Abstract
Study Objectives:
To examine the association between modifiable lifestyle factors, and the risk of developing restless legs syndrome (RLS).
Methods:
This is a Prospective Cohort study of population including 12,812 men participating in Health Professionals Follow-up Study and 42,728 women participating in the Nurses' Health study II. The participants were free of RLS at baseline (2002 for the HPFS and 2005 for the NHS II) and free of diabetes and arthritis through follow-up. RLS was assessed via a set of questions recommended by International Restless Legs Syndrome Study group. The Information was collected on height, weight, level of physical activity, dietary intake, and smoking status via questionnaires.
Results:
During 4–6 years of follow-up, we identified 1,538 incident RLS cases. Participants with normal weight, and who were physically active, non-smoker, and had some alcohol consumption had a lower risk of developing RLS. When we combined the effects of these four factors together, we observed a dose response relationship between the increased number of healthy lifestyle factors and a low risk of RLS: after adjusting for potential confounders the pooled odds ratio was 0.67 (95% CI: 0.47–0.97) for 4 vs.0 healthy factors (p trend < 0.001). In contrast, we did not observe significant associations between caffeine consumption or diet quality as assessed by the Alternate Healthy Eating Index, and altered RLS risk in men and women.
Conclusions:
Several modifiable lifestyle factors may play an important role in RLS risk.
Citation:
Batool-Anwar S, Li Y, De Vito K, Malhotra A, Winkelman J, Gao X. Lifestyle factors and risk of restless legs syndrome: prospective cohort study. J Clin Sleep Med 2016;12(2):187–194.
Keywords: behavioral modifications, epidemiology, lifestyle factors, restless legs syndrome, risk factors
INTRODUCTION
Restless legs syndrome (RLS), a debilitating illness that has affected people over the centuries,1 is characterized by unpleasant sensations and an irresistible urge to move the legs.2–4 A prevalence of 5% to 15% has been reported in United States and Europe5–7with lower prevalence rates (< 5%) in Asian populations.8 Epidemiologic studies have suggested an association between RLS and cardiovascular diseases, Parkinson disease, erectile dysfunction, poor sleep, and depressive symptoms.9–11 The burden of RLS on quality of life12 is comparable to that of other chronic illnesses such as diabetes, arthritis, hypertension, and acute myocardial infarction.3 The etiology of RLS is not known; however, several pathophysiologic mechanisms have been reported. Age and genetics are important determinants of clinical expression for primary RLS,13–15 whereas secondary RLS is present in a variety of conditions including iron deficiency, diabetes, renal failure, Parkinson disease, and pregnancy. Lifestyle factors such as lack of physical activity, obesity, cigarette smoking, alcohol intake, and consumption of coffee have also been postulated to have an effect on the risk or severity of RLS.13 However, the evidence linking these factors to RLS is based on small studies (n = 41),16 and the use of different methodologic and diagnostic criteria, and the lack of control for covariates, has resulted in conflicting results. We thus prospectively examined the associations between lifestyle factors (i.e., obesity, physical activity, overall diet quality, smoking, and alcohol intake) and risk of developing RLS in two large US cohorts including more than 65,000 men and women. This information may generate novel strategies regarding the prevention and management of RLS.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Lifestyle factors have been postulated to have an effect on the risk and severity of RLS. Since current treatment for RLS largely depends on pharmacologic interventions, we prospectively examined the associations between lifestyle factors and the risk of developing RLS.
Study Impact: The results of this study suggest that certain lifestyle factors are associated with the risk of developing RLS. The study underscore the importance of behavioral modification in treating RLS.
METHODS
Study Population
The study population included participants from the Health Professionals' Follow-up Study (HPFS) and the Nurses' Health Study (NHS) II cohorts.
The HPFS cohort is a prospective cohort study that began in 1986, and included 51,529 US male dentists, optometrists, pharmacists, osteopaths, podiatrists, and veterinarians aged 40–75 y. The NHS II is a large prospective cohort of 116,430 female registered nurses who were 25–42 y old at the start of the study in 1989. The study participants from both cohorts were mailed a detailed questionnaire that included items on lifestyle practices and medical history.
Follow-up questionnaires are mailed to the participants every 2 y to update information on potential risk factors and new diagnoses. The Partners HealthCare institutional review board approved this study and completion of the questionnaires was considered participant's consent. (Founded in 1994 by Brigham and Women's Hospital and Massachusetts General Hospital, Partners HealthCare, a not-for-profit health care system, includes community and specialty hospitals and other health-related entities.)
Assessment of RLS
The RLS questions were based on the International Restless Legs Study Group Criteria (IRLSSG) criteria.11,17 The following question was asked:
“Do you have unpleasant leg sensations like crawling, paresthesia, or pain combined with motor restlessness and an urge to move?” The possible responses were as follows: no, less than once/month, 2 to 4 times per month, 5–14 times per month, and ≥ 15 times per month. The participants who answered yes were asked the following two questions:
Do these symptoms occur only at rest, and does moving improve them?
Are these symptoms worse in the evening/night compared with the morning?
This set of three questions was used in a previous study of 369 Germans aged 65–83 y. The sensitivity, specificity, and kappa statistic of the three-question set compared to physician diagnoses was 87.5%, 96%, and 0.67, respectively.4,18,19
In 2002 and 2008, the HPFS participants were asked these RLS diagnostic and severity questions (n = 37,431, mean age 68.7 ± 9 y). The questions on RLS were completed by 31,729 men (85%). After excluding participants with RLS at baseline, or those who either did not respond to the RLS question or who were deceased, the cohort consisted of 21,542 men (mean age 67 y, mean body mass index [BMI] 26 kg/m2).
For the NHS II cohort, we asked the same questions (n = 97,642, mean age 50.4 y) about RLS symptoms in 2005 and 2009, and 79,992 women (82%) completed the questions. Again after excluding the participants who had reported RLS at baseline, or who either did not respond to the RLS question or were deceased, or who were pregnant, the cohort consisted of 61,555 (mean age 50 y, mean BMI 27 kg/m2).
Participants who did not complete the RLS questions had similar age (mean 50.4 versus. 50.4 y in women and 69.0 versus 68.6 y in men) and BMI (27.1 versus 26.5 kg/m2 in women and 26.5 versus 26.2 kg/m2 in men) as those with RLS information.11 To reduce the misclassification of RLS, participants with diabetes and arthritis, through the end of follow-up, were also excluded from the primary analysis. Our final study population consisted of 12,812 men and 42,728 women. No significant difference in lifestyle factors was noted among those included and excluded from the analysis (Appendix 1, supplemental material).
Ascertainment of Lifestyle Factors
Both cohorts are followed through biennial questionnaires. Using the information collected on height and weight, BMI is calculated as weight (in kg) divided by height (in meters) squared. Smoking status is expressed as never smoker, past smoker, and current smoker. Physical activity is expressed in metabolic equivalents (MET)-hours and we examined the association between physical activity (low versus high based on median level of activity) and RLS. Information on food and alcohol consumption was collected every 4 y via a validated semiquantitative food frequency questionnaire.20,21 We used the median value as a cutoff for defining high versus low alcohol consumption. Diet quality was assessed by the Alternate Healthy Eating Index (AHEI), which has been shown to be associated with a lower risk of major chronic diseases.22,23 We included eight of the nine components of the AHEI: vegetables, fruits, nuts, soy, cereal fiber, white-red meat ratio, polyunsaturated: saturated fat ratio, trans-fat, and multivitamin use. For the purpose of this analysis, alcohol, the ninth component of AHEI, was considered a separate lifestyle factor. For the remaining components, the possible scores ranged from 0–10, where 10 being the healthy dietary behavior. Thus, the total AHEI score in this study ranged from 6.8 (worst) to 7.9 (best).
The reliability and validity of self-reported BMI and level of physical activity has been previously investigated.24,25 Self-reported weight and physical activity have suggested a correlation of 0.97 and 0.79, respectively.26 Alcohol consumption measured by questionnaires, administered 1 y apart, also provided highly reproducible results with a correlation of 0.90.27 Similarly, caffeine and dietary patterns assessed by the food frequency questionnaire we used have been validated before.20,28 Although the information on lifestyle factors is collected biennially, the questions about RLS were asked in 2002 and 2008 for HPFS and 2005 and 2009 for NHS. Thus, for the purpose of this study we used the lifestyle factors at baseline (2002 for men, and 2005 for women) when participants were free of RLS symptoms. To address this limitation and take advantage of repeatedly collected information on lifestyle factors before the baseline, we performed 4- and 6-y lag analysis.
Ascertainment of Covariates
Other potential covariates included in the analysis were caffeine intake, menopausal status (for the NHS cohort), the Crown Crisp phobic anxiety index, aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and iron-specific supplement use, and diagnoses of major chronic diseases (hypertension, myocardial infarction, or stroke).
Statistical Analyses
Statistical analyses were completed using SAS version 9.1 (SAS Institute, Inc, Cary, NC). The lifestyle factors used in this analysis include BMI, level of physical activity, smoking status, AHEI, caffeine intake, and alcohol use. Logistic regression models are used to calculate odds ratio (ORs) and their 95% confidence intervals (CIs) for the risk of RLS. In order to minimize the effect of extreme values in regression models, we categorized the variables. We modeled BMI as five different categories in kg/m2; < 23, 23–24.9, 25–26.9, 27–29.9, and > 30 kg/m2. We categorized alcohol intake into five categories of g/day (0, 1–9.9, 10–19.9, 20–29.9, or > 30) and used median value as cut-off for defining high versus low alcohol consumption. We categorized caffeine intake (g/d) and physical activity (METs/w) into quintiles. We categorized smoking as never smoker, past smoker (1–14 and 15 cigarettes/d), and current smoker (1–14 and > 15 cigarettes/d).
We also created a lifestyle risk score and examined the overall effects of these lifestyle factors on RLS risk. For each lifestyle factor, we created a priori binary variables where they received 0 if they met the criteria for low risk and 1 otherwise. We defined optimal weight as BMI < 25 kg/m2. For physical activity and alcohol intake, we used the median values. For smoking, we defined low risk as not currently smoking. Using these binary variables we then calculated a lifestyle risk score by summing the total number of factors ranging from 0 to 4. The analyses were adjusted for age (y), ethnicity, use of antidepressants (yes/no), iron supplements (yes/no, as a surrogate for presence of iron deficiency anemia), Crown-Crisp phobia index (0–1, 2, 3, or > 4), presence of stroke, myocardial infarction, or hypertension (yes/no), and menopausal status for women. To test the robustness of our analysis, we conducted sensitivity analyses by (1) excluding participants with myocardial infarction and stroke, and (2) including the participants with diabetes and arthritis who were excluded in the primary analysis. We performed meta analyses by pooling the ORs from two cohorts using a fixed-effects model. Finally, we performed 4- to 6-y lagged analysis to investigate the association between lifestyle changes and RLS, as detailed previously.
RESULTS
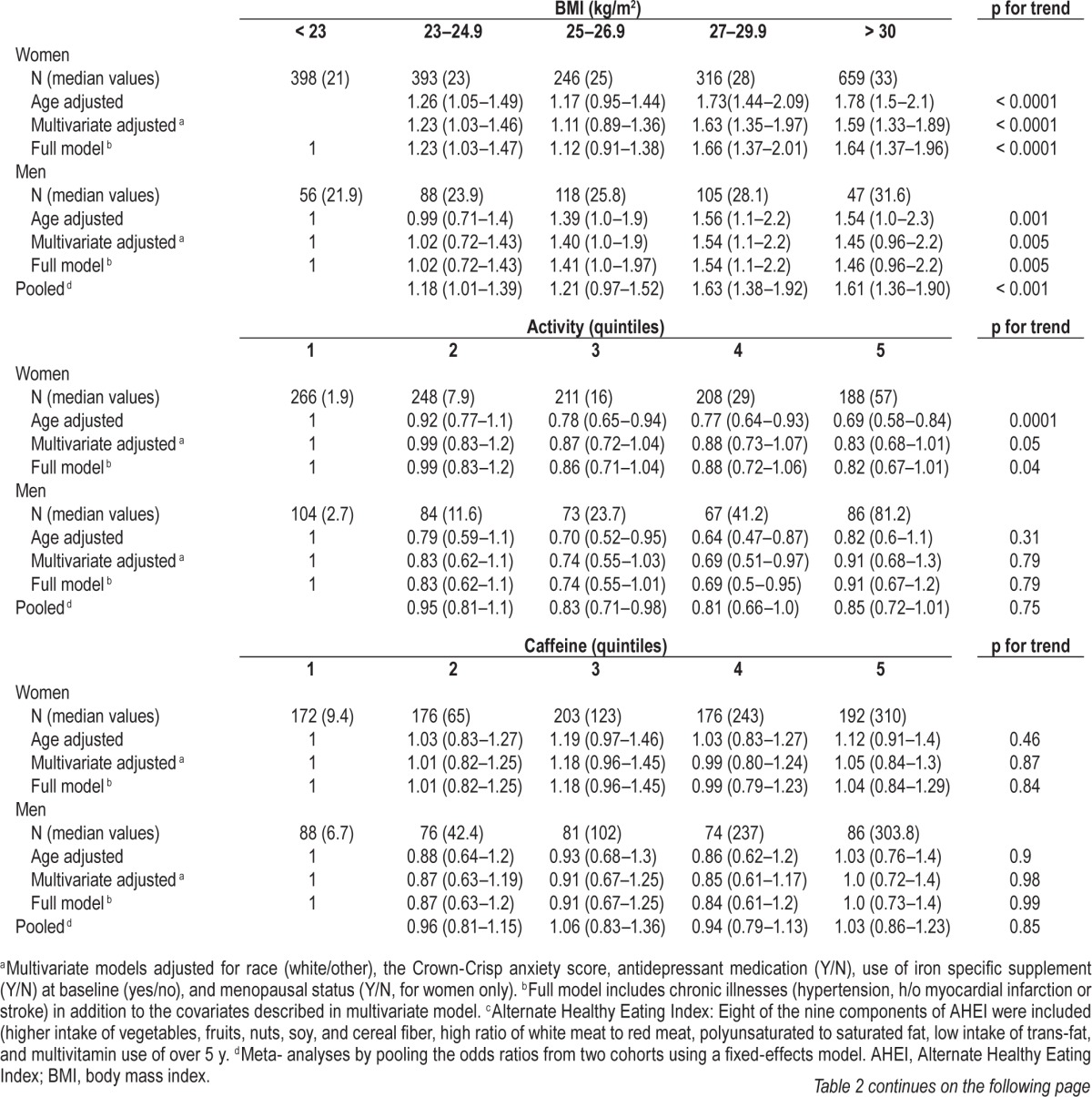
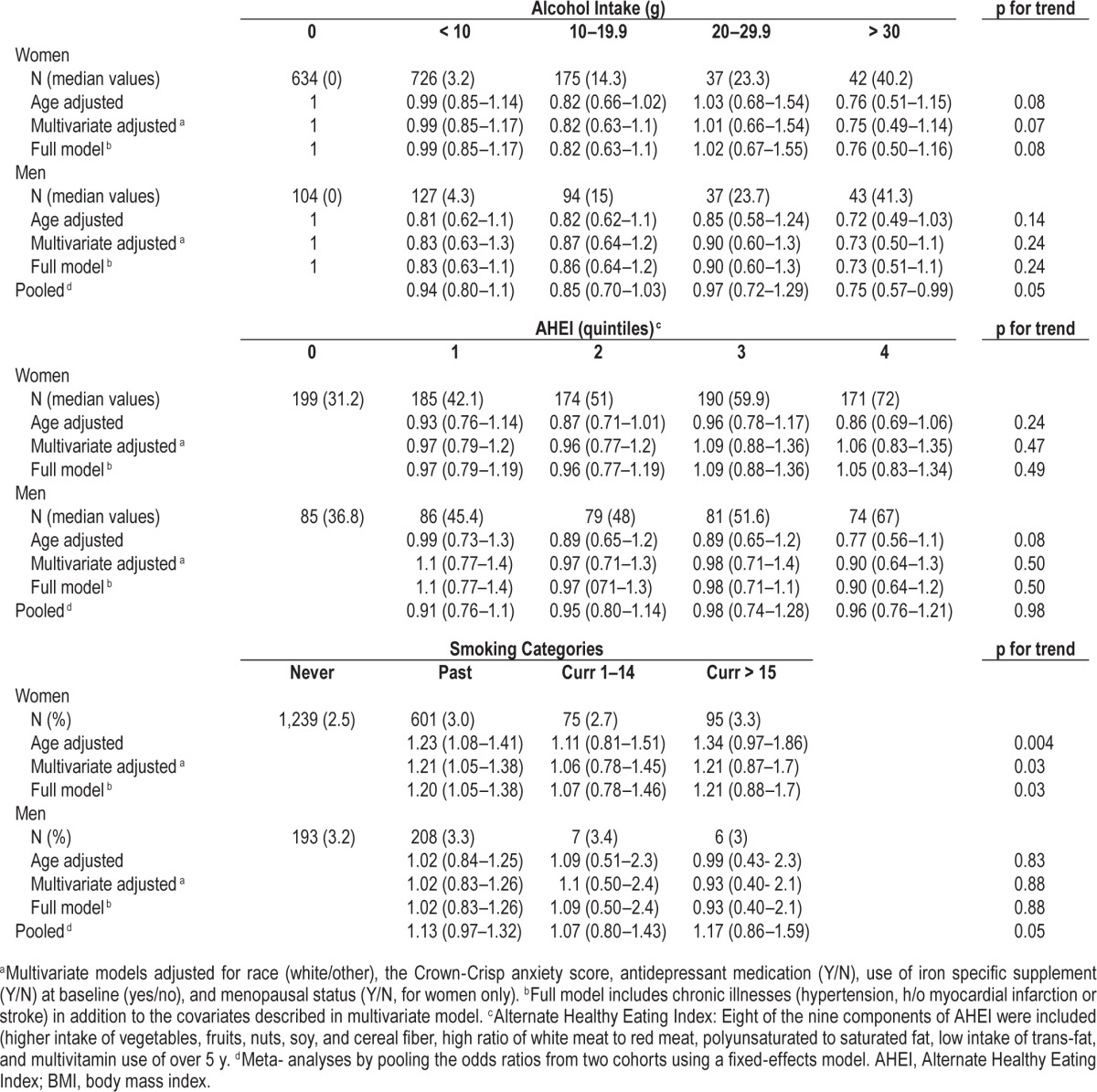
Average age for women and men was 50 and 66 y, respectively. The majority of the participants in both cohorts were white (∼95%). Participants with high BMI were less active, consumed more caffeine and tobacco, had less alcohol intake, and had unhealthy eating habits as assessed by AHEI (Table 1). The 6 y incident rate of RLS was 4.6% among men, and 4 y incident rate was 5.6% among women. Obesity was associated with increased risk of having RLS in both the cohorts (Table 2). The multivariate-adjusted OR for women with BMI > 30 versus < 25 kg/m2 was 1.64 (95% CI: 1.37–1.96, p for trend < 0.0001). The corresponding OR for men was 1.46 (95% CI: 0.96–2.2, p for trend 0.005), after adjusting for race, smoking, physical activity, caffeine intake, use of alcohol, antidepressants, anxiety score, and presence of chronic diseases such as hypertension, stroke, and myocardial infarction. We observed an inverse association between physical activity and the likelihood of developing RLS, particularly among women (Table 2). The multiple-adjusted OR for women who reported the highest quintiles of physical activity (METs/w) was 0.82 (95% CI: 0.67–1.01, p for trend 0.04) compared to those with lower quintiles of physical activity. The multiple adjusted OR for men in the highest category of physical activity was 0.91 (95% CI: 0.67–1.2, p for trend 0.79). Compared to women who never smoked, those who reported current heavy smoking demonstrated increased risk of the development of RLS after adjusting for potential covariates (OR 1.21; 95% CI: 0. 88–1.70, p trend 0.03). In contrast, no significant association was noted between smoking and the risk of RLS among men (OR 0.93; 95% CI: 0.40–2.1, p trend 0.88). Additionally, we found a nonsignificant trend with greater alcohol consumption and the lower risk of RLS. The OR for the highest versus lowest intake quartiles was 0.76; 95% CI 0.50–1.16 for women, and 0.73; 95% CI 0.51–1.1 for men, pooled p trend 0.05. Interestingly, we did not find a significant association between RLS and heavy consumption of caffeine.
Table 1.
Baseline characteristics according to the categories of lifestyle factors.
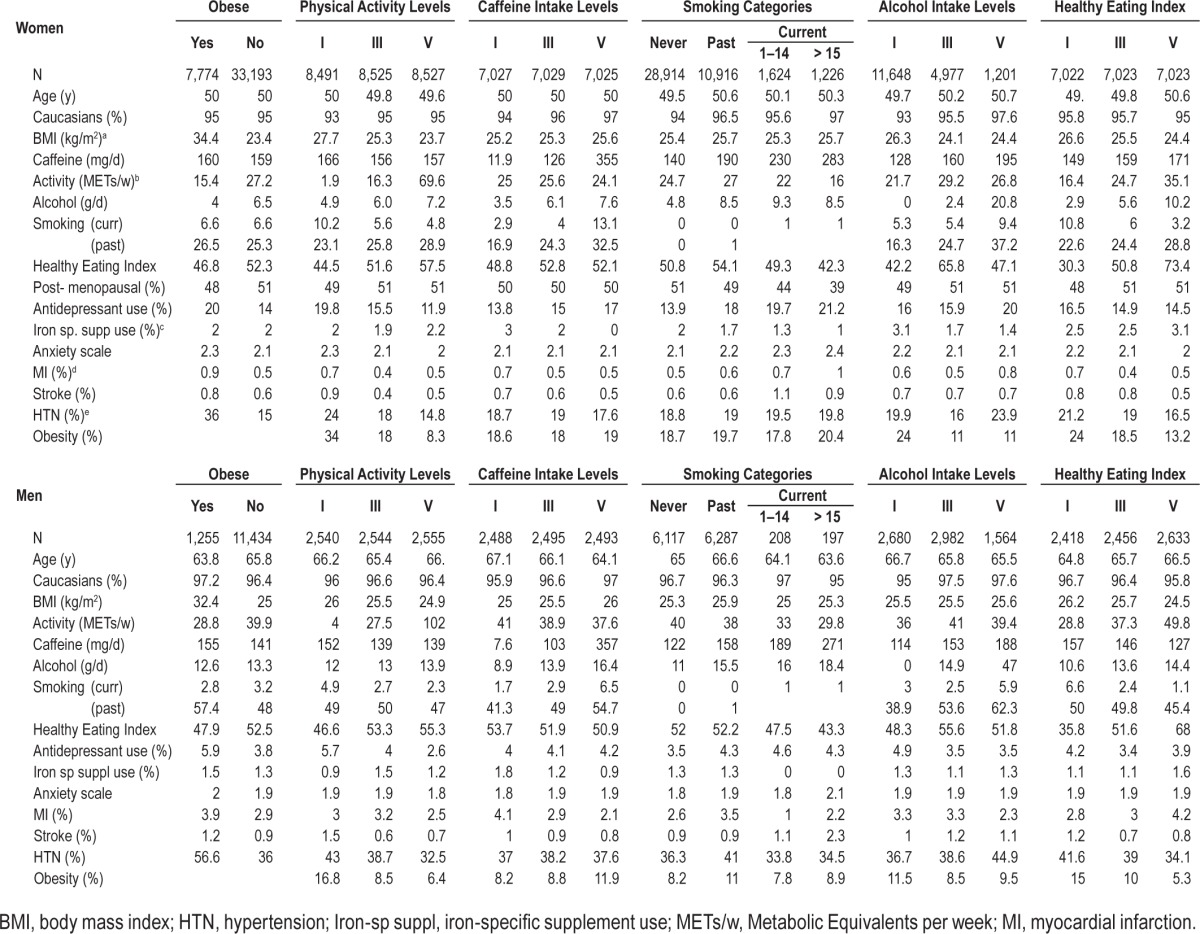
Table 2.
Odds ratios for developing restless legs syndrome according to lifestyle factors.
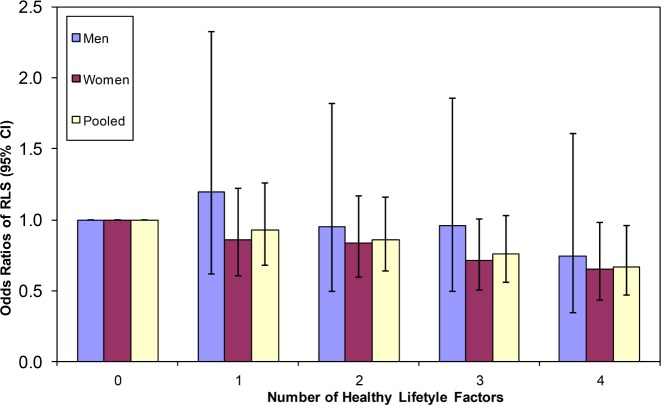
The healthy lifestyle score, including normal weight, physical activity, some alcohol intake, and nonsmoking, was associated with less risk for developing RLS (Figure 1). Similar inverse associations between the increase in number of healthy lifestyle factors and lower risk of RLS were observed in both men and women. After combining the ORs from both cohorts participants with all four healthy lifestyle factors demonstrated a lower risk for RLS compared to those with zero healthy factors (pooled OR 0.67; 95% CI 0.47–0.96; p for trend < 0.001).
Figure 1. Risk of RLS according to healthy lifestyle factors.
Further adjustment for iron supplements, antidepressant use, Crown-Crisp phobic anxiety index, and history of myocardial infarction or stroke did not materially change the results (data not shown). In the sensitivity analyses, we obtained similar results after excluding participants with myocardial infarction and stroke, and after including the participants with diabetes and arthritis (Appendix 2, supplemental material).
Using the 4-y lagged analysis did not significantly alter the association between BMI, caffeine/alcohol intake, and RLS. However a strong association was found between healthy eating habits, smoking, and RLS (Appendix 3, supplemental material).
DISCUSSION
In two large prospective cohorts of men and women, we found an association between overall lifestyle factors and RLS risk. The association was independent of age, race, sex, presence of stroke or myocardial infarction, and other potential confounders.
The findings of this study are in agreement with previous work demonstrating a strong cross-sectional association between obesity and the likelihood of having RLS.11 In another study (n = 1,803), the researchers found that each increase of BMI by 5 kg/m2 increased the odds of having RLS (OR 1.31, 95% CI: 1.11–1.53).13 Similarly, another study demonstrated that a higher percentage of participants with BMI > 27 kg/m2 reported RLS symptoms compared to leaner individuals (28% versus 21%).14 Although the pathogenesis of RLS is poorly understood, potential underlying mechanisms suggested by previous research include vascular pathology and reduced dopamine metabolism.11
We also found physical activity to have a protective effect on the risk of RLS. The mechanism(s) by which exercise could improve RLS symptoms is not known, but several theories have been postulated. Increased lower extremity blood flow, increased nitric oxide synthase activity, release of endorphins, and increased release of dopamine brought on by exercise are among the possible mechanisms by which exercise could improve RLS symptoms.1,29–31 In addition, the likelihood of reporting RLS symptoms (which are experienced at rest) may be reduced in people who exercise.
The relationship between cigarette smoking and RLS is also important to note. Our study suggests an increased association between smoking and RLS only among women. These findings were confirmed in another study suggesting a statistically significant association between RLS and smoking at least one pack per day.13 Nicotine has been found to have dopamine-stimulating effects and it is possible that smoking reduces RLS symptoms.32 Because the prevalence of RLS is high among women, it could explain the sex differences in our study. Possible mechanisms relating to sex difference and an association between smoking and RLS are unclear but may represent the effect of female hormones, regular menses, and parity. Interestingly, in the current study after adjusting for the potential timing effect during lag analysis, a significant association between smoking and RLS was noted among both men and women. The current study suggested a potentially protective effect of alcohol on the risk of RLS and these findings are in agreement with previous research. Phillips et al.13 have suggested an association between restless legs and alcohol abstinence.13 However, caution must be exercised in recommending alcohol use because it may lead to problems with overuse.
Studies have suggested that eating a healthy diet reduces risk for chronic illnesses and markedly lowers mortality.33 In a previous study we found higher AHEI scores to be protective against Parkinson disease.34 Although, both RLS and Parkinson disease respond to dopaminergic treatment and possibly share a common pathophysiologic mechanism, we did not find a similar association between AHEI scores and RLS. However, a significant association was noted between AHEI and subsequent RLS when we performed lagged analyses, suggesting that patients with RLS might have changed their dietary habits before the onset of classic symptoms. However, we cannot exclude the possibility of a finding based on chance alone. In this context, these finding must be interpreted with caution and examined by other prospective studies in different populations.
Because of the central nervous system stimulant and direct peripheral contractile properties of caffeine on the striated muscles, increased consumption of coffee or caffeinated beverages has been linked to RLS.35 The current study did not find a statistically significant association between caffeine consumption and RLS risk, even after controlling for potential confounders. To our knowledge this is the first prospective study examining the link between caffeine and the risk of RLS.
Strengths of this study include its large sample size in a population-based cohort using standardized questions to assess the presence of RLS. Because of the prospective nature of the study, the results are unlikely to be affected by recall bias. We acknowledge a number of limitations. Because the participants in these cohorts are health professionals, and almost all are white and older in age, the results of this study may lack generalizability. Further, selection bias cannot be excluded because the incident RLS assessment depends on self-reports 4 to 6 y after the baseline survey and that some participants with chronic diseases, which are generally associated with unhealthy lifestyle pattern, may not have been able to complete the second RLS questionnaire due to loss of follow-up. However, after further adjustment for presence of major chronic diseases or excluding those with these diseases, we obtained similar significant results. We acknowledge that our results may have differed if all of our participants had undergone a rigorous neurological history and physical examination. However, the participants of this study were health professionals who would be expected to report more accurately than the general population. Finally, there is a possibility of misdiagnosis of RLS due to RLS mimics. Although we did exclude diabetes and arthritis (RLS mimics) from the study population, we did not collect information on other RLS mimics such as positional discomfort and nocturnal cramps. Also, our study was also limited by the lack of data on iron deficiency in both cohorts; and to overcome this limitation the use of iron supplements was considered a surrogate of iron deficiency. Thus, despite our efforts at controlling for a number of potential confounders, residual confounding cannot be excluded.
CONCLUSION
The results of this large prospective study suggest that certain lifestyle factors, such as obesity, physical inactivity, and smoking, are associated with the risk of developing RLS. These results underscore the link between lifestyle factors and the risk of developing RLS, and suggest that lifestyle modifications may have an effect on RLS risk.
DISCLOSURE STATEMENT
This was not an industry supported study. Funding was provided by government grant: 5R01NS062879-02 (PI: Xiang Gao) NHSII: UM1 CA176726 and HPFS: UM1 CA167552. Dr. Winkelman has received consulting fees from UCB Pharma, Xenoport, Sunovion, and Impax Laboratories. He has provided expert testimony for Axinn Veltrop Harkrider, and received grant support from Impax Pharmaceuticals, GlaxoSmithKline, and UCB Pharma. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Salma Batool-Anwar, Yanping Li, Katerina De Vito -Drafting/ revising the manuscript for content, including medical writing for content, study concept or design and analysis or interpretation of data; Atul Malhotra, John Winkelman - Study concept or design, drafting/revising the manuscript for content, including medical writing for content; Xiang Gao - Drafting/revising the manuscript for content, including medical writing for content, study concept or design, analysis or interpretation of data, and study supervision and obtaining funding.
ABBREVIATIONS
- AHEI
Alternate Healthy Eating Index
- BMI
body mass index
- CIs
confidence intervals
- HPFS
Health Professionals' Follow-up Study
- IRLSSG
International Restless Legs Study Group Criteria
- MET
metabolic equivalents
- NHS II
Nurses' Health Study II
- NSAIDs
nonsteroidal anti-inflammatory drugs
- ORs
odds ratio
- RLS
restless legs syndrome
REFERENCES
- 1.Mitchell UH. Nondrug-related aspect of treating Ekbom disease, formerly known as restless legs syndrome. Neuropsychiatr Dis Treat. 2011;7:251–7. doi: 10.2147/NDT.S19177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghorayeb I, Tison F. Epidemiology of restless legs syndrome. Rev Neurol (Paris) 2009;165:641–9. doi: 10.1016/j.neurol.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Smith JE, Tolson JM. Recognition, diagnosis, and treatment of restless legs syndrome. J Am Acad Nurse Pract. 2008;20:396–401. doi: 10.1111/j.1745-7599.2008.00337.x. [DOI] [PubMed] [Google Scholar]
- 4.Allen RP, Picchietti D, Hening WA, et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10:153–67. doi: 10.1016/j.smrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 7.Hogl B, Kiechl S, Willeit J, et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64:1920–4. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 8.Tan EK, Seah A, See SJ, Lim E, Wong MC, Koh KK. Restless legs syndrome in an Asian population: a study in Singapore. Mov Disord. 2001;16:577–9. doi: 10.1002/mds.1102. [DOI] [PubMed] [Google Scholar]
- 9.Winkelman JW, Shahar E, Sharief I, Gottlieb DJ. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70:35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 10.Schlesinger I, Erikh I, Avizohar O, Sprecher E, Yarnitsky D. Cardiovascular risk factors in restless legs syndrome. Mov Disord. 2009;24:1587–92. doi: 10.1002/mds.22486. [DOI] [PubMed] [Google Scholar]
- 11.Gao X, Schwarzschild MA, Wang H, Ascherio A. Obesity and restless legs syndrome in men and women. Neurology. 2009;72:1255–61. doi: 10.1212/01.wnl.0000345673.35676.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10:295–305. doi: 10.1016/j.sleep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Phillips B, Young T, Finn L, Asher K, Hening WA, Purvis C. Epidemiology of restless legs symptoms in adults. Arch Intern Med. 2000;160:2137–41. doi: 10.1001/archinte.160.14.2137. [DOI] [PubMed] [Google Scholar]
- 14.Ohayon MM, Roth T. Prevalence of restless legs syndrome and periodic limb movement disorder in the general population. J Psychosom Res. 2002;53:547–54. doi: 10.1016/s0022-3999(02)00443-9. [DOI] [PubMed] [Google Scholar]
- 15.Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164:196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- 16.Aukerman MMM, Aukerman D, Bayard M, Tudiver F, Thorp L, Bailey B. Exercise and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med. 2006;19:487–93. doi: 10.3122/jabfm.19.5.487. [DOI] [PubMed] [Google Scholar]
- 17.Allen Rp KCAAMJ. The international restless legs syndrome study group validation of the international restless legs group rating scale. Sleep Med. 2003:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 18.Berger K, von Eckardstein A, Trenkwalder C, Rothdach A, Junker R, Weiland SK. Iron metabolism and the risk of restless legs syndrome in an elderly general population-the MEMO-Study. j Neurol. 2002;249:1195–9. doi: 10.1007/s00415-002-0805-2. [DOI] [PubMed] [Google Scholar]
- 19.Rothdach AJ, Trenkwalder C, Haberstock J, Keil U, Berger K. Prevalence and risk factors of RLS in an elderly population: the MEMO study. Memory and Morbidity in Augsburg Elderly. Neurology. 2000;54:1064–8. doi: 10.1212/wnl.54.5.1064. [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 21.Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol. 1989;18:858–67. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 22.McCullough ML, Feskanich D, Stampfer MJ, et al. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. Am J Clin Nutr. 2002;76:1261–71. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 23.Stampfer MJ, Hu FB, Manson JAE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122:327–34. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 25.Wolf AM, Hunter DJ, Colditz GA, et al. Reproducibility and validity of a self-administered physical activity questionnaire. Int J Epidemiol. 1994;23:991–99. doi: 10.1093/ije/23.5.991. [DOI] [PubMed] [Google Scholar]
- 26.Forman JP, Rimm EB, Stampfer MJ, Curhan GC. Folate intake and the risk of incident hypertension among US women. JAMA. 2005;293:320–9. doi: 10.1001/jama.293.3.320. [DOI] [PubMed] [Google Scholar]
- 27.Glovannucci E, Colditz G, Stampfer MJ, et al. The assessment of alcohol consumption by a simple self-administered questionnaire. Am J Epidemiol. 1991;133:810–17. doi: 10.1093/oxfordjournals.aje.a115960. [DOI] [PubMed] [Google Scholar]
- 28.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 29.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol. 2004;97:393–403. doi: 10.1152/japplphysiol.00179.2004. [DOI] [PubMed] [Google Scholar]
- 30.Buga GM, Gold ME, Fukuto JM, Ignarro LJ. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991;17:187–93. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- 31.Mello MTD, Lauro FAA, Silva AC, Tufik A. Incidence of periodic leg movements and of the restless legs syndrome during sleep following acute physical activity in spinal cord injury subjects. Spinal Cord. 1996;34:294–6. doi: 10.1038/sc.1996.53. [DOI] [PubMed] [Google Scholar]
- 32.Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–84. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 33.Van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in US women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao X, Chen H, Fung TT, et al. Prospective study of dietary pattern and risk of Parkinson disease. Am J Clin Nutr. 2007;86:1486–94. doi: 10.1093/ajcn/86.5.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lutz EG. Restless legs, anxiety and caffeinism. J Clin Psychiatry. 1978;39:693–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.