Abstract
OBJECTIVES
To assess objectively measured daytime physical activity and sleep duration and efficiency in hospitalized older adults and explore associations with demographic characteristics and disease severity.
DESIGN
Prospective cohort study.
SETTING
University of Chicago Medical Center general medicine wards.
PARTICIPANTS
Community-dwelling inpatients aged 50 and older (N = 120)
MEASUREMENTS
Physical activity and sleep were measured using wrist accelerometers. Information on Charlson Comorbidity Index and length of stay was collected from charts. Random-effects linear regression analysis was used to examine the association between in-hospital sleep and physical activity.
RESULTS
From March 2010 to May 2013, 120 participants wore wrist actigraphy monitors for at least 2 nights and 1 intervening day. Median activity level over the waking period was 77 counts/min (interquartile range 51–121 counts/min), an activity level that approximately corresponds to sitting while watching television (65 counts/min). Mean sleep duration the night before the activity interval was 289 ± 157 minutes, and mean sleep efficiency the night before the activity interval was 65.2 ± 26.9%. Mean activity counts/min were lowest for the oldest participants (oldest quartile 62, 95% confidence interval (CI) = 50–75; youngest quartile 121, 95% CI = 98–145, trend test P < .001) and those with highest Charlson Comorbidity Index (highest tertile 71, 95% CI = 60–83; lowest tertile 125, 95% CI = 104–147, trend test P = .01). Controlling for severity of illness and demographic characteristics, activity declined by 3 counts/min (95% CI = −5.65 to −0.43, P = .02) for each additional hour of inpatient sleep.
CONCLUSION
Older, sicker adults are less physically active during hospitalization. In contrast to studies in the community, inpatients who slept more were not more active. This may highlight that need for sleep is greater in the hospital than in the community.
Keywords: physical activity, sleep, older hospitalized adults, low mobility
Hospitalization is a period of low physical activity, particularly for older adults.1–3 The most common reasons for the limitation of physical activity include symptoms of illness, lack of support from medical staff, intravenous lines, and fear of injury.4 Moreover, approximately one-third of older hospitalized adults are placed on total bed rest,5 often without valid medical reason.6 Restriction to complete bed rest is at odds with the recommendation for early mobility to reduce complications.7,8 Low levels of activity6 and older age9 are both predictors of functional deterioration during hospitalization. Combined, these characteristics can have severe but often preventable consequences, such as loss of muscle mass,5,10 deep vein thrombosis, orthostatic intolerance,5,11 nursing home placement, and death,12–14 in hospitalized older adults.
Because physical activity is important for older adults, it has been assessed in older hospitalized adults. Recent studies have used continuous wireless monitoring in older hospitalized adults to determine body position, such as lying, sitting, and standing or walking. The results demonstrated that people spend the majority of time in bed even if they are able to walk independently,2,19 but these studies did not use activity counts (ACs), a measure of accelerometry, which may help distinguish gradations of activity, such as sitting passively versus sitting while actively using the arms. The studies examining ACs encompass a population restricted to specific health conditions, such as stroke or anorexia nervosa.15,16
One novel reason for limited activity in hospitalized individuals that has not been investigated is the role of sleep loss. Studies have demonstrated that sleep is markedly disturbed in older hospitalized adults, with sleep duration equivalent to that of severe sleep debt in healthy community-dwelling adults and with sleep efficiency similar to individuals with insomnia, often because of hospital noise and environmental disruptions.18,19 In addition, sleep loss in the hospital is linked to negative health outcomes, such as high blood pressure,20 delirium,21,22 and reduced immunity.23 Prior studies outside the hospital have demonstrated an association between poor sleep and diminished daytime physical activity. Nursing home studies have also shown that better sleep is associated with more physical activity and less agitation, whereas poor sleep is associated with difficulty with activities of daily living.24,25 Moreover, a community-based study demonstrated that individuals with more activity enrolled in a 12-month moderate-intensity exercise program had fewer awakenings and better subjective sleep quality than underactive subjects.26 Last, in a cohort of individuals undergoing cardiac surgery, better sleep efficiency and self-reported sleep quality postoperatively at 4 and 8 weeks was associated with greater physical function.27 To the knowledge of the authors of the current study, no study has examined the relationship between objectively measured daytime activity and sleep in older, hospitalized adults not restricted to certain diseases, conditions, or recent procedures. The present study aimed to characterize the association between objective daytime physical activity and sleep duration in hospitalized older adults, assess associations with demographic characteristics and disease severity, and quantify reference thresholds of physical activity over a minimum of 24 hours. Examining these associations is especially important because older adults are predisposed to sleep disturbances in the hospital and functional decline after discharge.28
METHODS
Study Design
Subjects in this study were identified as part of a larger study of sleep in hospitalized adults in a general medicine ward at the University of Chicago Medical Center.29 Eligible participants of the larger sleep study were ambulatory adults aged 50 and older living in the community before admission. To be considered ambulatory, individuals had to report that they were able to walk across the room independently or with a walker. Exclusion criteria included previously diagnosed sleep disorders such as obstructive sleep apnea and narcolepsy, cognitive impairment (Mini-Mental State Examination telephone version score <17),30 hospital stay of more than 72 hours before enrollment, complete bed rest, and admission to the general medicine ward from an intensive care unit. The exclusion criteria were designed to enroll an ambulatory population without significant sleep disturbances at baseline.
To be eligible for this substudy on sleep and physical activity, participants had to have provided 40 continuous hours of actigraphy recordings spanning 2 nights in the hospital and the intervening day under the parent study (Appendix 1). Daytime physical activity could be reliably measured from the moment a participant reported waking up to the moment they stated they went to bed. The University of Chicago institutional review board approved the study, and participants were provided written consent.
Data Collection
Wrist Actigraphy: Physical Activity and Sleep
Wrist actigraphy monitors (Actiwatch2, Respironics, Inc., Murrysville, PA) were worn for at least 40 hours, which spanned 2 nocturnal sleep periods; the intervening daytime period was the assumed waking period. The wrist-worn monitor contains a piezoelectric element and seismic mass that measures acceleration due to movement.31 The data were translated to an activity score for each 15- and 60-second interval, and these activity scores were used to determine sleep and activity values.32,33 The data were downloaded and analyzed using Actiware 5 (Respironics, Inc.).34
Average ACs/min were analyzed over the assumed wake period to quantify the participant’s degree of physical activity. The assumed wake period was derived from the time that participants reported that they woke up that morning to the time they reported that they went to bed that night. Two other activity variables were also examined: maximum ACs/min and total ACs.
To reference ACs for the wrist actigraphy monitoring system to known levels of daily activities, five healthy research volunteers aged 50 and older were asked to wear the Actiwatch while keeping a log of standardized activities.35,36 Activities were adapted from other studies to be relevant for hospitalized individuals and included sleeping, lying down awake, sitting passively watching television, sitting while eating, sitting with active arm movement writing a letter, casual walking, and brisk walking.35,36 Using Actiware5, the interval of each activity was set based on the self-reported logs of the volunteers, and the program calculated the mean ACs over the specified intervals. These values were then averaged across the five volunteers. This method enabled the mean ACs of hospitalized older adults to be compared with the reference activities.
To quantify sleep duration and efficiency in the hospital for each participant, the assumed sleep period was calculated based on the times that participants reported that they went to bed and the time they awoke the next morning.37,54 Objective sleep duration and sleep efficiency were then derived using the Actiware software based on when ACs were minimal over all 15-second epochs included in the assumed sleep period. Sleep duration is defined as total time spent asleep, and sleep efficiency is the ratio of sleep duration over the assumed sleep period (Appendix 1).
Sleep
To assess baseline sleep duration, participants were asked to self-report their sleep duration at home over the month before admission using the Karolinska Sleep Log. Participants’ charts were assessed for an established diagnosis of insomnia. On admission, baseline insomnia was calculated using the Insomnia Severity Index, which is a validated measure of insomnia based on a seven-question survey (total score range 0–28). Participants with scores of 15 or greater were considered to have clinical insomnia.53 In-hospital individual sleep debt was calculated by using the difference between self-reported sleep duration at home the month before admission and objective sleep duration using actigraphy. In a survey each morning, participants were asked whether pain disrupted their sleep (1 = not disrupted to 5 = extremely disrupted).
Physical Function and Physical Therapy
Because physical function before admission may predict physical activity, which inpatient physical therapy sessions are also likely to affect, data were collected on these factors as potential covariates in modeling the relationship between sleep and activity in older hospitalized adults. Participants were asked to report whether they felt limited in work or activities because of physical health during the month before hospitalization,38 on admission and again after 1 month of follow-up. Charts were reviewed to determine whether participants received physical therapy while hospitalized.
Demographic Information and Disease Severity
Information on demographic characteristics (age, race, ethnicity, sex) and markers of disease severity, including Charlson Comorbidity index (range 0–37) and length of hospital stay, was collected from participants charts through an ongoing study of individuals admitted to the University of Chicago Medical Center inpatient general medicine service.29 Age was divided into quartiles and centered by subtracting the mean age from each participant’s age. The Charlson Comorbidity Index and length of stay were divided into tertiles before analysis.39,40
Medications and Delirium
Charts were reviewed to determine whether participants received sleep or sedating medications. These medications include estazolam, eszopiclone, flurazepam, ramelteon, temazepam, trazodone, zaleplon, zolpidem, diphenhydramine, or lorazepam while hospitalized. In addition, each day that an individual was in the study, the interviewer administered the short version of the Confusion Assessment Method—a validated method to detect delirium by assessing mental change, behavior fluctuation, inattention, disorganized thinking, and altered level of consciousness.41
Agitation
Charts were reviewed to determine whether hospital staff observed that participants were agitated. Agitation was defined as repetitive verbal or physical actions, general restlessness, pacing, requests for attention, negativism, and verbal or physical aggression.24,42
Data Analysis
All data were entered into REDCap, which is a secure web application that allows research teams to create a secure database.43 Descriptive statistics were used to summarize physical activity and sleep duration and efficiency in the hospital. The primary physical activity outcome used for this analysis was average ACs/min over the assumed wake period, a frequently reported activity measure from actigraphy.31,44 Maximum ACs/min and total ACs were also examined.
Two-sample t-tests and nonparametric tests of trend were used to test the associations between average ACs/min and participant demographic characteristics (age in quartiles, sex, race), disease severity (Charlson Comorbidity Index, length of stay in tertiles), and physical function (baseline limited function, 1-month follow-up limited function, physical therapy).
Because there were multiple observations per subject, random-effects linear regression was performed to characterize the association between sleep duration the preceding night and mean physical ACs. Based on similar prior studies on sleep and physical function, models were adjusted for participant demographic characteristics (race, sex, age), disease severity (Charlson Comorbidity Index in tertiles, length of stay in tertiles), and physical function (baseline physical limitation 1 month before discharge, receipt of physical therapy).24,25
Next, analysis was performed to characterize the association between mean daytime physical activity (average ACs/min) and distal outcomes, namely physical function 1 month after discharge. Descriptive statistics were used to determine the number of participants reached for a follow-up telephone interview and the number who reported feeling limited in work or activities because of physical health. Logistic regression was used to characterize the association between mean ACs and physical function 1 month after discharge.
Last, data analysis was repeated for the different permutations of sleep characteristic and daytime physical activity models. This included examining the relationship between sleep duration and efficiency the night before daytime activity and average ACs/min, maximum ACs/min, and total ACs and between sleep duration and efficiency the night after daytime activity and average ACs/min, maximum ACs/min, and total ACs. Statistical significance was defined as P < .05, and statistical analysis was performed using Stata 12.0 (Stata Corp., College Station, TX).
RESULTS
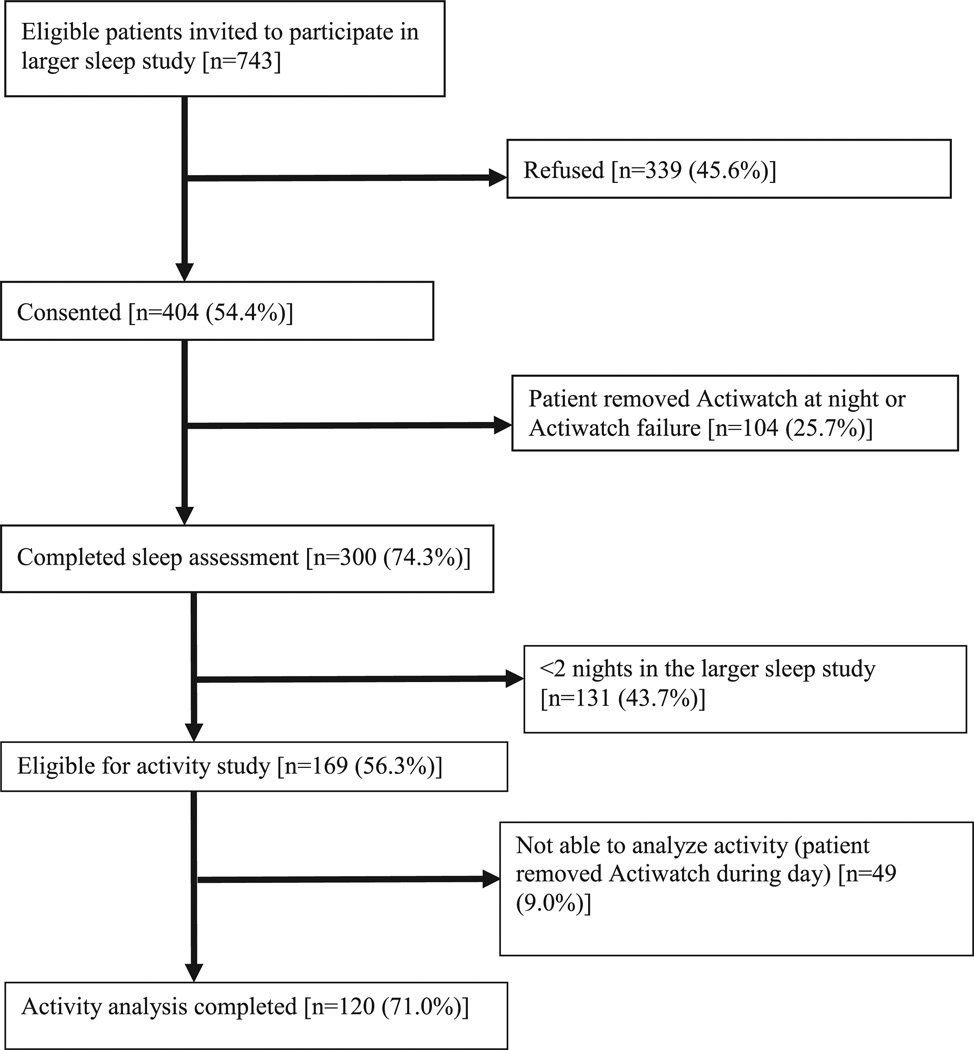
From March 2010 to May 2013, of 743 eligible individuals invited to participate in the parent sleep study, 404 (54.4%) consented; 169 of these were in the study for 2 nights or more, making them eligible for the activity analysis, and 120 of these (71.0%) had complete data for those 2 nights of actigraphy and completed all the subjective assessments for a total of 191 recorded events of daytime activity surrounded by 2 nights of sleep (Figure 1).
Figure 1.
Flow of participants through study.
The majority of participants were African American (65.8%), non-Hispanic (94.2%), and female (55.0%). The mean age was 65.5 ± 11.1 and the median length of stay was 5 days (interquartile range (IQR) 3–7). The study population had a median Charlson Comorbidity Index of 2 (IQR 0–3). Upon discharge, 87.5% of participants returned home, and 11.7% went to a rehabilitation center or were transferred to another medical facility; one participant died in the hospital (0.8%). Almost 62% of the participants reported feeling limited in the type of work or activities they were able to conduct during the month before hospitalization because of physical health.39 During their hospital stay, 49.2% of participants received physical therapy, 5.8% were prescribed a sleep medication, and 2.5% developed delirium. In-hospital sleep duration and efficiency were low. Average sleep the night before the activity interval was 289 ± 157 minutes, and mean sleep efficiency the night before the activity interval was 65.2 ± 26.9%. Twenty-one percent reported extreme disruption of sleep from pain, and 29.4% screened positive for clinical insomnia (Table 1).
Table 1.
Participant and Sleep Characteristics (N = 120)
| Characteristic | Value |
|---|---|
| Race n (%) | |
| African American | 79 (65.8) |
| Non-Hispanic | 113 (94.2) |
| Female, n (%) | 66 (55.0) |
| Age, mean ± SD | 65.5 ± 11.1 |
| Length of stay, days, median (IQR) | 5 (3–7) |
| Charlson Comorbidity Index, median (IQR) (range 0–37)a | 2 (0–3) |
| Discharge destination, n (%) | |
| Home | 105 (87.5) |
| Rehabilitation center | 10 (8.3) |
| Transferred | 4 (3.3) |
| Deceased | 1 (0.8) |
| Limitation before hospitalization, n (%)b | 74 (61.7) |
| Physical therapy, n (%) | 59 (49.2) |
| Sleep medication, n (%) | 7 (5.8) |
| Developed delirium, n (%) | 3 (2.5) |
| Sleep characteristic | |
| Sleep extremely disrupted by pain, n (%)c | 25.7 (21.4) |
| Clinical insomnia, n (%)d | 25 (29.4) |
| Sleep duration before admission, minutes, mean ± SDe | 390 ± 2.1 |
| Sleep duration before activity interval, minutes, mean ± SDf | 289 ± 157 |
| Sleep efficiency before activity interval, %, mean ± SDf | 65.2 ± 26.9 |
N = 116.
N = 119.
Based on self-report from daily survey while enrolled in study.
Based on Insomnia Severity Index ≥15, survey given upon admission. N = 85 given survey after original study began.
Based on self-reported sleep from month before admission.
Obtained using actigraphy, and efficiency analyzed using self-reported bed time and awake time during hospitalization as rest interval.
SD = standard deviation; IQR = interquartile range.
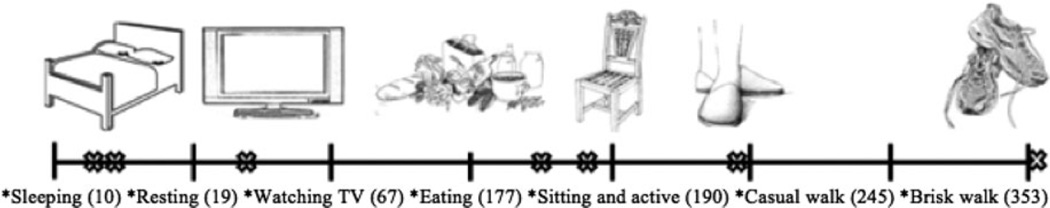
To have reference thresholds, average ACs/min were calculated from five research assistants performing certain routine activities: sleeping (10), lying down resting (19), sitting and watching television (67), sitting and eating (177), sitting and being active with hands (190), casually walking (245), and briskly walking (353) (Appendix 2).
Participants had a low level of activity (median 77 (IQR 51–121) average ACs/min, corresponding closely to sitting and watching television (67 average ACs/min). The average ACs of 90.1% of participants were less than 180 average ACs/min during their hospital stay, which corresponds to sitting and eating (177 average ACs/min). Mean maximum ACs was 446 ± 295, with a median of 371 (IQR 253–523), corresponding to a casual (245 average ACs/min) or brisk (353 average ACs/min) walk.
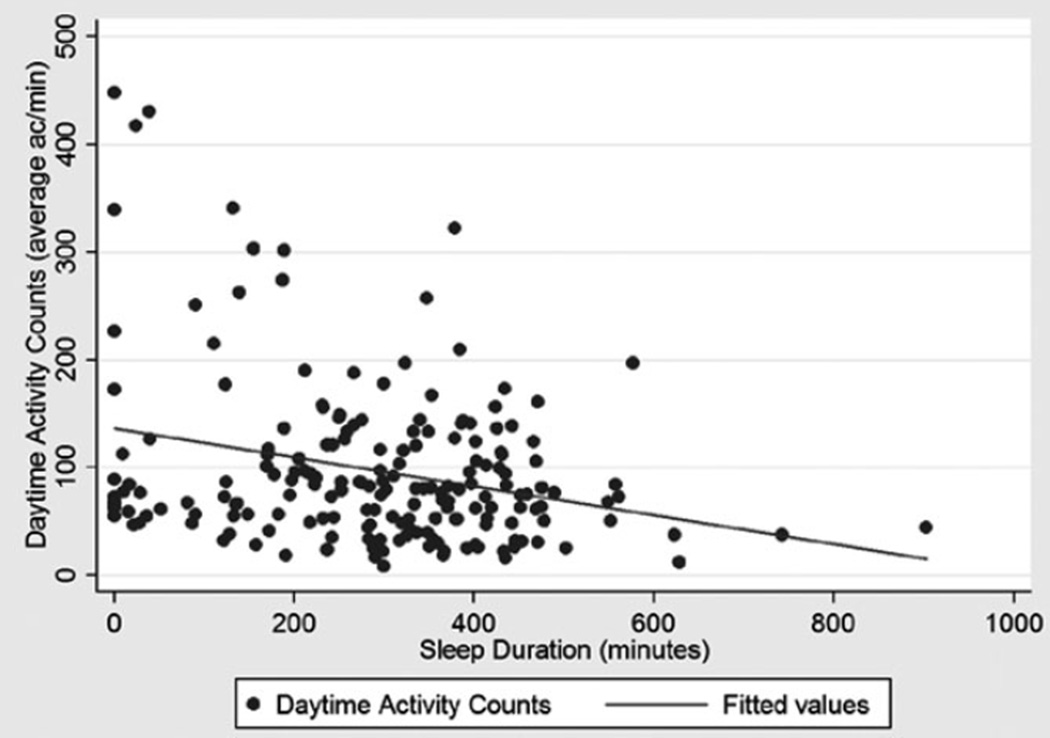
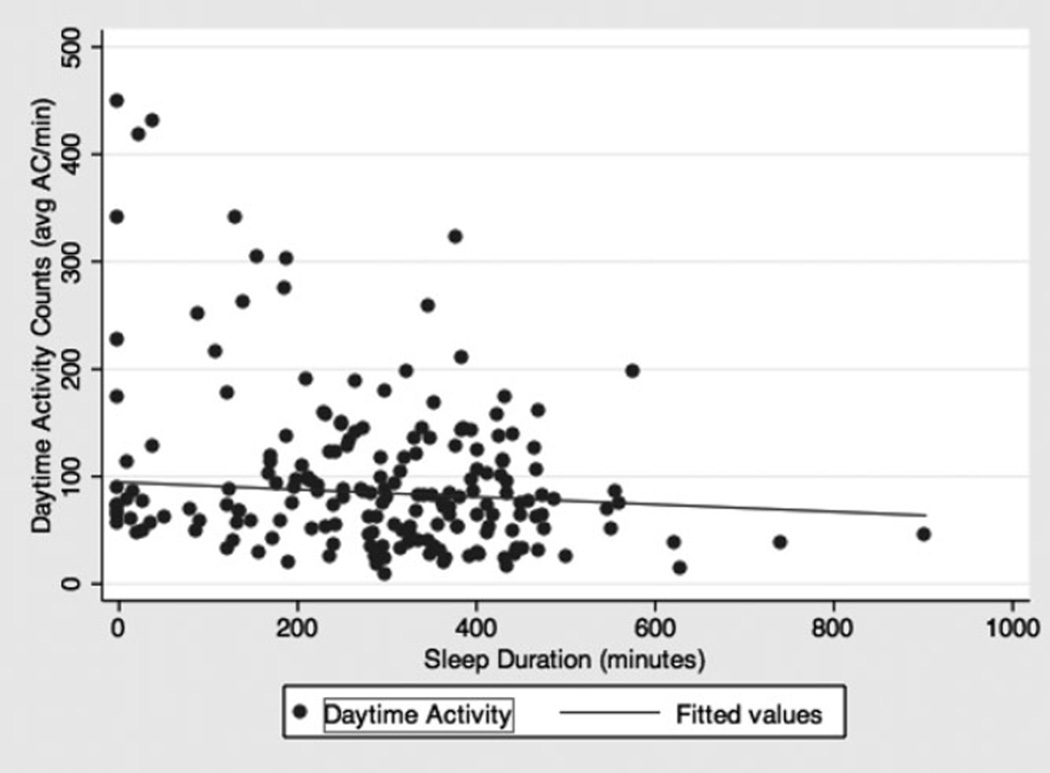
A scatter plot was derived for sleep duration the night before daytime activity according to actigraphy (minutes) versus daytime activity according to actigraphy (average ACs/min) (Appendix 3) (correlation coefficient = −0.27, P < .001). Most data points were clustered with daytime activity (average ACs/min) less than 200. Nine participants appeared to be outliers, because their average ACs/min were greater than 200, and sleep duration was less than 400 minutes according to actigraphy. A chart review of those nine participants revealed that four had no notes on agitation, and five had notes observing agitation (repetitive vomiting, dysuria and urinary frequency, uncooperative and negativism, general restlessness, repetitive itching). When those five participants with agitation were excluded from a scatter plot, the correlation coefficient was −0.03 (P = .11) (Appendix 4).
The degree of activity differed depending on age quartile, race, and sex (Table 2). Participants in the oldest quartile had lower mean ACs than those in the youngest quartile (average ACs/min 62, 95% CI = 50–74 vs 121, 95% CI = 98–145, P < .001 according to nonparametric test of trend). African-American participants also had a lower mean average ACs/min (88, 95% CI = 76–100) than those who were not African American (117, 95% CI = 95–138) (P = .01). Men had lower mean average ACs/min (82, 95% CI = 68–96) than women (112, 95% CI = 96–128) (P = .007). Average ACs/min were higher for participants in the lowest Charlson Comorbidity Index tertile (125, 95% CI = 104–147) than for those in the highest tertile (71, 95% CI = 60–83) (P = .01). Participants in the longest stay tertile also had lower average ACs/min (88, 95% CI = 76–100) than those in the shortest tertile (112, 95% CI = 87–136) (P = .03).
Table 2.
Association Between Demographic, Disease Severity, and Sleep Characteristics and Daytime Activity (N = 120)
| Characteristic | Average Activity Counts/Min, Mean (95% Confidence Interval) |
P-Value |
|---|---|---|
| Racea | ||
| African American | 88 (76–100) | .01 |
| Other | 117 (95–138) | |
| Sexa | ||
| Female | 112 (96–128) | .007 |
| Male | 82 (68–96) | |
| Ageb | ||
| 50–59 | 121 (98–145) | <.001 |
| 60–69 | 107 (88–126) | |
| 70–79 | 72 (53–90) | |
| ≥80 | 62 (50–75) | |
| Length of stay, daysb,c | ||
| 0–3 | 112 (87–138) | .03 |
| 4–5 | 110 (81–139) | |
| ≥6 | 88 (76–100) | |
| Charlson Comorbidity Indexb,d,e | ||
| 0–1 | 125 (104–147) | .01 |
| 2–3 | 79 (66–91) | |
| ≥4 | 71 (60–83) | |
| Objective sleep duration, minutesf | ||
| 0–232.8 | 127 (100–153) | .02 |
| 232.9–365.4 | 83 (70–96) | |
| >365.4 | 84 (70–98) | |
| Objective sleep efficiency, %f | ||
| 0–62.6 | 121 (97–145) | <.001 |
| 62.7–82.2 | 102 (84–119) | |
| >82.2 | 70 (58–82) | |
| Self-reported rest period, minutesg | ||
| <300 | 111 (79–142) | .56 |
| 300–450 | 86 (74–98) | |
| >450 | 107 (83–130) | |
| Individual sleep debt, minutesh | ||
| <300 | 87 (73–100) | .22 |
| 525–10,620 | 89 (72–106) | |
| >10,620 | 121 (95–147) | |
| Sleep disrupted by paine | ||
| 1 | 95 (81–109) | .74 |
| 2–4 | 119 (91–147) | |
| 5 | 98 (69–127) |
Two-sample t-test with equal variances.
Nonparametric test for trend across ordered groups.
N = 119.
N = 116.
Based on daily survey while hospitalized and in the study (1 = not disrupted to 5 = extremely disrupted).
Obtained from actigraphy.
Based on self-reported sleep duration from month before hospital admission.
Obtained from difference between objective sleep duration and self-reported rest period before hospital admission.
Participants with worse sleep in the hospital were marginally more active during the day. Average ACs/min were higher for participants in the shortest in-hospital objective sleep duration tertile (127, 95% CI = 101–153) than for those in the longest (84, 95% CI = 70–98) (P = .02) (Table 2). Likewise, participants in the worst inhospital sleep efficiency tertile had higher average ACs/min (121, 95% CI = 97–145) than those in the best tertile (70, 95% CI = 58–82) (P < .001) (Appendix 5). There was no association between self-reported sleep duration at home before hospitalization or in-hospital sleep debt and daytime physical activity while hospitalized. There was no association between self-reported sleep disruption from pain and daytime activity (Table 2).
There was no relationship between average ACs/min and self-reported limitation due to physical health 1 month before admission. Participants who received physical therapy had lower ACs/min (71, 95% CI = 62–80) than those who did not (127, 95% CI = 108–147) (P < .001). One month after discharge, 60% of participants completed a telephone interview assessing physical function, at which time, 60% reported feeling limited in work or activities because of physical health. Participants who reported physical limitation 1 month after discharge had lower mean ACs in the hospital (96.5, 95% CI = 80.1–112.9) than those who reported no limitation (129.7, 95% CI = 100.6–158.9) (P = .03) (Table 3).
Table 3.
Association Between Physical Function and Daytime Activity (N = 120)
| Physical Function and Activity |
Activity Counts/Min, Mean (95% Confidence Interval) |
P-Value |
|---|---|---|
| Limitation before hospitalizationa,b | ||
| Yes | 100 (86–114) | .62 |
| No | 95 (76–113) | |
| Physical therapya | ||
| Yes | 71 (62–80) | <.001 |
| No | 127 (108–147) | |
| Limitation at 1-month follow-upa,c | ||
| Yes | 97 (80–113) | .03 |
| No | 130 (101–159) |
Two-sample t-test with equal variances.
N = 119.
N = 72 (60%) responded to 1-month telephone follow-up survey.
More sleep in the hospital was associated with marginally less activity. Random-effects linear regression showed that, for each additional hour of sleep in the hospital, there was a marginal decrease of 2.68 ACs/min the following day (95% CI = −5.27 to −0.11) (P = .04). This relationship remained unchanged after controlling for demographic characteristics, comorbidities, length of stay, physical therapy, and physical limitation 1 month before hospitalization (−3.04%, 95% CI = −5.65 to −0.43) (P = .02) (Table 4). Likewise, better sleep efficiency was associated with marginally less daytime physical activity. A similar association was seen in the other models when examining sleep characteristics (sleep duration and efficiency the night before and after daytime activity) and the other physical activity variables (average ACs/min, maximum ACs/min, total ACs) (data available on request).
Table 4.
Regression Model for Physical Activity and Sleep (N = 115)
| Characteristic | Model 1 | Model 2 |
|---|---|---|
| Daytime Physical Activity, Mean Average Activity Counts/Min Beta (95% Confidence Interval) | ||
| Sleep night before, hours | −2.68 (−5.27 to −0.11) | −3.04 (−5.65 to −0.43)a |
| Charlson index tertile 3 | −55.7 (−88.2 to −23.3)a | |
| Charlson index tertile 2 | −33.3 (−59.8 to −6.80)a | |
| Physical therapy | −29.8 (−55.9 to −3.73)a | |
| Centered age (per year) | −1.24 (−2.40 to −0.07)a | |
| Male | −18.1 (−41.8−5.60) | |
| African American | −23.6 (−49.3−2.17) | |
| Baseline limitation | 20.6 (−5.70−46.9) | |
| Length-of-stay tertile 2 | −11.7 (−43.3−19.8) | |
| Length-of-stay tertile 3 | −17.5 (−48.0−12.9) | |
Random effects linear regression analyses were performed to assess the relationship between average activity counts per minute and sleep duration, with and without Charlson Comorbidity Index tertiles, length-of-stay tertiles, physical therapy, baseline physical limitation due to physical health, and demographic characteristics as covariates.
P < .05.
DISCUSSION
This study is the first, to the knowledge of the authors, to characterize the association between objectively measured daytime physical activity and sleep in older hospitalized adults not restricted to specific conditions. Although most participants had mean maximum ACs that corresponded to a casual walk, they spent the majority of their time in bed with low sleep duration and mean sleep efficiency falling below the clinical threshold of insomnia.45,46 Not surprisingly, older adults with higher comorbidity burden and longer stays were less physically active during hospitalization. Participants receiving physical therapy were less active than those not receiving physical therapy, although in contrast to nursing home residents and community-living individuals, better sleep in the hospital (longer sleep duration or better sleep efficiency) was not associated with greater daytime physical activity.24–27 Instead, more sleep was associated with marginally less daytime physical activity, but because the effect size in the study was small, the clinical significance is unclear. The association remained unchanged after controlling for demographic characteristics, comorbidities, length of stay, physical therapy, and physical limitation 1 month before hospitalization. Participants who were less physically active during hospitalization were more likely to report physical limitation 1 month after discharge.
It is important to consider the reason that longer sleep duration and greater sleep efficiency in the hospital is not associated with more activity, a finding that is not consistent with prior studies of community-dwelling adults.24–27 Two important differences between these groups are that hospitalized adults are acutely ill and more chronically debilitated. It is possible that sicker hospitalized adults are not only less physically active, but could also be in greater need of sleep in the hospital. Although it was attempted to account for disease severity in multivariate analyses, there could be unmeasured confounders of illness. Moreover, longer sleep duration in the hospital may be a marker of more-severe illness. In contrast, short sleep duration and high ACs while hospitalized appear to reflect agitation. This highlights the importance of accounting for agitation when using actigraphy in hospitalized individuals to measure physical activity.
Use of sleep medications and rates of delirium were low, which may have been a function of the exclusion criteria to select a healthier population without baseline sleep disturbances. In addition, there may be greater awareness of the need to minimize the prescription of sleep medications, especially in older adults.47–50 Likewise, individuals were excluded from the study if they were transferred from the intensive care unit, experiencing cognitive impairment, or bed-bound, which are predictors of delirium.51,52
There are several limitations to this study. It was conducted with a select group of individuals on a general medicine ward at a single institution. A prospective cohort study allows characterization of associations but not the establishment of causality. Unlike other accelerometers, the Actiwatch2 cannot calculate time above threshold or proportional integral mode, which would be useful measures to have in conjunction with ACs. Actiwatch2 was placed on the wrist; placing monitors on the waist or thigh can provide additional information. There were no previously published data on activity thresholds for this model, resulting in creating reference counts with a small group of research assistants. The effect size in this study was small, and it is unlikely that the decreases in ACs observed for each 1 hour of sleep were clinically significant. There could be differences in demographic characteristics and disease severity between participants who consistently wore the Actiwatch2 and those who removed them. Approximately one-fourth of participants who consented to the larger parent study were excluded and unable to complete a sleep assessment because of removal of the Actiwatch or failed battery at night. Approximately one-tenth of individuals who were able to complete sleep assessments were excluded from the activity analysis because of removal of the Actiwatch during the day for various reasons including necessary medical studies and comfort. Hospitalized individuals could also be hypersomnolent, and daytime napping during the assumed wake time cannot be excluded. Last, the assumed sleep period was based on self-reported times.
Older, sicker individuals are less physically active during hospitalization. In contrast to community-dwelling individuals and nursing home residents, hospitalized individuals who slept more in the hospital were not more active but were slightly less active, which may reflect the greater need for sleep of individuals in the hospital than of community-dwelling adults. These findings could have implications for future interventions to improve activity and sleep for older hospitalized adults. Optimizing the hospital environment, which is characterized by noise disruptions and sleep loss, may be critical for the sickest older adults. Last, understanding trajectories of sleep and activity after hospitalization may also help reveal how these relationships change after people recover.
Acknowledgments
Financial Disclosure: National Institute on Aging, 1T35 AG029795 Short-Term Aging-Related Research Program National Institute on Aging, K23AG033763 Career Development Award 1K24AG031326, Midcareer Career Development Award P01AG-11412, Program Project National Institutes on Aging, UL1 RR024999 Clinical Translational Sciences Award.
Sponsor’s Role: The sponsors did not have a role in the conception, design, analyses, interpretation of data, or in drafting, review, or approval of the article.
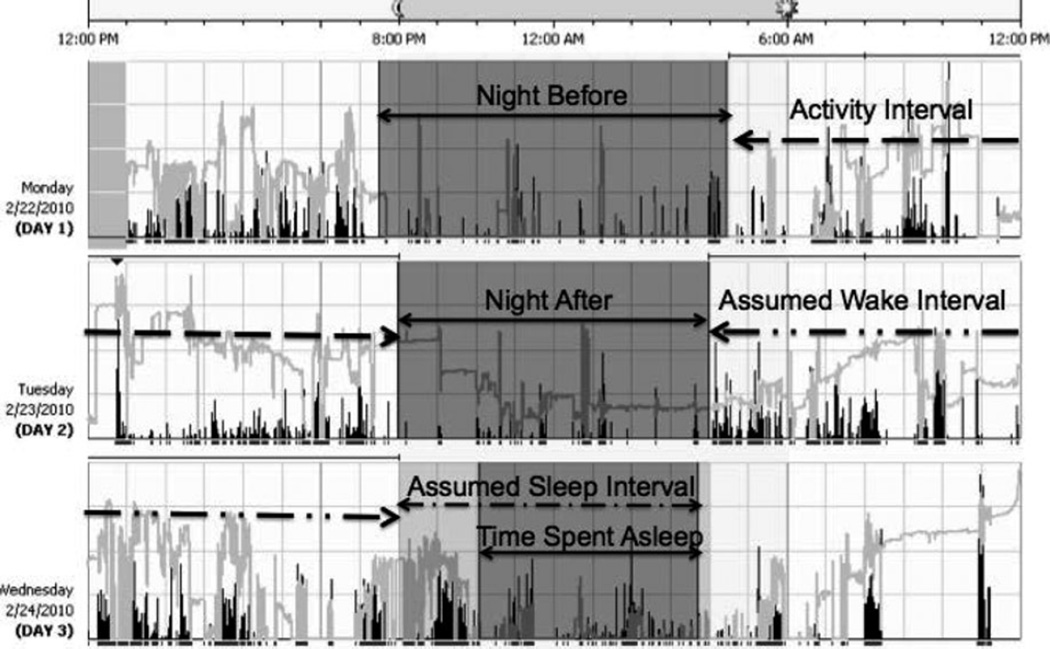
APPENDIX 1
Data collected from a participant using the accelerometer. This actigram shows 3 days in the hospital. Nights before and after the activity interval are measured to encompass a full day of physical activity. The watch measures movement, represented as black bars, and light, represented as lighter bars (not analyzed in this study). Assumed sleep interval is set based on when subjects report going to bed and waking up. This allows the program to set the time-spent-asleep interval based on when there is minimal movement.
APPENDIX 2
Baseline measurements of activity. Thresholds were determined using five research volunteers who wore the Actiwatches during routine activities: sleeping, lying down while resting, sitting passively (e.g., watching television), sitting while eating, sitting with active arm movement, casual walk, and brisk walk. Volunteers kept a physical activity record, noting the time interval they performed certain activities. Using Actiware5 (Respironics, Inc.), the interval of each activity was set based on the self-reported physical activity record, calculating the activity counts. These values were then averaged among the five volunteers.
APPENDIX 3
Scatter plot of sleep duration the night before daytime activity according to actigraphy (minutes) versus daytime physical activity according to actigraphy (average activity counts per minute). Fitted line shows correlation of −0.27, P < .001.
APPENDIX 4
Scatter plot of sleep duration the night before daytime activity according to actigraphy (minutes) versus daytime physical activity according to actigraphy (average activity counts per minute) excluding five participants with agitation. Fitted line shows correlation of −0.03, P = .11.
Appendix 5
Association between Age, Sleep Efficiency, and Daytime Physical Activity
| N = 120 | |||
|---|---|---|---|
| Mean Average Activity Counts per Minuteb (95% CI) |
P-value | ||
| Age (years)a | <65 | 120 (102, 138) | <0.001 |
| ≥65 | 73 (63, 84) | ||
| Objective Sleep Efficiencyb,c (%) | 0–62.6% | 121 (97, 145) | <0.001 |
| 62.6–82.2% | 102 (84, 119) | ||
| >82.2% | 70 (58, 85) | ||
Two-sample t-test with equal variances
Obtained from actigraphy
Nonparametric test for trend across ordered groups
Footnotes
Conflict of Interest: None of the authors have conflicts of interest to report.
Author Contributions: All authors had roles for important intellectual content and reviewed the manuscript for approval of final version. Beveridge: data collection, data analysis, interpretation of results, manuscript preparation and review. Knutson: study conception, design, data analysis, data interpretation, manuscript review. Spampinato: data collection, data analysis, manuscript review. Flores: data collection, data analysis, manuscript review. Meltzer: study conception, design, data collection, data interpretation, manuscript review. Van Cauter: study conception, design, data analysis, manuscript review. Arora: study conception, design, data analysis, interpretation of data, manuscript preparation.
REFERENCES
- 1.Fisher SR, Goodwin JS, Protas EJ, et al. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc. 2011;59:91–95. doi: 10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown CJ, Redden DT, Flood KL, et al. The underrecognized epidemic of low mobility during hospitalization of older adults. J Am Geriatr Soc. 2009;57:1660–1665. doi: 10.1111/j.1532-5415.2009.02393.x. [DOI] [PubMed] [Google Scholar]
- 3.Callen BL, Mahoney JE, Grieves CB, et al. Frequency of hallway ambulation by hospitalized older adults on medical units of an academic hospital. Geriatr Nurs. 2004;25:212–217. doi: 10.1016/j.gerinurse.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 4.So C, Pierluissi E. Attitudes and expectations regarding exercise in the hospital of hospitalized older adults: A qualitative study. J Am Geriatr Soc. 2012;60:713–718. doi: 10.1111/j.1532-5415.2012.03900.x. [DOI] [PubMed] [Google Scholar]
- 5.King BD. Functional decline in hospitalized elders. Medsurg Nurs. 2006;15:265–271. [PubMed] [Google Scholar]
- 6.Brown CJ, Friedkin RJ, Inouye SK. Prevalence and outcomes of low mobility in hospitalized older patients. J Am Geriatr Soc. 2004;52:1263–1270. doi: 10.1111/j.1532-5415.2004.52354.x. [DOI] [PubMed] [Google Scholar]
- 7.Sennour Y, Counsell SR, Jones J, et al. Development and implementation of a proactive geriatrics consultation model in collaboration with hospitalists. J Am Geriatr Soc. 2009;57:2139–2145. doi: 10.1111/j.1532-5415.2009.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scaffidi M, Vulpiani MC, Vetrano M, et al. Early rehabilitation reduces the onset of complications in the upper limb following breast cancer surgery. Eur J Phys Rehabil Med. 2012;48:601–611. [PubMed] [Google Scholar]
- 9.Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: Increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. doi: 10.1046/j.1532-5415.2003.51152.x. [DOI] [PubMed] [Google Scholar]
- 10.Mallery LH, MacDonald EA, Hubley-Kozey CL, et al. The feasibility of performing resistance exercise with acutely ill hospitalized older adults. BMC Geriatr. 2003;3:3. doi: 10.1186/1471-2318-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boltz M, Resnick B, Capezuti E, et al. Functional decline in hospitalized older adults: Can nursing make a difference? Geriatr Nurs. 2012;33:272–279. doi: 10.1016/j.gerinurse.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Rudberg MA, Sager MA, Zhang J. Risk factors for nursing home use after hospitalization for medical illness. J Gerentol A Biol Sci Med Sci. 1996;5A:M189–M194. doi: 10.1093/gerona/51a.5.m189. [DOI] [PubMed] [Google Scholar]
- 13.Walter LC, Brand RJ, Counsell SR, et al. Development and validation of a prognostic index for 1-year mortality in older adults after hospitalization. JAMA. 2001;285:2987–2994. doi: 10.1001/jama.285.23.2987. [DOI] [PubMed] [Google Scholar]
- 14.Inouye SK, Peduzzi PN, Robison JT, et al. Importance of functional measures in predicting mortality among older hospitalized patients. JAMA. 1998;279:1187–1189. doi: 10.1001/jama.279.15.1187. [DOI] [PubMed] [Google Scholar]
- 15.Reiterer V, Sauter C, Klosch G, et al. Actigraphy—a useful tool for motor activity monitoring in stroke patients. Eur Neurol. 2008;60:285–291. doi: 10.1159/000157882. [DOI] [PubMed] [Google Scholar]
- 16.Klein DA, Mayer LE, Schebendach JE, et al. Physical activity and cortisol in anorexia nervosa. Psychoneuroendocrinology. 2007;32:539–547. doi: 10.1016/j.psyneuen.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen MM, Bodilsen AC, Petersen J, et al. Twenty-four-hour mobility during acute hospitalization in older medical patients. J Gerontol A Biol Sci Med Sci. 2013;68A:331–337. doi: 10.1093/gerona/gls165. [DOI] [PubMed] [Google Scholar]
- 18.Yoder JC, Staisiunas PG, Meltzer DO, et al. Noise and sleep among adult medical inpatients: Far from a quiet night. Arch Intern Med. 2012;172:68–70. doi: 10.1001/archinternmed.2011.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Missildine K, Bergstrom N, Meininger J, et al. Sleep in hospitalized elders: A pilot study. Geriatr Nurs. 2010;31:263–271. doi: 10.1016/j.gerinurse.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 20.Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: Associations with blood pressure and mood. J Am Geriatr Soc. 2011;59:2185–2186. doi: 10.1111/j.1532-5415.2011.03644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inouye SK, Bogardus ST, Jr, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 22.Rosenbloom-Brunton DA, Henneman EA, Inouye SK. Feasibility of family participation in a delirium prevention program for hospitalized older adults. J Gerontol Nurs. 2010;36:22–33. doi: 10.3928/00989134-20100330-02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prather AA, Hall M, Fury JM, et al. Sleep and antibody response to hepatitis B vaccination. Sleep. 2012;35:1063–1069. doi: 10.5665/sleep.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alessi CA, Yoon EJ, Schnelle JF, et al. A randomized trial of a combined physical activity and environmental intervention in nursing home residents: Do sleep and agitation improve? J Am Geriatr Soc. 1999;47:784–791. doi: 10.1111/j.1532-5415.1999.tb03833.x. [DOI] [PubMed] [Google Scholar]
- 25.Martin JL, Fiorentino L, Jouldjian S, et al. Sleep quality in residents of assisted living facilities: Effect on quality of life, functional status, and depression. J Am Geriatr Soc. 2010;58:829–836. doi: 10.1111/j.1532-5415.2010.02815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King AC, Pruitt LA, Woo S, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. J Gerontol A Biol Sci Med Sci. 2008;63A:997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Redeker NS, Ruggiero JS, Hedges C. Sleep is related to physical function and emotional well-being after cardiac surgery. Nurs Res. 2004;53:154–162. doi: 10.1097/00006199-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Van Someren EJW. Circadian and sleep disturbances in the elderly. Exp Gerontol. 2000;35:1229–1237. doi: 10.1016/s0531-5565(00)00191-1. [DOI] [PubMed] [Google Scholar]
- 29.Meltzer D, Manning WG, Morrison J, et al. Effects of physician experience on costs and outcomes on an academic general medicine service: Results of a trial of hospitalists. Ann Intern Med. 2002;137:866–874. doi: 10.7326/0003-4819-137-11-200212030-00007. [DOI] [PubMed] [Google Scholar]
- 30.Roccaforte WH, Burke WJ, Bayer BL, et al. Validation of a telephone version of the Mini-Mental State Examination. J Am Geriatr Soc. 1992;40:697–702. doi: 10.1111/j.1532-5415.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- 31.Murphy SL. Review of physical activity measurement using accelerometers in older adults: Considerations for research design and conduct. Prev Med. 2009;48:108–114. doi: 10.1016/j.ypmed.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 33.Sadeh A. The role and validity of actigraphy in sleep medicine: An update. Sleep. 2011;15:259–267. doi: 10.1016/j.smrv.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Chae KY, Kripke DF, Poceta JS, et al. Evaluation of immobility time for sleep latency in actigraphy. Sleep Med. 2009;10:621–625. doi: 10.1016/j.sleep.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Warms CA, Belza BL. Actigraphy as a measure of physical activity for wheelchair users with spinal cord injury. Nurs Res. 2004;53:136–143. doi: 10.1097/00006199-200403000-00010. [DOI] [PubMed] [Google Scholar]
- 36.Crouter SE, Clowers KG, Bassett DR., Jr A novel method for using accelerometer data to predict energy expenditure. J Appl Physiol. 2006;100:1324–1331. doi: 10.1152/japplphysiol.00818.2005. [DOI] [PubMed] [Google Scholar]
- 37.Fung CH, Martin JL, Chung C, et al. Sleep disturbance among older adults in assisted living facilities. Am J Geriatr Psychiatry. 2012;20:485–493. doi: 10.1097/JGP.0b013e318252e3e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179–186. doi: 10.1093/oxfordjournals.pubmed.a024606. [DOI] [PubMed] [Google Scholar]
- 39.Li B, Evans D, Faris P, et al. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 42.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44:M77–M84. doi: 10.1093/geronj/44.3.m77. [DOI] [PubMed] [Google Scholar]
- 43.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Troiano RP, Berrigan D, Dodd KW, et al. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 45.Lichstein K, Durrence HH, Taylor DJ, et al. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 46.Edinger J, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 47.Tamblyn R, Abrahamowicz M, du Berger R, et al. A 5-year prospective assessment of the risk associated with individual benzodiazepines and doses in new elderly users. J Am Geriatr Soc. 2005;53:233–241. doi: 10.1111/j.1532-5415.2005.53108.x. [DOI] [PubMed] [Google Scholar]
- 48.Kolla BP, Lovely JK, Mansukhani MP, et al. Zolpidem is independently associated with increased risk of inpatient falls. J Hosp Med. 2013;8:1–6. doi: 10.1002/jhm.1985. [DOI] [PubMed] [Google Scholar]
- 49.Gray SL, LaCroix AZ, Hanlon JT, et al. Benzodiazepine use and physical disability in community-dwelling older adults. J Am Geriatr Soc. 2006;54:224–230. doi: 10.1111/j.1532-5415.2005.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pariente A, Dartigues JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging. 2008;25:61–70. doi: 10.2165/00002512-200825010-00007. [DOI] [PubMed] [Google Scholar]
- 51.Isfandiaty R, Harimurti K, Setiati S, et al. Incidence and predictors for delirium in hospitalized elderly patients: A retrospective cohort study. Acta Med Indones. 2012;44:290–297. [PubMed] [Google Scholar]
- 52.Schuurmans MJ, Duursma SA, Shortridge-Baggett LM. Early recognition of delirium: Review of the literature. J Clin Nurs. 2001;10:721–729. doi: 10.1046/j.1365-2702.2001.00548.x. [DOI] [PubMed] [Google Scholar]
- 53.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 54.Alessi CA, Martin JL, Webber AP, et al. More daytime sleeping predicts less functional recovery among older people undergoing inpatient post-acute rehabilitation. Sleep. 2008;31:1291–1300. [PMC free article] [PubMed] [Google Scholar]