Abstract
Background
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition that can be reliably diagnosed as early as 24 months. Immunological phenomena, including skewed cytokine production, have been observed among children with ASD. Little is known about whether immune dysregulation is present before diagnosis of ASD.
Methods
We utilized neonatal blood spots from 214 children with ASD (141 severe, 73 mild/moderate), 62 typically developing (TD), and 27 developmental delayed controls who participated in CHARGE (Childhood Autism Risks from Genetics and the Environment), a population-based case-control study. Levels of 17 cytokines/chemokines were compared across groups and in relation to developmental/behavioral domains.
Results
Interleukin (IL)-1β and IL-4 were independently associated with ASD vs. TD although these relationships varied by ASD symptom intensity. Elevated IL-4 associated with increased odds of severe ASD (ASDsev) (odds ratio[OR]=1.40, 95% confidence interval[CI] 1.03, 1.91) whereas IL-1β associated with increased odds of mild/moderate ASD (ASDmild) (OR=3.02, 95% CI 1.43, 6.38). Additionally, IL-4 was associated with a higher likelihood of ASDsev vs. ASDmild (OR=1.35, 95% CI 1.04, 1.75). In male ASD cases, IL-4 was negatively associated with non-verbal cognitive ability (β=−3.63, SE=1.33, P=0.04).
Conclusions
This study is part of a growing effort to identify early biological markers for ASD. We demonstrate that peripheral cytokine profiles at birth are associated with ASD later in childhood and that cytokine profiles vary depending on ASD severity. Cytokines have complex roles in neurodevelopment, and dysregulated levels may be indicative of genetic differences and environmental exposures or their interactions that relate to ASD.
Keywords: neonatal cytokines, chemokines; autism spectrum disorder; developmental delays; bloodspot; neurodevelopment
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by social deficits and restricted or stereotyped behavior patterns that manifest by age three.(1-3) The biological basis of ASD is unclear but likely involves a complex interplay between genetic susceptibility and environmental exposures.(4) There are no consistent biological markers for ASD, and diagnosis is based upon behavioral traits and developmental history.(5-7) The most consistently beneficial treatment for ASD is behavioral therapy, which is most effective when administered early in life.(8) Therefore, two major goals in autism research are characterization of biological signatures for ASD and early identification of children at risk.
Immunological factors may be involved in ASD. This includes alterations in the prenatal immune environment that may contribute to the risk of developing ASD.(9-11) Further, children diagnosed with ASD have evidence of immune dysfunction that is detected at young ages and near to diagnoses.(12-17) However, it is unclear whether these immunological differences arise in children before or after diagnosis. Resolving this issue is critical to understanding the temporal relationship between immune anomalies and the development of ASD.
Two recent studies found differences in cytokine/chemokine levels measured in newborn blood spots of children later diagnosed with ASD compared with controls.(18-20) Abdallah et al. found decreased levels of many cytokines (i.e., interferon-γ, interleukin [IL]-2, IL-4, IL-6, IL-10) in neonatal samples from children with ASD, and no differences in chemokine levels.(18, 19) In contrast, Zerbo et al. reported increased monocyte chemotactic protein (MCP)-1 and decreased Regulated upon Activation Normal T-Cell Expressed and Secreted (RANTES) in ASD cases versus controls.(20) While these studies present novel data, they are limited by lack of clinical confirmation of ASD and older diagnostic tools due to the timing of specimen collection. The current study utilizes archived neonatal dried blood spot samples to explore whether early immunological signs, specifically cytokine profiles, are predictive of neurodevelopmental outcomes in a well-defined study population of children with a confirmed diagnosis of ASD, developmental delays (DD) without ASD, or typical development (TD).
METHODS AND MATERIALS
Participants
This study utilized neonatal blood spots archived by the State of California and obtained for a subset of 303 children (214 ASD, 27 DD, and 62 TD) enrolled in the CHARGE (Childhood Autism Risks from Genetics and the Environment) Study(21) between January 2003 and October 2005. CHARGE is an ongoing population-based case-control study investigating risk factors for neurodevelopmental disorders, with participants selected from 3 groups: ASD, DD, and TD from the general population. Eligible children were 2-5 years old, born in California, living with a biological parent who speaks English or Spanish, and residing in selected regional center catchment areas. Children were identified as described previously.(21) CHARGE protocol was approved by the institutional review boards at the University of California in Davis and Los Angeles and the State of California Committee for the Protection of Human Subjects. Written informed consent was obtained prior to participation.
Diagnostic Confirmation
Children were assessed at the UC Davis MIND (Medical Investigation of Neurodevelopmental Disorders) Institute at study enrollment (2-5 years old). Cognitive and adaptive function were evaluated in all children with Mullen Scales of Early Learning (MSEL)(22) and Vineland Adaptive Behavior Scales (VABS)(23), respectively. ASD diagnosis was confirmed with the Autism Diagnostic Interview–Revised (ADI-R)(24) and the Autism Diagnostic Observation Schedule (ADOS)(6, 25) using criteria described by Risi et al.(26) and in accordance with the Diagnostic and Statistical Manual of Mental Disorders–5 (DSM-5).(3) Children with ASD (n=214) were subdivided into those exhibiting severe ASD symptoms (ASDsev [n=141]) and those with mild to moderate symptoms (ASDmild [n=73]) using ADOS comparison scores ≥7 and <7, respectively.(25)
Controls were screened for ASD using the Social Communication Questionnaire (SCQ)(27); those with SCQ ≥15 were evaluated with ADI-R and ADOS and reclassified if they met criteria for ASD. TD controls had no prior diagnosis of ASD or DD and composite scores ≥70 on both MSEL and VABS. DD controls had composite scores <70 on both MSEL and VABS.
Behavioral and Developmental Assessments
Aberrant Behavior Checklist (ABC)(28) measures maladaptive behavior using subscales: Irritability (15 items), Lethargy/Social Withdrawal (16 items), Stereotypy (7 items), and Hyperactivity (16 items). Each item is rated on a 4-point Likert scale ranging from 0 = Not at all a problem to 3 = Problem severe in degree.
Mullen Scales of Early Learning (MSEL) is a standardized assessment of cognitive development in young children and includes scales: Visual Reception (non-verbal cognitive ability), Fine Motor, Receptive Language (language comprehension), and Expressive Language (language production). Developmental quotients (DQ; [age equivalent / chronological age] × 100) were calculated for each scale and composite to overcome the floor effect.
Vineland Adaptive Behavior Scales (VABS) is a standardized assessment that measures personal and social skills needed for everyday living on these domains: Communication, Daily Living Skills, Socialization, and Motor Skills. DQs were calculated as described above.
Blood Spot Specimens
In California, capillary blood is collected at birth by heel-prick, spotted onto standardized filter paper, and tested for various disorders as part of the Genetic Disease Screening Program. The remaining specimen is stored through the California Department of Public Health.(29)
Blood Spot Elution
Three 3mm punches of each dried blood spot specimen were placed together into a single well in a 96-well plate and stored at −80°C until elution. The blood spot elution protocol is as follows: 200μl of elution buffer (Phosphate buffered saline, 0.5% Bovine serum albumin, and protease inhibitors (Roche Complete Protease Inhibitor Cocktail, Roche Applied Science, Indianapolis, IN)) was added to each well, and plates were placed on a plate shaker overnight at 4°C. The eluates were analyzed immediately following elution.
Cytokine/Chemokine Measurement
Blood spot cytokine and chemokine levels were measured using Luminex multiplex technology. Cytokines interleukin (IL)-1β, IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, IL-13, interferon (IFN)-γ, and tumor necrosis factor (TNF)-α were measured using high sensitivity Bio-Plex Precision Pro human cytokine kits (Bio-Rad, Hercules, CA). Chemokines IL-8, MCP-1, macrophage inflammatory protein (MIP)-1α, MIP-1β, Eotaxin, interferon-γ induced protein (IP)-10, and RANTES were measured using Milliplex human chemokine kits according to directions (Millipore, Billerica, MA). Briefly, 25μl of eluate was incubated with the kit beads with shaking overnight at 4°C. The beads were then washed using a vacuum manifold, and biotin-conjugated detection antibodies were added for a 1-hour incubation. After washing, the beads were incubated with streptavidin-PE for 30 minutes, the plates read on a Bio-Plex 100 (BioRad, Hercules, CA), and the signal analyzed using Bio-Plex Manager software using a 5-point standard curve. Because eluted blood spots contain rehydrated components of whole blood, including plasma and lysed cells, the cytokine/chemokine profiles are derived from both plasma and cellular sources. Total protein was measured in each eluted blood spot sample using a Pierce BCA protein assay (Rockford, IL). Cytokine/chemokine concentrations were normalized for total protein content and presented in pg/mg total protein.
Statistical Analysis
All statistical analyses were limited to 303 children with a confirmed diagnosis of ASD (n=214; ASDsev n=141 and ASDmild n=73), DD (n=27) or TD (n=62) whose blood spots were collected $48 hours of birth. Excluded from the initial sample of 459 children with blood spots were 77 children with other (n=58) or incomplete (n=19) diagnoses, and 79 children with blood collected >48 hours after delivery. Cytokine/chemokine concentrations were ln-transformed to normalize their distributions. All analyses were carried out using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Multiple imputation of cytokines/chemokines with values below level of detection (LOD)
We used PROC MI with the Fully Conditional Specification (FCS) method in SAS to impute values <LOD for each ln-transformed cytokine/chemokine (pg/ml eluate) separately and included the following variables: (1) ln-transformed cytokines/chemokines without missing values <LOD, (2) covariates: age at blood spot collection, years between sample collection and elution, birth month, gestational age, (3) child’s diagnosis, and (4) the cytokine/chemokine being imputed with values <LOD replaced by LOD/2 and adjusted for total protein (to keep imputed values in the low range of distribution). Ten imputation datasets were created in the first PROC MI step; the output dataset from the first step was used as the input dataset for the next PROC MI step (with ‘by imputation’ statement) to impute the next cytokine/chemokine, and so on until all values <LOD were imputed. The upper bound for imputed values was restricted to the lowest detectable value of a given cytokine/chemokine but could not be set for IFN-γ, IL-10, IL-5, IL-13, IL-4, MIP-1α, IL-6, and Eotaxin (due to convergence problems); nonetheless, there was little to no overlap between the distributions of imputed values (<LOD) and detectable values (Figure S1). Analyte concentrations were then exponentiated, corrected for total protein, and ln-transformed again for analysis. We performed all statistical analyses on the imputed datasets and combined the results using PROC MIANALYZE (with edf= option specifying degrees of freedom) to obtain inferences.
Principal Components Analysis (PCA)
Using imputed data, we conducted a PCA to examine the patterns of all cytokines/chemokines simultaneously in cases and controls and to visually inspect whether any clusters emerged by diagnostic group. Orthogonal rotations were performed, and only components that explained ≥10% of total variance were retained and verified by Horn’s parallel analysis.(30) Analytes with loadings <0.40 were suppressed in the final component loading matrices.
Logistic Regression Analyses
Our outcome of interest was child’s diagnostic group, with (ln-transformed) cytokines/chemokines as the predictors. We fitted separate models for each predictor and also examined multiple cytokines in one model. Covariates included child’s age (hours) at blood spot collection, race/ethnicity, season of birth, gestational age (weeks), maternal allergies and asthma, years between sample collection and elution, cytokine/chemokine plate numbers, and frequency-matching variables: child’s age at enrollment (months), sex, and regional center catchment area (5 geographic regions). All covariates were assessed for potential confounding by examining associations of each covariate with the predictor (cytokines/chemokines) and outcome (diagnostic group); covariates broadly associated (P <0.20) with at least the outcome included season of birth, gestational age, child’s sex and age at study enrollment, and years between sample collection and elution. They were included in initial models, and a change of ~10% in β-coefficient of the predictor was used to determine which covariates remained in the model; final multinomial logistic regression models were adjusted for season of birth, gestational age, child’s sex, and years between sample collection and elution. Odds ratios (OR) and 95% confidence intervals (CI) were calculated to measure the association between each analyte and a given diagnosis relative to a referent diagnosis. P-values were corrected for multiple comparisons (16 biomarkers) using the Benjamini-Hochberg procedure.(31)
Linear and Negative Binomial Regression Analyses
To further characterize ASD cases, we performed linear regression models adjusted for maternal education level (high school or less, some college, Bachelor degree or higher) to determine whether selected cytokines were associated with specific domains of cognitive (visual reception, receptive and expressive language) and adaptive (communication, socialization) development. Negative binomial regression models adjusted for maternal education and child’s age at study visit were carried out to examine associations between selected cytokines and maladaptive behavior (irritability, lethargy, stereotypy, hyperactivity) counts while accounting for the overdispersed behavior scores. β-coefficients and standard errors (SE) were calculated to measure the association between each analyte and the expected value of either a developmental score (linear regression) or log count of behavior (negative binomial regression). Associations with P-values <0.05 were considered significant and were corrected for multiple comparisons (5 developmental and 4 behavior scores).
RESULTS
Participants
TD controls were frequency-matched to ASD cases (4:1 male-to-female ratio) resulting in a skewed sex distribution and similar proportions of males in both groups (Table 1); 59% were male in the DD group, as they were not sex-matched. A higher proportion of TD was born in the Summer (39% vs. 24% and 30%) and fewer born in the Spring (14% vs. 24% and 22%) compared to cases and DD controls, respectively. Children with DD were born one to two weeks earlier, on average, than cases or TD controls (38 vs. 39 and 40 weeks, respectively). The length of time between blood spot collection and elution was approximately ½ year longer for cases than TD (7.8 vs. 7.4 years). There were also differences in regional catchment areas between cases and DD (33% vs. 59% were from Alta). Cases did not differ from TD or DD controls on race/ethnicity, age at blood spot collection or study enrollment, and maternal allergies and asthma. Also, cases and controls were similarly distributed across cytokine/chemokine plate numbers (Table S1).
Table 1.
Participant demographic and clinical characteristics, N=303
| ASD (n=214) |
DD (n=27) |
TD (n=62) |
|||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | P- valueg |
|
| Sexa | 0.0004 | ||||||
| Male | 189 | 88 | 16 | 59 | 50 | 81 | |
| Female | 25 | 12 | 11 | 41 | 12 | 19 | |
| Race/Ethnicity | 0.81 | ||||||
| White | 100 | 47 | 11 | 41 | 27 | 44 | |
| Hispanic | 71 | 33 | 11 | 41 | 25 | 40 | |
| Otherb | 43 | 20 | 5 | 18 | 10 | 16 | |
| Season of birthc | 0.29 | ||||||
| Winter | 47 | 22 | 4 | 15 | 15 | 24 | |
| Spring | 50 | 24 | 6 | 22 | 9 | 14 | |
| Summer | 52 | 24 | 8 | 30 | 24 | 39 | |
| Fall | 65 | 30 | 9 | 33 | 14 | 23 | |
| Maternal allergies or asthmad |
90 | 45 | 10 | 42 | 26 | 46 | 0.95 |
| Any allergye | 80 | 40 | 7 | 29 | 22 | 39 | 0.60 |
| Environmental allergy |
54 | 27 | 6 | 25 | 13 | 23 | 0.82 |
| Asthma | 24 | 12 | 4 | 17 | 10 | 18 | 0.50 |
| Regional Center catchment areaa |
0.20 | ||||||
| Alta, Far | 70 | 33 | 16 | 59 | 26 | 42 | |
| Northern, and Redwood Coast |
|||||||
| North Bay | 27 | 13 | 0 | 0 | 8 | 13 | |
| East Bay, San Andreas, and Golden Gate |
25 | 12 | 1 | 4 | 6 | 10 | |
| Valley Mountain, Central Valley, and Kern |
31 | 14 | 4 | 15 | 8 | 13 | |
| All Los Angeles, Orange, San Diego, Tri- counties, and Inland |
61 | 28 | 6 | 22 | 14 | 22 | |
|
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | Mean | SD | Range | P- valueg |
|
|
| ||||||||||
| ASD (n=214) |
DD (n=27) |
TD (n=62) |
||||||||
| n | % | n | % | n | % | P- valueg |
||||
| Gestational age (weeks)f |
39.3 | 1.8 | 31-45 | 38.2 | 3.4 | 30-43 | 39.9 | 1.6 | 35-45 | 0.001 |
| Age (hours) at blood spot collection |
29.3 | 9.9 | 3-48 | 29.5 | 10.2 | 13-47 | 28.6 | 8.2 | 13-47 | 0.88 |
| Age (months) at study enrollmenta |
43.4 | 10.0 | 24-65 | 45.3 | 9.5 | 24-58 | 40.8 | 8.6 | 26-60 | 0.08 |
| Years between collection and elution |
7.8 | 1.4 | 4.7- 10.7 |
7.6 | 1.2 | 4.9- 9.5 |
7.4 | 1.3 | 4.8- 9.9 |
0.13 |
TD controls were frequency-matched to a projected distribution of ASD cases on age, sex, and regional center catchment area
Includes Black/African American, American Indian/Alaska Native, Asian, Pacific Islander/Native Hawaiian, Multi-racial
Months grouped by season as follows: Winter = December to February, Spring = March to May, Summer = June to August, Fall = September to November
22 participants were missing maternal allergies/asthma (14 ASD, 3 DD, 5 TD)
Allergies include the following types: environmental (e.g., seasonal, pet, mold), food, skin, medication, or other
7 participants were missing gestational age (5 ASD, 2 TD)
P-values for categorical and continuous variables calculated with Chi-square test and one-way analysis of variance (ANOVA), respectively
Cytokine/Chemokine Distributions
Cytokine/chemokine concentrations measured in blood spots are shown in Table 2. Most analytes (11 of 17) were detectable in >75% of samples; IL-4, IL-5, and IL-13 were detectable in 60-70% of samples; MIP-1α and IL-6 were detected in 50% and 44% of samples, respectively. Eotaxin was detectable in only 10% of the samples and was excluded from analyses. Overall, the percentage of samples that fell below levels of detection (LOD) of a given immune biomarker was evenly distributed between cases and controls; one exception was IL-4 (<LOD in 37% cases vs. 45% TD controls), indicating that TD controls, on average, had lower concentrations of IL-4 than ASD cases. Values <LOD were imputed by multiple imputation methods described earlier.
Table 2.
Median concentrations and ranges of cytokines and chemokines in eluted blood spots, N=303
| ASD (n=214) | DD (n=27) | TD (n=62) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| Cytokinea | Rangeb | Med | IQR | <LOD | Med | IQR | <LOD | Med | IQR | <LOD |
| IL-1β | 0.03 – 2.03 |
0.31 | 0.23 – 0.40 |
-- | 0.32 | 0.27 – 0.46 |
-- | 0.28 | 0.20 – 0.38 |
-- |
| IL-2 | 0.04 – 9.75 |
1.14 | 0.29 – 2.30 |
14.5% | 1.22 | 0.21 – 1.91 |
14.8% | 1.13 | 0.19 – 2.00 |
16.1% |
| IL-4 | 0.01 – 1.38 |
0.04 | <lod – 0.07 |
37.4% | 0.05 | <lod – 0.07 |
25.9% | 0.04 | <lod – 0.08 |
45.2% |
| IL-5 | 0.03 – 7.81 |
0.40 | <lod – 0.74 |
29.4% | 0.36 | <lod – 0.50 |
25.9% | 0.44 | <lod – 0.66 |
29.0% |
| IL-6 | 0.01 – 585.2 |
<lod | <lod – 1.46 |
56.1% | <lod | <lod – 1.80 |
59.3% | <lod | <lod – 0.45 |
54.8% |
| IL-10 | 0.01 – 4.93 |
0.19 | 0.04 – 0.37 |
20.1% | 0.29 | 0.06 – 0.42 |
18.5% | 0.24 | <lod – 0.45 |
27.4% |
| IL-12 | 0.01 – 213.3 |
0.27 | 0.19 – 0.37 |
0.9% | 0.23 | 0.16 – 0.36 |
-- | 0.25 | 0.17 – 0.36 |
4.8% |
| IL-13 | 0.01 – 2.26 |
0.05 | <lod – 0.13 |
38.3% | 0.10 | 0.01 – 0.21 |
22.2% | 0.05 | <lod – 0.14 |
40.3% |
| IFN-γ | 0.01 – 5.86 |
0.25 | 0.13 – 0.38 |
8.4% | 0.18 | 0.12 – 0.31 |
3.7% | 0.23 | 0.06 – 0.38 |
16.1% |
| TNF-α | 0.01 – 0.84 |
0.08 | 0.05 – 0.12 |
5.6% | 0.10 | 0.05 – 0.13 |
3.7% | 0.10 | 0.05 – 0.13 |
4.8% |
| IL-8 | 4.26 – 356.5 |
18.22 | 12.59 – 27.94 |
-- | 22.01 | 12.01 – 36.05 |
-- | 22.24 | 12.51 – 30.22 |
-- |
| MCP-1 | 9.75 – 523.1 |
104.6 | 109.0 – 192.1 |
-- | 126.7 | 96.48 – 156.1 |
-- | 155.7 | 114.2 – 207.6 |
-- |
| MIP-1α | 0.35 – 437.8 |
1.25 | <lod – 3.11 |
48.6% | <lod | <lod – 3.11 |
51.9% | <lod | <lod – 3.11 |
54.8% |
| MIP-1β | 1.17 – 24.44 |
5.89 | 3.91 – 7.54 |
-- | 5.49 | 3.91 – 7.37 |
-- | 5.31 | 3.88 – 7.44 |
-- |
| Eotaxin | 1.30 – 12.11 |
<lod | <lod – <lod |
89.7% | <lod | <lod – <lod |
92.6% | <lod | <lod – <lod |
88.7% |
| IP-10 | 0.12 – 118.6 |
6.96 | 4.70 – 10.67 |
-- | 5.88 | 4.75 – 9.45 |
-- | 6.85 | 4.99 – 9.45 |
-- |
| RANTES | 493.1 – 21244 |
1951 | 1434 - 2500 |
-- | 1653 | 1108 - 2440 |
-- | 1810 | 1492 - 2278 |
-- |
Abbreviations: Med = Median, IQR = Interquartile range, LOD = Level of detection; IL = interleukin, IFN = interferon, TNF = tumor necrosis factor, MCP = monocyte chemotactic protein, MIP = macrophage inflammatory protein, IP = interferon-gamma induced protein, RANTES = regulated on activation normal T-cell expressed and secreted
Cytokine and chemokine concentrations are presented in pg/ml eluate (not corrected for total protein)
Range of detected values in the study population
Cytokine/Chemokine Profiles
All immune biomarkers, except Eotaxin, were included in the initial PCA to examine global cytokine/chemokine expression patterns in cases and controls; three components were generated, each explaining ≥10% of the total variance of the analytes. However, MCP-1 and IP-10 did not correlate with these components (loadings <0.40) and were excluded from the subsequent PCA. The final PCA, with 14 immune biomarkers, produced three components that collectively explained 67% of the total variance (Table S2). Component 1 consisted of cytokines largely associated with adaptive immune responses (IL-2, IL-4, IL-5, IL-12, IL-13, IFN-γ, TNF-α); Component 2 included chemokines (MIP-1α, MIP-1β, RANTES, IL-8); and Component 3 included pro-inflammatory cytokines IL-1β and IL-6, and the anti-inflammatory cytokine IL-10. Overall, plots of the principal component scores showed similar distributions across diagnostic groups with no overt clustering patterns that were predictive of case status (Figure S2).
Cytokines/Chemokines and Diagnosis
First, we conducted multinomial logistic regression models adjusted for season of birth, gestational age, child’s sex, and years between sample collection and analysis to determine whether the concentrations of individual cytokines/chemokines were associated with ASD, irrespective of symptom severity, relative to DD and TD. Levels of (ln-transformed) IL-1β and IL-4 were elevated in ASD compared with TD but did not reach statistical significance after multiple comparison correction (Table S3). No other cytokines/chemokines differed significantly between ASD and DD groups or between DD and TD controls.
Next, we examined ASD cases as two groups, defined by symptom intensity, in relation to each other and to TD and DD controls in models adjusted for the same covariates. Interestingly, elevated levels of IL-1β (OR=0.45, 95% CI 0.25, 0.82) and several other pro-inflammatory cytokines/chemokines were associated with lower odds of severe ASD (ASDsev) compared with mild-to-moderate ASD (ASDmild) (Table 3). IL-1β (OR=3.03, 95% CI 1.48, 6.23) was also associated with an increased likelihood of ASDmild relative to TD. Conversely, elevated IL-4 was associated with increased odds of ASDsev vs. TD but not statistically significant after multiple comparisons correction. No cytokines/chemokines differed significantly between the two case groups (ASDsev and ASDmild) and DD controls (Table S4).
Table 3.
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD with severe symptoms, ASD with mild to moderate symptoms, and TD, N=276a
| ASDsev vs. ASDmild |
ASDsev vs. TD |
ASDmild vs. TD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine or Chemokine |
OR | 95% CI | P- valueb |
OR | 95% CI |
P- valueb |
OR | 95% CI |
P- valueb |
| IL-1β |
0.4
5 |
(0.25,
0.82) |
0.045
9 |
1.3 7 |
(0.75 , 2.49) |
0.877 3 |
3.0
3 |
(1.48
, 6.23) |
0.041
6 |
| IL-2 | 1.1 0 |
(0.91, 1.31) |
0.570 8 |
1.2 1 |
(0.99 , 1.47) |
0.479 2 |
1.1 0 |
(0.89 , 1.37) |
0.601 1 |
| IL-4 | 1.1 4 |
(0.90, 1.44) |
0.570 8 |
1.3
9 |
(1.04
, 1.85) |
0.404 8 |
1.2 2 |
(0.89 , 1.68) |
0.436 2 |
| IL-5 | 1.0 2 |
(0.83, 1.24) |
0.941 4 |
1.0 6 |
(0.86 , 1.31) |
0.902 7 |
1.0 5 |
(0.82 , 1.33) |
0.763 7 |
| IL-6 |
0.8
6 |
(0.77,
0.96) |
0.045
9 |
0.9 4 |
(0.84 , 1.07) |
0.877 3 |
1.1 0 |
(0.96 , 1.26) |
0.393 4 |
| IL-10 | 0.9 0 |
(0.75, 1.10) |
0.570 8 |
0.9 8 |
(0.80 , 1.21) |
0.940 4 |
1.0 9 |
(0.86 , 1.38) |
0.648 4 |
| IL-12 | 0.9 3 |
(0.69, 1.26) |
0.867 3 |
1.2 9 |
(0.93 , 1.81) |
0.697 6 |
1.3 9 |
(0.95 , 2.04) |
0.294 7 |
| IL-13 | 0.9 8 |
(0.79, 1.21) |
0.941 4 |
1.0 5 |
(0.84 , 1.32) |
0.902 7 |
1.0 7 |
(0.83 , 1.39) |
0.691 7 |
| IFN-γ |
0.7
4 |
(0.58,
0.95) |
0.082 0 |
1.1 1 |
(0.88 , 1.39) |
0.877 3 |
1.4
9 |
(1.11
, 2.00) |
0.064 8 |
| TNF-α | 1.0 0 |
(0.71, 1.42) |
0.989 5 |
1.1 2 |
(0.77 , 1.64) |
0.902 7 |
1.1 2 |
(0.73 , 1.72) |
0.691 7 |
| IL-8 | 0.8 7 |
(0.59, 1.27) |
0.742 4 |
1.1 5 |
(0.72 , 1.83) |
0.902 7 |
1.3 2 |
(0.79 , 2.20) |
0.502 4 |
| MCP-1 | 0.9 6 |
(0.57, 1.62) |
0.941 4 |
0.8 7 |
(0.49 , 1.54) |
0.902 7 |
0.9 0 |
(0.47 , 1.74) |
0.763 7 |
| MIP-1α | 0.9 4 |
(0.79, 1.12) |
0.747 6 |
1.0 2 |
(0.84 , 1.24) |
0.931 3 |
1.0 8 |
(0.87 , 1.35) |
0.648 4 |
| MIP-1β | 0.6 7 |
(0.39, 1.16) |
0.495 7 |
1.1 0 |
(0.58 , 2.06) |
0.931 3 |
1.6 3 |
(0.81 , 3.28) |
0.393 4 |
| IP-10 |
0.5
7 |
(0.37,
0.86) |
0.045
9 |
1.0 1 |
(0.65 , 1.59) |
0.950 2 |
1.7
9 |
(1.06
, 3.02) |
0.154 1 |
| RANTES | 0.7 3 |
(0.45, 1.17) |
0.513 3 |
1.3 3 |
(0.72 , 2.46) |
0.877 3 |
1.8 2 |
(0.93 , 3.55) |
0.294 7 |
Logistic regression models were adjusted for season of birth, gestational age, years from blood spot collection to elution, and child’s sex; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); values below levels of detection were imputed by multiple imputation; 276 participants comprised the following groups: 141 ASD (severe), 73 ASD (mild), and 62 TD; ASD severity was defined using ADOS comparison scores, where ≥7 indicated severe and <7 indicated mild to moderate symptoms; OR = adjusted odds ratio, CI = confidence interval
P-values were corrected for multiple comparisons (16 biomarkers)
When IL-1β, IL-2, IL-4, IL-6, IFN-γ, and IP-10 were modeled together and adjusted for covariates, only IL-1β and IL-4 emerged as independently associated with ASDmild and ASDsev, respectively, relative to TD and each other (Model 1, Table 4). We then retained only IL-1β, IL-4, and covariates (Model 2); Relative to TD controls, elevated IL-1β was associated with a 3-fold increased likelihood of ASDmild (OR=3.02, 95% CI 1.43, 6.38) while IL-4 was associated a 1.4-fold increased likelihood of ASDsev (OR=1.40, 95% CI 1.03, 1.91). Compared with ASDmild, IL-1β associated with lower odds of ASDsev (OR=0.37, 95% CI 0.20, 0.67) whereas IL-4 associated with higher odds of ASDsev (OR=1.35, 95% CI 1.04, 1.75).
Table 4.
Adjusted odds ratios comparing neonatal cytokine and chemokine concentrations in ASD with severe symptoms, ASD with mild to moderate symptoms, and TD in one model, N=276a
| ASDsev vs. ASDmild |
ASDsev vs. TD |
ASDmild vs. TD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine or Chemokine |
OR | 95% CI | P- valu e |
OR | 95% CI |
P- valu e |
OR | 95% CI |
P- valu e |
| Model 1: | |||||||||
| IL-1β | 0.5 9 |
(0.29, 1.21) |
0.15 | 1.4 4 |
(0.68 , 3.04) |
0.34 |
2.4
3 |
(0.99
, 5.96) |
0.05 |
| IL-4 |
1.4
4 |
(1.08,
1.91) |
0.01 |
1.3
9 |
(1.00
, 1.93) |
0.05 | 0.9 6 |
(0.67 , 1.39) |
0.84 |
| IL-6 | 0.9 2 |
(0.81, 1.04) |
0.19 | 0.9 0 |
(0.78 , 1.04) |
0.17 | 0.9 9 |
(0.84 , 1.15) |
0.85 |
| IFN-γ | 0.7 6 |
(0.57, 1.01) |
0.06 | 0.9 9 |
(0.75 , 1.30) |
0.92 | 1.3 0 |
(0.94 , 1.81) |
0.12 |
| IP-10 | 0.6 9 |
(0.43, 1.10) |
0.11 | 1.0 5 |
(0.64 , 1.71) |
0.85 | 1.5 2 |
(0.86 , 2.68) |
0.15 |
| Model 2: | |||||||||
| IL-1β |
0.3
7 |
(0.20,
0.67) |
0.00
1 |
1.1 0 |
(0.59 , 2.07) |
0.76 |
3.0
2 |
(1.43
, 6.38) |
0.00
4 |
| IL-4 |
1.3
5 |
(1.04,
1.75) |
0.02 |
1.4
0 |
(1.03
, 1.91) |
0.03 | 1.0 4 |
(0.74 , 1.46) |
0.83 |
Logistic regression model included selected cytokines/chemokines modeled together and was adjusted for season of birth, gestational age, years from blood spot collection to elution, and child’s sex; cytokines/chemokines were ln-transformed and normalized for total protein (pg/mg total protein); values below levels of detection were imputed by multiple imputation; 276 participants comprised the following groups: 141 ASD (severe), 73 ASD (mild), and 62 TD; ASD severity was defined using ADOS comparison scores, where ≥7 indicated severe and <7 indicated mild to moderate symptoms; OR = adjusted odds ratio, CI = confidence interval
Selected Cytokines and Autism Severity, Behavior, and Development –Linear Regression
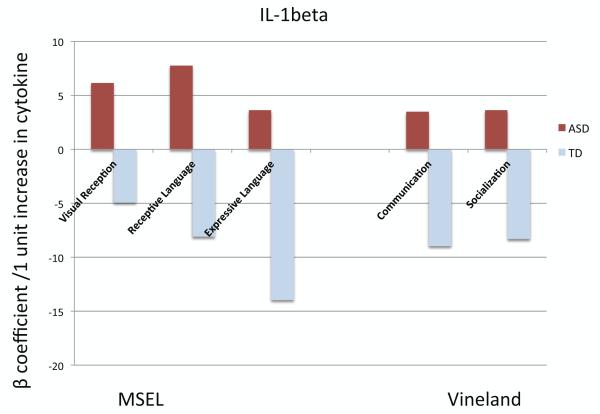
We examined whether neonatal IL-1β and IL-4 concentrations were associated with any particular developmental or behavioral patterns assessed at age 2-5 years. The developmental scores (MSEL, VABS) were individually modeled using linear regression restricted to cases and adjusted for maternal education level, with both IL-1β and IL-4 as the predictors. Neonatal IL-1β concentrations were positively associated with MSEL visual reception (β=7.13, SE=2.54, P=0.01) and receptive language (language comprehension; β=8.32, SE=2.90, P=0.01), indicating better performance; conversely, IL-4 concentrations were negatively associated with MSEL visual reception, but this association was not significant after multiple comparisons adjustment (Figure 1, Table S5). One should note that the mean developmental quotients (DQ) on all MSEL scales for both case groups were well below cutoffs for typical cognitive function (DQ <70; i.e., >2 standard deviations below the mean of 100).
Figure 1. Cognitive and adaptive scores of 2-5 year old children with ASD in relation to their neonatal IL-1β and IL-4 concentrations.
This figure illustrates the mean change in cognitive and adaptive scores for a 1-unit increase in ln-transformed cytokine (pg/mg total protein). Bars with an asterisk (*) denote statistically significant associations (P <0.05); P-values were adjusted for multiple comparisons. Mullen Scales of Early Learning (MSEL) measures cognitive development and subscales include Visual Reception (non-verbal cognitive ability), Receptive Language (language comprehension), and Expressive Language (language production); Vineland Adaptive Behavior Scales (VABS) subscales include Communication and Socialization. The scores have been converted to developmental quotients, and higher scores represent better performance.
The magnitude of the associations between IL-1β and MSEL visual reception (β= 7.97, SE=2.66, P=0.01) and receptive language subscales (β= 9.64, SE=3.03, P=0.01) was augmented when we examined male cases only (Figure S3, Table S6). Additionally, IL-4 levels were now significantly negatively associated with visual reception (β= −3.63, SE=1.33, P=0.04). No other behavioral or developmental scores associated with IL-1β or IL-4 concentrations. Among controls (DD+TD), there was a trend suggesting an association between higher IL-1β levels and lower MSEL expressive language (language production) (Figure S4, Table S7). IL-4 was not associated with any developmental domains.
DISCUSSION
The current study explores whether early cytokine/chemokine profiles are predictive of ASD. Elevated neonatal IL-4 and IL-1β concentrations measured in blood spots collected within 48 hours of birth were independently associated with ASD relative to TD controls. IL-1β levels were associated with a 3-fold increased likelihood of ASD with mild to moderately severe symptoms, while elevated IL-4 levels were associated with a 1.4-fold increase in the likelihood of severe ASD. IL-4 levels were also higher in children with severe ASD compared to those with mild-to-moderate ASD symptoms. We did not observe any significant differences in the levels of cytokines/chemokines between ASD cases and DD controls, although the DD sample size was likely too small to make definitive conclusions at this time. Among children with ASD, increased neonatal IL-4 concentrations were associated with greater impairments in non-verbal cognitive ability and language comprehension, particularly in male cases, but not in TD or DD controls.
The neonatal immune system is extremely immature, and cytokine production is much lower than in adults.(32) This was accurately reflected by the number of subjects whose whole blood cytokine levels were near or below the limit of detection. We expect the majority of the analytes we measured to be of neonatal origin because maternal cytokines and chemokines are largely blocked from placental passage, with the possible exception of IL-6 and GM-CSF.(33, 34)
Neonatal blood spots have been used to measure cytokine profiles in several studies,(35, 36) and to identify immune markers for a variety of disorders, including type 1 diabetes,(37) toxoplasmosis,(38) Down syndrome, and ASD.(18-20, 39) A 2006 study by Nelson et al. measured cytokine levels in neonatal blood spots from children with ASD, though only IL-8 was within the detectable range. The current study utilized a highly optimized procedure capable of detecting extremely low levels of 17 cytokines/chemokines. In a similar study, Abdallah et al.(19) reported decreased levels of many cytokines among children with ASD, including IFN-γ, IL-2, IL-4, IL-6, and IL-1β. While there were several analytes that overlapped between our study and that of Abdallah et al., the methodology and analysis differed significantly. One major limitation of the Abdallah study was that the diagnoses of cases and controls were not confirmed clinically by standardized measures. In the current study, we had access to detailed behavioral and developmental characteristics of our participants. This proved to be especially important as the severity of ASD symptoms associated with cytokine levels. Our cytokine measurement methods differed slightly from Abdallah et al. as well; we corrected cytokine levels based on total protein content to account for differences in collection efficiency between samples, while Abdallah et al. assumed that each dried blood spot contained the same volume of blood. The study by Zerbo et al.(20) had similar limitations as the Abdallah study. Further, the assays used for the Zerbo study were not as sensitive as those used for the current study.
IL-1β, IL-4, and their receptors are expressed in the developing brain,(40-43) and they have been shown to impact aspects of neuronal and glial cell development and function in cell culture models.(44) IL-1β is an inflammatory cytokine produced by many cell types early during an immune response. Beyond its role in inflammation, IL-1β has extensive neurological significance. It is capable of crossing the blood brain barrier and is involved in fever and sickness behavior.(45-47) Furthermore, genes for IL-1β and its receptor-associated proteins have been associated with cognitive disabilities, schizophrenia, and ASD.(48-50) Elevated IL-1β has been previously reported in older children with ASD as well.(12, 51-54)
IL-4 is a cytokine largely produced by T-helper type 2 (Th2) cells. It is involved in shaping the adaptive immune response, and is often associated with parasite defense, asthma, and atopy. IL-4 has been shown to impact neuronal proliferation, differentiation, survival, and synapse formation(55-58). Elevated IL-4 has also been linked to ASD with increased levels reported in maternal serum during the second trimester of pregnancy(59), and in amniotic fluid (60) collected from mothers giving birth to a child later diagnosed with ASD.
The increase in IL-1β and IL-4 is likely reflective of a prenatal immune challenge. At birth, the immune system is skewed towards a Th2, or more regulatory phenotype (versus an inflammatory T-helper type 1 (Th1) phenotype), compared to adults(61, 62). Prenatal immune activation can impact many aspects of fetal development and is associated with adverse outcomes including preterm birth, cerebral palsy, and miscarriage.(63-66) More subtly, maternal atopic disorders such as asthma and allergy as well as increased IL-4 and IFN-γ during pregnancy have been shown to be associated with an increased risk of ASD.(10, 67, 68) While many studies have examined the maternal gestational immune profile, newborn blood spots are primarily reflective of the infant’s immune status. Early immune dysregulation in the infant may have an impact on the developing nervous system as well. Of particular interest were our findings that demonstrated an improvement in the visual reception and receptive language scores with elevated IL-1β at birth, while these same scores were negatively associated with IL-4 levels in children with ASD. This apparent dichotomous finding suggests that skewing of the early immune environment towards a Th2 (IL-4 producing) phenotype could negatively impact the developmental trajectory of visual reception and receptive language while an increase in an opposing inflammatory cytokine associated with innate immune cell (and microglial) function alternatively protects or enhances these domains. Further mechanistic studies would need to be conducted to fully define the role of these two cytokines in this specific area of neurodevelopment.
It is of course possible that peripheral cytokine levels do not alter neurodevelopmental outcome independent of other factors. The immune and central nervous systems share many genes and signaling pathways, so disruption in one system can be reflected in the other. Altered cytokine profiles might simply be an epiphenomenon of genetic and/or environmental factors that impact neurodevelopment. Our data suggest, however, that a very early alteration in cytokine regulation is associated with a subsequent diagnosis of ASD. Ongoing studies will further address the relationship between neonatal cytokine profiles and immune disorders in children with ASD.
Collectively, our observations suggest that immune factors measured at birth could provide early markers of aberrant neurodevelopmental changes associated with ASD. Further, we demonstrate that differences in early neonatal cytokine profiles relate to ASD severity, which suggests that behavioral subphenotypic differences in the ASD population might have an immunologic component. In particular, the finding that the same cytokines are associating with both ASD risk and cognitive developmental outcomes (in the same or opposite directions) suggests a more global impact of early cytokine dysregulation. Finally, continued efforts to identify early markers of ASD will lead to a greater understanding of the disorder, and may ultimately suggest therapeutic options to improve the developmental trajectory and quality of life for affected individuals and their families.
Supplementary Material
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health (P01 ES11269 and R01 ES015359), the U.S. Environmental Protection Agency through the Science to Achieve Results (STAR) program (R829388 and R833292) and by the MIND Institute, University of California, Davis. The authors would like to thank the CHARGE Study participants and staff for their dedication and effort.
Footnotes
FINANCIAL DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lord C, Cook EH, Leventhal BL, Amaral DG. Autism spectrum disorders. Neuron. 2000;28:355–363. doi: 10.1016/s0896-6273(00)00115-x. [DOI] [PubMed] [Google Scholar]
- 2.World Heath Organization International Statistical Classification of Diseases and Related Health Problems (ICD-10) 10th edition 2006.
- 3.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) 5th ed American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- 4.Herbert MR. Contributions of the environment and environmentally vulnerable physiology to autism spectrum disorders. Curr Opin Neurol. 2010;23:103–110. doi: 10.1097/WCO.0b013e328336a01f. [DOI] [PubMed] [Google Scholar]
- 5.Le Couteur A, Haden G, Hammal D, McConachie H. Diagnosing autism spectrum disorders in pre-school children using two standardised assessment instruments: the ADI-R and the ADOS. J Autism Dev Disord. 2008;38:362–372. doi: 10.1007/s10803-007-0403-3. [DOI] [PubMed] [Google Scholar]
- 6.Lord C, Risi S, Lambrecht L, Cook EH, Jr., Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30:205–223. [PubMed] [Google Scholar]
- 7.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 8.Dawson G, Jones EJ, Merkle K, Venema K, Lowy R, Faja S, et al. Early behavioral intervention is associated with normalized brain activity in young children with autism. J Am Acad Child Adolesc Psychiatry. 2012;51:1150–1159. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goines P, Croen LA, Braunschweig D, Yoshida C, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased mid-gestational IFN-γ, IL-4, and IL-5 in women giving birth to a child with autism: a case-control study. Molecular Autism-submitted. 2011. [DOI] [PMC free article] [PubMed]
- 10.Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, et al. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64:583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Careaga M, Van de Water J, Ashwood P. Immune dysfunction in autism: a pathway to treatment. Neurotherapeutics. 2010;7:283–292. doi: 10.1016/j.nurt.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah I, Van de Water J. Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav Immun. 2011;25:40–45. doi: 10.1016/j.bbi.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Onore C, Enstrom A, Krakowiak P, Hertz-Picciotto I, Hansen R, Van de Water J, et al. Decreased cellular IL-23 but not IL-17 production in children with autism spectrum disorders. J Neuroimmunol. 2009;216:126–129. doi: 10.1016/j.jneuroim.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral D, Van de Water J. Autoantibodies in autism spectrum disorders (ASD) Ann N Y Acad Sci. 2007;1107:79–91. doi: 10.1196/annals.1381.009. [DOI] [PubMed] [Google Scholar]
- 15.Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, et al. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. J Neuroimmunol. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Goines PE, Ashwood P. Cytokine dysregulation in autism spectrum disorders (ASD): possible role of the environment. Neurotoxicol Teratol. 2013;36:67–81. doi: 10.1016/j.ntt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdallah MW, Larsen N, Grove J, Bonefeld-Jorgensen EC, Norgaard-Pedersen B, Hougaard DM, et al. Neonatal chemokine levels and risk of autism spectrum disorders: findings from a Danish historic birth cohort follow-up study. Cytokine. 2013;61:370–376. doi: 10.1016/j.cyto.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Abdallah MW, Larsen N, Mortensen EL, Atladottir HO, Norgaard-Pedersen B, Bonefeld-Jorgensen EC, et al. Neonatal levels of cytokines and risk of autism spectrum disorders: an exploratory register-based historic birth cohort study utilizing the Danish Newborn Screening Biobank. J Neuroimmunol. 2012;252:75–82. doi: 10.1016/j.jneuroim.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 20.Zerbo O, Yoshida C, Grether JK, Van de Water J, Ashwood P, Delorenze GN, et al. Neonatal cytokines and chemokines and risk of Autism Spectrum Disorder: the Early Markers for Autism (EMA) study: a case-control study. J Neuroinflammation. 2014;11:113. doi: 10.1186/1742-2094-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114:1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen EM. Mullen Scales of Early Early Learning. American Guidance Service, Inc; Circle Pines, MN: 1995. [Google Scholar]
- 23.Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales Interview Edition Expanded from Manual. American Guidance Services, Inc; Circle Pines, MN: 1984. [Google Scholar]
- 24.Le Couteur A, Lord C, Rutter M. Autism Diagnostic Interview–Revised (ADI-R) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 25.Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, Bishop SL. Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) Western Psychological Services; Los Angeles, CA: 2012. [Google Scholar]
- 26.Risi S, Lord C, Gotham K, Corsello C, Chrysler C, Szatmari P, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- 27.Rutter M, Bailey A, Berument SK, Lord C, Pickles A. Social Communication Questionnaire (SCQ) Western Psychological Services; Los Angeles, CA: 2003. [Google Scholar]
- 28.Aman MG, Singh NN. Aberrant Behavior Checklist—Community. Slosson Educational Publications; East Aurora, NY: 1994. [Google Scholar]
- 29.Kharrazi M, Pearl M, Yang J, DeLorenze GN, Bean CJ, Callaghan WM, et al. California Very Preterm Birth Study: design and characteristics of the population- and biospecimen bank-based nested case-control study. Paediatr Perinat Epidemiol. 2012;26:250–263. doi: 10.1111/j.1365-3016.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kabacoff RI. Determining the Dimensionality of Data: A SAS® Macro for Parallel Analysis. Proceedings of the Twenty Eighth Annual SAS Users Group International (SUGI 28); Seattle, WA. 2003. [Google Scholar]
- 31.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B. 1995;57:289–300. [Google Scholar]
- 32.Jaspan HB, Lawn SD, Safrit JT, Bekker LG. The maturing immune system: implications for development and testing HIV-1 vaccines for children and adolescents. AIDS. 2006;20:483–494. doi: 10.1097/01.aids.0000210602.40267.60. [DOI] [PubMed] [Google Scholar]
- 33.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103:546–550. doi: 10.1097/01.AOG.0000114980.40445.83. [DOI] [PubMed] [Google Scholar]
- 34.Gregor H, Egarter C, Levin D, Sternberger B, Heinze G, Leitich H, et al. The passage of granulocyte-macrophage colony-stimulating factor across the human placenta perfused in vitro. J Soc Gynecol Investig. 1999;6:307–310. doi: 10.1016/s1071-5576(99)00042-8. [DOI] [PubMed] [Google Scholar]
- 35.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–1866. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 36.Skogstrand K, Ekelund CK, Thorsen P, Vogel I, Jacobsson B, Norgaard-Pedersen B, et al. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J Immunol Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 37.Eising S, Svensson J, Skogstrand K, Nilsson A, Lynch K, Andersen PS, et al. Type 1 diabetes risk analysis on dried blood spot samples from population-based newborns: design and feasibility of an unselected case-control study. Paediatr Perinat Epidemiol. 2007;21:507–517. doi: 10.1111/j.1365-3016.2007.00846.x. [DOI] [PubMed] [Google Scholar]
- 38.Neto EC, Rubin R, Schulte J, Giugliani R. Newborn screening for congenital infectious diseases. Emerg Infect Dis. 2004;10:1068–1073. doi: 10.3201/eid1006.030830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nelson PG, Kuddo T, Song EY, Dambrosia JM, Kohler S, Satyanarayana G, et al. Selected neurotrophins, neuropeptides, and cytokines: developmental trajectory and concentrations in neonatal blood of children with autism or Down syndrome. Int J Dev Neurosci. 2006;24:73–80. doi: 10.1016/j.ijdevneu.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Lovett-Racke AE, Smith ME, Arredondo LR, Bittner PS, Ratts RB, Shive CL, et al. Developmentally regulated gene expression of Th2 cytokines in the brain. Brain Res. 2000;870:27–35. doi: 10.1016/s0006-8993(00)02398-2. [DOI] [PubMed] [Google Scholar]
- 41.Sawada M, Itoh Y, Suzumura A, Marunouchi T. Expression of cytokine receptors in cultured neuronal and glial cells. Neurosci Lett. 1993;160:131–134. doi: 10.1016/0304-3940(93)90396-3. [DOI] [PubMed] [Google Scholar]
- 42.Sawada M, Suzumura A, Marunouchi T. Cytokine network in the central nervous system and its roles in growth and differentiation of glial and neuronal cells. Int J Dev Neurosci. 1995;13:253–264. doi: 10.1016/0736-5748(94)00076-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nolan Y, Maher FO, Martin DS, Clarke RM, Brady MT, Bolton AE, et al. Role of interleukin-4 in regulation of age-related inflammatory changes in the hippocampus. The Journal of biological chemistry. 2005;280:9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- 44.de Araujo EG, da Silva GM, Dos Santos AA. Neuronal cell survival: the role of interleukins. Ann N Y Acad Sci. 2009;1153:57–64. doi: 10.1111/j.1749-6632.2008.03974.x. [DOI] [PubMed] [Google Scholar]
- 45.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1 alpha and murine IL-1 beta are transported from blood to brain in the mouse by a shared saturable mechanism. The Journal of pharmacology and experimental therapeutics. 1991;259:988–996. [PubMed] [Google Scholar]
- 46.Skurlova M, Stofkova A, Jurcovicova J. Exogenous IL-1beta induces its own expression, but not that of IL-6 in the hypothalamus and activates HPA axis and prolactin release. Endocrine regulations. 2006;40:125–128. [PubMed] [Google Scholar]
- 47.Dantzer R. Cytokine-induced sickness behavior: where do we stand? Brain Behav Immun. 2001;15:7–24. doi: 10.1006/brbi.2000.0613. [DOI] [PubMed] [Google Scholar]
- 48.Katila H, Hanninen K, Hurme M. Polymorphisms of the interleukin-1 gene complex in schizophrenia. Molecular psychiatry. 1999;4:179–181. doi: 10.1038/sj.mp.4000483. [DOI] [PubMed] [Google Scholar]
- 49.Piton A, Michaud JL, Peng H, Aradhya S, Gauthier J, Mottron L, et al. Mutations in the calcium-related gene IL1RAPL1 are associated with autism. Human molecular genetics. 2008;17:3965–3974. doi: 10.1093/hmg/ddn300. [DOI] [PubMed] [Google Scholar]
- 50.Handley MT, Lian LY, Haynes LP, Burgoyne RD. Structural and functional deficits in a neuronal calcium sensor-1 mutant identified in a case of autistic spectrum disorder. PloS one. 2010;5:e10534. doi: 10.1371/journal.pone.0010534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Suzuki K, Matsuzaki H, Iwata K, Kameno Y, Shimmura C, Kawai S, et al. Plasma cytokine profiles in subjects with high-functioning autism spectrum disorders. PloS one. 2011;6:e20470. doi: 10.1371/journal.pone.0020470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Emanuele E, Orsi P, Boso M, Broglia D, Brondino N, Barale F, et al. Low-grade endotoxemia in patients with severe autism. Neurosci Lett. 2010;471:162–165. doi: 10.1016/j.neulet.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 53.Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120:170–179. doi: 10.1016/s0165-5728(01)00421-0. [DOI] [PubMed] [Google Scholar]
- 54.Enstrom AM, Onore CE, Van de Water JA, Ashwood P. Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immun. 2010;24:64–71. doi: 10.1016/j.bbi.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Butovsky O, Ziv Y, Schwartz A, Landa G, Talpalar AE, Pluchino S, et al. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Molecular and cellular neurosciences. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 56.Araujo DM, Cotman CW. Trophic effects of interleukin-4, -7 and -8 on hippocampal neuronal cultures: potential involvement of glial-derived factors. Brain Res. 1993;600:49–55. doi: 10.1016/0006-8993(93)90400-h. [DOI] [PubMed] [Google Scholar]
- 57.da Silva AG, Campello-Costa P, Linden R, Sholl-Franco A. Interleukin-4 blocks proliferation of retinal progenitor cells and increases rod photoreceptor differentiation through distinct signaling pathways. J Neuroimmunol. 2008;196:82–93. doi: 10.1016/j.jneuroim.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 58.Sholl-Franco A, Marques PM, Ferreira CM, de Araujo EG. IL-4 increases GABAergic phenotype in rat retinal cell cultures: involvement of muscarinic receptors and protein kinase C. J Neuroimmunol. 2002;133:20–29. doi: 10.1016/s0165-5728(02)00327-2. [DOI] [PubMed] [Google Scholar]
- 59.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, et al. Increased midgestational IFN-gamma, IL-4 and IL-5 in women bearing a child with autism: A case-control study. Molecular autism. 2011;2:13. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abdallah MW, Larsen N, Grove J, Norgaard-Pedersen B, Thorsen P, Mortensen EL, et al. Amniotic fluid inflammatory cytokines: Potential markers of immunologic dysfunction in autism spectrum disorders. The world journal of biological psychiatry : the official journal of the World Federation of Societies of Biological Psychiatry. 2011 doi: 10.3109/15622975.2011.639803. [DOI] [PubMed] [Google Scholar]
- 61.Zaghouani H, Hoeman CM, Adkins B. Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009;30:585–591. doi: 10.1016/j.it.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rose S, Lichtenheld M, Foote MR, Adkins B. Murine neonatal CD4+ cells are poised for rapid Th2 effector-like function. J Immunol. 2007;178:2667–2678. doi: 10.4049/jimmunol.178.5.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Skogstrand K, Hougaard DM, Schendel DE, Bent NP, Svaerke C, Thorsen P. Association of preterm birth with sustained postnatal inflammatory response. Obstet Gynecol. 2008;111:1118–1128. doi: 10.1097/AOG.0b013e31817057fb. [DOI] [PubMed] [Google Scholar]
- 64.Minagawa K, Tsuji Y, Ueda H, Koyama K, Tanizawa K, Okamura H, et al. Possible correlation between high levels of IL-18 in the cord blood of pre-term infants and neonatal development of periventricular leukomalacia and cerebral palsy. Cytokine. 2002;17:164–170. doi: 10.1006/cyto.2001.0988. [DOI] [PubMed] [Google Scholar]
- 65.Lyon D, Cheng CY, Howland L, Rattican D, Jallo N, Pickler R, et al. Integrated review of cytokines in maternal, cord, and newborn blood: part I--associations with preterm birth. Biol Res Nurs. 2010;11:371–376. doi: 10.1177/1099800409344620. [DOI] [PubMed] [Google Scholar]
- 66.Pickler R, Brown L, McGrath J, Lyon D, Rattican D, Cheng CY, et al. Integrated review of cytokines in maternal, cord, and newborn blood: part II-- associations with early infection and increased risk of neurologic damage in preterm infants. Biol Res Nurs. 2010;11:377–386. doi: 10.1177/1099800409344619. [DOI] [PubMed] [Google Scholar]
- 67.Croen LA, Grether JK, Yoshida CK, Odouli R, Van de Water J. Maternal autoimmune diseases, asthma and allergies, and childhood autism spectrum disorders: a case-control study. Archives of pediatrics & adolescent medicine. 2005;159:151–157. doi: 10.1001/archpedi.159.2.151. [DOI] [PubMed] [Google Scholar]
- 68.Lyall K, Ashwood P, Van de Water J, Hertz-Picciotto I. Maternal immune-mediated conditions, autism spectrum disorders, and developmental delay. J Autism Dev Disord. 2014;44:1546–1555. doi: 10.1007/s10803-013-2017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.