Abstract
Relief from pain in humans is rewarding and pleasurable. Primary rewards, or reward predictive cues, are encoded in brain reward/motivational circuits. While considerable advances have been made in our understanding of reward circuits underlying positive reinforcement, less is known about the circuits underlying the hedonic and reinforcing actions of pain relief. We review findings from electrophysiological, neuroimaging and behavioral studies supporting the concept that the rewarding effect of pain relief requires opioid signaling in the anterior cingulate cortex, activation of midbrain dopamine neurons and release of dopamine in the nucleus accumbens. Understanding of circuits that govern the reward of pain relief may allow the discovery of more effective and satisfying therapies for patients with acute and chronic pain.
Relief of pain is a reward
Substantial scientific progress in the past century has deepened our understanding of somatosensation, including the neurobiology of pain that often follows from activation of nociceptors (see Glossary) (1, 2). Pain is commonly categorized along with other sensations and relief of pain is thus often interpreted simply as termination of nociceptive transmission. Early Greek philosophers, however, grouped pain with emotions and appetites, rather than sensation. Pain was considered to be the opposite of pleasure (3). This elegant and deep insight is consistent with emerging data revealing mechanisms underlying the emotional and motivational features of pain. Relief of aversive states, including pain, often promotes a positive emotional state. Thus, relief of an acute pain stimulus has been appropriately described as a reward (4, 5). Rewarding features of pain relief likely facilitate learning during the healing process about actions leading to relief. The neural mechanisms within reward and motivational circuits underlying reward from pain relief may therefore drive decisions promoting pain relief-motivated behavior that accelerate recovery.
Patients with chronic pain often suffer from co-morbid emotional disorders and cognitive deficits including anxiety, depression and attention or memory impairments, suggesting altered neuronal processing in the brain. Indeed, structural and functional remodeling of the brain has been demonstrated in chronic pain patients (6-8). Clinically, it is well known that the duration of pain positively correlates with the difficulty of achieving satisfactory pain relief with currently available treatments (9). Brain maladaptations during chronification of pain, specifically in circuits underlying the reward of pain relief, may be partly responsible for decreased analgesic efficacy (10, 11). It is therefore important to determine how reward from relief of pain is encoded in the brain and whether functional adaptations may occur in these circuits in the setting of chronic pain.
The neural encoding of rewarding and aversive events and circuits that generate behavioral responses has been of intense interest to neuroscientists. Until recently, however, little was known about the motivational circuits engaged by the relief of pain (12). We review current literature supporting the concept that relief of pain aversiveness can be considered a natural reward that requires neural signaling within brain circuits underlying motivation. We suggest that reward from pain relief is mediated, in part, by opioidergic transmission in the cingulate cortex, a region encoding aversiveness of pain, and dopaminergic signaling in the mesolimbic reward/valuation circuit. This model is based on findings from electrophysiological, neuroimaging and behavioral studies that demonstrate: 1) phasic activity and signaling of mesolimbic dopamine neurons at pain offset; 2) enhanced BOLD fMRI activity in corticolimbic regions following pain relief; 3) increased opioid and dopamine activity in these regions during placebo analgesia; and 4) dependence of pain relief-motivated behavior on opioidergic and dopaminergic signaling in the ACC and NAc, respectively. The putative role of pain relief signaling in motivated behavior is also briefly discussed.
Mesolimbic dopaminergic responses to the onset and offset of pain
Mesolimbic dopamine neurons are well recognized for their responses to primary rewards and reward predicting cues, as well as for their role in approach behavior, learning, reinforcement, decision-making and expression of positive emotions such as pleasure and happiness (reviewed in 13, 14, 15). Electrophysiological characterization of midbrain dopamine neurons in monkeys and rodents have established that in addition to tonic neural activity, most neurons display phasic activations following unpredicted, or better than expected, rewards and depressed neural activity in response to rewards that are worse than expected (16). It is generally accepted that the phasic dopamine response represents quantitative reward prediction error (14) serving as a teaching signal in reinforcement learning to promote behavior that will maximize future rewards (17).
Consistent with their role in reward prediction error, dopamine neurons are typically inhibited by aversive events including noxious stimulation (18-20). Some midbrain neurons, however, increase their firing in response to a noxious stimulus (21, 22). Neurons that are excited by a noxious foot shock were found to be located primarily in the ventral part of the ventral tegmental area (VTA) while neurons inhibited by the same stimulus were located in the dorsal VTA (22). Additionally, it was discovered that a proportion of dopamine neurons can be excited by alerting stimuli related to saliency, novelty or surprise (13). Midbrain dopamine neurons are therefore anatomically and functionally heterogeneous. Thus, different classes of dopamine neurons likely transmit motivational value, salience and alerting signals to target brain regions in the striatum, amygdala, prefrontal cortex and hippocampus to initiate motivated behavior and learning underlying future decisions.
Remarkably, a large proportion of dopamine neurons that are initially inhibited or unresponsive to noxious stimulation exhibit “rebound” activations at the offset of the stimulus (17, 23) (Fig. 1A). For example, Brischoux and colleagues found that dopamine neurons in the dorsal VTA that exhibited phasic inhibition following noxious foot shock, often show significant excitation at the termination of the stimulus (22). These phasic dopamine excitations were maximal between 100 and 150 ms after termination of the stimulus. These findings demonstrate that a specific subset of mesolimbic dopamine neurons can transmit information about cessation of a noxious stimulus. Similar to dopamine reward signals, these pain offset excitations, could reinforce actions and context that immediately precede relief from pain and may therefore represent a rewarding signal related to relief of pain (17).
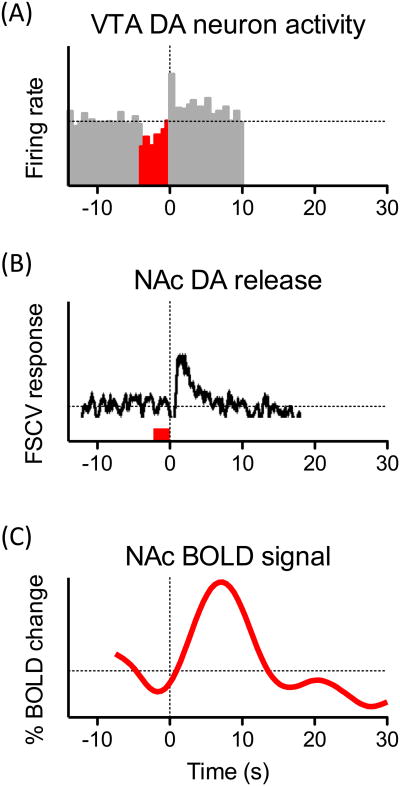
Figure 1.
Termination of a noxious stimulus (pain relief) results in activation of VTA dopamine neurons, dopamine release in the NAc and increased fMRI BOLD activity in the NAc. (A) Some dopamine neurons in the rat VTA show an inhibition of their firing rate following the onset of a noxious electrical stimulation of the hind paw (4 s duration, highlighted in red) and an excitation following its offset. Population peristimulus time histograms (500-ms bins) for the 5 identified dopamine neurons that exhibited an excitation at the offset of the footshocks. Adapted from (22). (B) Dopamine release in the rat NAc shell at the termination of a noxious tail pinch. A representative concentration– time plot of dopamine release evoked by a tail-pinch (3 s duration, red line) measured in rat NAc shell in real time by FSCV. Adapted from (32). (C) Time course of average fMRI BOLD responses for NAc in healthy human subjects following removal of a brief (12-30 s) thermal noxious stimulus to the participants' backs. Adapted from (81).
In the nucleus accumbens, a major target of mesolimbic neurons, dopamine is released at synaptic terminals either in a slow, irregular, tonic mode or a burst, phasic mode (24). Phasic dopamine transmission triggered by rewarding signals is expected to play an important role in reward-related associative learning and facilitate behavioral conditioning (25, 26). Tonic dopamine release, on the other hand, may modulate responsiveness of NAc neurons to inputs from other corticolimbic regions (24). Earlier microdialysis studies investigating effects of aversive nociceptive stimuli on extracellular dopamine concentrations in the striatum produced variable results demonstrating increases (27-30), decreases (31) or no change (30). Recent investigations using fast scan cyclic voltammetry (FSCV, see Box 1), a technique that allows measurements of brief sub-second dopamine transients, have suggested that sub-regions of the striatum have varying responses to painful stimulation. In this study, a painful tail pinch performed on anesthetized rats elicited dopamine release in the dorsal striatum and in the core region of the nucleus accumbens, which are implicated in coding stimulus saliency (32). In contrast, in the nucleus accumbens shell, the most prominent region linked to reward coding, dopamine concentration was suppressed throughout the duration of the stimulus and increased when the tail pinch was removed (32, 33) (Fig. 1B). Increased release of dopamine in the NAc shell at the pain offset is consistent with the observed pain offset excitation of VTA dopamine neurons. It should be noted, however, that dopamine release from terminals does not necessarily correlate with changes in the activity of VTA dopamine neurons but could be influenced by presynaptic mechanisms (34, 35). Nevertheless, neuronal excitation and dopamine release at the offset of noxious stimulation suggest a potential reward-related mechanism by which termination of pain promotes behavior.
Brain activity in response to pain and pain relief
Major advances in elucidating the brain mechanisms of pain have been achieved using neuroimaging techniques in humans and animals. Blood–oxygen–level dependent (BOLD) functional magnetic resonance imaging (fMRI) studies identified brain regions frequently activated by acute noxious stimulation, including the thalamus, the primary and secondary somatosensory cortices (S1, S2), mid/posterior insula and the anterior cingulate cortex (36). These regions interact with a broad network of corticolimbic structures encoding reward/motivation related information to shape behavioral responses (37). In addition to mesolimbic dopamine circuit described above, this reward/motivation network encompasses cortical areas including the anterior cingulate cortex (ACC), lateral orbitofrontal cortex (OFC), and ventromedial and anterior prefrontal cortices (PFC) (for review, see 38). Positron emission tomography (PET) imaging with radiotracers provide a powerful non-invasive method to study the roles of the brain endogenous neurotransmitter systems during behavioral task performance. PET imaging in humans demonstrates the involvement of endogenous dopamine and opioid neurotransmission in response to a noxious stimulation (39-42).
Following removal of an acute noxious stimulation, there is an expected attenuation of the pain-induced activations in most pain-related brain regions. However, in healthy human subjects, the offset of a brief thermal noxious stimulus resulted in increased BOLD activity in the nucleus accumbens (43, 44) (Fig. 1C). Similar activations in the NAc were also found following a cue signaling safety from an expected noxious stimulation (e.g., relief from expected pain) (5). Increased BOLD-fMRI signals have been shown to closely reflect increased local neuronal activity (45). Thus, even though this technique does not identify specific neurotransmitters, a positive NAc BOLD activity with relief of pain may correspond to the reward value prediction error encoded by mesolimbic dopamine neurons. In a comparative fMRI study between humans and awake rats, analogous excitations at the offset of a thermal noxious stimulus were observed in the NAc of both species (46) supporting the likelihood that pain relief reward coding within the NAc represents an evolutionarily conserved mechanism.
In addition to the NAc, increased activity at the termination of a noxious heat stimulation was also observed in the ACC in both human volunteers and in rats (46). This is not surprising, as prefrontal cortical areas encode many aspects of reward processing, decision-making and learning (38). Furthermore, the ACC receives a strong nociceptive input from the medial thalamic nuclei allowing integration and processing of multiple rewarding and aversive motivational signals. In conscious rats, many neurons in the ACC are activated at the onset of a noxious stimulation (47, 48). These neurons typically have very large or whole body receptive fields (49), supporting the idea that the ACC plays a role in the aversive/motivational aspects of pain rather than the sensory/discriminative aspects. (50, 51). Whether individual ACC neurons respond to the termination of a painful stimulus has not been systematically investigated, however, fMRI investigations revealed ACC activation at the offset of thermal noxious stimulus (46) and with omission of expected pain (5).
Role of endogenous opioid circuits in pain and pain relief
Earlier studies with opioid antagonists established a critical role of endogenous opioid system for innate analgesia during traumatic and stressful situations or during “runners high” (52, 53). ACC neurons express high levels of endogenous opioid neuropeptides and their receptors (54) suggesting that at least part of the endogenous analgesic effects could be mediated by opioid activity in this region (55). Interestingly, in rats, systemic administration of morphine is able to inhibit pain-induced firing of ACC nociceptive neurons (47, 48). PET imaging studies with opioid radiotracers have demonstrated increased endogenous opioid release in the ACC in response to painful stimulation (40, 42), suggesting a compensatory analgesic role during the pain experience. Importantly, activation of the opioid system in the ACC negatively correlated with pain-specific affective scores (40) supporting the notion that during pain the opioid system in the ACC is involved in regulating the affective rather than sensory aspects of the pain experience (56).
These observations raised the possibility that endogenous opioid mechanisms might also be involved in the rewarding experience of pain relief. It has been proposed, that increased opioidergic tone due to pain modulatory systems will persist briefly after termination of the noxious stimulus, promoting positive hedonic and motivational response to pain relief (4). Release of opioid neuropeptides at the termination of acute noxious stimulation has not yet been demonstrated. The opioidergic system, however, can be actively engaged without changes in pain intensity by emotional and cognitive tasks and during expectations, such as placebo analgesia (57, 58). Behavioral experiments outlined below indicate that endogenous opioid release may indeed be required for relief of ongoing pain.
Relief of pain provides a motivational and learning signal
A series of studies conducted in Drosophila, rodents and humans were implemented to investigate the mechanisms of learning associated with the relieving cessation of pain (reviewed by 59). To assess both fear and relief conditioning, these studies used a paradigm in which a conditioning stimulus either preceded or followed a brief noxious electric shock, respectively. In Drosophila, when a neutral olfactory cue preceded the foot shock (fear conditioning), the odor acquired aversive conditioned valence. However, when flies received an odor just after an electric shock (relief conditioning), they subsequently showed preference for that odor presumably due to prediction of reward (60). In rats and humans, modulation of the startle response was used as a behavioral measure to assess fear or relief learning. In both species, a cue could acquire either a negative (potentiation of the startle response) or positive (decreased amplitude of the startle response) conditioned valence depending on whether it was presented before or after a foot shock (61). Using fMRI imaging in humans the authors demonstrated that the relief-conditioned cue induced activations in the striatum. Correspondingly, in rats, inactivation of the ventral striatum abolished conditioned relief (61). These experiments suggest that termination of a noxious stimulus provides a positive motivational and learning signal mediated by activation of the ventral striatum.
Nucleus accumbens dopamine signaling and relief of ongoing pain
The pain relief signals discussed above have employed paradigms involving rapid offset of an acute noxious stimulus. Given the known reorganization of brain circuits in chronic pain, it is important to determine if relief of ongoing or chronic pain analogously produces a motivationally salient pain relief signal and how this signal is mediated. This was investigated in rodent models of ongoing or chronic pain using the conditioned place preference (CPP) learning paradigm. Relief of aversiveness of ongoing pain provides a powerful teaching signal allowing association of a context with relief-inducing treatment. Associative relief learning is revealed by motivation to seek that context in the future. We and others have demonstrated CPP with pain relieving treatments in multiple experimental pain models including: neuropathic (SNL, spinal nerve injury; SNI, partial or complete axotomy, spinal cord lesions, cisplatin-induced polyneuropathy) (62-65), inflammatory (Complete Freund's Adjuvant, CFA) (66, 67), post-surgical (incisional) (68), bone cancer (69) and osteoarthritic (mono-iodoacetate, MIA) (70, 71) pain. Importantly, treatments that are not intrinsically rewarding but alleviate pain in humans (e.g., lidocaine-induced peripheral nerve blockade or intrathecal administration of ω-conotoxin (ziconotide) (72) or clonidine (73, 74) do not produce CPP in uninjured animals but can do so in the presence of ongoing pain. Observation of CPP with a pain-relieving treatment ultimately reflects relief of affective features of pain (i.e., pain aversiveness) that are essential in pain perception. CPP following pain-relieving treatments however, do not necessarily require reduction of reflexive pain responses.
To test the hypothesis that dopamine neurotransmission in the mesolimbic circuit mediates reward from relief of ongoing pain we used in vivo microdialysis to measure tonic levels of dopamine in the NAc of conscious behaving rats. Increased extracellular dopamine levels in the NAc shell were measured in rats with post-surgical or neuropathic pain following pain relief with intrinsically non-rewarding treatments (68, 75). Microinjection of dopamine receptor antagonist, α-flupenthixol, into the NAc prevented pain relief induced CPP (68). Moreover, an increased number of activated, cFOS-expressing, dopaminergic neurons in the ventral tegmental area (VTA) was found in rats following pain relief. Accordingly, blockade of VTA dopamine neurons with a GABAB receptor agonist, baclofen, prevented pain relief mediated CPP. Thus, dopamine signaling in the NAc is necessary for CPP produced by relief of ongoing pain suggesting that like appetitive rewards, relief of pain also activates the mesolimbic dopamine circuit to facilitate learning and promote behavior.
Opioid signaling in the anterior cingulate cortex and relief of ongoing pain
Because endogenous as well as administered opioids are able to inhibit nociceptive neurons in the ACC, it is plausible to speculate that opioid activity in this region may be involved in relief of pain aversiveness and activation of the reward circuit. LaGraize et al., have demonstrated that in neuropathic rats, microinjection of morphine into the ACC produced a selective decrease of affective/motivational aspects of pain with no alteration of mechanical paw withdrawal threshold (76). Similarly, we have shown that ACC morphine produced CPP in rats with post-surgical and neuropathic pain but not in uninjured animals (77) (Fig. 2A). Additionally, this treatment elicited release of dopamine in the NAc only in injured rats (Fig. 2A), suggesting that opioid activity in the ACC relieves the aversiveness of pain and subsequently activates reward/motivation circuits. Accordingly, ACC morphine induced CPP in rats with neuropathic pain was blocked by pretreatment of NAc with a non-selective dopamine receptor blocker, α-flupenthixol (Fig. 2A). These findings were extended to determine whether non-opioid pain relieving treatments would also require the ACC opioid activity to promote CPP and activate reward circuits. Blockade of opioid neurotransmission in the ACC prevented CPP as well as NAc dopamine release following non-opioid analgesic treatments (e.g., peripheral nerve block in incised rats or i.th. clonidine in neuropathic rats) (Fig. 2B). Opioid blockade in the ACC had no effect on behavioral thresholds to evoked stimuli. Thus, endogenous opioidergic circuits within the ACC are both necessary and sufficient for reward from pain relief (77). Because activation of opioid receptors in the ACC has no effect on motivated behavior in uninjured rats, it is likely that increased ACC opioid activity in the setting of ongoing pain produces reward by alleviating aversiveness.
Figure 2.
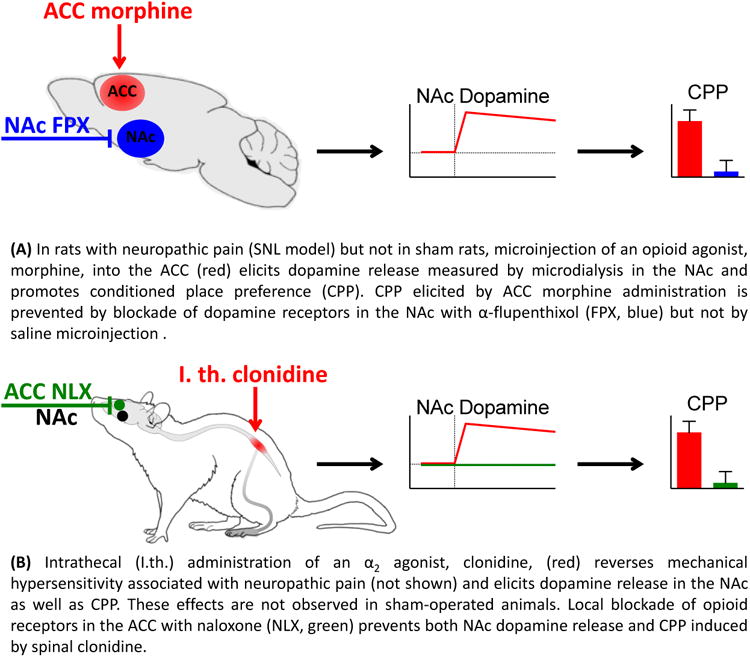
Opioid activity in the ACC and dopamine signaling in the NAc mediate rewarding/motivational aspects of pain relief. The figure summarizes the data from (77).
Conclusion: Brain circuits for pain relief reward
In addition to nociceptive (sensory) information, the pain experience is significantly dependent on emotional and cognitive processing in the brain (50). Onset of pain or increasing pain is a motivationally salient event that often supersedes other competing motivational conditions to generate an appropriate behavioral response (e.g., avoidance, guarding, decreased mobility) (78). Likewise, pain offset or reducing pain is also a motivationally relevant event leading to approach behavior. Thus, relief of pain is rewarding and could promote a positive emotional state (37). Neuronal excitation and neurotransmitter release coupled with neuroimaging and behavioral studies support the notion that mesolimbic dopamine signals and opioid signals in the cingulate cortex may represent the neuronal bases for the positive motivational and hedonic component of pain relief (Fig. 3).
Figure 3.
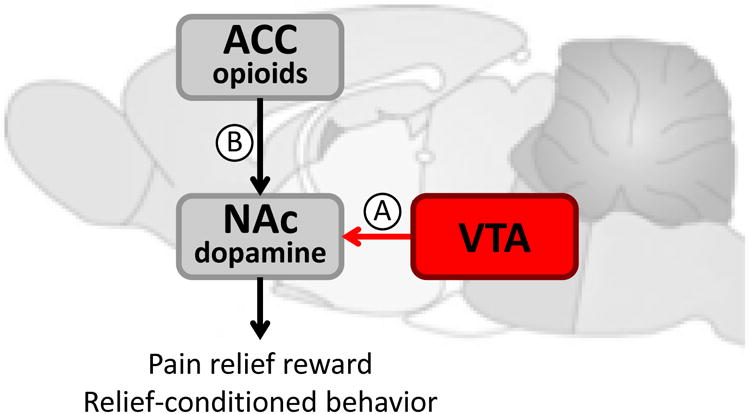
Proposed circuitry for pain relief reward signaling. (A) Activation of VTA dopamine neurons and phasic dopamine release in the NAc occur within milliseconds following the termination of an acute noxious stimulation and may therefore represent a reward signal for pain relief. Relief-conditioned behavior is dependent on activity in the striatum. (B) Relief of ongoing pain can also be considered a reward. In animals, relief of ongoing pain produces CPP that is dependent on dopamine release in the NAc. This effect, however, involves increased tonic dopamine levels in the NAc. Opioid activity in the ACC is both sufficient and necessary for tonic DA increases and CPP in injured animals. Therefore, ACC opioid activation is functionally linked to dopamine release in the NAc to promote pain relief-motivated behavior. Exact neuronal connectivity for the pain relief reward signal remains to be determined.
As in the case of primary rewards and reward predicting cues, dopamine signals for pain relief occur at different time courses (79). Thus, phasic activation of mesolimbic dopaminergic neurons reaches maximum 100-150 ms following the offset of a noxious stimulus (22). Dopamine transients in the NAc measured in rats with FSCV occur within milliseconds of tail pinch termination and last for several seconds (32). Similarly, in human subjects, BOLD fMRI activations in the NAc coincided with the falling phase of the reported pain intensity and continued for several seconds (44) (see Fig. 1). In contrast, tonic dopamine increases in the NAc measured by microdialysis in conscious rats following relief of sustained ongoing pain typically persist for 60-120 min (68). Whether dopamine activations occurring at different time scales serve similar functions is a continuous debate in the reward literature (79) and will require further investigations to delineate the reward from pain relief.
The functions of endogenous opioid signaling in the brain are much less characterized mainly due to the difficulties in directly measuring opioid fluxes (80). PET imaging with opioid radiotracers are most suitable for human investigation, but to our knowledge, no study has directly monitored opioid responses to the offset of a noxious stimulus or relief of chronic pain. Preclinical studies allow the use of invasive methods including microdialysis, but because of complications in opioid peptide quantification, no measurements of opioid peptide release in response to pain relief have been published. Thus, indirect methods of using blockade of opioid receptors in the ACC have been employed. In injured animals, microinjection of opioid receptor antagonists in the ACC prevented motivated behavior for pain relief (CPP) as well as relief-induced tonic dopamine release in the NAc (77). This study is the first demonstration that endogenous opioid signaling in the ACC is required for pain relief-motivated behavior.
Multiple lines of evidence support the conclusion that reward from pain relief requires corticolimbic neural processing in the brain. First, mesolimbic dopamine circuitry is activated at the offset of noxious stimuli and by relief of ongoing pain. Second, increased opioid activity in the cingulate cortex is associated with reduction of the aversiveness of pain. Third, blockade of either opioid transmission in the ACC or dopamine transmission in the NAc prevents pain relief-mediated behavior. Thus, NAc dopamine and ACC opioid neurotransmission may represent pain relief signals that are necessary for rewarding and motivational aspects of pain relief. Such signaling processes likely serve important learning functions that promote actions leading to recovery from pain. Importantly, neurotransmitter deficits in brain regions encoding pain relief reward might be partially responsible for the lack of analgesic efficacy of current therapies observed in some chronic pain patients. Targeting of the neural circuits underlying reward from pain relief may lead to the discovery of new treatment options for chronic pain.
Trends Box.
Electrophysiological studies in rats demonstrate that a subset of mesolimbic dopamine neurons that are initially inhibited by a noxious stimulation, show a “rebound” excitation at the offset of the stimulus.
Recent investigations using fast scan cyclic voltammetry in rats show phasic dopamine release in the nucleus accumbens shell at the termination of a noxious tail pinch.
Using neuroimaging in humans and rats, increased BOLD activity was detected at the offset of a brief noxious stimulus in the nucleus accumbens and in the anterior cingulate cortex.
In Drosophila, rodents and humans, relief of an acute painful stimulus is associated with conditioned reward learning.
In rats, relief of ongoing pain promotes conditioned place preference that requires opioid signaling in the anterior cingulate cortex and subsequent release of dopamine in the nucleus accumbens.
Outstanding Questions Box.
Which dopamine receptors in the NAc shell and core are important for pain relief mediated behavior?
Which endogenous opioid neurotransmitters and receptors are important in relief of pain within ACC circuits?
Are other neurotransmitters (e.g., enkephalins, dynorphins, orexins) in the NAc involved in pain relief reward?
What is the neural connection between endogenous opioid signaling in the ACC and dopamine release in the NAc?
Although PET imaging in humans shows increased dopamine release in the NAc at the offset of an acute pain, will relief of ongoing pain also elicit dopamine neurotransmission in the NAc?
Given that in human PET imaging, opioid binding availability in the ACC negatively correlates with pain affective rating, does relief of ongoing pain with non-opioid treatments result in increased levels of endogenous opioids in this region?
Acknowledgments
The authors thank Professor Howard Fields, UCSF for helpful comments on the manuscript and the support of the NIDA (DA 034975).
Glossary
- Noxious stimulus
a stimulus that is damaging or has the potential to damage tissues. Noxious mechanical, thermal or chemical stimuli are detected by specialized nerve endings, called nociceptors, and transduced from the site of injury via these peripheral nerves to the spinal cord and the brain. This neural process, termed nociception, triggers a variety of reflexive and autonomic responses and can result in subjective experience of pain. Pain is composed of sensory, affective and cognitive aspects. Affective or emotional features of pain motivate behavior
- Ongoing pain
Injury typically results in hypersensitivity to evoked mechanical or thermal stimuli and ongoing (or “spontaneous”) pain that is present without an apparent external stimulation. Although evoked hypersensitivity is a problem in some patients, aversive (i.e., affective) features of ongoing pain are most bothersome. Affective features of pain are tightly linked to sensory input (3) however, modulation of pain affect can be achieved without altering nociceptive input suggesting that affective and sensory qualities of pain are partially separable. Relief of pain aversiveness is sufficient to relieve “pain” perception. In non-verbal animals, relief of aversiveness of ongoing pain can be inferred from motivated behavior (CPP)
- Conditioned Place Preference (CPP)
an operant learning paradigm used to evaluate motivational effects of different experiences in laboratory animals. Typically, animals are conditioned to associate two different treatments (usually control and test treatment) with two conditioning chambers distinguished by sensory cues. Animals are then given access to both chambers and time spent within each chamber is recorded and analyzed for chamber preference. The paradigm can be used in animals with ongoing pain to investigate efficacy of analgesics to alleviate aversive aspects of pain
- Hedonic
related to a pleasant or rewarding feeling
- Fast Scan Cyclic Voltammetry (FSCV)
an electroanalytical method that enables real-time measurements of chemical activity. Modern in vivo FSCV involves applying a triangle waveform to monitor oxidation and reduction of neurotransmitters absorbed to the surface of a carbon-fiber microelectrode (88). This highly sensitive and selective technique has been successfully used to measure rapid sub-second changes of dopamine concentrations in the extracellular space in response to a variety of pharmacological and behavioral stimuli
- Blood-Oxygen-Level Dependent (BOLD) imaging
a technique used in functional magnetic resonance imaging (fMRI) that measures neuronal activity in different areas of the brain based on changes in the ratio of oxygenated to deoxygenated hemoglobin. In research, the method is used to determine which regions of the brain are activated during a specific task such as during application of a painful stimulus
- Positron Emission Tomography (PET)
a functional imaging technique that detects gamma rays emitted from a positron emitting radionuclide. Radionuclide containing molecules (radiotracers) that bind to specific receptors in the brain, such as dopamine and opioid receptors, have been used to investigate release and binding of endogenous neurotransmitters to these receptors. As the endogenous neurotransmitters are released, they compete for the receptor binding sites and displace the radiotracer. Thus reduced radiotracer binding is usually interpreted as increased release of the endogenous ligand
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Woolf CJ, Ma Q. Nociceptors--noxious stimulus detectors. Neuron. 2007 Aug 2;55:353. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 2.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009 Oct 16;139:267. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fields HL. Pain: an unpleasant topic. Pain. 1999 Aug;(6):S61. doi: 10.1016/S0304-3959(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 4.Leknes S, Brooks JC, Wiech K, Tracey I. Pain relief as an opponent process: a psychophysical investigation. Eur J Neurosci. 2008 Aug;28:794. doi: 10.1111/j.1460-9568.2008.06380.x. [DOI] [PubMed] [Google Scholar]
- 5.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS One. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seminowicz DA, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011 May 18;31:7540. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tracey I, Bushnell MC. How neuroimaging studies have challenged us to rethink: is chronic pain a disease? J Pain. 2009 Nov;10:1113. doi: 10.1016/j.jpain.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Wood P, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25:3576. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 9.Percie du Sert N, Rice AS. Improving the translation of analgesic drugs to the clinic: animal models of neuropathic pain. Br J Pharmacol. 2014 Jun;171:2951. doi: 10.1111/bph.12645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Apkarian AV, Baliki MN, Farmer MA. Predicting transition to chronic pain. Curr Opin Neurol. 2013 Aug;26:360. doi: 10.1097/WCO.0b013e32836336ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martikainen IK, et al. Chronic Back Pain Is Associated with Alterations in Dopamine Neurotransmission in the Ventral Striatum. J Neurosci. 2015 Jul 8;35:9957. doi: 10.1523/JNEUROSCI.4605-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014 Oct;17:1304. doi: 10.1038/nn.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010 Dec 9;68:815. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013 Apr;23:229. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014 Jan;76 Pt B:351. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungless MA. Dopamine: the salient issue. Trends Neurosci. 2004 Dec;27:702. doi: 10.1016/j.tins.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Redgrave P, Gurney K, Reynolds J. What is reinforced by phasic dopamine signals? Brain Res Rev. 2008 Aug;58:322. doi: 10.1016/j.brainresrev.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009 Jun 11;459:837. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mileykovskiy B, Morales M. Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear. J Neurosci. 2011 May 18;31:7471. doi: 10.1523/JNEUROSCI.5731-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012 Feb 2;482:85. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zweifel LS, et al. Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat Neurosci. 2011 May;14:620. doi: 10.1038/nn.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brischoux F, Chakraborty S, Brierley DI, Ungless MA. Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc Natl Acad Sci U S A. 2009 Mar 24;106:4894. doi: 10.1073/pnas.0811507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coizet V, Dommett EJ, Redgrave P, Overton PG. Nociceptive responses of midbrain dopaminergic neurones are modulated by the superior colliculus in the rat. Neuroscience. 2006;139:1479. doi: 10.1016/j.neuroscience.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 24.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007 May;30:220. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Zweifel LS, et al. Disruption of NMDAR-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009 May 5;106:7281. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai HC, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science. 2009 May 22;324:1080. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klitenick MA, Taber MT, Fibiger HC. Effects of chronic haloperidol on stress- and stimulation-induced increases in dopamine release: tests of the depolarization block hypothesis. Neuropsychopharmacology. 1996 Oct;15:424. doi: 10.1016/0893-133X(96)00017-6. [DOI] [PubMed] [Google Scholar]
- 28.Rouge-Pont F, Deroche V, Le Moal M, Piazza PV. Individual differences in stress-induced dopamine release in the nucleus accumbens are influenced by corticosterone. Eur J Neurosci. 1998 Dec;10:3903. doi: 10.1046/j.1460-9568.1998.00438.x. [DOI] [PubMed] [Google Scholar]
- 29.Amato D, Natesan S, Yavich L, Kapur S, Muller CP. Dynamic regulation of dopamine and serotonin responses to salient stimuli during chronic haloperidol treatment. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2011 Nov;14:1327. doi: 10.1017/S1461145711000010. [DOI] [PubMed] [Google Scholar]
- 30.Giorgi O, Lecca D, Piras G, Driscoll P, Corda MG. Dissociation between mesocortical dopamine release and fear-related behaviours in two psychogenetically selected lines of rats that differ in coping strategies to aversive conditions. Eur J Neurosci. 2003 Jun;17:2716. doi: 10.1046/j.1460-9568.2003.02689.x. [DOI] [PubMed] [Google Scholar]
- 31.Di Chiara G, Loddo P, Tanda G. Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: implications for the psychobiology of depression. Biol Psychiatry. 1999 Dec 15;46:1624. doi: 10.1016/s0006-3223(99)00236-x. [DOI] [PubMed] [Google Scholar]
- 32.Budygin EA, et al. Aversive stimulus differentially triggers subsecond dopamine release in reward regions. Neuroscience. 2012 Jan 10;201:331. doi: 10.1016/j.neuroscience.2011.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J, Bucher ES, Budygin EA, Wightman RM. Norepinephrine and dopamine transmission in 2 limbic regions differentially respond to acute noxious stimulation. Pain. 2015 Feb;156:318. doi: 10.1097/01.j.pain.0000460312.79195.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lammel S, et al. Unique properties of mesoprefrontal neurons within a dual mesocorticolimbic dopamine system. Neuron. 2008 Mar 13;57:760. doi: 10.1016/j.neuron.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Wightman RM, et al. Dopamine release is heterogeneous within microenvironments of the rat nucleus accumbens. Eur J Neurosci. 2007 Oct;26:2046. doi: 10.1111/j.1460-9568.2007.05772.x. [DOI] [PubMed] [Google Scholar]
- 36.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005 Aug;9:463. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Leknes S, Tracey I. A common neurobiology for pain and pleasure. Nat Rev Neurosci. 2008 Apr;9:314. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- 38.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011 Jun 23;70:1054. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 39.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006 Oct 18;26:10789. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zubieta JK, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001 Jul 13;293:311. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 41.Scott DJ, Stohler CS, Koeppe RA, Zubieta JK. Time-course of change in [11C]carfentanil and [11C]raclopride binding potential after a nonpharmacological challenge. Synapse. 2007 Sep;61:707. doi: 10.1002/syn.20404. [DOI] [PubMed] [Google Scholar]
- 42.Sprenger T, et al. Opioidergic activation in the medial pain system after heat pain. Pain. 2006 May;122:63. doi: 10.1016/j.pain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Becerra L, Borsook D. Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain. 2008 Oct;12:866. doi: 10.1016/j.ejpain.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010 Apr 15;66:149. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005 Aug 5;309:951. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 46.Becerra L, Navratilova E, Porreca F, Borsook D. Analogous Responses in the Nucleus Accumbens and Cingulate Cortex to Pain Onset (Aversion) and Offset (Relief) in Rats and Humans. J Neurophysiol. 2013 Jun 19; doi: 10.1152/jn.00284.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo CC, Yen CT. Comparison of anterior cingulate and primary somatosensory neuronal responses to noxious laser-heat stimuli in conscious, behaving rats. J Neurophysiol. 2005 Sep;94:1825. doi: 10.1152/jn.00294.2005. [DOI] [PubMed] [Google Scholar]
- 48.Wang JY, Huang J, Chang JY, Woodward DJ, Luo F. Morphine modulation of pain processing in medial and lateral pain pathways. Mol Pain. 2009;5:60. doi: 10.1186/1744-8069-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamura H, et al. Morphological and electrophysiological properties of ACCx nociceptive neurons in rats. Brain Res. 1996 Sep 30;735:83. doi: 10.1016/0006-8993(96)00561-6. [DOI] [PubMed] [Google Scholar]
- 50.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci. 2013 Jun;14:502. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004 Apr;7:398. doi: 10.1038/nn1207. [DOI] [PubMed] [Google Scholar]
- 52.Surbey GD, Andrew GM, Cervenko FW, Hamilton PP. Effects of naloxone on exercise performance. J Appl Physiol Respir Environ Exerc Physiol. 1984 Sep;57:674. doi: 10.1152/jappl.1984.57.3.674. [DOI] [PubMed] [Google Scholar]
- 53.Sgherza AL, et al. Effect of naloxone on perceived exertion and exercise capacity during maximal cycle ergometry. J Appl Physiol (1985) 2002 Dec;93:2023. doi: 10.1152/japplphysiol.00521.2002. [DOI] [PubMed] [Google Scholar]
- 54.Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005 Jul;6:533. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tuominen L, et al. Temperament trait Harm Avoidance associates with mu-opioid receptor availability in frontal cortex: a PET study using [(11)C]carfentanil. Neuroimage. 2012 Jul 2;61:670. doi: 10.1016/j.neuroimage.2012.03.063. [DOI] [PubMed] [Google Scholar]
- 56.Lee MC, Wanigasekera V, Tracey I. Imaging opioid analgesia in the human brain and its potential relevance for understanding opioid use in chronic pain. Neuropharmacology. 2013 Jul 25; doi: 10.1016/j.neuropharm.2013.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007 Jun 26;104:11056. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zubieta JK, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. J Neurosci. 2005 Aug 24;25:7754. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gerber B, et al. Pain-relief learning in flies, rats, and man: basic research and applied perspectives. Learn Mem. 2014;21:232. doi: 10.1101/lm.032995.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tanimoto H, Heisenberg M, Gerber B. Experimental psychology: event timing turns punishment to reward. Nature. 2004 Aug 26;430:983. doi: 10.1038/430983a. [DOI] [PubMed] [Google Scholar]
- 61.Andreatta M, et al. Onset and offset of aversive events establish distinct memories requiring fear and reward networks. Learn Mem. 2012;19:518. doi: 10.1101/lm.026864.112. [DOI] [PubMed] [Google Scholar]
- 62.King T, et al. Contribution of afferent pathways to nerve injury-induced spontaneous pain and evoked hypersensitivity. Pain. 2011 Sep;152:1997. doi: 10.1016/j.pain.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.King T, et al. Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci. 2009;12:1364. doi: 10.1038/nn.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qu C, et al. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. 2011 Jul;152:1641. doi: 10.1016/j.pain.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davoody L, et al. Conditioned place preference reveals tonic pain in an animal model of central pain. J Pain. 2011 Aug;12:868. doi: 10.1016/j.jpain.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.He Y, Tian X, Hu X, Porreca F, Wang ZJ. Negative reinforcement reveals non-evoked ongoing pain in mice with tissue or nerve injury. J Pain. 2012 Jun;13:598. doi: 10.1016/j.jpain.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Okun A, et al. Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol Pain. 2011;7:4. doi: 10.1186/1744-8069-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Navratilova E, et al. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012 Dec 11;109:20709. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Remeniuk B, et al. Behavioral and Neurochemical Analysis of Ongoing Bone Cancer Pain in Rats. Pain. 2015 May 4; doi: 10.1097/j.pain.0000000000000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu P, et al. Ongoing pain in the MIA model of osteoarthritis. Neurosci Lett. 2011 Apr 15;493:72. doi: 10.1016/j.neulet.2011.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Okun A, et al. Afferent drive elicits ongoing pain in a model of advanced osteoarthritis. Pain. 2012 Apr;153:924. doi: 10.1016/j.pain.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rauck RL, Wallace MS, Burton AW, Kapural L, North JM. Intrathecal ziconotide for neuropathic pain: a review. Pain Pract. 2009 Sep-Oct;9:327. doi: 10.1111/j.1533-2500.2009.00303.x. [DOI] [PubMed] [Google Scholar]
- 73.Andrieu G, et al. The efficacy of intrathecal morphine with or without clonidine for postoperative analgesia after radical prostatectomy. Anesth Analg. 2009 Jun;108:1954. doi: 10.1213/ane.0b013e3181a30182. [DOI] [PubMed] [Google Scholar]
- 74.Lavand'homme PM, Roelants F, Waterloos H, Collet V, De Kock MF. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008 Sep;107:948. doi: 10.1213/ane.0b013e31817f1595. [DOI] [PubMed] [Google Scholar]
- 75.Xie JY, et al. Activation of mesocorticolimbic reward circuits for assessment of relief of ongoing pain: A potential biomarker of efficacy. Pain. 2014 Aug;155:1659. doi: 10.1016/j.pain.2014.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol. 2006 Jan;197:22. doi: 10.1016/j.expneurol.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 77.Navratilova E, et al. Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci. 2015 May 6;35:7264. doi: 10.1523/JNEUROSCI.3862-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fields HL. In: 11th World Congress on Pain. F H, K E, Dostrovsky JO, editors. Seattle: 2006. pp. 449–459. [Google Scholar]
- 79.Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- 80.Murphy NP. Dynamic measurement of extracellular opioid activity: status quo, challenges, and significance in rewarded behaviors. ACS Chem Neurosci. 2015 Jan 21;6:94. doi: 10.1021/cn500295q. [DOI] [PubMed] [Google Scholar]
- 81.Baliki M, Geha P, Fields H, Apkarian A. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Westerink BH. Brain microdialysis and its application for the study of animal behaviour. Behav Brain Res. 1995 Oct;70:103. doi: 10.1016/0166-4328(95)80001-8. [DOI] [PubMed] [Google Scholar]
- 83.Stuart JN, Hummon AB, Sweedler JV. The chemistry of thought: neurotransmitters in the brain. Anal Chem. 2004 Apr 1;76:121A. [PubMed] [Google Scholar]
- 84.Venton BJ, Wightman RM. Psychoanalytical Electrochemistry: Dopamine and Behavior. Anal Chem. 2003;75:414 A. [Google Scholar]
- 85.Chefer VI, Thompson AC, Zapata A, Shippenberg TS. Overview of brain microdialysis Current Protocols in Neuroscience. J N C Editorial Board, Ed; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bucher ES, Wightman RM. Electrochemical Analysis of Neurotransmitters. Annu Rev Anal Chem (Palo Alto Calif) 2015 Jul 22;8:239. doi: 10.1146/annurev-anchem-071114-040426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Atcherley CW, Wood KM, Parent KL, Hashemi P, Heien ML. The coaction of tonic and phasic dopamine dynamics. Chem Commun (Camb) 2015 Feb 11;51:2235. doi: 10.1039/c4cc06165a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wightman RM. Detection technologies. Probing cellular chemistry in biological systems with microelectrodes. Science. 2006 Mar 17;311:1570. doi: 10.1126/science.1120027. [DOI] [PubMed] [Google Scholar]