Abstract
Background
Primary prevention guidelines focus on risk, often assuming negligible aversion to medication. Yet most subjects discontinue primary prevention statins within 3 years. We quantify real-world distribution of medication disutility, and separately calculate average utilities for a range of risk strata.
Method and Results
We randomly sampled 360 members of the general public in London. Medication aversion was quantified as the gain in lifespan required by each individual to offset the inconvenience (disutility) of taking an idealised daily preventative tablet.
In parallel, we constructed tables of expected gain in lifespan (utility) from initiating statin therapy for each age group, sex and cardiovascular risk profile in the population. This allowed comparison of the widths of the distributions of medication disutility and of group-average expectation of longevity gain.
Observed medication disutility ranged from 1 day to >10 years of life being required by subjects (median 6 months, inter-quartile range 1 to 36 months) to make daily preventative therapy worthwhile. Average expected longevity benefit from statins at ages ≥50 years ranges from 3.6 months (low-risk women) to 24.3 months (high-risk men).
Conclusion
We can no longer assume that medication disutility is almost zero. Over a quarter of subjects had disutility exceeding the group-average longevity gain from statins expected even for the highest-risk (i.e. highest-gain) group. Future primary prevention studies might explore medication disutility in larger populations.
Patients may differ more in disutility than in prospectively-definable utility (which provides only group-average estimates). Consultations could be enriched by assessing disutility, and exploring its reasons.
Keywords: Primary prevention, Statins, Disutility, Compliance
Introduction
Initiation of lifelong primary prevention therapy for cardiovascular disease in a high risk patient should be based on a shared decision-making process between patient and doctor following clear presentation of appropriate information, including quantification of the risks and benefits expected from treatment and the cost and inconvenience (disutility) to the patient. This ideal scenario is almost never achieved.
Currently, primary prevention practice focuses on risk stratification using population-based statistical estimates to determine which individuals would have most to gain from preventative therapy.1 Doctors are documented to view risks differently from patients, and both have difficulty in evaluating, perceiving and conveying risks and benefits in an easily understood manner 2-5. The benefits of primary prevention are thus often presented to the patient without formal quantification of the cost, harms or inconvenience they might incur. However patients do understand risks and trade-offs3 and trust doctors more when presented with numerical information than given vague interpretations of risk6.
Previous interventions, aimed at improving adherence, have used new methods to convey cardiovascular risk rather than tackling the underlying reasons why people stop medication. The focus has been on individual counselling, and on quantitative and graphical displays, or the use of imaging techniques such as coronary CT scans to improve risk perception.7-9 These are based on the principle that better risk perception will lead to higher adherence and persistence with primary prevention therapy.10-12
Patient inconvenience, or medication disutility, has rarely been taken into consideration when initiating therapy. Knowing one’s risk to be high does not necessarily mean that one will, must, or even should take a preventive step. Taking action is dependent on many factors, and a large part of a patient’s resistance to treatment involves the reluctance to embark on a lifetime of medication. Statins are cost-effective for most persons with even modestly elevated cholesterol or any coronary heart disease risk factors if they do not mind taking a pill daily13-17.
When medication disutility is incorporated into the risk-benefit equation, it becomes clear that cost-effectiveness of statins is extremely sensitive to medication disutility. However, in spite of its crucial importance in determining the incremental cost-effectiveness ratio (ICER), medication disutility data is very scarce14. Owing to lack of data, guideline-writers have had to work on the basis that medication disutility is negligible. Cost-effectiveness analyses have typically used base case estimate of zero disutility, and covered up to 0.01 or 0.02 in sensitivity analyses13, 14, 17. Expressed as an absolute lifespan gain, for current English life expectancy at age 50 years, this translates to covering in sensitivity analyses the possibility that patients may be willing to give up a lifespan as large as 3.6 (or at most 7.2 months) to avoid medication. The analyses highlight that conclusions are exquisitely sensitive to this value, but there is limited data on which to base an estimate.
We do not know how close to zero medication disutility is. Nor do we know whether its distribution is fairly narrow, in which case a single value may be suitable for use in disease prevention decisions for all, or whether the distribution is wide, in which case it may be advisable to assess disutility within individuals in clinical practice.
Our study is the first to attempt to quantify the spectrum of individual medication disutility for primary prevention in a sample of the general population. We juxtapose it against the spectrum of expected longevity gain from initiation of statin therapy across the same general population.
Methods
Medication disutility
Medication disutility was assessed in a random sample of the general population of London by face-to-face interview using a structured questionnaire. Medication disutility has been assumed to lie between 0 - 0.001 in Time Trade-Off studies used in previous economic calculations,15-17 which roughly translates to being willing to give up between 0 -5 months of life to avoid taking daily medication. We designed our study to be able to estimate the proportion of subjects having medication disutility greater than 6 months, with 95% precision and a confidence interval of ±2%, even if the actual proportion of subjects in the population with this level of medication disutility was as small as 5%. Power calculations were based on the assumption that medication disutility would be normally distributed in the population. The sample size required on this basis was a minimum of 300 participants. We planned to recruit 360.
Study Population
Survey participants were approached in public thoroughfares in London, on the basis that this would potentially be the target population for cardiovascular screening and primary prevention. Participants were approached and recruited on three days in October and November 2010. Members of the public were approached until 360 agreed to participate.
Disutility Survey
In order to focus the survey on medication disutility and minimize other potential sources of low compliance such as cost, subjects were asked to imagine an idealised tablet that was available at negligible cost, with no need for prescription, nor medical supervision, nor follow up blood tests. They were also asked to assume that the tablet would have no side effects and could be started or stopped at will with no consequence other than receiving only partial benefit.
Disutility was assessed by initially asking subjects whether gaining an additional day of expected life would be sufficient benefit for them to commence lifelong therapy with the idealised tablet. If the answer was negative, then the subjects were asked if an additional 10 years of expected life would suffice. If the answer was positive, medication disutility was assumed to lie in the interval between 1 day and 10 years. This range was progressively narrowed using a binary tree (maximum 6 further steps) to reach the benefit required by each subject to offset their personal medication disutility.
The algorithm was constructed to approximately halve the time interval at each step, thus aligning the time points approximately evenly on a log scale. Speed of completion of the algorithm was confirmed by pilot testing and on average took less than 1 minute. Subjects who indicated that 10 years of longevity benefit would be insufficient were classed as having an extreme medication disutility. Demographic information on age, sex, employment status, current use of medication and previous heart attack or stroke were also sought. The full questionnaire is shown in Appendix 1.
Statistics
Survey data were summarised using simple measures of central tendency (mean and median) and spread across quartiles for each age and sex group. The distribution of medication disutility was also examined visually to assess whether it followed a normal distribution, and whether it had the same shape in each age and sex group. Differences on tablet disutility across gender and age were tested using parametric and non parametric tests for both.
The survey was indicated by the local Ethical Committee chair to not require Ethical Committee Approval, because it assessed attitudes to an imaginary medication and was carried out on members of the general public without collection of personally-identifiable information.
Paddington life expectancy gain charts
We calculated the expected average increase in life expectancy due to initiation of statin therapy for men and women with different levels of baseline risk using standard multiple decrement life table methods.18 Baseline life expectancy was based on all-cause and cardiovascular mortality rates for England and Wales in 200519, 20 obtained from the Office of National Statistics UK. These were then decremented for high-risk groups according to the risk level induced by different permutations and combinations of the following risk factors: tobacco exposure, systolic blood pressure, total cholesterol, age and sex. The size of the decrement for each age-sex- risk combination was calculated by entering values into the SCORE algorithm21 recommended by the European Heart Association for risk stratification, and obtaining the percentage increase in mortality for each group. The SCORE algorithm compares each risk factor combination to the national average. Data on the national average mean, and the distribution of blood pressure, smoking status and cholesterol were obtained from the QRESEARCH database (2005) which includes data on over 13 million patients spread throughout the UK.22
Data on diabetes has not been collected uniformly in SCORE study cohorts. Thus people with diabetes were included in the general SCORE database used for the development of risk functions. However, because of non-uniformity in the ascertainment of diabetes, diabetes was not included as a dichotomous variable into the SCORE risk function.21 We have followed the same method for decrementation of life expectancy in diabetics in this study.
Blood pressure, total cholesterol and smoking status above the national average level were considered to act multiplicatively to increase cardiovascular risk as per the algorithm C. All in all, 40 different age-sex-risk combination tables were calculated to obtain values of expected longevity benefit for a full spectrum of risk groups (See Appendix 2 and Web table 1 for details). The design of the Paddington tables was kept as similar as possible to the SCORE charts, displaying, instead of 10-year risk of fatal cardiovascular disease, the average longevity benefit (in months) that a patient can expect to gain by starting lifelong therapy with statin.
Percentage reduction in cardiovascular mortality with statin therapy was obtained from a meta-analysis of trials of lipid lowering agents in primary prevention populations.23 For each cardiovascular risk group, life tables were then recalculated with the statin effect. The difference between baseline life expectancy, and life expectancy with the statin therapy, was taken as the average expected longevity benefit. The youngest age at which initiation of statin therapy was modelled was 50 years. The spectrum of cardiovascular risk modelled was based on the distributions of blood pressure, cholesterol and smoking in the UK population, thus the spectrum of longevity benefit represents the average distribution of life years gained from statin therapy in the UK population.
Results
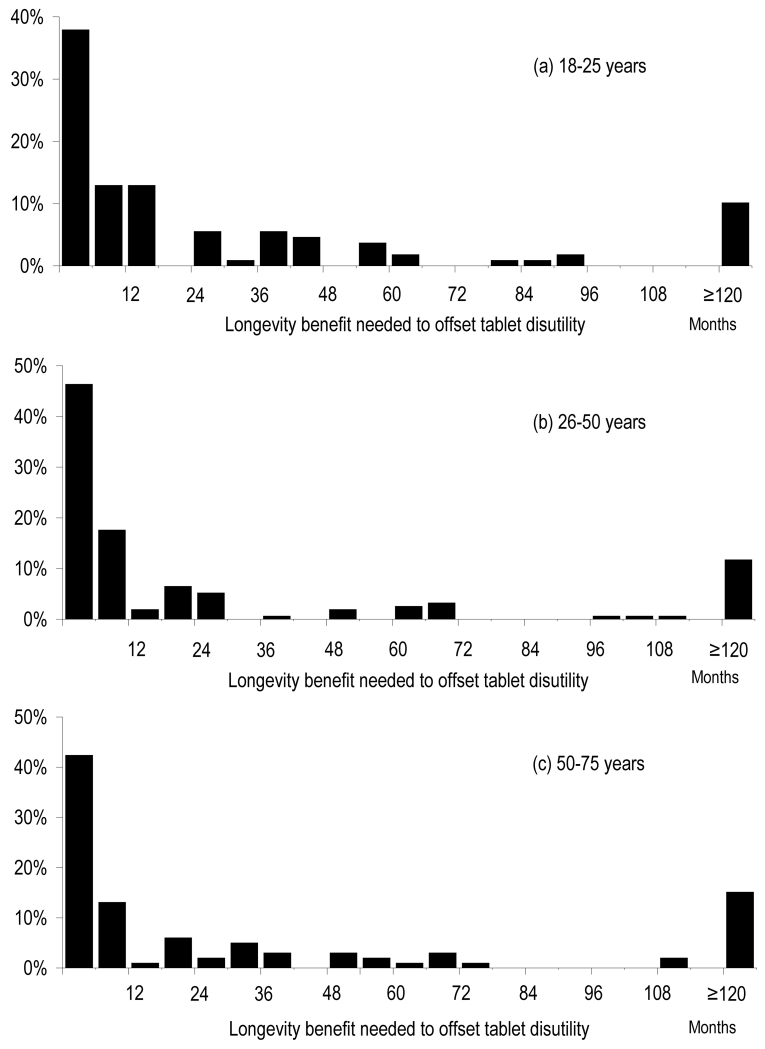
Table 1 shows the baseline characteristics of survey respondents. 360 participants were recruited after approaching 379 members of the public. The distribution of medication disutility expressed as longevity gain desired by an individual to offset the inconvenience of taking a lifelong preventative tablet, is shown in Figures 1 and 2 and Appendix 3. Two-thirds of subjects had medication disutility greater than 1 month and 12% had extreme medication disutility (desiring ≥10 years predicted increase in life expectancy to adhere to therapy). Near-zero medication disutility (<1 month longevity benefit required) was expressed by 34% of subjects. There was no relationship between sex and disutility (31±42 months in males versus 26±38 months in females, p=0.30 by t-test, p=0.40 by Mann-Whitney U test). There was no relationship between age and disutility: Pearson correlation with age was 0.04 (p=0.42), with sqrt(age) was −0.01 (p=0.79), Spearman rank correlation with age was −0.01 (p=0.79) (figure 1 and 2).
Table 1. Baseline Survey population characteristics.
| Population (n=360) | |
|---|---|
| Male gender (%) | 50 |
| Age (mean±SD) | 38±17 |
| Regular use of any medication (%) | 22 |
| Previous CVD History (%) | 1 |
Figure 1. Distribution of medication disutility by sex.
This figure illustrates the distribution of medication disutility for an idealised tablet, expressed as lifespan gain in months needed to offset taking daily therapy
Figure 2. Distribution of mediation disutility by age.
The distribution of medication disutility for an idealised tablet, expressed as number of months needed to make therapy worthwhile.
Tables of expected lifespan gain according to age, sex, smoking status, blood pressure and cholesterol level of the subject are shown in Figure 3. The shading on the chart corresponds to the increase in group-average life expectancy for a notional large group of patients with that specified cardiovascular risk profile starting lifelong statin therapy. These life expectancy gains are meaningful only for the group as a whole, as is the case for risk percentages that are also sometimes displayed in this way. In practice, a small proportion of patients will gain the lion’s share of the extra lifespan, while a large proportion will gain no extra lifespan, as shown in Appendix 4. From the age, sex, smoking status, blood pressure and cholesterol, it is not possible to be more specific as to whether one particular patient will gain. Even if a trial were conducted, each individual patient could only be in one arm, and it would not be possible to pinpoint if an individual patient had personally gained or not. The value represents only the mean for patients with that particular risk factor profile.
Figure 3. Paddington tables. Months of longevity benefit obtained from statin therapy.
Each chart can be read by looking up the patient age, sex, blood pressure, cholesterol level and smoking status. The shading corresponds to the increase in group-average life expectancy for a notional large group of patients with that specified cardiovascular risk profile starting lifelong statin therapy
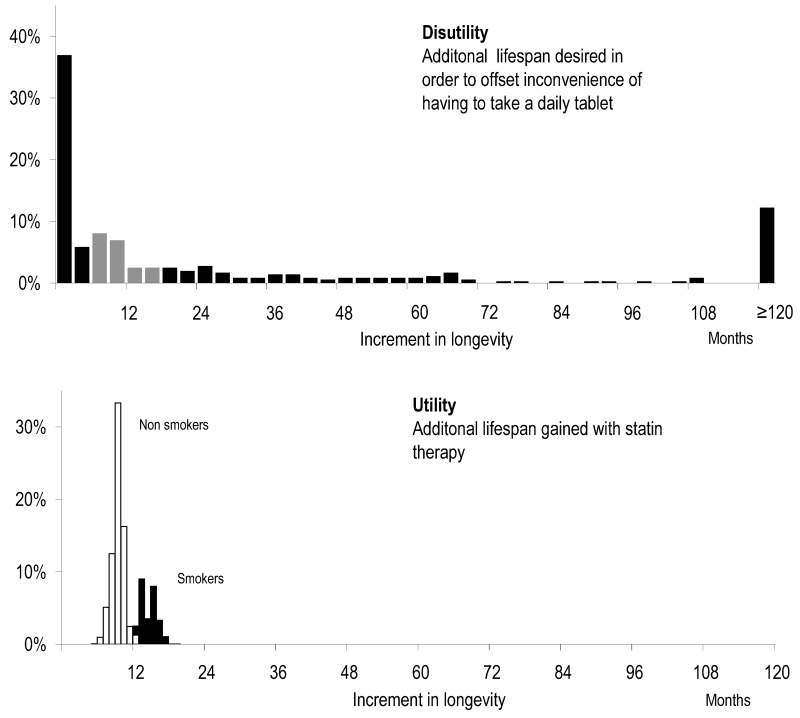
Figure 4 shows the frequency distribution of medication disutility (top panel), juxtaposed against longevity benefit from statin therapy (bottom panel). The calculated longevity benefit with statin therapy ranges from 5.5 months to 24.3 months in males, and from 3.6 to 18.2 months in females depending on individual cardiovascular risk profile. 99% of the UK population will gain less than 24.3 months of additional life as a result of lifelong primary prevention with a statin, whilst 1% has a risk profile which allows them to gain more than this. Individual-subject medication disutility has a wide distribution in our survey population ranging from less than 1 day to more than 10 years.
Figure 4. Disutility versus utility.
Frequency distribution of disutility, longevity benefit that subjects expressed a desire for in order to make tablet therapy worthwhile (top panel), and the frequency distribution of utility, actual expected gain in lifespan from statin therapy in the English population (bottom panel). The difference between the two values is the net benefit of tablet therapy. Because utility has a very much narrower spectrum than disutility, for those with a high disutility, regardless of utility statins are a net harm; for those with low disutility, regardless of utility statins are a net benefit. It is only for those in the middle grey zone of the top panel that sex, smoking status, blood pressure and cholesterol are the deciding factors.
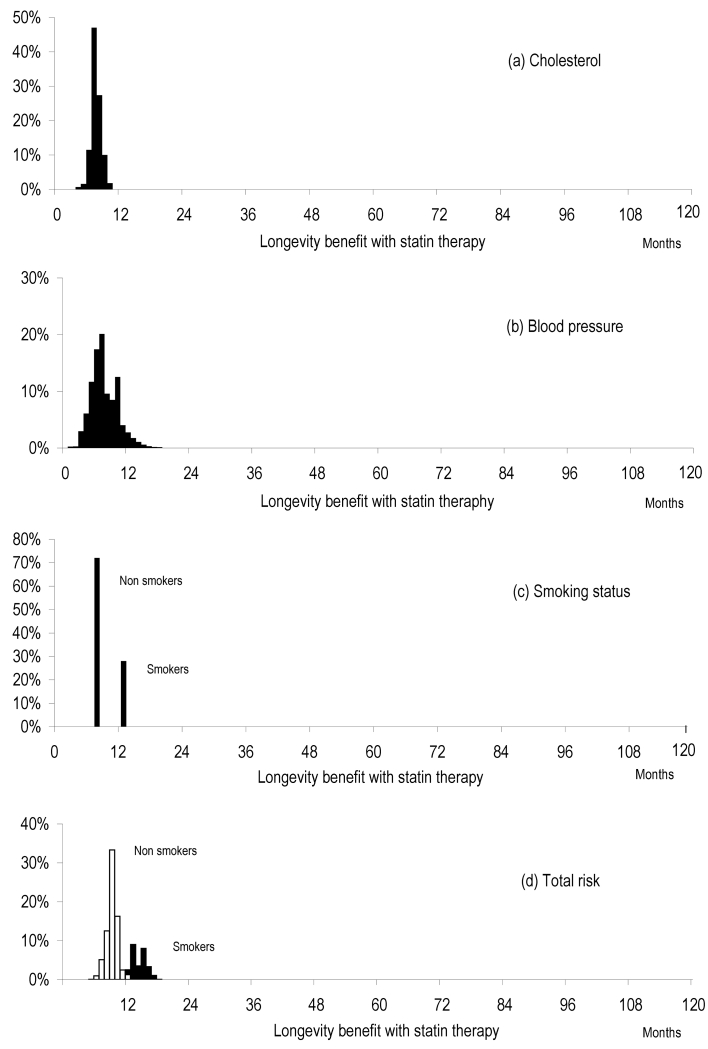
Figure 5 shows the expected distribution of longevity benefit in the English population resulting from distribution of (A) total serum cholesterol (B) systolic blood pressure (C) smoking in the general population with all other risk factors held constant and (D) the distribution of total cardiovascular risk using all 3 variables combined. For each panel (A, B, C), the distribution of longevity benefit with statin therapy was calculated allowing that particular risk factor to vary with a pre-specified distribution (the distribution of that risk factor in the population in the UK) whilst the other risk factors were held constant at the population mean. The distribution of longevity benefit for total cardiovascular risk was calculated using all 3 variables combined in an aggregate risk score using the SCORE algorithm21.
Figure 5. Expected distribution of longevity benefit in the English population.
Distribution of longevity benefit with statin therapy resulting from distribution of a) total serum cholesterol b) systolic blood pressure c) smoking in the general population with all other risk factors held constant. 4 d) shows the distribution of longevity benefit for total cardiovascular risk using all 3 variables combined in an aggregate risk score using the SCORE algorithm.21
Discussion
The implicit assumption in guideline development and clinical protocols for primary prevention of cardiovascular disease, namely that medication disutility is zero or near zero, may not be sound. Much more work remains to be done to develop evidence-based approaches to account for medication aversion during clinical encounters. In our simple study, even for an idealised tablet, more than a quarter of individuals have medication disutility which exceeds the group-mean lifespan gain from statin therapy calculated for a very high cardiovascular risk group.
A simple calculation of averaged expectation of benefit versus disutility might suggest that addition of even such an idealised agent would not be perceived by that individual patient to present a net gain. Whether they would judge the situation differently, if it were made clear that some patients would gain a great deal of lifespan while many gained none, is unknown and might be an important question to explore in future studies.
Prevalence of medication disutility in the general population
The prevalence and degree of significant medication disutility in the general population, which is the target population of primary prevention, may often be much greater than previously assumed. The medication disutility curves (Figures 1 and 2) are not normally distributed but centrifugal, with a standard deviation 1.5 times the mean. Nearly half of the population have disutility greater than double the median or less than half the median. The shape of the medication disutility distribution curve seems similar across age groups, suggesting that its shape is genuine and that ageing with associated perceived nearness of mortality did not have a large effect (Figures 1 and 2).
Medication disutility varies dramatically from person to person, to a much greater extent than estimated cardiovascular risk between individuals. Clinical practice evaluates risk factors using statistical estimates to determine whether taking a statin is worthwhile, but the inter-individual variation in medication disutility, which appears to have a greater effect on net benefit for individuals, is rarely addressed. This variation between individuals in the size of medication disutility is greater than the effect of variation in any one of the common risk factors used to determine thresholds for treatment (Figure 5).
Even if primary prevention guidelines were revised to incorporate a non-zero value for medication disutility, there is no single value that could reasonably be entered because disutility varies to such an extent between individuals; much more so than utility. If our data is representative, then alongside assessing blood pressure, cholesterol and smoking status, it may be informative to assess individual medication disutility and explore its reasons.
Faced with a patient with high expected lifespan gain from preventative therapy but even higher medication disutility, the clinician should not simply withhold therapy. Equally, however, they should not simply prescribe and assume the medication will be taken. High disutility could instead initiate exploration of its underlying reasons.
Use of an idealised tablet to assess medication disutility
We were keen to determine the lower limit of medication disutility and therefore used a hypothetical intervention to assess disutility rather than a real one that might have an adverse reputation. The hypothetical medication enhanced lifespan without having the four principal drawbacks of primary prevention medications: cost to the patient, inconvenience of obtaining a prescription, perceived loss of autonomy to stop and start at will, and adverse symptoms. Removal of these barriers improves compliance with medical therapy for chronic diseases.24 With real drugs, the possibility of side effects, the inconvenience of having to obtain prescriptions and the non-zero cost mean that the distribution of disutility is likely to be greater than the values we obtained, and the spectrum of values might be wider.
Our study should therefore be considered only a lower limit on medication disutility. Nevertheless it identifies that disutility is not near zero, and is not trivial in comparison to the benefits offered by a medication such as a statin. In order to translate this concept into clinical practice further studies with questionnaires specifically designed to investigate real medications used in primary prevention would be needed. Such a design, specific to the individual agent, and a particular cost and arrangement for prescription, will give a more complete picture of real-life medication disutility in a given particular clinical context.
Study limitations and future study design
We did not collect individualised risk factor data on the subjects in our survey and therefore are unable to plot a joint utility–versus–disutility distribution at an individual level. This would only have been possible with detailed background information (including measurement of blood pressure and measurement of blood lipids). We did not impose this step because we wanted this survey to be broadly representative of the general population and not only those willing to undergo these for a research study. Thus it is important to note that the longevity benefit distributions in Figure 5 describe the general population and not the particular subjects in this survey. We cannot exclude the possibility that our sample of subjects might be unrepresentative of the general population. Furthermore, comparing the individual medication disutility with expected life year gain can be problematic as the difference becomes significant especially at individual level. When used in real life a physician should make clear that a calculated increase of 1 year in life expectancy is an estimated based on an average of lifespan gain between subjects. To make this difficult concept easy to understand for every individual the physician could offer a page with 3 examples of how, amongst a group of 10 people with an average increase of 1 year, individual gain may vary from the mean (Appendix 4).
Our questionnaire was a very simple form of the time trade-off method. This was aimed to be brief to allow us to sample the general population and minimise the possibility of examining only an unrepresentative subset biased towards interest in health. Our choice of survey design achieved a 95% participation rate. In ultimate clinical practice, with a patient voluntarily engaging in a consultation and therefore already showing some level of commitment to the questioner, a more comprehensive tool would be appropriate.
We assessed medication disutility without assessing the individualised expected lifespan. It is possible that people who are formally told that their remaining expected lifespan is short, might have less medication disutility. However, in our dataset, age – known to the public to be the most powerful determinant of mortality risk - did not affect medication disutility. Future studies using individualised utility calculations would be able to test this hypothesis.
It is likely that a participant’s personal disutility may be influenced by context and situation25. For example, if we had questioned patients within a GP surgery or a hospital outpatient department, then their response may have been influenced by the many health related cues nearby. We cannot assume that disutility assessed in a public space is equivalent to disutility that would be assessed in a primary prevention scenario. Future studies are needed to assess medication disutility in patients attending a primary care service for screening and being considered for preventative treatment.
Despite our request to imagine an ideal medication accessible without effort and causing no side effects, participants’ responses may nevertheless have been coloured by an expectation of a high rate and magnitude of side effects, for example through non-placebo-controlled reports in the mass media.
Our survey had an upper limit on medication disutility of “>10 years”, which prevents us from being able to sub-classify subjects beyond this ceiling. However, from a practical point of view knowing exact disutility numerically when it is already above the maximum achievable longevity benefit may not be so important as recognising that subjects with such strong medication disutility do exist.
Since mortality rates change over time, the survival depicted in any period life table will not perfectly reflect the true survival experience of a cohort. For example, secular improvements in health mean that actual life expectancy of cohorts is often longer than that predicted using period life tables constructed by applying present-day survival rates across age groups. Furthermore, life expectancy varies from country to country and cohort to cohort, so that Paddington tables might need to be reconstructed for different countries and cohorts.
Our sample is limited to North West London, which may not be representative of other areas in the UK. However survey participants were drawn from the general population, which is the target population of primary prevention therapy. To minimise intrusiveness we did not ask subjects their ethnicity, but we did approach subjects without regard to their apparent ethnicity. Census data show that the general population of London is more ethnically diverse than most of the rest of the UK, with 58% being white British; 11.3% other white, 13.3% South Asian, 10.6% black, 1.5% Chinese, and 5.5% mixed or other.26 The consistently large variation in medication disutility in both sexes across all age groups suggests that distribution is genuinely wide. Interviewing subjects in other cities is likely to make the distribution not narrower but wider.
Individual medication disutility may be fluid over time, for example being influenced by a personal ‘heart scare’ or a cardiovascular event in a friend or family member. Mass media reports may also be unhelpful because, without the benefit of placebo control comparison, the extent of genuine incremental side effects can easily be overestimated.
Finally, our data reflect medication disutility in a primary prevention cohort and we did not assess the impact of cardiovascular events on medication disutility in secondary prevention. Only one individual in the survey had a previous cardiovascular event. Further studies are needed to investigate the longitudinal behaviour of medication disutility in order to determine how often medication disutility should be re-assessed.
Conclusions
The tables presented in this study are designed to allow both patient and doctor to compare risk and benefit of preventative tablet therapy to determine an average expected net benefit for a notional group of similar patients, using a mutually understood metric of lifespan gain. High disutility in an individual might prompt exploration of the underlying reasons, and enhancing the interaction between patient and clinician in this way might strengthen the consultation.
Guidelines specifying a risk threshold for treatment may have been derived from a tacit assumption of near-zero medication disutility, which may not be representative for many subjects. Future public health research could explore more advanced methodologies, since our simple medication disutility assessment takes only a minute - less than the time taken to measure cholesterol and blood pressure.
Although still at an early stage, individualised quantification and discussion of medication disutility, and parallel methods of describing group-average preventative benefits, might bring us closer to primary prevention that is truly personalised.
Supplementary Material
Footnotes
No conflict of interest to disclose.
REFERENCES
- 1.Robson J. Lipid modification: Cardiovascular risk assessment and the modification of blood lipids for the primary and secondary prevention of cardiovascular disease. Heart. 2008;94:1331–1332. doi: 10.1136/hrt.2008.150979. [DOI] [PubMed] [Google Scholar]
- 2.Nexoe J, Gyrd-Hansen D, Kragstrup J, Kristiansen IS, Nielsen JB. Danish gps’ perception of disease risk and benefit of prevention. Family practice. 2002;19:3–6. doi: 10.1093/fampra/19.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Hembroff LA, Holmes-Rovner M, Wills CE. Treatment decision-making and the form of risk communication: Results of a factorial survey. BMC medical informatics and decision making. 2004;4:20. doi: 10.1186/1472-6947-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spiegelhalter D, Pearson M, Short I. Visualizing uncertainty about the future. Science. 2011;333:1393–1400. doi: 10.1126/science.1191181. [DOI] [PubMed] [Google Scholar]
- 5.Gigerenzer G, Edwards A. Simple tools for understanding risks: From innumeracy to insight. Bmj. 2003;327:741–744. doi: 10.1136/bmj.327.7417.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gurmankin AD, Baron J, Armstrong K. The effect of numerical statements of risk on trust and comfort with hypothetical physician risk communication. Medical decision making : an international journal of the Society for Medical Decision Making. 2004;24:265–271. doi: 10.1177/0272989X04265482. [DOI] [PubMed] [Google Scholar]
- 7.Asaria P, Francis DP. Heart forecast for cardiovascular risk assessment. Heart. 2011;97:173–174. doi: 10.1136/hrt.2010.207100. [DOI] [PubMed] [Google Scholar]
- 8.Lipkus IM. Numeric, verbal, and visual formats of conveying health risks: Suggested best practices and future recommendations. Medical decision making : an international journal of the Society for Medical Decision Making. 2007;27:696–713. doi: 10.1177/0272989X07307271. [DOI] [PubMed] [Google Scholar]
- 9.Wells S, Kerr A, Eadie S, Wiltshire C, Jackson R. ‘Your heart forecast’: A new approach for describing and communicating cardiovascular risk? Heart. 2010;96:708–713. doi: 10.1136/hrt.2009.191320. [DOI] [PubMed] [Google Scholar]
- 10.Casebeer L, Huber C, Bennett N, Shillman R, Abdolrasulnia M, Salinas GD, Zhang S. Improving the physician-patient cardiovascular risk dialogue to improve statin adherence. BMC family practice. 2009;10:48. doi: 10.1186/1471-2296-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond GA, Kaul S. The things to come of shape: Cost and effectiveness of cardiovascular prevention. The American journal of cardiology. 2007;99:1013–1015. doi: 10.1016/j.amjcard.2006.10.070. [DOI] [PubMed] [Google Scholar]
- 12.Stacy JN, Schwartz SM, Ershoff D, Shreve MS. Incorporating tailored interactive patient solutions using interactive voice response technology to improve statin adherence: Results of a randomized clinical trial in a managed care setting. Population health management. 2009;12:241–254. doi: 10.1089/pop.2008.0046. [DOI] [PubMed] [Google Scholar]
- 13.Choudhry NK, Patrick AR, Glynn RJ, Avorn J. The cost-effectiveness of c-reactive protein testing and rosuvastatin treatment for patients with normal cholesterol levels. Journal of the American College of Cardiology. 2011;57:784–791. doi: 10.1016/j.jacc.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 14.Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation. 2011;124:146–153. doi: 10.1161/CIRCULATIONAHA.110.986349. [DOI] [PubMed] [Google Scholar]
- 15.Greving JP, Visseren FL, de Wit GA, Algra A. Statin treatment for primary prevention of vascular disease: Whom to treat? Cost-effectiveness analysis. Bmj. 2011;342:d1672. doi: 10.1136/bmj.d1672. [DOI] [PubMed] [Google Scholar]
- 16.Pignone M, Earnshaw S, Tice JA, Pletcher MJ. Aspirin, statins, or both drugs for the primary prevention of coronary heart disease events in men: A cost-utility analysis. Annals of internal medicine. 2006;144:326–336. doi: 10.7326/0003-4819-144-5-200603070-00007. [DOI] [PubMed] [Google Scholar]
- 17.Pletcher MJ, Lazar L, Bibbins-Domingo K, Moran A, Rodondi N, Coxson P, Lightwood J, Williams L, Goldman L. Comparing impact and cost-effectiveness of primary prevention strategies for lipid-lowering. Annals of internal medicine. 2009;150:243–254. doi: 10.7326/0003-4819-150-4-200902170-00005. [DOI] [PubMed] [Google Scholar]
- 18.Preston SH, H P, Guillot M. Multiple decrement processes in demography: Measuring and modelling population processes. 2001. pp. 71–91. Publishers OB. [Google Scholar]
- 19.Mortality statistics: Cause, england and wales, series dh2, no. 32, 2005 [internet] Table 1 estimated resident population: Single years of age and sex. 2005 Available from: Http://www.Ons.Gov.Uk/ons/publications/re-reference-tables.Html?Edition=tcm%3a77-39675.
- 20.Mortality statistics: Cause, england and wales, series dh2, no. 32, 2005 [internet] Table 2.9 deaths: Underlying cause, sex and age-group: Chapter ix diseases of the circulatory system. 2005 Available from: Http://www.Ons.Gov.Uk/ons/publications/re-reference-tables.Html?Edition=tcm%3a77-39675.
- 21.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetiere P, Jousilahti P, Keil U, Njolstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM. Estimation of ten-year risk of fatal cardiovascular disease in europe: The score project. Eur. Heart J. 2003;24:987–1003. doi: 10.1016/s0195-668x(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 22.Hippisley-Cox J, Coupland C, Vinogradova Y, Robson J, May M, Brindle P. Derivation and validation of qrisk, a new cardiovascular disease risk score for the united kingdom: Prospective open cohort study. Bmj. 2007;335:136. doi: 10.1136/bmj.39261.471806.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor F, Ward K, Moore TH, Burke M, Davey Smith G, Casas JP, Ebrahim S. Statins for the primary prevention of cardiovascular disease. The Cochrane database of systematic reviews. 2011:CD004816. doi: 10.1002/14651858.CD004816.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvert SB, Kramer JM, Anstrom KJ, Kaltenbach LA, Stafford JA, Allen LaPointe NM. Patient-focused intervention to improve long-term adherence to evidence-based medications: A randomized trial. American heart journal. 2012;163:657–665. e651. doi: 10.1016/j.ahj.2012.01.019. [DOI] [PubMed] [Google Scholar]
- 25.Lichtenstein S, Slovic P. The construction of preference. Cambridge University Press; Cambridge ; New York: 2006. [Google Scholar]
- 26. [Accessed 5 august 2012];Her majesty’s government: Office for national statistics. Http://www.Ons.Gov.Uk/ons/rel/regional-trends/regional-trends-online-tables/june-2011-release/topic-workbook-10--population-and-migration.Xls.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.