Abstract
Background
Transforming growth factor beta 1 (TGFβ1) is strongly induced following brain injury and polarises microglia to an anti-inflammatory phenotype. Augmentation of TGFβ1 responses may therefore be beneficial in preventing inflammation in neurological disorders including stroke and neurodegenerative diseases. However, several other cell types display immunogenic potential and identifying the effect of TGFβ1 on these cells is required to more fully understand its effects on brain inflammation. Pericytes are multifunctional cells which ensheath the brain vasculature and have garnered recent attention with respect to their immunomodulatory potential. Here, we sought to investigate the inflammatory phenotype adopted by TGFβ1-stimulated human brain pericytes.
Methods
Microarray analysis was performed to examine transcriptome-wide changes in TGFβ1-stimulated pericytes, and results were validated by qRT-PCR and cytometric bead arrays. Flow cytometry, immunocytochemistry and LDH/Alamar Blue® viability assays were utilised to examine phagocytic capacity of human brain pericytes, transcription factor modulation and pericyte health.
Results
TGFβ1 treatment of primary human brain pericytes induced the expression of several inflammatory-related genes (NOX4, COX2, IL6 and MMP2) and attenuated others (IL8, CX3CL1, MCP1 and VCAM1). A synergistic induction of IL-6 was seen with IL-1β/TGFβ1 treatment whilst TGFβ1 attenuated the IL-1β-induced expression of CX3CL1, MCP-1 and sVCAM-1. TGFβ1 was found to signal through SMAD2/3 transcription factors but did not modify nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) translocation. Furthermore, TGFβ1 attenuated the phagocytic ability of pericytes, possibly through downregulation of the scavenger receptors CD36, CD47 and CD68. Whilst TGFβ did decrease pericyte number, this was due to a reduction in proliferation, not apoptotic death or compromised cell viability.
Conclusions
TGFβ1 attenuated pericyte expression of key chemokines and adhesion molecules involved in CNS leukocyte trafficking and the modulation of microglial function, as well as reduced the phagocytic ability of pericytes. However, TGFβ1 also enhanced the expression of classical pro-inflammatory cytokines and enzymes which can disrupt BBB functioning, suggesting that pericytes adopt a phenotype which is neither solely pro- nor anti-inflammatory. Whilst the effects of pericyte modulation by TGFβ1 in vivo are difficult to infer, the reduction in pericyte proliferation together with the elevated IL-6, MMP-2 and NOX4 and reduced phagocytosis suggests a detrimental action of TGFβ1 on neurovasculature.
Electronic supplementary material
The online version of this article (doi:10.1186/s12974-016-0503-0) contains supplementary material, which is available to authorized users.
Keywords: Phagocytosis, Inflammation, Cytokine, Chemokine, BBB, Alzheimers, NOX4, MMP-2, IL-6, SMAD2/3
Background
Neuroinflammation contributes to the development and progression of epilepsy [1], traumatic brain injuries [2], stroke [3] and many neurodegenerative diseases [4]. Inflammatory cytokines are key mediators of the brain’s immune response and associated neurodegeneration [5]. Transforming growth factor beta (TGFβ) is a pleiotropic cytokine in the brain with roles in regulating cell proliferation, differentiation, survival and scar formation [6–9]. Furthermore, TGFβ orchestrates both pro- and anti-inflammatory responses in a cell and context-dependent manner [10–12]. TGFβ exists in three isoforms (TGFβ1–3), of which TGFB1 was the first identified and is the most widely studied. TGFβ exerts its actions through the serine/threonine kinase receptors, transforming growth factor beta receptor 1 (TGFBR1), TGFBR2 and TGFBR3. Activation of TGFBRs by TGFβ ligand binding initiates a signal transduction pathway predominantly through SMAD transcription factors [13].
Several cell types in the central nervous system (CNS) are capable of producing TGFβ1. Endothelial cells secrete this growth factor, and this is enhanced by co-culture with pericytes [14, 15]. Following brain injury, microglia and astrocytes produce large concentrations of TGFβ1 [8, 16, 17], whilst glioblastoma multiforme enhances TGFβ1 expression, possibly due to the increased and abnormal angiogenesis [18–20]. Furthermore, TGFβ1 is elevated in several inflammatory conditions including Alzheimer’s disease, type 1 diabetes mellitus and acute brain injuries such as ischaemic stroke [21–24].
A number of immunologically active brain cells express TGFBRs and can therefore respond to secretions of this growth factor. Microglia are the brain’s predominant immune cell and the most widely studied with respect to their inflammatory response [25]. TGFβ1 promotes a strong anti-inflammatory phenotype in microglia through attenuated cytokine, chemokine, adhesion molecule and reactive oxidative species (ROS) production; however, it has no effect on human leukocyte antigen (HLA) antigen presentation complexes [10, 26–28]. Furthermore, TGFβ1 enhances microglial phagocytosis of amyloid beta, thereby reducing plaque burden [29]. Astrocytes show a more complex regulation of immune responses by TGFβ1 stimulation, with reports of both enhancement and inhibition of chemokine and cytokine production [11, 12, 30, 31]. Other cells, including those derived from the choroid plexus and leptomeninges, also display attenuated inflammatory responses with TGFβ1 treatment [27, 32].
Previous work in our lab using human brain pericytes demonstrated a reduction in interferon gamma (IFNγ)-induced HLA-DR, HLA-DP and HLA-DQ expression with TGFβ1 treatment, suggesting an anti-inflammatory role [27]. Pericytes are multifunctional cells that surround endothelial cells and contact numerous parenchymal brain cells including astrocytes, neurons and microglia to form a neurovascular unit [33]. Pericyte coverage of brain vasculature is vital with respect to blood-brain barrier (BBB) formation and maintenance [34–36]. Several labs, including our own, have shown that pericytes can contribute to the brain’s immune response through cytokine, chemokine and adhesion molecule expression and that they display phagocytic potential [37–42].
The function of TGFβ1 on pericytes has been briefly studied, however, largely with respect to differentiation, proliferation or angiogenesis [43, 44]. Little is known about the role of TGFβ1 in the context of human brain pericyte immune function. We therefore sought to investigate how TGFβ1 modifies the human brain pericyte inflammatory response.
Methods
Tissue source
Human middle temporal gyrus brain tissue was obtained, with informed consent, from surgeries of patients with drug-resistant temporal lobe epilepsy. All specimens were collected with written patient consent. All protocols used in this study were approved by the Northern Regional Ethics Committee (New Zealand), and all methods were carried out in accordance with the approved guidelines.
Mixed glial cultures isolated from human brain tissue
Mixed glial cultures containing astrocytes, pericytes and microglia were isolated from adult human brain tissue with minor modifications as described previously [45]. As per [42], cells were grown until passage five before use in order to dilute out non-proliferating microglia and astrocytes and obtain a pure pericyte population. Pericyte cultures at passages five-eight were used for all experiments. Detailed characterisation of pericyte cultures has been performed previously [37, 42].
Cytokine and drug treatments
To examine the effect of cytokines on pericyte responses, cells were treated with 0.1–10 ng/mL TGFβ1 (PeproTech, NJ, USA; vehicle, 1 mM citric acid, pH 3 with 0.1 % bovine serum albumin (BSA)) or interleukin 1 beta (IL-1β; Peprotech, NJ, USA; vehicle, 0.1 % BSA in phosphate buffered saline (PBS)) for 0–72 h. Details for each experiment are noted in appropriate figure legends. To induce apoptosis as a positive control for cleaved caspase 3 (CC3) staining, pericytes were treated with 50 nM okadaic acid (Sigma-Aldrich, MO, USA) for 24 h. Cells were treated with drugs/cytokines by addition of a 1:100 dilution of a 100 × stock.
Collection of conditioned media and cytokine measurement by cytometric bead array
Conditioned media was collected from cells grown in a 96-well plate. Media was spun at 160×g for 5 min to collect any detached cells or debris. Supernatant was obtained and stored at −20 °C. The concentration of cytokines was measured using a cytometric bead array (CBA; BD Biosciences, CA, USA) as per manufacturer’s instructions. CBA samples were run on an Accuri C6 flow cytometer (BD Biosciences, CA, USA). Data was analysed using FCAP-array software (version 3.1; BD Biosciences, CA, USA) to convert fluorescent intensity values to concentrations using a ten-point standard curve (0–5000 pg/mL) as described previously [46].
Immunocytochemistry
Cells were fixed in 4 % paraformaldehyde (PFA) for 15 min and washed in PBS with 0.1 % triton X-100 (PBS-T). Cells were incubated with primary antibodies (Additional file 1: Table S1) overnight at 4 °C in immunobuffer containing 1 % goat serum, 0.2 % Triton X-100 and 0.04 % thimerosal in PBS. Cells were washed in PBS-T and incubated with appropriate anti-species fluorescently conjugated secondary antibodies overnight at 4 °C. Cells were washed again and incubated with Hoechst 33258 (Sigma-Aldrich, MO, USA) for 20 min. Images were acquired at ×10 magnification using the automated fluorescence microscope ImageXpress® Micro XLS (version 5.3.0.1, Molecular Devices, CA, USA). Quantitative analysis of intensity measures and positively stained cells was performed using the Cell Scoring and Show Region Statistics analysis modules on MetaXpress® software (version 5.3.0.1, Molecular Devices, CA, USA).
Phagocytosis assays
To evaluate phagocytosis by microscopy, cells were treated with 0–10 ng/mL TGFβ1 for 24 h, followed by a further 24-h incubation with Fluoresbrite® YG carboxylate microspheres of 1 μm diameter (Polysciences Inc, PA, USA; 1:1000 dilution) at 37 °C, 5 % CO2. At completion, cells were washed twice with PBS to remove un-phagocytosed beads and fixed in 4 % PFA as per immunocytochemistry. Nuclear staining was visualised by a 30-min incubation with the DNA-specific dye DRAQ5 (BioStatus, UK). Images were obtained using the ImageXpress® Micro XLS microscope and the percentage of phagocytic cells determined using the Cell Scoring module on MetaXpress® software.
To evaluate phagocytosis by flow cytometry, cells were treated with 0–10 ng/mL TGFβ1 for 24 h, followed by a further 2-h incubation with Fluoresbrite® YG carboxylate microspheres of 1-μm diameter (1:1000 dilution) at 37 °C, 5 % CO2. At completion, cells were washed twice with PBS, and 0.25 % trypsin-ethylenediaminetetraacetic acid (EDTA) was added to remove beads bound to the cell surface and bring cells into suspension. Selected samples were incubated for 10 min with 7-aminoactinomycin D (7-AAD; BD Biosciences, CA, USA) to assess viability. Samples were run on an Accuri C6 flow cytometer and viable cells gated based on forward scatter and side scatter. Mean fluorescent intensity (MFI) of the live cells was detected, indicative of the quantity of beads internalised.
Confocal laser scanning microscopy
Cells destined for confocal microscopy were plated at 5000 cells/well on 8-mm #1.5 glass coverslips (Menzel Gläser, Germany) within a 48-well plate. Fluoresbrite® YG carboxylate microspheres of 1-μm diameter (1:10,000 dilution) were added to cells for 24 h at 37 °C, 5 % CO2 and at completion washed twice in PBS to remove un-phagocytosed beads. Cells were fixed in 4 % PFA and immunostained for platelet-derived growth factor receptor beta (PDGFRβ) as per immunocytochemistry, with the exception of diluting primary and secondary antibodies in donkey immunobuffer (1 % donkey serum, 0.2 % Triton X-100 and 0.04 % thimerosal in PBS). Coverslips were mounted onto glass slides using fluorescent mounting medium (DAKO, Denmark). Confocal images were acquired using an oil immersion lens (×63 magnification, 1.4NA) in a Z-series with a gap of 0.8 μm using a Zeiss LSM 710 inverted confocal microscope (Biomedical Imaging Research Unit, University of Auckland) with ZEN 2010 software (Carl Zeiss, Germany).
EdU proliferation assay
5-Ethynyl-2′-deoxyuridine (EdU; 10 μM) was added to pericyte cultures 24 h prior to completion of experiment. Cells were fixed in 4 % PFA for 15 min and EdU visualised as per manufacturer’s instructions (Life Technologies, CA, USA). Cell nuclei were counterstained with Hoechst 33258 as per immunocytochemistry.
Alamar Blue® viability assay
To determine cell viability, Alamar Blue® (1:10 final dilution in culture medium; Life Technologies, CA, USA) was added to cells in a 96-well plate for 2 h at 37 °C, 5 % CO2. Fluorescence (Ex 544/ Em 590) was read on a FLUOStar Optima plate reader (BMG Labtech, Germany). Background fluorescence of wells with media but no cells was subtracted from all values, and fluorescence intensity values were normalised to cell number using imaging of Hoechst positive cells. Fluorescence intensity values were then normalised to vehicle control.
LDH cytotoxicity assay
To determine cellular toxicity, extracellular lactate dehydrogenase (LDH) was detected as per manufacturer’s instructions (Roche, Switzerland). Briefly, a positive control was first prepared by lysing cells through addition of DMEM/F12 media containing 1 % Triton X-100. The culture media from all samples was then transferred to a new plate and centrifuged at 160×g for 10 min at room temperature. To a new 96-well plate, 50 μL of centrifuged media was transferred and 50 μL of LDH reaction mixture was added. The plate was incubated at room temperature for 30 min and absorbance measured at 540 nm on a FLUOStar Optima plate reader. Absorbance of culture media containing no cells was subtracted from all values and the absorbance normalised to that of the positive control (100 % cytotoxicity).
Quantitative real-time reverse transcriptase PCR
Cells destined for quantitative real-time reverse transcriptase PCR (qRT-PCR) were plated at 100,000 cells/well in a six-well plate. At completion of the experiment, cells were washed in PBS and RNA extraction, and purification was performed using the RNeasy® mini kit (Qiagen, Netherlands) as per manufacturer’s instructions from duplicate wells. RNA was treated with DNase I (1 μg DNase I/1 μg RNA) using the RQ1 RNase-free DNAse kit (Promega, WI, USA) and cDNA made using the Superscript® III First-Strand Synthesis kit (Life Technologies, CA, USA). Quantitative real-time PCR was performed using Platinum® SYBR® Green qPCR SuperMix-UDG with Rox (Life Technologies, CA, USA) on a 7900HT Fast Real-Time PCR system (Applied Biosystems, Life Technologies, CA, USA). Standard curves were run for all primers, and efficiencies were all 100 ± 10 % (Additional file 2: Table S2). Relative gene expression analysis was performed using the 2−ΔΔCt method to the housekeeping gene GAPDH.
Microarray
Microarray analysis of TGFβ1-treated pericytes was conducted on technical triplicates of a single patient donor. Cells destined for microarray were treated for 72 h with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1. Cells were washed in PBS and lysed in 1 mL of TRIzol reagent (Life Technologies, CA, USA). To this, 200 μL of chloroform was added, and samples were vigorously shaken and centrifuged at 12,000×g for 15 min at 4 °C. The upper aqueous phase was transferred to a new tube and an equal volume of 70 % ethanol added. RNA purification was performed using the RNeasy® mini kit as per qRT-PCR experiments. RNA quality was analysed using a 2100 Bioanalyzer (Agilent Technologies, CA, USA) with all samples having a RIN value of 10. RNA was labelled and hybridised to Affymetrix Genechip® Primeview™ Human Gene Expression Arrays (Santa Clara, CA, USA) according to manufacturer’s instructions. Microarray was performed and analysed by New Zealand Genomics Limited (NZGL). Candidate microarray hits for qRT-PCR and CBA validation were selected based on the magnitude of change and prior evidence for involvement in neuro-immune responses but do not include all modified inflammatory genes.
Statistical analysis
Unless otherwise stated, all experiments were performed at least three independent times on tissue from three different donors. Statistical analysis was carried out using an unpaired Student’s t test, one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test to compare treatments versus vehicle control, ANOVA with Newman-Keuls multiple comparison test to compare all conditions, or a two-way ANOVA to compare the effect of both time and cytokine stimulation (Graphpad Prism 5.02). For statistical analysis of qRT-PCR data, ΔCt values were used. Statistical analysis of microarray data was performed as described previously [37].
Results
TGFβ1 modifies inflammatory gene expression in human brain pericytes
The role of TGFβ1 in modifying pericyte phenotype and associated functional responses including scarring, proliferation and differentiation has been previously studied [43, 44]. However, its effect on pericyte-mediated immune responses has not been well characterised. Previous work from our lab has identified a potential anti-inflammatory role for TGFβ1 in pericytes through prevention of IFNγ-induced HLA-DR expression [27]. In order to further understand the inflammatory phenotype adopted by TGFβ1-stimulated brain pericytes, microarray analysis was undertaken. As expected, several inflammatory genes showed attenuated expression, including interleukin 8 (IL8), p = 8.06−4; CX3CL1, p = 3.09−6; monocyte chemoattractant protein 1 (MCP1), p = 2.98−6; and vascular cell adhesion molecule 1 (VCAM1), p = 5.57−7 (Fig. 1a). Surprisingly, however, a number of inflammatory-related genes were also found to be induced by TGFβ1 treatment, including nicotinamide adenine dinucleotide phosphate-oxidase 4 (NOX4), p = 1.12−6; cyclooxygenase 2 (COX2), p = 1.35−6; matrix metalloproteinase 2 (MMP2), p = 1.09−4; and interleukin 6 (IL6), p = 2.03−4 (Fig. 1a). These changes were confirmed by qRT-PCR with TGFβ1-induced up-regulation of NOX4 (p < 0.001; Fig. 1b), COX2 (p < 0.001; Fig. 1c), MMP2 (p < 0.05; Fig. 1d) and IL6 (p < 0.05; Fig. 1e) and attenuation of IL8 (p < 0.05; Fig. 1f), CX3CL1 (p < 0.01; Fig. 1g), MCP1 (p < 0.001; Fig. 1h) and VCAM1 (p < 0.001; Fig. 1i) at 72 h. Furthermore, several changes were apparent as early as 24 h after TGFβ1 treatment (NOX4, p < 0.001, Fig. 1b; COX2, p < 0.001, Fig. 1c; IL8, p < 0.01, Fig. 1f; MCP1, p < 0.001, Fig. 1h and VCAM1, p < 0.001, Fig. 1i).
Fig. 1.
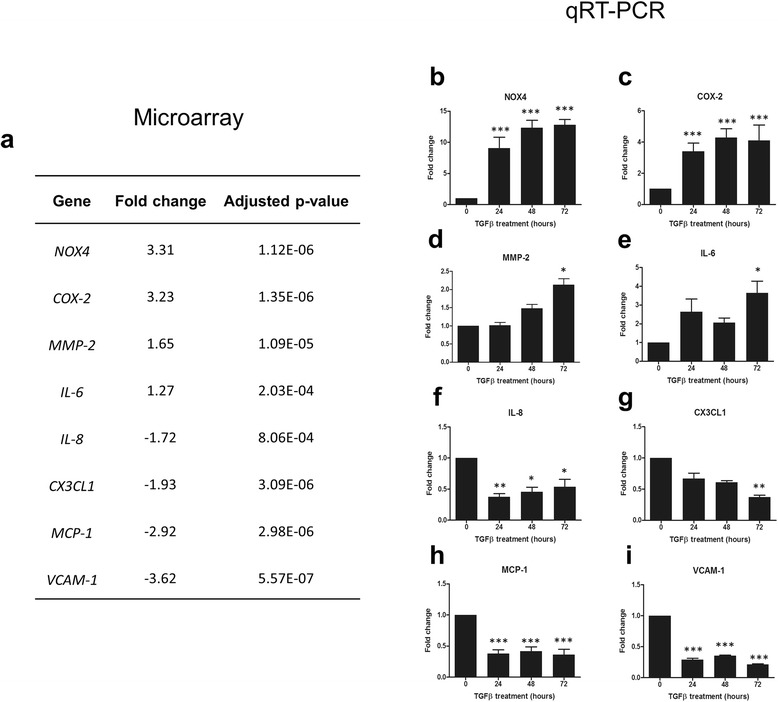
TGFβ1 modifies inflammatory gene expression in human brain pericytes. Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 72 h. RNA was extracted and expression of inflammation-related genes determined by microarray (a). Changes in gene expression (NOX4 (b), COX2 (c), MMP2 (d), IL6 (e), IL8 (f), CX3CL1 (g), MCP1 (h) and VCAM1 (i)) were confirmed by qRT-PCR from three independent experiments with 0–72 h treatments with 10 ng/mL TGFβ1. *p < 0.05, **p < 0.01, ***p < 0.001 versus 0 h time point (ANOVA)
TGFβ1 modifies inflammatory protein secretion in human brain pericytes
Having identified a role for TGFβ1 in modifying pericyte inflammatory gene expression, we sought to ascertain whether this translated to a functional change in protein levels. Several of the genes affected by TGFβ1 are secreted cytokines, chemokines or cleavable/secreted adhesion molecules. We therefore determined the concentration of these proteins in conditioned media from TGFβ1-treated pericytes. All tested inflammatory mediators were found to be secreted basally by pericytes and, consistent with microarray and qRT-PCR results, a 72-h treatment with TGFβ1 attenuated this secretion of soluble VCAM-1 (sVCAM-1; p < 0.001; Fig. 2a), CX3CL1 (p < 0.001; Fig. 2b) and MCP-1 (p < 0.001; Fig. 2c). Interestingly, the expression of IL-8 was found to be unchanged (p > 0.05, Fig. 2d). Furthermore, IL-6 secretion was significantly enhanced by TGFβ1 treatment (p < 0.001; Fig. 2e). Secretions of sVCAM-1 (p < 0.001; Fig. 2a) and CX3CL1 (p < 0.05; Fig. 2b) were significantly decreased after a 24-h treatment with TGFβ1, whilst MCP-1 attenuation (p < 0.001; Fig. 2c) and IL-6 induction (p < 0.001; Fig. 2e) was apparent at 48 h.
Fig. 2.
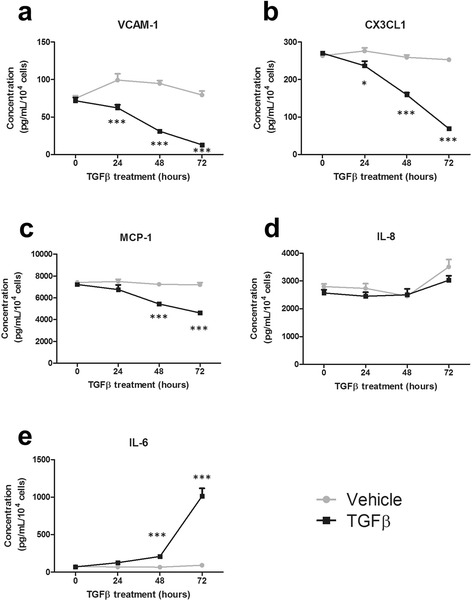
TGFβ1 modifies inflammatory protein secretion in human brain pericytes. Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 0–72 h, and conditioned media was collected. Concentration of secreted proteins (sVCAM-1 (a), CX3CL1 (b), MCP-1 (c), IL-8 (d) and IL-6 (e)) was determined by a multiplex cytometric bead array. One representative experiment (N = 4 wells) from three independent experiments is shown. *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle control at each time point (two-way ANOVA)
Synergistic and preventative effects of TGFβ1 on IL-1β-induced inflammatory protein secretion
As TGFβ1 appears to be a critical modifier of the pericyte immune phenotype, we sought to investigate its ability to attenuate or synergize with IL-1β, another pro-inflammatory cytokine. IL-1β alone significantly enhanced the basal secretion of sVCAM-1 (p < 0.001; Fig. 3a), CX3CL1 (p < 0.001; Fig. 3b), MCP-1 (p < 0.001; Fig. 3c), IL-8 (p < 0.001; Fig. 3d) and IL-6 (p < 0.001; Fig. 3e). TGFβ1 was found to significantly reduce IL-1β-induced sVCAM-1 (p < 0.001; Fig. 3a), CX3CL1 (p < 0.001; Fig. 3b) and MCP-1 (p < 0.001; Fig. 3c) secretions; however, it had no effect on IL-8 expression (p > 0.05, Fig. 3d). Furthermore, co-stimulation of pericytes with IL-1β and TGFβ1 showed a synergistic release of IL-6, well above that seen with IL-1β treatment alone (p < 0.001, Fig. 3e).
Fig. 3.
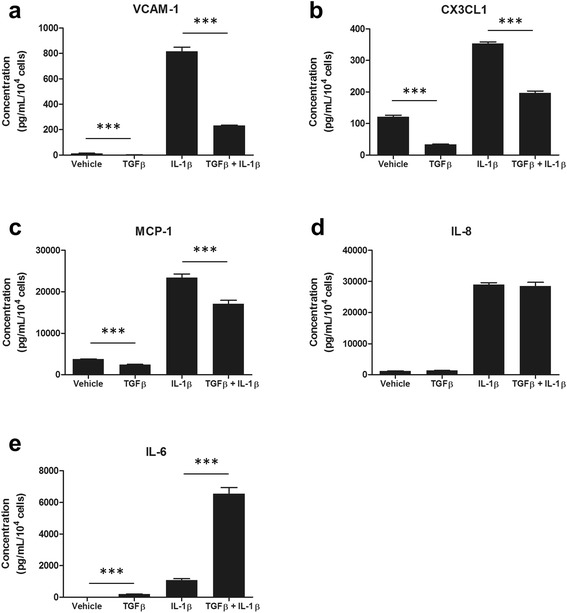
Synergistic and preventative effects of TGFβ1 on IL-1β-induced inflammatory protein secretion. Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 24 h followed by a further 24 h in the presence of 10 ng/mL IL-1β or vehicle (0.1 % BSA in PBS). Conditioned media was collected and the concentration of secreted proteins (sVCAM-1 (a), CX3CL1 (b), MCP-1 (c), IL-8 (d) and IL-6 (e)) was determined by a multiplex cytometric bead array. One representative experiment (N = 4 wells) from three independent experiments is shown. ***p < 0.001 (ANOVA)
TGFβ1 signals through SMAD2/3 transcription factors but does not affect NF-kB translocation
Inflammatory protein expression is largely controlled by transcription factor-mediated gene transcription. In order to further understand how TGFβ1 modifies the pericyte immune response, we investigated the ability of this cytokine to modify the localisation of two transcription factors, SMAD2/3 and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB). Whilst SMAD2/3 showed a low level of basal nuclear localisation, TGFβ1 treatment significantly enhanced this at 2 h (p < 0.001; Fig. 4a, b), and remained slightly elevated (albeit not significantly) for up to 48 h (p > 0.05; Fig. 4a, b). IL-1β was found to have no effect on SMAD2/3 nuclear localisation at 2, 24 or 48 h (p > 0.05) alone and did not alter TGFβ1-induced nuclear localisation at any time point (p > 0.05; Fig. 4a, b). IL-1β significantly enhanced nuclear translocation of NF-kB at 2 h (p < 0.001) but not 24 h (p > 0.05) or 48 h (p > 0.05), whilst TGFβ1 has no effect at any time point (p > 0.05; Fig. 4a, c). Furthermore, TGFβ1 did not modify IL-1β-induced NF-kB translocation at 2 h (p > 0.05), 24 h (p > 0.05) or 48 h (p > 0.05; Fig. 4a, c).
Fig. 4.
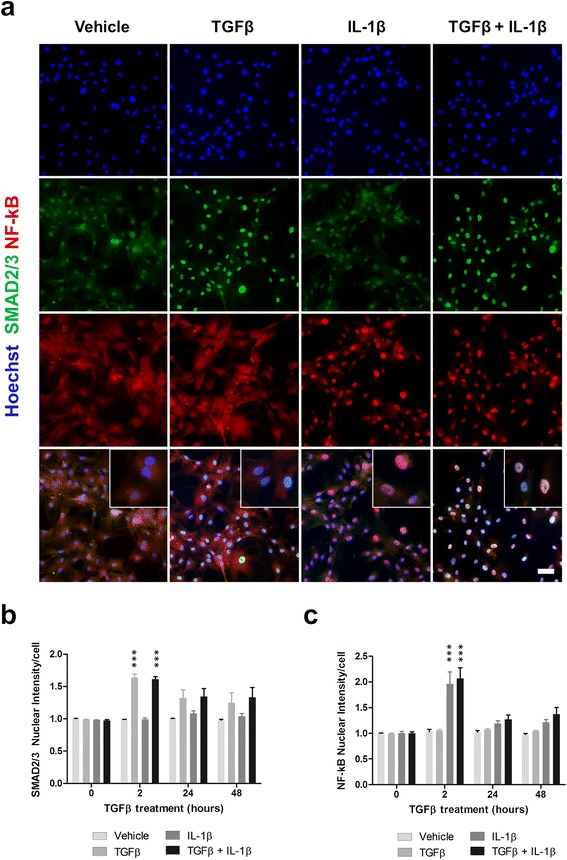
TGFβ1 signals through SMAD2/3 transcription factors but does not affect NF-kB translocation. Primary human brain pericytes were treated for 0–48 h with combinations of vehicle (1 mM citric acid, pH 3 with 0.1 % BSA), 10 ng/mL TGFβ1 and 10 ng/mL IL-1β. Cells were fixed and immunostained for NF-kB p65 and SMAD2/3. Representative images at 2 h are shown (a). The intensity of nuclear NF-kB (b) and nuclear SMAD2/3 (c) was determined by automated image analysis from four independent experiments. ***p < 0.001 versus vehicle control at each time point (two-way ANOVA). Scale bar = 50 μm
TGFβ1 attenuates phagocytosis by pericytes
Phagocytosis is a critical function of specific immune cells and is necessary to clear pathogenic substances from the brain [47]. Inflammatory cytokines, including TGFβ1, have previously been shown to modify the phagocytic ability of parenchymal brain cells, particularly microglia and astrocytes. Given the ability of TGFβ1 to modify pericyte inflammatory responses, we queried whether it could influence phagocytosis by these cells. TGFβ1 significantly attenuated pericyte phagocytosis of fluorescent latex beads at 1 ng/mL (p < 0.01) or 10 ng/mL (p < 0.001) as determined by an automated imaging-based assay (Fig. 5a, b). To confirm that beads were internalised and not simply bound to the cell membrane confocal microscopy was performed (Fig. 5c). A flow cytometry based assay was also employed to measure phagocytosis which again revealed a significant reduction in phagocytic ability by pericytes following a 10 ng/mL TGFβ1 treatment (p < 0.05, Fig. 5d, e) whilst lower concentrations trended towards a reduction.
Fig. 5.
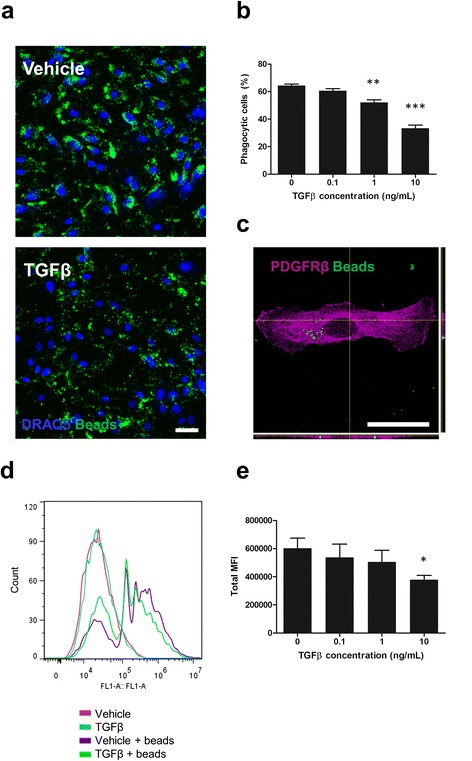
TGFβ1 attenuates phagocytosis by pericytes. Pericytes were treated for 24 h with 0–10 ng/mL TGFβ1 in 1 mM citric acid, pH 3 with 0.1 % BSA, followed by a further 24 h in the presence or absence of a 1:1000 dilution of fluorescent latex beads. Cells were fixed and nuclei counterstained with DRAQ5. Representative images for vehicle and 10 ng/mL TGFβ1 treatments are shown (a). The percentage of phagocytic pericytes was determined by automated image analysis from five independent experiments (b). Confocal microscopy of pericytes plated on glass coverslips, incubated for 24 h with a 1:10,000 dilution of fluorescent beads and immunostained with PDGFRβ confirmed internalisation of beads (c). Pericytes were treated for 24 h with 0–10 ng/mL TGFβ1 in 1 mM citric acid, pH 3 with 0.1 % BSA. For an additional two hours cells were incubated in the presence or absence of a 1:1000 dilution of fluorescent latex beads. Phagocytosis was determined by flow cytometry. One representative plot is shown (d) and the mean fluorescent intensity (MFI) of five independent experiments was determined (e). *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle control (ANOVA). Scale bar = 50 μm
TGFβ1 reduces gene expression of scavenger receptors
Scavenger receptors have a key role in recognising and coordinating the uptake of a wide range of macromolecules, including fluorescent beads and disease related proteins such as amyloid beta [48, 49]. As TGFβ1 was found to inhibit the phagocytic ability of pericytes, we investigated its ability to modify the expression of receptors involved in scavenging macromolecules. A 24-h treatment with TGFβ1 was found to significantly attenuate pericyte expression, as determined by qRT-PCR, of the receptors CD36 (p < 0.001, Fig. 6a), CD68 (p < 0.05, Fig. 6b) and CD47 (p < 0.05, Fig. 6c), highlighting a possible mechanism behind TGFβ1-reduced phagocytic ability.
Fig. 6.
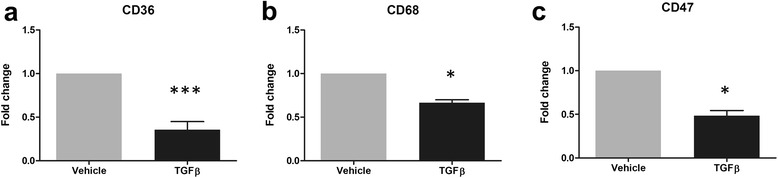
TGFβ1 reduces gene expression of scavenger receptors. Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 24 h. RNA was extracted and expression of CD36 (a), CD68 (b) and CD47 (c) and determined by qRT-PCR from three independent experiments. *p < 0.05, ***p < 0.001 versus vehicle control (Student’s t test)
TGFβ1 reduces pericyte proliferation but does not affect their viability
Functional responses of cells, including phagocytosis, can be influenced by general changes in cell viability. In order to exclude the possibility that TGFβ1 modifies phagocytosis through compromising pericyte viability, we undertook a range of assays with respect to examining cell health. TGFβ1 was unable to stimulate pericyte apoptosis, as determined by antibody labelling and imaging of the early apoptotic marker cleaved caspase 3 (CC3) at any time point (p > 0.05; Fig. 7a, b). Due to the low level of basal pericyte apoptosis, validation of the CC3 antibody was determined by okadaic acid-induced apoptosis (Fig. 7a, b). TGFβ1 treatment did result in a significant reduction of EdU positive cells, indicative of reduced cell proliferation, at 24, 48 and 72 h (p < 0.001; Fig. 7c, d). However, these proliferative changes resulted in only small, albeit significant (48 h, p < 0.01; 72 h, p < 0.001), reductions in cell number (Fig. 7e). Cellular cytotoxicity, as determined by LDH release, revealed no change with TGFβ1 treatment at any time point (p > 0.05; Fig. 7f) whilst an Alamar Blue® viability assay showed an increase at 24, 48 and 72 h (p < 0.001; Fig. 7g).
Fig. 7.
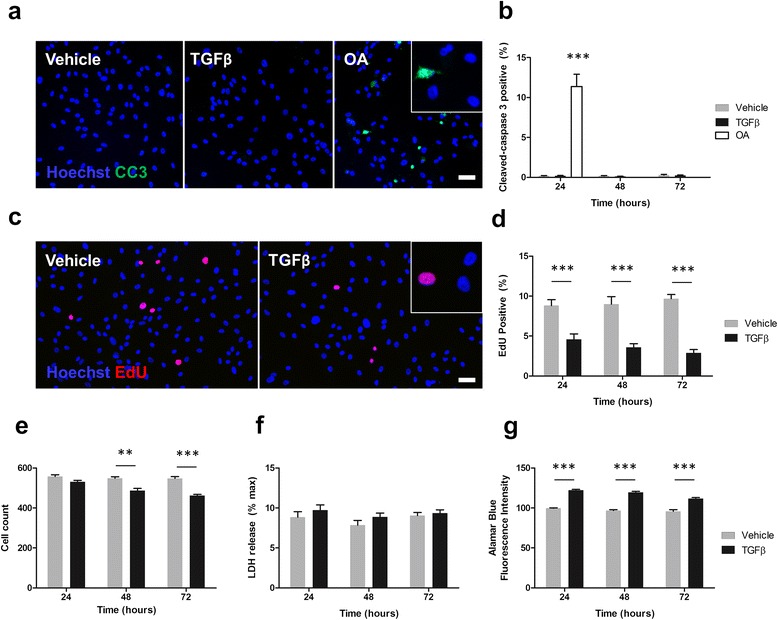
TGFβ1 decreases pericyte proliferation but does not affect their viability. Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 0–72 h. Cells were fixed and immunostained for cleaved caspase 3 (CC3). Nuclei were counterstained with Hoechst. Cells treated for 24 h with 50 nM okadaic acid (OA) were used as a positive control. Representative images from the 24 h time point are shown (a). The percentage of CC3 positive cells was determined by automated image analysis from three independent experiments (b). Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 0–72 h. For the final 24 h, cells were incubated with 10 μM EdU. Cells were fixed, EdU visualised and nuclei counterstained with Hoechst. Representative images from the 72-h time point are shown (c). The percentage of EdU positive cells (d), and the number of cells/field of view was determined by automated image analysis from three independent experiments (e). Primary human brain pericytes were treated with vehicle (1 mM citric acid, pH 3 with 0.1 % BSA) or 10 ng/mL TGFβ1 for 0–72 h. Cytotoxicity was determined from three independent experiments by an LDH release assay using cells lysed with DMEM/F12 containing 1 % Triton-X 100 as a positive control (f). Viability was determined from three independent experiments by a two hour treatment with Alamar Blue (g). *p < 0.05, **p < 0.01, ***p < 0.001 versus vehicle control for each time point (two-way ANOVA). Scale bar = 50 μm
Discussion
TGFβ1 is a pleotropic cytokine that has been widely studied with respect to CNS inflammatory responses. Due to a ubiquitous anti-inflammatory microglial phenotype in response to TGFβ1 stimulation, several studies have suggested utilisation of TGFβ1 in limiting brain inflammation [10, 26–28]. However, microglia are not the only immunologically active parenchymal brain cell, and therefore, investigating the effect of TGFβ1 treatment on other cell types is warranted. Several groups, including our own, have shown that human brain pericytes can produce inflammatory responses in vitro and that these responses do not necessarily mimic microglial responses [27, 50].
To investigate transcriptome-wide changes in inflammatory mediators, microarray analysis of human brain pericytes treated with TGFβ1 was first performed. Whilst several inflammatory mediators displayed an attenuated expression as expected, a number of inflammatory genes were also found to be up-regulated following TGFβ1 stimulation. Eight genes were chosen for qRT-PCR validation consisting of four up-regulated genes (IL6, MMP2, NOX4 and COX2) and four down-regulated genes (VCAM1, IL8, MCP1 and CX3CL1). As observed previously, qRT-PCR data correlated strongly with microarray analysis, with all genes examined showing consistent trends [37]. In order to confirm mRNA changes at the protein level, a cytometric bead array was employed to measure soluble and secreted concentrations of the aforementioned cytokines, chemokines and adhesion molecules. With the exception of IL-8, TGFβ1 was found to modify inflammatory mediator secretions in a manner consistent with microarray and qRT-PCR data. Furthermore, when co-stimulated with TGFβ1 and the pro-inflammatory cytokine IL-1β, IL-6 expression was synergistically induced whilst MCP-1, CX3CL1, VCAM-1 and MCP-1 all demonstrated reduced secretions.
Like TGFβ1, the function of IL-6 in the brain has been widely studied but is not well defined. In certain instances, IL-6 appears to act as a pro-inflammatory cytokine further enhancing CNS immune responses [51, 52]. Paradoxically, IL-6 also appears to have anti-inflammatory functions and can aid neuronal survival in the presence of inflammation [51, 53]. Similarly, COX-2 induction has been widely debated with regards to its inflammatory phenotype. Whilst initially perceived to have a solely pro-inflammatory function, many of the COX-2-derived prostaglandins can have anti-inflammatory effects in a cell- and context-dependent manner [54–56].
MMP-2 is a matrix metalloproteinase involved in the breakdown of extracellular matrix (ECM) proteins, including type IV collagen, a major constituent of the basement membrane [57]. MMP-2 expression in the vasculature promotes BBB breakdown through reduced basement membrane expression and endothelial tight junction deregulation, subsequently enhancing leukocyte infiltration [58–60]. MMP-2 also facilitates cancer cell invasion in glioblastoma multiforme, a brain tumour in which TGFβ1 is highly expressed [61–63]. Furthermore, MMP-2 can cleave non ECM substrates, including latent TGFβ1 which can promote an increased release of its active form, potentially creating a positive feedback loop [64]. TGFβ1-enhanced MMP-2 expression has been recently observed in brain pericytes, corroborating our data [65]. NOX4 is a member of the NADPH oxidase family and contributes to cellular superoxide production. NOX family members are highly expressed in professional phagocytic cells and reactive oxidative species (ROS) are crucial in the killing of micro-organisms [66]. However, NOX-derived ROS have also been implicated in arteriosclerosis, disturbance of vascular tone, disruption of the BBB and endothelial cell apoptosis through oxidative stress [67–69]. NOX4 induction is a characteristic feature in the vasculature, showing expression in the endothelium [70, 71], vascular smooth muscle cells [72, 73] and more recently human brain pericytes [74]. TGFβ1-mediated MMP-2 and NOX4 induction may therefore have a significant impact in the disruption of CNS vasculature following injury.
Cytometric bead array was also performed to examine soluble VCAM-1, MCP-1, IL-8 and CX3CL1 secretions. Whilst VCAM-1, MCP-1, and CX3CL1 all showed attenuated secretions with TGFβ1, the level of IL-8 was not significantly altered. A similar response has been observed previously in our lab whereby changes in IL-8 mRNA did not correlate with IL-8 secretions [42]. The reasons for this discrepancy are currently unclear.
VCAM-1 is an adhesion molecule expressed by cells of the vasculature, including endothelial cells and pericytes [40, 75]. Unlike the related adhesion molecule intracellular adhesion molecule 1 (ICAM-1), VCAM-1 was found to be expressed under basal conditions by human brain pericytes [42]. VCAM-1 aids the adhesion and subsequent transmigration of leukocytes across brain vasculature [76]. This interaction occurs via binding of very late antigen-4 (VLA-4), an integrin which undergoes conformational changes to allow VCAM-1 mediated adhesion after chemotactic activation [75–78]. Whilst typically implicated in endothelial-immune cell interactions, pericytes can also control immune cell adhesion and migration across the BBB [40, 79]. MCP-1 is a monocyte chemokine that attracts peripheral immune cells through a concentration-dependent gradient. Elevated MCP-1 expression is observed in the brain following an insult and chemoattracts parenchymal microglia as well as peripheral circulating leukocytes to the injured site, thereby contributing to a pro-inflammatory CNS phenotype [80–82]. Attenuated VCAM-1 and MCP-1 expression could therefore represent an anti-inflammatory function of TGFβ1 through limiting peripheral immune cell infiltration.
Fractalkine (CX3CL1) is a unique protein which functions as an adhesion molecule when membrane-bound, or a leukocyte chemokine in its soluble form [83–86]. Like MCP-1 and VCAM-1, attenuated expression of this mediator could thereby limit peripheral immune cell CNS entry. However, the effects of CX3CL1 are exerted specifically through the fractalkine receptor (CX3CR1) and whilst circulating leukocytes express this receptor [83, 87], in the brain, CX3CR1 expression is limited predominantly to microglia [88]. CX3CL1-CX3CR1 interactions in the CNS promote a strong anti-inflammatory microglial phenotype, whilst CX3CR1 knockout mice showed microglial-mediated neurotoxicity [88–90]. Fractalkine signalling therefore appears to be vital in controlling microglial inflammatory responses. CX3CL1 is believed to be expressed predominantly by neurons in the CNS [90], and to our knowledge, this is the first evidence of fractalkine secretion by brain pericytes and this highlights a possible role for these cells in modulating microglial inflammation. TGFβ1-attenuated CX3CL1 expression in pericytes may therefore deregulate microglial-mediated immune responses.
Modifications of cellular inflammatory responses are largely controlled through transcription factor-mediated gene transcription. Consistent with previous literature, TGFβ1 produced a pronounced induction of nuclear SMAD2/3 [91, 92]. However, it failed to induce nuclear translocation of NF-kB alone or modify the IL-1β-induced localisation of NF-kB. Previous studies, albeit in different cell types, report both TGFβ1-mediated NF-kB translocation or lack thereof [93, 94]. This discrepancy highlights the cell-specific effects of TGFβ1. Indeed, cell type-dependent responses with TGFβ1 have been observed previously in our lab with respect to astrocyte and microglial inflammatory responses [27]. Surprisingly, although TGFβ1-induced SMAD2/3 nuclear localisation was largely absent after 24 h, the induction of several inflammatory markers approached their peak 72 h after TGFβ1 treatment. It is therefore unlikely that SMAD2/3-modified gene transcription was directly involved in stimulating certain TGFβ1-induced inflammatory changes. Instead, it is possible that the early induction of select markers, particularly NOX4, could influence the expression of other inflammatory proteins. Indeed, pericyte-derived NOX4 has been previously implicated in BBB breakdown through enhanced neuroinflammatory responses, including MMP-9 induction [95]. Whilst it is currently unclear precisely how TGFβ1 modifies pericyte inflammatory responses, it appears that it is not through the prototypical inflammatory transcription factor NF-kB. Further studies examining a role for SMAD transcription factors in pericyte immune responses are therefore required
Aside from their role in expressing inflammatory mediators, brain pericytes also possess phagocytic ability with respect to latex beads and other particulate matter [41, 96]. Using confocal microscopy, we confirmed that our pericyte cultures were capable of phagocytosis in vitro and this function was able to be quantitatively determined by microscopy- and flow cytometry-based assays. TGFβ1 was found to significantly reduce the phagocytic uptake of latex beads in a concentration-dependent fashion. Interestingly, enhanced expression of TGFβ1 in the brain significantly elevates fibrillar amyloid beta deposition, exclusively in the vasculature [29, 97]. Our results suggest that TGFβ1-attenuated phagocytic function could contribute to the increased vasculature-associated amyloid. Due to their phagocytic ability and anatomical location, it is possible that pericytes have a key role in clearing pathogenic substances from the brain parenchyma. Indeed, pericytes have recently been shown to internalise amyloid beta, whilst their loss accelerates cerebral amyloid angiopathy [98]. Despite showing phagocytic capability, it is important to note that their rate of uptake was notably lower than that of microglia, the brains resident macrophage [99].
Whilst it is currently unclear precisely how TGFβ1 reduced pericyte phagocytic function, it was found to decrease the expression of the scavenger receptors CD36, CD47 and CD68. These receptors recognise a range of macromolecules, including latex beads and amyloid beta, and stimulate their internalisation [100, 101]. Inhibition of CD36 and CD47 can prevent phagocytic function [48, 102, 103] whilst TGFβ1 has previously been shown to attenuate CD36 expression in macrophages [104, 105].
As well as specific receptor involvement, alterations in phagocytosis may be achieved simply by modifying cell viability. Due to the range of inflammatory mediators induced by TGFβ1, this was certainly a possibility. Indeed, TGFβ1-induced NOX4 has been shown to precipitate endothelial cell apoptosis through oxidative stress [106]. In order to exclude a generalised loss of pericyte viability in this phagocytic response, we examined the effect of TGFβ1 on pericyte health. Whilst a decrease in cell number was observed with TGFβ1 treatment, this was explained by a reduction in pericyte proliferation and not apoptotic or necrotic death or alterations in viability.
Aside from pericytes, several other cells of the neurovasculature unit, including microglia [25], astrocytes [11] and endothelial cells [106], contain TGFβR’s and may therefore contribute to TGFβ1-induced immune responses. Whilst TGFβ1 has been previously studied with respect to in vivo neurovasculature function, the ability of numerous cell types to respond to this growth factor compromises the ability to study cell type-specific functions, particularly secreted cytokines, chemokines and ROS production. Indeed in differing in vivo models, TGFβ1 has been shown to exacerbate BBB permeability through MMP-9 induction [107] as well as preventing BBB breakdown through MMP9 suppression [108]. Whilst understanding the role of TGFβ1 in each cell type is vital in understanding whole-organism changes, it should be emphasised that isolated pericyte cultures lack the paracrine signalling with other parenchymal brain cells present in vivo, and the functional outcomes of TGFβ1 expression in the neurovasculature could differ as a result.
Conclusions
TGFβ1 attenuated the expression of key chemokines and adhesion molecules involved in CNS leukocyte trafficking and control of microglial function, as well as reducing their phagocytic ability. However, it also enhanced the expression of classical pro-inflammatory cytokines and enzymes which can disrupt BBB function. Together, these data suggest that TGFβ1 induction following brain injury stimulates a unique phenotype in brain pericytes which is neither specifically pro- nor anti-inflammatory. The functional in vivo outcome of TGFβ1-stimulation on brain pericytes is therefore difficult to predict. However, the reduction in pericyte proliferation, combined with elevated IL-6, MMP-2 and NOX4 expression, as well as attenuated phagocytic functioning, suggests a detrimental action of TGFβ1 on the neurovasculature.
Acknowledgements
We would like to thank the donors for their generous gift of brain tissue for research. We also thank the staff at Auckland Hospital (Lynair Roberts, Marcia Greenaway) as well as Inna Semenyajenko and Marika Eszes, Research Technicians at the Hugh Green Biobank and Neurological Foundation of New Zealand Human Brain Bank, respectively. This work was supported by a Programme Grant from the Health Research Council of New Zealand, the Hugh Green Foundation and the Neurological Foundation of New Zealand.
Additional files
List of antibodies used for immunocytochemistry. List of antibodies, suppliers and dilutions used for immunocytochemistry studies. (16.9 kb)
List of primers used for qRT-PCR. List of primer sequences and amplicon sizes for qRT-PCR studies. (15.7 kb)
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JR wrote the manuscript. JR, TIHP and MD designed the experiments. JR performed the majority of experiments. MA performed the microarray experiment. ELS assisted with flow cytometry and confocal microscopy. RLO, PSB, EWM, ESG, RLMF, MAC and MD contributed materials and relevant expertise as well as assisted in the manuscript preparation. All authors have read and approved the final manuscript.
References
- 1.Kleen JK, Holmes GL. Brain inflammation initiates seizures. Nat Med. 2008;14(12):1309–10. doi: 10.1038/nm1208-1309. [DOI] [PubMed] [Google Scholar]
- 2.Morganti-Kossmann MC, Rancan M, Stahel PF, Kossmann T. Inflammatory response in acute traumatic brain injury: a double-edged sword. Curr Opin Crit Care. 2002;8(2):101–5. doi: 10.1097/00075198-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 3.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10(1):95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–34. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:480739. doi: 10.1155/2013/480739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tesseur I, Zou K, Esposito L, Bard F, Berber E, Can JV, et al. Deficiency in neuronal TGF-beta signaling promotes neurodegeneration and Alzheimer’s pathology. J Clin Invest. 2006;116(11):3060–9. doi: 10.1172/JCI27341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A. Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci. 1994;6(3):355–63. doi: 10.1111/j.1460-9568.1994.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 8.Lindholm D, Castren E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-beta 1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117(2):395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falk S, Wurdak H, Ittner LM, Ille F, Sumara G, Schmid MT, et al. Brain area-specific effect of TGF-beta signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell. 2008;2(5):472–83. doi: 10.1016/j.stem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Paglinawan R, Malipiero U, Schlapbach R, Frei K, Reith W, Fontana A. TGFbeta directs gene expression of activated microglia to an anti-inflammatory phenotype strongly focusing on chemokine genes and cell migratory genes. Glia. 2003;44(3):219–31. doi: 10.1002/glia.10286. [DOI] [PubMed] [Google Scholar]
- 11.Benveniste EN, Kwon J, Chung WJ, Sampson J, Pandya K, Tang LP. Differential modulation of astrocyte cytokine gene expression by TGF-beta. J Immunol. 1994;153(11):5210–21. [PubMed] [Google Scholar]
- 12.Hurwitz AA, Lyman WD, Berman JW. Tumor necrosis factor alpha and transforming growth factor beta upregulate astrocyte expression of monocyte chemoattractant protein-1. J Neuroimmunol. 1995;57(1-2):193–8. doi: 10.1016/0165-5728(95)00011-P. [DOI] [PubMed] [Google Scholar]
- 13.Moustakas A, Souchelnytskyi S, Heldin CH. Smad regulation in TGF-beta signal transduction. J Cell Sci. 2001;114(Pt 24):4359–69. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, Tsuboi R, Lyons R, Moses H, Rifkin DB. Characterization of the activation of latent TGF-beta by co-cultures of endothelial cells and pericytes or smooth muscle cells: a self-regulating system. J Cell Biol. 1990;111(2):757–63. doi: 10.1083/jcb.111.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonelli-Orlidge A, Saunders KB, Smith SR, D’Amore PA. An activated form of transforming growth factor beta is produced by cocultures of endothelial cells and pericytes. Proc Natl Acad Sci U S A. 1989;86(12):4544–8. doi: 10.1073/pnas.86.12.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morgan TE, Nichols NR, Pasinetti GM, Finch CE. TGF-beta 1 mRNA increases in macrophage/microglial cells of the hippocampus in response to deafferentation and kainic acid-induced neurodegeneration. Exp Neurol. 1993;120(2):291–301. doi: 10.1006/exnr.1993.1063. [DOI] [PubMed] [Google Scholar]
- 17.McNeill H, Williams C, Guan J, Dragunow M, Lawlor P, Sirimanne E, et al. Neuronal rescue with transforming growth factor-[beta] 1 after hypoxic-ischaemic brain injury. Neuroreport. 1994;5(8):901–4. doi: 10.1097/00001756-199404000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Merrilees MJ, Sodek J. Synthesis of TGF-beta 1 by vascular endothelial cells is correlated with cell spreading. J Vasc Res. 1992;29(5):376–84. doi: 10.1159/000158954. [DOI] [PubMed] [Google Scholar]
- 19.Kaminska B, Kocyk M, Kijewska M. TGF beta signaling and its role in glioma pathogenesis. Adv Exp Med Biol. 2013;986:171–87. doi: 10.1007/978-94-007-4719-7_9. [DOI] [PubMed] [Google Scholar]
- 20.Wesolowska A, Kwiatkowska A, Slomnicki L, Dembinski M, Master A, Sliwa M, et al. Microglia-derived TGF-beta as an important regulator of glioblastoma invasion—an inhibition of TGF-beta-dependent effects by shRNA against human TGF-beta type II receptor. Oncogene. 2008;27(7):918–30. doi: 10.1038/sj.onc.1210683. [DOI] [PubMed] [Google Scholar]
- 21.Krupinski J, Kumar P, Kumar S, Kaluza J. Increased expression of TGF-beta 1 in brain tissue after ischemic stroke in humans. Stroke. 1996;27(5):852–7. doi: 10.1161/01.STR.27.5.852. [DOI] [PubMed] [Google Scholar]
- 22.Zorena K, Malinowska E, Raczynska D, Mysliwiec M, Raczynska K. Serum concentrations of transforming growth factor-Beta 1 in predicting the occurrence of diabetic retinopathy in juvenile patients with type 1 diabetes mellitus. J Diabetes Res. 2013;2013:614908. doi: 10.1155/2013/614908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao CC, Hu S, Frey WH, 2nd, Ala TA, Tourtellotte WW, Peterson PK. Transforming growth factor beta in Alzheimer’s disease. Clin Diagn Lab Immunol. 1994;1(1):109–10. doi: 10.1128/cdli.1.1.109-110.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chao CC, Ala TA, Hu S, Crossley KB, Sherman RE, Peterson PK, et al. Serum cytokine levels in patients with Alzheimer’s disease. Clin Diagn Lab Immunol. 1994;1(4):433–6. doi: 10.1128/cdli.1.4.433-436.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76(2):77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Lodge PA, Sriram S. Regulation of microglial activation by TGF-beta, IL-10, and CSF-1. J Leukoc Biol. 1996;60(4):502–8. doi: 10.1002/jlb.60.4.502. [DOI] [PubMed] [Google Scholar]
- 27.Smith AM, Graham ES, Feng SX, Oldfield RL, Bergin PM, Mee EW, et al. Adult human glia, pericytes and meningeal fibroblasts respond similarly to IFNy but not to TGFbeta1 or M-CSF. PLoS One. 2013;8(12) doi: 10.1371/journal.pone.0080463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40(6):1133–45. doi: 10.1016/S0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- 29.Wyss-Coray T, Lin C, Yan F, Yu GQ, Rohde M, McConlogue L, et al. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat Med. 2001;7(5):612–8. doi: 10.1038/87945. [DOI] [PubMed] [Google Scholar]
- 30.Cekanaviciute E, Dietrich HK, Axtell RC, Williams AM, Egusquiza R, Wai KM, et al. Astrocytic TGF-beta signaling limits inflammation and reduces neuronal damage during central nervous system toxoplasma infection. J Immunol. 2014;193(1):139–49. doi: 10.4049/jimmunol.1303284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wyss-Coray T, Lin C, Sanan DA, Mucke L, Masliah E. Chronic overproduction of transforming growth factor-beta1 by astrocytes promotes Alzheimer’s disease-like microvascular degeneration in transgenic mice. Am J Pathol. 2000;156(1):139–50. doi: 10.1016/S0002-9440(10)64713-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragunow M, Feng S, Rustenhoven J, Curtis M, Faull R. Studying human brain inflammation in leptomeningeal and choroid plexus explant cultures. Neurochemi Res. 2015;1–10. [DOI] [PubMed]
- 33.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75(3):388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 34.Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468(7323):562–6. doi: 10.1038/nature09513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Armulik A, Genove G, Mae M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468(7323):557–61. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 37.Jansson D, Rustenhoven J, Feng S, Hurley D, Oldfield RL, Bergin PS, et al. A role for human brain pericytes in neuroinflammation. J Neuroinflammation. 2014;11(1):104. doi: 10.1186/1742-2094-11-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pieper C, Marek JJ, Unterberg M, Schwerdtle T, Galla HJ. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014;1550:1-8. [DOI] [PubMed]
- 39.Kovac A, Erickson MA, Banks WA. Brain microvascular pericytes are immunoactive in culture: cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J Neuroinflammation. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guijarro-Munoz I, Compte M, Alvarez-Cienfuegos A, Alvarez-Vallina L, Sanz L. Lipopolysaccharide activates TLR4-mediated NF-kappaB signaling pathway and proinflammatory response in human pericytes. J Biol Chem. 2013. [DOI] [PMC free article] [PubMed]
- 41.Balabanov R, Washington R, Wagnerova J, Dore-Duffy P. CNS microvascular pericytes express macrophage-like function, cell surface integrin alpha M, and macrophage marker ED-2. Microvasc Res. 1996;52(2):127–42. doi: 10.1006/mvre.1996.0049. [DOI] [PubMed] [Google Scholar]
- 42.Rustenhoven J, Scotter EL, Jansson D, Kho DT, Oldfield RL, Bergin PS, et al. An anti-inflammatory role for C/EBPdelta in human brain pericytes. Sci Rep. 2015;5:12132. doi: 10.1038/srep12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CF, Chiang WC, Lai CF, Chang FC, Chen YT, Chou YH, et al. Transforming growth factor beta-1 stimulates profibrotic epithelial signaling to activate pericyte-myofibroblast transition in obstructive kidney fibrosis. Am J Pathol. 2013;182(1):118–31. doi: 10.1016/j.ajpath.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sieczkiewicz GJ, Herman IM. TGF-beta 1 signaling controls retinal pericyte contractile protein expression. Microvasc Res. 2003;66(3):190–6. doi: 10.1016/S0026-2862(03)00055-4. [DOI] [PubMed] [Google Scholar]
- 45.Gibbons HM, Hughes SM, Van Roon-Mom W, Greenwood JM, Narayan PJ, Teoh HH, et al. Cellular composition of human glial cultures from adult biopsy brain tissue. J Neurosci Methods. 2007;166(1):89–98. doi: 10.1016/j.jneumeth.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 46.O’Carroll SJ, Kho DT, Wiltshire R, Nelson V, Rotimi O, Johnson R, et al. Pro-inflammatory TNFα and IL-1β differentially regulate the inflammatory phenotype of brain microvascular endothelial cells. J Neuroinflammation. 2015;12(1):131. doi: 10.1186/s12974-015-0346-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sokolowski JD, Mandell JW. Phagocytic clearance in neurodegeneration. Am J Pathol. 2011;178(4):1416–28. doi: 10.1016/j.ajpath.2010.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones RS, Minogue AM, Connor TJ, Lynch MA. Amyloid-β-induced astrocytic phagocytosis is mediated by CD36, CD47 and RAGE. J Neuroimmune Pharmacol. 2013;8(1):301–11. doi: 10.1007/s11481-012-9427-3. [DOI] [PubMed] [Google Scholar]
- 49.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid β-protein via a scavenger receptor. Neuron. 1996;17(3):553–65. doi: 10.1016/S0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 50.Matsumoto J, Takata F, Machida T, Takahashi H, Soejima Y, Funakoshi M et al. Tumor necrosis factor-alpha-stimulated brain pericytes possess a unique cytokine and chemokine release profile and enhance microglial activation. Neurosci Lett. 2014;578:133-8 . [DOI] [PubMed]
- 51.Gadient RA, Otten UH. Interleukin-6 (IL-6)—a molecule with both beneficial and destructive potentials. Prog Neurobiol. 1997;52(5):379–90. doi: 10.1016/S0301-0082(97)00021-X. [DOI] [PubMed] [Google Scholar]
- 52.Penkowa M, Moos T, Carrasco J, Hadberg H, Molinero A, Bluethmann H, et al. Strongly compromised inflammatory response to brain injury in interleukin-6-deficient mice. Glia. 1999;25(4):343–57. doi: 10.1002/(SICI)1098-1136(19990215)25:4<343::AID-GLIA4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 53.Hama T, Miyamoto M, Tsukui H, Nishio C, Hatanaka H. Interleukin-6 as a neurotrophic factor for promoting the survival of cultured basal forebrain cholinergic neurons from postnatal rats. Neurosci Lett. 1989;104(3):340–4. doi: 10.1016/0304-3940(89)90600-9. [DOI] [PubMed] [Google Scholar]
- 54.Tzeng SF, Hsiao HY, Mak OT. Prostaglandins and cyclooxygenases in glial cells during brain inflammation. Curr Drug Targets Inflamm Allergy. 2005;4(3):335–40. doi: 10.2174/1568010054022051. [DOI] [PubMed] [Google Scholar]
- 55.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Minghetti L. Cyclooxygenase-2 (COX-2) in inflammatory and degenerative brain diseases. J Neuropathol Exp Neurol. 2004;63(9):901–10. doi: 10.1093/jnen/63.9.901. [DOI] [PubMed] [Google Scholar]
- 57.Liabakk N-B, Talbot I, Smith RA, Wilkinson K, Balkwill F. Matrix metalloprotease 2 (MMP-2) and matrix metalloprotease 9 (MMP-9) type IV collagenases in colorectal cancer. Cancer Res. 1996;56(1):190–6. [PubMed] [Google Scholar]
- 58.Liu J, Jin X, Liu KJ, Liu W. Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci. 2012;32(9):3044–57. doi: 10.1523/JNEUROSCI.6409-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song J, Wu C, Korpos E, Zhang X, Agrawal SM, Wang Y, et al. Focal MMP-2 and MMP-9 activity at the blood-brain barrier promotes chemokine-induced leukocyte migration. Cell Rep. 2015;10(7):1040–54. doi: 10.1016/j.celrep.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 60.Dal-Pizzol F, Rojas HA, dos Santos EM, Vuolo F, Constantino L, Feier G, et al. Matrix metalloproteinase-2 and metalloproteinase-9 activities are associated with blood-brain barrier dysfunction in an animal model of severe sepsis. Mol Neurobiol. 2013;48(1):62–70. doi: 10.1007/s12035-013-8433-7. [DOI] [PubMed] [Google Scholar]
- 61.Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-β. J Neurooncol. 2001;53(2):177–85. doi: 10.1023/A:1012209518843. [DOI] [PubMed] [Google Scholar]
- 62.Forsyth P, Wong H, Laing T, Rewcastle N, Morris D, Muzik H, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79(11-12):1828. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Joseph JV, Balasubramaniyan V, Walenkamp A, Kruyt FA. TGF-β as a therapeutic target in high grade gliomas—promises and challenges. Biochem Pharmacol. 2013;85(4):478–85. doi: 10.1016/j.bcp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 64.Wang M, Zhao D, Spinetti G, Zhang J, Jiang LQ, Pintus G, et al. Matrix metalloproteinase 2 activation of transforming growth factor-beta1 (TGF-beta1) and TGF-beta1-type II receptor signaling within the aged arterial wall. Arterioscler Thromb Vasc Biol. 2006;26(7):1503–9. doi: 10.1161/01.ATV.0000225777.58488.f2. [DOI] [PubMed] [Google Scholar]
- 65.Takahashi Y, Maki T, Liang AC, Itoh K, Lok J, Osumi N, et al. p38 MAP kinase mediates transforming-growth factor-beta1-induced upregulation of matrix metalloproteinase-9 but not -2 in human brain pericytes. Brain Res. 2014;1593:1–8. doi: 10.1016/j.brainres.2014.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87(1):245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 67.Sorescu D, Weiss D, Lassègue B, Clempus RE, Szöcs K, Sorescu GP, et al. Superoxide production and expression of nox family proteins in human atherosclerosis. Circulation. 2002;105(12):1429–35. doi: 10.1161/01.CIR.0000012917.74432.66. [DOI] [PubMed] [Google Scholar]
- 68.Kahles T, Luedike P, Endres M, Galla H-J, Steinmetz H, Busse R, et al. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38(11):3000–6. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- 69.Basuroy S, Bhattacharya S, Leffler CW, Parfenova H. Nox4 NADPH oxidase mediates oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol. 2009;296(3):C422–32. doi: 10.1152/ajpcell.00381.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, et al. Nox4 as the major catalytic component of an endothelial NAD (P) H oxidase. Circulation. 2004;109(2):227–33. doi: 10.1161/01.CIR.0000105680.92873.70. [DOI] [PubMed] [Google Scholar]
- 71.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol. 2007;27(11):2319–24. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 72.Hilenski LL, Clempus RE, Quinn MT, Lambeth JD, Griendling KK. Distinct subcellular localizations of Nox1 and Nox4 in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2004;24(4):677–83. doi: 10.1161/01.ATV.0000112024.13727.2c. [DOI] [PubMed] [Google Scholar]
- 73.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, et al. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2007;27(1):42–8. doi: 10.1161/01.ATV.0000251500.94478.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuroda J, Ago T, Nishimura A, Nakamura K, Matsuo R, Wakisaka Y, et al. Nox4 is a major source of superoxide production in human brain pericytes. J Vasc Res. 2014;51(6):429–38. doi: 10.1159/000369930. [DOI] [PubMed] [Google Scholar]
- 75.Elices MJ, Osborn L, Takada Y, Crouse C, Luhowskyj S, Hemler ME, et al. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990;60(4):577–84. doi: 10.1016/0092-8674(90)90661-W. [DOI] [PubMed] [Google Scholar]
- 76.Greenwood J, Wang Y, Calder V. Lymphocyte adhesion and transendothelial migration in the central nervous system: the role of LFA-1, ICAM-1, VLA-4 and VCAM-1. Immunology. 1995;86(3):408. [PMC free article] [PubMed] [Google Scholar]
- 77.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–89. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 78.Chigaev A, Waller A, Zwartz GJ, Buranda T, Sklar LA. Regulation of cell adhesion by affinity and conformational unbending of α4β1 integrin. J Immunol. 2007;178(11):6828–39. doi: 10.4049/jimmunol.178.11.6828. [DOI] [PubMed] [Google Scholar]
- 79.Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, et al. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and ‘instruct’ them with pattern-recognition and motility programs. Nat Immunol. 2013;14(1):41–51. doi: 10.1038/ni.2477. [DOI] [PubMed] [Google Scholar]
- 80.Stamatovic SM, Shakui P, Keep RF, Moore BB, Kunkel SL, Van Rooijen N, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25(5):593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]
- 81.Sagar D, Lamontagne A, Foss CA, Khan ZK, Pomper MG, Jain P. Dendritic cell CNS recruitment correlates with disease severity in EAE via CCL2 chemotaxis at the blood-brain barrier through paracellular transmigration and ERK activation. J Neuroinflammation. 2012;9:245. doi: 10.1186/1742-2094-9-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hinojosa AE, Garcia-Bueno B, Leza JC, Madrigal JL. CCL2/MCP-1 modulation of microglial activation and proliferation. J Neuroinflammation. 2011;8:77. doi: 10.1186/1742-2094-8-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, et al. Identification and molecular characterization of fractalkine receptor CX 3 CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91(4):521–30. doi: 10.1016/S0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]
- 84.Umehara H, Goda S, Imai T, Nagano Y, Minami Y, Tanaka Y, et al. Fractalkine, a CX3C-chemokine, functions predominantly as an adhesion molecule in monocytic cell line THP-1. Immunol Cell Biol. 2001;79(3):298–302. doi: 10.1046/j.1440-1711.2001.01004.x. [DOI] [PubMed] [Google Scholar]
- 85.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo J-A, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387(6633):611–6. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 86.Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–4. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 87.Fong AM, Robinson LA, Steeber DA, Tedder TF, Yoshie O, Imai T, et al. Fractalkine and CX3CR1 mediate a novel mechanism of leukocyte capture, firm adhesion, and activation under physiologic flow. J Exp Med. 1998;188(8):1413–9. doi: 10.1084/jem.188.8.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9(7):917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 89.Sheridan GK, Murphy KJ. Neuron–glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3(12):130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, et al. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429(2):167–72. doi: 10.1016/S0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 91.Town T, Laouar Y, Pittenger C, Mori T, Szekely CA, Tan J, et al. Blocking TGF-β–Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat Med. 2008;14(6):681–7. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Papetti M, Shujath J, Riley KN, Herman IM. FGF-2 antagonizes the TGF-beta1-mediated induction of pericyte alpha-smooth muscle actin expression: a role for myf-5 and Smad-mediated signaling pathways. Invest Ophthalmol Vis Sci. 2003;44(11):4994–5005. [DOI] [PubMed]
- 93.Hsieh H-L, Wang H-H, Wu W-B, Chu P-J, Yang C-M. Transforming growth factor-b1 induces matrix metalloproteinase-9 and cell migration in astrocytes: roles of ROS-dependent ERK-and JNK-NF-kB pathways. J Neuroinflammation. 2010;7:88. doi: 10.1186/1742-2094-7-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Obata H, Biro S, Arima N, Kaieda H, Kihara T, Eto H, et al. NF-κB is induced in the nuclei of cultured rat aortic smooth muscle cells by stimulation of various growth factors. Biochem Biophys Res Commun. 1996;224(1):27–32. doi: 10.1006/bbrc.1996.0979. [DOI] [PubMed] [Google Scholar]
- 95.Nishimura A, Ago T, Kuroda J, Arimura K, Tachibana M, Nakamura K et al. Detrimental role of pericyte Nox4 in the acute phase of brain ischemia. J Cereb Blood Flow Metab. 2015;0271678X15606456. [DOI] [PMC free article] [PubMed]
- 96.Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Rev. 1999;31(1):42–57. doi: 10.1016/S0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 97.Wyss-Coray T, Masliah E, Mallory M, McConlogue L, Johnson-Wood K, Lin C, et al. Amyloidogenic role of cytokine TGF-β1 in transgenic mice and in Alzheimer’s disease. Nature. 1997;389(6651):603–6. doi: 10.1038/39321. [DOI] [PubMed] [Google Scholar]
- 98.Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, et al. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun. 2013;4. [DOI] [PMC free article] [PubMed] [Retracted]
- 99.Rustenhoven J, Park TI, Schweder P, Scotter J, Correia J, Smith AM, et al. Isolation of highly enriched primary human microglia for functional studies. Sci Rep. 2016;6:19371. doi: 10.1038/srep19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Husemann J, Loike JD, Anankov R, Febbraio M, Silverstein SC. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 101.Gough PJ, Gordon S. The role of scavenger receptors in the innate immune system. Microbes Infect. 2000;2(3):305–11. doi: 10.1016/S1286-4579(00)00297-5. [DOI] [PubMed] [Google Scholar]
- 102.Koenigsknecht J, Landreth G. Microglial phagocytosis of fibrillar β-amyloid through a β1 integrin-dependent mechanism. J Neurosci. 2004;24(44):9838–46. doi: 10.1523/JNEUROSCI.2557-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bamberger ME, Harris ME, McDonald DR, Husemann J, Landreth GE. A cell surface receptor complex for fibrillar β-amyloid mediates microglial activation. J Neurosci. 2003;23(7):2665–74. doi: 10.1523/JNEUROSCI.23-07-02665.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Draude G, Lorenz RL. TGF-β1 downregulates CD36 and scavenger receptor A but upregulates LOX-1 in human macrophages. Am J Physiol Heart Circ Physiol. 2000;278(4):H1042–8. doi: 10.1152/ajpheart.2000.278.4.H1042. [DOI] [PubMed] [Google Scholar]
- 105.Han J, Hajjar DP, Tauras JM, Feng J, Gotto AM, Nicholson AC. Transforming growth factor-β1 (TGF-β1) and TGF-β2 decrease expression of CD36, the type B scavenger receptor, through mitogen-activated protein kinase phosphorylation of peroxisome proliferator-activated receptor-γ. J Biol Chem. 2000;275(2):1241–6. doi: 10.1074/jbc.275.2.1241. [DOI] [PubMed] [Google Scholar]
- 106.Yan F, Wang Y, Wu X, Peshavariya HM, Dusting GJ, Zhang M, et al. Nox4 and redox signaling mediate TGF-beta-induced endothelial cell apoptosis and phenotypic switch. Cell Death Dis. 2014;5 doi: 10.1038/cddis.2013.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McMillin MA, Frampton GA, Seiwell AP, Patel NS, Jacobs AN, DeMorrow S. TGFβ1 exacerbates blood–brain barrier permeability in a mouse model of hepatic encephalopathy via upregulation of MMP9 and downregulation of claudin-5. Lab Investig. 2015. [DOI] [PMC free article] [PubMed]
- 108.Cai Y, Liu X, Chen W, Wang Z, Xu G, Zeng Y, et al. TGF-β1 prevents blood–brain barrier damage and hemorrhagic transformation after thrombolysis in rats. Exp Neurol. 2015;266:120–6. doi: 10.1016/j.expneurol.2015.02.013. [DOI] [PubMed] [Google Scholar]


