Abstract
African Americans suffer a higher prevalence of hypertension compared to other racial/ethnic groups. In this study, we performed a pharmacogenomics genome-wide association study of blood pressure response to β-blockers in African Americans with uncomplicated hypertension. Genome-wide meta-analysis was performed in 318 African American hypertensive participants in the two Pharmacogenomic Evaluation of Antihypertensive Responses studies: 150 treated with atenolol monotherapy and 168 treated with metoprolol monotherapy. The analysis adjusted for age, gender, baseline BP and principal components for ancestry. Genome-wide significant variants with p < 5*10−8 and suggestive variants with p < 5*10−7 were evaluated in an additional cohort of 141 African Americans treated with the addition of atenolol to hydrochlorothiazide treatment. The validated variants were then meta-analyzed in these 3 groups of African Americans. Two variants discovered in the monotherapy meta-analysis were validated in the add-on therapy. African American participants heterozygous for SLC25A31 rs201279313 deletion vs wild-type genotype had better diastolic blood pressure response to atenolol monotherapy, metoprolol monotherapy and atenolol add-on therapy: −9.3 vs. −4.6, −9.6 vs. −4.8 and −9.7 vs. −6.4 mmHg, respectively (3-group meta-analysis p= 2.5*10−8, beta = −4.42 mmHg per variant allele). Similarly, LRRC15 rs11313667 was validated for systolic blood pressure response to β-blocker therapy with 3-group meta-analysis p = 7.2 *10−8 and beta = −3.65 mmHg per variant allele. In this first pharmacogenomics genome-wide meta-analysis of BP response to β-blockers in African Americans, we identified novel variants that may provide valuable information for personalized antihypertensive treatment in this group.
Keywords: pharmacogenomics, GWAS, beta-blockers, PEAR, blood pressure response, African Americans
Introduction
Hypertension is the most common modifiable risk factor for cardiovascular disease and death 1. It affects about one in three adults of ≥ 20 years of age in the United States, representing about 80 million American adults 2. The most recent estimate of the population of non-Hispanic African Americans in the United States is 40.8 million 3. African American adults have among the highest prevalence of hypertension in the world, with age-adjusted prevalence of 44.9% and 46.1% for men and women, respectively 2. Compared with European Americans, hypertension in African Americans has an earlier onset, greater severity and higher rate of target organ damage, contributing to decreased longevity 4. Lowering blood pressure (BP) with antihypertensive therapy has been shown to be beneficial even in patients with mild hypertension 5. According to the NHANES 2007-2012 data, the BP control rates were 41.4% and 55.9% in non-Hispanic black males and females, compared to 54.0% and 58.7% in non-Hispanic white males and females, respectively 2. There is considerable inter-individual variability in blood pressure (BP) response to all classes of antihypertensive drugs 6, 7. Current selection of the initial antihypertensive therapy for uncomplicated hypertension individuals is essentially by trial-and-error. Pharmacogenomics has the potential to individualize antihypertensive drug therapy and lead to better BP control and improve the long term outcomes of those with hypertension.
Among all individuals in the US over age 65, approximately 32% are prescribed a β-blocker 8. β-blockers are often viewed as a suboptimal antihypertensive choice in African Americans overall despite recognition that BP response to any single antihypertensive drug is characterized by large inter-individual variation 9, 10. In this study, we performed the first pharmacogenomics genome-wide meta-analysis of BP response to β-blockers in African Americans to shed light on pharmacogenomics signals that may explain this observed variability in response.
Methods
Study Participants
The Pharmacogenomic Evaluation of Antihypertensive Responses (PEAR): PEAR-1 and PEAR-2 Studies
The PEAR-1 study (clinicaltrials.gov identifier NCT00246519) was a multicenter randomized controlled clinical trial evaluating genetic determinants of BP responses to atenolol and hydrochlorothiazide (HCTZ) monotherapy and combination therapy of the two drugs11. The PEAR-2 study was a multicenter sequential monotherapy clinical trial (clinicaltrials.gov identifier NCT01203852) 12 evaluating genetic determinants of BP response to metoprolol monotherapy and chlorthalidone monotherapy. Both PEAR-1 and PEAR-2 studies included individuals with mild to moderate essential hypertension. Individuals with diabetes and known cardiovascular disease were excluded. The details of these two studies are summarized in the Supplemental materials. The study protocols for both the PEAR-1 and PEAR 2 studies were reviewed and approved by the Institutional Review Boards at all sites recruiting subjects (University of Florida, Gainesville, FL; Emory University, Atlanta, GA; Mayo Clinic, Rochester, MN and East Coast Institute for Research, Jacksonville, FL (PEAR-2 only)) and all participants provided voluntary, written informed consent prior to participation. This analysis focuses on the African American BP response to atenolol mono and add-on therapy in the PEAR-1 study and the BP response to metoprolol monotherapy in the PEAR-2 study.
Blood pressure phenotype
In the PEAR-1 study, BP was measured using three different methods: home, office and ambulatory 11. For genome-wide association analysis, we used the most precise phenotype available, which is a composite weighted average of the office, home, ambulatory daytime and nighttime BP responses calculated based on the row sums of the inverse of the inter-method covariance matrices 13. This weighted average blood pressure had higher signal-to-noise ratio and therefore provides greater power to detect genetic differences compared to any single component measurement of the composite BP response phenotype 13.
In the PEAR-2 study, home and office BP measurements were collected. We have previously demonstrated that home BP is the more informative single BP measurement compared to the office BP measurement 13. Therefore, home BP is the phenotype used for the genome-wide association analysis for PEAR-2. We have demonstrated 13 that different methods of measure BP response to antihypertensive drug therapy measure the same signal but with different signal-to-noise ratios. This allows us to perform the meta-analysis of PEAR1 and PEAR2 to identify the real BP response signal.
Genotyping and Imputation
The details on the genome-wide genotyping, quality control and imputation performed on PEAR-1 and PEAR-2 participants are presented in the Supplemental Materials.
Statistical Analysis
Continuous variables were presented as mean and standard deviation and categorical variables were presented as numbers and percentages. t test, Wilcoxon rank sum test or chi-square test were used to compare the individuals treated with atenolol or metoprolol monotherapy as appropriate. Spearman correlation was performed between baseline plasma renin activity (PRA) and BP response. Genome-wide association analysis for BP response was performed in African American hypertensive patients treated with atenolol monotherapy (n = 150) and metoprolol monotherapy (n = 168) separately. Multiple linear regression analysis was performed adjusting for baseline BP, age, gender and the first two principal components for ancestry. Variables such as body mass index and smoking were evaluated but were not associated with the BP response, therefore not included in the regression model. The additive mode of inheritance was assumed, where imputed dosages of the SNPs were used in the linear regression model. Meta-analysis of monotherapy response was then performed using METAL 14. SNPs with p < 5*10−8 were considered genome-wide significant and those with p < 5*10−7 were considered suggestive. To validate the genome-wide significant and the suggestive SNPs in an independent group of participants, we assessed the association of these SNPs and BP response in PEAR African American participants treated with atenolol add-on therapy (n = 141). We considered SNPs with p < 0.05 with same direction of association as was observed in the monotherapy analysis to be validated, and performed 3-group meta-analysis for the SNPs that achieved that level. As a sensitivity analysis, the association was also adjusted for pre-treatment PRA.
RESULTS
The relevant baseline characteristics of the African American participants treated with atenolol monotherapy and those treated with metoprolol monotherapy are summarized in Table 1. The participants treated with atenolol monotherapy were significantly younger (47 vs. 50 years old on average) than those treated with metoprolol (p = 0.006). Atenolol-treated participants were predominantly female (71%), while females represented 53% of those treated with metoprolol (p = 0.0008). The pre-treatment BP (145.1/93.9 mmHg) in the atenolol group was lower than in the metoprolol group (147.5/95.6 mmHg, p = 0.045/0.017). The baseline PRA was similar between atenolol monotherapy (median of 0.37 ng/ml/h) and metoprolol monotherapy (median of 0.41 ng/ml/h).
Table 1.
Baseline characteristics of PEAR African American participants.
| Characteristics* | Atenolol monotherapy (n = 150) |
Metoprolol monotherapy (n = 168) |
Atenolol add- on (n = 141) |
|---|---|---|---|
| Age (mean ± SD) | 47.2 ± 8.5 | 50.0 ± 9.2 | 47.4 ± 8.5 |
| Female gender (n, %) | 107 (71.3%) | 89 (53.0%) | 103 (72.5%) |
| BMI (kg/m2) | 31.6 ± 6.3 | 30.8 ± 5.2 | 31.8 ± 6.2 |
| Duration of hypertension (years) | 6.4 ± 6.8 | 6.8 ± 5.5 | 6.5 ± 6.7 |
| Taking at least 1 antihypertensive drug at entry | 105 (70.0%) | 122 (72.6%) | 100 (70.4%) |
| Smoker (ever) | 52 (34.1%) | 70 (41.7%) | 18 (13%) |
| Baseline office blood pressure | |||
| Systolic (mmHg) | 151.3 ± 12.1 | 150.8 ± 13.2 | 142.4 ± 16.9 |
| Diastolic (mmHg) | 99.0 ± 5.7 | 98.7 ± 6.1 | 90.8 ± 10.1 |
| Baseline Home Blood pressure |
|||
| Systolic (mmHg) | 145.1 ± 10.5 | 147.5 ± 10.7 | 141.9 ± 12.9 |
| Diastolic (mmHg) | 93.9 ± 6.5 | 95.6 ± 6.1 | 89.5 ± 8.1 |
| Plasm renin activity (ng/ml/h), median (interquartile range) |
0.37 (0.21- 0.73) |
0.41 (0.26- 0.74) |
0.93 (0.42- 2.21) |
Numeric characteristics were presented as mean± standard deviation or median and interquartile range if not normally distributed; categorical variables were presented as number and percentages.
Abbreviation: SD: standard deviation; BMI: body mass index.
BP response to atenolol and metoprolol
In the PEAR-1 study, the median length of atenolol monotherapy was 70 days (with interquartile range of 64 – 77days). Eighty-nine percent of patients had the atenolol dose titrated to 100 mg/day; in those who did not have the dose titrated from 50 mg/day, the most common reason was a heart rate < 55 bpm. The mean BP response was −3.2/−4.2 mmHg after atenolol monotherapy. There was no significant difference in BP response between those treated with higher dose (−3.3/−4.1 mmHg) vs. with lower dose (−2.4/−5.0 mmHg) (p = 0.75 and 0.60 for SBP and DBP response, respectively). Also there was no correlation between the length of monotherapy and the BP response (p = 0.94 for SBP response vs. length of treatment correlation and p = 0.89 for DBP response vs. length of treatment correlation).
In the PEAR-2 study, approximately 96% of the patients had their metoprolol dose titrated and the median length of monotherapy was 58 days (interquartile range: 56 - 64 days). The average BP response was −4.7/−5.8 mmHg after metoprolol monotherapy. Similar to response to atenolol, the BP response was not correlated with the length of the treatment (p = 0.77 for SBP response and p = 0.66 for DBP response) or the dose of metoprolol (p = 0.39 for SBP response and p = 0.11 for DBP response).
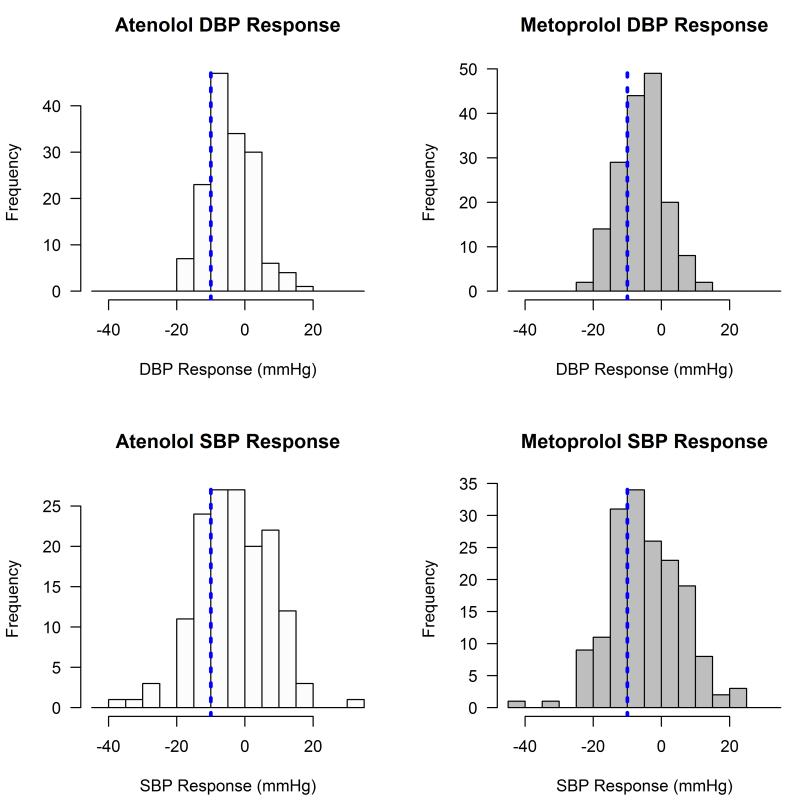
Figure 1 demonstrates the substantial inter-individual variability in BP response to both atenolol and metoprolol observed in our African American hypertensive participants. While the average SBP reduction was around 3 ~ 5 mmHg, approximately 26% and 32% of the African American participants had a greater than 10 mmHg SBP reduction in response to atenolol and metoprolol, respectively.
Figure 1.
Distribution of blood pressure response to atenolol and metoprolol in African American participants in PEAR. Vertical reference lines: −10mmHg in blood pressure response.
In both groups of individuals treated with atenolol monotherapy and metoprolol monotherapy, the BP response was negatively correlated with the baseline PRA. The Spearman correlation coefficients were −0.25 (p = 0.0017) between baseline PRA and DBP response to atenolol and −0.20 (p = 0.0098) between baseline PRA and DBP response to metoprolol. The tertile of individuals with most DBP reduction in response to atenolol had significantly higher baseline PRA (median of 0.54 and interquartile range of 0.28-1.05 ng/ml/h) than those with least DBP reduction with median baseline PRA of 0.30 (0.16 – 0.50) ng/ml/h (p = 0.002). Similarly, the baseline PRA for those with most DBP lowering in response to metoprolol monotherapy had significantly higher baseline PRA (0.45 (0.30 −0.85) ng/ml/h than those with least DBP lowering (0.31 (0.23-0.58) ng/ml/h) (p = 0.008).
Genome-wide Analysis of BP response to beta-blockers in African Americans
SNPs associated with BP response to atenolol in the genome-wide analysis with suggestive significance (p < 5*10−7) are listed in Table S1. One SNP (rs61128812) on Chromosome 20p12.1 reached genome-wide significance, with p = 1.68*10−8 for association with DBP response to atenolol. Top SNPs associated with BP response to metoprolol are summarized in Table S2. One insertion/deletion variant (rs138781672) on chromosome 12 was associated with SBP response to metoprolol with p = 2.76*10−8, which also achieved genome wide significance.
In the meta-analysis of BP response to atenolol or metoprolol monotherapy, 1 SNP was genome-wide significant with p < 5*10−8. The Manhattan plots are shown in Figure S1. The SNPs with p < 5*10−7 are summarized in Table 2 for DBP response and Table 3 for SBP response, which represented 8 unique signals/regions. An intronic SNP, rs1367094 in the ZMAT4 (zinc finger matrix type 4) gene was associated DBP response to β-blocker monotherapy (meta-analysis p = 2.82*10−8) (Figure S2). African American hypertensive participants with the TT genotype of rs1367094 had a DBP response of −6.0 mmHg when treated with atenolol and −6.6 mmHg when treated with metoprolol, while those with CT genotype had +1.1 mmHg in response to atenolol and −2.3 mmHg in response to metoprolol (p = 1.62*10−6 for atenolol and 0.0027 for metoprolol) (Figure S3). This SNP was also associated with SBP response to these specific β-blockers in the meta-analysis (p = 1.55*10−7) (Table 3).
Table 2.
Top SNPs associated with diastolic blood pressure response to beta-blocker monotherapy in the meta-analysis with p < 5*10−7.
| SNP | CHR: Position | Nearest Gene | function | A1 | A2 | Freq_A1 | Beta | SE | P |
|---|---|---|---|---|---|---|---|---|---|
| rs1367094 | 8:40402374 | ZMAT4 | Intronic | T | C | 0.91 | −5.34 | 0.96 | 2.82E-08 |
| rs75165759 | 12:23922338 | SOX5 | intronic | T | C | 0.98 | −10.03 | 1.89 | 1.07E-07 |
| rs201279313 | 4:128657040:TTTA_ | SLC25A31 | intronic | D | R | 0.09 | −5.13 | 0.97 | 1.10E-07 |
| rs10456462 | 6:38166511 | BTBD9 | intronic | A | G | 0.18 | 3.32 | 0.64 | 2.33E-07 |
| rs7160625 | 14:35812484 | RP11- 561B11.3 |
intergenic | T | C | 0.16 | 3.36 | 0.65 | 2.60E-07 |
| rs143451596 | 3:175443589 | NAALADL2 | intronic | T | C | 0.97 | 7.88 | 1.55 | 3.98E-07 |
| rs3125734 | 10:63958112 | RTKN2 | missense | T | C | 0.64 | 2.58 | 0.51 | 4.38E-07 |
| rs7832003 | 8:10782004 | XKR6 | intronic | C | G | 0.13 | −3.51 | 0.70 | 4.81E-07 |
CHR: chromosome; Position: hg19 position. A1: allele 1; A2: allele 2. Freq: frequency; beta: regression coefficient for allele 1; SE: standard error of the beta coefficient. D: deletion; R: reference allele
Table 3.
Top SNPs associated with systolic blood pressure response to beta-blocker monotherapy in the meta-analysis with p < 5*10−7.
| SNP | CHR:position | Nearest Gene |
function | A1 | A2 | Freq_A1 | Beta | SE | P |
|---|---|---|---|---|---|---|---|---|---|
| rs76558488 | 5:10131285 | CTD- 2199O4.1 |
intergenic | A | G | 0.04 | 12.11 | 2.26 | 8.60E-08 |
| rs6663367 | 1:167147189 | RP11- 277B15.2 |
intergenic | A | C | 0.03 | 11.76 | 2.21 | 1.09E-07 |
| rs11313667 | 3:194082161:TC_T | LRRC15 | intronic | D | R | 0.74 | −4.90 | 0.93 | 1.49E-07 |
| rs1367094 | 8:40402374 | ZMAT4 | intronic | T | C | 0.91 | −7.92 | 1.51 | 1.55E-07 |
| rs142849141 | 11:87432794:TA_T | RP11- 720D4.3 |
intergenic | D | R | 0.07 | 7.58 | 1.48 | 2.99E-07 |
| rs114380097 | 3:30873418 | GADL1 | intronic | T | C | 0.95 | −10.76 | 2.10 | 3.04E-07 |
| rs35909283 | 8:70897741 | RN5S270 | intergenic | A | C | 0.04 | 11.67 | 2.31 | 4.19E-07 |
| rs7807739 | 7:50699557 | GRB10 | intronic | T | C | 0.05 | −9.08 | 1.80 | 4.61E-07 |
CHR: chromosome; Position: hg19 position. A1: allele 1; A2: allele 2. D: deletion; R: reference allele; Freq: frequency; Beta: regression coefficient for allele 1; SE: standard error of the beta coefficient
Validation in BP response to atenolol add-on therapy
In order to validate the association of the SNPs in an independent patient group, we evaluated the association of the suggestive SNPs/regions (8 regions for DBP association and 8 regions for SBP association, respectively) in the only available African American β-blocker treated clinical trial cohort, to our knowledge: the 141 African Americans hypertensive patients in PEAR study who had atenolol added to HCTZ monotherapy (demographics presented in Table 1).
The ZMAT4 SNP (rs1367094) that reached genome-wide significance in African American DBP response association in the monotherapy meta-analysis was not associated with the BP response to the atenolol add-on therapy. The p value was 0.71 for rs1367094 in DBP response to atenolol add-on therapy, with a beta of −0.37 mmHg/allele, the same direction as in the monotherapy. The result for SBP response was similar, with p of 0.66 and beta of −0.63 mmHg/allele.
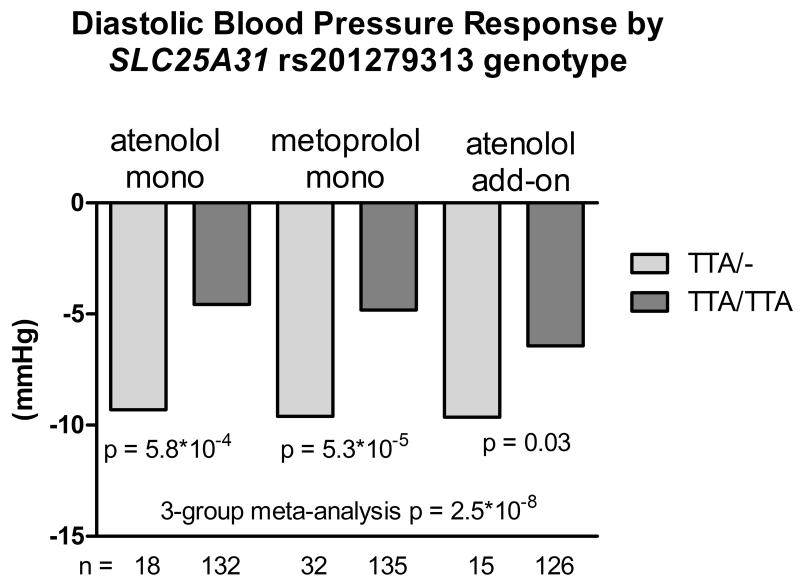
One variant (rs201279313) in the SLC25A31 gene was validated in the DBP response to the atenolol add-on therapy, with the same direction of effect compared to the monotherapy group. The regional plot of this variant is shown in Figure S4. This variant is a deletion of ‘TTA’ in the intronic region. The deletion allele was associated with greater BP reduction after β-blocker treatment. In the African American participants treated with atenolol monotherapy, those with the heterozygous genotype had a DBP response of −9.3 mmHg compared to −4.6 mmHg in those with the wild-type genotype of this variant (p = 5.8*10−4). The DBP responses to metoprolol monotherapy were −9.6 and −4.8 mmHg in heterozygous and wild-type participants, respectively (p = 5.3*10−5). In the atenolol add-on therapy, the DBP responses were −9.7 vs. −6.4 mmHg for the heterozygotes and wild-type individuals, respectively (p = 0.033). The meta-analysis of the atenolol monotherapy, metoprolol monotherapy and atenolol add-on therapy yielded p value of 2.5*10−8 and beta of −4.42 mmHg per deletion allele (Figure 2). The p value of meta-analysis remained significant (4.81*10−8) after controlling for pre-treatment PRA.
Figure 2.
Diastolic blood pressure response to atenolol monotherapy, metoprolol monotherapy and atenolol add-on therapy in African Americans by SLC25A31 rs201279313 genotype.
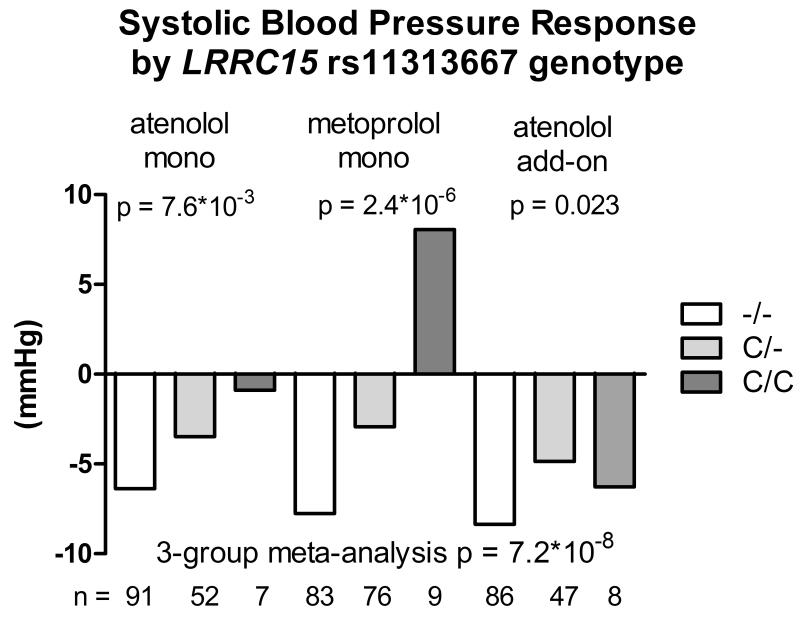
Likewise, one variant validated for SBP response in the atenolol add-on therapy. The deletion allele of rs11313667 (C/-, MAF=16%), located in the intronic region of LRRC15 gene on chromosome 3 (regional plot shown in Figure S5), was associated with better SBP response to β-blocker monotherapy (beta = −4.9 mmHg, p = 1.49*10−7) and was nominally associated with better SBP response to atenolol add-on therapy (beta = −2.3 mmHg, p = 0.023). The p value for the 3-group meta-analysis was 7.16*10−8 with a beta of −3.65 mmHg per deletion allele (Figure 3). After controlling for pre-treatment PRA, the p value for the meta-analysis was slightly weaker (1.58*10−7).
Figure 3.
Systolic blood pressure response to atenolol monotherapy, metoprolol monotherapy and atenolol add-on therapy in African Americans by LRRC15 rs11313667 genotype.
DISCUSSION
This is the first pharmacogenomic genome-wide association study of BP response to β-blockers reported in African American hypertensive patients. Two variants that were selected for follow-up based on meta-analysis of responses to atenolol and metoprolol validated in the analysis of African American hypertensive participants treated with atenolol add-on therapy, with meta-analysis p value of one achieving genome-wide significance. The variants were both deletion alleles, rs201279313 in SLC25A31 for DBP response (p = 2.5*10−8 in the 3-group meta-analysis) and rs11313667 in LRRC15 for SBP response (p = 7.2*10−8 in the 3-group meta-analysis). We also identified 1 SNP with genome-wide significance in the meta-analysis of BP response to β-blocker monotherapy: rs1367094 (an intronic SNP in gene ZMAT4) for DBP response, but this SNP did not validate when tested in the atenolol add-on therapy cohort.
Atenolol and metoprolol are both cardioselective β-blockers that block the action of the sympathetic nervous system, in particular on β1 adrenergic receptors (β1 adrenoceptors) that are mainly expressed in the heart and kidneys. β-blockers are often overlooked as potent suppressors of renin secretion and presumably of angiotensin II formation 15. Sympathetic nervous system activation is usually accompanied by increased renin secretion 16. Accordingly, the BP lowering of β-blockers is greatest in patients with high PRA and least in those with low PRA 17-19. Hypertensive patients of African descent on average have lower PRA and therefore less BP lowering when treated with β-blockers compared to those of European ancestry 20. However, we have shown that 20-30% of African Americans hypertensive participants responded well to β-blocker treatment. Consistent with prior reports, we found that those with higher baseline PRA responded better to atenolol or metoprolol. This highlights that β-blocker therapy can be a very effective antihypertensive drug class for a portion of African Americans. Identifying these patients a priori (based on not only PRA but also independent genetic variants) could provide valuable information on the optimal antihypertensive treatment for this group of African Americans.
In this genome-wide analysis study, two variants were validated across 3 cohorts (and two β-blockers) as associated with BP response to beta-blockers, one associated with DBP response and the other associated with SBP response. The variant rs201279313 that was validated for DBP response is the deletion of a TTA in the intronic region of SLC25A31, solute carrier family 25 member 31, on chromosome 4q28.1. The SLC25A31 gene encodes ADP/ATP translocase 4 (ANT4), which inhibits apoptosis by catalyzing ADP/ATP exchange across the mitochondrial membranes, regulating membrane potential 21. To date, there is no evidence of SLC25A31 gene mutations being associated with a human disease 22. Other genes in this region include INTU (inturned planar cell polarity protein), HSPA4L (heat shock 70kDa protein 4-like), PLK4 (polo-like kinase 4), MFSD8 (major facilitator superfamily domain containing 8), and LARP1B (La ribonucleoprotein domain family, member 1B). It is not immediately clear how this variant could be involved with response to BP response to β-blockers. The HaploReg database 23 indicates there is no other variant in high linkage disequilibrium with this variant in African populations in the 1000 Genomes database, and this variant was found to alter 14 regulatory motifs including Evi-1_4, Fox and HDAC2_disc6. Further study is warranted to understand the functional underpinning of this association.
The variant validated for SBP response to β-blockers, rs11313667, is an insertion/deletion variant (C/-) in the intronic region of LRRC15 (leucine rich repeat containing 15) gene. In the 1000 Genomes African populations, this variant is in high linkage disequilibrium with 3 synonymous SNPs in the same gene: rs4974533 (r2 = 0.8, D’ = −0.97), rs9682540 (r2 = 0.82 and D’=−0.98) and rs923935 (r2 = 0.8, D’=−0.97). Synonymous SNPs have been found to affect mRNA splicing, stability and structure as well as protein folding 24. LRRC15 is a tumor antigen, a cell surface glycoprotein normally expressed only in the invasive cytotrophoblast layer of the placenta and it was found to be overly expressed in the androgen-independent metastatic prostate cancer 25. There is no apparent link between LRRC15 and BP in the literature. Other genes in this region include CPN2, GP5 and ATP13A3. The most potentially interesting gene in this region related to blood pressure is GP5 encoding platelet glycoprotein V, which mediates the adhesion of platelets to injured vascular surfaces in the arterial circulation, a critical initiating event in hemostasis. Soluble platelet glycoprotein V is a plasma marker of thrombosis released from the platelet surface by thrombin and was found to be a marker of thrombosis in patients with ischemic stroke 26. Hypertension is a major risk factor for thrombotic events such as myocardial infraction and stroke, reflecting a prothrombotic state that is present in hypertensive patients. There is evidence that this prothrombolitc state can be induced by the activated renin-angiotensin system 27. A study by Remkova and colleagues demonstrated that soluble platelet glycoprotein V level was elevated in hypertensive patients compared to healthy individuals and more importantly, treatment with the ACE inhibitor perindopril (but not the angiotensin II receptor blocker telmisartan) significantly decreased the level of platelet glycoprotein V in hypertensive individuals 28. It is plausible that β-blockers, as suppressors of renin secretion, could be involved with expression of platelet glycoprotein V. We observed that the association of this variant was only slightly weaker after adjusting for baseline PRA, indicating that a very small portion of this association might be explained by PRA but essentially the mechanism of this association remains to be elucidated. It is important to carry out further functional studies to elucidate the mechanisms of these findings.
We acknowledge at least two limitations of this study. Firstly, we recognize that the BP response to add-on therapy may not be the optimal validation for association with BP response to β-blocker monotherapy and lack of association in the add-on therapy should not be regarded as evidence to dismiss their association with the BP response to the monotherapy. One potential reason that the significant SNP in the β-blocker monotherapy was not validated in the add-on therapy is that the pre-treatment PRA was much higher compared to those in the monotherapy as a result of HCTZ monotherapy which is known to increase PRA. Even though our sensitivity analysis that adjust for pre-treatment PRA did not show different results, it is possible that statistical adjustment might not be sufficient to control for such difference. Thus, in particular the variant that achieved genome-wide significance in the monotherapy meta-analysis remains of interest. Secondly, both PEAR-1 and PEAR-2 studies have active treatment for all arms, the phenotype of changed in BP might not be easy to interpret without a placebo run-in or double blind cross-over design.
In conclusion, we performed the first genome-wide meta-analysis of BP response to β-blockers in hypertensive African American individuals and identified several potentially important variants that deserve additional study. Further investigation regarding the functional underpinning of these associations could advance our current understanding of β-blocker use in African Americans and potentially serve as a basis of precision medicine in treating hypertension in this special group of individuals.
PERSPECTIVE
Hypertension is the most important risk factor for global disease burden and it is particularly problematic in African Americans. Hypertension is also the most common chronic disease for which medications are prescribed. Pharmacogenomics offers the clinical promise of individualized antihypertensive therapy on the basis of matching a patient’s genetic makeup and the pharmacological action of the drugs prescribed. This study performed the first pharmacogenomics genome-wide association study of BP response to β-blockers in African American hypertensive individuals and identified two variants that validated across three different cohorts, with clinically-relevant differences in BP response by genotype. Further investigations of the two associated regions may provide valuable information for personalized antihypertensive treatment in African Americans.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) What Is New?
This is the first pharmacogenomic genome-wide association analysis of BP response to β-blockers in African American hypertensive patients. We performed meta-analysis of BP response in the African Americans treated with atenolol monotherapy and metoprolol monotherapy. SNPs with suggestive associations were then validated in African Americans treated with atenolol add-on therapy. Two variants, SLC25A31 rs201279313 and LRRC15 rs11313667, were validated, with clinically-relevant effect sizes. In this first pharmacogenomics genome-wide meta-analysis of BP response to β-blockers in African Americans, we identified novel variants that may provide valuable information for personalized antihypertensive treatment in this group.
2) What Is Relevant?
The two identified variants have clinically relevant effects on blood pressure response to β-blockers in African American hypertensive patients.
Summary
Genome-wide association analyses of BP response to β-blockers in African American hypertensive patients identified two novel regions with clinically relevant effect sizes. These variants may be useful to identify the African Americans who are responders to β-blockers.
ACKNOWLEDGEMENTS
We acknowledge and thank the valuable contributions of the PEAR and PEAR-2 study participants, support staff, and study physicians: Frederic Rabari-Oskoui and Dan Rubin.
SOURCES OF FUNDING
Both PEAR studies were supported by the National Institute of Health Pharmacogenomics Research Network grant U01-GM074492 and the National Center for Advancing Translational Sciences under the award numbers UL1 TR000064 (University of Florida); UL1 TR000454 (Emory University) and UL1 TR000135 (Mayo Clinic). PEAR was also supported by funds from the Mayo Foundation.
Footnotes
CONFLICT OF INTEREST/DISCLOSURE
YG, ALB, ABC, JGG, EB, STT, RMC-D and JAJ received funding from NIH. JGG is a consultant for Boehringer-Ingelheim. EB received honoraria from the Foundation of Rome. Other authors declare no conflict of interest.
REFERENCES
- 1.Oparil S, Schmieder RE. New approaches in the treatment of hypertension. Circ Res. 2015;116:1074–1095. doi: 10.1161/CIRCRESAHA.116.303603. [DOI] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation. 2015;131:e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 3.CDC [Accessed September 20, 2015];Health of black or African American Non-hispanic population. 2015 Available at: http://www.cdc.gov/nchs/fastats/black-health.htm.
- 4.CDC Racial/ethnic disparities in the awareness, treatment, and control of hypertension - United States, 2003-2010. MMWR Morb Mortal Wkly Rep. 2013;62:351–355. [PMC free article] [PubMed] [Google Scholar]
- 5.Sundström J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M, Neal B, Collaboration BPLTT. Effects of blood pressure reduction in mild hypertension: A systematic review and meta-analysis. Ann Intern Med. 2015;162:184–191. doi: 10.7326/M14-0773. [DOI] [PubMed] [Google Scholar]
- 6.Bidiville J, Nussberger J, Waeber G, Porchet M, Waeber B, Brunner HR. Individual responses to converting enzyme inhibitors and calcium antagonists. Hypertension. 1988;11:166–173. doi: 10.1161/01.hyp.11.2.166. [DOI] [PubMed] [Google Scholar]
- 7.Freis ED, Materson BJ, Flamenbaum V. Comparison of propranolol or hydrochlorothiazide alone for treatment of hypertension. Iii. Evaluation of the renin-angiotensin system. Am J Med. 1983;74:1029–1041. doi: 10.1016/0002-9343(83)90812-4. [DOI] [PubMed] [Google Scholar]
- 8.National Center for Health Statistics (US) Health, United States, 2013: With special feature on prescription drugs. Center for Disease Control and Prevention; Atlanta: 2014. [PubMed] [Google Scholar]
- 9.Materson BJ, Reda DJ, Cushman WC, Massie BM, Freis ED, Kochar MS, Hamburger RJ, Fye C, Lakshman R, Gottdiener J. Single-drug therapy for hypertension in men. A comparison of six antihypertensive agents with placebo. The department of veterans affairs cooperative study group on antihypertensive agents. N Engl J Med. 1993;328:914–921. doi: 10.1056/NEJM199304013281303. [DOI] [PubMed] [Google Scholar]
- 10.Materson BJ, Reda DJ, Cushman WC. Department of veterans affairs single-drug therapy of hypertension study. Revised figures and new data. Department of veterans affairs cooperative study group on antihypertensive agents. Am J Hypertens. 1995;8:189–192. doi: 10.1016/0895-7061(94)00196-i. [DOI] [PubMed] [Google Scholar]
- 11.Johnson JA, Boerwinkle E, Zineh I, Chapman AB, Bailey K, Cooper-DeHoff RM, Gums J, Curry RW, Gong Y, Beitelshees AL, Schwartz G, Turner ST. Pharmacogenomics of antihypertensive drugs: Rationale and design of the pharmacogenomic evaluation of antihypertensive responses (pear) study. Am Heart J. 2009;157:442–449. doi: 10.1016/j.ahj.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamadeh IS, Langaee TY, Dwivedi R, Garcia S, Burkley BM, Skaar TC, Chapman AB, Gums JG, Turner ST, Gong Y, Cooper-DeHoff RM, Johnson JA. Impact of cyp2d6 polymorphisms on clinical efficacy and tolerability of metoprolol tartrate. Clin Pharmacol Ther. 2014;96:175–181. doi: 10.1038/clpt.2014.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner ST, Schwartz GL, Chapman AB, Beitelshees AL, Gums JG, Cooper-Dehoff RM, Boerwinkle E, Johnson JA, Bailey KR. Power to identify a genetic predictor of antihypertensive drug response using different methods to measure blood pressure response. J Transl Med. 2012;10:47. doi: 10.1186/1479-5876-10-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willer CJ, Li Y, Abecasis GR. Metal: Fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blumenfeld JD, Sealey JE, Mann SJ, Bragat A, Marion R, Pecker MS, Sotelo J, August P, Pickering TG, Laragh JH. Beta-adrenergic receptor blockade as a therapeutic approach for suppressing the renin-angiotensin-aldosterone system in normotensive and hypertensive subjects. Am J Hypertens. 1999;12:451–459. doi: 10.1016/s0895-7061(99)00005-9. [DOI] [PubMed] [Google Scholar]
- 16.Zayas VM, Blumenfeld JD, Bading B, McDonald M, James GD, Lin YF, Sharrock NE, Sealey JE, Laragh JH. Adrenergic regulation of renin secretion and renal hemodynamics during deliberate hypotension in humans. Am J Physiol. 1993;265:F686–692. doi: 10.1152/ajprenal.1993.265.5.F686. [DOI] [PubMed] [Google Scholar]
- 17.Bühler FR, Laragh JH, Baer L, Vaughan ED, Brunner HR. Propranolol inhibition of renin secretion. A specific approach to diagnosis and treatment of renin-dependent hypertensive diseases. N Engl J Med. 1972;287:1209–1214. doi: 10.1056/NEJM197212142872401. [DOI] [PubMed] [Google Scholar]
- 18.Hollifield JW, Sherman K, Zwagg RV, Shand DG. Proposed mechanisms of propranolol's antihypertensive effect in essential hypertension. N Engl J Med. 1976;295:68–73. doi: 10.1056/NEJM197607082950203. [DOI] [PubMed] [Google Scholar]
- 19.Stumpe KO, Kolloch R, Vetter H, Gramann W, Krück F, Ressel C, Higuchi M. Acute and long-term studies of the mechanisms of action of beta-blocking drugs in lowering blood pressure. Am J Med. 1976;60:853–865. doi: 10.1016/0002-9343(76)90905-0. [DOI] [PubMed] [Google Scholar]
- 20.Turner ST, Schwartz GL, Chapman AB, Beitelshees AL, Gums JG, Cooper-DeHoff RM, Boerwinkle E, Johnson JA, Bailey KR. Plasma renin activity predicts blood pressure responses to beta-blocker and thiazide diuretic as monotherapy and add-on therapy for hypertension. Am J Hypertens. 2010;23:1014–1022. doi: 10.1038/ajh.2010.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallerne C, Touat Z, Chen ZX, Martel C, Mayola E, Sharaf el dein O, Buron N, Le Bras M, Jacotot E, Borgne-Sanchez A, Lemoine A, Lemaire C, Pervaiz S, Brenner C. The fourth isoform of the adenine nucleotide translocator inhibits mitochondrial apoptosis in cancer cells. Int J Biochem Cell Biol. 2010;42:623–629. doi: 10.1016/j.biocel.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 22.Clémençon B, Babot M, Trézéguet V. The mitochondrial adp/atp carrier (slc25 family): Pathological implications of its dysfunction. Mol Aspects Med. 2013;34:485–493. doi: 10.1016/j.mam.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 23.Ward LD, Kellis M. Haploreg: A resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt R, Sauna ZE, Ambudkar SV, Gottesman MM, Kimchi-Sarfaty C. Silent (synonymous) snps: Should we care about them? Methods Mol Biol. 2009;578:23–39. doi: 10.1007/978-1-60327-411-1_2. [DOI] [PubMed] [Google Scholar]
- 25.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 26.Wolff V, Aleil B, Giroud M, Lorenzini JL, Meyer N, Wiesel ML, Cazenave JP, Lanza F. Soluble platelet glycoprotein v is a marker of thrombosis in patients with ischemic stroke. Stroke. 2005;36:e17–19. doi: 10.1161/01.STR.0000155738.02753.4d. [DOI] [PubMed] [Google Scholar]
- 27.Remková A, Remko M. The role of renin-angiotensin system in prothrombotic state in essential hypertension. Physiol Res. 2010;59:13–23. doi: 10.33549/physiolres.931525. [DOI] [PubMed] [Google Scholar]
- 28.Remková A, Kratochvíl'ová H, Durina J. Impact of the therapy by renin-angiotensin system targeting antihypertensive agents perindopril versus telmisartan on prothrombotic state in essential hypertension. J Hum Hypertens. 2008;22:338–345. doi: 10.1038/sj.jhh.1002328. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.