Abstract
Signaling pathways regulated by the receptor CDCP1 play central roles in promoting cancer and in mediating resistance to chemo- and targeted-therapies. In this perspective we briefly summarize these findings as well as data demonstrating poorer outcomes for several malignancies that exhibit elevated CDCP1 expression. Promising data from preclinical studies suggest that CDCP1 targeted agents, including therapeutic antibodies, could be useful in the treatment of cancer patients selected on the basis of activation of CDCP1 and its signaling partners including EGFR, HER2, Met and Src.
Keywords: CDCP1, EGFR, HER2, Met, Src, antibody
INTRODUCTION
Over the last decade the membrane spanning glycoprotein CUB Domain Containing Protein 1 (CDCP1) has emerged as a potential therapeutic target for several cancers [1-3]. Consistent with roles in malignant progression its elevated expression correlates with poor outcome in malignancies of the kidney [4, 5], lung [6], colon and rectum [7], pancreas [8] and ovary [9]. Similarly there is accumulating evidence that CDCP1 is important in processes generic to cancer progression such as cell survival, migration and anchorage-independent growth, and that it is critical for dissemination of cancers that spread via vascular and transcoelomic routes [1-3]. Its potential as a target is supported by reports that five anti-CDCP1 antibodies, or antibody fragments, have demonstrated the ability, in in vivo models, to inhibit tumor growth or metastasis [10-13], with one of these antibodies successfully tested in a breast cancer tumor regression model [12].
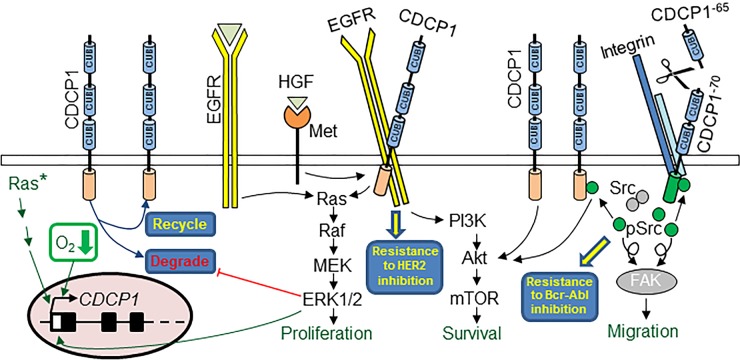
As highlighted in Figure 1, an emerging feature of CDCP1 biology is its intersection with pathways that are critical for cancer progression, and that dual targeting of CDCP1 and these other pathways could be beneficial against a range of malignancies. For example, EGF signalling, via EGFR to RAS/RAF/MEK/ERK, simultaneously stimulates CDCP1 expression and promotes CDCP1-mediated in vitro migration of ovarian cancer cells [14]. It has also been shown that EGF activation of EGFR further accentuates CDCP1-induced pro-cancer effects by inhibiting proteasome-mediated, palmitoylation-dependent constitutive degradation of CDCP1. This promotes recycling of CDCP1 to the cell surface, increasing its physical availability to mediate increased cell migration and, potentially, as a target for therapeutic antibodies [15]. CDCP1-mediated pro-cancer effects can also occur via ligand/receptor-independent activation of pathways downstream of cell surface EGFR family members. For example, mutations in the most frequently activated oncogenes, the Ras gene family, activate RAF/MEK/ERK which robustly induce CDCP1 mRNA and protein expression in non-small cell lung cancer (NSCLC) cells [16]. At least in NSCLC cells, p-Y-CDCP1 is required for Ras oncogenic functions including cell migration, invasion, colony formation in soft agar and resistance to anoikis in vitro [16]. Signaling via CDCP1, potentially via growth factor receptor axes, can be intensified under hypoxic conditions, a common microenvironment for cancer cells that also contributes to chemoresistance. In clear cell renal cell carcinoma (ccRCC) cells under hypoxic conditions, hypoxia-inducible factor (HIF)-2α induces the expression and activation of CDCP1, which relays pro-invasion and pro-tumor growth signals via PKCδ [5, 17]. These effects are potentially via the hypoxia-regulated and known HIF-2α targets EGFR and the HGF receptor, Met [5]. Induction of CDCP1 by HIF-2α under hypoxic conditions has also been demonstrated in hepatocellular carcinoma where elevated CDCP1 expression also correlates with poor patient survival [18].
Figure 1. CDCP1-mediated and targetable pathways in cancer.
CDCP1 promotes cancer cell proliferation (generally under non-adherent conditions), survival and migration, and mediates resistance to chemo- and targeted-therapies. CDCP1 expression at the cell surface is increased by ligand activation of EGFR signaling, constitutively activated mutant Ras (Ras*), and hypoxia (O2↓). Scissors represent arginine/lysine specific serine proteases that cleave and activate CDCP1.
Recent literature indicates that CDCP1 may also be a key mediator of resistance to therapies targeting EGFR family members. It has recently been shown that Met mediates resistance to the EGFR targeted therapies gefitinib and cetuximab [19]. HGF induced signalling efficiently confers this resistance in squamous cell carcinoma cells, with analysis of HGF-induced EGFR binding partners identifying direct interaction between EGFR and CDCP1 [19]. Although the role of EGFR/CDCP1 binding in resistance was not directly tested, interactions between these proteins were impervious to EGFR inhibition [19]. Consistent with a role in resistance to therapy, doxorubicin-induced apoptosis of prostate cancer cells in vitro is disrupted by CDCP1, with antibody blockade of CDCP1 restoring chemosensitivity [10]. Antibody targeting of CDCP1 can also markedly improve responsiveness to chemotherapy in vivo, as demonstrated for an intraperitoneal mouse xenograft of a clear cell ovarian carcinoma cell line treated with carboplatin [9]. Another powerful example of the role of CDCP1 in mediating poor response to therapy involves breast cancer resistance to the HER2 targeted therapeutic antibody trastuzumab. A quantitative proteomics screen comparing the phospho-tyrosine (p-Y) content of trastuzumab-sensitive and -resistant breast cancer cell lines identified 12 fold higher levels of p-Y-CDCP1 in resistant cells [20]. Confirming the role of CDCP1 in resistance in this model, siRNA mediated silencing of CDCP1 restored cell line responsiveness in vitro to trastuzumab [20]. Further supporting that CDCP1 is important in breast cancer resistance to trastuzumab, a more recent study demonstrated that CDCP1 and HER2 are co-overexpressed in 12% of primary and 30% of metastatic breast tumors, and that co-expression correlates with poorest disease free and overall survival [21]. Functionally, co-expression markedly increased orthotopic xenograft tumor growth in mice, and also drastically accentuated activation of both HER2 and HER3 in vitro as well as ligand-independent HER2 homodimerization, and heterodimerization of HER2/HER3 and HER2/EGFR [21]. Mechanistically the role of CDCP1 in recruiting Src to the plasma membrane appears to be a key contributor to trastuzumab resistance. CDCP1 binds directly to HER2 which is required for Src-mediated phosphorylation of HER2-Y877 and -Y1248 and EGFR-Y1068, as well as reciprocal HER2 phosphorylation of Src [21]. Importantly, both in vitro and in vivo the CDCP1 enhanced Src-HER2 interaction drove breast cancer trastuzumab resistance, with dual targeting of HER2 and Src able to overcome resistance in a mouse model [21]. The data provide a rationale for development of CDCP1-targeting agents that can be used in combination with anti-HER2 therapies for tumors double positive for HER2 and CDCP1 [21].
Interestingly, CDCP1-mediated activation of Src can be initiated by other distinct events. CDCP1 is synthesized as a 135 kDa transmembrane protein (CDCP1−135) that in cell lines in vitro and in vivo, and in human tumors, can be proteolytically cleaved producing a 70 kDa cell retained fragment (CDCP1−70), and two 65 kDa cell released fragments (CDCP1−65) [9, 22, 23]. While the roles of the CDCP1−65 fragments are unknown, both CDCP1−70 and CDCP1−135 interact with Src to transduce signals to a range of other proteins, generally via activation of PKCδ. In settings where cancer cells are required to survive without the support of a matrix, including growth in suspension, growth as spheroids or during dissemination, Src-dependent and -independent pathways activate PI3K/Akt signaling to promote cell survival and movement [8, 24–28]. For example, in a mouse intraperitoneal model of ovarian cancer CDCP1 pro-survival signalling to PI3K/Akt is Src independent [9], whereas in animal models of vascular metastasis, proteolytic cleavage of CDCP1 induces Src phosphorylation of CDCP1−70 which complexes with activated β1-integrin to signal via Src to FAK-PI3K-Akt to suppress PARP-1-induced apoptosis [10, 22, 29]. It is apparent that in these, and potentially other oncogenic settings, CDCP1 is the major substrate of Src family kinases (SFKs) and that it actively competes for Src with other mediators of pro-cancer phenotypes such as FAK [30]. There is evidence that interactions between CDCP1 and SFKs can also mediate resistance to therapy. A recent study demonstrated that CDCP1 is increased ∼9 fold in chronic myeloid leukemia cells that are resistant to the selective Bcr-Abl kinase inhibitor nilotinib, and that silencing of CDCP1 markedly improved responses to nilotinib [31].
In summary, CDCP1 has important and targetable roles in cancer. The growing literature demonstrating that CDCP1 expression is often elevated in cancer and that this overexpression correlates with poor outcome for various malignancies, sets the challenge for the development of effective CDCP1 targeted therapies. In particular, such agents have potential, in carefully selected patient cohorts, to improve responsiveness to therapies that target EGFR family members and Src family kinases, and to delay or even circumvent development of resistance to these drugs.
Footnotes
CONFLICTS OF INTEREST
JDH is an inventor on a patent describing CDCP1 as an anti-cancer target. All other authors declare no conflict of interest.
REFERENCES
- 1.Karachaliou N, Rosell R, Molina MA, Viteri S. Predicting resistance by selection of signaling pathways. Transl Lung Cancer Res. 2014;3:107–115. doi: 10.3978/j.issn.2218-6751.2014.02.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Uekita T, Sakai R. Roles of CUB domain-containing protein 1 signaling in cancer invasion and metastasis. Cancer Sci. 2011;102:1943–1948. doi: 10.1111/j.1349-7006.2011.02052.x. [DOI] [PubMed] [Google Scholar]
- 3.Wortmann A, He Y, Deryugina EI, Quigley JP, Hooper JD. The cell surface glycoprotein CDCP1 in cancer—insights, opportunities, and challenges. IUBMB Life. 2009;61:723–730. doi: 10.1002/iub.198. [DOI] [PubMed] [Google Scholar]
- 4.Awakura Y, Nakamura E, Takahashi T, Kotani H, Mikami Y, Kadowaki T, Myoumoto A, Akiyama H, Ito N, Kamoto T, Manabe T, Nobumasa H, Tsujimoto G, Ogawa O. Microarray-based identification of CUB-domain containing protein 1 as a potential prognostic marker in conventional renal cell carcinoma. J Cancer Res Clin Oncol. 2008;134:1363–1369. doi: 10.1007/s00432-008-0412-4. [DOI] [PubMed] [Google Scholar]
- 5.Emerling BM, Benes CH, Poulogiannis G, Bell EL, Courtney K, Liu H, Choo-Wing R, Bellinger G, Tsukazawa KS, Brown V, Signoretti S, Soltoff SP, Cantley LC. Identification of CDCP1 as a hypoxia-inducible factor 2alpha (HIF-2alpha) target gene that is associated with survival in clear cell renal cell carcinoma patients. Proc Natl Acad Sci U S A. 2013;110:3483–3488. doi: 10.1073/pnas.1222435110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda J, Oda T, Inoue M, Uekita T, Sakai R, Okumura M, Aozasa K, Morii E. Expression of CUB domain containing protein (CDCP1) is correlated with prognosis and survival of patients with adenocarcinoma of lung. Cancer Sci. 2009;100:429–433. doi: 10.1111/j.1349-7006.2008.01066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gao W, Chen L, Ma Z, Du Z, Zhao Z, Hu Z, Li Q. Isolation and phenotypic characterization of colorectal cancer stem cells with organ-specific metastatic potential. Gastroenterology. 2013;145:636–646. e635. doi: 10.1053/j.gastro.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 8.Miyazawa Y, Uekita T, Hiraoka N, Fujii S, Kosuge T, Kanai Y, Nojima Y, Sakai R. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010;70:5136–5146. doi: 10.1158/0008-5472.CAN-10-0220. [DOI] [PubMed] [Google Scholar]
- 9.He Y, Wu AC, Harrington BS, Davies CM, Wallace SJ, Adams MN, Palmer JS, Roche DK, Hollier BG, Westbrook TF, Hamidi H, Konecny GE, Winterhoff B, Chetty NP, Crandon AJ, Oliveira NB, et al. Elevated CDCP1 predicts poor patient outcome and mediates ovarian clear cell carcinoma by promoting tumor spheroid formation, cell migration and chemoresistance. Oncogene. 2016;35(4):468–78. doi: 10.1038/onc.2015.101. [DOI] [PubMed] [Google Scholar]
- 10.Deryugina EI, Conn EM, Wortmann A, Partridge JJ, Kupriyanova TA, Ardi VC, Hooper JD, Quigley JP. Functional role of cell surface CUB domain-containing protein 1 in tumor cell dissemination. Mol Cancer Res. 2009;7:1197–1211. doi: 10.1158/1541-7786.MCR-09-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukuchi K, Steiniger SC, Deryugina E, Liu Y, Lowery CA, Gloeckner C, Zhou B, Kaufmann GF, Quigley JP, Ja KD. Inhibition of tumor metastasis: functional immune modulation of the CUB domain containing protein 1. Mol Pharm. 2010;7:245–253. doi: 10.1021/mp900236t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollmorgen G, Niederfellner G, Lifke A, Spohn GJ, Rieder N, Harring SV, Bauss F, Burtscher H, Lammers R, Bossenmaier B. Antibody mediated CDCP1 degradation as mode of action for cancer targeted therapy. Mol Oncol. 2013;7:1142–1151. doi: 10.1016/j.molonc.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siva AC, Wild MA, Kirkland RE, Nolan MJ, Lin B, Maruyama T, Yantiri-Wernimont F, Frederickson S, Bowdish KS, Xin H. Targeting CUB domain-containing protein 1 with a monoclonal antibody inhibits metastasis in a prostate cancer model. Cancer Res. 2008;68:3759–3766. doi: 10.1158/0008-5472.CAN-07-1657. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, He Y, de Boer L, Stack MS, Lumley JW, Clements JA, Hooper JD. The cell surface glycoprotein CUB domain-containing protein 1 (CDCP1) contributes to epidermal growth factor receptor-mediated cell migration. J Biol Chem. 2012;287:9792–9803. doi: 10.1074/jbc.M111.335448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adams MN, Harrington BS, He Y, Davies CM, Wallace SJ, Chetty NP, Crandon AJ, Oliveira NB, Shannon CM, Coward JI, Lumley JW, Perrin LC, Armes JE, Hooper JD. EGF inhibits constitutive internalization and palmitoylation-dependent degradation of membrane-spanning procancer CDCP1 promoting its availability on the cell surface. Oncogene. 2015;34:1375–1383. doi: 10.1038/onc.2014.88. [DOI] [PubMed] [Google Scholar]
- 16.Uekita T, Fujii S, Miyazawa Y, Iwakawa R, Narisawa-Saito M, Nakashima K, Tsuta K, Tsuda H, Kiyono T, Yokota J, Sakai R. Oncogenic Ras/ERK signaling activates CDCP1 to promote tumor invasion and metastasis. Mol Cancer Res. 2014;12:1449–1459. doi: 10.1158/1541-7786.MCR-13-0587. [DOI] [PubMed] [Google Scholar]
- 17.Razorenova OV, Finger EC, Colavitti R, Chernikova SB, Boiko AD, Chan CK, Krieg A, Bedogni B, LaGory E, Weissman IL, Broome-Powell M, Giaccia AJ. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKC{delta}- driven migration. Proc Natl Acad Sci U S A. 2011;108:1931–1936. doi: 10.1073/pnas.1011777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao M, Gao J, Zhou H, Huang J, You A, Guo Z, Fang F, Zhang W, Song T, Zhang T. HIF-2alpha regulates CDCP1 to promote PKCdelta-mediated migration in hepatocellular carcinoma. Tumour Biol. 2015 doi: 10.1007/s13277-015-3527-7. [DOI] [PubMed] [Google Scholar]
- 19.Gusenbauer S, Vlaicu P, Ullrich A. HGF induces novel EGFR functions involved in resistance formation to tyrosine kinase inhibitors. Oncogene. 2013;32:3846–3856. doi: 10.1038/onc.2012.396. [DOI] [PubMed] [Google Scholar]
- 20.Boyer AP, Collier TS, Vidavsky I, Bose R. Quantitative proteomics with siRNA screening identifies novel mechanisms of trastuzumab resistance in HER2 amplified breast cancers. Mol Cell Proteomics. 2013;12:180–193. doi: 10.1074/mcp.M112.020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alajati A, Guccini I, Pinton S, Garcia-Escudero R, Bernasocchi T, Sarti M, Montani E, Rinaldi A, Montemurro F, Catapano C, Bertoni F, Alimonti A. Interaction of CDCP1 with HER2 enhances HER2-driven tumorigenesis and promotes trastuzumab resistance in breast cancer. Cell Rep. 2015;11:564–576. doi: 10.1016/j.celrep.2015.03.044. [DOI] [PubMed] [Google Scholar]
- 22.Casar B, He Y, Iconomou M, Hooper JD, Quigley JP, Deryugina EI. Blocking of CDCP1 cleavage in vivo prevents Akt-dependent survival and inhibits metastatic colonization through PARP1-mediated apoptosis of cancer cells. Oncogene. 2012;31:3924–3938. doi: 10.1038/onc.2011.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Wortmann A, Burke LJ, Reid JC, Adams MN, Abdul- Jabbar I, Quigley JP, Leduc R, Kirchhofer D, Hooper JD. Proteolysis-induced N-terminal ectodomain shedding of the integral membrane glycoprotein CUB domain-containing protein 1 (CDCP1) is accompanied by tyrosine phosphorylation of its C-terminal domain and recruitment of Src and PKCdelta. J Biol Chem. 2010;285:26162–26173. doi: 10.1074/jbc.M109.096453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kollmorgen G, Bossenmaier B, Niederfellner G, Haring HU, Lammers R. Structural requirements for cub domain containing protein 1 (CDCP1) and Src dependent cell transformation. PLoS One. 2012;7:e53050. doi: 10.1371/journal.pone.0053050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu H, Ong SE, Badu-Nkansah K, Schindler J, White FM, Hynes RO. CUB-domain-containing protein 1 (CDCP1) activates Src to promote melanoma metastasis. Proc Natl Acad Sci U S A. 2011;108:1379–1384. doi: 10.1073/pnas.1017228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spassov DS, Wong CH, Moasser MM. Trask phosphorylation defines the reverse mode of a phosphotyrosine signaling switch that underlies cell anchorage state. Cell Cycle. 2011;10:1225–1232. doi: 10.4161/cc.10.8.15343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uekita T, Jia L, Narisawa-Saito M, Yokota J, Kiyono T, Sakai R. CUB domain-containing protein 1 is a novel regulator of anoikis resistance in lung adenocarcinoma. Mol Cell Biol. 2007;27:7649–7660. doi: 10.1128/MCB.01246-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uekita T, Tanaka M, Takigahira M, Miyazawa Y, Nakanishi Y, Kanai Y, Yanagihara K, Sakai R. CUB-domain-containing protein 1 regulates peritoneal dissemination of gastric scirrhous carcinoma. Am J Pathol. 2008;172:1729–1739. doi: 10.2353/ajpath.2008.070981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casar B, Rimann I, Kato H, Shattil SJ, Quigley JP, Deryugina EI. In vivo cleaved CDCP1 promotes early tumor dissemination via complexing with activated beta1 integrin and induction of FAK/PI3K/Akt motility signaling. Oncogene. 2014;33(2):255–268. doi: 10.1038/onc.2012.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wortmann A, He Y, Christensen ME, Linn M, Lumley JW, Pollock PM, Waterhouse NJ, Hooper JD. Cellular settings mediating Src Substrate switching between focal adhesion kinase tyrosine 861 and CUB-domain-containing protein 1 (CDCP1) tyrosine 734. J Biol Chem. 2011;286:42303–42315. doi: 10.1074/jbc.M111.227462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gioia R, Leroy C, Drullion C, Lagarde V, Etienne G, Dulucq S, Lippert E, Roche S, Mahon FX, Pasquet JM. Quantitative phosphoproteomics revealed interplay between Syk and Lyn in the resistance to nilotinib in chronic myeloid leukemia cells. Blood. 2011;118:2211–2221. doi: 10.1182/blood-2010-10-313692. [DOI] [PubMed] [Google Scholar]