28). Briefly, the spleens were taken out and mildly glass ground, residual erythrocytes were lysed with hypotonic buffers, splenocytes were washed, counted, and 1 × 107 cells/ml were then seeded on tissue culture plates and incubated at 37 °C for 2 h. Fresh Dulbecco's modified Eagle's medium (DMEM) was then used to wash the adherent splenic macrophage. The adherent murine macrophage cell line RAW 264.7 was cultured in 5% CO2 atmosphere at 37 °C in presence of RPMI 1640 (Invitrogen) supplemented with 10% heat-inactivated FBS, 100 μg/ml of streptomycin, and 100 units/ml of penicillin. In vitro infection of macrophages was carried out with L. donovani promastigotes at a parasite:cell ratio of 10:1 (27) for specific periods of incubation.
Reagents and Antibodies
Procaspase-3, procaspase-9, caspase-3, caspase-9, TOM70, BAK, BAX, CREB, phospho-CREB, and MCL-1(S-19)-specific mAb were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Actinomycin D was purchased from Calbiochem. Alkaline phosphatase-conjugated anti-mouse, anti-goat, and anti-rabbit secondary antibodies were purchased from Santa Cruz Biotechnology. Phospho-STAT3 and STAT3 antibodies were purchased from Cell Signaling (Danvers, MA). Cytochrome c mAb was obtained from BD Biosciences (San Jose, CA).
Analysis of Cell Death
Percentage of apoptotic cell was quantified by detecting phosphatidylserine in the outer layer of the plasma membrane using the Annexin V-FLUOS staining kit (Roche Applied Science, Indianapolis, IN) as per the manufacturer's instructions. Briefly, both RAW 264.7 and BMDM (2 × 106) were infected with L. donovani promastigotes for different time periods. One group of infected macrophages was treated with actinomycin D for each time point of infection. After 7 h of incubation, cells were washed with phosphate-buffered saline (PBS) and stained with fluorescein isothiocyanate (FITC)-conjugated annexin V and propidium iodide for 15 min at room temperature in the dark. The stained cells were then analyzed by a FACS Canto IITM cell sorter using 488 nm excitation and 530 nm emissions for FITC and 600 nm for PI fluorescence using FACS Diva software.
Caspase 3 Assay
2 × 106 cells (RAW 264.7 as well as BMDM) after infection and/or treatment with actinomycin D were harvested and washed twice with ice-cold PBS. Activity of caspase-3 was then assessed using the caspase-3 Apoptosis Detection kit (Santa Cruz Biotechnology) according to the manufacturer's instructions.
Immunoblotting and Immunoprecipitation
RAW 264.7 as well as BMDM were harvested and lysed using ice-cold lysis buffer (Cell Signaling Technology) supplemented with 2 mm PMSF (phenylmethylsulfonyl fluoride) and 3 mm protease inhibitor mixture. Protein concentration in the clear supernatant was determined using a Bio-Rad protein assay. From each sample an equal amount of protein (50 μg) was resolved by 10% SDS-PAGE, transferred to nitrocellulose membrane followed by blocking the membrane with 5% BSA and incubation overnight with primary antibody. After washing, membranes were incubated with alkaline phosphatase-conjugated secondary antibody and detected by hydrolysis of 5 bromo-4-chloro-3′-indolylphosphate chromogenic substrate according to the manufacturer's instructions.
For immunoprecipitation studies, 500 μg of protein lysates were pre-cleared using A/G plus agarose (Santa Cruz Biotechnology). The pre-cleared lysates were then incubated overnight at 4 °C with a specific antibody against the protein to be precipitated in the presence of protease inhibitor mixture. The immunoprecipitated proteins were then washed with PBS and resolved and blotted as described.
Analysis of Cross-linked Protein Complexes
Cells were extracted using 2% CHAPS, 10 mm HEPES, 137 mm NaCl, 2 mm EDTA, and 1 mm sodium orthovanadate in complete protease inhibitor mixture at pH 7.4 on ice for 30 min to preserve the formed oligomeric complexes. Soluble proteins were then cross-linked using EGS (ethylene glycol-bis(succinimidylsuccinate)) at room temperature. The cross-linking reaction was stopped after 30 min by adding Tris-HCl, pH 7.4. The desired cross-linked proteins were then analyzed using specific antibodies by immunoblotting as described.
Real-time PCR
Total RNA from macrophages was extracted with RNeasy mini kit (Qiagen, Valencia, CA) as per the manufacturer's instructions and the RNA (1 μg) was reverse transcribed using the SuperScript first-strand synthesis system for the RT-PCR kit (Invitrogen). The synthesized cDNA was used for real-time PCR analysis using SYBR Green on a ABI 7500 Fast real-time PCR system (Applied Biosystems, Foster City, CA). The PCR amplification conditions that maintained throughout the whole amplification process were: 40 cycles of 95 °C for 15 s and 60 °C for 1 min. β-Actin mRNA was used as endogenous control to normalize target mRNA values and data were expressed relative to normalized values of corresponding controls. Samples were analyzed in three independent experiments in triplicates. Primers used were: MCL-1: sense, 5′-GACGACCTATACCGCCAGTC-3′, antisense, 5′-TCCTGCCCCAGTTTGTTACG-3′; BCL-XL: sense, 5′-CCTGCTTGCTGGTCGCC-3′, antisense, 5′-CCGCAGTTCAAACTCATCGC-3′; A1: sense, 5′-AGGGAAGATGGCTGAGTCTGA-3′, antisense, 5′-GGGCAATCTGCTCTTGTGGA-3′; BCL-2: sense, 5′-AGCATGCGACCTCTGTTTGA-3′, antisense, 5′-GCCACACGTTTCTTGGCAAT-3′; and BCL-W: sense, 5′-ACCAGGAAACAACCGGATTC-3′, antisense, 5′-CCACCATCCAATCCTGCACT-3′.
Confocal Microscopy
RAW 264.7 cells (5 × 105) infected with L. donovani or transfected with siRNA specific for MCL-1 were washed twice with PBS and fixed in paraformaldehyde for 15 min at room temperature. Cells were permeabilized with 0.1% Triton X-100 for 5 min, blocked with 2% BSA in wash buffer (PBS, 0.1% Tween 20) for 2 h, washed with PBST, and incubated with primary antibody (1:100) overnight. Cells were then washed with wash buffer, incubated with secondary antibody (1:200), and nuclei were stained with DAPI (4′,6-diamidino-2-phenylindole, 1 μg/ml). For mitochondrial staining, cells were incubated with MitoTracker dye (Molecular Probes) in DMEM for 15 min, followed by immunostaining. Cells were then mounted with Prolong Gold anti-fade medium (P36930) (Invitrogen). Cell images were taken by an Olympus IX81 microscope equipped with a FV1000 confocal system using ×100 oil immersion Plan Apo (N.A. 1.45) objectives. After section scanning images were analyzed by Olympus Fluoview (version 3.1a; Tokyo, Japan). Quantitative analysis of microscopic data were performed using ImageJ 1.42I software.
Chromatin Immunoprecipitation (ChIP) Assay
A ChIP assay was carried out using ChIP reagents from Santa Cruz Biotechnology in accordance with the manufacturer's protocol. 4 μg of rabbit anti-CREB or rabbit anti-STAT3 antibody was used for immunoprecipitation reactions. PCR was performed with primers to amplify the 193-bp region comprising −155 to +38 in the MCL-1 promoter region. Non-immunoprecipitated chromatin served as input control and immunoprecipitation with a normal rabbit IgG was used for the negative control.
siRNA Transfection
MCL-1, CREB, and control (scrambled) siRNA were purchased from Santa Cruz Biotechnology. 2 × 106 RAW 264.7 cells were seeded per plate in antibiotic and serum-free normal growth medium. Transfection of siRNA into RAW cells was conducted using siRNA at a concentration of 15 pmol/100 μl of siRNA transfection medium (Santa Cruz Biotechnology) as per the manufacturer's instruction.
Preparation of Cytosolic, Mitochondrial, and Nuclear Fractions
Cytosolic, nuclear, and mitochondrial fractions were isolated using the EN-Qproteome kit as per the manufacturer's instructions. Briefly, 2 × 107 cells were centrifuged at 500 × g, the supernatant was discarded, and the pellet was washed with 0.9% sodium chloride. The cell pellet was resuspended in lysis buffer and incubated for 10 min at 4 °C. After centrifuging the lysate at 1000 × g for 10 min the supernatant was removed. This supernatant contained the cytosolic fraction. The remaining cell pellet was then resuspended in ice-cold disruption buffer and disrupted completely using blunt ended needle. Cell lysate was then centrifuged at 1000 × g for 10 min and the supernatant was taken. The pellet containing the nuclear fraction was then resuspended in buffer (10 mm HEPES, pH 7.6, 10 mm KCl, 0.1 mm EDTA, 1 mm DTT, and 0.5 mm PMSF) and centrifuged at 16,000 × g for 30 min. The resultant pellet was resuspended in buffer (20 mm HEPES, pH 7.6, 25% glycerol, 0.4 m NaCl, 1 mm EDTA, 1 mm DTT, and 0.5 mm PMSF). The supernatant containing the nuclear fraction was collected by centrifugation at 12,000 × g for 2 min and stored at −80 °C. The supernatant obtained at the previous step was centrifuged at 6000 × g for 10 min at 4 °C and the pellet containing the mitochondrial fraction was then washed with mitochondrial storage buffer and centrifuged at 6000 × g for 20 min. The pellet was then resuspended in mitochondrial storage buffer. Digitonin-soluble and -insoluble fractions were prepared as previously described (29). Briefly, 2 × 106 cells were suspended in PBS and mixed properly with an equal volume of digitonin (150 μg/ml) in sucrose (0.5 m). After 30 s of an ice incubation, the cell suspension was centrifuged at 14,000 × g for 60 s. The supernatants contained the soluble fraction and the pellet obtained was lysed with lysis buffer (Cell Signaling) and centrifuged at 13,000 × g for 15 min. The resultant supernatant was taken as the digitonin-insoluble mitochondrial fraction.
In Vivo Infection and Injection of Lentivirus Particle for MCL-1 Silencing
To perform in vivo infection, female BALB/c mice were injected with 107 L. donovani promastigotes via the tail vein. Parasite burden was ascertained by Giemsa-stained impression smears and expressed as Leishman-Donovan units, measured as the number of amastigotes/1000 nucleated cells × organ weight. For in vivo stable knockdown of MCL-1, spleen tissue of an anesthetized BALB/c mice were separately injected with murine MCL-1-specific short hairpin RNA (shRNA) expression vectors as well as control shRNA vectors (Open Biosystems, Huntsville, AL) 3 days prior to being injected with L. donovani. All animal care and experimental studies were carried out in strict accordance with the recommendations of the United States National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. The protocol has been approved by the Committee on the Ethics of Animal Experiments of Indian Institute of Chemical Biology (permit 147–1999).
Densitometric Analysis
Densitometric analysis was carried out using QUANTITY ONE software (Bio-Rad). Band intensities were quantitated densitometrically and normalized to endogenous control and expressed in arbitrary units. The ratios of density of particular bands to endogenous control are represented as bar graphs.
Statistical Analysis
Data are shown as mean ± S.D. of at least three independent experiments. Student's t test was selected to assess the statistical differences among pairs of data sets with a p value of <0.05 considered to be significant.
Results
Leishmania Infection Renders Macrophages Resistant to Apoptosis
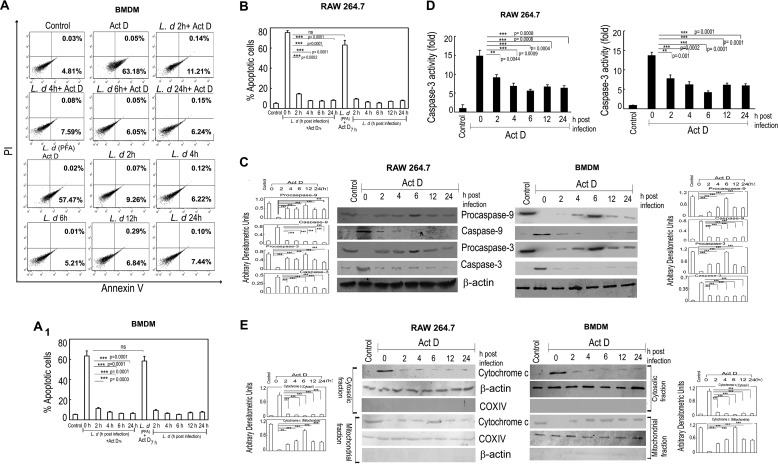
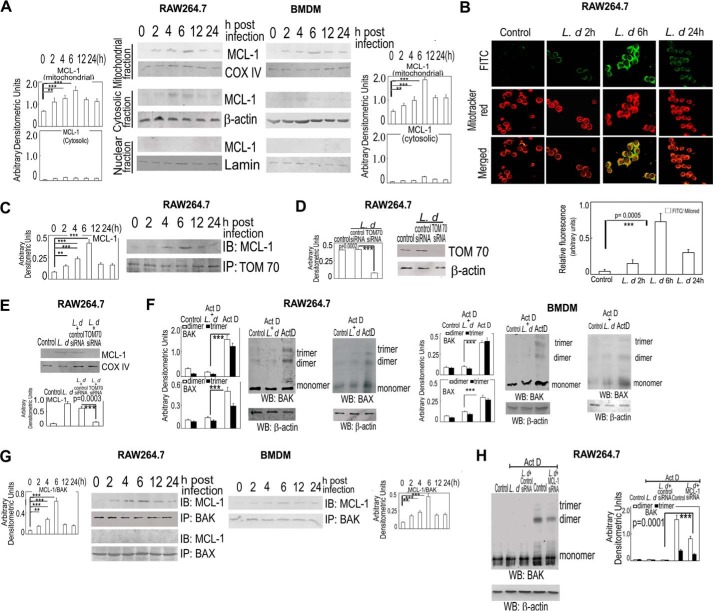
To successfully survive within host cells, L. donovani employs various strategies that render macrophage defense inactive (30). Because apoptosis could be one such means used by macrophages to eliminate intracellular infection, we wanted to see the effect of L. donovani infection on actinomycin D-induced macrophage apoptosis. To this end, RAW 264.7 cells and BMDM were infected with live parasites or paraformaldehyde-fixed parasites, washed with PBS, and subjected to actinomycin D treatment for 7 h. The percentage of apoptotic cells were measured by annexin V-PI flow cytometric study. Treatment with actinomycin D resulted in 63.2 ± 5.3 and 75.1 ± 6.8% annexin V-positive cells in the control BMDM and RAW cells, respectively (Fig. 1, A and B). In contrast, L. donovani-infected cells showed a much lower extent of apoptosis (11.3 ± 0.8, 7.6 ± 0.6, 6.1 ± 0.6, and 6.3 ± 0.5% annexin V-positive cells at 2, 4, 6, and 24 h after infection) in BMDM (Fig. 1A) and (14.1 ± 0.9, 7.4 ± 0.6, 7.2 ± 0.6, and 7.9 ± 0.7% annexin V-positive cells at 2, 4, 6, and 24 h after infection) in RAW cells (Fig. 1B). On the other hand, paraformaldehyde-fixed parasite-treated macrophages showed an almost similar extent of apoptosis as uninfected normal macrophages on exposure to actinomycin D (Fig. 1, A and B). A similar trend was observed in the absence of actinomycin D also, where cells infected with Leishmania showed very little extent of apoptosis (9.3 ± 0.8, 6.3 ± 0.6, 5.2 ± 0.4, 6.9 ± 0.6, and 7.5 ± 0.7% annexin V-positive cells at 2, 4, 6, 12, and 24 h after infection) in BMDM (Fig. 1A) and (12.1 ± 0.9, 7.8 ± 0.8, 6.2 ± 0.6, 7.9 ± 0.7, and 8.3 ± 0.8% annexin V-positive cells at 2, 4, 6, 12, and 24 h after infection) in RAW macrophages (Fig. 1B). We next checked the activation of caspases in actinomycin D-treated uninfected and infected macrophages. Uninfected RAW and BMDM cells following actinomycin D treatment showed high levels of active initiator caspase-9, which was significantly reduced (maximum 82.6 and 86.4% reduction in RAW and BMDM, respectively, at 6 h post-infection, p < 0.001, compared with actinomycin D-treated uninfected cells) during infection (Fig. 1C). Expression of caspase 3, the main effector caspase involved in apoptotic signaling cascade, was also found to be significantly reduced (maximum 63.4 and 72.8% reduction in RAW and BMDM, respectively, at 6 h post-infection, p < 0.001, as compared with uninfected cells after actinomycin D treatment) (Fig. 1C). Activity of caspase 3 was also found to be inhibited (maximum 62.9 and 67.4% reduction at 6 h post-infection in RAW and BMDM, respectively, p < 0.001, compared with actinomycin D-treated cells) (Fig. 1D). Cytochrome c release from mitochondria, the hallmark of caspase activation, was also studied by Western blot analysis in mitochondria and cytosolic fractions using anti-cytochrome c antibody. Cytochrome c was mostly found to be present in the mitochondrial fraction in L. donovani-infected actinomycin D-treated RAW 264.7 cells in contrast to uninfected cells, where it was mostly present in the cytosolic fraction (Fig. 1E, left panel). BMDM also showed a similar trend (Fig. 1E, right panel). All these results suggest that L. donovani successfully circumvents the apoptotic machinery in macrophages induced by actinomycin D.
FIGURE 1.
L. donovani infection renders macrophages resistant to apoptosis. A and B, both BMDM and RAW 264.7 macrophages were either infected with L. donovani (L.d) or treated with paraformaldehyde-fixed L. donovani with a parasite/macrophage ratio of 10:1. After incubation for the indicated time periods (0–24 h), cells were either treated with actinomycin D (Act D) (5 μg/ml) for 7 h or left untreated. Cells were then washed and apoptosis were analyzed by annexin-V-tagged FITC-PI flow cytometry. Dual parameter dot plot of FITC fluorescence (x axis) versus PI fluorescence (y axis) is expressed as logarithmic fluorescence intensity for BMDM (A). Quadrants: lower left, live cells; upper left, necrotic cells; upper right, necrotic or late phase of apoptosis; and lower right, apoptotic cells. Individual bar graph represents the mean percentage ± S.D. of apoptotic cells in RAW 264.7 (B) and BMDM (A1). C, L. donovani-infected macrophage cells (both RAW 264.7 and BMDM) were treated with actinomycin D and expression of pro- and active forms of caspase-9 and caspase-3 were detected by immunoblotting. D, whole cell extract (10 μg) prepared from L. donovani-infected actinomycin D-treated cells (both RAW 264.7 and BMDM) were subjected to caspase-3 activity assay as described under “Experimental Procedures.” E, expression of cytochrome c (both cytosolic and membrane fractions) was evaluated in L. donovani-infected actinomycin D-treated RAW 264.7 and BMDM cells by immunoblotting. β-Actin and cytochrome c oxidase (COX) subunit IV were used as internal controls for cytosolic and mitochondrial fractions, respectively. Bands were analyzed densitometrically and expressed as mean ± S.D., n = 3, **, p < 0.01; ***, p < 0.001; ns, nonsignificant; Student's t test.
Role of MCL-1 on Leishmania-mediated Inhibition of Apoptosis
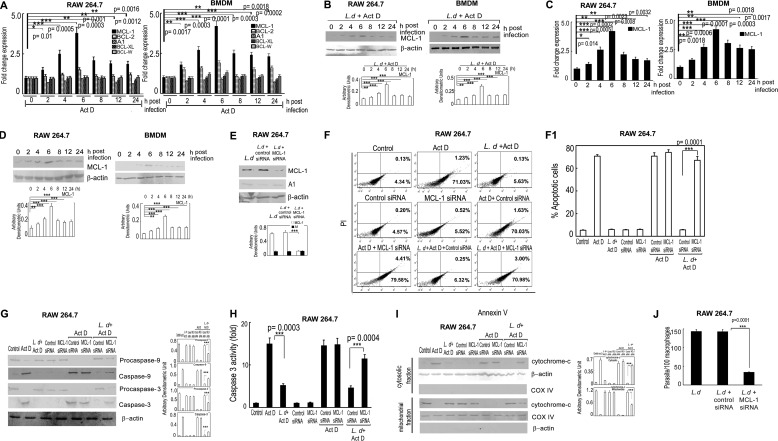
Because BCL-2 family proteins play major roles in modulating apoptosis (31), we studied the expression of anti-apoptotic members BCL-2, BCL-W, BCL-XL, MCL-1, and A1 in actinomycin D-treated infected cells by real-time PCR analysis. Among all five members, the level of MCL-1 mRNA was preferentially up-regulated with a maximum 3.8-fold at 6 h post-infection compared with uninfected RAW cells, p < 0.001, and a steady level was maintained as observed up to 24 h post-infection (Fig. 2A, left panel). On the other hand, levels of other anti-apoptotic proteins, BCL-XL, BCL-W, and A1, remained almost unchanged throughout all the time points except BCL-2, which showed a moderate increase (1.5-fold at 6 h post-infection compared with uninfected actinomycin D-treated cells) (Fig. 2A, left panel). This was further validated in BMDM, which also yielded similar results with a maximum induction of 4.3-fold MCL-1 mRNA expression over uninfected control at 6 h post-infection, p < 0.001 (Fig. 2A, right panel). Increased expression of MCL-1 was also reflected at the protein level with comparable kinetics (maximum increase of 3.1- and 3.6-fold in RAW and BMDM, respectively, at 6 h post-infection compared with control actinomycin D-treated cells, p < 0.001) (Fig. 2B). Even in the absence of actinomycin D treatment, MCL-1 was found to be up-regulated both at mRNA (maximum increase of 4.2- and 4.7-fold in RAW and BMDM cells, respectively, at 6 h post-infection compared with uninfected control, p < 0.001) and protein levels (maximum increase of 3.6- and 3.5-fold in RAW and BMDM cells, respectively, at 6 h post-infection compared with uninfected control, p < 0.001) in L. donovani-infected cells (Fig. 2, C and D). To investigate whether induction of MCL-1 was associated with inhibition of apoptosis, the siRNA-mediated knockdown system was used. The efficacy of siRNA treatment was determined by Western blotting, which showed 70.4% reduction of MCL-1 expression in siRNA-treated RAW cells as compared with control siRNA, p < 0.001 (Fig. 2E). The expression level of A1, another anti-apoptotic protein, did not show any significant change in MCL-1 siRNA-transfected cells (Fig. 2E). When MCL-1-silenced RAW cells were subjected to infection followed by actinomycin D treatment, significantly increased apoptosis was observed (68.9 ± 4.2% of annexin V positive cells compared with 6.2 ± 0.5% in scrambled siRNA control) (Fig. 2F). MCL-1 knocked down infected actinomycin D-treated cells also showed higher levels of cleaved caspase-9 and caspase-3 (3.2- and 2.6-fold increase, respectively, compared with control siRNA treatment, p < 0.001) (Fig. 2G). Activity of caspase 3 was also found to be increased in MCL-1-silenced cells (2.5-fold increase compared with control siRNA-transfected cells, p < 0.001) (Fig. 2H). Marked reduction of the cytochrome c level in the mitochondrial fraction (53.1% reduction compared with control siRNA treatment, p < 0.001) along with an increase in the cytosolic extract was also found in MCL-1 knocked down actinomycin D-treated infected cells (Fig. 2I). Effect of increased apoptosis in MCL-1 knocked down macrophages was also reflected on parasite survival where the intra-macrophage parasite number was significantly reduced in MCL-1 knocked down cells (76.2% reduction compared with control siRNA-treated cells, p < 0.001) (Fig. 2J). All these observations suggest that MCL-1 might have an important role in L. donovani-induced cytoprotection of host cells.
FIGURE 2.
Role of MCL-1 on L. donovani-mediated inhibition of apoptosis. A and B, both RAW 264.7 and BMDM were infected with L. donovani (L.d) for the indicated time periods (0–24 h) and subjected to actinomycin D (Act D) treatment for 7 h. Expression of MCL-1, BCL-2, BCL-W, A1, and BCL-XL were evaluated at the mRNA level by real-time PCR (A) and at the protein level by Western blotting (B). C and D, RAW 264.7 and BMDM were infected with L. donovani for the indicated time periods and expression of MCL-1 at the mRNA (C) and protein (D) levels were determined by real-time PCR and immunoblotting, respectively. E, RAW 264.7 cells were first transfected with control or MCL-1 siRNA followed by infection with L. donovani. The protein level expression of MCL-1 and A1 was determined by immunoblotting. F-I, RAW 264.7 cells were either treated with actinomycin D or infected with L. donovani and then treated with actinomycin D or first transfected with control or MCL-1 siRNA followed by actinomycin D treatment or L. donovani infection for 6 h followed by actinomycin D treatment. The percentage of apoptotic cells were analyzed by flow cytometry (F). Individual bar graph represents the mean percentage ± S.D. of apoptotic cells (F1). Expression of pro- and active forms of caspase-9 and caspase-3 were detected by immunoblotting (G). Caspase-3 activity was determined from the whole cell extract (10 μg of protein per sample) as described under “Experimental Procedures” using DEVD-AFC as substrate (H). Expression of cytochrome c (both cytosolic and membrane fractions) were analyzed by immunoblotting (I). J, macrophage cells were first transfected with control or MCL-1 siRNA followed by infection with L. donovani. The intracellular parasite number was then determined by Giemsa staining. Results are representative of three independent experiments performed in triplicate. Bands were analyzed densitometrically and expressed as mean ± S.D., n = 3, *, p < 0.05; **, p < 0.01; ***, p < 0.001; Student's t test.
Transcriptional Regulation of MCL-1 in L. donovani Infection
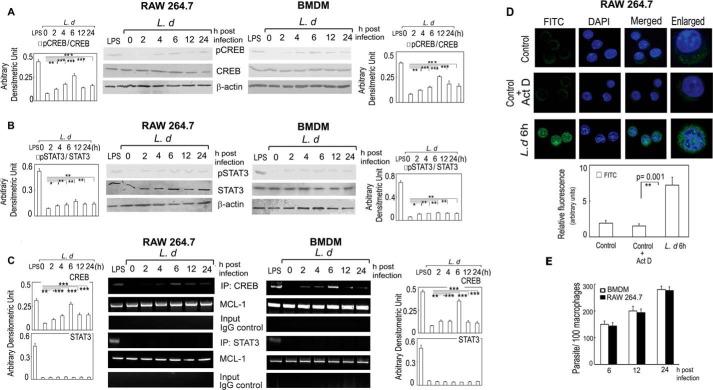
Earlier studies revealed putative binding sites for signal transducer and activator of transcription (STAT) 3, NFκB, and cAMP response element-binding (CREB) protein in the promoter region of MCL-1 (32). Because CREB and STAT3 need to get phosphorylated for promoter binding (33, 34), we therefore, checked the phosphorylation status of both proteins after infection in RAW 264.7 cells. LPS was used as a positive control as it is a strong inducer of CREB and STAT3 activation (35–38). We ruled out NF-κB as a putative transcription factor for MCL-1, because previous reports documented that NF-κB was not activated during L. donovani infection (39). Time course analysis (0–24 h) demonstrated an increase in the phosphorylation status of CREB with a maximum induction of 2.8-fold in RAW and 3.1-fold in BMDM at 6 h post-infection as compared with uninfected cells (p < 0.001) (Fig. 3A). A relatively low level of induction (1.9- and 1.8-fold increase in RAW and BMDM, respectively) was observed for phospho-STAT3 (Fig. 3B). We then analyzed the binding of STAT3 and CREB to MCL-1, the promoter region by ChIP assay, and observed strong CREB binding with a maximum of 3.8- and 4.3-fold in RAW and BMDM, respectively, compared with uninfected cells (p < 0.001) at 6 h post-infection (Fig. 3C), indicating that CREB might have a role in increased expression of MCL-1 following L. donovani infection. Interestingly, no detectable binding of STAT3 to the MCL-1 promoter was obtained (Fig. 3C), suggesting thereby that STAT3 may not be directly involved in MCL-1 gene regulation. The activation of CREB was further ascertained by nuclear translocation of phospho-CREB as observed by fluorescence microscopy using anti-pCREB antibody. In uninfected macrophages, with or without actinomycin D treatment, the weak signal for pCREB was distributed throughout the cell but did not co-localize with DAPI-stained nuclei indicating its cytosolic localization (Fig. 3D). In contrast, L. donovani infection for 6 h resulted in an increase in the nuclear localization of pCREB as evident by markedly enhanced co-localization of the pCREB signal (green) with DAPI-stained nuclei (blue) (Fig. 3D). These results suggest that L. donovani infection might lead to activation and nuclear translocation of CREB, which regulates the expression of MCL-1 even in the absence of actinomycin D.
FIGURE 3.
Transcriptional regulation of MCL-1 in L. donovani infection. A and B, both RAW 264.7 and BMDM were infected with L. donovani (L.d) for the indicated time periods (0–24 h). LPS was used as a positive control. Expressions of pCREB and CREB (A) and pSTAT3 and STAT3 (B) were analyzed by immunoblotting. C, for ChIP assay, DNA from L. donovani-infected or LPS-treated RAW 264.7 and BMDM were immunoprecipitated using either anti-CREB or anti-STAT3 or normal IgG. Immunoprecipitated DNA was then analyzed using MCL-1 promoter-specific primers by PCR followed by agarose gel electrophoresis. D, RAW 264.7 cells were either infected with L. donovani for 6 h or treated with actinomycin D (Act D) for 7 h and then stained with anti-CREB monoclonal antibody followed by secondary FITC-conjugated antibody. Nuclei were stained with DAPI and the cells were analyzed under a confocal microscope. The intensity of staining for antibody was measured in each cell using ImageJ 1.42I software and expressed as relative fluorescence intensity. E, RAW 264.7 and BMDM were infected with L. donovani promastigotes at a macrophage:parasite ratio of 1:10 for the indicated time periods, and the intracellular parasite number was determined by Giemsa staining. The data shown are representative from three independent experiments. Results are expressed as mean ± S.D., n = 3, *, p < 0.05; **, p < 0.01; ***, p < 0.001; Student's t test.
Role of CREB on MCL-1-mediated Caspase Activation, Macrophage Apoptosis, and Parasite Survival
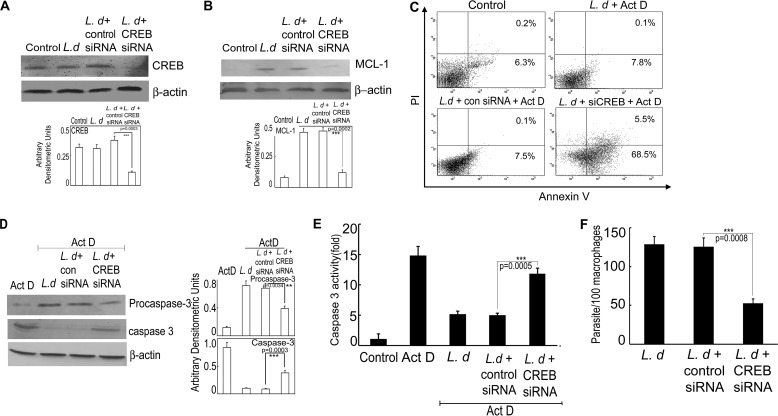
To ascertain the role of CREB in regulating MCL-1 transcription, we knocked down CREB by siRNA-mediated gene silencing, which resulted in 69.1% reduction in protein expression in infected cells compared with control siRNA-treated infected cells (p < 0.001) as revealed by immunoblot analysis (Fig. 4A). CREB-silenced cells showed markedly decreased expression of MCL-1 (74.6% reduction as compared with control siRNA-treated cells, p < 0.001) at 6 h post-infection (Fig. 4B). The role of CREB in L. donovani-induced cytoprotection of host cells was further investigated by studying apoptosis in CREB-silenced cells. CREB silencing resulted in increased population of apoptotic cells upon actinomycin D treatment (68.5 ± 5.8% apoptotic cells in case of CREB knocked down cells compared with 7.5 ± 0.7% in control siRNA-treated cells) (Fig. 4C). CREB knocked down cells also showed a marked increase in the level of cleaved caspase-3 (2.9-fold as compared with control siRNA-treated cells, p < 0.001) at 6 h post-infection and the level was comparable with actinomycin D-treated uninfected cells (Fig. 4D). CREB silencing in L. donovani-infected cells also showed a significant increase in caspase 3 activity (2.7-fold more than control siRNA-treated cells, p < 0.001) (Fig. 4E). Silencing of CREB resulted in a significant decrease in intra-macrophage survival of parasites (57.8% reduction in case of CREB knockdown as compared with control siRNA treatment, p < 0.001) (Fig. 4F). All these results suggest that induction of MCL-1 may be mediated by activation of CREB, which results in prevention of apoptosis in actinomycin D-treated infected macrophages.
FIGURE 4.
Role of CREB on MCL-1-mediated caspase activation, macrophage apoptosis, and parasite survival. A and B, RAW 264.7 cells were transfected with either control siRNA or CREB siRNA followed by L. donovani (L.d) infection for 6 h and expressions of CREB (A) and MCL-1 (B) were determined by immunoblotting. C–E, macrophage cells were transfected with control or CREB siRNA followed by L. donovani infection and actinomycin D (Act D) treatment. The percentage of apoptotic cells were measured by flow cytometry (C), whereas expressions of pro- and active caspase-3 were determined by immunoblotting (D) and caspase-3 activity was determined in 10 μg of whole cell lysate using DEVD-AFC as substrate (E). F, macrophage cells were first transfected with control or CREB siRNA followed by infection with L. donovani. The intracellular parasite number was then determined by Giemsa staining. Results are representative of three independent experiments performed in triplicate. Bands were analyzed densitometrically and expressed as mean ± S.D., n = 3, **, p < 0.01; ***, p < 0.001; Student's t test.
Role of MCL-1 on Mitochondria-dependent Apoptosis
Because MCL-1 is involved in mitochondria-dependent apoptosis, we wanted to study subcellular localization of MCL-1 during L. donovani infection. Immunoblot analysis with anti-MCL-1 antibody revealed the presence of MCL-1 mostly in the mitochondrial fraction (Fig. 5A). This was further confirmed by a co-localization study using anti-MCL-1 antibody (FITC conjugated, green) and mitochondria-specific dye MitoTracker (red), which resulted in co-localization with a yellow signal (Fig. 5B). Because TOM70 is important for mitochondrial translocation of proteins (40), we next examined the involvement of TOM70 in the mitochondrial import of MCL-1. TOM70 was immunoprecipitated from infected cells at different time points and subjected to Western blot analysis using anti-MCL-1 antibody. MCL-1 was found to co-immunoprecipitate with TOM70 with a maximum association observed at 6 h post-infection (Fig. 5C). To further ascertain the role of TOM70 in the mitochondrial import of MCL-1, we transfected cells with TOM70 siRNA, which resulted in 82.3% inhibition of TOM70 protein expression in infected cells compared with control siRNA-treated infected cells (Fig. 5D). A significant decrease of the MCL-1 protein level (70.4%) was observed in the mitochondrial fraction in knocked down TOM70-infected cells compared with the level found in control siRNA-treated infected cells (p < 0.001) (Fig. 5E). Two major pro-apoptotic proteins, BAK and BAX, play regulatory factors for mitochondrial dysfunction and BAK/BAK or BAX/BAX oligomerization leads to pore formation in the mitochondrial membrane and subsequent release of cytochrome c (41, 42). We, therefore, carried out immunoblot analysis with anti-BAK or anti-BAX antibodies to detect whether BAK/BAK or BAX/BAX oligomer formation was prevented during infection. Studies with 6-h infected actinomycin D-treated cells revealed that both BAK/BAK and BAX/BAX oligomerization were significantly reduced compared with actinomycin D-treated uninfected control cells (78.2 and 80.5% reduction in BAK/BAK dimer and trimer, respectively, and 70.3 and 65.5% reduction in BAX/BAX dimer and trimer, respectively (p < 0.001)) (Fig. 5F). We further validated these findings in BMDM, which also depicted a similar pattern of inhibition in both BAK/BAK and BAX/BAX oligomerization during infection (74.3 and 81.4% reduction in BAK/BAK dimer and trimer, respectively, and 57.3 and 64.8% reduction in BAX/BAX dimer and trimer respectively, p < 0.001) (Fig. 5F, right panel). Because MCL-1 is involved in mitochondria-dependent apoptosis and contains a BH3 homology domain, we wanted to determine whether MCL-1 interacts with BAK or BAX and prevents their oligomerization. Co-immunoprecipitation studies with both anti-MCL-1 and anti-BAK/BAX antibodies revealed a strong association between MCL-1 and BAK in L. donovani-infected cells, whereas no interaction was observed between MCL-1 and BAX (Fig. 5G). A strong interaction between MCL-1 and BAK was also observed in BMDM (Fig. 5G, right panel). To further ascertain the role of MCL-1 in BAK/BAK oligomerization, we studied the effect of siRNA-mediated silencing of MCL-1. When MCL-1-silenced cells were subjected to infection followed by actinomycin D treatment, significantly increased BAK/BAK dimer and trimer formation was observed, which is comparable with that in actinomycin D-treated cells (Fig. 5H). Collectively, these results suggest that mitochondrial transportation of up-regulated MCL-1 during L. donovani infection may be associated with the prevention of BAK-mediated mitochondrial dysfunction leading to inhibition of host cell apoptosis.
FIGURE 5.
Role of MCL-1 on mitochondria-dependent apoptosis. A, expression of MCL-1 was analyzed in the mitochondrial, cytosolic, and nuclear fractions of L. donovani (L.d.)-infected (0–24 h) RAW 264.7 and BMDM by immunoblotting. COX-IV, β-actin, and lamin were used as internal control for mitochondrial, cytosolic, and nuclear fractions, respectively. B, infected cells were stained with anti-MCL-1 monoclonal antibody followed by secondary FITC-conjugated antibody. Mitochondria were stained with MitoTracker Red and localization of MCL-1 was analyzed by confocal microscope. Top row, FITC stained cells; middle row, MitoTracker Red-stained cells; bottom row, merged. The intensity of staining for MCL-1 was measured in each cell using ImageJ 1.42I software, normalized to mitored stain used to mark mitochondria and expressed as relative fluorescence intensity. C, association of MCL-1 with TOM70 was determined by immunoprecipitating (IP) TOM70 with anti-TOM70 antibody from the mitochondrial fraction followed by immunoblotting (IB) with MCL-1 specific antibody. D, RAW 264.7 cells were first transfected with control or TOM70 siRNA followed by infection with L. donovani. Efficacy of the TOM70 siRNA was determined by immunoblotting. E, macrophage cells were transfected with control or TOM70 siRNA followed by infection with L. donovani. Levels of MCL-1 were then determined by immunoblotting. F, RAW 264.7 and BMDM were either treated with actinomycin D for 7 h or first infected with L. donovani for 6 h followed by actinomycin D treatment for 7 h. Cellular lysates were prepared and treated with EGS to keep the protein-protein interaction intact. To detect monomer, dimer, or trimer formation of BAK or BAX, immunoblotting was done using anti-BAK or anti-BAX monoclonal antibody. G, both RAW 264.7 and BMDM were infected with L. donovani for the indicated time periods (0–24 h) and association of MCL-1 with BAK and BAX were determined by immunoprecipitating BAK or BAX with anti-BAK or anti-BAX antibody from the mitochondrial fraction followed by immunoblotting with MCL-1 specific antibody. H, RAW 264.7 cells were either treated with actinomycin D or first transfected with control or MCL-1 siRNA followed by L. donovani infection. Cellular lysates were prepared and treated with EGS to keep the protein-protein interaction intact. Immunoblotting was done with BAK specific antibody to detect monomer, dimer, and trimer formation of BAK. The data shown are representative from three independent experiments. Results are expressed as mean ± S.D., n = 3; **, p < 0.01; ***, p < 0.001; Student's t test.
Role of MCL-1 in in Vivo Infection
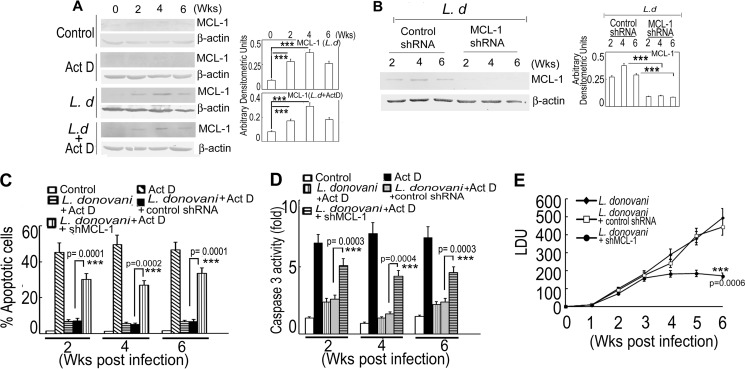
The in vitro experiments suggesting prime involvement of MCL-1 in L. donovani-induced inhibition of macrophage apoptosis led us to investigate the role of MCL-1 in the disease progression of visceral leishmaniasis in the BALB/c mouse model. To this end, we isolated splenic macrophages from both control and L. donovani-infected mice after 2, 4, and 6 weeks of infection, treated with actinomycin D for 7 h, and evaluated MCL-1 expression at protein level by immunoblot analysis. Although no significant induction of MCL-1 was observed in uninfected control as well as in actinomycin D-treated control splenic macrophages, but a marked up-regulation was observed in infected mice with a maximum at 4 weeks post-infection both in the case of actinomycin D-treated and -untreated splenic macrophages (4.1- and 3.7-fold, respectively, p < 0.001, compared with control mice) (Fig. 6A). We then sought to determine whether inhibition of MCL-1 could reverse the parasite-induced anti-apoptotic response and to this end lentiviral vector-mediated MCL-1-specific shRNA or control shRNA was transfected into spleen tissue in anesthetized BALB/c mice followed by L. donovani infection up to 6 weeks. shRNA treatment apparently did not cause any visible physiological disorder among the group of mice. The specificity of MCL-1 shRNA was confirmed by Western blotting, which showed a significant reduction (73.9 and 68.6% compared with control shRNA at 4 and 6 weeks post-infection, respectively, p < 0.001) (Fig. 6B). Splenic macrophages were then isolated from infected control, control shRNA- and MCL-1 shRNA-administered infected mice, treated with actinomycin D, and the percentage of apoptotic cells was analyzed by FACS. Splenic macrophages from MCL-1-silenced infected mice showed significantly increased levels of apoptosis (26.8 ± 2.4% apoptotic cells as compared with 5.2 ± 0.5% in control shRNA-treated mice, p < 0.001) (Fig. 6C) at 4 weeks post-infection. Moreover, the activity of caspase 3 was also induced in actinomycin D-treated splenic macrophages from MCL-1 knocked down mice (2.9-fold more than control shRNA-treated mice, p < 0.001) (Fig. 6D) at 4 weeks post-infection. Finally, to ascertain whether L. donovani-induced prevention of host cell apoptosis indeed leads to increased parasite survival, spleen parasite burden was determined by Giemsa staining. MCL-1 knock down in the spleen of infected mice resulted in markedly reduced parasite burden (61.3% reduction compared with control shRNA-treated infected animal, p < 0.001) at 6 weeks post-infection (Fig. 6E). These results suggest that inhibition of MCL-1 following L. donovani infection facilitates induction of apoptosis leading to parasite suppression, which specifies MCL-1 as an anti-apoptotic protein exploited by Leishmania for disease progression.
FIGURE 6.
Role of MCL-1 in in vivo infection. A, splenic macrophages from control or 2-, 4-, and 6-week L. donovani (L.d)-infected BALB/c mice were treated with actinomycin D (Act D) for 7 h and expression of MCL-1 in splenic macrophage lysates were analyzed by immunoblotting. B–E, MCL-1 knockdown in vivo was achieved by transfecting lentiviral vector (∼108 transducing units/ml, 0.5 μl) specific for MCL-1 or control shRNA (GFP-encoding shRNA) into spleen tissue in anesthetized BALB/c mice and then infected with L. donovani for the indicated time periods (0–6 weeks). Splenic macrophages were isolated from control or MCL-1 shRNA-transfected L. donovani-infected mice and expression of MCL-1 was measured in cell lysates by immunoblot analysis (B). One set of control shRNA or MCL-1 shRNA-transfected L. donovani-infected mouse splenic macrophages from each system was further treated with actinomycin D for 7 h and the percentage of apoptotic cells were measured by annexin V-PI flow cytometry (C). Caspase-3 activity was evaluated in splenic macrophage lysates of different groups using DEVD-AFC as substrate (D). Spleen parasite burden was determined weekly in different groups of infected mice as described under “Experimental Procedures” expressed as Leishman-Donovan units (LDU) ± S.D. for five animals (E). Results are representative of three individual experiments and are expressed as mean ± S.D. ***, p < 0.001; Student's t test.
Discussion
Suppression of apoptosis of the host cell is a necessary pre-requisite for successful infection of many intracellular pathogens including Leishmania (10). In this study we attempted to elucidate the signaling mechanisms employed by L. donovani to circumvent host cell apoptosis and establish infection.
We demonstrated that actinomycin D-mediated apoptosis of macrophages was largely suppressed upon Leishmania infection indicating attenuation of host cell apoptosis for infection establishment. Caspases, which are activated due to some pro-apoptotic stimuli, perform a critical role in executing apoptosis by cleaving a wide range of cellular proteins, which are essential for maintaining cellular integrity (43). The significant repression of actinomycin D-mediated activation of initiator and effector caspases as well as release of cytochrome c to the cytosol upon infection further supported the attenuation of mitochondria-dependent apoptosis by Leishmania (6). The intrinsic mitochondrial apoptotic pathway is counteracted by a complex interplay of anti-apoptotic proteins of the BCL-2 family, which includes BCL-2, BCL-XL, BCL-W, A1, and MCL-1 (15–18). Some of these anti-apoptotic proteins have been reported to be exploited by the intracellular pathogens for protecting their niche for the establishment as well as progression of infection (6, 44). The significant up-regulation of the anti-apoptotic protein MCL-1 at both the mRNA and protein levels, in the presence or absence of actinomycin D in our study corroborated well with the role of MCL-1 in the infection scenario to prevent apoptosis as in the case of Toxoplasma gondii and S. aureus (6, 24). Although a previous report documented the induction of BCL-XL in Leishmania-infected RAW 264.7 cells (45), we did not observe any noticeable increase in the expression of this protein, which may be because of the different strain used in that study (MHOM/IN/95/9515). siRNA-mediated silencing of MCL-1, which significantly induced apoptotic death of the host cell, further ascertained the functional role of the anti-apoptotic protein MCL-1 in intracellular survival and/or proliferation of Leishmania.
MCL-1 has been reported to localize in different cellular compartments including cytosol, nuclear envelope, and mitochondria, depending on the cell type and the function that it has to perform (20). Although most of the studies reported mitochondrial localization, a very few reported cytosolic or nuclear localization of this protein (46, 47). Leishmania induced MCL-1 mostly translocated to the mitochondria via the mitochondrial import protein TOM70. Silencing of TOM70 by siRNA prevented mitochondrial translocation of MCL-1, which is in line with the evidence where MCL-1 failed to localize in mitochondria in TOM70 knocked down cells (48). Homo-oligomerization of BCL-2 family proteins BAK and BAX in response to various apoptotic stimuli leads to mitochondrial permeabilization and consequently cytochrome c efflux from the mitochondria to the cytosol resulting in cellular apoptosis (41, 42). MCL-1 has been found to possess a strong affinity for BAK and BAX and by binding with BAK or BAX it prevents their oligomer formation leading to inhibition of apoptosis (20). Although we found prevention of homo-oligomerization of both BAK and BAX during Leishmania infection, a co-immunoprecipitation study revealed a strong association of MCL-1 with only BAK. A strong binding between MCL-1 and BAK was also found in 293T and HeLa cells to keep pro-apoptotic BAK in check (18). Neither oligomeric formation of BAX nor its association with MCL-1 was observed in our study, which could be because of its impaired translocation to mitochondria during infection. According to Quan et al. (50), the pro-apoptotic protein BAD activated through the PI3K/AKT pathway during T. gondii infection prevents host cell apoptosis via inhibition of mitochondrial translocation of BAX. However, an in depth study is required to unravel the mechanism of L. donovani-mediated inactivation of BAX.
The transcription factors that regulate MCL-1 synthesis during Leishmania infection have important roles to play as MCL-1 is labile in nature due to the presence of PEST sequence (20). Previous reports suggested that CREB, activated via the PI3K/AKT pathway and STAT3, activated via the JAK/STAT pathway, are the two major transcriptional regulators of MCL-1 (51, 52). Moreover, the MCL-1 promoter has been reported to possess the binding sites of other transcriptional factors like NF-κB, ETS, Sp-1, and SRE (32). We ruled out the possible involvement of NF-κB in MCL-1 transcription as NF-κB deactivation is well documented during Leishmania infection (39). We selectively studied the role of STAT-3 and CREB as earlier reports suggested their activation during Leishmania infection in the context of intra-macrophage survival (53, 54). CREB was found to be the major transcriptional regulator of MCL-1 although L. donovani infection induced the phosphorylation of both STAT3 and CREB. Activation and nuclear translocation of CREB along with its binding with the MCL-1 promoter region are supported by the reports that CREB independently regulates MCL-1 expression in several other instances (49, 51). The phosphorylation of CREB at Ser-133 upon Leishmania infection has also been well documented in RAW 264.7 and human myeloid cells for macrophage survival and regulating the synthesis of IL-10 (49). The phosphorylation of STAT3 has been implicated in switching the Th1/Th2 cytokine balance toward disease progressing Th2 cytokine during Leishmania infection (53). The decreased level of MCL-1 and increased induction of apoptotic death of infected macrophages upon siRNA-mediated silencing of CREB suggest the important role played by CREB as a MCL-1 transcription factor in helping Leishmania to protect their niche. Splenic macrophages of L. donovani-infected mice showed up-regulation of MCL-1 and shRNA-mediated knockdown of MCL-1 in the infected mouse spleen not only increased host cell apoptosis but also substantially decreased spleen parasite burden thereby validating the involvement of MCL-1 in the in vivo situation also. In summary, the present study demonstrates that MCL-1 has a crucial function in regulating L. donovani-mediated inhibition of apoptotic machinery of the host cell, thus helping in dissemination of this parasite using macrophage cells as the Trojan horse and identifies MCL-1 as a potent therapeutic target in visceral leishmaniasis.
Author Contributions
A. U., J. G., and S. S. conceived and designed the experiments. J. G., S. S., M. B., S. P., and P. G. performed the experiments. A. U., J. G., and S. P. analyzed the data. A. U., J. G., and S. S. wrote the paper.
Acknowledgment
We thank Boni Haldar (DBT-CU-IPLS Core Facility) for microscopic analysis.
This work was supported by the Department of Science and Technology Grants SB/WEA-013/2013 and SB/SO/BB-0055/2013, the Council of Scientific and Industrial Research (CSIR), University Grants Commission (UGC), and Indian Council of Medical Research (ICMR), Government of India. The authors declare that they have no conflicts of interest with the contents of this article.
- BMDM
- bone marrow-derived macrophage
- MCL-1
- myeloid cell leukemia 1
- BAK
- Bcl-2 homologous antagonist/killer
- BAX
- Bcl-2-associated X protein
- CREB
- cAMP response element-binding protein
- STAT3
- signal transducer and activator of transcription 3
- EGS
- ethylene glycol-bis(succinimidylsuccinate).
References
- 1. Shadab M., and Ali N. (2011) Evasion of host defence by Leishmania donovani: subversion of signaling pathways. Mol. Biol. Int. 2011, 343961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang K. P., and Dwyer D. M. (1976) Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science 193, 678–680 [DOI] [PubMed] [Google Scholar]
- 3. Olivier M., Gregory D. J., and Forget G. (2005) Subversion mechanisms by which Leishmania parasites can escape the host immune response: a signaling point of view. Clin. Microbiol. Rev. 18, 293–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Griffin D. E., and Hardwick J. M. (1999) Perspective: virus infections and the death of neurons. Trends Microbiol. 7, 155–160 [DOI] [PubMed] [Google Scholar]
- 5. Williams G. T. (1994) Programmed cell death: a fundamental protective response to pathogens. Trends Microbiol. 2, 463–464 [DOI] [PubMed] [Google Scholar]
- 6. Goebel S., Gross U., and Lüder C. G. (2001) Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J. Cell Sci. 114, 3495–3505 [DOI] [PubMed] [Google Scholar]
- 7. Cahir-McFarland E. D., Davidson D. M., Schauer S. L., Duong J., and Kieff E. (2000) NF-κB inhibition causes spontaneous apoptosis in Epstein-Barr virus-transformed lymphoblastoid cells. Proc. Natl. Acad. Sci. U.S.A. 97, 6055–6060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Alli O. A., Gao L. Y., Pedersen L. L., Zink S., Radulic M., Doric M., and Abu Kwaik Y. (2000) Temporal pore formation-mediated egress from macrophages and alveolar epithelial cells by Legionella pneumophila. Infect. Immun. 68, 6431–6440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Heussler V. T., Küenzi P., and Rottenberg S. (2001) Inhibition of apoptosis by intracellular protozoan parasites. Int. J. Parasitol. 31, 1166–1176 [DOI] [PubMed] [Google Scholar]
- 10. Moore K. J., and Matlashewski G. (1994) Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J. Immunol. 152, 2930–2937 [PubMed] [Google Scholar]
- 11. Lisi S., Sisto M., Acquafredda A., Spinelli R., Schiavone M., Mitolo V., Brandonisio O., and Panaro M. (2005) Infection with Leishmania infantum inhibits actinomycin D-induced apoptosis of human monocytic cell line U-937. J. Eukaryot. Microbiol. 52, 211–217 [DOI] [PubMed] [Google Scholar]
- 12. Akarid K., Arnoult D., Micic-Polianski J., Sif J., Estaquier J., and Ameisen J. C. (2004) Leishmania major-mediated prevention of programmed cell death induction in infected macrophages is associated with the repression of mitochondrial release of cytochrome c. J. Leukoc. Biol. 76, 95–103 [DOI] [PubMed] [Google Scholar]
- 13. Ruhland A., Leal N., and Kima P. E. (2007) Leishmania promastigotes activate PI3K/Akt signalling to confer host cell resistance to apoptosis. Cell Microbiol. 9, 84–96 [DOI] [PubMed] [Google Scholar]
- 14. Srivastav S., Basu Ball W., Gupta P., Giri J., Ukil A., and Das P. K. (2014) Leishmania donovani prevents oxidative burst-mediated apoptosis of host macrophages through selective induction of suppressors of cytokine signaling (SOCS) proteins. J. Biol. Chem. 289, 1092–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Willis S. N., Fletcher J. I., Kaufmann T., van Delft M. F., Chen L., Czabotar P. E., Ierino H., Lee E. F., Fairlie W. D., Bouillet P., Strasser A., Kluck R. M., Adams J. M., and Huang D. C. (2007) Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315, 856–859 [DOI] [PubMed] [Google Scholar]
- 16. Willis S. N., and Adams J. M. (2005) Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17, 617–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Leu J. I., Dumont P., Hafey M., Murphy M. E., and George D. L. (2004) Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell. Biol. 6, 443–450 [DOI] [PubMed] [Google Scholar]
- 18. Willis S. N., Chen L., Dewson G., Wei A., Naik E., Fletcher J. I., Adams J. M., and Huang D. C. (2005) Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 19, 1294–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chao J. R., Wang J. M., Lee S. F., Peng H. W., Lin Y. H., Chou C. H., Li J. C., Huang H. M., Chou C. K., Kuo M. L., Yen J. J., and Yang-Yen H. F. (1998) Mcl-1 is an immediate-early gene activated by the granulocyte-macrophage colony-stimulating factor (GM-CSF) signaling pathway and is one component of the GM-CSF viability response. Mol. Cell. Biol. 18, 4883–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas L. W., Lam C., and Edwards S. W. (2010) Mcl-1: the molecular regulation of protein function. FEBS Lett. 584, 2981–2989 [DOI] [PubMed] [Google Scholar]
- 21. Opferman J. T., Letai A., Beard C., Sorcinelli M. D., Ong C. C., and Korsmeyer S. J. (2003) Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature 426, 671–676 [DOI] [PubMed] [Google Scholar]
- 22. Opferman J. T., Iwasaki H., Ong C. C., Suh H., Mizuno S., Akashi K., and Korsmeyer S. J. (2005) Obligate role of anti-apoptotic MCL-1 in the survival of hematopoietic stem cells. Science 307, 1101–1104 [DOI] [PubMed] [Google Scholar]
- 23. Milot E., and Filep J. G. (2011) Regulation of neutrophil survival/apoptosis by Mcl-1. ScientificWorldJournal 11, 1948–1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koziel J., Kmiecik K., Chmiest D., Maresz K., Mizgalska D., Maciag-Gudowska A., Mydel P., and Potempa J. (2013) The role of Mcl-1 in S. aureus-induced cytoprotection of infected macrophages. Mediators Inflamm. 2013, 427021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rajalingam K., Sharma M., Lohmann C., Oswald M., Thieck O., Froelich C. J., and Rudel T. (2008) Mcl-1 is a key regulator of apoptosis resistance in Chlamydia trachomatis-infected cells. PLoS ONE 3, e3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sly L. M., Hingley-Wilson S. M., Reiner N. E., and McMaster W. R. (2003) Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 170, 430–437 [DOI] [PubMed] [Google Scholar]
- 27. Kar S., Ukil A., Sharma G., and Das P. K. (2010) MAPK-directed phosphatases preferentially regulate pro- and anti-inflammatory cytokines in experimental visceral leishmaniasis: involvement of distinct protein kinase C isoforms. J. Leukoc. Biol. 88, 9–20 [DOI] [PubMed] [Google Scholar]
- 28. Ayala A., Perrin M. M., Wang P., Ertel W., and Chaudry I. H. (1991) Hemorrhage induces enhanced Kupffer cell cytotoxicity while decreasing peritoneal or splenic macrophage capacity: involvement of cell-associated tumor necrosis factor and reactive nitrogen. J. Immunol. 147, 4147–4154 [PubMed] [Google Scholar]
- 29. Single B., Leist M., and Nicotera P. (1998) Simultaneous release of adenylate kinase and cytochrome c in cell death. Cell Death Differ. 5, 1001–1003 [DOI] [PubMed] [Google Scholar]
- 30. Handman E., and Bullen D. V. (2002) Interaction of Leishmania with the host macrophage. Trends Parasitol. 18, 332–334 [DOI] [PubMed] [Google Scholar]
- 31. Yang J., Liu X., Bhalla K., Kim C. N., Ibrado A. M., Cai J., Peng T. I., Jones D. P., and Wang X. (1997) Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275, 1129–1132 [DOI] [PubMed] [Google Scholar]
- 32. Moshynska O., Sankaran K., Pahwa P., and Saxena A. (2004) Prognostic significance of a short sequence insertion in the MCL-1 promoter in chronic lymphocytic leukemia. J. Natl. Cancer Inst. 96, 673–682 [DOI] [PubMed] [Google Scholar]
- 33. Wen A. Y., Sakamoto K. M., and Miller L. S. (2010) The role of the transcription factor CREB in immune function. J. Immunol. 185, 6413–6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wen Z., Zhong Z., and Darnell J. E. Jr. (1995) Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell 82, 241–250 [DOI] [PubMed] [Google Scholar]
- 35. Avni D., Ernst O., Philosoph A., and Zor T. (2010) Role of CREB in modulation of TNFγ and IL-10 expression in LPS-stimulated RAW264.7 macrophages. Mol. Immunol. 47, 1396–1403 [DOI] [PubMed] [Google Scholar]
- 36. Mellett M., Atzei P., Jackson R., O'Neill L. A., and Moynagh P. N. (2011) Mal mediates TLR induced activation of CREB and expression of IL-10. J. Immunol. 186, 4925–4935 [DOI] [PubMed] [Google Scholar]
- 37. Samavati L., Rastogi R., Du W., Hüttemann M., Fite A., and Franchi L. (2009) STAT3 tyrosine phosphorylation is critical for interleukin 1β and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol. Immunol. 46, 1867–1877 [DOI] [PubMed] [Google Scholar]
- 38. Carl V. S., Gautam J. K., Comeau L. D., and Smith M. F. Jr. (2004) Role of endogenous IL-10 in LPS-induced STAT3 activation and IL-1 receptor antagonist gene expression. J. Leukoc. Biol. 76, 735–742 [DOI] [PubMed] [Google Scholar]
- 39. Srivastav S., Kar S., Chande A. G., Mukhopadhyaya R., and Das P. K. (2012) Leishmania donovani exploits host deubiquitinating enzyme A20, a negative regulator of TLR signaling, to subvert host immune response. J. Immunol. 189, 924–934 [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto H., Fukui K., Takahashi H., Kitamura S., Shiota T., Terao K., Uchida M., Esaki M., Nishikawa S., Yoshihisa T., Yamano K., and Endo T. (2009) Roles of Tom70 in import of presequence-containing mitochondrial proteins. J. Biol. Chem. 284, 31635–31646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dewson G., Kratina T., Czabotar P., Day C. L., Adams J. M., and Kluck R. M. (2009) Bak activation for apoptosis involves oligomerization of dimers via their α6 helices. Mol. Cell 36, 696–703 [DOI] [PubMed] [Google Scholar]
- 42. Bleicken S., Classen M., Padmavathi P. V., Ishikawa T., Zeth K., Steinhoff H. J., and Bordignon E. (2010) Molecular details of Bax activation, oligomerization, and membrane insertion. J. Biol. Chem. 285, 6636–6647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McIlwain D. R., Berger T., and Mak T. W. (2015) Caspase functions in cell death and disease. Cold Spring Harb. Perspect. Biol. 7, a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kausalya S., Somogyi R., Orlofsky A., and Prystowsky M. B. (2001) Requirement of A1-a for Bacillus Calmette-Guerin-mediated protection of macrophages against nitric oxide-induced apoptosis. J. Immunol. 166, 4721–4727 [DOI] [PubMed] [Google Scholar]
- 45. Donovan M. J., Maciuba B. Z., Mahan C. E., and McDowell M. A. (2009) Leishmania infection inhibits cycloheximide-induced macrophage apoptosis in a strain-dependent manner. Exp. Parasitol. 123, 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fujise K., Zhang D., Liu J., and Yeh E. T. (2000) Regulation of apoptosis and cell cycle progression by MCL1: differential role of proliferating cell nuclear antigen. J. Biol. Chem. 275, 39458–39465 [DOI] [PubMed] [Google Scholar]
- 47. Jamil S., Sobouti R., Hojabrpour P., Raj M., Kast J., and Duronio V. (2005) A proteolytic fragment of Mcl-1 exhibits nuclear localization and regulates cell growth by interaction with Cdk1. Biochem. J. 387, 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chou C. H., Lee R. S., and Yang-Yen H. F. (2006) An internal EELD domain facilitates mitochondrial targeting of Mcl-1 via a Tom70-dependent pathway. Mol. Biol. Cell 17, 3952–3963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Liu H., Perlman H., Pagliari L. J., and Pope R. M. (2001) Constitutively activated Akt-1 is vital for the survival of human monocyte-differentiated macrophages. Role of Mcl-1, independent of nuclear factor (NF)-κB, Bad, or caspase activation. J. Exp. Med. 194, 113–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Quan J. H., Cha G. H., Zhou W., Chu J. Q., Nishikawa Y., and Lee Y. H. (2013) Involvement of PI 3 kinase/Akt-dependent Bad phosphorylation in Toxoplasma gondii-mediated inhibition of host cell apoptosis. Exp. Parasitol. 133, 462–471 [DOI] [PubMed] [Google Scholar]
- 51. Wang J. M., Chao J. R., Chen W., Kuo M. L., Yen J. J., and Yang-Yen H. F. (1999) The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol. Cell. Biol. 19, 6195–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Epling-Burnette P. K., Liu J. H., Catlett-Falcone R., Turkson J., Oshiro M., Kothapalli R., Li Y., Wang J. M., Yang-Yen H. F., Karras J., Jove R., and Loughran T. P. Jr. (2001) Inhibition of STAT3 signaling leads to apoptosis of leukemic large granular lymphocytes and decreased Mcl-1 expression. J. Clin. Invest. 107, 351–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bhattacharya P., Gupta G., Majumder S., Adhikari A., Banerjee S., Halder K., Majumdar S. B., Ghosh M., Chaudhuri S., Roy S., and Majumdar S. (2011) Arabinosylated lipoarabinomannan skews Th2 phenotype towards Th1 during Leishmania infection by chromatin modification: involvement of MAPK signaling. PLoS ONE 6, e24141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nandan D., Camargo de Oliveira C., Moeenrezakhanlou A., Lopez M., Silverman J. M., Subek J., and Reiner N. E. (2012) Myeloid cell IL-10 production in response to leishmania involves inactivation of glycogen synthase kinase-3β downstream of phosphatidylinositol-3 kinase. J. Immunol. 188, 367–378 [DOI] [PubMed] [Google Scholar]