Abstract
Chikungunya virus (CHIKV) was recently introduced into the Americas. In Nicaragua, the first endogenous transmission of CHIKV was recognized in September 2014. We used an ongoing dengue cohort study of children aged 2–14 years in Managua, Nicaragua, to document the attack rate of symptomatic chikungunya in a presumably naive population. From September 2014 through March 2015, the overall clinical attack rate of laboratory-confirmed CHIKV infection was 2.9% (95% confidence interval [CI]: 2.3%, 3.4%). The attack rate was greater in children ≥ 8 years of age (4.1%; 95% CI: 3.2%, 5.1%) than in those < 8 years of age (1.5%; 95% CI: 0.9%, 2.1%). The mean age of CHIKV cases presenting with typical chikungunya symptoms was 9.8 years, compared with 7.8 years for cases presenting with undifferentiated fever (P = 0.04). Our data suggest that the clinical attack rate in children may underestimate the true burden of disease as some children, especially young children, may experience more atypical symptoms (e.g., undifferentiated fever).
Chikungunya is a viral disease transmitted by Aedes aegypti and Aedes albopictus mosquitoes. Since its reemergence in 2004, chikungunya virus (CHIKV) has caused outbreaks in the Indian Ocean region, India, southeast Asia, the Pacific region, and, to a lesser extent, Europe.1–4 In December 2013, CHIKV was reported in the Caribbean island of St. Martin. Since then, over 1,955,000 chikungunya cases have been reported by the Pan American Health Organization, and most countries in the Americas are reporting autochthonous transmission of CHIKV.5 In Nicaragua, the first imported case was detected in July 2014 and the first autochthonous case in September 2014.
Symptoms of acute chikungunya include abrupt onset of high fever, arthralgia/arthritis, rash, back pain, headache, and myalgia. Clinically, the acute phases of chikungunya and dengue may be very similar, but, unlike dengue, chikungunya is rarely life threatening. Further, chikungunya is characterized by chronic joint pain that can last for weeks to years following acute infection.6 Fewer studies have examined clinical features of CHIKV infection in children than in adults; however, they support that children likely have a different clinical presentation than adults.7 A serosurvey in Mayotte found that children aged 2–14 years experienced more asymptomatic infections than adults.1,8 In addition, several studies have demonstrated severe manifestations of chikungunya predominately in children under 2 years of age.9–12
Here, we describe the attack rate of symptomatic CHIKV infection in children in our pediatric dengue and chikungunya cohort study in Managua, Nicaragua, during the first epidemic of chikungunya in the country.
The Pediatric Dengue Cohort Study (PDCS) began in August 2004 to study the incidence and natural history of dengue and dengue virus (DENV) infection.13 In 2014, we included CHIKV testing in the PDCS to study the emergence of chikungunya. The PDCS consists of ~3,500 children in Managua. Healthy children are eligible to participate in the study if they are 2–14 years of age, live in the area of District II served by the Health Center Sócrates Flores Vivas, and they and their guardian complete informed consent and assent procedures. Children are provided with medical care through the study, and data from all medical appointments are collected systematically. In addition, an annual healthy blood sample is collected from participants, and socioeconomic and risk factor surveys are completed in March/April. New 2-year-old children are enrolled each year, and children are withdrawn when they reach 15 years of age. Vector control is not instituted as a part of the PDCS; rather, children in the cohort receive vector control services from the government, as does the rest of Managua. The study was approved by the institutional review boards at the Nicaraguan Ministry of Health, the University of California, Berkeley, and the University of Michigan.
All children in the cohort meeting the testing criteria for chikungunya and/or dengue have an acute and a convalescent (days 14–21) blood sample collected. The testing criteria for both diseases are 1) fever or feverishness with ≥ 2 of the following symptoms: headache, muscle ache, joint pain, retro-orbital pain, rash, hemorrhagic manifestations, or leukopenia or 2) undifferentiated fever. Acute blood samples are tested for CHIKV and DENV by reverse transcription polymerase chain reaction (RT-PCR) using established protocols.14,15 RT-PCR is performed on fresh serum, or, when testing is delayed, serum is stored at −80°C until tested. RT-PCR for CHIKV was included in the dengue cohort in November 2014, and previously collected samples were tested retrospectively. A child is considered positive for chikungunya if CHIKV RNA is detected by RT-PCR. Positivity for DENV was determined as previously described.16
The primary outcome measure examined in this study is the clinical attack rate of chikungunya. Cohort participants included in the study of the clinical attack rate were limited to those who participated during the entire 7-month period of the first chikungunya epidemic. Wilcoxon rank-sum tests or a nonparametric test for trend were used to determine P values, as appropriate. Principal components analysis was used to create a wealth index to categorize participants into three quantiles: very poor, poor, and not poor. The wealth index was constructed using the following dichotomous variables: car, motorcycle, and/or home ownership, indoor water tap, non-dirt floor, use of a wood stove, and overcrowding, and the following continuous variables: number of fans, televisions, and/or refrigerators/freezers owned. All data were analyzed in STATA version 12.1 (Stata Corp., College Station, TX).
During the 7-month period between September 1, 2014, and March 31, 2015, 3,745 children participated in the cohort study, of which 3,377 (90.2%) were enrolled for the entire period. Children ranged in age from 2 to 14 years, with a roughly equal distribution among all 1-year age groups. Of the 368 children who did not participate for the entire period, 139 were 2-year-old children enrolled after September 2014, 131 were 15-year-old children who were withdrawn from the study, and 98 were lost to follow-up. Of the 3,377 children, 3,353 (99.3%) had an annual survey completed in 2014. Demographic characteristics are presented in Table 1.
Table 1.
Clinical attack rate of chikungunya according to demographic characteristics, Managua, Nicaragua, 2014–2015
| N (%) | Clinical attack rate (%) | 95% CI | |
|---|---|---|---|
| All participants | 3,377 | 2.9 | 2.3%, 3.4% |
| By sex | |||
| Female | 1,699 (50.3) | 2.6 | 1.8%, 3.6% |
| Male | 1,678 (49.7) | 3.1 | 2.3%, 3.9% |
| By age (years) | |||
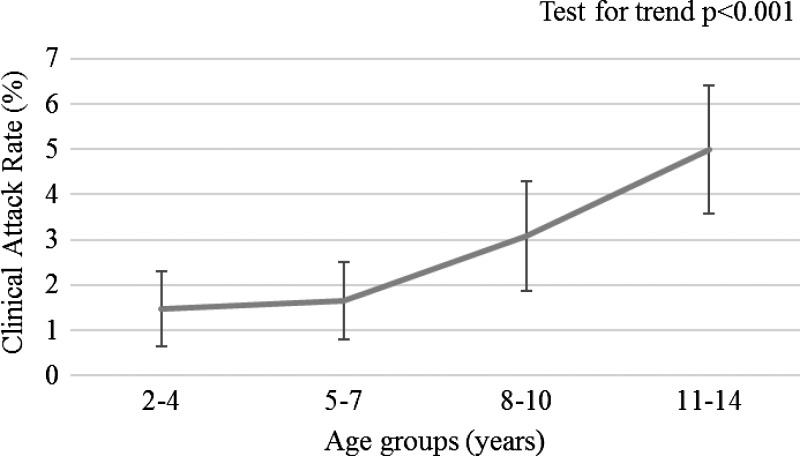
| 2–4 | 825 (24.4) | 1.5 | 0.6%, 2.3% |
| 5–7 | 848 (25.1) | 1.7 | 0.8%, 2.5% |
| 8–10 | 783 (23.2) | 3.1 | 1.9%, 4.3% |
| 11–14 | 921 (27.3) | 5.0 | 3.6%, 6.4% |
| By water tap | |||
| Not inside | 1,342 (40.2) | 3.2 | 2.3%, 4.3% |
| Inside | 2,011 (59.6) | 2.6 | 1.9%, 3.3% |
| By floor | |||
| Dirt floor | 364 (10.9) | 3.0 | 2.4%, 3.6% |
| Other floor | 2,989 (89.1) | 1.9 | 0.5%, 3.3% |
| By wealth index | |||
| Very poor | 1,326 (39.6) | 3.3 | 2.3%, 4.2% |
| Poor | 1,224 (36.5) | 2.7 | 1.8%, 3.6% |
| Not poor | 803 (24.0) | 2.5 | 1.4%, 3.6% |
CI = confidence interval.
A total of 96 RT-PCR-confirmed cases of chikungunya were detected among the 3,377 children. The first case of chikungunya in the cohort was detected the same month as the first reported autochthonous case in Nicaragua. One potential CHIKV–DENV coinfection was detected that was RT-PCR positive for CHIKV and showed a ≥ 4-fold increase in anti-DENV antibody titer between acute and convalescent blood samples. Of the 96 chikungunya cases, 85 (88.5%) met the study definition of a probable case of chikungunya and 11 (11.5%) presented with undifferentiated fever.
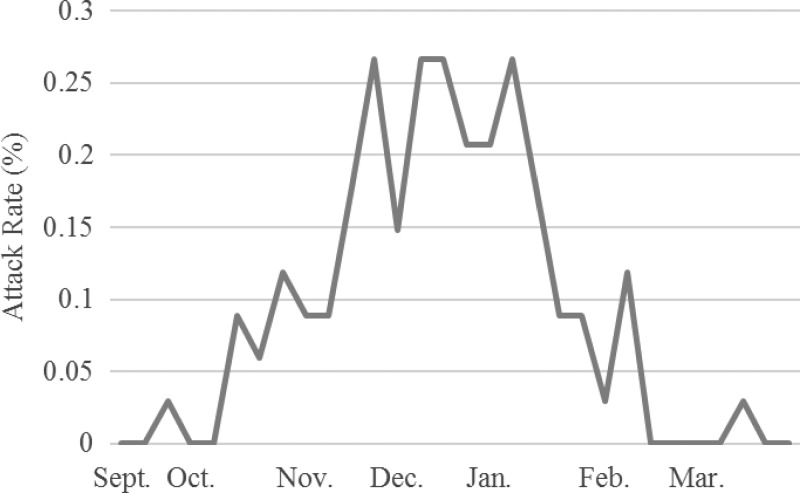
The clinical attack rate of chikungunya in the cohort was 2.9% and was similar in males and females (Table 1). Transmission began in September and continued through March (Figure 1 ). In children ≥ 8 years of age, the attack rate of 4.1% (95% confidence interval [CI]: 3.2%, 5.1%) was higher, based on nonoverlapping 95% CIs, than the attack rate in children < 8 years, 1.5% (95% CI: 0.9%, 2.1%). Among cohort children, age was strongly associated with clinical chikungunya infection (test for trend = 4.80, P < 0.001; Figure 2 ). The mean age of CHIKV cases meeting the case definition for a probable case of dengue or chikungunya was 9.8 years compared with 7.8 years of age for cases presenting with undifferentiated fever (P = 0.04). Socioeconomic status (SES) or factors associated with SES did not significantly affect the clinical attack rate in children in our cohort; however, we observed a trend toward increasing attack rate with increasing poverty, and our study may have been underpowered to detect an association.
Figure 1.
Weekly clinical attack rate (%) of chikungunya in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2014–2015.
Figure 2.
Clinical attack rate of RT-PCR–positive chikungunya by age in the Pediatric Dengue Cohort Study, Managua, Nicaragua, 2014–2015. Error bars represent 95% confidence intervals.
Here we find that in the 7 months after the introduction of CHIKV in Nicaragua, with a presumably chikungunya-naive population, the attack rate for symptomatic chikungunya was 2.9% in children aged 2–14 years in Managua. Among CHIKV-positive cases, older children were more likely to present with typical CHIKV symptoms than younger children, who were more likely to present with undifferentiated fever. Our study included children aged 2–14 years, and thus we were not able to assess atypical severe manifestations in children under 2 years of age, such as bullous rash or neurological manifestations, which have been observed in other studies.9–11 Of note, a recent study using a mouse model has indicated that the Asian lineage CHIKV circulating in the Americas is less virulent than the East Central South African (ECSA) genotype circulating in India and the Indian Ocean region; thus, our findings may not be generalizable to other CHIKV genotypes.17
We found a lower attack rate of clinical chikungunya infection in young children in the cohort. With DENV infection, we see the highest rates of DENV-positive undifferentiated fever in the youngest children and the highest rates of cases meeting the case definition in older children,18 leading us to postulate that CHIKV infections in young children may present with atypical mild symptoms or remain asymptomatic. To fully evaluate this question, further studies are needed to assess asymptomatic or subclinical infection in children. We are using healthy annual blood samples to examine the age-specific rate of CHIKV infection compared with clinical cases in our cohort.
A major strength of our study is the prospective cohort design, which allowed us to capture CHIKV cases from the beginning of the outbreak in Nicaragua. One limitation of this study is that young children have reduced verbal skills and thus may be less able to report symptoms of classical chikungunya, such as myalgia and headache. This could lead to overestimation of the percentage presenting with undifferentiated fever. Another limitation is that it is in a geographically limited area, and while the children in the cohort study are representative of the general population in Managua, outbreaks tend to be focal, thus, the attack rates reported here are specific to this setting.
In conclusion, we have found that ~3% of children experienced a clinical CHIKV infection during the first epidemic of the disease in Nicaragua. The attack rate in these children was lower than expected given the explosive chikungunya epidemics in other countries in the Americas after introduction.5 It is possible that transmission was lower due to environmental conditions or control efforts. However, our data also suggest that the clinical attack rate in children may underestimate the true burden of disease, as some children, especially younger children, may experience more mild atypical symptoms (e.g., undifferentiated fever) or subclinical CHIKV infections.
ACKNOWLEDGMENTS
We would like to thank the cohort participants and their families. We would also like to thank the superb study staff at the Health Center Sócrates Flores Vivas and the Centro Nacional de Diagnostico y Referencia.
Footnotes
Financial support: This work was supported by the National Institute of Allergy and Infectious Diseases, National Institutes of Health (grant no. R01AI099631 to Angel Balmaseda and K02TW009483 to Aubree Gordon).
Authors' addresses: Angel Balmaseda, Saira Saborio, and Yolanda Tellez, Laboratorio Nacional de Virología, Centro Nacional de Diagnóstico y Referencia, Ministerio de Salud, Managua, Nicaragua, E-mails: abalmaseda@minsa.gob.ni, ysaborio@minsa.gob.ni, and ytellez@minsa.gob.ni. Aubree Gordon, Department of Epidemiology, School of Public Health, University of Michigan, Ann Arbor, MI, E-mail: gordonal@umich.edu. Lionel Gresh, Sergio Ojeda, and Nery Sanchez, Sustainable Sciences Institute, Managua, Nicaragua, E-mails: lionelgresh@gmail.com, sojeda@icsnicaragua.org, and nsanchez@icsnicaragua.org. Guillermina Kuan, Centro de Salud Sócrates Flores Vivas, Ministerio de Salud, Managua, Nicaragua, E-mail: gkmontes@icsnicaragua.org. Eva Harris, Division of Infectious Diseases and Vaccinology, School of Public Health, University of California, Berkeley, Berkeley, CA, E-mail: eharris@berkeley.edu.
References
- 1.Sissoko D, Moendandze A, Malvy D, Giry C, Ezzedine K, Solet JL, Pierre V. Seroprevalence and risk factors of chikungunya virus infection in Mayotte, Indian Ocean, 2005–2006: a population-based survey. PLoS One. 2008;3:e3066. doi: 10.1371/journal.pone.0003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodriguez-Barraquer I, Solomon SS, Kuganantham P, Srikrishnan AK, Vasudevan CK, Iqbal SH, Balakrishnan P, Solomon S, Mehta SH, Cummings DA. The hidden burden of dengue and chikungunya in Chennai, India. PLoS Negl Trop Dis. 2015;9:e0003906. doi: 10.1371/journal.pntd.0003906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sergon K, Yahaya AA, Brown J, Bedja SA, Mlindasse M, Agata N, Allaranger Y, Ball MD, Powers AM, Ofula V, Onyango C, Konongoi LS, Sang R, Njenga MK, Breiman RF. Seroprevalence of chikungunya virus infection on Grande Comore Island, Union of the Comoros, 2005. Am J Trop Med Hyg. 2007;76:1189–1193. [PubMed] [Google Scholar]
- 4.AbuBakar S, Sam I-C, Wong P-F, Hooi P-S, Roslan N, MatRahim N. Reemergence of endemic Chikungunya, Malaysia. Emerg Infect Dis. 2007;13:147–149. doi: 10.3201/eid1301.060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan American Health Organization 2015. http://www.paho.org/hq/index.php?Itemid=40931 Accessed November 20, 2015.
- 6.Burt FJ, Rolph MS, Rulli NE, Mahalingam S, Heise MT. Chikungunya: a re-emerging virus. Lancet. 2012;379:662–671. doi: 10.1016/S0140-6736(11)60281-X. [DOI] [PubMed] [Google Scholar]
- 7.Ritz N, Hufnagel M, Gerardin P. Chikungunya in children. Pediatr Infect Dis J. 2015;34:789–791. doi: 10.1097/INF.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 8.Sissoko D, Ezzedine K, Moendandze A, Giry C, Renault P, Malvy D. Field evaluation of clinical features during chikungunya outbreak in Mayotte, 2005–2006. Trop Med Int Health. 2010;15:600–607. doi: 10.1111/j.1365-3156.2010.02485.x. [DOI] [PubMed] [Google Scholar]
- 9.Lewthwaite P, Vasanthapuram R, Osborne JC, Begum A, Plank JL, Shankar MV, Hewson R, Desai A, Beeching NJ, Ravikumar R, Solomon T. Chikungunya virus and central nervous system infections in children, India. Emerg Infect Dis. 2009;15:329–331. doi: 10.3201/eid1502.080902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robin S, Ramful D, Le Seach F, Jaffar-Bandjee MC, Rigou G, Alessandri JL. Neurologic manifestations of pediatric chikungunya infection. J Child Neurol. 2008;23:1028–1035. doi: 10.1177/0883073808314151. [DOI] [PubMed] [Google Scholar]
- 11.Robin S, Ramful D, Zettor J, Benhamou L, Jaffar-Bandjee MC, Riviere JP, Marichy J, Ezzedine K, Alessandri JL. Severe bullous skin lesions associated with chikungunya virus infection in small infants. Eur J Pediatr. 2010;169:67–72. doi: 10.1007/s00431-009-0986-0. [DOI] [PubMed] [Google Scholar]
- 12.Gerardin P, Barau G, Michault A, Bintner M, Randrianaivo H, Choker G, Lenglet Y, Touret Y, Bouveret A, Grivard P, Le Roux K, Blanc S, Schuffenecker I, Couderc T, Arenzana-Seisdedos F, Lecuit M, Robillard PY. Multidisciplinary prospective study of mother-to-child chikungunya virus infections on the island of La Reunion. PLoS Med. 2008;5:e60. doi: 10.1371/journal.pmed.0050060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuan G, Gordon A, Aviles W, Ortega O, Hammond SN, Elizondo D, Nunez A, Coloma J, Balmaseda A, Harris E. The Nicaraguan pediatric dengue cohort study: study design, methods, use of information technology, and extension to other infectious diseases. Am J Epidemiol. 2009;170:120–129. doi: 10.1093/aje/kwp092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balmaseda A, Sandoval E, Perez L, Gutierrez CM, Harris E. Application of molecular typing techniques in the 1998 dengue epidemic in Nicaragua. Am J Trop Med Hyg. 1999;61:893–897. doi: 10.4269/ajtmh.1999.61.893. [DOI] [PubMed] [Google Scholar]
- 15.Lanciotti RS, Kosoy OL, Laven JJ, Panella AJ, Velez JO, Lambert AJ, Campbell GL. Chikungunya virus in US travelers returning from India, 2006. Emerg Infect Dis. 2007;13:764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balmaseda A, Standish K, Mercado JC, Matute JC, Tellez Y, Saborio S, Hammond SN, Nunez A, Aviles W, Henn MR, Holmes EC, Gordon A, Coloma J, Kuan G, Harris E. Trends in patterns of dengue transmission over 4 years in a pediatric cohort study in Nicaragua. J Infect Dis. 2010;201:5–14. doi: 10.1086/648592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teo TH, Her Z, Tan JJ, Lum FM, Lee WW, Chan YH, Ong RY, Kam YW, Leparc-Goffart I, Gallian P, Renia L, de Lamballerie X, Ng LF. Caribbean and La Reunion chikungunya virus isolates differ in their capacity to induce proinflammatory Th1 and NK cell responses and acute joint pathology. J Virol. 2015;89:7955–7969. doi: 10.1128/JVI.00909-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biswas HH, Ortega O, Gordon A, Standish K, Balmaseda A, Kuan G, Harris E. Early clinical features of dengue virus infection in Nicaraguan children: a longitudinal analysis. PLoS Negl Trop Dis. 2012;6:e1562. doi: 10.1371/journal.pntd.0001562. [DOI] [PMC free article] [PubMed] [Google Scholar]