Abstract
Study Objectives:
To investigate whether administration of an oral dose of 6 mg melatonin before bedtime perioperatively in breast cancer surgery could change sleep outcomes measured by actigraphy.
Methods:
This paper reports secondary outcomes from a double-blind, placebo-controlled, randomized clinical trial where patients received 6 mg melatonin (n = 27) or placebo (n = 21) approximately 60 minutes before bedtime 3 nights preoperatively until at least one week postoperatively. Participants were monitored in the entire period with actigraphy, and were instructed to complete visual analogue scale (VAS) for sleep, and the Karolinska Sleepiness Scale (KSS) each morning.
Results:
Administration of 6 mg oral melatonin approximately 1 hour before bedtime resulted in significantly increased sleep efficiency and reduced wake after sleep onset for the entire 2-week postoperative period. No other significant differences for actigraphy determined sleep outcomes or subjective outcome parameters in the perioperative period were found between the groups. Overall, the patients sleep outcomes were within normal ranges and no participants had pathological sleep disturbances.
Conclusions:
Melatonin significantly changed sleep efficiency and wake after sleep onset after surgery, but had no effects on other objective sleep outcomes or on subjective sleep quality (VAS and KSS).
Clinical Trial Registration:
The trial was registered on www.clinicaltrials.gov (NCT01355523) before inclusion of the first patient.
Citation:
Madsen MT, Hansen MV, Andersen LT, Hageman I, Rasmussen LS, Bokmand S, Rosenberg J, Gögenur I. Effect of melatonin on sleep in the perioperative period after breast cancer surgery: a randomized, double-blind, placebo-controlled trial. J Clin Sleep Med 2016;12(2):225–233.
Keywords: actigraphy, sleep, melatonin, breast cancer surgery, randomized clinical trial
INTRODUCTION
Melatonin is the pineal hormone with the main function of regulating the circadian rhythm of mammals.1 As an exogenous supplement it has been extensively researched as a treatment for jetlag and insomnia.2 Recently the use of melatonin in the perioperative period has been reviewed3 and has shown an effect on reducing anxiety and pain. A minor effect on improving sleep has been shown in the perioperative period,3 but sleep was predominantly quantified via questionnaires,4–7 and only one study used an objective measure of actigraphy.5 In light of this, high quality studies with appropriate measuring techniques are warranted.
Sleep disturbances are serious and overlooked problems in patients with cancer.8,9 A prevalence of sleep problems up to 80% in cancer patients has been shown.10 Patients with breast cancer represent a vulnerable population.11 In these patients, sleep disturbances are present throughout the entire course of cancer disease, i.e., before oncological treatment,12 during oncological treatment,10,13 and even among breast cancer survivors.14,15 Sleep disturbances in patients with breast cancer are also present in the perioperative period.16,17 In the immediate postoperative period, REM sleep is reduced and light sleep is increased, with a subsequent normalization two weeks postoperatively in patients undergoing breast cancer surgery.17
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disturbances are severe and overlooked problems in patients with breast cancer. Melatonin can potentially alleviate these sleep disturbances; hence this study was undertaken to investigate this issue.
Study Impact: Melatonin was shown to improve objectively determined sleep as measured via actigraphy, which has never before been shown in patients with breast cancer.
The primary recommended treatment of insomnia in a healthy population is cognitive behavioural therapy,18 which has also been investigated in patients with breast cancer. both during oncological treatment19 and in breast cancer survivors.20 Although the studies have shown promising results, the improvements have predominantly been shown in subjective sleep outcomes (i.e., sleep questionnaires and diaries),13 but not in the objective sleep measurements (actigraphy). An intervention should ideally improve both subjective and objective sleep outcomes (actigraphy or polysomnography).
We investigated whether administration of an oral dose of 6 mg of melatonin at bedtime before and after surgery could change sleep outcomes measured objectively by actigraphy. Furthermore, we sought to evaluate the effect of melatonin on subjective sleep (visual analogue scale and Karolinska Sleepiness Scale).
METHODS
This is a report of secondary endpoints from a previously published study.21 The reporting conforms to the CONSORT statement.22
Design Overview
The MELODY trial23 was a randomized (1:1), double-blind, placebo-controlled trial with the primary outcome being the effect of melatonin on depressive symptoms as reported elsewhere.21 Other secondary outcomes on postoperative cognitive dysfunction (POCD), sleep diary and VAS data have also been reported previously.24 Subjective sleep outcomes (sleep diary and VAS) were reported as summarized data in 2 periods (peri-operative period and long-term postoperative period), which is different from the current paper. The study was approved by the Local Ethics Committee (H-4-2011-007), the Danish Medicines Agency (EudraCT nr. 2010-022460-12) and the Danish Data Protection Agency (2007-58-0015/HEH.750.89-12) and was registered on www.clinicaltrials.gov (NCT01355523) before inclusion of the first patient. All participants gave oral and written informed consent before inclusion and the study was monitored by the Good Clinical Practice Unit at Copenhagen University Hospital.
Setting and Participants
Patients were recruited from the Department of Breast Surgery, Herlev Hospital, Copenhagen, Denmark. Eligible patients were women aged 30–75 years, scheduled for lumpectomy or mastectomy for breast cancer, with American Society of Anesthesiologists (ASA) class I-III. At the time of enrolment, patients were screened for signs of depression with the Major Depression Inventory (MDI).25 We excluded those who had mild, moderate or severe depression using the ICD-10 criteria. A further comprehensive list of exclusion criteria can be seen in the previously published protocol article.23 After initiation of the trial, the upper limit of the age criteria was raised from 70 to 75 years on October 19, 2011, because of slow recruitment. Otherwise no changes were made to the inclusion or exclusion criteria.
Randomization and Interventions
At least 3 days before surgery, patients were assessed and screened for inclusion. The screening consisted of administration of the Mini Mental State Examination (MMSE),26 a neuropsychological test battery,27 and the MDI.25
On inclusion, patients were randomly assigned 1:1, in blocks of 6, to melatonin or placebo. The intervention group received 6 mg oral melatonin daily approximately 1 h before bedtime for at least 3 days preoperatively until 12 weeks postoperatively. The control group received placebo tablets in the same time period. The randomization list was computer generated using dedicated software (http://www.randomization.com). To assure blinding, this procedure was completed by the hospital pharmacy, who received the medicine directly from Pharma Nord (Vejle, Denmark). The pharmacy packed the melatonin/placebo in identical, sequentially numbered, sealed boxes. The participants, the health care providers, the research staff, and the investigators assessing the outcomes were all blinded to allocation and the allocation sequence by taking the sequentially numbered sealed boxes. The melatonin/placebo was supplied by Pharma Nord, and the tablets and packages were physically identical.
Right after inclusion, patients wore an Actigraph (Micro Motionlogger sleep watch, Ambulatory Monitoring Inc., New York, USA) and continued to do so for the first 2 weeks postoperatively. Patients completed a sleep diary daily in the same time period (data not published). Furthermore, patients recorded the subjective parameter of sleep using visual analogue scales (VAS), and the Karolinska Sleepiness Scale (KSS) every morning.28
Outcomes and Follow-up
The primary outcome was the incidence of depressive symptoms, and this is reported elsewhere.21 The data in the current paper represent secondary outcomes from the MELODY trial.23
Actigraphy Data
An Actigraph (Micro Motionlogger sleep watch, ambulatory monitoring, Inc, New York, USA) is a small wrist-worn device containing an accelerometer in the form of a piezoelectric transducer.29,30 The data from the accelerometer are stored on an internal memory in the device, which can be downloaded to a personal computer for analysis. On the computer, dedicated software ActionW 2.6 (ambulatory monitoring, Inc, New York, USA) can transform the accelerometer data into relevant sleep outcomes using the Cole-Kripke sleep algorithm.31 Before the analysis was performed the raw data was cleaned with data from the sleep and activity diaries serving as a guide to “lights off” (i.e. the time the patient tried to sleep) and “lights on” (the time the patient awakened) and when the patient was in the bath (the actigraph was taken off). In this way the sleep diaries were used to define sleep and wake periods and for filtering periods where the actigraph was not worn. The actigraph in this study was set in the zero crossing mode (ZCM) recommended for sleep analysis,29 and an epoch length of 1 min was the basis for the data analysis.
The actigraph was placed on the wrist of the opposite side of the breast to be operated on, as this ensures the highest overall level of accuracy. In case of bilateral mastectomy the Acti-graph was placed on the non-dominant arm.32
As recommended by Berger et al.,33 a complete set of actigraphy sleep outcomes were reported to avoid selective reporting. Of the multiple actigraphy outcomes sleep efficiency (SE) was chosen as the primary sleep outcome. Sleep efficiency (SE) is the percentage of actual sleep while being in bed at night (total sleep time/time in bed). The remaining actigraphy outcomes were considered secondary outcomes. Time in bed (TIB) is the time spent physically in bed and this is based on sleep diary correction of actigraphy measurements. Total sleep time (TST) is the duration from “lights off” to the time of awakening and as such is shorter than TIB. Wake after sleep onset (WASO) is the number of wake minutes during the sleep period after sleep onset. Sleep latency (SL) is the time from “lights-off” until the actual onset of sleep. Number of awakenings (NOA > 5 min) is the number of awakenings with a duration ≥ 5 min during the sleep period. In the wake period a total of 3 actigraphy outcomes were reported. Number of naps (NON > 5 min) are the number of naps during the wake period > 5 minutes. Sleep time (ST) is the total number of minutes spent sleeping (napping) during the wake period. Wake time (WT) is the total number of minutes spent awake during the wake period.
The sleep data were divided into relevant time frames to yield meaningful information about the sleep changes in the perioperative period. Nights three and two before (PRE2/3) surgery all participants slept in their own beds, and therefore this time period was taken as the patient's habitual sleep. The night before surgery a few patients slept in the hospital due to a long commute, and in addition the night before surgery can be associated with anxiety which can affect sleep.16 We therefore chose to look at sleep on the preoperative night (PRE1) as a separate data point. A subset of 3 patients had missing data on a couple of postoperative nights and last observation carried forward (LOCF) was used to compute the missing values. We chose to look at postoperative sleep in 3-day periods (i.e., postoperative nights 1, 2, and 3 (PO123) and postoperative nights 4, 5, and 6 (PO456), as has previously been recommended.34 Lastly, the night before the histology answer (PREMIK) approximately 2 weeks postoperatively was analyzed as a single data point for the same reasons as the preoperative night.
VAS and KSS
The visual analogue scale was used to quantify subjective experiences of sleep. The sleep quality visual analogue scale (VAS, 0 mm = best possible sleep and 100 mm = worst possible sleep) was completed every morning, reflecting the previous night's sleep.6 The Karolinska Sleepiness Scale (KSS) was used to assed the level of sleepiness and is a 9-point ordinal scale (1 = very alert and 9 = very sleepy) and was also assessed every morning.28 These subjective outcomes were grouped as the above mentioned time periods for sleep determined with actigraphy. The VAS data in the current publication represent a detailed analysis of VAS data measured in the perioperative period which has previously been published.24
Statistical Analysis
For statistical analyses, IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA) was used. A p value of ≤ 0.05 was considered statistically significant.
The sample size estimation was based on a primary outcome of reduction in depressive symptoms.23 Due to non-normal distribution of data, nonparametric statistics were used and data are presented as frequencies or median and range (minimum–maximum). When comparing the baseline characteristics of the 2 groups, Fisher exact test was used for binominal data and otherwise Mann-Whitney test was used. Comparisons in outcome parameters were made with Mann-Whitney test. No corrections for multiple comparisons were applied.
RESULTS
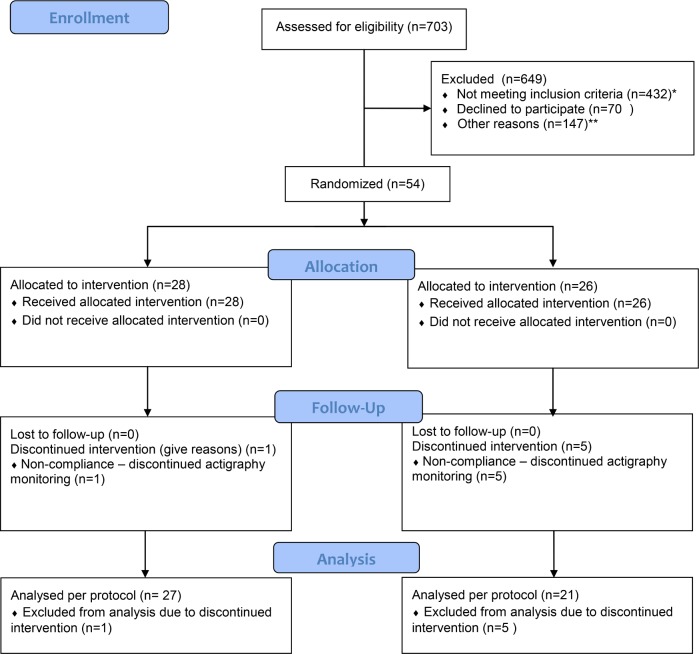
Patients were recruited from July 2011 to December 2012. The CONSORT trial profile (Figure 1) shows the screening, randomization, and follow-up of the patients; 27 patients were available for data analysis in the melatonin group and 21 in the placebo group.
Figure 1. CONSORT flow diagram.
*Age > 70–75 years = 134; ductus carcinoma in situ (DCIS) or Mb. Paget = 30; preoperative chemotherapy = 51; selective serotonin reuptake inhibitors, antithrombotic drug therapy, monoaminoxidase inhibitors, calcium channel blockers = 31; epilepsy = 1; know or treated sleep apnea = 1; diabetes mellitus treated with insulin = 4; ongoing or pervious medically treated depression or bipolar disorder = 44; known autoimmune disease = 9; severe kidney disease = 1; previous or other current cancer = 75; known medically treated sleep disorder = 1; daily intake of > units (1 unit= 8 g pure alcohol) = 3; preoperative, continues treatment with psychopharmacological drugs of any kind, opioids, anxiolytics or hypnotics = 35; predicted poor compliance (e.g. not fluent in native language of the country) = 12.
**Logistics = 50; < 3 days until the day of surgery = 82; patient participating in other trials = 15.
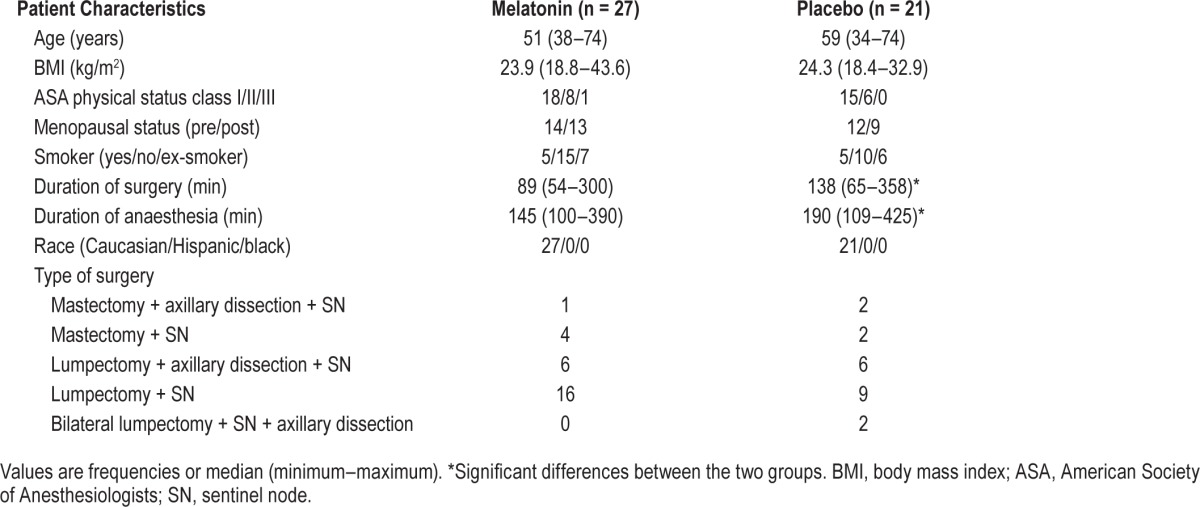
Baseline, perioperative and demographic characteristics were similar between the 2 groups (Table 1), except median duration of surgery was 89 and 138 min, and duration of anaesthesia was 145 and 190 min in the melatonin and placebo groups, respectively.
Table 1.
Demographics.
Actigraphy Sleep Outcomes
When looking at the preoperative sleep outcomes PRE2/3, we found no significant differences for any of the reported outcomes during the sleep period (TIB, TST, SE, WASO, NOA, and SL), nor during the wake period (NON, WT, and ST) between the groups (Table 2A). Analyzing the preoperative night's sleep (PRE1) alone, the 2 groups did not differ (Table 2B).
Table 2A.
Comparison for actigraphy parameters in breast cancer patients receiving melatonin of placebo.
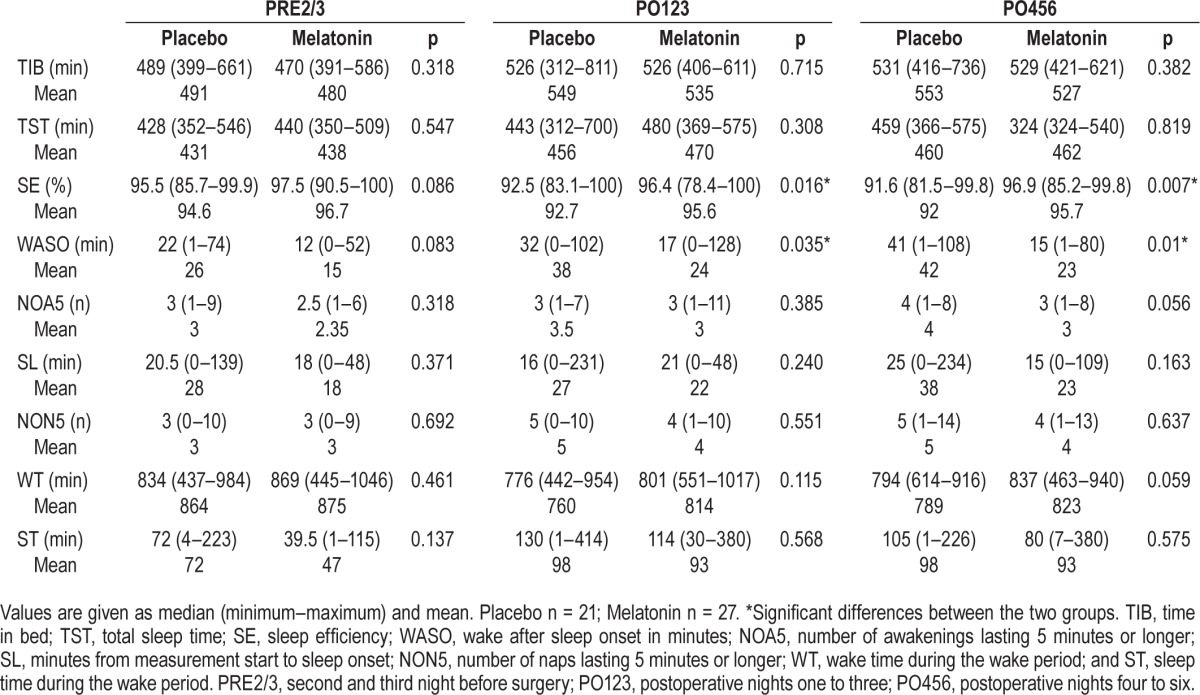
Table 2B.
Comparison for actigraphy parameters in breast cancer patients receiving melatonin of placebo.
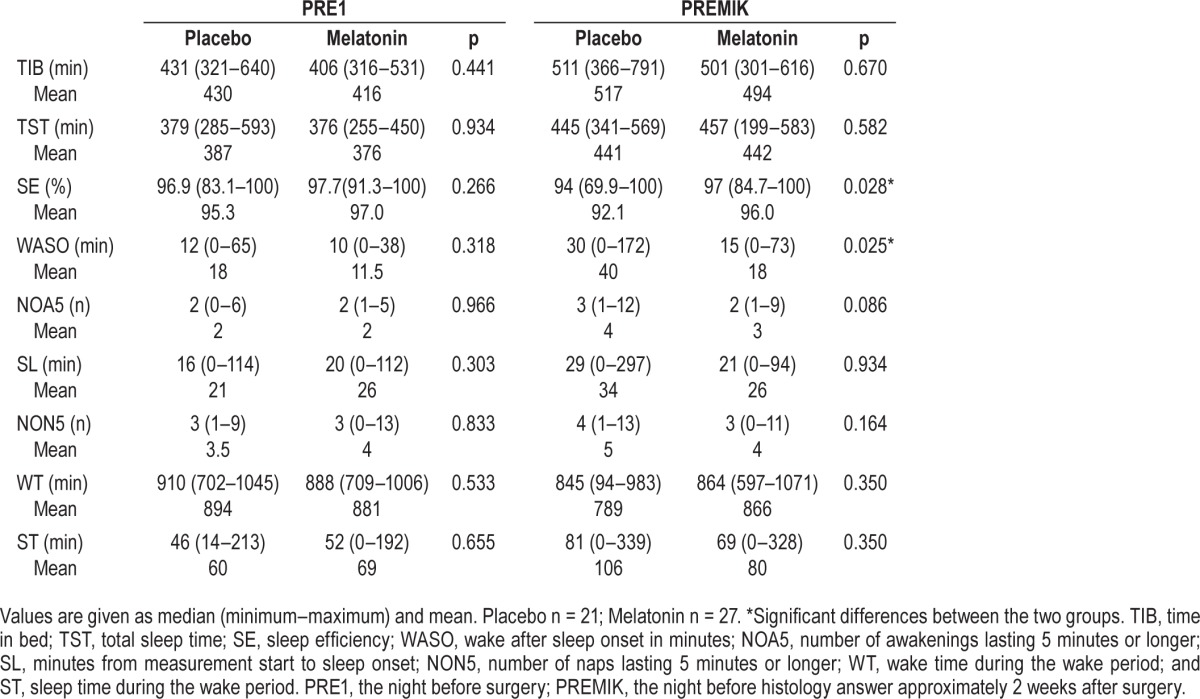
In the postoperative period (PO123 and PO456) SE was significantly higher in the melatonin group (p = 0.016 and p = 0.007, respectively). On PO123 the melatonin had an SE of 96.4% (range: 78.4% to 100%) and the placebo group 92.5% (range: 83.1% to 100%), corresponding to a difference of approximately 4% (range: 0% to 21.5%). On PO456 the melatonin group had a SE of 96.9% (range: 85.2% to 99.8%) and the placebo group 91.6% (range: 81.5% to 99.8%), corresponding to a difference of approximately 5% (range: 0.2% to 18.5%) (Table 2A). A significantly higher SE was also found in the melatonin group on the PREMIK night, 97% (range: 84.7% to 100%) vs. placebo, 94% (range: 69.9% to 100%) (p = 0.028, Table 2B).
Furthermore, in the postoperative period (PO123 and PO456) WASO was significantly lower in the melatonin group (p = 0.035 and p = 0.01, respectively), and WASO was also lower at the PREMIK time point (p = 0.025, Tables 2A and 2B).
No other significant differences in sleep outcomes between the groups were found in the postoperative period (PO123 and PO456) or PREMIK sleep period outcomes (Tables 2A and 2B).
Visual Analogue Scale and Karolinska Sleepiness Scale Outcomes
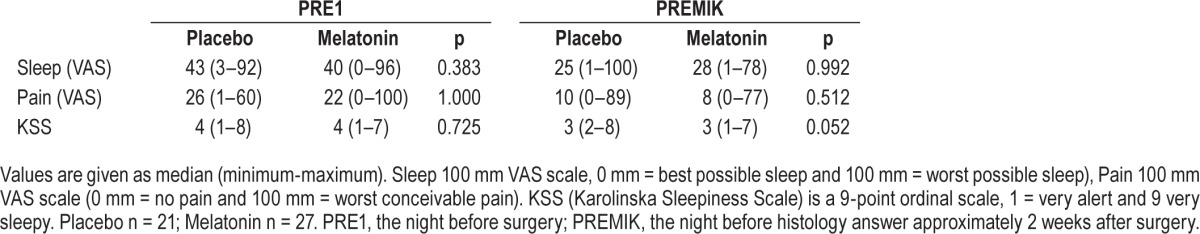
For the subjectively measured parameters (sleep, pain and KSS) no significant differences were present between the melatonin and placebo groups on PRE2/3, PRE1, PO123 and PO456 (Tables 3A and 3B).
Table 3A.
Comparisons of subjective sleep quality (visual analogue scale [VAS]) and pain in the perioperative period for breast cancer patients.
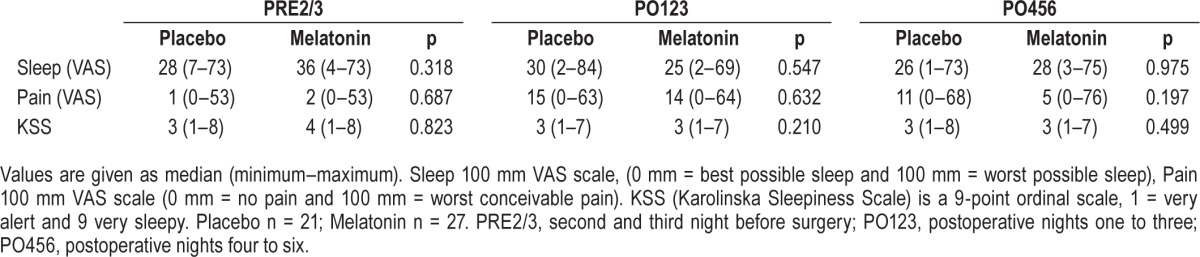
Table 3B.
Comparisons of subjective sleep quality (visual analogue scale [VAS]) and pain in the perioperative period for breast cancer patients.
DISCUSSION
Administration of 6 mg oral melatonin approximately one hour before bedtime resulted in a significantly higher sleep efficiency between the melatonin and placebo group throughout the first two postoperative weeks. Furthermore, the duration of wake after sleep onset was significantly lower in the melatonin group. No other differences were found between the two groups for objective or subjective outcomes (VAS or KSS) in the perioperative period. As sleep measured by actigraphy was not the endpoint on which the sample size calculation was based, these results should be interpreted with caution.
A recent review3 looked at randomized clinical trials investigating the use of perioperative melatonin. A total of four studies in this review investigated sleep in the perioperative period.4–7 Three studies showed a subjectively assessed sleep improving effect of melatonin,4,5,7 whereas one study6 failed to show an improvement in subjective sleep.
All studies4–7 had design limitations leading to a risk of bias.3 Three studies4,5,7 had incomplete outcome data, and four studies were at risk of selective reporting.4–7 Samarkandi et al.7 furthermore failed to properly define a primary outcome. Apart from these limitations, the studies were all randomized, double-blind, and placebo-controlled in different surgical populations. A considerable difference between these studies was in the timing of the administration of melatonin as in three studies4,5,7 melatonin was administered preoperatively (night before surgery and 1 h before surgery), whereas in another study,6 melatonin was only administered on the postoperative nights (30 min before bedtime). In this perspective, our study is similar to the mentioned studies,4,5,7 as we administered at least three nights of preoperative melatonin. Administration of melatonin the night before surgery seems to be of some importance when investigating the sleep promoting effect of melatonin in the perioperative period.
The method chosen to quantify sleep in all four studies4–7 was subjective sleep outcomes (VAS or sleep diary). One study5 reported the use of actigraphy but only reported the rest-activity rhythm (r24), which is a circadian rhythm outcome and not a sleep outcome.33 Our results showed a significant improvement in objective sleep outcomes as measured by actigraphy, which lasted at least 1 week postoperatively. We did not show this effect in the subjective sleep outcome as measured by VAS, which the other studies have shown. However, our study did not administer a pre-surgery (60 min before surgery) dose of melatonin and in this way differed from the other studies.4,5,7 We, however, found a similar increase in SE which was also reported in the sleep diary24 from the same study population. Overall, subjective and objective sleep outcomes seem to describe two different dimensions of sleep.9,13 As previously recommended,9,13 both objective and subjective outcomes should be used to quantify sleep in a cancer population.
A bidirectional relationship exists between postoperative sleep and pain.35 Furthermore, patients undergoing breast cancer surgery with preoperative disrupted sleep may experience increased postoperative pain.16 Our study showed no difference in pain preoperatively or postoperatively between the two groups, hence we could not show an analgesic effect of melatonin. Postoperative opioid use has been shown to negatively affect postoperative sleep.35 Pain management in the current population consisted of paracetamol and ibuprofen and therefore our population was not exposed to the negative effect of opioids. Previous studies showing an analgesic effect of melatonin administered melatonin immediately preoperatively or postoperatively and this was not performed in the current study. Pain measured by VAS was not the primary outcome of the study and further investigation into the relationship between postoperative pain and sleep is warranted.
The dosage of melatonin used and the timing of the administration is also of importance for its hypnotic effect. In the most recent update2 from 2008 (Cochrane review) doses of 0.5–5 mg showed similar hypnotic effects, although 5 mg had a more pronounced effect on the treatment of jetlag. Generally, the other studies4–6 administered doses of 5–6 mg of melatonin before bedtime, which was similar to our choice of 6 mg. Samarkandi et al.7 administered varying amounts (0.1–0.5 mg/kg) only as a pre-surgery dose. In spite of the relevant design (dose-response),7 no differences were shown between the various doses of melatonin. One should note that the study was performed in children and generally children respond stronger to melatonin administration than the elderly.36 With increasing age the endogenous production of melatonin decreases, and a correlation between decreased production of melatonin and insomnia has been reported.37 Furthermore, increased age may also be associated with reduced receptor sensitivity for melatonin.36 All of this makes an extrapolation of results found in children to adults difficult and of little clinical importance to our study. Some evidence shows a considerable inter-individual variability of circulating melatonin and overlap between ages.36 Administration of 5 mg of oral melatonin results in supra-physiological levels of melatonin, meaning that our population (age range of 34–74 years) all received supra-physiological doses. Regarding the timing of administration, the acute hypnotic effects of melatonin can promote sleep within 1–2 hours.36 Therefore, the choice of administration of melatonin 1 hour before intended bedtime seems rational.36
The studies4–7 investigating sleep in the perioperative period were not performed in patients undergoing breast cancer surgery. The studies investigated children,7 abdominal,6 urological,4 and gynecological5 patients undergoing different types of procedures. Surgery itself can cause sleep disturbances,17 however the cancer per se is an important contributing factor to sleep disturbances in women with breast cancer.11 None of the other studies4–7 included patients with cancer, although it was not specifically stated that the patients explicitly underwent benign surgery. To our knowledge our study is the first to investigate melatonin administration in the perioperative period in patients undergoing breast cancer surgery. We know of one other randomized clinical trial investigating melatonin intervention in the same diagnose population,14,15 but in breast cancer survivors. However, patients being categorized as breast cancer survivors have no ongoing oncological treatment and the patients were principally disease free. The studies mentioned14,15 investigated a 3 mg intervention administered before bedtime for a period of 4 months. The primary findings were that melatonin was tolerated with no grade 3/4 toxicity, and no influence on circulating estradiol, IGF-1, or IGFBP-3 blood levels.15 More interestingly, melatonin was shown to improve subjective sleep quality (Pittsburgh Sleep Quality Index) through a 4-month period. At baseline, 43% to 56% of the patients were defined as poor sleepers (i.e., PSQI > 5, not treatment demanding i.e., PSQI > 8); after the intervention total PSQI was reduced with 1.9 point equivalent to 1 standard deviation in the population.
Our intervention of 6 mg melatonin showed an improvement in SE of 4% to 5% and a reduction of 15–26 min WASO in the postoperative period. The question is whether this is clinically relevant. Overall the absolute values of actigraphy measurement throughout the study were all within what could be considered normal ranges in healthy female patients of the same age.38 Sleep efficiency ranged from 91% to 97% and was never under 85%, which is an established value for pathological sleep.38 In a descriptive sleep study17 in the same population using polysomnography, we showed WASO to increase postoperatively and fall before the PO14. A similar trend was present in the current placebo group and WASO in the melatonin group was significantly lower in the entire postoperative period. An absolute improvement of 4–5% SE and 15–26 min WASO might not seem clinically relevant, although we were able to show a significant effect in a study population which could be categorized as “good sleepers.” A possible explanation for our study population being “good sleepers” may very well be our very strict exclusion criteria of no previous sleep disorder, no use of hypnotics, or no previous depression, as these factors could have resulted in poorer overall sleep in the study population. Our strict exclusion criteria made our study population homogeneous but made patient recruitment difficult and the external validity limited. A future trial should apply less strict inclusion criteria (e.g., no age restrictions, allow preoperative chemotherapy, previous depression, and other current cancer).
Our study has several limitations. Overall, we only included approximately 7% (48/703) of those assed for eligibility, and 61% (432/703) were excluded due to exclusion criteria. The internal validity of our study was influenced by the distribution of dropouts; 5:1 (placebo: melatonin). We chose not to complete an intention-to-treat analyses due to the high rate of dropouts (i.e., missing data problem), which we were not able to solve by any type of imputation. The use of actigraphy can be viewed as a limitation, as it is not the golden standard of sleep evaluation. Polysomnography (PSG) is the golden standard; however, actigraphy has high accuracy in comparison to PSG,30 and we have previously shown high levels of sensitivity and specificity compared to (PSG) in a similar population.36 Furthermore, contrary to PSG actigraphy is a mobile and noninvasive methodology, and we have adhered to the guidelines put forth by Berger et al.33 The discontinuation of the actigraphy measurement is a possible risk of bias. Future studies should apply attention to reduce the dropout rate which could consist of a more intense follow-up in the ambulatory setting, e.g., calling the patients more extensively by phone. Furthermore, a future trial should use sleep outcomes as the basis for the power calculation so sleep determined by actigraphy could serve as the primary outcome.
Overall the use of melatonin in cancer treatment is not new,39 and along with the beneficial results from previous studies,14,15,21 and the non-toxic nature of melatonin,2 we believe that melatonin may hold a central place in the treatment of sleep disturbances and/or depression in patients with breast cancer.
CONCLUSION
In conclusion, the overall population's sleep was within what could be considered normal sleep. We showed, however, that melatonin significantly changed sleep efficiency and wake after sleep onset in the two-week postoperative period as measured by actigraphy. The data presented represent secondary outcomes from the primary study and should therefore be interpreted with caution. The results need to be verified in other clinical trials. Further investigations of melatonin administered in cancer populations are warranted.
DISCLOSURE STATEMENT
This work was supported by grants from the University of Copenhagen, The Danish Medical Society of Copenhagen, The Aase and Ejnar Danielsens Foundation, The A.P.Møller Foundation for the Advancement of Medical Science, The Else and Mogens Wedell Wedellborgs Foundation, The Beckett Foundation, The Hede Nielsen Family Foundation, The Dagmar Marshalls Foundation, The Lundbeck Foundation, TrygFonden and Manufacturer Einar Willumsen's Memorial Scholarship. These abovementioned funders, as well as Pharma Nord who provided the melatonin and placebo tablets, had no influence on the design and conduct of the study, collection, management, analysis, or interpretation of data, preparation, review, or approval of the manuscript, or decision to submit the manuscript for publication. This study received no financial support from the industry. Dr. Gögenur received a fee for speaking at a convention on behalf of Ethicon divion of Johnson & Johnson. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Authors made the following contributions: Mr. Madison - Collected the data, analysed and interpreted most of the data, prepared the first draft of the manuscript, revised and coordinated revision of the manuscript, and approved the final manuscript; Dr. Hansen - planned the study, designed the study, initiated the study, collected the data, interpreted the data, revised the manuscript, and approved the final manuscript; Dr. Andersen - collected the data, revised the manuscript, and approved the final manuscript; Dr. Hageman - planned the study, designed the study, revised the manuscript, and approved the final manuscript; Professor Rasmussen - planned the study, designed the study, initiated the study, revised the manuscript, and approved the final manuscript; Dr. Bokmand - planned the study, revised the manuscript, and approved the final manuscript; Professor Rosenberg - planned the study, designed the study, initiated the study, revised the manuscript, and approved the final manuscript; Professor Gögenur - planned the study, designed the study, initiated the study, interpreted the data, revised the manuscript, and approved the final manuscript.
ABBREVIATIONS
- ASA
American Society of Anesthesiologists
- MDI
Major Depression Inventory
- MMSE
Mini Mental State Examination
- PO123
postoperative nights 1–3
- PO456
postoperative nights 4–6
- PRE1
preoperative night
- PRE2/3
2 and 3 nights preoperatively
- PREMIK
night before histology answer
REFERENCES
- 1.Claustrat B, Brun J, Chazot G. The basic physiology and pathophysiology of melatonin. Sleep Med Rev. 2005;9:11–24. doi: 10.1016/j.smrv.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Herxheimer A, Petrie KJ. Melatonin for the prevention and treatment of jet lag. Cochrane Database Syst Rev. 2002;2:CD001520. doi: 10.1002/14651858.CD001520. [DOI] [PubMed] [Google Scholar]
- 3.Andersen LP, Werner MU, Rosenberg J, Gogenur I. A systematic review of peri-operative melatonin. Anaesthesia. 2014;69:1163–71. doi: 10.1111/anae.12717. [DOI] [PubMed] [Google Scholar]
- 4.Borazan H, Tuncer S, Yalcin N, Erol A, Otelcioglu S. Effects of preoperative oral melatonin medication on postoperative analgesia, sleep quality, and sedation in patients undergoing elective prostatectomy: a randomized clinical trial. J Anesth. 2010;24:155–60. doi: 10.1007/s00540-010-0891-8. [DOI] [PubMed] [Google Scholar]
- 5.Caumo W, Torres F, Moreira NL, Jr., et al. The clinical impact of preoperative melatonin on postoperative outcomes in patients undergoing abdominal hysterectomy. Anesth Analg. 2007;105:1263–71. doi: 10.1213/01.ane.0000282834.78456.90. [DOI] [PubMed] [Google Scholar]
- 6.Gogenur I, Kucukakin B, Bisgaard T, et al. The effect of melatonin on sleep quality after laparoscopic cholecystectomy: a randomized, placebo-controlled trial. Anesth Analg. 2009;108:1152–6. doi: 10.1213/ane.0b013e31819a6cf0. [DOI] [PubMed] [Google Scholar]
- 7.Samarkandi A, Naguib M, Riad W, et al. Melatonin vs. midazolam premedication in children: a double-blind, placebo-controlled study. Eur J Anaesthesiol. 2005;22:189–96. doi: 10.1017/s0265021505000335. [DOI] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864–6. doi: 10.1200/JCO.2009.24.5993. [DOI] [PubMed] [Google Scholar]
- 9.Langford DJ, Lee K, Miaskowski C. Sleep disturbance interventions in oncology patients and family caregivers: a comprehensive review and meta-analysis. Sleep Med Rev. 2012;16:397–414. doi: 10.1016/j.smrv.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Palesh O, Peppone L, Innominato PF, et al. Prevalence, putative mechanisms, and current management of sleep problems during chemotherapy for cancer. Nat Sci Sleep. 2012;4:151–62. doi: 10.2147/NSS.S18895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10:419–29. doi: 10.1016/j.smrv.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Liu L, Marler MR, et al. Fatigue, sleep, and circadian rhythms prior to chemotherapy for breast cancer. Support Care Cancer. 2006;14:201–9. doi: 10.1007/s00520-005-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen MT, Huang C, Gogenur I. Actigraphy for measurements of sleep in relation to oncological treatment of patients with cancer: a systematic review. Sleep Med Rev. 2015;20:73–83. doi: 10.1016/j.smrv.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Chen WY, Giobbie-Hurder A, Gantman K, et al. A randomized, placebo-controlled trial of melatonin on breast cancer survivors: impact on sleep, mood, and hot flashes. Breast Cancer Res Treat. 2014;145:381–8. doi: 10.1007/s10549-014-2944-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schernhammer ES, Giobbie-Hurder A, Gantman K, et al. A randomized controlled trial of oral melatonin supplementation and breast cancer biomarkers. Cancer Causes Control. 2012;23:609–16. doi: 10.1007/s10552-012-9927-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CE, Bovbjerg DH, Montgomery GH, et al. Disrupted sleep the night before breast surgery is associated with increased postoperative pain. J Pain Symptom Manage. 2009;37:352–62. doi: 10.1016/j.jpainsymman.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hansen MV, Madsen MT, Wildschiodtz G, Rosenberg J, Gogenur I. Sleep disturbances and changes in urinary 6-sulphatoxymelatonin levels in patients with breast cancer undergoing lumpectomy. Acta Anaesthesiol Scand. 2013;57:1146–53. doi: 10.1111/aas.12157. [DOI] [PubMed] [Google Scholar]
- 18.Harvey AG, Tang NK. Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep Med Rev. 2003;7:237–62. doi: 10.1053/smrv.2002.0266. [DOI] [PubMed] [Google Scholar]
- 19.Barsevick A, Beck SL, Dudley WN, et al. Efficacy of an intervention for fatigue and sleep disturbance during cancer chemotherapy. J Pain Symptom Manage. 2010;40:200–16. doi: 10.1016/j.jpainsymman.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palesh O, Aldridge-Gerry A, Ulusakarya A, Ortiz-Tudela E, Capuron L, Innominato PF. Sleep disruption in breast cancer patients and survivors. J Natl Compr Canc Netw. 2013;11:1523–30. doi: 10.6004/jnccn.2013.0179. [DOI] [PubMed] [Google Scholar]
- 21.Hansen MV, Andersen LT, Madsen MT, et al. Effect of melatonin on depressive symptoms and anxiety in patients undergoing breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Breast Cancer Res Treat. 2014;145:683–95. doi: 10.1007/s10549-014-2962-2. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. doi: 10.1136/bmj.c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansen MV, Madsen MT, Hageman I, et al. The effect of melatonin on depression, anxiety, cognitive function and sleep disturbances in patients with breast cancer. The MELODY trial: protocol for a randomised, placebo-controlled, double-blinded trial. BMJ Open. 2012;2(1):e000647. doi: 10.1136/bmjopen-2011-000647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen MV, Madsen MT, Andersen LT, et al. Effect of melatonin on cognitive function and sleep in relation to breast cancer surgery: a randomized, double-blind, placebo-controlled trial. Int J Breast Cancer. 2014;2014:416531. doi: 10.1155/2014/416531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bech P, Rasmussen NA, Olsen LR, Noerholm V, Abildgaard W. The sensitivity and specificity of the Major Depression Inventory, using the Present State Examination as the index of diagnostic validity. J Affect Disord. 2001;66:159–64. doi: 10.1016/s0165-0327(00)00309-8. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–61. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 28.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 29.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 30.Sadeh A. The role and validity of actigraphy in sleep medicine: an update. Sleep Med Rev. 2011;15:259–67. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–9. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 32.Madsen MT, Hansen VH, Wildschiødtz G, Rosenberg J, Gögenur I. Actigraphy can be used to quantify sleep in the perioperative period in women undergoing breast cancer surgery: a validation study. J Sleep Disord Treat Care. 2014;3:4. [Google Scholar]
- 33.Berger AM, Wielgus KK, Young-McCaughan S, Fischer P, Farr L, Lee KA. Methodological challenges when using actigraphy in research. J Pain Symptom Manage. 2008;36:191–9. doi: 10.1016/j.jpainsymman.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Littner M, Kushida CA, Anderson WM, et al. Practice parameters for the role of actigraphy in the study of sleep and circadian rhythms: an update for 2002. Sleep. 2003;26:337–41. doi: 10.1093/sleep/26.3.337. [DOI] [PubMed] [Google Scholar]
- 35.Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. 2014;18:273–82. doi: 10.1016/j.smrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Zhdanova IV. Melatonin as a hypnotic: pro. Sleep Med Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 37.Haimov I, Laudon M, Zisapel N, et al. Sleep disorders and melatonin rhythms in elderly people. BMJ. 1994;309:167. doi: 10.1136/bmj.309.6948.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young T, Rabago D, Zgierska A, Austin D, Laurel F. Objective and subjective sleep quality in premenopausal, perimenopausal, and postmenopausal women in the Wisconsin Sleep Cohort Study. Sleep. 2003;26:667–72. doi: 10.1093/sleep/26.6.667. [DOI] [PubMed] [Google Scholar]
- 39.Mills E, Wu P, Seely D, Guyatt G. Melatonin in the treatment of cancer: a systematic review of randomized controlled trials and meta-analysis. J Pineal Res. 2005;39:360–6. doi: 10.1111/j.1600-079X.2005.00258.x. [DOI] [PubMed] [Google Scholar]