Summary
Background
Alectinib, a highly selective, central nervous system (CNS)-active anaplastic lymphoma kinase (ALK) inhibitor, demonstrated promising clinical activity in crizotinib-naïve and crizotinib-resistant ALK-positive non-small-cell lung cancer (NSCLC). This phase 2 study evaluated the safety and efficacy of alectinib in ALK-positive NSCLC patients who progressed on previous crizotinib.
Methods
This ongoing North American study (NCT01871805) enrolled patients with stage IIIB/IV ALK-positive NSCLC, who had progressed following crizotinib. Patients were treated with oral alectinib 600 mg twice daily until progression, death or withdrawal. Primary endpoint was overall response rate (ORR) by independent review committee (IRC) using RECIST v1.1. Secondary endpoints included progression-free survival (PFS), duration of response (DOR), intracranial ORR and DOR, safety, and patient-reported outcomes. The intent-to-treat population was used for efficacy and safety analyses, with the response evaluable population used for response endpoints.
Findings
A total of 87 patients were enrolled in the intent-to-treat population. All patients had received prior crizotinib therapy, and 64 patients (74%) had also received prior chemotherapy. Fifty-two patients (60%) had baseline CNS metastases, of whom 18 (35%) had received no prior brain radiation therapy. At the time of primary analysis (median follow-up 4.8 months), ORR by IRC was 48% (95% CI 36–60). Adverse events were predominantly grade 1 or 2, most commonly constipation, fatigue, myalgia and peripheral edema. The most common grade ≥3 AEs were changes in laboratory values, including increased blood creatine phosphokinase (in 8%, n=7), increased alanine aminotransferase (in 6% n=5), and increased aspartate aminotransferase (in 5% n=4).
Interpretation
Alectinib demonstrated clinical efficacy and was well tolerated in patients with ALK-positive NSCLC who had progressed on crizotinib. Alectinib was active in the CNS, as demonstrated by durable responses in the majority of crizotinib-resistant patients with CNS disease. Therefore, alectinib could be a suitable treatment for patients with ALK-positive disease who have progressed on crizotinib.
Introduction
Chromosomal rearrangements of anaplastic lymphoma kinase (ALK) define a distinct molecular subset of non-small cell lung cancer (NSCLC).1 Present in 3–7% of NSCLC patients, ALK rearrangements lead to expression of oncogenic ALK fusions like echinoderm microtubule-associated protein-like 4 (EML4)-ALK. Patients with ALK-rearranged NSCLC (ALK-positive) are highly responsive to small molecule tyrosine kinase inhibitors of ALK. Crizotinib, the first ALK inhibitor tested in the clinic,2 demonstrated superiority to cytotoxic chemotherapy in advanced ALK-positive NSCLC, with a response rate of 65–74% and a median progression-free survival (PFS) of 7·7–10·9 months.3,4
Despite the efficacy of crizotinib in ALK-positive NSCLC, most patients relapse within the first year of treatment. One of the most common sites of relapse is the central nervous system (CNS). In a retrospective analysis of two clinical trials of crizotinib, the CNS was the most common site of progression in patients with brain metastases at enrollment and with non-target or new lesions at progression.5 The propensity to relapse in the CNS likely reflects poor penetration of crizotinib through the blood-brain barrier and failure to achieve therapeutic drug concentrations in the CNS.6 In contrast, systemic or extracranial relapses appear to be mediated by a variety of different mechanisms of resistance.7 In approximately one-third of cases, resistance is due to amplification of the ALK fusion gene or to a secondary mutation within the ALK tyrosine kinase domain, like the gatekeeper L1196M mutation.8–10 Resistance can also be mediated by activation of alternative signaling pathways, including the epidermal growth factor pathway, insulin-like growth factor pathway and SRC, among others.9,11,12
To address the issue of crizotinib resistance, numerous next generation ALK inhibitors have entered the clinic. In general, these inhibitors are more potent ALK inhibitors than crizotinib and can overcome the most common ALK resistance mutations like L1196M. Ceritinib (Novartis) has shown efficacy in patients previously treated with crizotinib, with a response rate of 56% and a median PFS of 6.9 months.13 Ceritinib is now an approved agent in many countries based on these results. Like ceritinib, alectinib (F. Hoffmann-La Roche, Basel, Switzerland) has also shown antitumour activity in crizotinib-resistant patients. In a phase 1 dose-escalation study conducted in the US, the response rate with alectinib was 55%.14 Among those patients with brain metastases, objective responses in the CNS were observed in 52%, including 29% with complete responses. Alectinib has also been shown to be highly active in crizotinib-naïve ALK-positive NSCLC Japanese patients,15 leading to the recent approval of alectinib in Japan.
The US phase 1 study of alectinib established the recommended phase 2 dose (600 mg twice daily) and demonstrated preliminary activity of alectinib in crizotinib-treated patients.14 Here we report efficacy and safety results from the phase 2 portion of the study of alectinib (NCT01871805) conducted in US and Canadian patients with crizotinib-resistant, ALK-positive NSCLC.
Methods
Study design and participants
This single-arm, open-label, multicentre study (28 centers; see appendix page 1) enrolled US and Canadian patients ≥18 years old with an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0, 1, or 2,16 and locally advanced or metastatic ALK-positive NSCLC, determined by a locally-performed, FDA-approved fluorescence in situ hybridization (FISH) test. Patients had to have progressed on crizotinib (with a minimum 1-week washout period), and may have had prior chemotherapy. Patients with untreated or treated brain or leptomeningeal metastases were eligible, providing they were asymptomatic and neurologically stable. Exclusion criteria included chemotherapy within 4 weeks or radiotherapy within 2 weeks of study start, or prior treatment with an ALK inhibitor other than crizotinib. Patients with a history of myocardial infarction, congestive heart failure, unstable angina or cardiac arrhythmia were also excluded. Patients were required to have measurable disease at baseline according to Response Evaluation Criteria in Solid Tumors (RECIST),17 version 1.1, as assessed by the investigators. Patients were also required to have adequate hematological, hepatic and renal function, aspartate aminotransferase and alanine aminotransferase ≤2.5 × ULN(≤5XULN in patients with liver metastases) and calculated creatinine clearance of ≥60 mL/min. This study was conducted in conformance with the principles of the Declaration of Helsinki and the Good Clinical Practice ICH Tripartite Guideline. The study was approved by the local institutional review boards at each participating site. Written informed consent was obtained from all patients prior to screening.
Procedures
All patients received alectinib 600 mg orally twice daily in 21-day cycles. The dose of alectinib could be reduced by no more than two dose levels, if necessary. If further dose reduction was indicated, the patient was to be considered for withdrawal from the study. Treatment was continued until disease progression, withdrawal, or death. Patients with disease progression were to be withdrawn from the study unless there was reasonable evidence of ongoing clinical benefit in the opinion of the treating investigator. Biopsies at the time of study entry and at progression on alectinib were not mandatory. Acceptable samples (in order of preference) were core biopsies, fine needle aspirates (FNA), and bronchoalveolar lavage (only for patients with lung lesions). ’All patients underwent tumour imaging at baseline, including computed tomography (CT) chest/abdomen/pelvis and magnetic resonance imaging (MRI) brain scans. If MRI imaging was not possible, CT head scan was acceptable. The IRC made an independent evaluation of measurable disease at baseline. This IRC evaluated both systemic and CNS disease. A separate IRC consisting of neuroradiologists assessed CNS responses and progressions.18 With regards to identification of CNS lesions, disease was assessed by both investigators and the IRC according to RECIST 1.1. If multiple non-target lesions were present in a given organ, they may have been reported as a single entry (as allowed per RECIST 1.1 – for example ‘multiple brain metastases’). For all patients, restaging scans including brain scans were obtained every 6 weeks through cycle six, then every 9 weeks thereafter. Tumour responses were evaluated using RECIST v1.1. Laboratory tests (hematology, serum chemistry, blood coagulation tests, urinalysis and ECGs) were conducted on day 1 of every cycle and at the end of treatment. For the independent review, all scans were read by two different IRC readers. If there was no discordance between the two assessments, data from the first reader was used. Any discrepancies between the readers were independently adjudicated by a third reader. With the updated analysis, additional restaging scans were available to the IRC. For some patients, the additional assessments triggered the need for adjudication which led to selection of a different reader’s assessment, including assessment of baseline measurable disease. Therefore, the number of patients with measurable disease at baseline was slightly different at the primary analysis compared with the updated analysis.
Outcomes
The primary endpoint of the study was objective response rate (ORR) according to RECIST v1.1, as assessed by an Independent Review Committee (IRC). The IRC was BioClinica, an established provider of Independent Review with a pool of specialised and expert Radiologists (readers) that were assigned to the study (including neuroradiologists, who were used to assess CNS-specific endpoints). Secondary endpoints included ORR in the CNS, disease control rate (DCR; percentage of patients with confirmed CR, PR or SD lasting ≥5 weeks) in the CNS, and CNS progression rate, all assessed by a separate IRC using RECIST v1.1, overall survival, safety, ORR based on investigator assessment, and patient-reported outcomes. Other secondary endpoints included DCR, duration of response (DOR), and PFS, as assessed by IRC and investigators.
Adverse events (AEs) were graded according to Common Terminology Criteria for Adverse Events, version 4.0. AEs were assessed from the start of treatment until 28 days after the final administration of alectinib. Quality of life (QoL) was assessed on day one of cycle one and on day one of all subsequent odd cycles using the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire (QLQ-C30) and its corresponding module for lung cancer (QLQ-LC13). The cut-off date for the primary analysis (i.e., date when all patients have been followed for a minimum of 12 weeks) was October 24, 2014. The cut-off date for the updated analysis was April 27, 2015.
Statistical analysis
The planned sample size of this single-arm study (NCT01871805) was 85 patients. A response rate of at least 35% was considered clinically relevant. With 85 patients, an observed ORR of 46% (39 responses) would have a lower limit of the two-sided 95% confidence interval (CI) (using an exact Clopper-Pearson CI) of 35% with a power of 80% to detect an increase of the ORR to 50%. The primary analysis population for the primary endpoint and other response endpoints was prospectively defined as the response evaluable (RE) population (patients with measurable disease at baseline by IRC who received at least one dose of study drug). All other endpoints were assessed in the intent-to-treat population. Time-to-event endpoints, such as PFS, OS and DOR were estimated using Kaplan–Meier methodology. SAS (version 9.2) was used for all statistical analysis.
Role of the funding source
The study was designed by F.Hoffmann-La Roche Ltd. together with the study investigators. Data were collected, analysed and interpreted by F. Hoffmann-La Roche Ltd, with input from the authors and investigators. All authors had access to the data. The first author wrote the first draft of the manuscript, had full access to all of the data, and had final responsibility to submit for publication. Editorial and graphic support was provided by Gardiner-Caldwell Communications and funded by the sponsor.
Results
Patients
This phase 2 study enrolled a total of 87 ALK-positive NSCLC patients who had developed disease progression following crizotinib (first patient started on 04 September 2013, last patient on 04 August 2014). A total of 125 patients were screened for entry. There were 38 screen failures with the most common reasons being inadequate renal function, negative ALK-rearrangement test and brain or leptomeningeal metastases that were symptomatic or required treatment. Baseline characteristics of the patients are shown in table 1. The majority of patients were Caucasian (84%, n=73/87), had an ECOG PS of 0 or 1 (90%, n=78/87), and were never smokers (62%, n=54/87). Of the 87 patients, 64 (74%, n=64/87) had received prior chemotherapy in addition to crizotinib. Fifty-two patients (60%, n=52/87) had CNS metastases at the time of enrollment, of whom 34 (65%, n=34/52) had received prior brain radiation. According to the IRC, there were two patients enrolled in the study with brain metastases only. No patients enrolled had leptomeningeal disease. All patients except for three had their CNS disease consistently evaluated using serial MRI brain imaging. Sixteen of the 34 patients had received brain radiation greater than 6 months prior to the start of alectinib. Median time from last dose of crizotinib to first dose of alectinib was 15 days (range 7–733). At the time of primary analysis, median duration of follow up for all 87 patients was 4·8 months (range, 1·1–13·6). In the updated analysis, median duration of follow-up was 9·9 months (range, 1·1–19·9).
Table 1.
Baseline characteristics of the patients
| Baseline characteristic | Alectinib 600 mg BID (n=87) | |
|---|---|---|
| Median age, years (range) | 54·0 (29–79) | |
| Sex, n (%) | Male | 39 (45) |
| Female | 48 (55) | |
| Race, n (%) | White | 73 (84) |
| Asian | 7 (8) | |
| Other* | 7 (8) | |
| ECOG PS, n (%) | 0 | 30 (35) |
| 1 | 48 (55) | |
| 2 | 9 (10) | |
| Smoking status, n (%) | Never smoker | 54 (62) |
| Former smoker | 33 (38) | |
| Stage of disease, n (%) | IIIB | 1 (1) |
| IV | 86 (99) | |
| Histology, n (%) | Adenocarcinoma | 82 (94) |
| Other† | 5 (6) | |
| Baseline CNS metastases, n (%) | Yes | 52 (60) |
| No | 35 (40) | |
| Prior chemotherapy, n (%) | Yes | 64 (74) |
| No | 23 (26) |
Other race includes Black or African American (n=3), and multiple or other races (n=4).
Other histology includes large cell carcinoma (n=1), squamous cell carcinoma (n=1), adenosquamous carcinoma (n=2), and poorly differentiated carcinoma (n=1). ECOG PS=Eastern Cooperative Oncology Group; BID=twice daily; CNS=central nervous system.
Efficacy
Systemic Efficacy
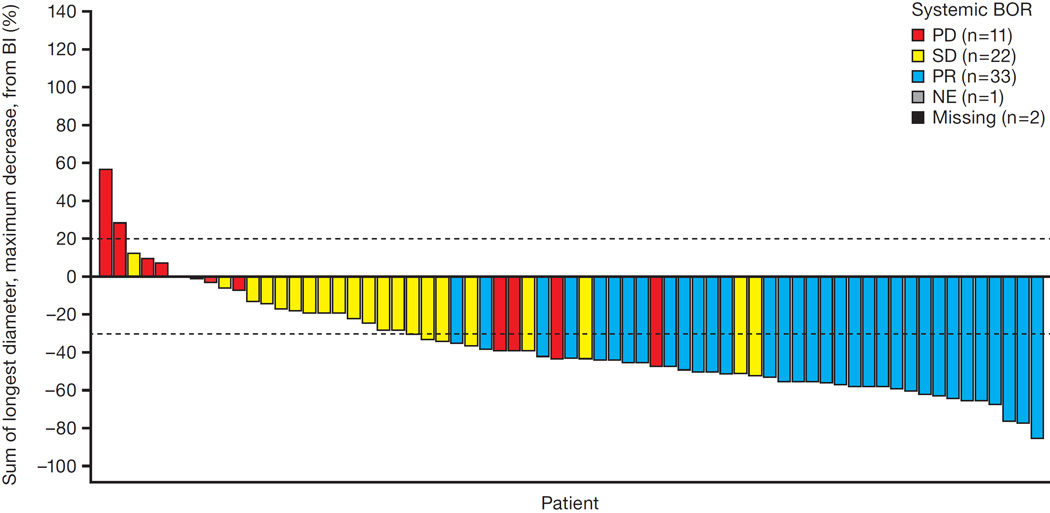
Median follow-up for the primary analysis was 20·7 weeks (interquartile range 14·1–31·0).Of the 87 patients enrolled (intent-to-treat population), 69 had measurable disease at baseline according to the IRC (response evaluable population). At the time of primary analysis, 33 of the 69 patients had a confirmed partial response to alectinib (figure 1), yielding an ORR of 48% (95% CI 36–60; n=33/69). Twenty two patients (32%, n=22/69) had stable disease and 11 patients (16%, n=11/69) had progressive disease as their best response. For three patients (4%, n=3/69), response was not known due to missing or unevaluable post-baseline response assessments. The ORR among all 87 patients as assessed by investigators was 46% (95% CI 35–57; n=40/87), similar to the IRC ORR of 48%.
Figure 1. Best overall systemic responses according to IRC at the time of primary analysis.
This waterfall plot illustrates the best overall responses among 69 patients with baseline measurable disease according to the IRC. A total of 33 patients achieved a partial response (PR) as their best response, and 22 patients achieved stable disease (SD). Eleven patients had progressive disease (PD) as their best response. The responses of three patients are unknown due to unavailable or unevaluable post-baseline restaging scans.
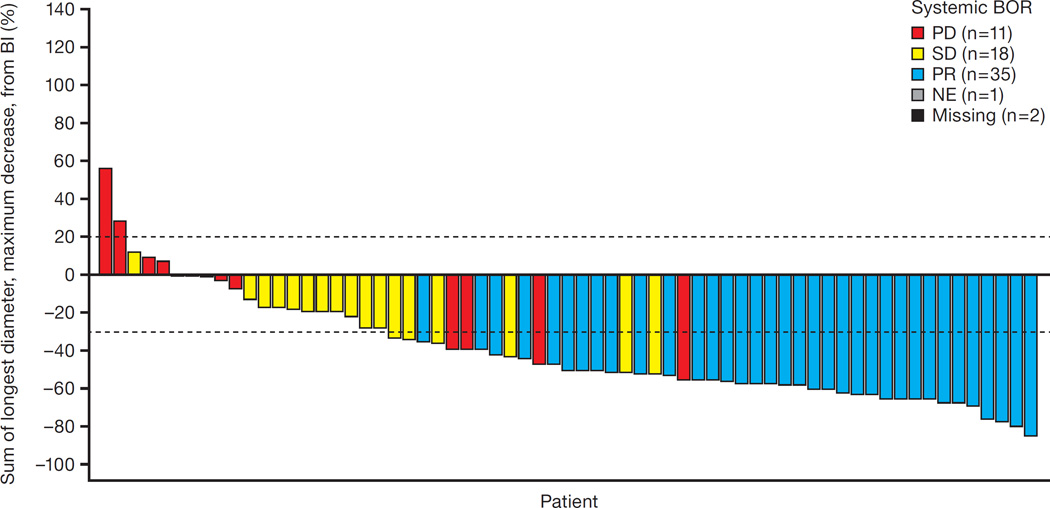
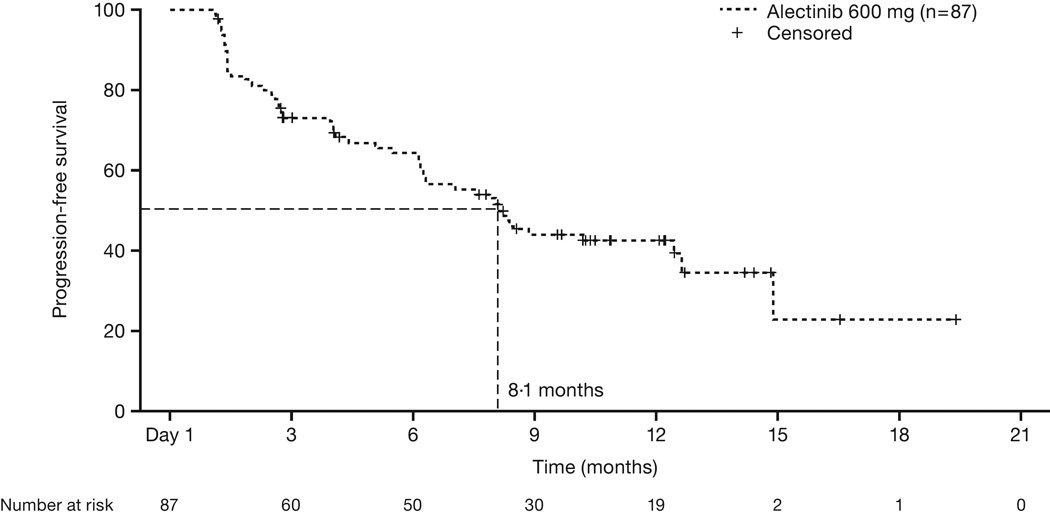
In the updated analysis with longer follow-up (Median follow-up for the updated analyses was 43 weeks [interquartile range 26·9–55·9]), ORR by the IRC among 67 patients with measurable disease was 52% (n=35/67; figure 2), and ORR among all 87 patients as assessed by investigators was 51% (95% CI 40–61; n=44/87). Among those patients with a response, median duration of response was 13·5 months (95% CI 6·7–not estimable) (see appendix page 2). However, data for 21 of the 35 responding patients (60%, n=21/35) was censored at the time of data cut-off. The estimated median PFS among all 87 patients was 8·1 months (95 CI 6·2–12·6) (figure 3). At the time of data cut-off, there were 24 deaths. The estimated overall survival rate at 12 months was 71% (95% CI 61–81) based on Kaplan–Meier methodology.
Figure 2. Best overall systemic responses according to IRC at the updated analysis.
This waterfall plot illustrates the best overall responses among 67 patients with baseline measurable disease according to the IRC. A total of 35 patients achieved a partial response (PR) as their best response, and 18 patients achieved stable disease (SD). Eleven patients had progressive disease (PD) as their best response. The responses of three patients are unknown due to unavailable or unevaluable post-baseline restaging scans.
Figure 3. Progression-free survival by IRC.
Shown is the Kaplan-Meier curve for estimated PFS among 87 patients treated with alectinib. PFS is defined as the time from first alectinib dose to disease progression or death from any cause. 44% of patients were censored at the time of data cut-off. Vertical lines on the PFS curve indicate censored patients.
CNS efficacy
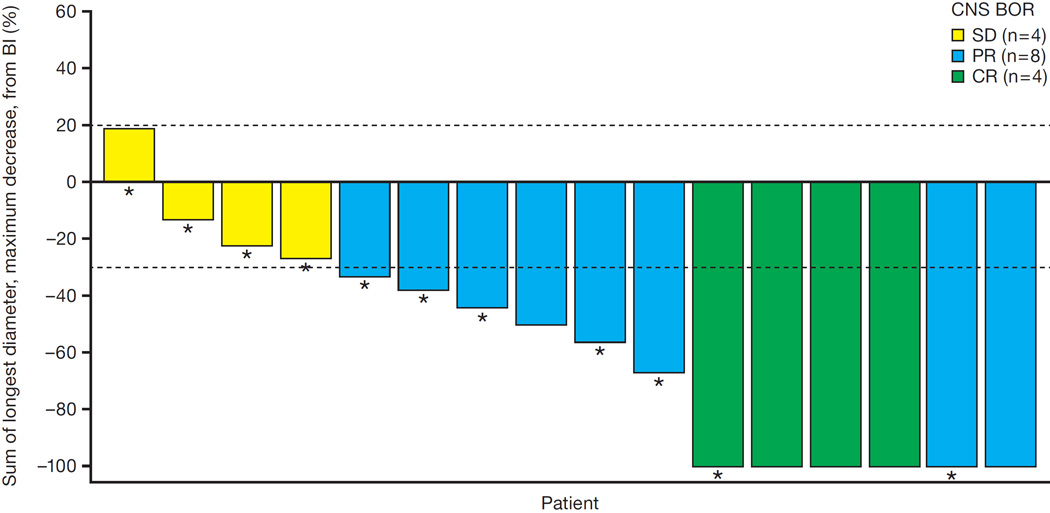
A total of 16 patients had measurable CNS disease at baseline according to RECIST v1.1, of whom 11 had received prior radiation therapy. At the time of the updated analysis, 4 of the 16 patients (25%, n=4/16) had a complete response, and 8 (50%, n=8/16) had a partial response based on IRC assessment, for an intracranial ORR of 75% (95% CI 48–93; n=12/16) (figure 4). Median duration of CNS response was 11·1 months (95% CI 5·8–11·1). CNS DCR was 100% (95% CI 79–100; n=16/16).
Figure 4. Best overall CNS response to alectinib.
This waterfall plot illustrates the best overall intracranial responses among 16 patients with baseline measurable CNS disease according to the IRC. Four patients achieved a complete response (CR), and eight patients achieved a partial response (PR). The remaining four patients had stable disease (SD) as their best response. Asterisks indicate those patients who did not receive prior radiation therapy for their CNS disease.
Among 52 patients with either measurable or non-measurable CNS disease at baseline, 21 (40%, n=21/52) had a response, including 13 (25%, n=13/52) with a complete response. Thirty-three patients (63%, n=33/52) had a non-complete response or stable disease. In patients with non-measurable CNS disease, only complete resolution of metastatic lesions (a complete response) is considered a response, and there are no partial responses.. Median duration of CNS response in the 52 patients was 11·1 months (95% CI 10·8–not estimable), and CNS DCR was 89% (95% CI 77–96; n=46/52).
Of the 52 patients with measurable or non-measurable CNS metastases at baseline, 18 had not received prior radiation therapy for their CNS disease. CNS ORR among the 18 patients with previously untreated CNS lesions was 67% (n=12/18), with 10 complete responses and two partial responses. Five patients had stable CNS disease and one patient had progressive disease. These findings suggest that alectinib is active in the CNS in the absence of prior radiation therapy.
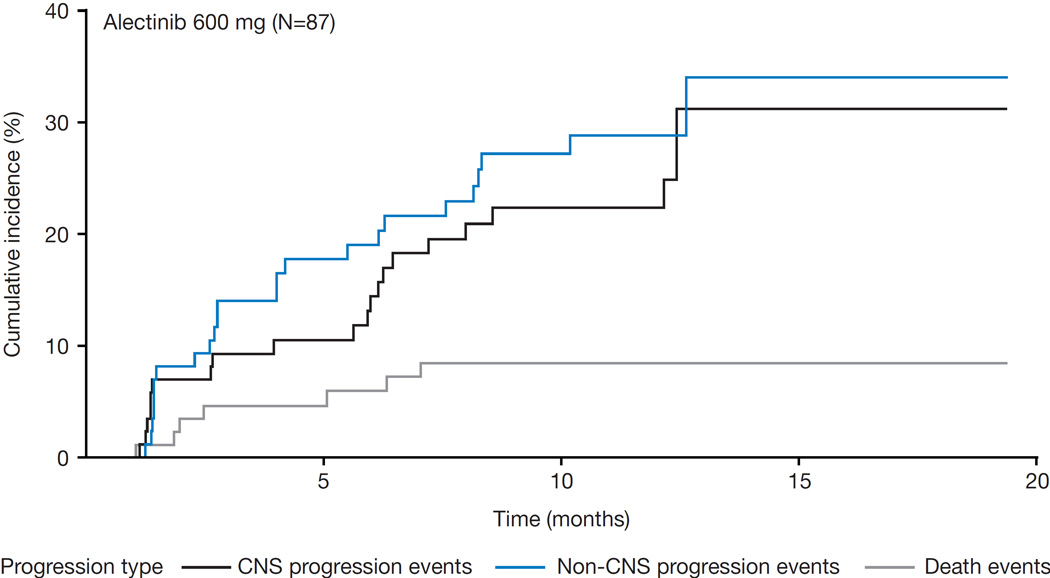
Finally, CNS progression rates were analyzed by cumulative incidence functions. The rates of CNS progression were below the non-CNS progression rates (figure 5).
Figure 5. Cumulative incidence curves of CNS and non-CNS progression rates.
Shown is the cumulative incidence of CNS and non-CNS progression, plotted using a cumulative incidence curve. CNS progression was defined as a new CNS lesion or progression of pre-existing CNS lesions, according to the IRC.
Safety
The safety profile of alectinib was similar to that reported in previous phase 1 studies.14,15 In the updated analysis, the most common side effects were constipation (in 36% of patients, n=31/87), fatigue (in 33%, n=29/87), myalgia (in 24%, n=21/87), and peripheral edema (in 23%, n=20/87). The most common grade ≥3 AEs were changes in laboratory values, including increased blood creatine phosphokinase (in 8%, n=7/87), increased alanine aminotransferase (in 6%, n=5/87), and increased aspartate aminotransferase (in 5%, n=4/87). AEs by highest grade reported are shown in Table 2. Serious adverse events (SAEs) were reported in 13 patients (15%, n=13/87). Two grade 5 events were reported, one in a patient with fatal hemorrhage who was also on anticoagulants, considered related to study treatment by the investigator, and one in a patient with fatal disease progression and recent history of stroke, which was not considered related to treatment. There were no cases of hepatic failure and no cases of interstitial lung disease reported. Overall, the rates of dose interruption and dose reduction were low (36% [n=31/87] and 16% [n=14/87], respectively), reflecting good tolerability with alectinib. The mean dose intensity was 92% (standard deviation 14%). Only two patients (2%, n=2/87) discontinued due to an AE.
Table 2.
Summary of all-causality grade 1–2 adverse events reported in ≥10%, and all grade 3, 4 or 5 events
| Alectinib 600 mg BID (n=87) | ||||
|---|---|---|---|---|
| AE, n (%) | Grade 1–2 | Grade 3 | Grade 4 | Grade 5 |
| Blood creatine phosphokinase increased | 12 (13.8) | 7 (8.0) | - | - |
| Aspartate Aminotransferase Increased | 14 (16.1) | 4 (4.6) | - | - |
| Alanine Aminotransferase Increased | 11 (12.6) | 5 (5.7) | - | - |
| Weight increased | 14 (16.1) | - | - | - |
| Blood Alkaline phospatase Increased | 11 (12.6) | - | - | - |
| Blood bilirubin increased | 6 (6.9) | 1 (1.1) | - | - |
| Activated Partial Thromboplastin Time Prolonged | 4 (4.6) | 1 (1.1) | - | - |
| Electrocardiogram Qt Prolonged | - | 1 (1.1) | - | - |
| Fatigue | 29 (33.3) | - | - | - |
| Peripheral oedema | 20 (23.0) | - | - | - |
| Death | - | - | - | 1 (1.1) |
| Generalised oedema | - | 1 (1.1) | - | - |
| Constipation | 31 (35.6) | - | - | - |
| Nausea | 19 (21.8) | - | - | - |
| Diarrhoea | 18 (20.7) | - | - | - |
| Vomiting | 10 (11.5) | - | - | - |
| Intestinal obstruction | 1 (1.1) | - | - | - |
| Myalgia | 21 (24.1) | - | - | - |
| Back pain | 9 (10.3) | - | - | - |
| Headache | 18 (20.7) | - | - | - |
| Dizziness | 9 (10.3) | - | - | |
| Seizure | 2 (2.3) | 1 (1.1) | - | - |
| Hemiparesis | 1 (1.1) | 1 (1.1) | - | - |
| Brain Oedema | - | - | 1 (1.1) | - |
| Cerebral Ventricle Dilatation | - | 1 (1.1) | - | - |
| Cerebrovascular Accident | - | 1 (1.1) | - | - |
| Embolic stroke | - | - | 1 (1.1) | - |
| Dyspnoea | 13 (14.9) | 3 (3.4) | - | - |
| Cough | 15 (17.2) | - | - | - |
| Obstructive Airways Disorder | 1 (1.1) | - | - | - |
| Upper Respiratory Tract Infection | 9 (10.3) | - | - | - |
| Lung infection | 1 (1.1) | 1 (1.1) | - | - |
| Influenza | 1 (1.1) | - | - | |
| Staphylococcal Sepsis | 1 (1.1) | - | - | |
| Hypokalaemia | 6 (6.9) | 2 (2.3) | - | - |
| Hypertriglyceridaemia | 5 (5.7) | 2 (2.3) | - | - |
| Hypoalbuminaemia | 4 (4.6) | 1 (1.1) | - | - |
| Hypophosphataemia | 2 (2.3) | 2 (2.3) | - | - |
| Hypocalcaemia | 2 (2.3) | 1 (1.1) | - | - |
| Hyponatraemia | 2 (2.3) | - | 1 (1.1) | - |
| Glucose Tolerance Impaired | - | 1 (1.1) | - | - |
| Hyperammonaemia | - | 1 (1.1) | - | - |
| Malnutrition | - | 1 (1.1) | - | - |
| Photosensitivity Reaction | 9 (10.3) | - | - | - |
| Anaemia | 15 (17.2) | - | 1 (1.1) | - |
| Neutropenia | 3 (3.4) | 1 (1.1) | - | - |
| Lymphopenia | 1 (1.1) | 1 (1.1) | - | - |
| Insomnia | 10 (11.5) | - | - | - |
| Confusional State | - | 1 (1.1) | - | - |
| Drug–Induced Liver Injury | - | 1 (1.1) | - | - |
| Haemorrhage | - | - | - | 1 (1.1) |
All events of dyspnea were grade 3 with no grade 4 and 5 events reported. All events were reported as non-serious, not related to alectinib and none led to modification of treatment with alectinib. AE=adverse event; BID=twice daily; ALT=alanine transaminase.
Patient-reported outcomes
Using EORTC QLQ-C30 and QLQ-LC13 questionnaires, clinically meaningful improvement is defined as a change from baseline of 10 or more points. There was a clinically meaningful improvement from baseline in global health status (supplementary figure S2A). This improvement was noted at the first assessment at 6 weeks, maintained for at least two consecutive visits and generally maintained until the end of treatment. Consistent with these results, there was also a reduction from baseline in the lung cancer symptom of fatigue (see appendix page 3).
Discussion
Based on the results of this phase 2 study, alectinib is an effective therapy in ALK-positive NSCLC after failure of crizotinib. At the time of primary analysis, the systemic response rate by IRC was 48% (n=33/69). In the updated analysis presented here, the systemic response rate was 52% (n=35/67) by IRC, and responses were durable with a median DOR of 13·5 months (6·7–not estimable). The IRC ORR was comparable to the investigator-assessed ORR, despite a difference in the number of patients included in the response evaluable population by investigator versus IRC. This difference was due to the absence of measurable disease at baseline in a number of patients according to the IRC as compared to the investigators’ assessment. Like the systemic response rate, the intracranial response rate was also high at 75% (48–93; n=12/16) according to the IRC, and median duration of intracranial response exceeded 11 months. Alectinib was well tolerated, with predominantly grade 1 or 2 AEs, and was associated with improved QoL. These results are consistent with a recently reported, global phase 2 study of alectinib19 and provide additional evidence of alectinib’s systemic and intracranial efficacy in crizotinib-resistant disease.
At present, patients with ALK-positive NSCLC who relapse on crizotinib have two major treatment options: cytotoxic chemotherapy or second-generation ALK inhibitors. While crizotinib has been shown to be superior to standard chemotherapy, the latter, particularly platinum-pemetrexed combination chemotherapy, does have documented activity in advanced ALK-positive NSCLC. In a randomised first-line study of crizotinib versus chemotherapy, the ORR with platinum-pemetrexed was 45%, and median PFS was 7 months.4 One major disadvantage of chemotherapy, however, is the potential for significant side effects, including fatigue, nausea and myelosuppression, which can compromise QoL. A second major disadvantage is that chemotherapy has shown limited efficacy in the CNS. For example, among patients with treated brain metastases at baseline, the intracranial disease control rate with first-line platinum/pemetrexed chemotherapy was only 45% at 12 weeks and 25% at 24 weeks.20 With crizotinib, which has relatively poor CNS penetration, the intracranial disease control rates were 85% and 56% at 12 and 24 weeks, respectively.20 By comparison, in this study of alectinib, where median followup was 43 weeks, the intracranial disease control rate among patients with baseline measurable CNS disease (including untreated disease) was 100% (n=16/16).
Next generation ALK inhibitors represent an alternative treatment option for patients who have progressed on crizotinib. Of the eight next generation ALK inhibitors that have entered the clinic, three – ceritinib, alectinib, and brigatinib, have demonstrated robust activity in ALK-positive NSCLC. In a number of countries, including the US, ceritinib is an approved agent for ALK-positive NSCLC previously treated with crizotinib. In the phase 1 registration study, ceritinib demonstrated an ORR of 56%, a median DOR of 8·2 months, and a median PFS of 6·9 months in patients who had received prior crizotinib.13 Brigatinib has also shown marked clinical activity in patients previously treated with crizotinib, with a reported response rate of 71% and a median PFS of 13·4 months.21 Taken together, the emerging efficacy data with ceritinib, alectinib, and brigatinib suggest that more potent ALK inhibition is a highly effective therapeutic strategy for patients who have relapsed on crizotinib.
To date, there are no randomized studies comparing next generation ALK inhibitors in the crizotinib-resistant setting, preventing direct comparisons of targeted agents. However, in reviewing the separate, single-arm trials of each next generation ALK inhibitor, alectinib may have several potential advantages in terms of both efficacy and tolerability. For example, duration of response was prolonged with alectinib in this study (median 13.5 months), as compared with ceritinib or brigatinib (median DOR 8.2 and 9.3 months, respectively)13,21. In addition, the CNS efficacy observed with alectinib is notable. Previous preclinical and clinical studies have shown that alectinib, which is not a substrate of P-glycoprotein, crosses the blood-brain barrier efficiently, with CSF Ctrough levels approaching unbound systemic Ctrough levels.14,22 Consistent with this finding, the intracranial response rate with alectinib in this study was remarkably high at 75% among patients with measurable CNS disease at baseline, including patients whose CNS disease was previously untreated. Additionally, among patients with measurable and nonmeasurable CNS lesions, one-quarter achieved a complete response. In contrast, preclinical studies with ceritinib have revealed a brain-to-blood exposure ratio of approximately 15%, and in the phase 1 study of ceritinib, the intracranial response rate among 24 patients with measurable brain metastases was 29%.23 The ability of alectinib to salvage CNS relapses, including leptomeningeal disease, after failure of crizotinib and ceritinib,24 further supports the superior intracranial activity of alectinib.
The second key feature of alectinib is its tolerability profile. Overall, alectinib was well tolerated as reflected by the low rates of dose reduction, interruption and withdrawal and by most AEs being grade 1 or 2. Nausea and diarrhoea were seen in 22% (n=19/87) and 21% (n=18/87) of patients, respectively. Transaminase elevation of grade ≥3 was observed in 5–6%(n=4–5/87) of patients, and there were no hepatic failure cases reported. In contrast, for ceritinib, over one-half of patients in the phase 1 study required at least one dose reduction.13 Gastrointestinal side effects were very common with ceritinib, with nausea and diarrhoea reported in 80% and 86% of patients, respectively. Alanine transaminase (ALT) elevation of grade ≥3 was seen in 27% of ceritinib-treated patients. With brigatinib, gastrointestinal side effects were less common than with ceritinib, but more common than with alectinib, with nausea and diarrhoea seen in 52% and 40% of patients, respectively.20 In addition, there were no reports of interstitial lung disease or pneumonitis with alectinib in this phase 2 study or in the preceding phase 1 study.14 There have also been no reports of early pulmonary toxicity with alectinib, as has been reported with brigatinib.21
The clinical activity of alectinib and other next-generation ALK inhibitors in crizotinib-resistant, ALK-positive NSCLC reinforces the current treatment paradigm of sequential therapy with crizotinib followed by a more potent ALK inhibitor. The high response rates observed with next-generation ALK inhibitors suggests that most patients remain ALK-dependent after relapsing on crizotinib. At present, the optimal next-generation ALK inhibitor to use in a crizotinib-resistant patient is uncertain, but based on this study, alectinib is highly effective in inducing durable responses both systemically and in the CNS. In preclinical models, alectinib has been shown to overcome many of the known ALK resistance mutations, including the two most common mutations ALK L1196M and G1269A, but is inactive against G1202R and I1171 mutations.25,26 Similarly, other ALK inhibitors also show variable potency against the known ALK resistance mutations.26 Whether selection of a next generation ALK inhibitor after failure of crizotinib should be tailored based on the specific crizotinib-resistance mutation remains to be determined.
In summary, alectinib is an effective and well-tolerated treatment for patients with advanced ALK-positive NSCLC who have failed crizotinib. Based on the earlier phase 1 study, alectinib received US FDA breakthrough therapy designation for this indication. Alectinib may also have a role in the crizotinib-naïve setting. In a small phase 1/2 study conducted in Japan, ORR was 94% and median PFS was estimated to be greater than 29 months among 46 ALK-positive NSCLC patients previously treated with chemotherapy but not crizotinib.15,27 A global, randomised, phase 3 trial comparing alectinib with crizotinib in newly diagnosed ALK-positive NSCLC is ongoing (NCT02075840) and will address the question of optimal first-line therapy for advanced ALK-positive NSCLC.
Supplementary Material
Research in context panel.
Evidence before this study
We reviewed PubMed and conference abstracts, searching for reports published in English, using the search terms ‘NSCLC’, ‘lung neoplasms’, ‘ALK’ and ‘anaplastic lymphoma kinase’. Numerous studies reported data on the ALK inhibitors crizotinib and ceritinib, showing that while crizotinib is clinically active, the majority of patients will relapse within one year, most commonly in the central nervous system (CNS). Therefore, this study of the new, CNS-penetrable ALK inhibitor alectinib was undertaken to assess both systemic and CNS efficacy in patients who had progressed on previous crizotinib treatment.
Added value of this study
This study demonstrates that alectinib has clinical efficacy in patients with ALK-positive NSCLC who have progressed on crizotinib. Alectinib is highly active in the CNS, as demonstrated by durable responses in the majority of crizotinib-resistant patients with progressive CNS disease. Alectinib is also well tolerated, with predominantly grade 1 or 2 adverse events, and is associated with improved quality of life. These data suggest that alectinib may be a suitable treatment option for patients who have progressed on crizotinib.
Implications of all available evidence
The emerging efficacy data from new ALK inhibitors suggest that more potent ALK inhibition is a highly effective therapeutic strategy for patients who have relapsed on crizotinib. As CNS relapses are common on crizotinib, the intracranial activity of these new agents is critical to establish. Tolerability is also an important consideration given the prolonged durations of treatment. The optimal sequencing of ALK inhibitor therapies will be determined in the future based on the results of randomized phase III studies.
Acknowledgments
AS is supported by NIH grant 5R01CA164273. Third-party editorial assistance, under the direction of the authors, was provided by Joanna Musgrove of Gardiner-Caldwell Communications, and was funded by F. Hoffmann-La Roche Ltd.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
S-HO received honoraria and participated in an advisory board for F.Hoffmann-La Roche Ltd. AZ is an employee at F.Hoffmann-La Roche Ltd. KG has receive personal fees from Bristol Myers Squibb and Pfizer. RC has received personal fees from F.Hoffmann-La Roche Ltd. LG has participated in an advisory board for F.Hoffmann-La Roche Ltd., participated in advisory boards and lectures for Merck, and advisory boards for Astra-Zeneca. WB is an employee and has stock ownership at F.Hoffmann-La Roche Ltd. HW has performed consultant roles for F.Hoffmann-La Roche Ltd., Novartis, Boehringer-Ingelheim, Astra-Zeneca, Bristol Myers Squibb, Abbvie, and Celgene. TM has participated in a speaker’s bureau and advisory board for F.Hoffmann-La Roche Ltd. HB has participated in an advisory board for Genentech, Eli Lilly, Bristol Myers Squibb, Celgene and Clovis. AC has participated in a speaker’s bureau for Genentech, Pfizer, Boehrigner-Ingelheim, Celgene and Novartis. GR has received grants from Chugai and F.Hoffmann-La Roche Ltd., has performed a consulting for Novartis, and his employer receives research funding from Pfizer, Novartis, and Millennium for support of research led by him. BC is an employee of Eli Lilly and Company. VH, OP and BB are employees of and have stock ownership at F.Hoffmann-La Roche Ltd. AS has participated in an advisory board for Genentech, F. Hoffmann-La Roche Ltd., Pfizer, Novartis, and Arlad, and performed a consultant role for F.Hoffmann-La Roche Ltd., Pfizer, Novartis, and Ignyta. SG has participated in advisory boards for Genentech, Norvartis and Ariad. JC, and MS have nothing to disclose.
Contributors
AS, LG, and AZ were responsible for designing the study, collection and assembly of data, and data analysis and interpretation. GR was responsible for designing the study and the collection, assembly, analysis and interpretation of data. SG, JC, HW, RC, AC, TM, HB, and VH were responsible for the collection, assembly, analysis and interpretation of data. MS was responsible for the study design, data collection, analysis and interpretation. OP was responsible for the study design, data collection and analysis/interpretation. KG was responsible for patient accrual, and analysis and interpretation of data. BC was responsible for literature research, and collection, analysis and interpretation of data. WB was responsible for designing the study and data analysis and interpretation. BB was responsible for conception and design of the study, and collection, assembly, analysis and interpretation of data. S-HO was responsible for patient accrual, conception of the study, and data analysis and interpretation. All authors contributed to the writing, review and approval of the final manuscript.
References
- 1.Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561–566. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 2.Kwak EL, Bang YJ, Camidge DR, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363:1693–1703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 4.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 5.Costa DB, Shaw AT, Ou SH, et al. Clinical experience with crizotinib in patients with advanced ALK-Rearranged non-small-cell lung cancer and brain metastases. J Clin Oncol. 2015;33:1881–1888. doi: 10.1200/JCO.2014.59.0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costa DB, Kobayashi S, Pandya SS, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29:e443–e445. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 7.Katayama R, Lovly CM, Shaw AT. Therapeutic targeting of anaplastic lymphoma kinase in lung cancer: a paradigm for precision cancer medicine. Clin Cancer Res. 2015;21:2227–2235. doi: 10.1158/1078-0432.CCR-14-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi YL, Soda M, Yamashita Y, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363:1734–1739. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 9.Katayama R, Shaw AT, Khan TM, et al. Mechanisms of Acquired Crizotinib Resistance in ALK-Rearranged Lung Cancers. Science Translational Medicine. 2012;4:120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doebele RC, Pilling AB, Aisner DL, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18:1472–1482. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crystal AS, Shaw AT, Sequist LV, et al. Patient-derived models of acquired resistance can identify effective drug combinations for cancer. Science. 2014;346:1480–1486. doi: 10.1126/science.1254721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovly CM, McDonald NT, Chen H, et al. Rationale for co-targeting IGF-1R and ALK in ALK fusion-positive lung cancer. Nat Med. 2014;20:1027–1034. doi: 10.1038/nm.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaw AT, Kim DW, Mehra R, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370:1189–1197. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15:1119–1128. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 15.Seto T, Kiura K, Nishio M, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14:590–598. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 16.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28:1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 19.Ou S, Ahn J, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2015 doi: 10.1200/jco.2015.63.9443. In press. [DOI] [PubMed] [Google Scholar]
- 20.Solomon BJ, Felip E, Blackhall FH, et al. Overall and intracranial efficacy results and time to symptom deterioration in PROFILE 1014: 1st line crizotinib vs pemetrexed-platinum chemotherapy in patients with advanced ALK-positive non-squamous non-small cell lung cancer. Presented at the ESMO Congress, Madrid, Spain. 2014 (abstr 1225O) [Google Scholar]
- 21.Camidge DR, Bazhenova L, Salgia R, et al. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non-small cell lung cancer (NSCLC) J Clin Oncol. 2015;33:8062. [Google Scholar]
- 22.Kodama T, Hasegawa M, Takanashi K, et al. Antitumor activity of the selective ALK inhibitor alectinib in models of intracranial metastases. Cancer Chemother Pharmacol. 2014;74:1023–1028. doi: 10.1007/s00280-014-2578-6. [DOI] [PubMed] [Google Scholar]
- 23.Shaw AT, Mehra R, Tan DS, et al. Evaluation of ceritinib-treated patients with anaplastic lymphoma kinase rearranged (ALK)+) non-small cell lung cancer (NSCLC) and brain metastases in the ASCEND-1 study. Annals Oncol. 2014;25(suppl 4):iv426–iv470. [Google Scholar]
- 24.Gainor JF, Sherman CA, Willoughby K, et al. Alectinib salvages CNS relapses in ALK-positive lung cancer patients previously treated with crizotinib and ceritinib. J Thorac Oncol. 2015;10:232–236. doi: 10.1097/JTO.0000000000000455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakamoto H, Tsukaguchi T, Hiroshima S, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19:679–690. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Zou HY, Friboulet L, Kodack DP, et al. PF-06463922, an ALK/ROS1 Inhibitor, Overcomes Resistance to First and Second Generation ALK Inhibitors in Preclinical Models. Cancer Cell. 2015;28:70–81. doi: 10.1016/j.ccell.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamura T, Seto T, Nakagawa K, et al. Updated data of a phase I/II study (AF-001JP) of alectinib, a CNS-penetrant, highly selective ALK inhibitor in ALK-rearranged advanced NSCLC, CMSTO. Chicago Multidisciplinary Symposium in Thoracic Oncology. 2014;90(suppl):S6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.