SUMMARY
T cells can be re-directed to kill cancer cells using chimeric antigen receptors (CARs) or T cell receptors (TCRs). This approach, however, is constrained by the rarity of tumor-specific single antigens. Targeting antigens also found on bystander tissues can cause life-threatening adverse effects. A powerful way to enhance ON-target activity of therapeutic T cells is to engineer them to require combinatorial antigens. Here we engineer a combinatorially activated T cell circuit in which a synthetic Notch receptor for one antigen induces the expression of a CAR for a second antigen. These dual receptor AND-gate T cells are only armed and activated in the presence of dual antigen tumor cells. These T cells show precise therapeutic discrimination in vivo – sparing single antigen “bystander” tumors while efficiently clearing combinatorial antigen “disease” tumors. This type of precision dual receptor circuit opens the door to immune recognition of a wider range of tumors.
INTRODUCTION
Recent advances in immunotherapy have demonstrated that T cells can be redirected to recognize and eliminate tumors using chimeric antigen receptors (CARs) or engineered T cell receptors (TCRs) that bind tumor specific antigens (Barrett et al., 2014a; June et al., 2009). The application of this therapeutic approach, however, is limited by the rarity of single, highly specific tumor-only antigens. Few antigens are absolutely tumor specific, and T cells targeted to antigens that are also found on normal bystander tissues can cause life-threatening adverse side effects. The most successful T cell therapies, to date, have been targeted to B cell malignancies, utilizing CARs directed to the B cell specific antigen CD19. Even in this successful treatment, however, normal B cells are targeted and eradicated (Brentjens et al., 2013; Grupp et al., 2013; Porter et al., 2011). Although patients can live without B cells, this type of treatment would be far more broadly applicable if T cell therapeutics could more reliably discriminate normal tissue from diseased (Lamers et al., 2006; Morgan et al., 2013; 2010; Sadelain et al., 2009).
Attempting to discriminate cancer cells via a single receptor that recognizes a single antigen is inherently a one-dimensional approach, and it would be a significant improvement if multiple receptors could be used to combinatorially detect multiple antigens (Figure 1A and 1B) (Barrett et al., 2014b). Such multi-antigen approaches would take full advantage of the capabilities of a cell-based therapy, as cells usually integrate multiple inputs to modulate their natural decisions in sophisticated ways.
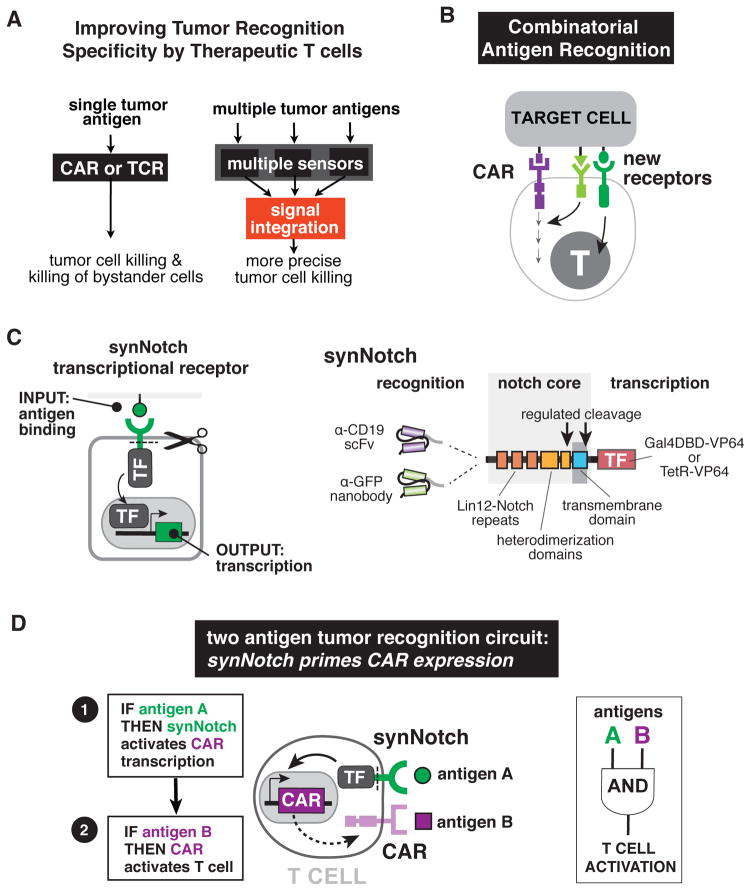
Figure 1. Design of Combinatorial Antigen Sensing Circuits in T cells Using Sequentially Regulated SynNotch and Chimeric Antigen Receptors.
(A) CAR or tumor-specific TCR T cells generally target single antigens often causing OFF-target tissue damage. Improved therapeutic T cells will require multiple sensors that recognize combinations of both tumor antigens and tissue-specific antigens, allowing the cells to assess their environment and make more precise decisions on when to activate. Such therapeutic cells would be better equipped to distinguish target diseased tissue from normal tissue.
(B) New types of receptors that sense combinations of antigens and regulate T cell signaling and transcription must be built to allow for sophisticated cellular decision-making and more precise therapeutic T cell responses.
(C) SynNotch receptors are engineered with a custom extracellular ligand-binding domain (e.g. scFv or nanobody) directed towards an antigen of interest (e.g. CD19 or surface GFP). Upon ligand recognition by the synNotch receptor, an orthogonal transcription factor (e.g. TetR-VP64 or Gal4-VP64) is cleaved from the cytoplasmic tail that regulates a custom genetic circuit.
(D) Design of a synNotch AND-gate circuit that requires T cells to sense two antigens to activate. This AND-gate signaling circuit works in two sequential steps: 1) A synNotch receptor allows the T cell to recognize the first antigen A, and 2) the T cell expresses a CAR directed towards a second tumor antigen B. If A and B are present, the T cells can activate and kill the target tumor.
Prior strategies to engineer multi-input control of T cells have focused on expressing two CARs in the same cell, each with partial signaling function and distinct extracellular antigen recognition domains (Kloss et al., 2013; Wilkie et al., 2012). While such cells show enhanced activation when both target antigens are present, success of this approach relies on delicately balancing the same set of coordinated signaling events that occur downstream from the CAR. Thus, behavior is highly dependent on the exact expression ratios and activities of the different receptor chains, leading to less robust and predictable behavior. Moreover, partial independent function of each receptor inherently limits the dynamic range of this approach – it remains challenging to obtain an AND-gate T cell that is both fully inhibited in the presence of either individual antigen, but fully activated in the presence of both antigens.
To construct more reliable multi-antigen responses, it would be ideal to have new receptors that function completely independently from the CAR/TCR pathway, but that can interface with CAR activity in a controlled manner (Figure 1B). Recently, we have developed a new class of modular receptors called synthetic Notch (synNotch) receptors (accompanying manuscript, Morsut et al.). SynNotch receptors use an extracellular recognition domain (e.g. single chain variable fragment, scFv) to recognize a target antigen, but unlike CARs, binding of the target antigen does not trigger T cell activation. Instead, ligand engagement leads to cleavage of the receptor and release of a transcriptional activator domain, which can in turn enter the nucleus and drive expression of user specified target genes (Figure 1C).
Here we show that we can construct combinatorial antigen recognition T cell circuits in which a synNotch receptor for one antigen drives the inducible expression of a CAR for a second antigen (Figure 1D). These dual receptor T cells are only armed and activated in the presence of dual antigen tumor cells (AND-gate). These combinatorially gated T cells show a remarkable degree of therapeutic discrimination both in vitro and in vivo – sparing single antigen “bystander” tumors while efficiently eradicating combinatorial antigen “disease” tumors. Here we are able to harness the computational power of a dynamic synthetic regulatory circuit to achieve much higher target cell discrimination, while still maintaining a high dynamic range of tumor killing. Given the modularity of both CARs and synNotch receptors, and the robust discrimination we observe, this type of dual receptor circuit could lead to precise immune recognition of a much larger set of tumors.
RESULTS
Design of a Two Antigen AND-gate Circuit: SynNotch Receptors Induce CAR Expression
The design of a simple two receptor AND gate circuit is outlined in Figure 1D. A T cell is engineered to constitutively express a synNotch receptor that recognizes antigen A. In addition, the gene for a CAR that recognizes antigen B would also be inserted into the cell, but it would be under the control of a promoter that requires activation by the synNotch induced transcription factor (synNotch engagement results in receptor cleavage and release of a transcriptional activation domain, Morsut et al., accompanying manuscript). Thus, no CAR expression or activity should be present in the cell until the synNotch receptor is activated. This sequential receptor activation circuit is highly modular in design since swapping extracellular domains can easily change the antigen recognition properties of both receptors.
Testing SynNotch-gated CAR Expression in Jurkat T cells – Combinatorial Antigen Requirement for Jurkat T cell Activation
To test the concept of utilizing synNotch receptors to control the expression of CARs, we first attempted to engineer combinatorial antigen control over the activation of Jurkat T cells. In these experiments, we targeted two model tumor antigens, CD19 and mesothelin. The Jurkat T cells were engineered with an α-CD19 synNotch receptor bearing an intracellular tetracycline-controlled transactivator (tTa) domain (TetR-VP64). We also inserted the α-mesothelin CAR gene (with 4-1BBζ costimulatory domain), under the control of a promoter with the corresponding tetracycline response elements (TRE) activated by the synNotch receptor (Figure 2A and 2B). The engineered Jurkats were co-cultured in vitro with target K562 myelogenous leukemia cells with ectopic expression of CD19, mesothelin, or both antigens together (Figure 2A and 2B). Since these engineered Jurkat T cells only express the α-mesothelin CAR in response to α-CD19 synNotch stimulation, the T cells should not activate in response to mesothelin alone. If the T cells are exposed to CD19, the α-mesothelin CAR is expressed and the T cells are primed for activation. The T cells can then sense mesothelin and activate (Figure 2A and 2B).
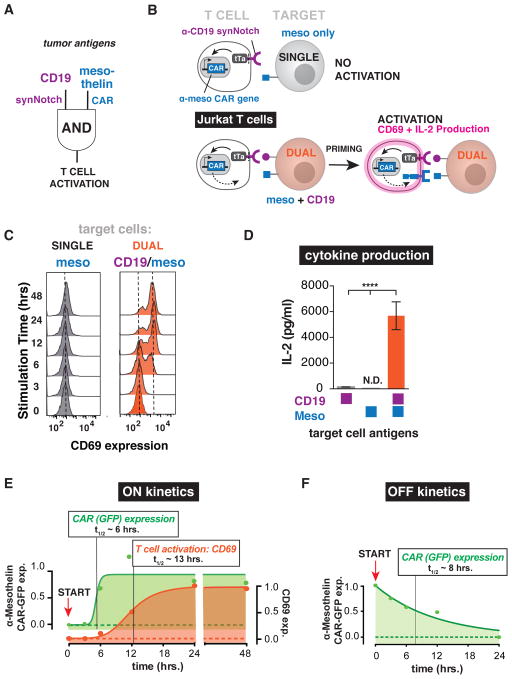
Figure 2. SynNotch Regulated CAR Expression – Combinatorial Antigen Requirement for Jurkat T cell Activation.
(A) Engineering a two-receptor AND-gate circuit: α-CD19 synNotch receptor induces α-mesothelin CAR expression.
(B) Jurkat T cells were engineered with the α-CD19 synNotch tTa receptor and the corresponding response elements controlling α-mesothelin 4-1BBζ CAR expression. The Jurkat T cells must first recognize CD19 on the target tumor via their synNotch receptor to initiate CAR expression. After the T cell is primed to activate by CD19, the α-mesothelin CAR can then bind mesothelin and activate the Jurkat cell. Two canonical markers of T cell activation are CD69 upregulation and IL-2 production. The synNotch AND gate Jurkat T cells should only activate when exposed to target tumor cells expressing both CD19 and mesothelin.
(C) Histograms of the activation marker CD69 in synNotch AND-gate Jurkat T cells co-cultured with single antigen (mesothelin only) or dual antigen (CD19/mesothelin) K562 tumor cells over a 48-hour timecourse. CD69 was only expressed when the T cells were exposed to dual antigen K562 cells (representative of 3 independent experiments).
(D) IL-2 ELISA showing IL-2 production by synNotch AND-gate Jurkat cells only when exposed to dual antigen K562 cells (n=3, error bars are SEM, significance determined by Student’s t-test, **** = P ≤ 0.0001)
(E) Timecourse of AND-gate T cell activation upon stimulation with dual antigen K562 cells. Expression of the GFP-tagged mesothelin CAR (green) occurs with a half-time of ~6 hours. Subsequently, activation of the T cell by CAR activation (monitored by CD69 expression) then occurs with a lag of several more hours (t1/2 ~13 hrs). FACS histograms for CAR expression are shown in Figure S1B.
(F) Timecourse of AND-gate T cell inactivation upon removal of synNotch ligand. Jurkat T cells expressing the AND-gate circuit were stimulated for 24 hours by plate-bound α-Myc antibody (synNotch receptor has extracellular Myc-tag). START indicates time at which cells were removed from the ligand, and the decay of GFP tagged CAR expression was monitored (t1/2 ~8 hrs). FACS histograms for CAR expression are shown in Figure S1C.
When we tested these cells, we indeed observed activation only by the tumor cells that expressed both CD19 and mesothelin, as measured by the upregulation of the activation marker CD69 and secretion of the cytokine IL-2. Tumor cells expressing either single antigen did not lead to activation (Figure 2C and 2D). We analyzed the dynamics of T cell activation when stimulated by dual antigens (Figure 2E and Figure S1A and S1B). The Jurkat synNotch AND-Gate T cells induce CAR expression in response to tumor cell stimulation with a t1/2 of ~6 hours, reaching steady state expression by 24 hours (Figure 2E and Figure S1A and S1B). The subsequent T cell activation (monitored via CD69 expression) occurs after CAR expression initiates, with an expected sequential delay of an additional ~6 hours. Thus, the effective composite half-time for T cell activation by this two step circuit is ~13 hours (Figure 2E).
We also characterized the decay dynamics of synNotch induced CAR expression (Figure 2F and Figure S2C). We first exposed the synNotch T cells to a surrogate of the priming antigen CD19: since the α-CD19 synNotch receptor has a Myc-tag on its extracellular domain, we previously found that the receptor could also be activated by exposure of the cells to α-Myc antibody coated plates. This activation approach allows for rapid cessation of synNotch activation by removing cells from the plate-bound antigen. After 24 hours of stimulus with α-Myc antibody, we removed the T cells and monitored the decay of α-mesothelin CAR expression over 24 hours. The half-life of CAR expression after removal of the synNotch stimulus was ~8 hours (Figure 2F and Figure S1C).
SynNotch-Gated CAR Expression in Human Primary T cells - Combinatorial Antigen Control Over T cell Activation and Tumor Killing
Given the success of the synNotch AND-gate in Jurkat T cells, we then tested whether the same type of synNotch-driven CAR expression circuit could function in primary T cells to discriminate multiple antigens. We tested various synNotch receptors in primary T cells, and found that the Gal4-VP64 transcriptional activation domain worked reliably, yielding good synNotch receptor expression and minimal basal transcriptional activity. This is an ideal scenario for the synNotch→CAR AND-gate because there should be no basal expression of the CAR until the T cells sense the synNotch antigen.
As a proof of principal demonstration of this approach, we first utilized an α-GFP synNotch receptor (α-GFP nanobody recognizes surface expressed GFP; intracellular Gal4VP64 is released) to drive expression of the α-CD19 4-1BBζ CAR (Figure 3A). Our rationale for choosing this model setup is that the α-CD19 CAR is a gold standard in the field of immunotherapy and it shows potent tumor clearance in vivo.
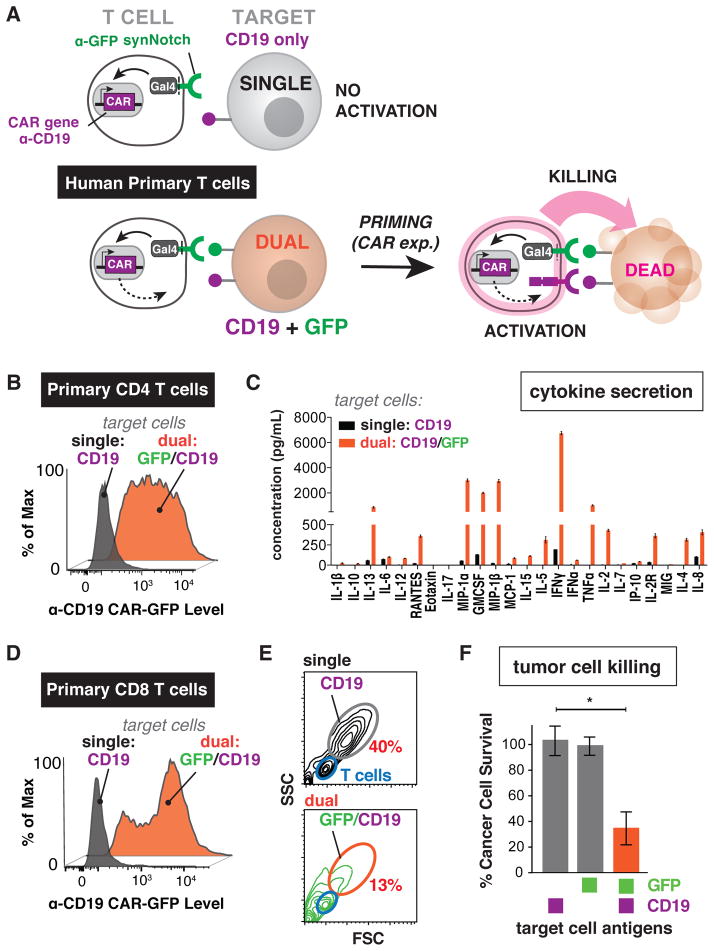
Figure 3. SynNotch Regulated CAR Expression in Human Primary T cells – Combinatorial Antigen Control Over Therapeutic T cell Activation and Tumor Killing.
(A) Human primary CD4+ and CD8+ T cells were engineered with the α-GFP nanobody synNotch Gal4VP64 receptor and the corresponding response elements controlling expression of the α-CD19 4-1BBζ CAR. These CD4+ or CD8+ synNotch AND gate T cells first must sense surface GFP via their synNotch receptor and only then do they express the α-CD19 CAR and are primed to activate. These AND-gate primary T cells should only activate and produce cytokine or kill target cells if they sense both GFP and CD19.
(B) Primary CD4+ synNotch AND gate T cells described in panel A were co-cultured with CD19 only or surface GFP/CD19 K562 cells. Histograms of α-CD19 CAR GFP receptor expression level show that the CAR is only expressed when GFP is present on the surface of the target cell (representative of at least 3 independent experiments).
(C) The supernatant from CD4+ synNotch AND gate T cells activated either by CD19 only or GFP/CD19 K562s was analyzed for the presence of 25 cytokines via Luminex. Cytokines were only produced when the T cells were exposed to GFP/CD19 T cells (error bars are SEM, n=3).
(D) CD8+ synNotch AND gate primary T cells were engineered as described in panel A. As with the CD4+ T cells, the histograms of α-CD19 CAR GFP receptor expression level show that the CAR is only expressed when GFP is present on the surface of the target cell (representative of at least 3 independent experiments).
(E) Forward and side scatter flow cytometry plots after 24 hour co-culture of CD8+ synNotch AND gate primary T cells with either CD19 only or GFP/CD19 tumors cells. The T cells fall within the blue gate and the target CD19 or the GFP/CD19 K562s are in the gray and orange gates, respectively. The synNotch AND gate T cells only killed the GFP/CD19 K562s, shown by the reduction of cells in the K562 gate (representative of 3 experiments).
(F) Quantification of replicate CD8+ synNotch AND gate primary T cell cytotoxicity data shown in panel E. (n=3, error bars are SEM, significance determined by Student’s t-test * = P ≤ 0.05). Other examples of synNotch→CAR circuits in primary T cells are shown in Figure S2.
Human primary CD4+ T cells were engineered with the α-GFP synNotch Gal4VP64 receptor and the corresponding response elements controlling α-CD19 4-1BBζ CAR expression. These T cells were then exposed to K562 target cells expressing CD19 only, GFP only, or GFP and CD19. The CD4+ T cells only displayed expression of the α-CD19 4-1BBζ CAR when stimulated with cells expressing the synNotch ligand, GFP (Figure 3B). Moreover, these T cells only showed activation, as assayed by cytokine production, when exposed to target cells expressing both GFP and CD19 on their surface (Figure 3B and 3C).
Human primary CD8+ cells containing the same dual receptor circuit also showed AND-gate behavior, only killing targets when GFP and CD19 were present on the target cell (Figure 3D–3F). Thus, the synNotch AND-gate is functional in the critical cell types required for T cell immunotherapy in humans. To show the versatility and modularity of this approach, we tested two other synNotch/CAR AND-gate configurations and all showed combinatorial antigen requirements for CD4+ and CD8+ T cell activation (Figure S2A–S2I).
SynNotch Receptors Drive Tumor Localized CAR Expression In vivo
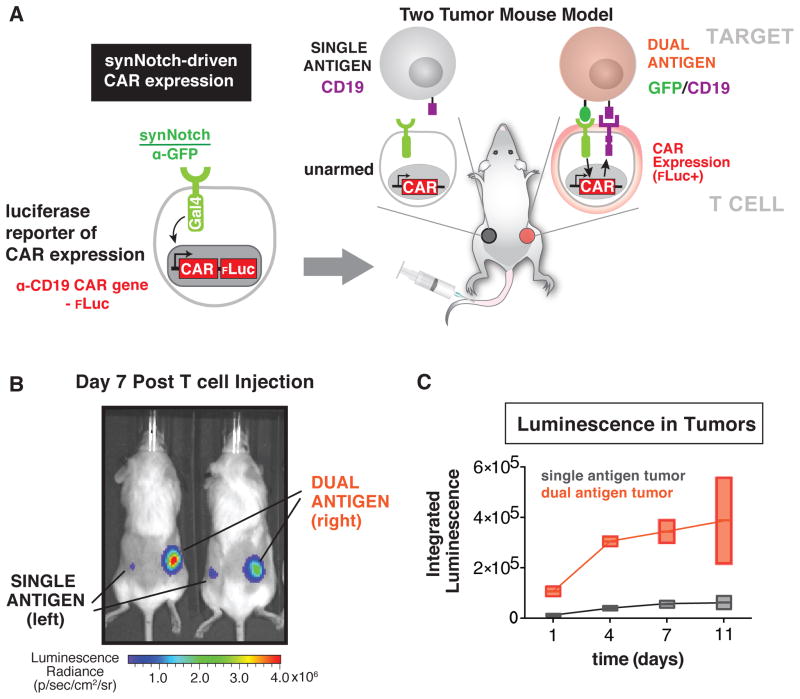
Since synNotch receptors reliably gate CAR expression in primary T cells in vitro, we next tested whether T cells could be targeted to tumors in vivo via synNotch receptors and only express the CAR when in the tumor microenvironment. For these experiments, we injected bilateral xenograft CD19+ Daudi B cell lymphoblast tumors in immunocompromised NOD scid IL-2Rγ−/− (NSG) mice. Wild-type Daudi cells (containing no synNotch ligand) were injected subcutaneously in the left flank, while Daudi tumor cells also expressing surface GFP were injected in the right flank. After giving the tumors ten days to implant, we injected primary CD4+ and CD8+ human T cells equipped with the α-GFP synNotch Gal4VP64 receptor and the corresponding response elements controlling the expression of the α-CD19 4-1BBζ CAR and an IRES enhanced firefly luciferase (effluc) reporter (Figure 4A and S3A and S3B) (Rabinovich et al., 2008). We then monitored luciferase expression as a reporter for CAR expression over the course of 11 days. The T cells started to express the CAR selectively in the GFP+ Daudi tumor by day 1 and continually increased local expression of the CAR over the 11 day period in the dual antigen tumor (Figure 4B and 4C). The increase in luciferase signal in the target tumor is likely the result of a combination of synNotch-driven CAR expression and expansion of cells in the dual antigen target tumor (Figure S3B and S3C). No increase in luciferase was observed in the control GFP− tumor.
Figure 4. SynNotch Receptors Drive Tumor Localized CAR Expression In vivo.
(A) Primary human CD4+ and CD8+ T cells were engineered with the α-GFP synNotch Gal4VP64 receptor and the corresponding response elements regulating α-CD19 4-1BBζ CAR IRES effluc expression and injected i.v. into NSG mice with a Daudi tumor (CD19 only) on the left flank and a surface GFP Daudi (GFP/CD19) tumor on the right flank. Luciferase expression was monitored over 11 days after i.v. injection of engineered T cells.
(B) A representative image of luciferase expression in mice treated as described in panel A at day 7 post T cell injection. Luciferase expression was high in the GFP/CD19 tumor indicating localized CAR expression only in the dual antigen tumor (n=2 mice).
(C) Quantification of integrated intensity of luciferase levels in the left flank Daudi tumor (CD19 only) and surface GFP Daudi tumor (GFP/CD19) in the right flank. Luciferase expression is enriched in the dual antigen tumor at all time points (error is SD n=2).
Selective Combinatorial Antigen Tumor Clearance In vivo by synNotch Gated CAR Expression
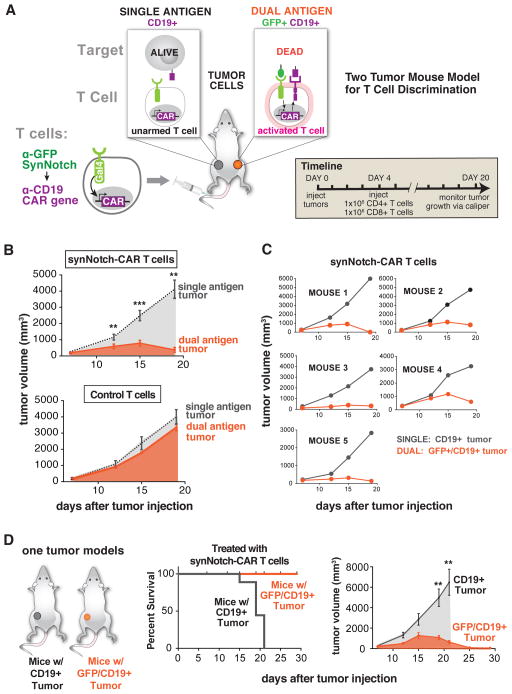
Now that we knew that the α-GFP synNotch receptor could target T cells to tumors and control local expression of the α-CD19 CAR, we tested whether the synNotch AND gate T cells could selectively clear a dual antigen tumor in vivo. For these experiments, we set up a similar bilateral tumor model with K562 tumor cells (Figure 5A and Figure S4A and S4B). We implanted the tumors and allowed 4 days for implantation (K562 tumors grow more rapidly and establish larger tumors compared to Daudi cells). At day 4, we injected CD4+ and CD8+ T cells bearing the α-GFP synNotch→α-CD19 CAR AND-gate circuit, and monitored tumor growth via caliper for 20 days (Figure 5A). We also treated a group of mice with untransduced control T cells to have a reference for tumor growth. In this experiment, the T cells are directly challenged to discriminate dual antigen “disease” tumors from single antigen “bystander” tissues within the same animal.
Figure 5. Selective Combinatorial Antigen Tumor Killing In vivo by SynNotch Gated CAR Expression.
(A) Primary human CD4+ and CD8+ T cells were engineered with the α-GFP synNotch Gal4VP64 receptor and the corresponding response elements regulating α-CD19 4-1BBζ CAR expression and were injected i.v. into NSG mice with a CD19 K562 tumor on the left flank and a surface GFP/CD19 K562 tumor on the right flank. Tumor size was monitored over 16 days after i.v. injection of engineered T cells or untransduced T cell controls.
(B) Graphs showing CD19 and GFP/CD19 tumor volumes for mice treated with synNotch AND gate T cells (top) and untransduced control T cells (bottom). synNotch AND gate T cells target the dual antigen tumor exclusively and the CD19 only tumor grew at the same rate as in mice treated with untransduced control T cells (n=5 mice, error bars are SEM, significance determine by Student’s t-test ** = P ≤ 0.01, *** = P ≤ 0.001).
(C) Tumor volume measurement for individual mice treated with synNotch AND gate T cells. All mice showed selective killing of the dual antigen tumor.
(D) Kaplan-Meier graphs showing synNotch AND gate T cells clear GFP/CD19 tumors with 100% of the mice surviving. Mice with CD19 only tumors are not cleared by synNotch AND gate T cells and have uncontrolled tumor growth. The corresponding tumor growth curves are given on the right of panel D (n=5 mice, error bars are SEM, significance determine by Student’s t-test ** = P ≤ 0.01).
The synNotch AND gate T cells displayed remarkably high and reproducible discriminatory action against the two tumors present in the same animal. In all animals, they selectively cleared the dual antigen “disease” tumor (GFP+/CD19+) while leaving the single antigen “bystander” tumor (CD19+ only) unperturbed. These bystander single antigen tumors grew at rates similar to the negative control tumor treated with untransduced T cells (Figure 5B and 5C and Figure S4B). Thus, there is little detectable OFF-target killing of the “bystander” single antigen tumor.
We also set up single tumor experiments where mice were implanted with either a single antigen (CD19+ only) or a dual antigen (GFP+/CD19+) K562 tumor. The mice were then treated with α-GFP synNotch→α-CD19 CAR AND-gate T cells or untransduced control T cells. The mice treated with control T cells all reached euthanasia criteria rapidly regardless of the tumor type. The mice with GFP/CD19 tumors treated with the AND-gate T cells all lived and the tumor was completely cleared by day 25 post tumor injection (Figure 5D). Mice with CD19 only tumors treated with synNotch AND gate T cells reached euthanasia criteria at the same rate as mice treated with untransduced T cells suggesting there is no OFF-target killing of single antigen tumors (Figure 5D). These in vivo data collectively show that synNotch-gated CAR expression is an effective AND-gate allowing T cells to confine their activity to the tumor microenvironment and only activate and kill in response to multiple antigens.
An important concern was whether the AND-gate T cells could engage a tumor expressing the synNotch ligand (GFP), become primed by expressing the α-CD19 CAR, and then migrate elsewhere to then kill single antigen (CD19+ only) bystander tissues. To test this, we performed experiments with a bilateral tumor model, but in this case one tumor contained CD19+ only cells and the other tumor contained GFP+ only cells (two single-antigen tumors) (Figure S4D). In these mice, it would in principle be possible for the T cells to be primed by the GFP+ only tumor, then migrate and kill the CD19+ only tumors. Nonetheless, when we monitored growth of the CD19+ tumor treated with AND-gate T cells, we found that it was identical to the growth observed when treated with negative control T cells (untransduced) (Figure S4E). Thus, there appears to be no evidence for priming of the AND-gate T cells and subsequent killing of bystander CD19+ cells elsewhere. Based on our prior data (Figure 2), the decay of induced CAR expression is on the order of several hours, which is likely faster than the composite time that would be required for migration out of the priming tumor, combined with the time required for CAR mediated T cell activation in the bystander tumor. These activation/inactivation dynamics could explain the requirement for highly local dual antigens.
DISCUSSION
The Discriminatory Power of Dual Antigen Sensing SynNotch→CAR Circuits in Therapeutic T cells
The general concept of utilizing synNotch receptors to regulate expression of receptors that drive T cell activation (e.g. CARs or TCRs) has great potential to improve the safety and effectiveness of T cell therapies by precisely and reliably localizing when and where the therapeutic (and toxic) action of T cells occurs. Here we have shown that synNotch AND gate T cells reliably discriminate tumors with two identifying antigens from tissues expressing only one of these antigens. The AND-gate circuit works in a sequential manner – the synNotch receptor first recognizes a tumor-localized antigen, thereby driving the expression of a CAR that recognizes a second tumor antigen. The CAR then mediates T cell activation only if the second cognate antigen is present. This strategy 1) restricts the expression of the CAR to the tumor microenvironment, and 2) has the potential to overcome the problem of OFF-target cross reaction that can occur with conventional CAR T cells when the target antigen is also present in bystander tissues (Figure 6A and 6B). Specific discrimination for a tumor could be achieved if the tumor has a unique combinatorial antigen profile that distinguishes it from other bystander tissues.
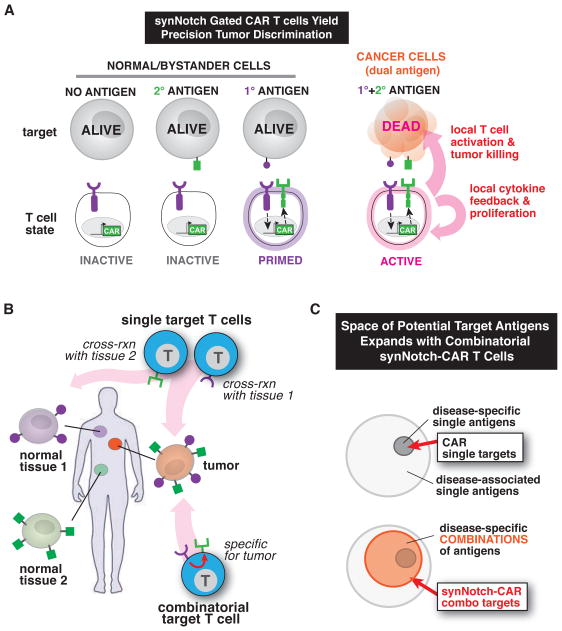
Figure 6. SynNotch Receptors Control and Localize CAR T cell Response for Precision Immunotherapy.
(A) Here we engineered T cells with synNotch receptors that sense tumor antigens and upregulate expression of a CAR to a second antigen. Thus, these synNotch AND gate T cells only activate in response to combinatorial antigen recognition in the tumor microenvironment, preventing OFF-target toxicity mediated by single antigen recognition.
(B) SynNotch AND gate T cells, unlike therapeutic T cells that target single antigens, can reliably discriminate combinatorial antigen targets from single antigen bystander tissue. Combinatorial antigen sensing by synNotch-CAR T cells could aid in precisely targeting T cells to tumors preventing OFF-target toxicity.
(C) synNotch receptors expand the targetable tumor antigen space. Tumor-specific antigens are rare compared to tumor-associated antigens (antigens that are expressed on normal tissue but are more highly expressed on tumors). Since CARs fully activate T cells resulting in the killing of target tissue, T cells engineered with a single CAR must be targeted to tumor-specific antigens in order to reduce fatal OFF-target toxicity (top venn diagram). SynNotch receptors can gate CAR expression and control where the T cells are armed. When targeting tumor specific antigen combinations, it may now be possible to use CAR receptors directed towards tumor-associated antigens. This should reduce OFF-target damage to tissues that express the CAR antigen in other parts of the body.
This approach for combinatorial antigen recognition is a critical advance for T cell therapies, as most other combinatorial antigen recognition strategies involve integrated signaling from multiple partially functional CARs that work cooperatively or antagonistically to control the activation of the T cell (Fedorov et al., 2013; Kloss et al., 2013; Wilkie et al., 2012). Combinatorial control over T cell activation with the synNotch-CAR AND gate has several benefits compared to the multiple CAR approach. Importantly, the synNotch receptor by itself does not directly trigger T cell activation at all – it is completely independent from CAR/TCR signaling. Therefore, synNotch receptor engagement by itself does not inflict any damage on the synNotch antigen bearing tissue – it simply results in the priming response of inducing CAR expression.
In contrast, when multiple CARs are expressed for combinatorial antigen gating of the T cell response, there are often scenarios where the partial signaling through one of the receptors can generate sufficient T cell activity to cause some OFF-target tissue damage (Wilkie et al., 2012). Multiple CAR strategies are dependent on achieving a delicate balance of signaling, and thus have inherent limitations on generating digital-like AND gate behavior (no activity towards single antigens, maximal activity towards dual antigens). Moreover, many parameters of multi-CAR systems must be precisely controlled, including the level and ratio of the complimentary receptors and their relative signaling strength. The amount of the target antigens present on different tissues in the body can also complicate the ability of the T cells to truly exhibit AND gate logic (Kloss et al., 2013; Wilkie et al., 2012). While these multi-CAR systems are an exciting and important approach to enhance tumor targeting, a wider range of combinatorial sensing strategies will improve our ability to treat a variety of tumors and diseases with T cell therapies. It is in principle possible to combine the dynamically controlled synNotch→CAR system described here with these other strategies.
Factors Contributing to Robust Dual Antigen Discrimination
There are also valid concerns for the synNotch gated CAR T cells, including the potential for cells to leave the ‘priming’ tissue and cause damage to OFF-target tissues that express only the CAR antigen. We have performed in vivo experiments to determine the extent to which this is a problem, and have found that for our dual tumor model, T cells do not migrate from the region of priming to then kill a ‘bystander’ tumor (Figure S4D and S4E). We hypothesize that once synNotch engagement is ceased, the decay of CAR expression is fast (hours) compared to the time required for effective migration and full T cell activation by the CAR, preventing such issues (Figure 2).
Moreover, there are other factors that are likely to strongly amplify T cell action within a dual antigen ‘disease’ tumor. First, induced expression of the CAR will result in two recognition domains that may more strongly retain the T cells in the dual antigen tumor. Second, and perhaps most importantly, the resulting T cell activation will induce both local IL-2 release and proliferation, leading to a strong positive feedback loop of local T cell expansion and activation. It is likely than these local positive feedback loops have multiplicative effects, contributing to the remarkable degree of discrimination that we observe with these AND-gate T cells.
It is also worth noting that one of the most powerful aspects of synNotch receptor circuits is their amenability to facile engineering. Although not explored here, in addition to engineering the selectivity of antigen recognition, it is possible to modulate the dynamics of CAR expression (e.g. using degrons, mRNA destabilization and feedback control), which could tune the duration and range of CAR activity (Lienert et al., 2014; Lim, 2010). In effect, such strategies would tune the degree of temporal coupling between the priming synNotch receptor and the effector CAR. The current synNotch-CAR circuits already function in a highly controlled manner with no observable OFF-target toxicity, but could likely be further improved or tailored for particular tumor contexts.
SynNotch Receptors Increase the Landscape of Targetable Antigens for T cell Therapies
Most engineered T cell strategies have focused on identifying and targeting a single tumor specific antigen with a CAR or engineered TCR. Thus the rarity of these truly tumor specific antigens has limited this approach. Although there are many tumor-associated antigens, few of them are truly tumor specific. Many tumors, particularly solid tumors, overexpress antigens that could be targeted, but are also expressed at lower levels in other bystander tissues.
The robust AND-gate dual antigen detection demonstrated by the synNotch → CAR T cells now opens the possibility that tumors could be targeted based on combinatorial antigen signatures (Figure 6C). It is far more likely that multiple antigens will provide higher discriminatory power between tumor and normal tissues. For example, it might be possible to target both a disease associated antigen and a tissue specific (normal) antigen to more precisely attack a particular type of cancer cell. Tissue specific antigen detection by synNotch receptors could restrict the priming of therapeutic T cells only to particular tissues. This is a unique way in which synNotch receptors could both increase the therapeutic effect and reduce systemic toxicity.
Dual antigen AND-gate T cells might allow more widespread use of CARs and TCRs directed to tumor-associated antigens that, when recognized by themselves, often yield toxic side effects (e.g. the α-Her2 CAR) (Barrett et al., 2014b; Dotti et al., 2014; Morgan et al., 2010). SynNotch receptors could be used to gate the expression of this class of CARs to confine their expression to the disease site, away from OFF-target tissue. SynNotch receptors thus increase the landscape of targetable antigens for CARs, and may facilitate the usage of CARs that have so far been thought to have high toxic potential (Figure 6C) (Dotti et al., 2014).
SynNotch receptors are a general and powerful platform to not only localize therapeutic T cell activity, but also to build combinatorial antigen sensing capabilities that enhance the ability of any therapeutic cell to recognize diseased target tissues with high precision and specificity. While much development remains, the versatility and modularity of the synNotch receptor system could in principle be used to program therapeutic cells to perform a large spectrum of combinatorial logical decisions extending beyond dual antigen sensing.
MATERIAL AND METHODS
SynNotch Receptor and Response Element Construct Design
SynNotch receptors were built by fusing the CD19 scFv (Porter et al., 2011), LaG17 (lower affinity GFP), or LaG16_2 (high affinity GFP) nanobody (Fridy et al., 2014) to the mouse Notch1 (NM_008714) minimal regulatory region (Ile1427 to Arg1752) and Gal4 DBD VP64 or TetR VP64 (tTa). All synNotch receptors contain an n-terminal CD8α signal peptide (MALPVTALLLPLALLLHAARP) for membrane targeting and a myc-tag (EQKLISEEDL) for easy determination of surface expression with α-myc A647 (cell-signaling #2233) (see accompanying manuscript, Morsut et al., for receptor synNotch receptor peptide sequences). The receptors were cloned into a modified pHR’SIN:CSW vector containing a PGK promoter for all primary T cell experiments. The pHR’SIN:CSW vector was also modified to make the response element plasmids. Five copies of the Gal4 DNA binding domain target sequence (GGAGCACTGTCCTCCGAACG) were cloned 5′ to a minimal CMV promoter. Also included in the response element plasmids is a PGK promoter that constitutively drives mCherry expression to easily identify transduced T cells. For all inducible CAR vectors, the CARs were tagged c-terminally with GFP and were cloned via a BamHI site in the multiple cloning site 3′ to the Gal4 response elements. All constructs were cloned via In fusion cloning (Clontech #ST0345).
Primary Human T cell Isolation and Culture
Primary CD4+ and CD8+ T cells were isolated from anonymous donor blood after apheresis by negative selection (STEMCELL Technologies #15062 & 15063). Blood was obtained from Blood Centers of the Pacific (San Francisco, CA) as approved by the University Institutional Review Board. T cells were cryopreserved in RPMI-1640 (UCSF cell culture core) with 20% human AB serum (Valley Biomedical Inc., #HP1022) and 10% DMSO. After thawing, T cells were cultured in human T cell medium consisting of X-VIVO 15 (Lonza #04-418Q), 5% Human AB serum and 10 mM neutralized N-acetyl L-Cysteine (Sigma-Aldrich #A9165) supplemented with 30 units/mL IL-2 (NCI BRB Preclinical Repository) for all experiments.
Lentiviral Transduction of Human T cells
Pantropic VSV-G pseudotyped lentivirus was produced via transfection of Lenti-X 293T cells (Clontech #11131D) with a pHR’SIN:CSW transgene expression vector and the viral packaging plasmids pCMVdR8.91 and pMD2.G using Fugene HD (Promega #E2312). Primary T cells were thawed the same day, and after 24 hours in culture, were stimulated with Human T-Activator CD3/CD28 Dynabeads (Life Technologies #11131D) at a 1:3 cell:bead ratio. At 48 hours, viral supernatant was harvested and the primary T cells were exposed to the virus for 24 hours. At day 4 post T cell stimulation, the Dynabeads were removed, and the T cells expanded until day 9 when they were rested and could be used in assays. T cells were sorted for assays with a Beckton Dickinson (BD) FACs ARIA II. AND-gate T cells exhibiting basal CAR expression were gated out during sorting.
Generation of SynNotch AND Gate Jurkat T cells
E6-1 Jurkat T cells (ATCC# TIB-152) were lentivirally transduced with the α-CD19 synNotch tTa receptor (Myc-tagged) and a TRE3GS inducible promoter controlling expression of the α-mesothelin 4-1BBζ CAR that also contains a constitutive cassette driving mCherry expression. After viral transduction, the Jurkat cells with expression of both the receptor and response elements were single cell sorted into 96 well plates with a FACs ARIA II. Single cell clones that grew out in culture were then assessed for retained expression of the constructs and used in assays.
Cancer Cell Lines
The cancer cell lines used were K562 myelogenous leukemia cells (ATCC #CCL-243) and Daudi B cell lymphoblasts (ATCC #CCL-213). K562s were lentivirally transduced to stably express human CD19 at equivalent levels as Daudi tumors. CD19 levels were determined by staining the cells with α-CD19 APC (Biolegend #302212). K562s and Daudi cells were also transduced to stably express surface GFP (GFP fused to the PDGF transmembrane domain). The CD19 and surface GFP peptide sequences can be found in the accompanying manuscript by Morsut et al. All cell lines were sorted for expression of the transgenes.
In vitro Stimulation of SynNotch T cells
For all in vitro synNotch T cell stimulations, 2×105 T cells were co-cultured with sender cells at a 1:1 ratio. After mixing the T cells and cancer cells in round bottom 96-well tissue culture plates, the cells were centrifuged for 1 min at 400×g to force interaction of the cells, and the cultures were analyzed at 24 hours for markers of activation (e.g. CD69) for CD4+ T cells and specific lysis of target tumor cells for CD8+ T cells with a BD LSR II. All flow cytometry analysis was performed in FlowJo software (TreeStar).
Luminex MAGPIX Cytokine Quantification
Primary CD4+ T cells expressing the α-CD19 synNotch Gal4VP64 receptor and 5× Gal4 response elements controlling the CAR were stimulated as described above with the indicated target cancer cell line. The supernatant was collected at 24 hours and analyzed with a Luminex MAGPIX (Luminex Corp.) Human Cytokine Magentic 25-plex Panel (Invitrogen ref#LHC0009M) according to the manufacturer’s protocol. All cytokine levels were calculated based on standard curves with xPONENT software (Luminex Corp.).
IL-2 ELISA and CD69 Staining
Primary CD4+ or Jurkat synNotch AND-Gate T cells were stimulated with the indicated cancer cell line as described above for 24 hours and supernatant was harvested. IL-2 levels in the supernatant were determined via IL-2 ELISA (eBiosciences #BMS2221HS). The T cells were also collected and stained with α-CD69 APC (Biolegend #310910) to determine if they were activated.
Assessment of SynNotch AND-Gate T cell Cytotoxicity
CD8+ synNotch AND-Gate T cells were stimulated for 24 hours as described above with target cells expressing the indicated antigens. The level of specific lysis of target cancer cells was determined by comparing the fraction of target cells alive in the culture compared to treatment with untransduced T cell controls. Cell death was monitored by uptake of the live/dead stain SYTOX Blue (Thermo Scientific #S34857) and shifting of the target cells out of the side scatter and forward scatter region normally populated by the target cells.
In vitro Quantification of Luciferase Reporter Activity in SynNotch T cells
Sorted CD4+ and CD8+ primary human T cells engineered to express the α-GFP nanobody (LaG17) synNotch Gal4VP64 receptor and the corresponding response elements controlling α-CD19 4-1BBζ CAR IRES effluc expression were stimulated with GFP+ or GFP− Daudi cells for 24 hours (2×105 T cells and 2×105 K562s). Production of effluc was assessed with the ONE-glo Luciferase Assay System (Promega #E6110). Bioluminescence was measured with a FlexStation 3 (Molecular Devices).
In vivo Luciferase Imaging of SynNotch T cells
Animal studies were conducted with the UCSF Preclinical Therapeutics Core under a protocol approved by the UCSF Institutional Animal Care and Use Committee. Ten days prior to T cell injection, Daudi tumors and surface GFP Daudi tumors were injected subcutaneously into the left and right flanks of NOD scid gamma (NSG) mice (female, 8~12 weeks old, Jackson Laboratory #005557). Sorted CD4+ and CD8+ primary human T cells engineered to express the α-GFP nanobody (LaG17) synNotch Gal4VP64 receptor and the corresponding response elements controlling α-CD19 4-1BBζ CAR IRES effluc expression were injected at a 1:1 CD4+ to CD8+ T cell ratio (1×106 of each T cell type) i.v. into the tumor bearing mice 10 days post tumor implantation. Luciferase expression was monitored over 11 days with bioluminescent imaging performed using the IVIS 100 (Xenogen) preclinical imaging system at the indicated time points. Images were acquired 10 min following i.p. injection with 150 mg/kg of D-luciferin (Gold Technology #LUCK-100). Quantification of integrated bioluminescence intensities was quantified in ImageJ (NIH).
In vivo Dual Antigen Tumor Targeting by SynNotch AND-gate T cells
NSG mice were implanted with two xenograft tumors -- 5×106 CD19+ and GFP+/CD19+ K562 tumor cells subcutaneously on the left and right flank, respectively. Four days post tumor implantation, 1×106 primary human CD4+ and CD8+ T cells (2×106 total T cells) were injected i.v. into the mice. These T cells were either untransduced (control) or engineered with the α-GFP synNotch Gal4VP64 receptor and the corresponding response elements regulating α-CD19 4-1BBζ CAR expression. Tumor size was monitored by the UCSF Preclinical Therapeutics Core staff via caliper over 20 days after T cell injection. For Kaplan-Meier experiments, the same protocol was used, but single tumors were injected into the mice. Mice were considered dead when the tumor size reached euthanasia criteria.
Statistical Analysis and Curve Fitting
Statistical significance was determined by Student’s t-test (two-tailed) unless otherwise noted. All statistical analysis and curve fitting was performed with Prism 6 (Graphpad) and P values are reported (n.s. = p > 0.05, * = p ≤ 0.05, ** = p ≤ 0.01, *** = p ≤ 0.001, **** = p ≤ 0.0001). All error bars represent either S.E.M. or S.D.
Supplementary Material
Acknowledgments
We would like to thank Scott Coyle and members of the Lim lab and Arthur Weiss and members of the Weiss lab for helpful discussions and comments on the manuscript. We also thank the Preclinical Therapeutics Core at UCSF for help with in vivo studies and Michael Milone and Carl June (UPenn) for the CAR constructs. This work was supported by a Jane Coffin Childs Memorial Fund Postdoctoral Fellowship A121505 (K.T.R.), a Human Frontiers of Science Program Postdoctoral Fellowship (L.M.), and NIH grants PN2 EY016546, P50FM081879, R01 GM055040, R01 CA196277, and the Howard Hughes Medical Institute (W.A.L).
Footnotes
CONTRIBUTIONS
K.T.R, L.J.R., L.M, and W.A.L conceived and designed the experiments. K.T.R. and L.J.R. performed experiments. K.T.R. analyzed the data. J.S.P. helped setup the Daudi tumor model in NSG mice. W.J.W. and K.A.M. provided technical assistance. K.T.R., L.M., and W.A.L. wrote and edited the paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barrett DM, Singh N, Porter DL, Grupp SA, June CH. Chimeric antigen receptor therapy for cancer. Annu Rev Med. 2014a;65:333–347. doi: 10.1146/annurev-med-060512-150254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett DM, Teachey DT, Grupp SA. Toxicity management for patients receiving novel T-cell engaging therapies. Curr Opin Pediatr. 2014b;26:43–49. doi: 10.1097/MOP.0000000000000043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens RJ, Davila ML, Rivière I, Park J, Wang X, Cowell LG, Bartido S, Stefanski J, Taylor C, Olszewska M, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med. 2013;5:177ra38–177ra38. doi: 10.1126/scitranslmed.3005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227–242. doi: 10.1038/nri3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnasamy D, Yu Z, Theoret MR, Zhao Y, Shrimali RK, Morgan RA, Feldman SA, Restifo NP, Rosenberg SA. Gene therapy using genetically modified lymphocytes targeting VEGFR-2 inhibits the growth of vascularized syngenic tumors in mice. J Clin Invest. 2010;120:3953–3968. doi: 10.1172/JCI43490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5:215ra172–215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridy PC, Li Y, Keegan S, Thompson MK, Nudelman I, Scheid JF, Oeffinger M, Nussenzweig MC, Fenyö D, Chait BT, et al. A robust pipeline for rapid production of versatile nanobody repertoires. Nat Methods. 2014;11:1253–1260. doi: 10.1038/nmeth.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June CH, Blazar BR, Riley JL. Engineering lymphocyte subsets: tools, trials and tribulations. Nat Rev Immunol. 2009;9:704–716. doi: 10.1038/nri2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarla S, Chow KKH, Mata M, Shaffer DR, Song XT, Wu MF, Liu H, Wang LL, Rowley DR, Pfizenmaier K, et al. Antitumor effects of chimeric receptor engineered human T cells directed to tumor stroma. Mol Ther. 2013;21:1611–1620. doi: 10.1038/mt.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31:71–75. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers CHJ, Sleijfer S, Vulto AG, Kruit WHJ, Kliffen M, Debets R, Gratama JW, Stoter G, Oosterwijk E. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24:e20–e22. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- Lienert F, Lohmueller JJ, Garg A, Silver PA. Synthetic biology in mammalian cells: next generation research tools and therapeutics. Nat Rev Mol Cell Biol. 2014;15:95–107. doi: 10.1038/nrm3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim WA. Designing customized cell signalling circuits. Nat Rev Mol Cell Biol. 2010;11:393–403. doi: 10.1038/nrm2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Chinnasamy N, Abate-Daga D, Gros A, Robbins PF, Zheng Z, Dudley ME, Feldman SA, Yang JC, Sherry RM, et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RA, Yang JC, Kitano M, Dudley ME, Laurencot CM, Rosenberg SA. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol Ther. 2010;18:843–851. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederman TMJ, Ghogawala Z, Carter BS, Tompkins HS, Russell MM, Mulligan RC. Antitumor activity of cytotoxic T lymphocytes engineered to target vascular endothelial growth factor receptors. Proc Natl Acad Sci USa. 2002;99:7009–7014. doi: 10.1073/pnas.092562399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich BA, Ye Y, Etto T, Chen JQ, Levitsky HI, Overwijk WW, Cooper LJN, Gelovani J, Hwu P. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USa. 2008;105:14342–14346. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie S, van Schalkwyk MCI, Hobbs S, Davies DM, van der Stegen SJC, Pereira ACP, Burbridge SE, Box C, Eccles SA, Maher J. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32:1059–1070. doi: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.