Abstract
Apoptosis is a carefully choreographed process of cellular self-destruction in the absence of inflammation. During the death process, apoptotic cells actively communicate with their environment, signaling to both their immediate neighbors as well as distant sentinels. Some of these signals direct the anti-inflammatory immune response, instructing specific subsets of phagocytes to participate in the limited and careful clearance of dying cellular debris. These immunomodulatory signals can also regulate the activation state of the engulfing phagocytes. Other signals derived from apoptotic cells contribute to tissue growth control with the common goal of maintaining tissue integrity. Derangements in these growth control signals during prolonged apoptosis can lead to excessive cell loss or proliferation. Here, we highlight some of the most intriguing signals produced by apoptotic cells during the course of normal development as well as during physiological disturbances such as atherosclerosis and cancer.
1. APOPTOSIS: A SILENT DEATH?
Apoptosis is a carefully choreographed process of cellular self-destruction, observed across the spectrum of metazoans from worms to flies to mammals (Abrams, White, Fessler, & Steller, 1993; Ellis & Horvitz, 1986; Kerr, Wyllie, & Currie, 1972). During development, apoptosis shapes developing tissues by removing superfluous cells, sculpting out defined structures, or regulating tissue size (Glucksmann, 1951) (more recently reviewed in Suzanne & Steller, 2013). In adult organisms, apoptosis can trigger death in cells that are no longer functioning properly such as those injured by toxins or transformed by genetic aberrations (reviewed in Fuchs & Steller, 2011). This removal is critical to maintaining tissue integrity and homeostasis, and it is the mechanism of removal that distinguishes apoptosis from other forms of cell death. Cells that are damaged, infected, or otherwise unwanted are capable of initiating a tightly controlled cascade of events, which leads to the cessation of normal cellular activity, the degradation of major macromolecules including DNA, and ultimately the contained fragmentation of the cell so that it may be cleared via phagocytosis (Kerr et al., 1972; Lockshin & Williams, 1965; Schwartz, Smith, Jones, & Osborne, 1993).
Apoptosis was initially distinguished from necrotic cell death based on the quiet nature of its cellular demise. Unlike necrosis where cells spill their contents causing secondary tissue damage and infiltrating immune cells react with such fervor they induce significant inflammation, apoptosis is characterized by an unassuming departure, contained cellular contents, few immune cells, and no detectable inflammation. This contrast earned apoptotic cell death the moniker of “altruistic cell suicide,” and so for a time, the characterization of apoptosis as the silent cell death prevailed (Bar, 1996).
To better understand how apoptotic cells can die without causing further damage, we will first review the basics of apoptotic cell death. From worms to humans, there are a variety of ways to initiate the apoptotic cascade—some cascades are triggered by intrinsic developmentally regulated transcriptional programs, others by extrinsic death signals; some are triggered by active induction, others by neglect; some depend on the release of cytochrome C from the mitochondria, others can be driven by accumulation of proapoptotic factors (reviewed in Bergmann, 2010; Conradt, 2009; Czabotar, Lessene, Strasser, & Adams, 2014; Danial & Korsmeyer, 2004; Domingos & Steller, 2007; Steller, 1995; Xu et al., 2009).
What all apoptotic deaths have in common, however, is the activation of caspases. These cysteine-dependent aspartate-directed proteases are the critical effectors of cell death (Miura, Zhu, Rotello, Hartwieg, & Yuan, 1993; Yuan, Shaham, Ledoux, Ellis, & Horvitz, 1993). Caspases are initially produced as zymogens, which are not active until they are proteolytically cleaved. Autocatalytic activation of the initiator Caspase-9 most typically occurs via complex formation with the adaptor protein Apaf-1, along with cytochrome C and dATP (Li et al., 1997). Activated initiator caspases can cleave and activate effector caspases such as Caspase-3 and Caspase-7 (Brustugun, Fladmark, Doskeland, Orrenius, & Zhivotovsky, 1998; Zou, Henzel, Liu, Lutschg, & Wang, 1997). Activated effector caspases carry out the methodical process of executing cell death, directly activating other death enzymes such as nucleases and kinases, inactivating proteins required to sustain normal cellular processes, or indirectly disrupting normal physiological processes by disassembling compartments such as the nucleus and the mitochondria (Coleman et al., 2001; Enari et al., 1998; Gavrieli, Sherman, & Ben-Sasson, 1992; Li, Luo, & Wang, 2001; Liu, Zou, Slaughter, & Wang, 1997; Sebbagh et al., 2001; Susin et al., 1999).
While only ten percent of specific caspase cleavage sites are conserved between worms and humans, there is incredible conservation of the biological pathways which are targeted by effector caspases (Crawford et al., 2012). Among these, there are a number of targets that do not seem to be involved in the actual disassembly of the dying cell, but instead are released into the surrounding microenvironment. Over the past decade, interest in these apoptosis-derived signals has led to the discovery of critical communications between dying cells and their environment. Generally these signals can be broken into two categories: (1) signals that act on immune cells to regulate the clearance of apoptotic debris, prevent inflammation, and limit fibrosis; and (2) signals that act directly on neighboring surviving cells to maintain tissue integrity via growth control. Taken together, it is becoming increasingly evident that apoptosis is not a silent death, but instead apoptotic cells actively instruct the many players involved in executing an efficient, non-disruptive death. Here, we discuss some of the most intriguing signals produced by apoptotic cells that direct these coordinated responses and their importance in development and disease.
2. APOPTOTIC SIGNALS DICTATE IMMUNOLOGICAL RESPONSES TO CELL DEATH
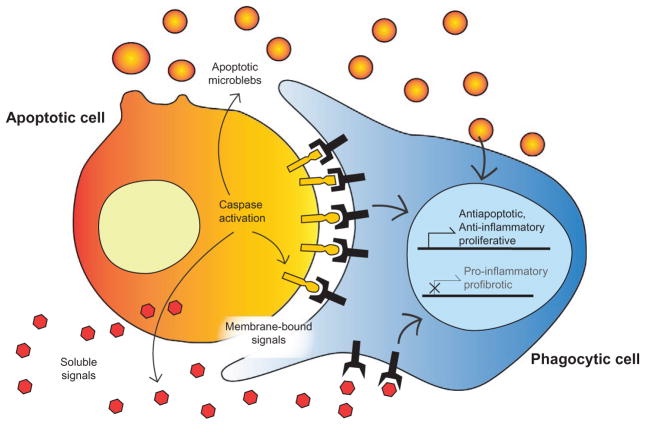
A key component of the apoptotic program is the efficient and controlled clearance of the dying cells before they become dangerous necrotic cells, spilling their contents haphazardly. The phagocytic removal of dying cells is often referred to as efferocytosis meaning “take to the grave” (Thorp, Subramanian, & Tabas, 2011). However, apoptotic cells do not go quietly to their final resting place. Instead, they communicate directly with professional phagocytes, such as macrophages, and nonprofessional phagocytes, such as engulfing epithelial cells. These signals are capable of directing appropriate prophagocytic and anti-inflammatory responses (Fig. 1).
Figure 1.
Apoptotic cells communicate with phagocytes to modulate immune responses to cell death. Apoptotic cells communicate with professional phagocytes such as macrophages and nonprofessional phagocytes such as engulfing epithelial cells. The apoptosis-derived signals include (1) soluable chemokines, (2) membrane-bound proteins and phospholipids, and (3) apoptotic microblebs. These signals are capable of directing appropriate anti-inflammatory responses promoting survival of neighboring healthy tissue.
2.1 “Find Me! Eat Me!”: Classic Chemoattractants and Phagocytic Signals
There are a myriad of classic signals that can lead phagocytic cells to sites of apoptotic cell death, which have been expertly review elsewhere (Chen, Zhao, & Liu, 2014; Hochreiter-Hufford & Ravichandran, 2013; Peter, Wesselborg, Herrmann, & Lauber, 2010; Poon, Lucas, Rossi, & Ravichandran, 2014). Here, we would like to highlight a few apoptosis-derived chemoattractant signals that are specifically produced via caspase activity.
2.1.1 Lysophosphatidylcholine
Lysophosphatidylcholine (LPC) is a phospholipid that was identified as one of the first truly caspase-derived “find me” signals. Caspase-3-mediated activation of calcium-independent phospholipase A2 (iPLA2) leads to the production of a variety of lipid-based signaling molecules. Production of LPC by iPLA2 specifically leads to the recruitment of phagocytes to the site of apoptosis (Lauber et al., 2003; Mueller, Sheriff, Gaipl, Wesselborg, & Lauber, 2007). Once phagocytes arrive, this phospholipid can also facilitate opsonization of apoptotic cells (Kim, Gershov, Ma, Brot, & Elkon, 2002).
2.1.2 Sphingosine-1-Phosphate
Sphingosine-1-phosphate (S1P) is another lipid-based chemoattractant that is produced directly following caspase activation (Gude et al., 2008). Upon triggering apoptosis, the expression of sphingosine kinase is upregulated in a caspase-dependent manner, leading to excess production of S1P. Gude et al. (2008) found that S1P is a potent attractant for monocytes and macrophages. Interestingly, S1P is also critically involved in regulating epithelial cell extrusion during apoptosis, as described later in this chapter (Gu, Forostyan, Sabbadini, & Rosenblatt, 2011; Gu et al., 2015).
2.1.3 Endothelial Monocyte-Activating Polypeptide II
Endothelial monocyte-activating polypeptide II (EMAP II) is a processed form of pro-EMAP/p43 that is shed by apoptotic cells and has the ability to serve as a monocyte chemoattractant (Knies et al., 1998). Interestingly, work by Behrensdorf, van de Craen, Knies, Vandenabeele, and Clauss (2000) suggested that this chemoattractant is released by caspases, specifically by Caspase-3 and Caspase-7-mediated cleavage (Behrensdorf et al., 2000). However, this study was limited to using mouse recombinant proteins in vitro. Later work by Zhang and Schwartz refuted the conclusion that pro-EMAP II is directly cleaved by caspases in humans, finding no evidence of caspase-3- or caspase-7-dependent release in human tumor cell cultures (Zhang & Schwarz, 2002). Adding to the controversy, other published reports have suggested that pro-EMAP II may be either cleaved or released in an unprocessed form, that it may come from either apoptotic cells or necrotic cells, and that it may be cleaved by elastases, metalloproteases, or calpains—all depending on which experimental parameters were tested (Martinet et al., 2010; Matschurat et al., 2003; van Horssen, Eggermont, & ten Hagen, 2006). These seeming conflicts in the literature might simply reflect a versatile “find me” signal and that EMAP II regulation is finely tuned by a variety of factors depending on the context of the dying cell. Future work may elucidate the full spectrum of EMAP II processing and function in recruiting professional phagocytes to sites of cell death, and may provide helpful clues to the apoptotic programs that direct this clearance signal.
2.2 “Listen to Me!”: Complex Modulatory Signals from Apoptotic Cells
In addition to the traditional functions of recruiting professional and non-professional phagocytes to the site of cell death, and promoting apoptotic cell removal, several of these apoptosis-derived immunoregulatory signals can also function in more nuanced modulatory pathways. Here, we highlight a few apoptosis-derived signals that were initially characterized in classic clearance pathways but have since been found to profoundly affect their surrounding environment by modulating the activities of engulfing phagocytes and surrounding progenitors.
2.2.1 Phosphatidylserine
Shortly following initiation of the apoptotic program, phosphatidylserine (PS) begins to accumulate on the outer leaflet of the dying cell’s membrane (Fadok et al., 1992; Martin et al., 1995). This exposure is critically required for recognition and engulfment of the dying cell by the phagocyte (Fadok, de Cathelineau, Daleke, Henson, & Bratton, 2001). In living cells, plasma membrane phospholipid asymmetry is maintained by flippases. Upon activation of effector caspases, these flippases are cleaved and inactivated, which contributes to PS accumulation on the outer membrane (Bratton et al., 1997; Chen, Mapes, Lee, Skeen-Gaar, & Xue, 2013; Mandal, Mazumder, Das, Kundu, & Basu, 2005; Martin, Finucane, Amarante-Mendes, O’Brien, & Green, 1996; Segawa et al., 2014). In addition, other enzymes such as the Xk-related family of scramblases are activated upon caspase cleavage and actively promote the transfer of PS to the outer leaflet (Suzuki, Imanishi, & Nagata, 2014). Interestingly, PS exposure on the apoptotic cell also dictates what type of immunological response should occur. Upon recognizing the apoptotic “eat me” signal, macrophages actively induce production of anti-inflammatory cytokines including TGFβ and prostaglandins, and suppress production of typical pro-inflammatory cytokines such as TNFα and IL-1β (Fadok et al., 1998). This prevents the further recruitment of other, potentially more damaging, immune cells such as neutrophils. These immunosuppressive programs are dependent of the activation of the PS receptor (Fadok et al., 2000; Huynh, Fadok, & Henson, 2002).
Strikingly, this finding can also be extended to nonprofessional phagocytes. Mammary gland involution involves the massive apoptosis, extrusion, and clearance of most milk-producing mammary epithelial cells within 72 h of ceasing lactation (Walker, Bennett, & Kerr, 1989). In this developmental context of physiological weaning, the dying milk cells are phagocytosed not by macrophages, but primarily by the remaining viable mammary epithelial cells (Monks et al., 2005; Monks, Smith-Steinhart, Kruk, Fadok, & Henson, 2008). Again, the apoptotic cells are capable of inducing this anti-inflammatory state in the tissue via activation of the PS receptor on the non-professional phagocytes. The engulfing epithelial cells then produce TGFβ and suppress the production of any pro-inflammatory cytokines (Monks et al., 2005).
2.2.2 Fractalkine
Fractalkine (FKN), also known as CX3CL1, is a membrane-bound chemokine that can facilitate intercellular interactions, or it can be cleaved by the TNFα-converting enzyme ADAM17 (Garton et al., 2001), diffusing away in search of cells bearing its receptor CX3CR1. Specifically, FKN is released by apoptotic cells to recruit professional phagocytes to the site of cell death (Sokolowski, Chabanon-Hicks, Han, Heffron, & Mandell, 2014; Truman et al., 2008; Tsai et al., 2014). Beyond simple recruitment, however, FKN can also enhance the ability of macrophages and microglia to execute their phagocytic functions (Miksa, Amin, Wu, Ravikumar, & Wang, 2007).
Neuronally derived FKN promotes survival of microglia under neurotoxic conditions, inhibiting Fas-mediated death via upregulation of anti-apoptotic Bcl family proteins (Boehme, Lio, Maciejewski-Lenoir, Bacon, & Conlon, 2000). At the same time, FKN also triggers activation of the phagocytic response to clear cellular debris and stress–response pathways to counteract any remaining neurotoxic molecules that caused the initial damage (Noda et al., 2011). In the cardiovascular system, FKN released from apoptotic cells exerts both antiapoptotic and mitogenic effects on neighboring vascular smooth muscle cells (White et al., 2010). These authors demonstrated that FKN released by apoptotic cells binds its receptor CX3CR1 on smooth muscle cells and induces the expression of epiregulin, an epidermal growth factor receptor ligand. FKN may also promote proper wound healing and regeneration by inhibiting fibrotic responses to cell death. Engel and colleagues found CX3CR1 in the kidneys to be important for inhibiting proliferation of profibrotic macrophages (Engel et al., 2015). Taken together, release of FKN from apoptotic cells appears to guide the immune response to minimize further tissue damage and preserve tissue function.
2.2.3 Apoptotic Microblebs
Blebbing of the apoptotic cellular membrane is easily seen on electron micrographs and was one of the early defining features that distinguished apoptotic cells from necrotic cells. Membrane blebbing is driven by caspase-mediated activation of the Rho-associated kinase ROCK I (Coleman et al., 2001; Sebbagh et al., 2001). These approximately five micron particles function as chemoattractants, recruiting monocytes to sites of apoptosis (Segundo et al., 1999) possibly based on the classic attractants they harbor on their membranes (Tsai et al., 2014). Upon calcium-activated release, these microblebs spread out through the intercellular spaces, potentially traveling great distances within the organism (Hoang, Rampon, Freyssinet, Vriz, & Kerbiriou-Nabias, 2011). For example, circulating apoptotic microblebs are found abundantly in patients with cardiovascular disease (Rautou et al., 2011), and directly correlate with the degree of disease-associated vascular endothelial dysfunction and cell death (Werner, Wassmann, Ahlers, Kosiol, & Nickenig, 2006). In an epidemiological study, higher levels of circulating microblebs are associated with greater risk of cardiovascular death, independent of other classic cardiovascular risk factors (Chistiakov, Orekhov, & Bobryshev, 2015; Sinning et al., 2011). Microblebs are therefore an intriguing potential biomarker for those patients in need of more aggressive therapy and risk management.
Beyond serving as a chemoattractant, and potential biomarker for disease, these circulating microblebs are also capable of altering the biological state of other cells, both near and far from the site of apoptosis. Jansen and colleagues reported that microblebs derived from apoptotic endothelial cells can be taken up by target surviving endothelial cells via the PS receptor. They are then capable of inhibiting p38 activity, thus promoting an anti-apoptotic state in the surviving cells (Jansen et al., 2012). The microblebs also carry the instructions to recruit endothelial progenitor cells to sites of excessive cell death, such as atherosclerotic plaques (Hristov, Erl, Linder, & Weber, 2004; Zernecke et al., 2009). These instructions include an enrichment of microRNA-126, which upon transfer to the recipient endothelial cell, promotes the increased production of CXCL12, a progenitor chemoattractant (Zernecke et al., 2009). Recruited progenitors in turn promote regeneration, stabilization of the plaque, and maintenance of vascular function. Therefore, apoptotic microblebs are versatile messengers, carrying whatever final instructions the apoptotic cell wished to communicate, allowing these signals to persist long after the dying cell corpse has been removed.
3. APOPTOTIC CELLS DIRECTLY INFLUENCE TISSUE HOMEOSTASIS AND GROWTH CONTROL
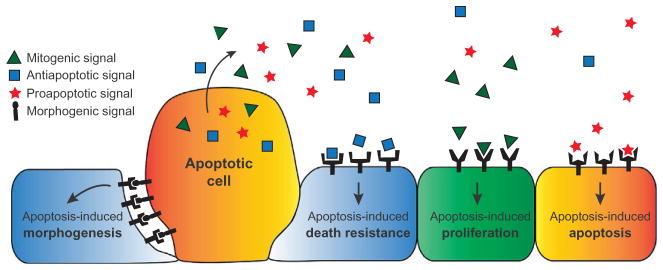
Kerr, Wylie, and Currie initially characterized apoptosis as the kinetic counterpoint to mitosis, a process that actively contributes to tissue homeostasis and maintenance of cell populations (Kerr et al., 1972). Yet, since that time, apoptosis has often been regarded as an altruistic cell death, indirectly affecting growth control through a passive death—a solitary and final action for the greater benefit of the tissue (Czabotar et al., 2014). An emerging field of study focuses on the pathways utilized by apoptotic cells to produce instructive signals influencing the growth state of neighboring survivor cells. Depending on the context, apoptotic cells are capable of producing proapoptotic, antiapoptotic, mitogenic, and morphogenetic signals that act directly on the surrounding tissue, without an immunological intermediate (Fig. 2).
Figure 2.
Apoptotic cells direct growth control. Apoptotic cells can produce a variety of signals that can have an effect on the growth state of the surviving tissue. The outcome of this communication can depend on both the amounts of signal produced and the receptivity of the surviving cell to these different stimuli.
3.1 Apoptosis-Induced Apoptosis: Communal Cell Death
One of the earliest descriptions of programmed cell death was the case of coordinated cell loss during embryogenesis (Glucksmann, 1951). Throughout development, there are several instances where entire populations of cells initiate apoptosis almost simultaneously. In certain contexts at least, this communal, or cohort, cell death is triggered by proapoptotic signals originating from the initial apoptotic cells (Perez-Garijo, Fuchs, & Steller, 2013). These authors found in developing Drosophila epithelial tissues (wing imaginal discs) that induction of cell death in one tissue compartment resulted in additional ectopic apoptosis in distant compartments. This apoptosis-induced apoptosis (AiA) is dependent on the production and release of the Drosophila TNFα ortholog, Eiger, from the initial population of dying cells. Interestingly, not all cells in the disc become apoptotic; healthy nonapoptotic cells persist between the two populations of dying cells. These intervening healthy cells are known to be particularly resistant to apoptotic stimuli, whereas the cells subject to AiA are known to be particularly susceptible (Milan, Campuzano, & Garcia-Bellido, 1997; Moon et al., 2005). This susceptibility requirement fits well with the observed phenomenon of rapid, complete, and specific cohort cell death.
Perez-Garijo et al. (2013) also investigated AiA in a mammalian system. The mammalian hair follicle experiences cohort cell death during the normal regressive phase of the hair cycle, and previous studies established that the coordination of this cell death is dependent on TNFα (Botchkareva, Ahluwalia, & Shander, 2006; Lindner et al., 1997; Tong & Coulombe, 2006). TNFα production was limited to apoptotic cells, and inhibition of TNFα signaling reduced the number of apoptotic cells. This apoptosis-derived feed-forward loop provides a mechanism for the observed cohort cell death in the hair follicle. However, the direct connection between caspase activation and TNFα production in both the fly and mammalian hair follicle remains to be determined.
AiA may also contribute to the complex cellular responses observed following radiation treatments or following certain pathological conditions. The radiation bystander effect includes the ability of irradiated cells to induce the death of healthy nonirradiated cells (Hei, Zhou, Chai, Ponnaiya, & Ivanov, 2011; Prise & O’Sullivan, 2009). Additionally, dying cells that are damaged by infection or ischemia are known to propagate death to neighboring cells (Barber, 2001). Most spreading death seems to be derived from necrotic cells, but it is possible in these cases there is a specific apoptotic component that drives AiA. Further research may determine if there are specific apoptosis-derived signals that mediate these pathological forms of cohort death.
3.2 Apoptosis-Induced Death Resistance: The Mahakali Effect
In contrast to the radiation bystander effect of AiA, recent studies in Drosophila have also found an apoptosis-induced death resistance (AiDR) program. Jaklevic et al. (2008) first noted that ionizing radiation of the developing wing disc generally increases the levels of bantam, a microRNA that stimulates cell proliferation and inhibits apoptosis by repressing the proapoptotic factor hid (Brennecke, Hipfner, Stark, Russell, & Cohen, 2003; Jaklevic et al., 2008). Additionally, bantam null animals were exceptionally susceptible to ionizing radiation, exhibiting increased apoptosis. However, follow-up studies by the same group revealed the surprising finding that when apoptosis is limited to a specific domain, the increase in bantam, and thus the antiapoptotic effect, was nonautonomous (Bilak, Uyetake, & Su, 2014). These authors found that AiDR, which they termed the “Mahakali” effect, was mediated by the receptor tyrosine kinase Tie on the surviving cell, and the apoptosis-dependent production of the Pvf1 ligand. Interestingly, Pvf1 was required, but not sufficient, to induce AiDR, suggesting that other apoptosis-derived signals may also be required to stimulate this prosurvival effect.
Angiopoeitin-1 is the mammalian ligand for Tie-2, and has been described as a nonautonomous apoptosis survival factor in human cell culture (Kwak, So, Lee, Kim, & Koh, 1999). Bilak et al. (2014) suggested that Angiopoeitin-1 may be released by neighboring cells to protect the endothelial cells; however, there is no evidence to date of AiDR in mammals.
3.3 Apoptosis-Induced Proliferation: Caspase-Driven Compensatory Proliferation
Compensatory proliferation can occur during regeneration of lost tissue via additional or accelerated cell divisions. In Drosophila, some of the first evidence for compensatory proliferation was uncovered by Haynie and Bryant when they demonstrated that up to sixty percent of cells in developing wing precursor tissue could be eliminated by radiation, and yet extra cell divisions within the surviving tissue resulted in a full-sized and normally functioning adult wing (Haynie & Bryant, 1977).
In 2004, it was reported that in the developing Drosophila epithelial tissues, induction of apoptotic cell death could induce nonautonomous proliferation in the surrounding cells (Huh, Guo, & Hay, 2004; Perez-Garijo, Martin, & Morata, 2004; Ryoo, Gorenc, & Steller, 2004). Since that time, there has been a mounting interest in the concept that this proliferation, under certain contexts, is driven explicitly by mitogenic signals produced by the dying cell. There is still controversy today regarding which signals are actually produced by the dying cell, versus which mitogenic factors may be produced elsewhere, but in the past decade numerous studies described below have validated the concept in both flies and mammals. Apoptosis-induced proliferation (AiP) therefore is a form of compensatory proliferation and is defined as the process by which apoptotic cells actively stimulate surviving cells to divide (Mollereau et al., 2013).
3.3.1 AiP in Drosophila Depends on the Environmental Context of the Dying Cell
In vivo studies of apoptotic cells and any apoptosis-derived signals during normal development are challenging due to the fleeting nature of the cell death and signal. However, in Drosophila, by capitalizing on the use of the effector caspase inhibitor P35, it is possible to examine the pathways driving AiP (Hay, Wolff, & Rubin, 1994; Huh et al., 2004; Perez-Garijo et al., 2004; Ryoo et al., 2004). Through misexpressing p35 in one compartment of the developing wing, at the same time as triggering an apoptotic stimulus, these researchers were able to uncouple the initiation of the apoptotic signaling cascade from the actual execution of cell death. This generates an “undead” state in which any apoptosis-derived signals are sustained. In this case, mitogen production was sustained, resulting in excessive proliferation and tissue overgrowth. Importantly, proliferation occurs in both the posterior compartment where the undead apoptotic cells exist, and in the genetically unaffected anterior region (Huh et al., 2004; Ryoo et al., 2004). This strongly suggested the presence of a secreted and diffusible mitogen. Two mitogens identified at the time included Wingless (Wg, an ortholog of Wnt) and Decapentaplegic (Dpp, ortholog of TGFβ) (Perez-Garijo et al., 2004; Ryoo et al., 2004). Follow-up work has also identified a role for Spitz (Spi, the EGF ortholog in Drosophila) (Fan et al., 2014). In the undead model, these mitogens are produced in response to c-Jun N-terminal kinase (JNK) activity in the undead cells and are required for tissue overgrowth (Bergantinos, Corominas, & Serras, 2010; Fan et al., 2014; Ryoo et al., 2004). However, in genuine models of AiP that do not rely on p35 expression, the requirement and source of these mitogens is still under debate (Martin, Perez-Garijo, & Morata, 2009; Perez-Garijo, Martin, Struhl, & Morata, 2005; Perez-Garijo, Shlevkov, & Morata, 2009; Smith-Bolton, Worley, Kanda, & Hariharan, 2009).
A common hypothesis in these studies had been that, following apoptosis induction, there is a bifurcation in the signaling cascade that ultimately results in both nonautonomous proliferation and autonomous execution of cell death. In this specific context, the bifurcation is at the level of the Drosophila initiator caspase, Dronc (Caspase-9) (Fan et al., 2014; Huh et al., 2004; Kondo, Senoo-Matsuda, Hiromi, & Miura, 2006; Wells, Yoshida, & Johnston, 2006). In genuine apoptotic cells, Dronc activates the effector caspases Drice and Dcp-1 (Caspase-3 and -7), as well as a currently unidentified target upstream of JNK activation, leading to compensatory proliferation (Fan et al., 2014). In undead cells, effector caspase activity is inhibited, and so Dronc continuously signals for compensatory proliferation, thus driving the tissue to hyperproliferation. Thus, the undead model is a potentially fascinating tool to understand how AiP may play a role in the development of cancers (Bergmann & Steller, 2010; Ryoo & Bergmann, 2012).
Interestingly, this Dronc-dependent signaling cascade only applies to epithelial cells that have not begun terminal differentiation. Undifferentiated, actively proliferating tissue exists in the wing and the anterior of the eye imaginal disc. However, in the posterior eye disc differentiated photoreceptors induce AiP by a completely different mechanism (Fan & Bergmann, 2008a, 2008b). Here, apoptotic photoreceptor cells trigger a Drice- and Dcp-1-dependent cascade that leads to release of Hedgehog (Hh). Hh secreted from these apical cells then stimulates the underlying unspecified progenitor cells to reenter the cell cycle and proliferate.
The idea of context-dependent apoptosis-derived mitogenic signals has opened up this field to a wealth of possibilities. Ongoing work is investigating the spectrum of AiP-derived mitogenic signals in the flies as well as the context-specific determinants of survivor receptivity to proliferative signals.
3.3.2 AiP in Mammals: Phoenix Rising and Tumor Repopulation
Unlike developmental apoptosis which is neatly confined to small cell populations or well-defined compartments, the cell death associated with trauma often cuts across large swaths of cell types at varying developmental stages. Therefore, one of the most visible characteristics of mammalian wound healing and tissue regeneration is the infiltration of immune cells to the wound site (Haertel, Werner, & Schafer, 2014; Muller, Meyer, & Werner, 2012). This inflammatory reaction precedes regeneration, and the infiltrating cells disperse after the wound resolves. Therefore, it was long assumed that this type of regeneration required proliferative signals from the inflammatory cells. Immune cells are certainly capable of producing a wide variety of cytokines and growth factors, which in many cases likely do contribute to healing growth. However, PU.1 knockout mice lack the inflammatory cells typically associated with wound healing, including macrophages and neutrophils (Scott, Simon, Anastasi, & Singh, 1994), yet these animals are capable of fully repairing damage to their skin (Martin et al., 2003). This demonstrated that there is something intrinsic to the wounded tissue that can also stimulate regrowth and healing. Based on this observation, Li et al. hypothesized that the initial proliferative signal could be derived directly from the damaged tissue, specifically apoptotic cells (Li et al., 2010). This group established a method to test in in vitro coculture assays the effect of a large number of irradiated dying cells on a smaller population of fluorescently labeled nonirradiated surviving cells. Excitingly, in these assays, dying cells stimulated proliferation of the fluorescently labeled cells via Caspase-3-dependent activation of calcium-independent phospholipase A2 (iPLA2). This enzyme had previously been identified as a caspase cleavage target important for the release of phospholipid derived “find me” signals (see Section 2.1.1) (Atsumi et al., 1998; Lauber et al., 2003). In this model of regeneration, however, the important signal produced by iPLA2 is prostaglandin E2 (PGE2) which promotes stem and progenitor cell proliferation (Hagedorn, Durand, Fast, & Zon, 2014). Li et al. (2010) termed this mammalian regenerative AiP pathway “Phoenix Rising.”
Following this critical mammalian study, a series of reports found that the hypothetical scenario posited by the invertebrate field does in fact exist in mammals: The Phoenix Rising pathway can be co-opted in cancer and can contribute to tumor repopulation following radiation and chemotherapy (Huang et al., 2011). These findings were validated in breast cancer, melanoma, and pancreatic ductal adenocarcinoma cell lines (Cheng et al., 2015; Donato et al., 2014; Kurtova et al., 2015).
This Caspase-3/iPLA2/PGE2 signaling cascade can be found in dying tumor cells promoting growth of surviving tumor cells, but also extends to dying vascular endothelial cells promoting tumor cell growth. One treatment considered for many solid tumors is antiangiogenic therapy to limit oxygen to the developing tumor mass (Sitohy, Nagy, & Dvorak, 2012). However, if dying vascular endothelial cells are also capable of activating the Caspase-3/iPLA2/PGE2 cascade, such as in one study of glioma cells, targeting this AiP pathway may be critically important for successful therapy (Mao, Smith, Xie, & Wang, 2013).
In addition to the Caspase-3/iPLA2/PGE2 signaling, in certain cancer cell lines such as the Panc1 line derived from ductal carcinoma, there is also a role for 7-PKCδ-Akt/p38 MAPK-stimulated mitogen production (Cheng et al., 2015). Also, there is evidence of Sonic Hedgehog modulating AiP, feeding back on dying cells in a paracrine manner to regulate WNT signaling via SFRP1 (secreted frizzled related protein 1). SFRP1 acts as an antagonist of WNT signaling, which allows for tumor repopulation (Ma, Cheng, Gong, Tian, & Huang, 2015).
These human cell line studies, taken with the in vivo work in Drosophila, would suggest that AiP is likely a commonly used mechanism to promote regeneration, whether normal or cancerous. Historically, cancer has been called a wound that will not heal (Dvorak, 1986). It is possible that the very pathways designed to promote wound healing are the ones promoting tumor growth. Most notably, given the diverse signals already cataloged, it is very likely that we have only begun to uncover the many paths to AiP, which will vary based on the dying cell, the surviving cell, the developmental context, and the surrounding microenvironment.
3.4 Apoptosis-Induced Morphogenesis: Directed Maintenance of Tissue Integrity
Finally, independent of the growth state of the surrounding environment, apoptotic cells can have a profound effect on the organization of the surviving tissue. Apoptosis-derived signals can direct tissue reorganization and promote maintenance of tissue integrity and function.
3.4.1 Apoptosis-Directed Extrusion from Epithelial Layers
When stromal cells or individual immune cells activate an apoptotic program, their clearance and subsequent absence has relatively little effect on the structure of their surrounding environment. However, if an epithelial cell activates apoptosis, its unexpected loss could result in a gap or discontinuity of the functional layer where it resides. Proper extrusion of the dying cell from this layer maintains epithelial integrity and preserves its barrier function. Work by Rosenblatt, Raff, and Cramer initially determined that proper extrusion was dependent on changes to the cytoskeleton in both the dying and neighboring cells, but that the process originated from the apoptotic cell (Rosenblatt, Raff, & Cramer, 2001). Follow-up studies determined that this coordinated cytoskeletal rearrangement is in fact dependent on a caspase-mediated signal from the apoptotic cell (Andrade & Rosenblatt, 2011). S1P produced by the apoptotic cell (see Section 2.1.2) binds to the G-protein-coupled receptor S1P2 on neighboring live cells (Gu et al., 2011). Interestingly, Gu et al. noted that S1P2 expression is reduced in a number of cancers (Gu et al., 2015). Their investigation centered on evaluating the effects of blocking S1P activity by (1) reducing S1P levels, (2) reducing S1P2 levels, or (3) blocking their interaction by neutralizing antibody. All of these conditions resulted in defects in proper apical extrusion, and commonly resulted in cells being extruded basally. In this scenario, Gu et al. proposed that transformed cells are more likely to persist in this basal compartment and potentially invade and metastasize. Enhancing proper apical extrusion could reduce tumorigenesis.
3.4.2 Apoptosis-Induced Sprouting Morphogenesis
An overlooked domain in cell biology, though not a signal in the usual sense, biophysical properties of dying cells can even serve to communicate with the surrounding environment. Apoptotic cells direct the sprouting morphogenesis of vascular endothelial cells based on the accumulated negative charges on the apoptotic cell membrane (Weihua, Tsan, Schroit, & Fidler, 2005). Specifically, negatively charged apoptotic membranes attract hyperpolarized endothelial cell membrane extensions, which then provide the scaffold for recruited proliferating endothelial cells. Revascularization following injury is important for wound healing, and angiogenesis is critically involved in tumor progression. Better understanding of the biophysical properties of apoptotic cells, and how these properties complement or contradict secreted signals could potentially provide mechanisms to enhance or inhibit these angiogenic events.
4. APOPTOSIS: A LOUD DEATH
Under normal physiological conditions, we should not think of dead apoptotic cells. Instead, there are actively dying apoptotic cells that interact quite extensively with their environment to coordinate a host of physiological processes. These dying cells are eventually cleared and digested by phagocytes, which cease their instructive signals and resolves the death process. Before this clearance, however, apoptotic cells actively produce signals, both secreted and membrane bound, that serve to dictate their own immunologic control and fate, as well as direct growth control to ensure tissue integrity and homeostasis.
A common concern often presented in the literature is the seemingly contradictory effects of dying cells on their neighbors. This has been especially true, when researchers are working in the same tissue such as the Drosophila wing discs. How can the same dying cells promote cell death and cell proliferation? The reality of the apoptotic cell is likely much more complex than we have outlined here. Dying cells may produce any combination of these signals depending on their predeath state, the specific mode of apoptotic induction, the presence or absence of immune cells as signals are being released, or feedback from the surrounding immune cells and micro-environment. Additionally, signals from dying apoptotic cells will only have as much effect on neighboring cells as those neighbors are receptive to hearing them. Progrowth and prodeath signals may originate from the same dying cell, with the winning signal dependent on the current state of the neighbor. While apoptotic death is rapid, it is not instantaneous, and in that dying moment, it is anything but silent.
Acknowledgments
We would like to thank our colleagues for helpful discussions during the course of this work. We apologize to those whose work may not have been cited due to space restrictions. We would like to acknowledge Christopher MacKay for his assistance with the artwork. C.E.F. would like to thank the UMMS MD/PhD program for ongoing support. This work was supported by the NIH (GM068016 and GM107789).
References
- Abrams JM, White K, Fessler LI, Steller H. Programmed cell death during Drosophila embryogenesis. Development. 1993;117(1):29–43. doi: 10.1242/dev.117.1.29. [DOI] [PubMed] [Google Scholar]
- Andrade D, Rosenblatt J. Apoptotic regulation of epithelial cellular extrusion. Apoptosis. 2011;16(5):491–501. doi: 10.1007/s10495-011-0587-z. http://dx.doi.org/10.1007/s10495-011-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsumi G, Tajima M, Hadano A, Nakatani Y, Murakami M, Kudo I. Fas-induced arachidonic acid release is mediated by Ca2+–independent phospholipase A2 but not cytosolic phospholipase A2, which undergoes proteolytic inactivation. The Journal of Biological Chemistry. 1998;273(22):13870–13877. doi: 10.1074/jbc.273.22.13870. [DOI] [PubMed] [Google Scholar]
- Bar PR. Apoptosis—The cell’s silent exit. Life Sciences. 1996;59(5–6):369–378. doi: 10.1016/0024-3205(96)00315-3. [DOI] [PubMed] [Google Scholar]
- Barber GN. Host defense, viruses and apoptosis. Cell Death and Differentiation. 2001;8(2):113–126. doi: 10.1038/sj.cdd.4400823. http://dx.doi.org/10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- Behrensdorf HA, van de Craen M, Knies UE, Vandenabeele P, Clauss M. The endothelial monocyte-activating polypeptide II (EMAP II) is a substrate for caspase-7. FEBS Letters. 2000;466(1):143–147. doi: 10.1016/s0014-5793(99)01777-9. [DOI] [PubMed] [Google Scholar]
- Bergantinos C, Corominas M, Serras F. Cell death-induced regeneration in wing imaginal discs requires JNK signalling. Development. 2010;137(7):1169–1179. doi: 10.1242/dev.045559. http://dx.doi.org/10.1242/dev.045559. 137/7/1169 [pii] [DOI] [PubMed] [Google Scholar]
- Bergmann A. The role of ubiquitylation for the control of cell death in Drosophila. Cell Death and Differentiation. 2010;17(1):61–67. doi: 10.1038/cdd.2009.70. http://dx.doi.org/10.1038/cdd.2009.70. cdd200970 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann A, Steller H. Apoptosis, stem cells, and tissue regeneration. Science Signaling. 2010;3(145):re8. doi: 10.1126/scisignal.3145re8. http://dx.doi.org/10.1126/scisignal.3145re8. scisignal.3145re8 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilak A, Uyetake L, Su TT. Dying cells protect survivors from radiation-induced cell death in Drosophila. PLoS Genetics. 2014;10(3):e1004220. doi: 10.1371/journal.pgen.1004220. http://dx.doi.org/10.1371/journal.pgen.1004220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehme SA, Lio FM, Maciejewski-Lenoir D, Bacon KB, Conlon PJ. The chemokine fractalkine inhibits Fas-mediated cell death of brain microglia. Journal of Immunology. 2000;165(1):397–403. doi: 10.4049/jimmunol.165.1.397. [DOI] [PubMed] [Google Scholar]
- Botchkareva NV, Ahluwalia G, Shander D. Apoptosis in the hair follicle. The Journal of Investigative Dermatology. 2006;126(2):258–264. doi: 10.1038/sj.jid.5700007. http://dx.doi.org/10.1038/sj.jid.5700007. [DOI] [PubMed] [Google Scholar]
- Bratton DL, Fadok VA, Richter DA, Kailey JM, Guthrie LA, Henson PM. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. The Journal of Biological Chemistry. 1997;272(42):26159–26165. doi: 10.1074/jbc.272.42.26159. [DOI] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113(1):25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Brustugun OT, Fladmark KE, Doskeland SO, Orrenius S, Zhivotovsky B. Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death and Differentiation. 1998;5(8):660–668. doi: 10.1038/sj.cdd.4400399. http://dx.doi.org/10.1038/sj.cdd.4400399. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Mapes J, Lee ES, Skeen-Gaar RR, Xue D. Caspase-mediated activation of Caenorhabditis elegans CED-8 promotes apoptosis and phosphatidylserine externalization. Nature Communications. 2013;4:2726. doi: 10.1038/ncomms3726. http://dx.doi.org/10.1038/ncomms3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhao Y, Liu Y. The role of nucleotides and purinergic signaling in apoptotic cell clearance—Implications for chronic inflammatory diseases. Frontiers in Immunology. 2014;5:656. doi: 10.3389/fimmu.2014.00656. http://dx.doi.org/10.3389/fimmu.2014.00656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, Tian L, Ma J, Gong Y, Zhang Z, Chen Z, et al. Dying tumor cells stimulate proliferation of living tumor cells via caspase-dependent protein kinase Cδ activation in pancreatic ductal adenocarcinoma. Molecular Oncology. 2015;9(1):105–114. doi: 10.1016/j.molonc.2014.07.024. http://dx.doi.org/10.1016/j.molonc.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chistiakov DA, Orekhov AN, Bobryshev YV. Extracellular vesicles and atherosclerotic disease. Cellular and Molecular Life Sciences. 2015;72:2697–2708. doi: 10.1007/s00018-015-1906-2. http://dx.doi.org/10.1007/s00018-015-1906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nature Cell Biology. 2001;3(4):339–345. doi: 10.1038/35070009. http://dx.doi.org/10.1038/35070009. [DOI] [PubMed] [Google Scholar]
- Conradt B. Genetic control of programmed cell death during animal development. Annual Review of Genetics. 2009;43:493–523. doi: 10.1146/annurev.genet.42.110807.091533. http://dx.doi.org/10.1146/annurev.genet.42.110807.091533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford ED, Seaman JE, Barber AE, 2nd, David DC, Babbitt PC, Burlingame AL, et al. Conservation of caspase substrates across metazoans suggests hierarchical importance of signaling pathways over specific targets and cleavage site motifs in apoptosis. Cell Death and Differentiation. 2012;19(12):2040–2048. doi: 10.1038/cdd.2012.99. http://dx.doi.org/10.1038/cdd.2012.99. cdd201299 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: Implications for physiology and therapy. Nature Reviews. Molecular Cell Biology. 2014;15(1):49–63. doi: 10.1038/nrm3722. http://dx.doi.org/10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Steller H. Pathways regulating apoptosis during patterning and development. Current Opinion in Genetics & Development. 2007;17(4):294–299. doi: 10.1016/j.gde.2007.05.009. http://dx.doi.org/10.1016/j.gde.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AL, Huang Q, Liu X, Li F, Zimmerman MA, Li CY. Caspase 3 promotes surviving melanoma tumor cell growth after cytotoxic therapy. The Journal of Investigative Dermatology. 2014;134(6):1686–1692. doi: 10.1038/jid.2014.18. http://dx.doi.org/10.1038/jid.2014.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. The New England Journal of Medicine. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. http://dx.doi.org/10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Ellis HM, Horvitz HR. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44(6):817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391(6662):43–50. doi: 10.1038/34112. http://dx.doi.org/10.1038/34112. [DOI] [PubMed] [Google Scholar]
- Engel DR, Krause TA, Snelgrove SL, Thiebes S, Hickey MJ, Boor P, et al. CX3CR1 reduces kidney fibrosis by inhibiting local proliferation of profibrotic macrophages. Journal of Immunology. 2015;194(4):1628–1638. doi: 10.4049/jimmunol.1402149. http://dx.doi.org/10.4049/jimmunol.1402149. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of Clinical Investigation. 1998;101(4):890–898. doi: 10.1172/JCI1112. http://dx.doi.org/10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok VA, Bratton DL, Rose DM, Pearson A, Ezekewitz RA, Henson PM. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000;405(6782):85–90. doi: 10.1038/35011084. http://dx.doi.org/10.1038/35011084. [DOI] [PubMed] [Google Scholar]
- Fadok VA, de Cathelineau A, Daleke DL, Henson PM, Bratton DL. Loss of phospholipid asymmetry and surface exposure of phosphatidylserine is required for phagocytosis of apoptotic cells by macrophages and fibroblasts. The Journal of Biological Chemistry. 2001;276(2):1071–1077. doi: 10.1074/jbc.M003649200. http://dx.doi.org/10.1074/jbc.M003649200. [DOI] [PubMed] [Google Scholar]
- Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. Journal of Immunology. 1992;148(7):2207–2216. [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Apoptosis-induced compensatory proliferation. The Cell is dead. Long live the Cell! Trends in Cell Biology. 2008a;18(10):467–473. doi: 10.1016/j.tcb.2008.08.001. http://dx.doi.org/10.1016/j.tcb.2008.08.001. S0962-8924(08)00212-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Bergmann A. Distinct mechanisms of apoptosis-induced compensatory proliferation in proliferating and differentiating tissues in the Drosophila eye. Developmental Cell. 2008b;14(3):399–410. doi: 10.1016/j.devcel.2008.01.003. http://dx.doi.org/10.1016/j.devcel.2008.01.003. S1534-5807(08)00031-2 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Wang S, Hernandez J, Yenigun VB, Hertlein G, Fogarty CE, et al. Genetic models of apoptosis-induced proliferation decipher activation of JNK and identify a requirement of EGFR signaling for tissue regenerative responses in Drosophila. PLoS Genetics. 2014;10(1):e1004131. doi: 10.1371/journal.pgen.1004131. http://dx.doi.org/10.1371/journal.pgen.1004131. PGENETICS-D-12-01255 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease (Research Support, Non-U.S. Gov’t Review) Cell. 2011;147(4):742–758. doi: 10.1016/j.cell.2011.10.033. http://dx.doi.org/10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton KJ, Gough PJ, Blobel CP, Murphy G, Greaves DR, Dempsey PJ, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1) The Journal of Biological Chemistry. 2001;276(41):37993–38001. doi: 10.1074/jbc.M106434200. http://dx.doi.org/10.1074/jbc.M106434200. [DOI] [PubMed] [Google Scholar]
- Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. The Journal of Cell Biology. 1992;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glucksmann A. Cell deaths in normal vertebrate ontogeny. Biological Reviews of the Cambridge Philosophical Society. 1951;26(1):59–86. doi: 10.1111/j.1469-185x.1951.tb00774.x. [DOI] [PubMed] [Google Scholar]
- Gu Y, Forostyan T, Sabbadini R, Rosenblatt J. Epithelial cell extrusion requires the sphingosine-1-phosphate receptor 2 pathway. The Journal of Cell Biology. 2011;193(4):667–676. doi: 10.1083/jcb.201010075. http://dx.doi.org/10.1083/jcb.201010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Shea J, Slattum G, Firpo MA, Alexander M, Mulvihill SJ, et al. Defective apical extrusion signaling contributes to aggressive tumor hallmarks. eLife. 2015;4:e04069. doi: 10.7554/eLife.04069. http://dx.doi.org/10.7554/eLife.04069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gude DR, Alvarez SE, Paugh SW, Mitra P, Yu J, Griffiths R, et al. Apoptosis induces expression of sphingosine kinase 1 to release sphingosine-1-phosphate as a “come-and-get-me” signal. The FASEB Journal. 2008;22(8):2629–2638. doi: 10.1096/fj.08-107169. http://dx.doi.org/10.1096/fj.08-107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haertel E, Werner S, Schafer M. Transcriptional regulation of wound inflammation. Seminars in Immunology. 2014;26(4):321–328. doi: 10.1016/j.smim.2014.01.005. http://dx.doi.org/10.1016/j.smim.2014.01.005. [DOI] [PubMed] [Google Scholar]
- Hagedorn EJ, Durand EM, Fast EM, Zon LI. Getting more for your marrow: Boosting hematopoietic stem cell numbers with PGE2. Experimental Cell Research. 2014;329(2):220–226. doi: 10.1016/j.yexcr.2014.07.030. http://dx.doi.org/10.1016/j.yexcr.2014.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994;120(8):2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Haynie JL, Bryant PJ. The effects of X-rays on the proliferation dynamics of cells in the imaginal wing disc of Drosophila melanogaster. Development Genes and Evolution. 1977;183(2):85–100. doi: 10.1007/BF00848779. [DOI] [PubMed] [Google Scholar]
- Hei TK, Zhou H, Chai Y, Ponnaiya B, Ivanov VN. Radiation induced non-targeted response: Mechanism and potential clinical implications. Current Molecular Pharmacology. 2011;4(2):96–105. doi: 10.2174/1874467211104020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TQ, Rampon C, Freyssinet JM, Vriz S, Kerbiriou-Nabias D. A method to assess the migration properties of cell-derived microparticles within a living tissue. Biochimica et Biophysica Acta. 2011;1810(9):863–866. doi: 10.1016/j.bbagen.2011.05.003. http://dx.doi.org/10.1016/j.bbagen.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Hochreiter-Hufford A, Ravichandran KS. Clearing the dead: Apoptotic cell sensing, recognition, engulfment, and digestion. Cold Spring Harbor Perspectives in Biology. 2013;5(1):a008748. doi: 10.1101/cshperspect.a008748. http://dx.doi.org/10.1101/cshperspect.a008748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood. 2004;104(9):2761–2766. doi: 10.1182/blood-2003-10-3614. http://dx.doi.org/10.1182/blood-2003-10-3614. [DOI] [PubMed] [Google Scholar]
- Huang Q, Li F, Liu X, Li W, Shi W, Liu FF, et al. Caspase 3-mediated stimulation of tumor cell repopulation during cancer radiotherapy. Nature Medicine. 2011;17(7):860–866. doi: 10.1038/nm.2385. http://dx.doi.org/10.1038/nm.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA. Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Current Biology. 2004;14(14):1262–1266. doi: 10.1016/j.cub.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Huynh ML, Fadok VA, Henson PM. Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. The Journal of Clinical Investigation. 2002;109(1):41–50. doi: 10.1172/JCI11638. http://dx.doi.org/10.1172/JCI11638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklevic B, Uyetake L, Wichmann A, Bilak A, English CN, Su TT. Modulation of ionizing radiation-induced apoptosis by bantam microRNA in Drosophila. Developmental Biology. 2008;320(1):122–130. doi: 10.1016/j.ydbio.2008.04.043. http://dx.doi.org/10.1016/j.ydbio.2008.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen F, Yang X, Hoyer FF, Paul K, Heiermann N, Becher MU, et al. Endothelial microparticle uptake in target cells is annexin I/phosphatidylserine receptor dependent and prevents apoptosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(8):1925–1935. doi: 10.1161/ATVBAHA.112.253229. http://dx.doi.org/10.1161/ATVBAHA.112.253229. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. British Journal of Cancer. 1972;26(4):239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Gershov D, Ma X, Brot N, Elkon KB. I-PLA(2) activation during apoptosis promotes the exposure of membrane lysophosphatidylcholine leading to binding by natural immunoglobulin M antibodies and complement activation. The Journal of Experimental Medicine. 2002;196(5):655–665. doi: 10.1084/jem.20020542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knies UE, Behrensdorf HA, Mitchell CA, Deutsch U, Risau W, Drexler HC, et al. Regulation of endothelial monocyte-activating polypeptide II release by apoptosis. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(21):12322–12327. doi: 10.1073/pnas.95.21.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Senoo-Matsuda N, Hiromi Y, Miura M. DRONC coordinates cell death and compensatory proliferation. Molecular and Cellular Biology. 2006;26(19):7258–7268. doi: 10.1128/MCB.00183-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtova AV, Xiao J, Mo Q, Pazhanisamy S, Krasnow R, Lerner SP, et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature. 2015;517(7533):209–213. doi: 10.1038/nature14034. http://dx.doi.org/10.1038/nature14034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HJ, So JN, Lee SJ, Kim I, Koh GY. Angiopoietin-1 is an apoptosis survival factor for endothelial cells. FEBS Letters. 1999;448(2–3):249–253. doi: 10.1016/s0014-5793(99)00378-6. [DOI] [PubMed] [Google Scholar]
- Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, et al. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell. 2003;113(6):717–730. doi: 10.1016/s0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Li F, Huang Q, Chen J, Peng Y, Roop DR, Bedford JS, et al. Apoptotic cells activate the “phoenix rising” pathway to promote wound healing and tissue regeneration. Science Signaling. 2010;3(110):ra13. doi: 10.1126/scisignal.2000634. http://dx.doi.org/10.1126/scisignal.2000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LY, Luo X, Wang X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature. 2001;412(6842):95–99. doi: 10.1038/35083620. http://dx.doi.org/10.1038/35083620. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Lindner G, Botchkarev VA, Botchkareva NV, Ling G, van der Veen C, Paus R. Analysis of apoptosis during hair follicle regression (catagen) The American Journal of Pathology. 1997;151(6):1601–1617. [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zou H, Slaughter C, Wang X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell. 1997;89(2):175–184. doi: 10.1016/s0092-8674(00)80197-x. [DOI] [PubMed] [Google Scholar]
- Lockshin RA, Williams CM. Programmed cell death—I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. Journal of Insect Physiology. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- Ma J, Cheng J, Gong Y, Tian L, Huang Q. Wnt signaling downregulated after sonic hedgehog activation negatively associates with tumor repopulation. Disease Models & Mechanisms. 2015;8(4):385–391. doi: 10.1242/dmm.018887. http://dx.doi.org/10.1242/dmm.018887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal D, Mazumder A, Das P, Kundu M, Basu J. Fas-, caspase 8-, and caspase 3-dependent signaling regulates the activity of the aminophospholipid translocase and phosphatidylserine externalization in human erythrocytes. The Journal of Biological Chemistry. 2005;280(47):39460–39467. doi: 10.1074/jbc.M506928200. http://dx.doi.org/10.1074/jbc.M506928200. [DOI] [PubMed] [Google Scholar]
- Mao P, Smith L, Xie W, Wang M. Dying endothelial cells stimulate proliferation of malignant glioma cells via a caspase 3-mediated pathway. Oncology Letters. 2013;5(5):1615–1620. doi: 10.3892/ol.2013.1223. http://dx.doi.org/10.3892/ol.2013.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P, D’Souza D, Martin J, Grose R, Cooper L, Maki R, et al. Wound healing in the PU.1 null mouse—Tissue repair is not dependent on inflammatory cells. Current Biology. 2003;13(13):1122–1128. doi: 10.1016/s0960-9822(03)00396-8. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Finucane DM, Amarante-Mendes GP, O’Brien GA, Green DR. Phosphatidylserine externalization during CD95-induced apoptosis of cells and cytoplasts requires ICE/CED-3 protease activity. The Journal of Biological Chemistry. 1996;271(46):28753–28756. doi: 10.1074/jbc.271.46.28753. [DOI] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Morata G. Apoptosis in Drosophila: Compensatory proliferation and undead cells. The International Journal of Developmental Biology. 2009;53(8–10):1341–1347. doi: 10.1387/ijdb.072447fm. http://dx.doi.org/10.1387/ijdb.072447fm. 072447fm [pii] [DOI] [PubMed] [Google Scholar]
- Martin SJ, Reutelingsperger CP, McGahon AJ, Rader JA, van Schie RC, LaFace DM, et al. Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: Inhibition by over-expression of Bcl-2 and Abl. The Journal of Experimental Medicine. 1995;182(5):1545–1556. doi: 10.1084/jem.182.5.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinet W, De Meyer I, Cools N, Timmerman V, Bult H, Bosmans J, et al. Cell death-mediated cleavage of the attraction signal p43 in human atherosclerosis: Implications for plaque destabilization. Arteriosclerosis, Thrombosis, and Vascular Biology. 2010;30(7):1415–1422. doi: 10.1161/ATVBAHA.110.206029. http://dx.doi.org/10.1161/ATVBAHA.110.206029. [DOI] [PubMed] [Google Scholar]
- Matschurat S, Knies UE, Person V, Fink L, Stoelcker B, Ebenebe C, et al. Regulation of EMAP II by hypoxia. The American Journal of Pathology. 2003;162(1):93–103. doi: 10.1016/S0002-9440(10)63801-1. http://dx.doi.org/10.1016/S0002-9440(10)63801-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksa M, Amin D, Wu R, Ravikumar TS, Wang P. Fractalkine-induced MFG-E8 leads to enhanced apoptotic cell clearance by macrophages. Molecular Medicine. 2007;13(11–12):553–560. doi: 10.2119/2007-00019.Miksa. http://dx.doi.org/10.2119/2007-00019.Miksa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellido A. Developmental parameters of cell death in the wing disc of Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(11):5691–5696. doi: 10.1073/pnas.94.11.5691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura M, Zhu H, Rotello R, Hartwieg EA, Yuan J. Induction of apoptosis in fibroblasts by IL-1 beta-converting enzyme, a mammalian homolog of the C. elegans cell death gene ced-3. Cell. 1993;75(4):653–660. doi: 10.1016/0092-8674(93)90486-a. [DOI] [PubMed] [Google Scholar]
- Mollereau B, Perez-Garijo A, Bergmann A, Miura M, Gerlitz O, Ryoo HD, et al. Compensatory proliferation and apoptosis-induced proliferation: A need for clarification. Cell Death and Differentiation. 2013;20(1):181. doi: 10.1038/cdd.2012.82. http://dx.doi.org/10.1038/cdd.2012.82. cdd201282 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks J, Rosner D, Geske FJ, Lehman L, Hanson L, Neville MC, et al. Epithelial cells as phagocytes: Apoptotic epithelial cells are engulfed by mammary alveolar epithelial cells and repress inflammatory mediator release. Cell Death and Differentiation. 2005;12(2):107–114. doi: 10.1038/sj.cdd.4401517. http://dx.doi.org/10.1038/sj.cdd.4401517. [DOI] [PubMed] [Google Scholar]
- Monks J, Smith-Steinhart C, Kruk ER, Fadok VA, Henson PM. Epithelial cells remove apoptotic epithelial cells during post-lactation involution of the mouse mammary gland. Biology of Reproduction. 2008;78(4):586–594. doi: 10.1095/biolreprod.107.065045. http://dx.doi.org/10.1095/biolreprod.107.065045. [DOI] [PubMed] [Google Scholar]
- Moon NS, Frolov MV, Kwon EJ, Di Stefano L, Dimova DK, Morris EJ, et al. Drosophila E2F1 has context-specific pro- and antiapoptotic properties during development. Developmental Cell. 2005;9(4):463–475. doi: 10.1016/j.devcel.2005.08.015. http://dx.doi.org/10.1016/j.devcel.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Mueller RB, Sheriff A, Gaipl US, Wesselborg S, Lauber K. Attraction of phagocytes by apoptotic cells is mediated by lysophosphatidylcholine. Autoimmunity. 2007;40(4):342–344. doi: 10.1080/08916930701356911. http://dx.doi.org/10.1080/08916930701356911. [DOI] [PubMed] [Google Scholar]
- Muller AK, Meyer M, Werner S. The roles of receptor tyrosine kinases and their ligands in the wound repair process. Seminars in Cell & Developmental Biology. 2012;23(9):963–970. doi: 10.1016/j.semcdb.2012.09.015. http://dx.doi.org/10.1016/j.semcdb.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Noda M, Doi Y, Liang J, Kawanokuchi J, Sonobe Y, Takeuchi H, et al. Fractalkine attenuates excito-neurotoxicity via microglial clearance of damaged neurons and antioxidant enzyme heme oxygenase-1 expression. The Journal of Biological Chemistry. 2011;286(3):2308–2319. doi: 10.1074/jbc.M110.169839. http://dx.doi.org/10.1074/jbc.M110.169839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Fuchs Y, Steller H. Apoptotic cells can induce non-autonomous apoptosis through the TNF pathway. eLife. 2013;2:e01004. doi: 10.7554/eLife.01004. http://dx.doi.org/10.7554/eLife.01004. 01004 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G. Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development. 2004;131(22):5591–5598. doi: 10.1242/dev.01432. [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Struhl G, Morata G. Dpp signaling and the induction of neoplastic tumors by caspase-inhibited apoptotic cells in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(49):17664–17669. doi: 10.1073/pnas.0508966102. http://dx.doi.org/10.1073/pnas.0508966102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Garijo A, Shlevkov E, Morata G. The role of Dpp and Wg in compensatory proliferation and in the formation of hyperplastic overgrowths caused by apoptotic cells in the Drosophila wing disc. Development. 2009;136(7):1169–1177. doi: 10.1242/dev.034017. http://dx.doi.org/10.1242/dev.034017. dev.034017 [pii] [DOI] [PubMed] [Google Scholar]
- Peter C, Wesselborg S, Herrmann M, Lauber K. Dangerous attraction: Phagocyte recruitment and danger signals of apoptotic and necrotic cells. Apoptosis. 2010;15(9):1007–1028. doi: 10.1007/s10495-010-0472-1. http://dx.doi.org/10.1007/s10495-010-0472-1. [DOI] [PubMed] [Google Scholar]
- Poon IK, Lucas CD, Rossi AG, Ravichandran KS. Apoptotic cell clearance: Basic biology and therapeutic potential. Nature Reviews. Immunology. 2014;14(3):166–180. doi: 10.1038/nri3607. http://dx.doi.org/10.1038/nri3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prise KM, O’Sullivan JM. Radiation-induced bystander signalling in cancer therapy. Nature Reviews. Cancer. 2009;9(5):351–360. doi: 10.1038/nrc2603. http://dx.doi.org/10.1038/nrc2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautou PE, Vion AC, Amabile N, Chironi G, Simon A, Tedgui A, et al. Microparticles, vascular function, and atherothrombosis. Circulation Research. 2011;109(5):593–606. doi: 10.1161/CIRCRESAHA.110.233163. http://dx.doi.org/10.1161/CIRCRESAHA.110.233163. [DOI] [PubMed] [Google Scholar]
- Rosenblatt J, Raff MC, Cramer LP. An epithelial cell destined for apoptosis signals its neighbors to extrude it by an actin- and myosin-dependent mechanism. Current Biology. 2001;11(23):1847–1857. doi: 10.1016/s0960-9822(01)00587-5. [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Bergmann A. The role of apoptosis-induced proliferation for regeneration and cancer. Cold Spring Harbor Perspectives in Biology. 2012;4(8):a008797. doi: 10.1101/cshperspect.a008797. http://dx.doi.org/10.1101/cshperspect.a008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Developmental Cell. 2004;7(4):491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Schwartz LM, Smith SW, Jones ME, Osborne BA. Do all programmed cell deaths occur via apoptosis? Proceedings of the National Academy of Sciences of the United States of America. 1993;90(3):980–984. doi: 10.1073/pnas.90.3.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott EW, Simon MC, Anastasi J, Singh H. Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science. 1994;265(5178):1573–1577. doi: 10.1126/science.8079170. [DOI] [PubMed] [Google Scholar]
- Sebbagh M, Renvoize C, Hamelin J, Riche N, Bertoglio J, Breard J. Caspase-3-mediated cleavage of ROCK I induces MLC phosphorylation and apoptotic membrane blebbing. Nature Cell Biology. 2001;3(4):346–352. doi: 10.1038/35070019. http://dx.doi.org/10.1038/35070019. [DOI] [PubMed] [Google Scholar]
- Segawa K, Kurata S, Yanagihashi Y, Brummelkamp TR, Matsuda F, Nagata S. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344(6188):1164–1168. doi: 10.1126/science.1252809. http://dx.doi.org/10.1126/science.1252809. [DOI] [PubMed] [Google Scholar]
- Segundo C, Medina F, Rodriguez C, Martinez-Palencia R, Leyva-Cobian F, Brieva JA. Surface molecule loss and bleb formation by human germinal center B cells undergoing apoptosis: Role of apoptotic blebs in monocyte chemotaxis. Blood. 1999;94(3):1012–1020. [PubMed] [Google Scholar]
- Sinning JM, Losch J, Walenta K, Bohm M, Nickenig G, Werner N. Circulating CD31+/Annexin V+ microparticles correlate with cardiovascular outcomes. European Heart Journal. 2011;32(16):2034–2041. doi: 10.1093/eurheartj/ehq478. http://dx.doi.org/10.1093/eurheartj/ehq478. [DOI] [PubMed] [Google Scholar]
- Sitohy B, Nagy JA, Dvorak HF. Anti-VEGF/VEGFR therapy for cancer: Reassessing the target. Cancer Research. 2012;72(8):1909–1914. doi: 10.1158/0008-5472.CAN-11-3406. http://dx.doi.org/10.1158/0008-5472.CAN-11-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Bolton RK, Worley MI, Kanda H, Hariharan IK. Regenerative growth in Drosophila imaginal discs is regulated by Wingless and Myc. Developmental Cell. 2009;16(6):797–809. doi: 10.1016/j.devcel.2009.04.015. http://dx.doi.org/10.1016/j.devcel.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolowski JD, Chabanon-Hicks CN, Han CZ, Heffron DS, Mandell JW. Fractalkine is a “find-me” signal released by neurons undergoing ethanol-induced apoptosis. Frontiers in Cellular Neuroscience. 2014;8:360. doi: 10.3389/fncel.2014.00360. http://dx.doi.org/10.3389/fncel.2014.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397(6718):441–446. doi: 10.1038/17135. http://dx.doi.org/10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Suzanne M, Steller H. Shaping organisms with apoptosis. Cell Death and Differentiation. 2013;20(5):669–675. doi: 10.1038/cdd.2013.11. http://dx.doi.org/10.1038/cdd.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki J, Imanishi E, Nagata S. Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. The Journal of Biological Chemistry. 2014;289(44):30257–30267. doi: 10.1074/jbc.M114.583419. http://dx.doi.org/10.1074/jbc.M114.583419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorp E, Subramanian M, Tabas I. The role of macrophages and dendritic cells in the clearance of apoptotic cells in advanced atherosclerosis. European Journal of Immunology. 2011;41(9):2515–2518. doi: 10.1002/eji.201141719. http://dx.doi.org/10.1002/eji.201141719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Coulombe PA. Keratin 17 modulates hair follicle cycling in a TNFalpha-dependent fashion. Genes & Development. 2006;20(10):1353–1364. doi: 10.1101/gad.1387406. http://dx.doi.org/10.1101/gad.1387406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truman LA, Ford CA, Pasikowska M, Pound JD, Wilkinson SJ, Dumitriu IE, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. doi: 10.1182/blood-2008-06-162404. http://dx.doi.org/10.1182/blood-2008-06-162404. [DOI] [PubMed] [Google Scholar]
- Tsai WH, Shih CH, Feng SY, Li IT, Chang SC, Lin YC, et al. CX3CL1(+) microparticles mediate the chemoattraction of alveolar macrophages toward apoptotic acute promyelocytic leukemic cells. Cellular Physiology and Biochemistry. 2014;33(3):594–604. doi: 10.1159/000358637. http://dx.doi.org/10.1159/000358637. [DOI] [PubMed] [Google Scholar]
- van Horssen R, Eggermont AM, ten Hagen TL. Endothelial monocyte-activating polypeptide-II and its functions in (patho)physiological processes. Cytokine & Growth Factor Reviews. 2006;17(5):339–348. doi: 10.1016/j.cytogfr.2006.08.001. http://dx.doi.org/10.1016/j.cytogfr.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Walker NI, Bennett RE, Kerr JF. Cell death by apoptosis during involution of the lactating breast in mice and rats. The American Journal of Anatomy. 1989;185(1):19–32. doi: 10.1002/aja.1001850104. http://dx.doi.org/10.1002/aja.1001850104. [DOI] [PubMed] [Google Scholar]
- Weihua Z, Tsan R, Schroit AJ, Fidler IJ. Apoptotic cells initiate endothelial cell sprouting via electrostatic signaling. Cancer Research. 2005;65(24):11529–11535. doi: 10.1158/0008-5472.CAN-05-2718. http://dx.doi.org/10.1158/0008-5472.CAN-05-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells BS, Yoshida E, Johnston LA. Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Current Biology. 2006;16(16):1606–1615. doi: 10.1016/j.cub2006.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner N, Wassmann S, Ahlers P, Kosiol S, Nickenig G. Circulating CD31 +/annexin V+ apoptotic microparticles correlate with coronary endothelial function in patients with coronary artery disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 2006;26(1):112–116. doi: 10.1161/01.ATV.0000191634.13057.15. http://dx.doi.org/10.1161/01.ATV.0000191634.13057.15. [DOI] [PubMed] [Google Scholar]
- White GE, Tan TC, John AE, Whatling C, McPheat WL, Greaves DR. Fractalkine has anti-apoptotic and proliferative effects on human vascular smooth muscle cells via epidermal growth factor receptor signalling. Cardiovascular Research. 2010;85(4):825–835. doi: 10.1093/cvr/cvp341. http://dx.doi.org/10.1093/cvr/cvp341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Woodfield SE, Lee TV, Fan Y, Antonio C, Bergmann A. Genetic control of programmed cell death (apoptosis) in Drosophila. Fly (Austin) 2009;3(1):78–90. doi: 10.4161/fly.3.1.7800. http://dx.doi.org/10.4161/fly.3.1.7800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 beta-converting enzyme. Cell. 1993;75(4):641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Science Signaling. 2009;2(100):ra81. doi: 10.1126/scisignal.2000610. http://dx.doi.org/10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- Zhang FR, Schwarz MA. Pro-EMAP II is not primarily cleaved by caspase-3 and –7. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2002;282(6):L1239–L1244. doi: 10.1152/ajplung.00141.2001. http://dx.doi.org/10.1152/ajplung.00141.2001. [DOI] [PubMed] [Google Scholar]
- Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90(3):405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]