Abstract
Elevated maternal testosterone levels are shown to cause fetal growth restriction, eventually culminating in sex-specific adult-onset hypertension that is more pronounced in males than females. In this study, we tested whether utero- and feto-placental disturbances underlie fetal growth restriction and if these changes vary in male and female placentas. Pregnant Sprague-Dawley rats were injected with vehicle (n=16) or testosterone propionate (0.5 mg/Kg/day from gestation day 15–19; n=16). On gestation day 20, we quantified uterine artery blood flow using microultrasound, visualized placental arterial network using x-ray microcomputed tomography, determined fetoplacental hypoxia using pimonidazole and hypoxia-inducible factor-1α, and used Affymetrix array to determine changes in placental expression of genes involved in vascular development. Plasma testosterone levels increased 2-fold in testosterone-injected rats. Placental and fetal weights were lower in rats with elevated testosterone. Uterine artery blood flow was lower and resistance index was higher in testosterone group. Radial and spiral artery diameter and length, number of fetoplacental arterial branches, and umbilical artery diameter were reduced in the testosterone group. In addition, markers of hypoxia in the placentas and fetuses were elevated in the testosterone group. The magnitude of changes in placental vasculature and hypoxia were greater in males than females and were associated with sex-specific alteration of unique sets of genes involved in angiogenesis and blood vessel morphogenesis. The results demonstrate that elevated testosterone during gestation induces a decrease in uterine arterial blood flow and fetal sex-related uteroplacental vascular changes, which may set the stage for subsequent sex differences in adult-onset diseases.
Keywords: testosterone, preeclampsia, placenta, hypoxia, vascular, angiogenesis, sexual dimorphism
INTRODUCTION
About 1 in every 12 babies in the United States1 and up to 15% of all pregnancies worldwide exhibit some degree of intrauterine growth restriction (IUGR) and are consequently at a higher risk of perinatal and childhood morbidity and mortality.2 Moreover, those IUGR babies develop chronic conditions in adult life, such as hypertension, dyslipidaemia, and diabetes mellitus.3–9 Maternal health factors, such as chronic hypertension, preeclampsia, diabetes mellitus, chronic renal disease, poor nutrition, smoking, and fetal genetic abnormalities increase the risk of IUGR.10 The maternal cardiovascular system undergoes tremendous adaptations to accommodate the increased circulatory requirements of the growing fetus. Nowhere are these adaptations more profound than in the uteroplacental vasculature, where a marked increase in uteroplacental blood flow is achieved by a large reduction in vascular resistance11;12 and pronounced enlargement and structural reorganization of the uterine and spiral arteries.13 These changes allow the expansion of blood volume in the uteroplacental unit and sufficient blood flow from the placenta to the fetus.13 Normal fetal growth is vastly dependent on adequate placental development, whereas increased vascular resistance and hypoxia are associated with reduced fetal growth.14 However, the molecular basis of uteroplacental dysfunction in IUGR is not well understood.
Epidemiological studies show human IUGR is associated with increased maternal testosterone (T) levels.15;16 Most pregnancy pathologies which cause fetal growth restriction also present with high androgen levels, such as preeclampsia,17–21 polycystic ovarian syndrome (PCOS),22 congenital adrenal hyperplasia (CAH),23;24 and maternal smoking or nicotine intake.25–27 Moreover, pregnant African-American women have higher serum T levels, with a greater frequency of low-birth-weight babies.28–30 Experimental studies show that increasing T levels in pregnant rats to concentrations similar to levels in preeclamptic pregnancies leads to fetal growth restriction.31;32 Furthermore, these low-birth-weight T offspring develop cardiovascular dysfunction in a sex-specific manner that is more pronounced in males than females.33;34 However, the pathophysiological mechanism by which T induces fetal growth restriction is unknown.
Elevated maternal T levels can produce at least 2 major potential effects: direct actions of T on the fetus and indirect actions on the uteroplacental unit. Numerous studies have demonstrated that T directly causes fetal damage.35–39 On the other hand, the adverse effects of T on fetal growth could be from the indirect action of T on the maternal-fetal unit and the uteroplacental circulation. Studies in humans have shown that elevated T, as observed in PCOS pregnancies, is associated with impaired decidual trophoblast invasion, increased uterine artery resistance index, and reduced blood flow.40;41 Further experimental studies have shown that elevated T decreases placental weight in rats,32 advances placental differentiation in sheep,42 decreases placental nutrient transport capacity in rats,32 and exaggerates vascular contractile response in uterine arteries of rats.43 However, in spite of the well-documented effects of elevated maternal T on placental growth and the available circumstantial evidence of its association with increased uterine artery resistance in PCOS pregnancy,40;41 the effect of T on placental vascular development has not been studied. Furthermore, the placenta is often considered an asexual organ, with most studies consistently pooling data derived from the placentas of male and female fetuses into a single group.44 It has been postulated that the placenta is a programming agent that can contribute to the sex differences in adult-onset diseases.45 Hence, in this study, we examined if the pronounced hypertensive responses reported in the prenatal T-exposed adult males compared to females33;34 relate to the underlying sex-specific utero- and feto-placental disturbances. Herein, we present evidence in an in vivo pregnancy rat model that elevated T, at concentrations similar to that observed in clinical conditions, promotes a decrease in uterine artery blood flow, increases resistance in uterine arteries, and reduces placental vascularization, leading to placental and fetal hypoxia. Interestingly, the magnitude of changes in placental vasculature and hypoxia appears to be sex-dependent, with a greater effect in males than females, and is associated with alteration in a unique set of sex-specific genes involved in angiogenesis and blood vessel morphogenesis.
MATERIALS AND METHODS
Animals
All procedures were approved by the Animal Care and Use Committee at the University of Texas Medical Branch and were in accordance with those guidelines published by the US National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85–23, revised 1996). As we described earlier,31;43;46;47 timed pregnant Sprague-Dawley rats (gestational day [GD] 12; presence of copulation plug is day 1), purchased from Charles River (Wilmington, MA), were used in this study. After acclimatization, on GD 14, dams were randomly divided into 2 groups. Dams in the treatment group were subcutaneously injected with T propionate (0.5 mg/Kg/day, n=16) for 5 days from GD 15–19. The control group received vehicle (sesame oil, n=16). This dose and duration of exposure is commonly used to mimic plasma T levels observed in preeclamptic and IUGR pregnancies.48;32;49;50 Standard rodent chow and water were available ad libitum throughout the experimental protocols. The animals were housed in a room with a controlled temperature and a 12-hour light-dark cycle. From 8–10 am on day 20 of pregnancy, rats were anaesthetized for ultrasound and then used for placental vascularization. Another set of animals were used to examine placental and fetal oxygenation. Additional animals were sacrificed with carbon dioxide inhalation and placentas and fetuses were isolated, blotted to remove fluids and blood, weighed, snap-frozen in liquid nitrogen, and stored at −80°C until analyzed. An expanded Methods section is available in the online-only Data Supplement, which includes, measurement of plasma T levels, ultrasound of uterine arteries, placental vascularization, assessment of placental and fetal hypoxia, placental and fetal sex determination, placental gene expression by microarray, and validation of gene expression by quantitative real-time PCR.
Statistical Analysis
All values are given as mean ±S.E.M, where n is the number of mothers. Statistical comparisons were made by ANOVA followed by the Bonferroni post hoc test on multiple observations and unpaired Student t test for comparison of single observations between control and T groups. Correlation between microarray and RT-qPCR data was assessed with a Spearmann correlation test. All tests were performed with GraphPad Prism (San Diego CA, USA). Statistical significance was assumed if P<.05.
RESULTS
Increased Plasma T levels in T dams
Plasma T levels were 2-fold higher in T dams (2.39±0.12 ng/mL; P<.05) compared to controls (1.19 ± 0.08 ng/mL).
Fetal, Placental, and Maternal Growth in dams with elevated T
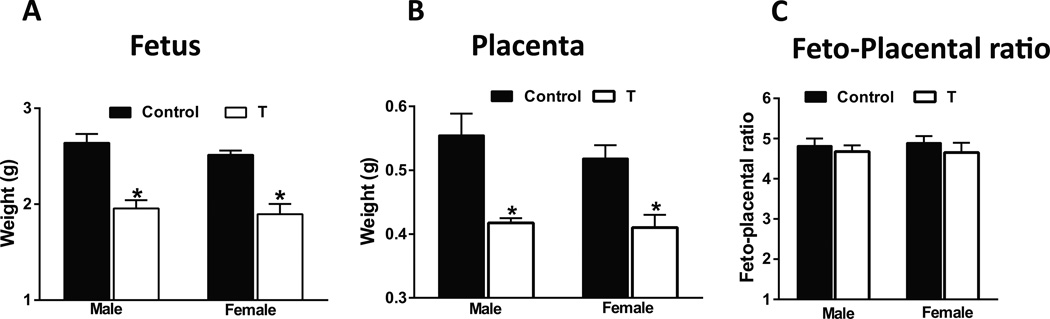
Fetal body weight was significantly lower (−26% in males and −25% in females) at 20 days’ gestation in T dams compared with controls (Fig 1A; P<.05). Sex ratios within the litters were not significantly different. No significant differences were noted in mean litter sizes between controls (13.4 ± 1.7) and T dams (12.7 ± 2.2). Placental weights of male and female fetuses were also significantly lower (−25% in males and −21% in females) compared to respective controls (Fig 1B; P<.05). The fetal to placental weight ratio remained unchanged (Fig 1C). Maternal body weight increased similarly in both groups and was not significantly different at 20 days’ gestation between T dams and controls (T dams: 318·5 ± 29 g; controls: 327·5 ± 33·3 g).
Figure 1.
Fetal and placental weight are reduced in pregnant rats with elevated T. Fetal (A), placental (B), and feto-placental ratio (C) at gestation day 20 in control and T dams. Data expressed as mean ± SEM of 6 rats in each group. *P<.05 vs respective controls.
Reduced Uteroplacental Blood Flow and Elevated Uteroplacental Vascular Resistance in T dams
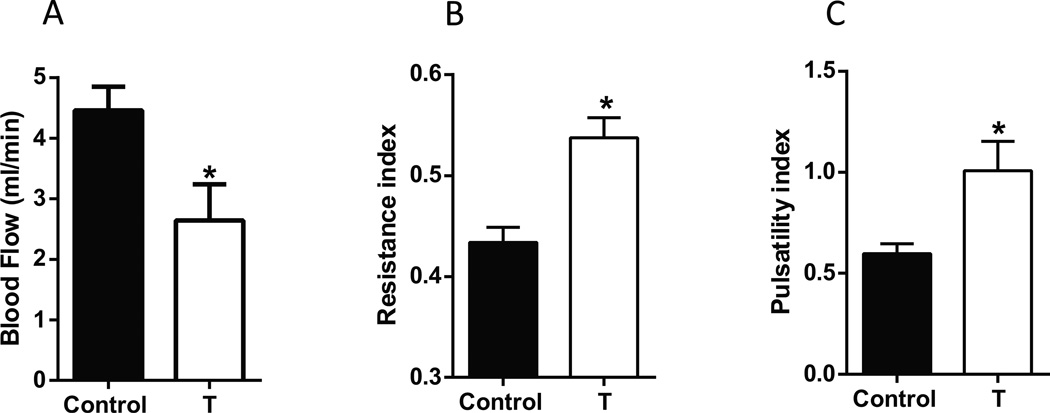
Uterine arterial blood flow measured using transcutaneous micro ultrasound was significantly reduced by 40% in T dams relative to controls (Fig 2A; P<.05). The uterine arterial diameter was significantly reduced in T dams (Control: 736.12± 19.4 µm and T: 547.23± 26 µm, P<.001, Table 1). We observed significant elevations in the resistance index (Fig 2B; P<.05) and pulsatility index (Fig 2C; P<.05) in T dams compared to controls. Thus, results indicate that elevated T decreases uterine artery diameter and blood flow and increases uterine vascular resistance.
Figure 2.
Uterine arterial blood flow and resistance are altered in pregnant rats with elevated T. Uterine arterial blood flow (A), resistance index (B), and pulsatility index (C) measured using micro-ultrasound gestation day 20 in control and T dams. Data expressed as mean ± SEM of 6 rats in each group. *P<.05 vs respective controls.
Table 1.
Uteroplacental arterial tree measurements
| Male | Female | ||||
|---|---|---|---|---|---|
| Artery | Parameter | Control | T | Control | T |
| Uterine artery | Diameter | 0.73±0.009 | 0.54±0.011* | 0.72±0.006 | 0.58±0.017* |
| Radial artery | Number of first order radial arteries | 4.75±0.411 | 2±0.267* | 4.5±0.308 | 3.5±0.327* |
| Length | 0.84±0.036 | 0.62±0.005* | 0.81±0.008 | 0.63±0.007* | |
| Diameter | 0.63±0.009 | 0.46±0.008* | 0.65±0.013 | 0.47±0.006* | |
| Spiral artery | Number of spiral arteries | 13.75±0.591 | 12±1.035 | 13.11±0.423 | 12.87±0.479 |
| Diameter | 0.15±0.006 | 0.11±0.002* | 0.16±0.007 | 0.12±0.002* | |
| Length | 0.54±0.015 | 0.42±0.029* | 0.57±0.016 | 0.62±0.011 | |
| Canal | Diameter | 0.12±0.0119 | 0.10±0.002 | 0.18±0.025 | 0.17±0.002 |
| Canal length | 7.82±0.047 | 6.65±0.18* | 7.17±0.118 | 6.5±0.056* | |
| Number of canals | 4.5±0.327 | 2.12±0.398* | 4.11±0.260 | 3.75±0.250 | |
| Canal branches | 5.25±0.366 | 4.25±0.45 | 3.88±0.260 | 3.87±0.295 | |
Values are expressed as mean±SEM, n=8 in each group
Diameter and length are presented in ‘mm’
P<.05 compared to respective control group
Reduced Growth of Spiral Arteries, Central Arterial Canals, Fetoplacental Arterial Branches, and Umbilical Arteries in T dams
Changes were observed in the uteroplacental arterial tree (Fig supplementary S1), beginning with a 23% reduction in uterine artery diameter relative to controls (Fig supplementary S2A; Table 1; P<.05). The uterine artery directly supplied a smaller number of preplacental radial arteries in the T dams (3 in males; 3.5 in females) compared to controls (4.75 in males, 4.5 in females) (Table 1). Also, the first segment of preplacental radial arteries in T dams were shorter (−26% in males; −22% in females; Table 1), branching sooner than controls, and were smaller in diameter (−26% in males; −27% in females; Table 1). Preplacental radial arteries gave rise to spiral arteries, which were less coiled and smaller in T mothers than controls. This reduction was not due to the number of spiral arteries, which was approximately 13 in each group (Table 1); rather, it was due to decreased diameters (−27% in males; −25% in females; Table 1) and reduced length (−22% in males; +8% in females; Table 1). Spiral arteries converge into canals, which were smaller in placentas of T dams. This reduction was not due to a decrease in canal diameters but was related to a decrease in canal length (−15% in males; −9% in females; Table 1). Trends toward reduced canal branches (P=.09) were also observed in male placentas of T dams (Table 1).
Examination of the fetoplacental arterial trees showed a centrally located umbilical artery branched across the chorionic plate before elaborately branching into smaller diameter intraplacental arteries. Measurements revealed a small but significant decrease (−5% in males; −3.2% in females; Table 2) in umbilical artery diameters in T placentas. Vascular segmentation further revealed that there was a significant decrease in the total number of arterial vessel segments in T placentas (−13% in males; −11% in females; Table 2) compared to controls. Also, the number of branching generations were significantly lower in T dams (−24% in males; −10% in females; Fig supplementary S2B; Table 2) compared to controls.
Table 2.
Fetoplacental arterial tree measurements
| Parameter | Male | Female | ||
|---|---|---|---|---|
| Control | T | Control | T | |
| Umbilical artery diameter | 0.62±0.056 | 0.59±0.036* | 0.62±0.036 | 0.60±0.034* |
| Number of vessel segments | 2136±76 | 1856±53* | 1986±48 | 1754±58* |
| Total length of vasculature | 562±11 | 465±15* | 492±9 | 451±11* |
| Number of branching generations | 46±4 | 35±3* | 51±3 | 46±2* |
Values are expressed as mean±SEM, n=8 in each group
Diameter and length are presented in ‘mm’
P<.05 compared to respective control group
Together, these changes in uteroplacental and fetoplacental vascularity suggest a generalized reduction in arterial enlargement and elaboration of the utero- and feto-placental circulation in T rats. Overall, the magnitude of changes appears to be similar in male and female placentas of T dams compared to their respective controls expect that the spiral artery length and number of canals were significantly decreased only in T males.
Increased Placental and Fetal Hypoxia in T dams
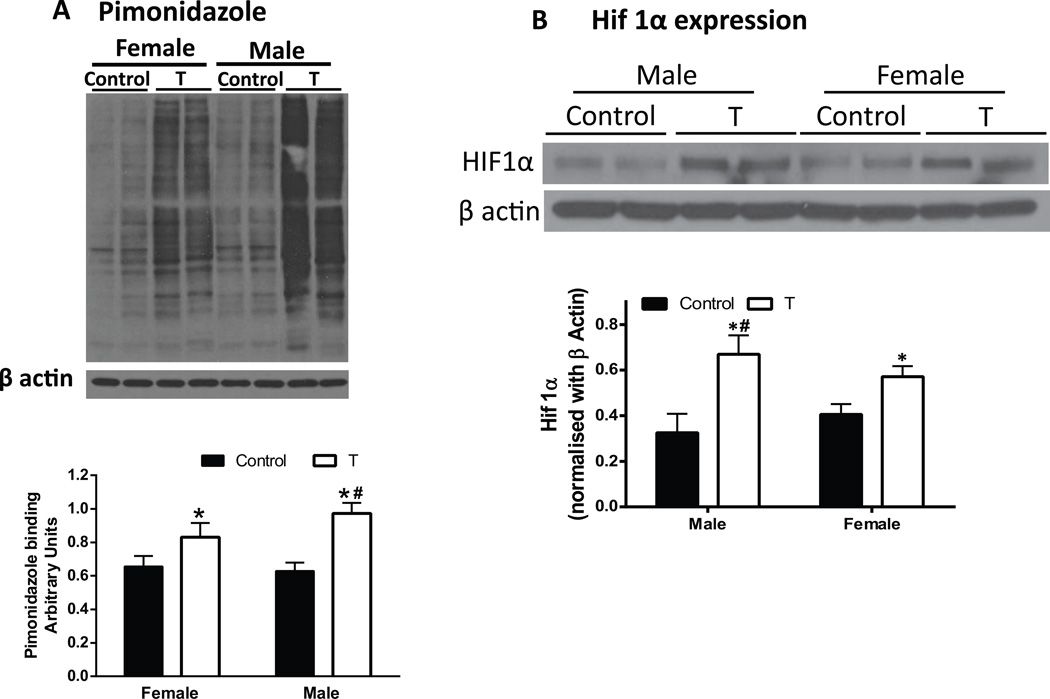
Decreased uterine arterial blood flow and improper placental vascularization were anticipated to decrease oxygen delivery to the placenta. We, therefore, predicted that the placentas in T dams would be hypoxic in vivo. To test this, we used the oxygen-sensitive hypoxia marker pimonidazole and hypoxia inducible factor (HIF) 1α. Western blotting revealed increased pimonidazole binding (+55% in males; +27% in females; Fig 3A; P<.05) and HIF 1α levels (+2.1 fold in males, +1.4 fold in females; Fig 3B; P<.05) in placentas of T dams compared to controls. The percent increase in pimonidazole binding and fold increase in HIF 1α levels in T males was significantly higher compared to T females (Fig 3A and B; P<.05).
Figure 3.
Increased markers of placental hypoxia in pregnant rats with elevated T. Pimonidazole (A) and hypoxia-inducible factor (HIF) 1α (B) immunoblotting were used to identify hypoxia in the placenta at gestation day 20 in control and T dams. Representative Western blots for Pimonidazole, HIF1α, and β-actin are shown at top; blot density obtained from densitometric scanning of Pimonidazole and HIF1α normalized to actin is shown at bottom. Values are given as means ± SEM of 6 rats in each group. *P≤.05 vs respective control. #P≤.05 vs T females.
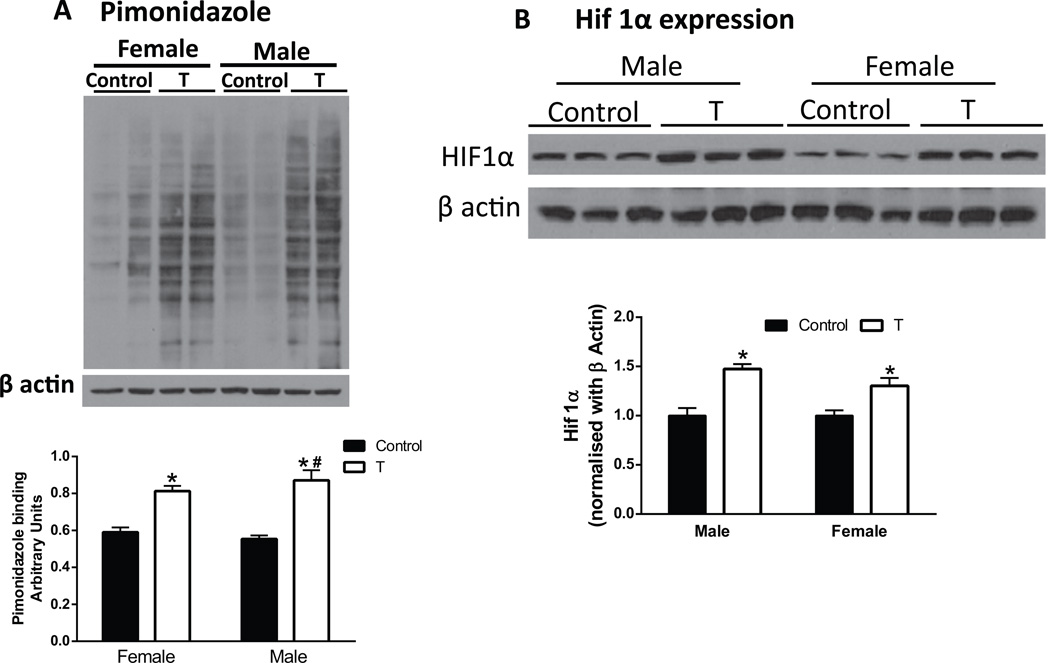
Since pimonidazole crosses the placenta51 and binds to hypoxic fetal cells, we next determined whether elevated maternal T levels affect oxygen delivery to the fetus. Pimonidazole binding (+57% in males; +38% in females; Fig 4A; P<.05) and HIF 1α levels (+1.5 fold in males, +1.3 fold in females; Fig 4B; P<.05) were greater in T fetuses than in controls. These findings suggest that placentas and fetuses of T dams are hypoxic with a more pronounced effect in the males that females. The percent increase in pimonidazole binding in T male fetuses was significantly higher compared to T female fetuses (Fig 4A; P<.05)..
Figure 4.
Increased markers of hypoxia in fetuses from pregnant rats with elevated T. Pimonidazole (A) and hypoxia-inducible factor (HIF) 1α (B) immunoblotting were used to identify hypoxia in the placenta at gestation day 20 in control and T fetuses. Representative Western blots for Pimonidazole, HIF1α, and β-actin are shown at top; blot density obtained from densitometric scanning of Pimonidazole and HIF1α normalized to β-actin is shown at bottom. Values are given as means ± SEM of 6 fetuses in each group. *P≤.05 vs respective control. #P≤.05 vs T female fetueses.
Placental Expression of Genes Involved in Blood Vessel Morphogenesis and Angiogenesis were Altered in T dams
To characterize the underlying changes in genes that could contribute to the altered vascular development, microarray analysis was performed on placental cDNA obtained from control and T dams. In order to characterize whether elevated maternal T triggers different changes in males and females, we analyzed the gene changes in both sexes separately. We compared the male T with the male control microarrays and female T with the female control microarrays. The microarray data are in compliance with the Minimum Information about Microarray Experiments and these microarray data have been submitted to Gene Omnibus and are accessible through accession number GSE61543 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=uxmlqiimhxalziv&acc=GSE61543). We found that 782 genes were affected (Fc >1·25, P < 0.05) in T male placentas with 486 being up-regulated and 296 down-regulated. In the T female placentas, 603 genes were affected with 394 up-regulated and 209 down-regulated.
To further analyze the observed gene expression changes, DAVID functional annotation clustering was used to identify pathways, physiological functions, cellular localization, or other meaningful commonalities among the regulated genes. The dysregulated genes in male placentas were involved principally in response to hormone stimulus, vasculature development and angiogenesis, response to nutrient levels, extracellular region, and cell adhesion (Supplementary Table S1). In female placentas, the biological functions associated with the dysregulated genes were principally involved in extracellular region, extracellular matrix, inflammatory response, response to hormone stimulus, response to nutrient levels, vasculature development and angiogenesis (Supplementary Table S2). There is no significant difference in the pathways affected between the T male s and females.
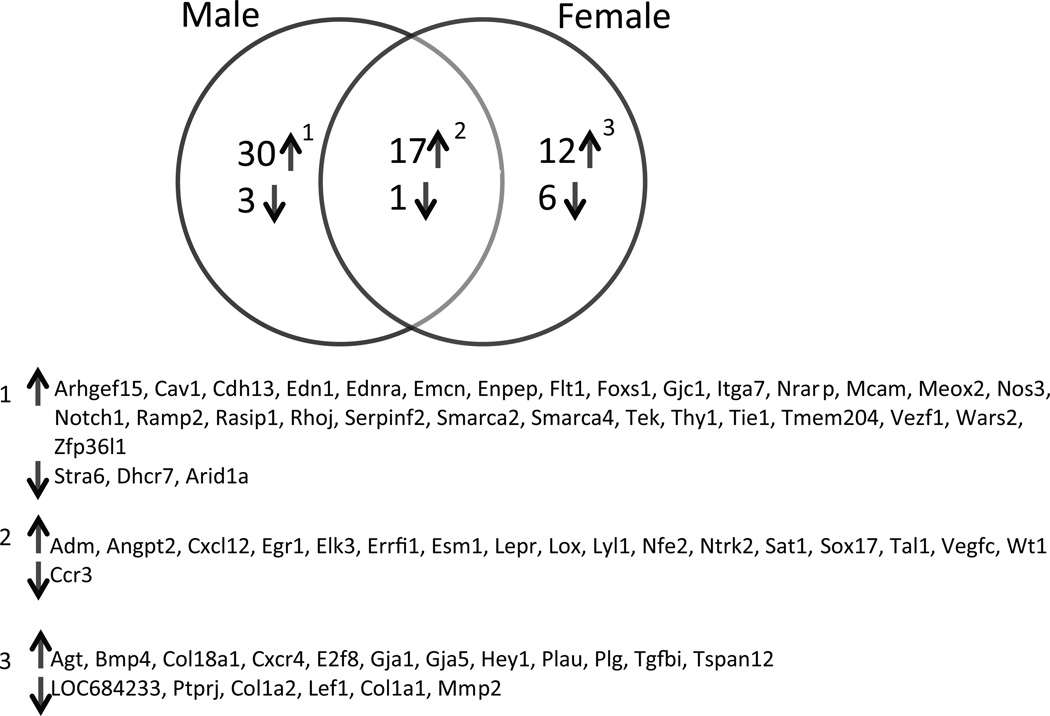
Since elevated T levels induced striking effects on the length, diameter and branching of placental vasculature, we further examined dysregulated genes within the vascular development pathway (GO:0048514 : blood vessel morphogenesis [412 gene products] and GO:0001525 : angiogenesis [333 gene products]). Compared to the controls, in the male placentas of T dams, 51 differentially expressed genes were identified, with 47 genes upregulated and 4 genes down regulated (Fig 5). In the female placentas of T dams, 36 genes were differentially expressed, with 29 upregulated and 7 downregulated (Fig 5). Among these altered genes, only 18 were dysregulated in both sexes: 17 upregulated and 1 downregulated. Interestingly, some genes showed sexual dimorphism: 30 were upregulated and 3 downregulated in males only, and 12 were upregulated and 6 downregulated in females only (Fig 5). The fold change in gene expression in presented in supplementary Table S3.
Figure 5.
Venn diagram displaying significant sexual dimorphism in the dysregulated genes. The list of dysregulated gens are presented below.
Quantitative RT-PCR Validation of Blood Vessel Morphogenic and Angiogenic Genes
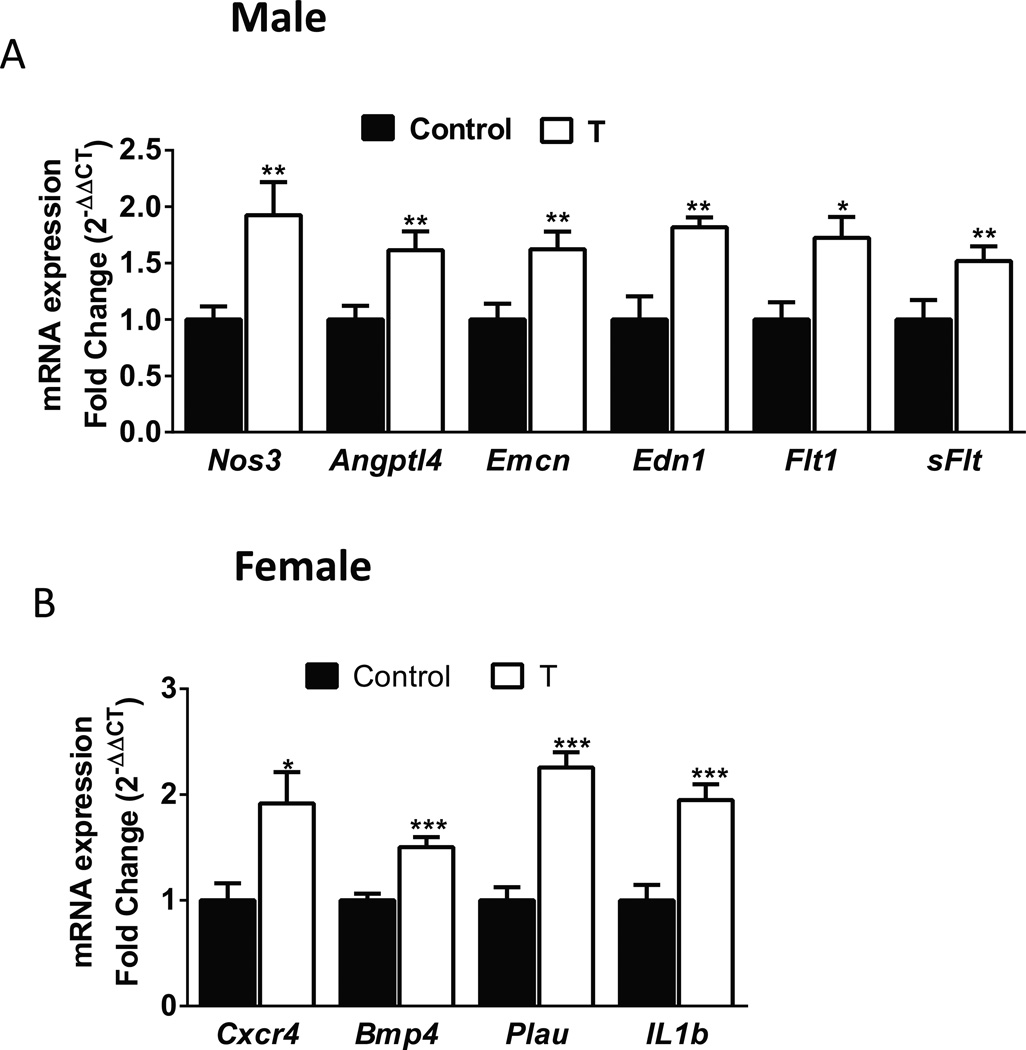
The differentially expressed of genes involved in blood vessel morphogenesis and angiogenesis in males (Nos3, Angptl4, Emcn, Edn1 Flt1, and sFlt) (Fig 6A) and females (Cxcr4 Bmp4, Plau, and IL1β) (Fig 6B) were confirmed by real-time PCR. For all genes examined, a significant correlation between microarray and qPCR data was established (Spearman’s Rank Correlation, P<.05).
Figure 6.
Validation of placental mRNA levels for candidate genes, determined by RT-qPCR, in the placentas of pregnant rats with elevated T. Real-time reverse transcriptase PCR was used to assess mRNA expression. Quantitation of candidate genes was normalized relative to β-actin levels. Values are given as means ± SEM of 6 placentas in each group. *P≤.05 vs control.
DISCUSSION
Human and animal studies reported that elevated maternal T levels are associated with reduced fetal weight52–55; however, the mechanisms underlying this growth-restricted phenotype are not known. In the present study, we found that the reduced fetal weight in pregnant rats with elevated maternal T is associated with uteroplacental dysfunction evidenced by greater uterine artery resistance index with reduced blood flow, abnormal placental vascularization, and hypoxia. Interestingly, these placental changes were associated with differential expression of unique sets of genes in males and female placentas.
During pregnancy, the development of uteroplacental circulation with low vascular tone accommodates a greater than 20-fold increase in uterine blood flow in near-term pregnant sheep and in humans, which ensures normal placental perfusion and fetal development.13;56 The adaptation of uterine artery contraction and relaxation mechanisms to pregnancy is complex. In addition to remodeling of the uterine vasculature and reduction in downstream resistance, decreased uterine artery resistance is accomplished by significantly blunted vascular contractility57;58 and enhanced endothelium-dependent relaxation of the uterine artery.59;60 We have previously shown that elevated T in pregnant rats enhances vascular smooth muscle contractile responses to angiotensin II and decreases endothelium-dependent relaxation in uterine arteries.43 This elevated T-induced enhancement of uterine artery contraction may contribute to the decrease in diameter and increase in resistance index, culminating eventually in decreased uterine artery blood flow. In addition, the increase in downstream vascular resistance due to poor placental vascularization may also contribute to elevated uterine artery resistance indices and the decrease in blood flow. This suggests that elevated maternal T can cause alterations in uterine artery hemodynamics, which could explain for the observed increase in uterine artery resistance index and decrease in blood flow in pregnant hyperandrogenic PCOS patients.61;62 These results are also consistent with the increase in uterine vascular resistance and decrease in blood flow in fetal growth-restricted human pregnancies.63
We found an important and previously unrecognized role of elevated maternal T on placental vascularization. This study showed about 25% reductions in radial and spiral artery diameters in rats with elevated T. The spiral arteries were also less elongated and less tortuous in rats with elevated T than in controls. Reduced diameters were limited to endothelial-lined vessels; diameters did not differ in trophoblast-lined maternal arterial canals. It is not clear if the effect of elevated T on endothelium contributes to this segmental difference. In contrast to the 25% reductions in diameter of endothelial-lined uteroplacental arteries in rats with elevated T, the diameter of fetoplacental umbilical vessels decreased by <5%, even though endothelium is present in umbilical vessels, at least in humans.64 This result suggests that elevated T plays a lesser role in control of arterial diameters of the fetoplacental arterial vasculature than the uteroplacental vasculature. Instead, changes in the fetoplacental arterial vasculature in rats with elevated T were mostly influenced on elaboration of arterioles as evidenced by decreased arterial length and branching generations. This is consistent with rarefaction of fetoplacental arteriole vessels in fetal growth-restricted human pregnancies.65 Although placental analysis did not reveal any significant sex differences, the magnitude of vascular disturbances in male placentas was marginally higher than in female placentas.
The placentas of T rats showed signs of increased hypoxia, as indicated by increased pimonidazole binding and HIF1α levels. Increased hypoxia could be attributed to reduced uteroplacental blood flow and impaired placental vascular development observed in rats with elevated T, which would tend to reduce oxygen delivery to placenta. The hypoxia is also extended to fetus. The decreased umbilical artery diameter in T dams, together with the decreased in placental vascularity (ie, decreased fetal-placental exchange surface), will also contribute to the decreased fetal oxygen delivery. Interestingly, the placental and fetal hypoxia appear to be sex-specific, with male placentas being more hypoxic than females. The underlying mechanisms for higher hypoxic effects in the male placentas and fetuses remain nebulous; however, the higher nutritional and oxygen demand by the faster growing male fetus in utero66 may predispose them to exhibit pronounced hypoxic effects following oxygen and/or nutrient deprivation.67
Vasculogenesis and angiogenesis are critical processes that lead to the formation of the placental vascular network necessary for adequate uteroplacental circulation.68;69 We observe that elevated T increases expression of angiopoietin2 (angpt2) and its receptor (tie1), which is known to disrupt vascular remodeling and induce endothelial cell apoptosis.70 In addition, we observed downregulation in several important vascular development and angiogenesis-related genes (Ccr3, Stra6, Dhcr7, Arid1a, Ptprj, Col1a2, Lef1, Col1a1, and Mmp2), suggesting an antivasculogenic gene expression profile was in placentas of rats with elevated T. Microarray studies on human placentas complicated with IUGR have shown aa similar antiangiogenic gene expression profile.71 However, T placentas also showed some upregulated angiogenesis genes that could be an effort to compensate for the decreased uteroplacental circulation and placental hypoxia. Although there is no significant quantitative difference in T-induced decrease in diameter, length and branching of placental vasculature between the male and female placentas, we show for the first time that there is striking difference in the gene sets altered by T. Indeed, the sets of genes affected by T differed markedly between the two sexes. It is unclear if the male placentas cope differently than the female placentas to the same maternal insult or if this T-induced difference in gene expression is driven by the genetic sex of the cells. Sexual dimorphism in placenta could result from differential effects of sex chromosomes. In bovine blastocysts, sex determines the level of expression of one third of the actively expressed genes.72 In human term placentas, Sood et al. showed that many of the sex-correlated genes were located on the sex chromosomes, but that some were autosomal.73 In our study, we cannot conclude that there are more genes on the Y or X chromosome directly accounting for sexually dimorphic placental gene expression in basic conditions. Differences in expression of androgen receptor levels between the male and female T placentas may also contribute for the gender differences in gene expressions. A similar difference in sex-specific placental gene expression has been reported in pregnant dams fed a high-fat74 or low-protein diet.75 Further, our transcriptomic analysis provides evidence for a greater perturbation in male than in female rat placentas of T dams in terms of the numbers of dysregulated genes. This evidence contrasts studies reported in mice in which high-fat-fed dams displayed more changes in placental gene expression in female than males.74 This discrepancy in reactivity between males and females placentas may be due to differences in maternal insult, species, and placental developmental stage. Although sex-specific alterations in the gene expression of the placenta were found, it remains to be established if these alterations are found in the fetus as well and if they play a underlying role in contributing to sex differences in the development of adult-life dysfunctions. The limitation of this study, like most expression studies in rats and mice, is that we analyzed whole placentas that contain mixed cell populations.74;76;77 Changes in the proportions of the different cell populations might generate the differences observed independently of changes in gene expression in a single cell population.
PERSPECTIVES
Elevated T during pregnancy is associated with abnormal fetal growth and development leading to adult-life diseases, yet the mechanisms underlying the detrimental effects of T remain unknown. In the present study, clinically relevant concentrations of testosterone produced a reduction in uterine arterial blood flow and disruption of uteroplacental arterial vasculature, resulting in placental hypoxia in pregnant rats. The effect of elevated T in maintaining high-vascular resistance and in disrupting fetoplacental vascularization most likely contributed to reduced fetal tissue oxygenation and reduced fetal growth at term. Further, current findings suggest that elevated maternal T-induced disruption of placental vascular development is associated with underlying alteration in expression of genes involved in blood vessel development and angiogenesis. Intriguingly, elevated T induced striking differences in altering different sets of genes in the male and female placentas. The results suggest a role for T as a possible mediator of increased vascular resistance and placental insufficiency during pregnancy. Therefore, some of the vascular effects observed during preeclampsia or PCOS pregnancies may indeed be androgen-mediated. The ability of elevated T to influence vascular and placental function during pregnancy may contribute to some of the negative effects of T on fetal growth and development. Understanding T’s influences on the vascular and placental system could lead to new therapeutic approaches to antagonize some placental dysfunctions during pregnancy. Furthermore, these results provide a novel approach to understanding the sex differences in the underlying factors that contribute to the gender differences in the pathogenesis of fetal origins of adult diseases.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is New?
In contrast to the well-studied beneficial roles of estrogen and progesterone in maternal cardiovascular adaptations to pregnancy, this study shows that elevated maternal plasma T, at levels similar to those observed in preeclampsia, PCOS mothers, and pregnant African-American women, leads to a reduction in uterine arterial blood flow, disruption in development of uteroplacental arterial vasculature, and placental hypoxia in pregnant rats.
Testosterone-mediated impairments in placental vascular development correlate with alteration in placental expression of genes involved in blood vessel development and angiogenesis.
Elevated T induces striking sexual dimorphism by inducing alterations in different sets of genes in the male and female placentas.
The enhanced uterine artery contraction and reduced relaxation mechanisms may decrease uterine arterial blood flow, and disruption of placental vascularization may contribute to reduced fetal tissue oxygenation and nutrient delivery, leading to reduced fetal growth.
What Is Relevant?
Most pregnancy pathologies, which cause fetal growth restriction, also present with high androgen levels, such as preeclampsia, PCOS, congenital adrenal hyperplasia, maternal smoking or nicotine intake, caffeine intake, obesity, or stress. Moreover, pregnant African-American women have higher serum T levels, with a greater frequency of low-birth-weight babies. Thus, it is of clinical significance to examine androgen’s role in fetal growth.
Numerous studies have demonstrated that T directly causes fetal damage. On the other hand, the adverse effects of T on fetal growth could be from indirect action of T on the maternal-fetal unit and the uteroplacental circulation.
Herein, we present evidence for the first time that elevated T levels induce a decrease in uterine arterial blood flow and fetal sex-related uteroplacental vascular changes, which may set the stage for subsequent sex-differences in adult-onset diseases.
Strategies that target excessive androgen action in the uteroplacental circulation could have an important therapeutic potential in treatment of pregnancies complicated by fetal growth restriction.
Summary
This article is the first to show how elevated maternal T, at concentrations relevant to those observed in abnormal pregnancy conditions, like preeclampsia and IUGR, affects uterine artery and placental function. Elevated maternal T increased vascular resistance, decreased uterine blood flow, and disrupted placental vascular development, which may lead to reduced fetal nutrient and oxygen delivery and growth restriction.
Acknowledgments
SOURCES OF FUNDING
Financial Support from the National Institute of Health (NIH) through grants HL119869 awarded to K. Sathishkumar is greatly appreciated.
Footnotes
CONFLICT OF INTEREST
None
Reference List
- 1.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Munson ML. Births: final data for 2005. Natl Vital Stat Rep. 2007;56:1–103. [PubMed] [Google Scholar]
- 2.Manning FAFA. Fetal Medicine: Principles and Practice. Norwalk, Conn: Appleton & Lange; 2010. Intrauterine Growth Retardation: Etiology, Pathophysiology, Diagnosis, and Treatment. [Google Scholar]
- 3.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman PD, Hanson MA, Pinal C. The developmental origins of adult disease. Matern Child Nutr. 2005;1:130–141. doi: 10.1111/j.1740-8709.2005.00020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ong KK, Dunger DB. Perinatal growth failure: the road to obesity, insulin resistance and cardiovascular disease in adults. Best Pract Res Clin Endocrinol Metab. 2002;16:191–207. doi: 10.1053/beem.2002.0195. [DOI] [PubMed] [Google Scholar]
- 6.Szymanowski K, Chmaj-Wierzchowska K, Florek E, Opala T. [Influence of tobacco smoking to development of the fetus, newborn and child--a review] Przegl Lek. 2006;63:1135–1137. [PubMed] [Google Scholar]
- 7.Zhang X, Sliwowska JH, Weinberg J. Prenatal alcohol exposure and fetal programming: effects on neuroendocrine and immune function. Exp Biol Med (Maywood) 2005;230:376–388. doi: 10.1177/15353702-0323006-05. [DOI] [PubMed] [Google Scholar]
- 8.Kajantie E. Fetal origins of stress-related adult disease. Ann N Y Acad Sci. 2006;1083:11–27. doi: 10.1196/annals.1367.026. [DOI] [PubMed] [Google Scholar]
- 9.Steckler TL, Roberts EK, Doop DD, Lee TM, Padmanabhan V. Developmental programming in sheep: administration of testosterone during 60–90 days of pregnancy reduces breeding success and pregnancy outcome. Theriogenology. 2007;67:459–467. doi: 10.1016/j.theriogenology.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Vandenbosche RC, Kirchner JT. Intrauterine growth retardation. Am Fam Physician. 1998;58:1384. [PubMed] [Google Scholar]
- 11.Thaler I, Manor D, Itskovitz J, Rottem S, Levit N, Timor-Tritsch I, Brandes JM. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162:121–125. doi: 10.1016/0002-9378(90)90834-t. [DOI] [PubMed] [Google Scholar]
- 12.Magness R, Bazer FW. The Endocrinology of Pregnancy. Totowa: Humana Press Inc; 1998. Maternal cardiovascular and other physiologic responses to the endocrinology of pregnancy; pp. 507–539. [Google Scholar]
- 13.Osol G, Mandala M. Maternal uterine vascular remodeling during pregnancy. Physiology (Bethesda ) 2009;24:58–71. doi: 10.1152/physiol.00033.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Croy BA, Yamada A, DeMayo FJ, Adamson SL. The Guide to Investigation of Mouse Pregnancy. 1st. Elsevier; 2014. 1 ed. 2014. [Google Scholar]
- 15.Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155:365–370. doi: 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- 16.Voegtline KM, Costigan KA, Kivlighan KT, Henderson JL, DiPietro JA. Sex-specific associations of maternal prenatal testosterone levels with birth weight and weight gain in infancy. J Dev Orig Health Dis. 2013;4:280–284. doi: 10.1017/S2040174413000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salamalekis E, Bakas P, Vitoratos N, Eleptheriadis M, Creatsas G. Androgen levels in the third trimester of pregnancy in patients with preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2006;126:16–19. doi: 10.1016/j.ejogrb.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Acromite MT, Mantzoros CS, Leach RE, Hurwitz J, Dorey LG. Androgens in preeclampsia. Am J Obstet Gynecol. 1999;180:60–63. doi: 10.1016/s0002-9378(99)70150-x. [DOI] [PubMed] [Google Scholar]
- 19.Ghorashi V, Sheikhvatan M. The relationship between serum concentration of free testosterone and pre-eclampsia. Endokrynol Pol. 2008;59:390–392. [PubMed] [Google Scholar]
- 20.Carlsen SM, Romundstad P, Jacobsen G. Early second-trimester maternal hyperandrogenemia and subsequent preeclampsia: a prospective study. Acta Obstet Gynecol Scand. 2005;84:117–121. doi: 10.1111/j.0001-6349.2005.00493.x. [DOI] [PubMed] [Google Scholar]
- 21.Troisi R, Potischman N, Roberts JM, Ness R, Crombleholme W, Lykins D, Siiteri P, Hoover RN. Maternal serum oestrogen and androgen concentrations in preeclamptic and uncomplicated pregnancies. Int J Epidemiol. 2003;32:455–460. doi: 10.1093/ije/dyg094. [DOI] [PubMed] [Google Scholar]
- 22.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Perez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 23.Mains LM, Lathi RB, Burney RO, Dahan MH. Serum total testosterone levels in a patient with late onset 21-hydroxylase deficiency and a twin gestation. Fertil Steril. 2007;87:1212–1218. doi: 10.1016/j.fertnstert.2006.07.1545. [DOI] [PubMed] [Google Scholar]
- 24.Spiro RP, Christian SL, Ledbetter DH, New MI, Wilson RC, Roizen N, Rosenfield RL. Intrauterine growth retardation associated with maternal uniparental disomy for chromosome 6 unmasked by congenital adrenal hyperplasia. Pediatr Res. 1999;46:510–513. doi: 10.1203/00006450-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Smith LM, Cloak CC, Poland RE, Torday J, Ross MG. Prenatal nicotine increases testosterone levels in the fetus and female offspring. Nicotine Tob Res. 2003;5:369–374. doi: 10.1080/146222031000094196. [DOI] [PubMed] [Google Scholar]
- 26.Kandel DB, Udry JR. Prenatal effects of maternal smoking on daughters’ smoking: nicotine or testosterone exposure? Am J Public Health. 1999;89:1377–1383. doi: 10.2105/ajph.89.9.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizwan S, Manning JT, Brabin BJ. Maternal smoking during pregnancy and possible effects of in utero testosterone: evidence from the 2D:4D finger length ratio. Early Hum Dev. 2007;83:87–90. doi: 10.1016/j.earlhumdev.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Rohrmann S, Sutcliffe CG, Bienstock JL, Monsegue D, Akereyeni F, Bradwin G, Rifai N, Pollak MN, Agurs-Collins T, Platz EA. Racial variation in sex steroid hormones and the insulin-like growth factor axis in umbilical cord blood of male neonates. Cancer Epidemiol Biomarkers Prev. 2009;18:1484–1491. doi: 10.1158/1055-9965.EPI-08-0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agurs-Collins T, Rohrmann S, Sutcliffe C, Bienstock JL, Monsegue D, Akereyeni F, Bradwin G, Rifai N, Pollak MN, Platz EA. Racial variation in umbilical cord blood sex steroid hormones and the insulin-like growth factor axis in African-American and white female neonates. Cancer Causes Control. 2012;23:445–454. doi: 10.1007/s10552-011-9893-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potischman N, Troisi R, Thadhani R, Hoover RN, Dodd K, Davis WW, Sluss PM, Hsieh CC, Ballard-Barbash R. Pregnancy hormone concentrations across ethnic groups: implications for later cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:1514–1520. doi: 10.1158/1055-9965.EPI-04-0869. [DOI] [PubMed] [Google Scholar]
- 31.Chinnathambi V, Balakrishnan M, Ramadoss J, Yallampalli C, Sathishkumar K. Testosterone Alters Maternal Vascular Adaptations: Role of the Endothelial NO System. Hypertension. 2013;61:647–654. doi: 10.1161/HYPERTENSIONAHA.111.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sathishkumar K, Elkins R, Chinnathambi V, Gao H, Hankins GD, Yallampalli C. Prenatal testosterone-induced fetal growth restriction is associated with down-regulation of rat placental amino acid transport. Reprod Biol Endocrinol. 2011;9:1–12. doi: 10.1186/1477-7827-9-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chinnathambi V, Balakrishnan M, Yallampalli C, Sathishkumar K. Prenatal Testosterone Exposure Leads to Hypertension That Is Gonadal Hormone-Dependent in Adult Rat Male and Female Offspring. Biol Reprod. 2012;206:507.e1–507.e10. doi: 10.1095/biolreprod.111.097550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chinnathambi V, Yallampalli C, Sathishkumar K. Prenatal testosterone induces sex-specific dysfunction in endothelium-dependent relaxation pathways in adult male and female rats. Biol Reprod. 2013;89:1–9. doi: 10.1095/biolreprod.113.111542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dean A, Smith LB, Macpherson S, Sharpe RM. The effect of dihydrotestosterone exposure during or prior to the masculinization programming window on reproductive development in male and female rats. Int J Androl. 2012;35:330–339. doi: 10.1111/j.1365-2605.2011.01236.x. [DOI] [PubMed] [Google Scholar]
- 36.Dean A, Sharpe RM. Clinical review: Anogenital distance or digit length ratio as measures of fetal androgen exposure: relationship to male reproductive development and its disorders. J Clin Endocrinol Metab. 2013;98:2230–2238. doi: 10.1210/jc.2012-4057. [DOI] [PubMed] [Google Scholar]
- 37.Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J Neurosci. 2012;32:674–680. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morisset AS, Dube MC, Drolet R, Pelletier M, Labrie F, Luu-The V, Tremblay Y, Robitaille J, John WS, Tchernof A. Androgens in the maternal and fetal circulation: association with insulin resistance. J Matern Fetal Neonatal Med. 2013;26:513–519. doi: 10.3109/14767058.2012.735725. [DOI] [PubMed] [Google Scholar]
- 39.Rae M, Grace C, Hogg K, Wilson LM, McHaffie SL, Ramaswamy S, MacCallum J, Connolly F, McNeilly AS, Duncan C. The pancreas is altered by in utero androgen exposure: implications for clinical conditions such as polycystic ovary syndrome (PCOS) PLoS One. 2013;8:e56263 1–e56263 13. doi: 10.1371/journal.pone.0056263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Battaglia C, Mancini F, Cianciosi A, Busacchi P, Facchinetti F, Marchesini GR, Marzocchi R, de AD. Vascular risk in young women with polycystic ovary and polycystic ovary syndrome. Obstet Gynecol. 2008;111:385–395. doi: 10.1097/01.AOG.0000296657.41236.10. [DOI] [PubMed] [Google Scholar]
- 41.Chekir C, Nakatsuka M, Kamada Y, Noguchi S, Sasaki A, Hiramatsu Y. Impaired uterine perfusion associated with metabolic disorders in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2005;84:189–195. doi: 10.1111/j.0001-6349.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 42.Beckett EM, Astapova O, Steckler TL, Veiga-Lopez A, Padmanabhan V. Developmental programing: impact of testosterone on placental differentiation. Reproduction. 2014;148:199–209. doi: 10.1530/REP-14-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chinnathambi V, Selvanesan BC, Vincent KL, Saade GR, Hankins GD, Yallampalli C, Sathishkumar K. Elevated testosterone levels during rat pregnancy cause hypersensitivity to angiotensin II and attenuation of endothelium-dependent vasodilation in uterine arteries. Hypertension. 2014;64:405–414. doi: 10.1161/HYPERTENSIONAHA.114.03283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4:5. doi: 10.1186/2042-6410-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chinnathambi V, More AS, Hankins GD, Yallampalli C, Sathishkumar K. Gestational exposure to elevated testosterone levels induces hypertension via heightened vascular angiotensin II type 1 receptor signaling in rats. Biol Reprod. 2014;91:6. doi: 10.1095/biolreprod.114.118968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blesson CS, Chinnathambi V, Hankins GD, Yallampalli C, Sathishkumar K. Prenatal Testosterone Exposure Induces Hypertension in Adult Females via Androgen Receptor-Dependent Protein Kinase Cdelta-Mediated Mechanism. Hypertension. 2014;65:683–690. doi: 10.1161/HYPERTENSIONAHA.114.04521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlsen SM, Jacobsen G, Romundstad P. Maternal testosterone levels during pregnancy are associated with offspring size at birth. Eur J Endocrinol. 2006;155:365–370. doi: 10.1530/eje.1.02200. [DOI] [PubMed] [Google Scholar]
- 49.Sathishkumar K, Elkins R, Yallampalli U, Balakrishnan M, Yallampalli C. Fetal programming of adult hypertension in female rat offspring exposed to androgens in utero. Early Hum Dev. 2011;87:407–414. doi: 10.1016/j.earlhumdev.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chinnathambi V, Balakrishnan M, Yallampalli C, Sathishkumar K. Prenatal testosterone exposure leads to hypertension that is gonadal hormone-dependent in adult rat male and female offspring. Biol Reprod. 2012;137:1–7. doi: 10.1095/biolreprod.111.097550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee YM, Jeong CH, Koo SY, Son MJ, Song HS, Bae SK, Raleigh JA, Chung HY, Yoo MA, Kim KW. Determination of hypoxic region by hypoxia marker in developing mouse embryos in vivo: a possible signal for vessel development. Dev Dyn. 2001;220:175–186. doi: 10.1002/1097-0177(20010201)220:2<175::AID-DVDY1101>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 52.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Elevated Androgen Levels During Pregnancy Impair Fetal Growth Due to Placental Insufficiency and Programs for Adult Hypertension in Rats. Biology of Reproduction. 2009:250. [Google Scholar]
- 53.Hotchkiss AK, Lambright CS, Ostby JS, Parks-Saldutti L, Vandenbergh JG, Gray LE., Jr Prenatal testosterone exposure permanently masculinizes anogenital distance, nipple development, and reproductive tract morphology in female Sprague-Dawley rats. Toxicol Sci. 2007;96:335–345. doi: 10.1093/toxsci/kfm002. [DOI] [PubMed] [Google Scholar]
- 54.Steckler T, Wang J, Bartol FF, Roy SK, Padmanabhan V. Fetal programming: prenatal testosterone treatment causes intrauterine growth retardation, reduces ovarian reserve and increases ovarian follicular recruitment. Endocrinology. 2005;146:3185–3193. doi: 10.1210/en.2004-1444. [DOI] [PubMed] [Google Scholar]
- 55.Manikkam M, Crespi EJ, Doop DD, Herkimer C, Lee JS, Yu S, Brown MB, Foster DL, Padmanabhan V. Fetal programming: prenatal testosterone excess leads to fetal growth retardation and postnatal catch-up growth in sheep. Endocrinology. 2004;145:790–798. doi: 10.1210/en.2003-0478. [DOI] [PubMed] [Google Scholar]
- 56.Rosenfeld CR, Morriss FH, Jr, Makowski EL, Meschia G, Battaglia FC. Circulatory changes in the reproductive tissues of ewes during pregnancy. Gynecol Invest. 1974;5:252–268. doi: 10.1159/000301658. [DOI] [PubMed] [Google Scholar]
- 57.Magness RR, Rosenfeld CR. Systemic and uterine responses to alpha-adrenergic stimulation in pregnant and nonpregnant ewes. Am J Obstet Gynecol. 1986;155:897–904. doi: 10.1016/s0002-9378(86)80047-3. [DOI] [PubMed] [Google Scholar]
- 58.Naden RP, Rosenfeld CR. Effect of angiotensin II on uterine and systemic vasculature in pregnant sheep. J Clin Invest. 1981;68:468–474. doi: 10.1172/JCI110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ross GR, Yallampalli U, Gangula PR, Reed L, Sathishkumar K, Gao H, Chauhan M, Yallampalli C. Adrenomedullin Relaxes Rat Uterine Artery: Mechanisms and Influence of Pregnancy and Estradiol. Endocrinology. 2010;151:4485–93. doi: 10.1210/en.2010-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xiao D, Pearce WJ, Zhang L. Pregnancy enhances endothelium-dependent relaxation of ovine uterine artery: role of NO and intracellular Ca(2+) Am J Physiol Heart Circ Physiol. 2001;281:H183–H190. doi: 10.1152/ajpheart.2001.281.1.H183. [DOI] [PubMed] [Google Scholar]
- 61.Palomba S, Falbo A, Russo T, Battista L, Tolino A, Orio F, Zullo F. Uterine blood flow in pregnant patients with polycystic ovary syndrome: relationships with clinical outcomes. BJOG. 2010;117:711–721. doi: 10.1111/j.1471-0528.2010.02525.x. [DOI] [PubMed] [Google Scholar]
- 62.Palomba S, Russo T, Falbo A, Di CA, Amendola G, Mazza R, Tolino A, Zullo F, Tucci L, La Sala GB. Decidual endovascular trophoblast invasion in women with polycystic ovary syndrome: an experimental case-control study. J Clin Endocrinol Metab. 2012;97:2441–2449. doi: 10.1210/jc.2012-1100. [DOI] [PubMed] [Google Scholar]
- 63.Krishna U, Bhalerao S. Placental insufficiency and fetal growth restriction. J Obstet Gynaecol India. 2011;61:505–511. doi: 10.1007/s13224-011-0092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hracsko Z, Hermesz E, Ferencz A, Orvos H, Novak Z, Pal A, Varga IS. Endothelial nitric oxide synthase is up-regulated in the umbilical cord in pregnancies complicated with intrauterine growth retardation. In Vivo. 2009;23:727–732. [PubMed] [Google Scholar]
- 65.Baykal C, Sargon MF, Esinler I, Onderoglu S, Onderoglu L. Placental microcirculation of intrauterine growth retarded fetuses: scanning electron microscopy of placental vascular casts. Arch Gynecol Obstet. 2004;270:99–103. doi: 10.1007/s00404-003-0511-z. [DOI] [PubMed] [Google Scholar]
- 66.Parker AJ, Davies P, Mayho AM, Newton JR. The ultrasound estimation of sex-related variations of intrauterine growth. Am J Obstet Gynecol. 1984;149:665–669. doi: 10.1016/0002-9378(84)90255-2. [DOI] [PubMed] [Google Scholar]
- 67.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huppertz B, Peeters LL. Vascular biology in implantation and placentation. Angiogenesis. 2005;8:157–167. doi: 10.1007/s10456-005-9007-8. [DOI] [PubMed] [Google Scholar]
- 69.Arroyo JA, Winn VD. Vasculogenesis and angiogenesis in the IUGR placenta. Semin Perinatol. 2008;32:172–177. doi: 10.1053/j.semperi.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 71.McMinn J, Wei M, Schupf N, Cusmai J, Johnson EB, Smith AC, Weksberg R, Thaker HM, Tycko B. Unbalanced placental expression of imprinted genes in human intrauterine growth restriction. Placenta. 2006;27:540–549. doi: 10.1016/j.placenta.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 72.Bermejo-Alvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Sex determines the expression level of one third of the actively expressed genes in bovine blastocysts. Proc Natl Acad Sci U S A. 2010;107:3394–3399. doi: 10.1073/pnas.0913843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sood R, Zehnder JL, Druzin ML, Brown PO. Gene expression patterns in human placenta. Proc Natl Acad Sci U S A. 2006;103:5478–5483. doi: 10.1073/pnas.0508035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci U S A. 2010;107:5557–5562. doi: 10.1073/pnas.1000440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shang Y, Jia Y, Sun Q, Shi W, Li R, Wang S, Sui S, Zhao R. Sexually dimorphic effects of maternal dietary protein restriction on fetal growth and placental expression of 11beta-HSD2 in the pig. Anim Reprod Sci. 2015;160:40–48. doi: 10.1016/j.anireprosci.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 76.Gheorghe CP, Goyal R, Mittal A, Longo LD. Gene expression in the placenta: maternal stress and epigenetic responses. Int J Dev Biol. 2010;54:507–523. doi: 10.1387/ijdb.082770cg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gheorghe C, Mohan S, Longo LD. Gene expression patterns in the developing murine placenta. J Soc Gynecol Investig. 2006;13:256–262. doi: 10.1016/j.jsgi.2006.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.