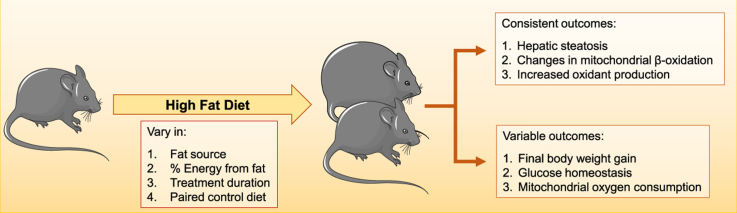
Abstract
Human metabolic diseases can be mimicked in rodents by using dietary interventions such as high fat diets (HFD). Nonalcoholic fatty liver disease (NAFLD) develops as a result of HFD and the disease may progress in a manner involving increased production of oxidants. The main intracellular source of these oxidants are mitochondria, which are also responsible for lipid metabolism and thus widely recognized as important players in the pathology and progression of steatosis. Here, we review publications that study redox and bioenergetic effects of HFD in the liver. We find that dietary composition and protocol implementations vary widely, as do the results of these dietary interventions. Overall, all HFD promote steatosis, changes in β-oxidation, generation and consequences of oxidants, while effects on body weight, insulin signaling and other bioenergetic parameters are more variable with the experimental models adopted. Our review provides a broad analysis of the bioenergetic and redox changes promoted by HFD as well as suggestions for changes and specifications in methodologies that may help explain apparent disparities in the current literature.
Abbreviations: ADP, adenosine diphosphate; ATP, adenosine triphosphate; CPT1a, carnitine palmitoyltransferase 1; DIO, diet induced obesity; ER, endoplasmic reticulum; ETC, electron transport chain; ETF-DH, electron transfer flavoprotein dehydrogenase; FAO, Food and Agriculture Organization of the United Nations; FFA, free fatty acid; HFD, high fat diet; MUFA, monounsaturated fatty acid; NAFLD, nonalcoholic fatty liver diseases; NASH, nonalcoholic steatohepatitis; NEFA, non-esterified fatty acid; PUFA, polyunsaturated fatty acid; RCR, respiratory control ratio; SFA, saturated fatty acid; TAG, triacylglycerides; UFA, unsaturated fatty acid; VLCAD, very long chain acyl CoA dehydrogenase
Keywords: Mitochondria, High fat diets, Liver, Metabolic diseases
Graphical abstract
Highlights
-
•
High fat diets (HFDs) induce steatosis, even with no weight changes .
-
•
HFDs activate β-oxidation.
-
•
HFDs promote oxidative imbalance.
1. Introduction
Human metabolic diseases such as obesity, nonalcoholic steatohepatitis (NASH), and the metabolic syndrome can be mimicked in rodents by using genetic and dietary interventions. Both strategies lead to body weight gain, hyperglycemia, hyperinsulinemia, hepatic steatosis and cardiac impairment [11], [32], [55]. Dietary models are considered more similar to human metabolic diseases, but there is no standard composition and duration for these diets currently: High fat diets (HFD) range from 30% to 60% energy content in fat, include saturated (SFA), monounsaturated (MUFA) and polyunsaturated fatty acids (PUFA) and last from a few days (short term) to over one week (long term) [11], [36]. As a result, very variable phenotypes are found in studies in the area, and data are often difficult to compare [11]. For example, the consumption of different types of lipids has a variable effect on the energy expenditure of mammals. Diets rich in SFAs lead to decreased basal metabolic rates, higher body weights and fat gain when compared to diets containing predominantly unsaturated fatty acids [41], [54], [68]. Furthermore, insulin resistance, an important step in the development of the metabolic syndrome, can be influenced by the lipid content of the diet, occurs more frequently in SFA and MUFA-rich diets and may be minimized by the consumption of PUFAs [10], [30], [44].
The nutrient overload generated by HFD in experimental animals resembles conditions of overnutrition and physical inactivity that are major risk factors for the development of metabolic syndrome in humans [32], [37]. These conditions, when combined, are able to exceed the adipose tissue’s ability to handle excessive energy, resulting in an increased efflux of non-esterified fatty acids (NEFAs) and release of proinflammatory cytokines and adipokines that may result in ectopic fat deposition in the liver, muscle and heart [18], [74].
In the liver, fat accumulation can be toxic [64], [65]. Nonalcoholic fatty liver disease (NAFLD) comprises several liver anomalies related to the accumulation of fat in hepatocytes, including simple steatosis, a benign condition that may progress to serious liver cirrhosis [12], [6], [65], [69]. Currently, the accepted pathophysiological model for NAFLD is the “two hits” model. First, there is accumulation of triacylglycerides (TAG) and free fatty acids (FFA) in the liver as a result of changes in the influx, synthesis, oxidation and transport of fatty acids. The second hit, triggered by the first hit, includes oxidative imbalance, decreased hepatic ATP production, insulin resistance and induction of proinflammatory cytokines as a result of mitochondrial dysfunction, lipid peroxidation and activation of inflammatory pathways as NF-kB and JNK [61], [69].
The progression from simple steatosis to NASH involves increased production of reactive oxygen and nitrogen species, lipotoxicity and pro-inflammatory cytokines [7]. The main cellular source of these oxidants is the mitochondrion [35], [59], an organelle also responsible for the lipid metabolism, which, consequently, plays an important role in the pathology and progression of steatosis [29], [61], [6], [66]. However, the parameters that characterize what is described as “mitochondrial dysfunction”, including changes in oxygen consumption, respiratory complex activity, oxidation of fatty acids and mitochondrial DNA content, differ widely in the literature. In addition to differences between genetic rodent strains of and human polymorphisms, part of the differences observed are probably due to the type and duration of the diet [7].
Given the growing obesity epidemic [53] and the importance of overnutrition in the development of diseases such as the metabolic syndrome and NAFLD [18], [37], [69], we sought to evaluate and to summarize changes in liver mitochondria related to the consumption of HFD. We collected and discuss papers published over the last 15 years that discussed metabolic changes in the livers of rodents under HFD, focusing on dietary differences and mitochondrial function.
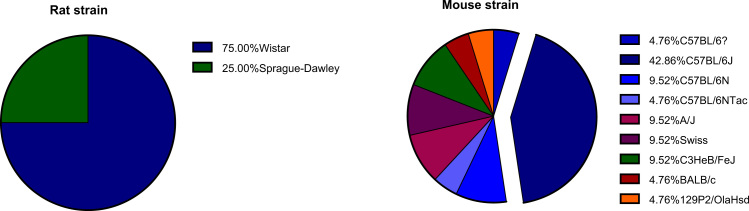
2. Rodents
Although there are some known metabolic differences between rodents and humans, most laboratory metabolic disease studies are conducted in rats or mice because of their inexpensive maintenance, small body size, relatively short lifespan, sequenced genome, and superior physiological characterization compared to other species such as pigs or dogs [2], [71]. Studies selected in this review were conducted in rodents, comprising mainly male animals and predominantly C57BL/6 mice (Table 1 and Fig. 1). Reuter [59] defined male C57BL/6J mice as the gold standard for a diet-induced obesity (DIO), and we indeed find it to be the strain used in most papers. These animals are known to develop obesity only under HFD, and metabolic parameters such as glucose intolerance and insulin resistance are worsened over the exposure time to these diets, as is seen in humans [43], [59]. A point of notice that should be considered when analyzing data in mice is that the C57BL/6J mice from the Jackson Laboratories, but not C57BL/6 from the National Institute of Health, Taconic Labs or Charles River, carry a spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene that has been implicated in several redox alterations that can affect mitochondrial function [23], [62]. C57BL/6N mice do not harbor this mutation.
Table 1.
Rodent studies using HFD: sex profiles.
| Mice (n=31) | Rats (n=15) | |
|---|---|---|
| Male | 24 | 14 |
| Female | 6 | 0 |
| Both | 1 | 1 |
Fig. 1.
: Rat and mouse strain distribution in HFD studies.
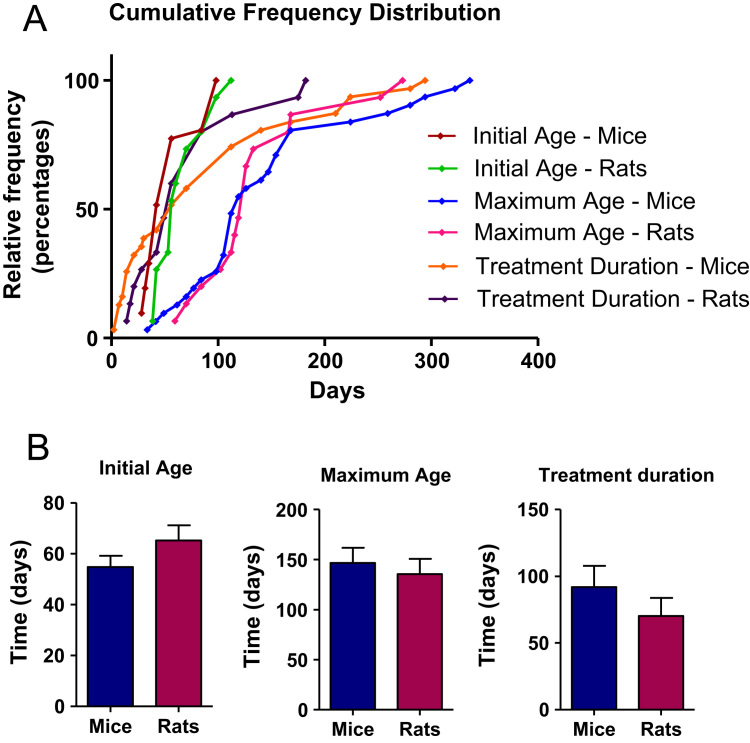
Mice and rats generally are started on the HFD at similar ages, and were kept on the diet during widely variable times (Fig. 2). Most of the papers analyzed used paired age groups. Ciapaite et al., 2011, described increased body weights and fasting plasmatic glucose and insulin levels as well as decreased oxidative phosphorylation capacity in 39-week-old animals fed with standard chow diet (9.2% kcal from fat, vegetable oils) in comparison to 16.5-week-old Wistar rats. In addition, Fontana et al. [25], observed that C57BL/6 aged animals (44 and 88 weeks old, fed during 16 weeks with a HFD) did not show increased steatosis compared to their younger counterparts, but exhibited increased inflammation and hepatocellular injury that could contribute to chronic liver disease development and progression. Thus, HFD groups should be paired by diet and also by age to avoid misinterpretation of data.
Fig. 2.
Rodent age and treatment duration distribution. A) Cumulative distribution of initial and maximum age and treatment duration; Averages+SEM are depicted in B. Results were not significantly different.
3. Diets and metabolic profiles
One important critique in HFD treatment is the difficulty to compare studies because of their variability. There are some updated reviews that compare metabolic findings between papers in order to identify the causes of variation and why there is an inability to unify the findings [11], [32], [43], [7]. In this review, we focused only on high energy diets containing fat as the main caloric source. The papers analyzed present a myriad of experimental designs of HFD interventions and significant variation in measured parameters. Diet composition description from controls and high fat groups is often overlooked by the authors and not fully provided in the papers. Due to the lack of information in some papers, a few assumptions were made to facilitate comparisons:
-
1)
Most authors refer to fat content as percentage of energy from fat, since ad libitum feeding will be determined by energy requirements [59], [60]. However, if the description was made by fat weight and the metabolized energy per weight of food was available, energy from fat was calculated by assuming (as defined by FAO) that 1 g of fat=9 kcal=37 kJ (FAO, 2013).
-
2)
Some authors describe the fat content of their diet, including individual fatty acid type. However, most only describe the main source of fat, i.e. lard, vegetable oil, or fish. In addition to the seasonality and origin of the fat, the proportionality trend of fat classes could be defined using the Fat and Oils Handbook [8].
In Table 2, we summarize the percentage of energy from fat of collected studies and in Fig. 3, the main source described by the authors or manufacturer. Most of the papers uncovered used manufactured purified diet that usually follows some of the requirements of AIN-93M defined by the American Institute of Nutrition in 1993 in order to obtain a global standard for rodent nutrition [58]. Ingredients can be added or removed from the recommended diet, but are always maintained in control animal groups [59]. In addition, purified HFD comparison against chow diet is not advisable: Regular chow diets are made from natural ingredients and this can cause variations in contents due to seasonality, origin or differences in metabolic responses due to phytoestrogen contents [11], [43], [5], [59]. As a result, changes other than those attributed to the high fat content may occur in the animals.
Table 2.
Dietary energy from fat (percentage).
| HFD (n=32) | Control (n=33) | Fold change (n=32) vs. Control | |
|---|---|---|---|
| Minimum | 40.0 | 8.0 | 2.0 |
| Median | 51.8 | 11.4 | 4.5 |
| Maximum | 71.0 | 35.0 | 6.8 |
| Mean | 52.6±9.9 | 13.5±7.1 | 4.3±1.2 |
Fig. 3.
Frequency distribution of fat sources and type of diet.
Lieber et al. [45], proposed a NAFLD model using a purified liquid diet containing 71% energy from fat (mainly corn oil, with a prevalence of omega 6 fatty acids) versus an isocaloric control diet containing the same ingredients, but 35% energy from fat. Buettner et al. [11] suggest that more than 30% energy from fat might already be a HFD and believe over 60% would present questionable results comparative to human diets, an observation that the authors of this review support. They fed Wistar rats for 3 weeks and observed increased lipid accumulation in the livers from both groups. The called “Liber-DeCarli” was diet used in two other papers analyzed [17], [46]. Mantena et al. [48] describe the development of steatosis that resembles human NASH [70] with this diet, including macro and microvesicular inclusions, Mallory bodies and hepatocyte ballooning. However, the protocol is criticized due lack of reproducibility [1], [50].
Enos et al. [20] described an interesting experimental design to evaluate the impact of differential fatty acid composition on cellular processes known to be obesity-related. Briefly, they used three purified HFDs with different concentrations of SFAs (6%, 12% and 24%) that are MUFA>omega 6>SFA>omega 3, MUFA>SFA>omega 6>omega 3 and SFA>MUFA>omega 6>omega 3 relations, respectively. In addition, they used two purified control diets in order to pair omega 3/omega 6 ratios with the HFDs. In Enos et al. [21] they describe that after 16 weeks the 12% SFA group gained more body and adipose tissue weight than controls and HFD groups, and presented the highest plasma insulin levels despite unchanged glucose plasmatic levels. This suggests more accentuated insulin resistance generated by MUFA>SFA>omega 6>omega 3 composition, a dietary intervention more closely related to the standard Western diet. In their 2014 paper, they show that only 6 and 12% HFDs induce steatosis and that only the 12% group had increased 4-HNE content in the liver, as well as JNK pathway activation and ER stress, suggesting a specific fatty acid sensitivity in the liver.
Even though HFDs are often used to promote obesity in rodents, some authors did not find statistical differences in body weight when compared to standard laboratory animals (Table 3). Rodents, unlike humans, are considered obese when increases in body weight or body fat content are seen, and are not usually evaluated by measurements such as body mass indexes [32]. The same lack of a homogeneous metabolic change is observed when measuring plasma glucose and insulin levels (Table 4). This lack of a universal body weight and metabolic change may be a reflection of the fact that “control” laboratory animals are sedentary and obese due to access to ad libitum diets [49], or due to differences in dietary protocols. Despite this lack of a homogeneous response in weight and insulin levels, the development of HFD-induced steatosis is consistently observed as changes in histology or by increased lipid content in the liver. Some other trends can be seen, such as the more favorable metabolic outcome when animals are fed with fish oil [10], [3], [47].
Table 3.
Changes in body weight after HFD.
| Authors | Strain | Sex | Age (w) | Duration (w) | HFD (% energy from fat) | Control (% energy from fat) | Main fat source |
|---|---|---|---|---|---|---|---|
| Decreased body weight (vs. indicated control group) | |||||||
| Buettner et al. [10] | Wistar | M | 6 | 12 | 42 | 11 | Fish |
| Increased body weight (vs. indicated control group) | |||||||
| Rats | |||||||
| Buettner et al. [10] | Wistar | M | 6 | 12 | 42 | 11 | Lard, coconut or olive oils |
| Nadal-Casellas et al. [51] | Wistar | Both | 10 | 26 | 54,5 | 8 | Cafeteria diet |
| Cipaite et al. [16] | Wistar | M | 14 | 2.5 or 25 | 45,7 | 9.2 | Lard |
| Flamment et al. [24] | Sprague Dawley | M | 10 | 2, 4 or 8 | – | 8.75 & | Lard |
| Herlein et al. [33] | Sprague Dawley | M | 7.5 | 16 | 60 | 13 | Lard |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Lard or fish |
| Mice | |||||||
| Gallou-Kabani et al. [28] | C57BL/6J or A/J | Both | 4 | 20 | 60 | 10 | Lard |
| Chan et al. [15] | C57BL/6J | F | 8 | 10 | 40 | 12 | Butter |
| Cardoso et al. [13] | Swiss | F | 6 | 8 or 40 | – | 14.3 & | Soybean oil |
| Poussin et al. [56] | C57BL/6J | M | 5 | 30 days | 58 | 12.70 & | Coconut oil |
| Satapati et al. [63] | C57BL/6J | M | 5 | 8, 16 or 32 | 60 | 10 | Lard |
| Kahle et al. [38] | C3HeB/FeJ, 129P2/OlaHsd | M | 14 | 1, 2 or 3 | 58 | 13 | Safflower oil |
| Kahle et al. [38] | C57BL/6NTac, C57BL/6J | M | 14 | 2 or 3 | 58 | 13 | Safflower oil |
| Yuzefovych et al. [75] | C57BL/6J | M | 6 | 16 | 60 | 10 | Lard |
| Nesteruk et al. [52] | C57BL/6J | M | 6 | 10 or 42 | 60 | 10 | Lard |
| Franko et al. [27] | C57BL/6N | M | 4 | 20 | 60 & | 13 & | Lard |
| Enos et al. [20] | C57BL/6 | M | 4 | 16 | 40 | 12.5 | Lard, coconut, corn, soybean and olive oil |
| Kahle et al. [39] | C3HeB/FeJ | M | 14 | 1, 2 or 3 | 58 | 13 | Safflower oil |
| Non-significant changes (vs. indicated control group) | |||||||
| Rats | |||||||
| Lieber et al. [45] | Sprague Dawley | M | – | 3 | 71 | 35 | Corn oil |
| Cipaite et al. [17] | Wistar | M | – | 7 | 50.4 | 16.4 | – |
| Vial et al. [72] | Wistar | M | 16 | 8 | – | – | Lard |
| Aoun et al. [3] | Wistar | M | 6 | 12 | 51.8 & | 11.4 & | Lard or Fish |
| Aoun et al. [4] | Wistar | M | 6 | 12 | 51.8 & | 11.4 & | Lard |
| Mice | |||||||
| Chan et al. [15] | C57BL/6J | F | 8 | 2 or 4 | 40 | 12 | Butter |
| Eccleston et al. [19] | C57BL/6J | M | 8 | 16 | 71 | 35 | Corn oil |
| Poussin et al. [56] | A/J | M | 5 | 2, 10 or 30 days | 58 | 12.70 & | Coconut oil |
| Poussin et al. [56] | C57BL/6J | M | 5 | 2 or 10 days | 58 | 12.70 & | Coconut oil |
| Kahle et al. [38] | C57BL/6J and C57BL/6NTac | M | 14 | 1 | 58 | 13 | Safflower oil |
| Cardoso et al. [14] | Swiss | F | 6 | 1 | – | 14.3 & | Soybean oil |
| Ho et al. [35] | BALB/c | M | 8 | 8, 24 or 32 | 60 | 10 | Lard |
&=calculated energy from fat based on information provided by the authors. 1 g fat=9 kcal=37 kJ [22].
Table 4.
Changes in plasma glucose and insulin levels after HFD.
| Authors | Strain | Sex | Age (w) | Duration (w) | HFD (% energy from fat) | Control (% energy from fat) | Main fat source | Glucose | Insulin |
|---|---|---|---|---|---|---|---|---|---|
| Rats | |||||||||
| Lieber et al. [45] | Sprague Dawley | M | – | 3 | 71 | 35 | Corn oil | – | Increased |
| Buettner et al. [10] | Wistar | M | 6 | 12 | 42 | 11 | Fish and coconut oil | ns | ns |
| Buettner et al. [10] | Wistar | M | 6 | 12 | 42 | 11 | Lard | ns | Increased |
| Buettner et al. [10] | Wistar | M | 6 | 12 | 42 | 11 | Olive oil | Increased | ns |
| Cipaite et al. [17] | Wistar | M | – | 7 | 50.4 | 16.4 | Increased | – | |
| Rafaella et al. [57] | Wistar | M | 12 | 7 | 50 | 10.6 | Butter | ns | |
| Cipaite et al. [16] | Wistar | M | 14 | 2.5 and 25 | 45.7 | 9.2 | Lard | ns | Increased |
| Flamment et al. [24] | Sprague Dawley | M | 10 | 2, 4 and 8 | 8.75 & | Lard | Increased | Increased | |
| Aoun et al. [3] | Wistar | M | 6 | 12 | 51.8 & | 11.4 & | Fish or lard | ns | Increased |
| Herlein et al. [33] | Sprague Dawley | M | 7,5 | 16 | 60 | 13 | Lard | ns | ns |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Lard | Increased | Increased |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Fish | Increased | ns |
| Mice | |||||||||
| Gallou-Kabani et al. [28] | C57BL/6J | M | 4 | 20 | 60 | 10 | Lard | Increased | Increased |
| Gallou-Kabani et al. [28] | A/J | M | 4 | 20 | 60 | 10 | Lard | ns | Increased |
| Gallou-Kabani et al. [28] | C57BL/6J | F | 4 | 20 | 60 | 10 | Lard | Increased | – |
| Gallou-Kabani et al. [28] | A/J | F | 4 | 20 | 60 | 10 | Lard | ns | – |
| Chan et al. [15] | C57BL/6J | F | 8 | 2 and 4 | 40 | 12 | Butter | ns | – |
| Chan et al. [15] | C57BL/6J | F | 8 | 10 | 40 | 12 | Butter | Increased | – |
| Chan et al. [15] | C57BL/6J | F | 8 | 4 | 40 | 12 | Butter | ns | – |
| Cardoso et al. [13] | Swiss | F | 6 | 8 and 40 | 14.3 & | Soybean oil | ns | – | |
| Eccleston et al. [19] | C57BL/6 | M | 8 | 16 | 71 | 35 | Corn oil | Increased | ns |
| Poussin et al. [56] | C57BL/6J | M | 5 | 2 d | 58 | 12.70 & | Coconut oil | Decreased | ns |
| Poussin et al. [56] | C57BL/6J | M | 5 | 10 d | 58 | 12.70 & | Coconut oil | ns | ns |
| Poussin et al. [56] | C57BL/6J | M | 5 | 30 d | 58 | 12.70 & | Coconut oil | Decreased | ns |
| Poussin et al. [56] | A/J | M | 5 | 2 d | 58 | 12.70 & | Coconut oil | ns | ns |
| Poussin et al. [56] | A/J | M | 5 | 10 d | 58 | 12.70 & | Coconut oil | Increased | ns |
| Poussin et al. [56] | A/J | M | 5 | 30 d | 58 | 12.70 & | Coconut oil | ns | ns |
| Satapati et al. [63] | C57BL/6 | M | 5 | 8, 16 and 32 | 60 | 10 | Lard | Increased | Increased |
| Kahle et al. [38] | C3HeB/FeJ | M | 14 | 1 and 2 | 58 | 13 | Safflower oil | ns | ns |
| Kahle et al. [38] | C3HeB/FeJ | M | 14 | 3 | 58 | 13 | Safflower oil | ns | Increased |
| Kahle et al. [38] | C57BL/6NTac | M | 14 | 1 and 2 | 58 | 13 | Safflower oil | ns | ns |
| Kahle et al. [38] | C57BL/6NTac | M | 14 | 3 | 58 | 13 | Safflower oil | Increased | Increased |
| Kahle et al. [38] | C57BL/6J | M | 14 | 1, 2 and 3 | 58 | 13 | Safflower oil | ns | ns |
| Kahle et al. [38] | 129P2/OlaHsd | M | 14 | 1 and 2 | 58 | 13 | Safflower oil | ns | ns |
| Kahle et al. [38] | 129P2/OlaHsd | M | 14 | 3 | 58 | 13 | Safflower oil | ns | Increased |
| Cardoso et al. [14] | Swiss | F | 6 | 1 | 14.3 & | Soybean oil | ns | – | |
| Ho et al. [35] | BALB/c | M | 8 | 8, 24 and 32 | 60 | 10 | Lard | ns | Increased |
| Yuzefovych et al. [75] | C57BL/6J | M | 6 | 16 | 60 | 10 | Lard | Increased | Increased |
| Franko et al. [27] | C57BL/6 | M | 4 | 20 | 60 & | 13 & | Lard | Increased | Increased |
| Enos et al. [20] | C57BL/6 | M | 4 | 16 | 40 | 12.5 | Lard, coconut, corn, soybean and olive oil | Increased | Increased |
| Kahle et al. [39] | C3HeB/FeJ | M | 14 | 1 and 2 | 58 | 13 | Safflower oil | ns | ns |
| Kahle et al. [39] | C3HeB/FeJ | M | 14 | 3 | 58 | 13 | Safflower oil | ns | Increased |
&=calculated energy from fat based on information provided by the authors. 1 g fat=9 kcal=37 kJ [22].
ns=authors did not find statistically significant differences.
4. Mitochondrial (Dys)function
Mitochondrial function is evaluated by assessing its ability to produce ATP under physiological conditions and under stress [34], [9]. In isolated mitochondria, the best and simplest way to assess function (and, thus, dysfunction) is by measuring respiratory control ratios. After mitochondrial isolation these organelles are challenged by the addition of substrates and pharmacological inhibitors in an isolated chamber containing an oxygen electrode, in order to modulate and record oxygen consumption rates. State 3 respiration is measured in the presence of ample substrates and ADP, and is related to its phosphorylation. State 4 occurs when ATP synthesis is inhibited by high ATP/ADP ratios or the inhibition of ATP synthesis. The respiratory control ratio (RCR) is calculated determining the state 3/state 4 ratio and functionally healthy mitochondria should have high RCRs [9].
Bioenergetic results of HFD varied highly with the highly variable dietary interventions (Table 5). Decreased or unchanged state 3 respiratory rates are often observed when using malate, glutamate and/or pyruvate, complex I related substrates, or succinate, a complex II substrate [67]. Studies using palmitoyl-carnitine, a lipid substrate [42], [73] had inconsistent results: some authors found an increase [14], [17], [47], [63] and others, a decrease [24], [56], [57], [72] in respiratory rates. There is no evident trend to explain this discrepancy considering the fat source, treatment duration, rodent species or strain, or initial age.
Table 5.
Oxygen consumption in isolated liver mitochondria.
| Authors | Strain | Sex | Age (w) | Duration (w) | HFD (% energy from fat) | Control (% energy from fat) | Main fat source | Main observations (vs. indicated control group) |
|---|---|---|---|---|---|---|---|---|
| Rats | ||||||||
| Cipaite et al. [17] | Wistar | M | – | 7 | 50.4 | 16.4 | – | Succinate+rotenone: |
| ns state 3 | ||||||||
| Rafaella et al. [57] | Wistar | M | 12 | 7 | 50 | 10.6 | Butter | Glutamate+malate: |
| Decreased states 3 and 4; ns RCR | ||||||||
| Palmitoyl-CoA+carnitine+malate | ||||||||
| Decreased states 3 and 4; ns RCR | ||||||||
| Succinate+rotenone: | ||||||||
| Decreased states 3 and 4; ns RCR | ||||||||
| Nadal-Casellas et al. [51] | Wistar | M | 10 | 26 | 54.5 | 8 | Cafeteria diet | Succinate: |
| Decreased states 3 and 4; ns RCR | ||||||||
| Vial et al. [72] | Wistar | M | 16 | 8 | – | – | Lard | Succinate+glutamate+malate: |
| Decreased state 3, ns state 4 | ||||||||
| Palmitoyl-carnitine: | ||||||||
| Decreased state 3, | ||||||||
| ns state 4 | ||||||||
| Glutamate+malate: | ||||||||
| ns state 3, | ||||||||
| increased state 4 | ||||||||
| Cipaite et al. [16] | Wistar | M | 14 | 2.5 and 25 | 45.7 | 9.2 | Lard | Pyruvate+malate: |
| Decreased states 3 and uncoupled, | ||||||||
| ns state 4 RCR decreased by diet and age. | ||||||||
| Palmitoyl-carnitine+malate: | ||||||||
| Increased states 3 and uncoupled; | ||||||||
| ns state 4 –the three parameters were decreased by age | ||||||||
| ns RCR | ||||||||
| Aoun et al. [4] | Wistar | M | 6 | 12 | 51.8 & | 11.4 & | Fish or Lard | Malate+glutamate+succinate: |
| ns states 3, 4 and uncoupled. | ||||||||
| Increased RCR in three HFDs groups | ||||||||
| Flamment et al. [24] | Sprague Dawley | M | 10 | 2, 4 or 8 w | 8.75 & | Lard | Pyruvate+malate | |
| ns states 3 and 4 | ||||||||
| ns RCR | ||||||||
| Succinate+rotenone | ||||||||
| ns States 3 and 4 | ||||||||
| ns RCR | ||||||||
| Palmitoyl-carnitine+malate | ||||||||
| Decreased at 8w | ||||||||
| RCR decreased at 8w | ||||||||
| Herlein et al. [33] | Sprague Dawley | M | 7,5 | 16 | 60 | 13 | Lard | Succinate: |
| ns State 4 | ||||||||
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Lard | Pyruvate+malate: |
| ns States 3 and 4 | ||||||||
| ns RCR | ||||||||
| Succinate+rotenone: | ||||||||
| Decreased states 3, 4 and uncoupled | ||||||||
| ns RCR. | ||||||||
| Palmitoyl-CoA+malate: | ||||||||
| Increased states 3 and 4. | ||||||||
| ns RCR. | ||||||||
| Decreased proton leak under palmitate. | ||||||||
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Fish | Pyruvate+malate: |
| ns States 3 and 4 | ||||||||
| ns RCR | ||||||||
| Succinate+rotenone: | ||||||||
| ns states 3, 4 and uncoupled. | ||||||||
| ns RCR. | ||||||||
| Palmitoyl-CoA+malate: | ||||||||
| Increased states 3 (higher than in lard) and 4. | ||||||||
| ns RCR. | ||||||||
| Increased proton leak under palmitate. | ||||||||
| Mice | ||||||||
| Mantena et al. [48] | C57BL/6J | M | 8 | 8 and 16 | 71 | 35 | Corn oil | Glutamate+malate: |
| Decreased states 3 and RCR at 16w. | ||||||||
| ns state 4 | ||||||||
| Succinate+rotenone: | ||||||||
| Decreased states 3 and uncoupled. | ||||||||
| ns state 4 | ||||||||
| Decreased RCR at 8 and 16w | ||||||||
| Cardoso et al. [13] | Swiss | F | 6 | 8 and 40 | – | 14.3 & | Soybean oil | Succinate: |
| ns state 3, 4 and RCR. | ||||||||
| Poussin et al. [56] | A/J | M | 5 | 2, 10 or 30 days | 58 | 12.70 & | Coconut oil | Succinate+rotenone: |
| Increased states 2, 3 and 4. | ||||||||
| Decreased RCR. | ||||||||
| Palmitoyl-carnitine: | ||||||||
| Decreased states 2 and 3. | ||||||||
| Decreased RCR. | ||||||||
| Poussin et al. [56] | C57BL/6 J | M | 5 | 2, 10 or 30 days | 58 | 12.70 & | Coconut oil | Succinate+Rotenone: |
| Decreased state 3. | ||||||||
| Palmitoyl-carnitine: | ||||||||
| Decreased state 2. | ||||||||
| Satapati et al. [63] | C57BL/6NCrl | M | 5 | 8, 16 or 32 | 60 | 10 | Lard | Glutamate+malate: |
| Increased states 2 and 4 at 32w. | ||||||||
| ns state 3 and RCR. | ||||||||
| Succinate+rotenone: | ||||||||
| Increased state 4 at 32w. | ||||||||
| ns state 2, 3 and RCR. | ||||||||
| Palmitoyl-carnitine: | ||||||||
| Increased state 2, 3 and 4 at 32w. | ||||||||
| Decreased RCR. | ||||||||
| Cardoso et al. [14] | Swiss | F | 6 | 1 | – | 14.3 & | Soybean oil | Succinate: |
| ns states 2, 3, 4, uncoupled and RCR. | ||||||||
| Palmitoyl-carnitine: | ||||||||
| ns states 2, 4 and RCR. | ||||||||
| Increased states 3 and uncoupled. | ||||||||
| Franko et al. [27] | C57BL/6N | M | 4 | 20 | 60 & | 13 & | Lard | Pyruvate+malate: |
| ns states 3 and 4. | ||||||||
| Malate+glutamate | ||||||||
| ns state 3 | ||||||||
| Succinate: | ||||||||
| ns state 3 | ||||||||
| Glycerol-3-phosphate: | ||||||||
| Increased state 3. | ||||||||
| Decreased proton leak under succinate. | ||||||||
&=calculated energy from fat from the information provided by the authors. 1 g fat=9 kcal=37 kJ [22].
ns=authors did not find statistically significant differences.
In agreement with the observed increase in respiration seen by some authors in samples energized with palmitoyl-carnitine, β-oxidation activities of long chain fatty acids were found to be increased after a 1 week HFD [14]. This increased activity was accompanied by increased expression of β-oxidation enzymes. Increased palmitate oxidation was also observed by Satapati et al. [63] after longer HFD treatment. Indeed, increased carnitine palmitate transferase 1a (CPT1a) mRNA, protein expression and/or activity was seen in other studies [15], [47], [52], [72]. Flamment et al. [24] suggest that an inversion of fatty acid metabolism adaptation occurs because they see an increased content of seric β-hydroxybutyrate, a β-oxidation biomarker, at 4 weeks on a HFD. This is abrogated at 8 weeks following a decrease in state 3 fatty acid-related respiration. They do not find any changes in CPT1a mRNA expression at any time point. Aoun et al. [4] saw a decrease in β-hydroxyacyl-CoA dehydrogenase (the last step in mitochondrial β-oxidation) in three HFD (basal, lard and fish). Vial et al. [72] hypothesized that lower fatty acid oxidation (despite higher capacity) could be related to inhibited oxidative phosphorylation at complex IV and a lower cellular redox state, with increased oxidant production. It should be noted that most of these contradictory results were obtained in HFD containing lard as the main source of fat (Table 3).
Lionetti et al. [47] compared mitochondrial function and dynamics in Wistar rats fed with a lard or fish-based diet (40% energy from fat, fish fat from cod liver) for six weeks. That evaluation was similar to the one published by Aoun et al. [4] using a different experimental design (12 weeks treatment, calculated 51.8% energy from fat; lard or fish fat from tuna and sardine oils). They observed that fat quantity, not quality, had a more prominent increased effect on mitochondrial membrane fluidity and membrane potentials, but not oxygen consumption. β-oxidation, however, was reduced in all three diets, although the effect was more pronounced in fish and basal (lard+mixed vegetable oils) diets than when using lard. Additionally, Aoun's fish HFD mice did not show changes in body weight, plasma glucose or insulin, despite steatotic livers [3]. Lionetti et al. [47], in contrast, observed a higher state 3 (palmitoyl-CoA energized) oxygen consumption on lard, but even higher on fish-based diets. Probably as a reflex of diet composition, a decreased proton leak was seen in the lard group, while it was increased in fish. In respect to the diet, both Aoun and Lionetti control diets were low fat purified and, thus, precise in all ingredients, avoiding chow vs. high fat misconceptions [32], [43]. The main probable differences are the fish species and the lard content in the “fish” Aoun diet.
Interestingly, Lionetti et al. [47] also observed that the lard group had shorter mitochondria, predominantly tubular, while the fish group mitochondria were more elongated, in equivalent proportions of tubular and round shapes. This suggests dominant fission and fusion processes, respectively. Adaptations in mitochondrial shape, morphology and dynamics have been discussed as sensors for the control of energy demand and supply; more fragmented mitochondria have been associated with decreased function (reviewed by [46]). Other papers discussed mitochondrial dynamics briefly; Guo et al. [31] also observed more fragmented mitochondria in HFD groups after 30 weeks treatment (60% energy from fat, containing mainly lard). However, Raffaella et al. [57] found increased mitochondrial volume density and area, despite the same number of organelles after 7 weeks (50% energy from fat, containing mainly butter).
Gallou-Kabani et al. [28] fed HFDs to a large cohort of A/J (obesity resistant) and C57BL/6J (obesity prone) mice for 20 weeks (60% energy from fat, mainly lard) and did not find differences in final body weights. All mice were glucose intolerant and insulin resistant, however, these findings were more pronounced in A/J males. The authors suggest both C57BL/6J and A/J are good models to evaluate disparities in the time course of glucose homeostasis impairment. Furthermore, Poussin et al. [56] compared the liver adaptive process of A/J mice versus C57BL/6J under HFD. They observed that after 10 days feeding with HFD containing 58% energy from fat, mainly coconut oil, liver mitochondria energized by succinate or palmitoyl-carnitine had increased oxygen consumption (higher state 3 and 4) compared to normal chow in A/J and C57BL/6 mice, although the ATP production rate was the same. A/J mice also had a transient upregulation of mitochondrial complexes at the second and tenth days. The authors hypothesized that these differences could be related to increased peroxisomal β-oxidation and uncoupled mitochondria. This would be one of the metabolic adaptation tools to protect A/J mice from developing obesity and related metabolic diseases. Despite this, 30 days on HFD did not caused glucose or insulin changes in A/J or C57BL6/J mice, although only obesity-prone mice had increased body weight (Table 4, Table 5).
Chan et al. [15] showed that during the first 4 weeks of HFD feeding (40% energy from fat, mainly butter), genes regulating lipogenesis and cholesterol metabolism are downregulated, while the ones related to fatty acid oxidation were upregulated. After 10 weeks, however, the trend was the opposite. The authors suggest that this reflects an attempt to maintain energy homeostasis, which failed after 4 weeks. In the same adaptation hypothesis line, Franko et al. [27] did not observe distinctive mitochondrial differences in HFD (60% calculated energy from fat, mainly lard) groups after 20 weeks (unchanged protein and mRNA levels of respiratory complexes, no changes in oxidative phosphorylation), despite the development of steatosis and blunted glucose homeostasis [26]. They suggest that steatosis and insulin resistance do not cause mitochondrial dysfunction in liver; instead, they elicit an adaptive response with increased ETC performance and coupling in order to handle the nutrient overload and insulin inability to suppress gluconeogenesis.
5. Oxidative imbalance
It is widely accepted that mitochondrial oxidative imbalance contributes toward the development and progression of NAFLD and related metabolic diseases ([61], [6]). Indeed, most HFD models lead to increased mitochondrial hydrogen peroxide production (Table 6), higher incidence of oxidative damage markers (for example increased lipid oxidation, carbonylated proteins, 3-nitrotyrosine levels, and reduced aconitase activity), and decreased antioxidant defenses (Table 7), suggesting oxidative imbalance occurs in HFD animals. Cardoso et al. [14] described a diet-sensitive source of hydrogen peroxide production related to long chain fatty acid metabolism: the first dehydrogenation of pamitoyl-CoA catalyzed by very long chain acyl CoA dehydrogenase (VLCAD) was identified as an important electron leak step in mitochondrial β-oxidation. In addition, our in vitro assays uncovered a residual oxidase activity for VLCAD, generating H2O2 [40].
Table 6.
Mitochondrial H2O2 generation.
| Authors | Strain | Sex | Age (w) | Duration (w) | HFD (% energy from fat) | Control (% energy from fat) | Main fat source | H2O2generation (vs. indicated control group) |
|---|---|---|---|---|---|---|---|---|
| Rats | ||||||||
| Nadal-Casellas et al. [51] | Wistar | Both | 10 | 26 | 54.5 | 8 | Cafeteria diet | Increased with succinate+Antimycin A |
| Vial et al. [72] | Wistar | M | 16 | 8 | – | – | Lard | Increased with palmitoyl-CoA and succinate+rotenone |
| ns malate+glutamate | ||||||||
| Herlein et al. [33] | Sprague Dawley | M | 7.5 | 16 | 60 | 13 | Lard | ns with: succinate, succinate+rotenone, glutamate+malate; glutamate+ malate+rotenone |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Lard | Increased with succinate+rotenone |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Fish | ns with succinate+rotenone |
| Mice | ||||||||
| Cardoso et al. [13] | Swiss | F | 6 | 8 | – | 14.3 & | Soybean oil | Increased with palmitoyl-carnitine, succinate, and malate+glutamate |
| Cardoso et al. [13] | Swiss | F | 6 | 40 | – | 14.3 & | Soybean oil | Increased with succinate |
| Eccleston et al. [19] | C57BL/6J | M | 8 | 8 | 71 | 35 | Corn oil | Increased with succinate |
| Eccleston et al. [19] | C57BL/6J | M | 8 | 16 | 71 | 35 | Corn oil | Decreased with succinate |
| Cardoso et a. [14] | Swiss | F | 6 | 1 | – | – | Soybean oil | Increased with palmitoyl-CoA; ns with succinate, malate+glutamate; α-ketoglutarate |
&=calculated energy from fat from the information provided by the authors. 1 g fat=9 kcal=37 kJ [22].
ns=authors did not find statistically significant differences.
Table 7.
Redox evaluation.
| Authors | Strain | Sex | Age (w) | Duration (w) | HFD (% energy from fat) | Control (% energy from fat) | Main fat source | Findings (vs. indicated control group) |
|---|---|---|---|---|---|---|---|---|
| Rats | ||||||||
| Rafaella et al. [57] | Wistar | M | 12 | 7 | 50 | 10.6 | Butter | ns SOD activity; |
| Decreased aconitase activity; | ||||||||
| Increased TBARS | ||||||||
| Nadal-Casellas et al. [51] | Wistar | Both | 10 | 26 | 54.5 | 8 | Cafeteria diet | Decreased GPx, MnSOD expression, and GSH content; |
| ns TBARS and Carbonylated protein | ||||||||
| Cipaite et al. [16] | Wistar | M | 14 | 2.5 | 45.7 | 9.2 | Lard | ns TBARS and Carbonylated protein |
| Cipaite et al. [16] | Wistar | M | 14 | 25 | 45.7 | 9.2 | Lard | Increased TBARS and Carbonylated protein |
| Herlein et al. [33] | Sprague Dawley | M | 7,5 | 16 | 60 | 13 | Lard | ns GPx and MnSOD expression |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Lard | Decreased aconitase activity; Increased 3-NT |
| Lionetti et al. [47] | Wistar | M | 8 | 6 | 40 | 10.6 | Fish | ns aconitase activity and 3-NT |
| Mice | ||||||||
| Mantena et al. [48] | C57BL/6J | M | 8 | 16 | 71 | 35 | Corn oil | Increased 3-NT |
| Satapati et al. [63] | C57BL/6NCrl | M | 5 | 8 | 60 | 10 | Lard | ns SOD activity and TBARS |
| Satapati et al. [63] | C57BL/6NCrl | M | 5 | 16 | 60 | 10 | Lard | ns SOD activity and TBARS |
| Satapati et al. [63] | C57BL/6NCrl | M | 5 | 32 | 60 | 10 | Lard | Increased SOD activity and TBARS |
| Cardoso et al. [14] | Swiss | F | 6 | 1 | – | 14.3 & | Soybean oil | Increased carbonylated protein |
| Yuzefovych et al. [75] | C57BL/6J | M | 6 | 16 | 60 | 10 | Lard | Decreased MnSOD content, GSH/GSSG ratio and SOD expression; Increased carbonylated protein |
| Franko et al. [27] | C57BL/6N | M | 4 | 20 | 60 & | 13 & | Lard | ns MnSOD content |
| ns Aconitase activity | ||||||||
| Enos et al. [21] | C57BL/6 | M | 4 | 16 | 40 | 12.5 | Lard, and coconut, corn, soybean and olive oils | Increased 4-HNE content in 12% SFA group |
&=calculated energy from fat from the information provided by the authors. 1 g fat=9 kcal=37 kJ [22].
ns=authors did not find statistically significant differences.
6. Conclusions
A broad look at publications studying bioenergetic and redox effects of HFD clearly shows that immense variability exists regarding how these dietary interventions are designed. Despite this variability, some findings are highly reproducible:
-
•
HFDs induce steatosis, even when no changes in insulin signaling or weight are found.
-
•
HFDs activate β-oxidation, as measured both by expression levels and activity of enzymes in this pathway.
-
•
HFDs promote oxidative imbalance, as evidenced both by the measurement of H2O2 production and changes in biomarkers.
On the other hand, many other comparisons are confounded by the variability in dietary interventions. In this sense, we would like to suggest points to be considered when designing HFD studies in order to increase future reproducibility in this area:
-
•
Avoid the use of unknown diets for the control groups. Pair the composition of the HFD and control diet so that the significant change in the diet is the fat content itself.
-
•
Specify the full dietary content for both control and HFD groups in publications. Specify the source and type of fat added.
-
•
Be aware of the fact that “control” ad libitum and sedentary animal group may present significant metabolic disorders and may not be a good model for a healthy human [49].
-
•
Care must be taken in the literature regarding different treatment times, since short and long term treatment definitions often vary significantly among groups. We propose that short term treatments comprise those of a week or less on the HFD. Treatment time and age when the diet was introduced should be specified in the text.
-
•
Choose the rodent species, strain (and in some cases, substrain), sex and initial age carefully based on prior studies.
Acknowledgments
This work was supported by Grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, 2010/51906-1) Centro de Pesquisa, Inovacão e Difusão de Processos Redox em Biomedicina (13/07937-8), Núcleo de Pesquisa em Processos Redox em Biomedicina (NAP-Redoxoma), Instituto Nacional de Ciência e Tecnologia de Processos Redox em Biomedicina (INCT Redoxoma) and Conselho Nacional de Pesquisa e Desenvolvimento (CNPq, 153560/2011-8, 302898/2013-1). P.A.K. is a recipient of a FAPESP fellowship (2013/03556-0).
References
- 1.Akin H., Deniz M., Tahan V., Can G., Kedrah A.E. High-fat liquid “Lieber-DeCarli” diet for an animal model of nonalcoholic steatohepatitis: does it really work? Hepatol. Int. 2007;1:449–450. doi: 10.1007/s12072-007-9028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.M.R. Anver, D.C. Haines, Chapter 21-Gerontology, in: The Laboratory Mouse, ed. PHJHG Bullock:327-43, Academic Press, London, 2004, pp. 327–343.
- 3.Aoun M., Feillet-Coudray C., Fouret G., Chabi B., Crouzier D. Rat liver mitochondrial membrane characteristics and mitochondrial functions are more profoundly altered by dietary lipid quantity than by dietary lipid quality: effect of different nutritional lipid patterns. Br. J. Nutr. 2012;107:647–659. doi: 10.1017/S000711451100331X. [DOI] [PubMed] [Google Scholar]
- 4.Aoun M., Fouret G., Michel F., Bonafos B., Ramos J. Dietary fatty acids modulate liver mitochondrial cardiolipin content and its fatty acid composition in rats with non alcoholic fatty liver disease. J. Bioenerg. Biomembr. 2012;44:439–452. doi: 10.1007/s10863-012-9448-x. [DOI] [PubMed] [Google Scholar]
- 5.Barnard D.E., Lewis S.M., Teter B.B., Thigpen J.E. Open- and closed-formula laboratory animal diets and their importance to research. J. Am. Assoc. Lab. Anim. Sci. 2009;48:709–713. [PMC free article] [PubMed] [Google Scholar]
- 6.Begriche K., Igoudjil A., Pessayre D., Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Begriche K., Massart J., Robin M.A., Bonnet F., Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 8.Bockisch M., Knovel . Fats and Oils Handbook. AOCS Press; Champaign, Ill: 1998. [Google Scholar]
- 9.Brand M.D., Nicholls D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buettner R., Parhofer K.G., Woenckhaus M., Wrede C.E., Kunz-Schughart L.A. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J. Mol. Endocrinol. 2006;36:485–501. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- 11.Buettner R., Scholmerich J., Bollheimer L.C. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity. 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 12.Byrne C.D. Fatty liver: role of inflammation and fatty acid nutrition. Prostaglandins Leukot. Essent. Fat. Acids. 2010;82:265–271. doi: 10.1016/j.plefa.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 13.Cardoso A.R., Cabral-Costa J.V., Kowaltowski A.J. Effects of a high fat diet on liver mitochondria: increased ATP-sensitive K+ channel activity and reactive oxygen species generation. J. Bioenerg. Biomembr. 2010;42(3):245–253. doi: 10.1007/s10863-010-9284-9. [DOI] [PubMed] [Google Scholar]
- 14.Cardoso A.R., Kakimoto P.A., Kowaltowski A.J. Diet-sensitive sources of reactive oxygen species in liver mitochondria: role of very long chain acyl-CoA dehydrogenases. PLoS One. 2013;8:e77088. doi: 10.1371/journal.pone.0077088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan M.Y., Zhao Y., Heng C.K. Sequential responses to high-fat and high-calorie feeding in an obese mouse model. Obesity. 2008;16:972–978. doi: 10.1038/oby.2008.32. [DOI] [PubMed] [Google Scholar]
- 16.Ciapaite J., van den Broek N.M., Te Brinke H., Nicolay K., Jeneson J.A., Houten S.M., Prompers J.J. Differential effects of short- and long-term high-fat diet feeding on hepatic fatty acid metabolism in rats. Biochim. Biophys. Acta. 2011;1811(7–8):441–451. doi: 10.1016/j.bbalip.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Ciapaite J., Bakker S.J., Van Eikenhorst G., Wagner M.J., Teerlink T. Functioning of oxidative phosphorylation in liver mitochondria of high-fat diet fed rats. Biochim. Biophys. Acta. 2007;1772:307–316. doi: 10.1016/j.bbadis.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 19.Eccleston H.B., Andringa K.K., Betancourt A.M., King A.L., Mantena S.K. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid. Redox Signal. 2011;15:447–459. doi: 10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enos R.T., Davis J.M., Velazquez K.T., McClellan J.L., Day S.D. Influence of dietary saturated fat content on adiposity, macrophage behavior, inflammation, and metabolism: composition matters. J. Lipid Res. 2013;54:152–163. doi: 10.1194/jlr.M030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Enos R.T., Velazquez K.T., Murphy E.A. Insight into the impact of dietary saturated fat on tissue-specific cellular processes underlying obesity-related diseases. J. Nutr. Biochem. 2014;25:600–612. doi: 10.1016/j.jnutbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Food and Agriculture Organization of the United Nations, Food energy – methods of analysis and conversion factors. FAO food and nutrition paper 77, Report of a Technical Workshop, Rome, 3–6 December 2002, FAO, Rome, 2003.
- 23.Figueira T.R. A word of caution concerning the use of Nnt-mutated C57BL/6 mice substrains as experimental models to study metabolism and mitochondrial pathophysiology. Exp. Physiol. 2013;98:1643. doi: 10.1113/expphysiol.2013.074682. [DOI] [PubMed] [Google Scholar]
- 24.Flamment M., Rieusset J., Vidal H., Simard G., Malthiery Y. Regulation of hepatic mitochondrial metabolism in response to a high fat diet: a longitudinal study in rats. J. Physiol. Biochem. 2012;68:335–344. doi: 10.1007/s13105-012-0145-3. [DOI] [PubMed] [Google Scholar]
- 25.Fontana L., Zhao E., Amir M., Dong H., Tanaka K., Czaja M.J. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57:995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franko A., von Kleist-Retzow J.C., Bose M., Sanchez-Lasheras C., Brodesser S. Complete failure of insulin-transmitted signaling, but not obesity-induced insulin resistance, impairs respiratory chain function in muscle. J. Mol. Med. 2012;90:1145–1160. doi: 10.1007/s00109-012-0887-y. [DOI] [PubMed] [Google Scholar]
- 27.Franko A., von Kleist-Retzow J.C., Neschen S., Wu M., Schommers P. Liver adapts mitochondrial function to insulin resistant and diabetic states in mice. J. Hepatol. 2014;60:816–823. doi: 10.1016/j.jhep.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 28.Gallou-Kabani C., Vige A., Gross M.S., Rabes J.P., Boileau C. C57BL/6J and A/J mice fed a high-fat diet delineate components of metabolic syndrome. Obesity. 2007;15:1996–2005. doi: 10.1038/oby.2007.238. [DOI] [PubMed] [Google Scholar]
- 29.Galloway C.A., Lee H., Brookes P.S., Yoon Y. Decreasing mitochondrial fission alleviates hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307:G632–G641. doi: 10.1152/ajpgi.00182.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Periz A., Horrillo R., Ferre N., Gronert K., Dong B. Obesity-induced insulin resistance and hepatic steatosis are alleviated by omega-3 fatty acids: a role for resolvins and protectins. FASEB J. 2009;23:1946–1957. doi: 10.1096/fj.08-125674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo Y., Darshi M., Ma Y., Perkins G.A., Shen Z. Quantitative proteomic and functional analysis of liver mitochondria from high fat diet (HFD) diabetic mice. Mol. Cell. Proteom. 2013;12:3744–3758. doi: 10.1074/mcp.M113.027441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hariri N., Thibault L. High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 33.Herlein J.A., Fink B.D., Sivitz W.I. Superoxide production by mitochondria of insulin-sensitive tissues: mechanistic differences and effect of early diabetes. Metabolism. 2010;59:247–257. doi: 10.1016/j.metabol.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hill B.G., Benavides G.A., Lancaster J.R., Jr., Ballinger S., Dell’Italia L. Integration of cellular bioenergetics with mitochondrial quality control and autophagy. Biol. Chem. 2012;393:1485–1512. doi: 10.1515/hsz-2012-0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ho J.H., Lee O.K., Fu Y.J., Shih H.T., Tseng C.Y. An iTRAQ proteomic study reveals an association between diet-induced enhanced fatty acid metabolism and the development of glucose intolerance in prediabetic mice. J. Proteome Res. 2013;12:1120–1133. doi: 10.1021/pr300662j. [DOI] [PubMed] [Google Scholar]
- 36.Hulbert A.J., Turner N., Storlien L.H., Else P.L. Dietary fats and membrane function: implications for metabolism and disease. Biol. Rev. Camb. Philos. Soc. 2005;80:155–169. doi: 10.1017/s1464793104006578. [DOI] [PubMed] [Google Scholar]
- 37.James A.M., Collins Y., Logan A., Murphy M.P. Mitochondrial oxidative stress and the metabolic syndrome. Trends Endocrinol. Metab. 2012;23:429–434. doi: 10.1016/j.tem.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Kahle M., Horsch M., Fridrich B., Seelig A., Schultheiss J. Phenotypic comparison of common mouse strains developing high-fat diet-induced hepatosteatosis. Mol. Metab. 2013;2:435–446. doi: 10.1016/j.molmet.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kahle M., Schafer A., Seelig A., Schultheiss J., Wu M. High fat diet-induced modifications in membrane lipid and mitochondrial-membrane protein signatures precede the development of hepatic insulin resistance in mice. Mol. Metab. 2015;4:39–50. doi: 10.1016/j.molmet.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kakimoto P.A., Tamaki F.K., Cardoso A.R., Marana S.R., Kowaltowski A.J. H2O2 release from the very long chain acyl-CoA dehydrogenase. Redox Biol. 2015;4C:375–380. doi: 10.1016/j.redox.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kien C.L., Bunn J.Y., Ugrasbul F. Increasing dietary palmitic acid decreases fat oxidation and daily energy expenditure. Am. J. Clin. Nutr. 2005;82:320–326. doi: 10.1093/ajcn.82.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kunau W.H., Dommes V., Schulz H. beta-oxidation of fatty acids in mitochondria, peroxisomes, and bacteria: a century of continued progress. Prog. Lipid Res. 1995;34:267–342. doi: 10.1016/0163-7827(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 43.Lai M., Chandrasekera P.C., Barnard N.D. You are what you eat, or are you? The challenges of translating high-fat-fed rodents to human obesity and diabetes. Nutr. Diabetes. 2014;4:e135. doi: 10.1038/nutd.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee J.S., Pinnamaneni S.K., Eo S.J., Cho I.H., Pyo J.H. Saturated, but not n-6 polyunsaturated, fatty acids induce insulin resistance: role of intramuscular accumulation of lipid metabolites. J. Appl. Physiol. 2006;100:1467–1474. doi: 10.1152/japplphysiol.01438.2005. [DOI] [PubMed] [Google Scholar]
- 45.Lieber C.S., Leo M.A., Mak K.M., Xu Y., Cao Q. Model of nonalcoholic steatohepatitis. Am. J. Clin. Nutr. 2004;79:502–509. doi: 10.1093/ajcn/79.3.502. [DOI] [PubMed] [Google Scholar]
- 46.Liesa M., Shirihai O.S. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell. Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lionetti L., Mollica M.P., Donizzetti I., Gifuni G., Sica R. High-lard and high-fish-oil diets differ in their effects on function and dynamic behaviour of rat hepatic mitochondria. PLoS One. 2014;9:e92753. doi: 10.1371/journal.pone.0092753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mantena S.K., Vaughn D.P., Andringa K.K., Eccleston H.B., King A.L. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem. J. 2009;417:183–193. doi: 10.1042/BJ20080868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martin B., Ji S., Maudsley S., Mattson M.P. “Control” laboratory rodents are metabolically morbid: why it matters. Proc. Natl. Acad. Sci. USA. 2010;107:6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marsman H.A., Al-Saady R.L., Heger M., van Gulik T.M. How reproducible are rat steatosis models using high-fat diets? J. Hepatol. 2009;51:822–823. doi: 10.1016/j.jhep.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 51.Nadal-Casellas A., Amengual-Cladera E., Proenza A.M., Llado I., Gianotti M. Long-term high-fat-diet feeding impairs mitochondrial biogenesis in liver of male and female rats. Cell. Physiol. Biochem. 2010;26:291–302. doi: 10.1159/000320552. [DOI] [PubMed] [Google Scholar]
- 52.Nesteruk M., Hennig E.E., Mikula M., Karczmarski J., Dzwonek A. Mitochondrial-related proteomic changes during obesity and fasting in mice are greater in the liver than skeletal muscles. Funct. Integr. Genom. 2014;14:245–259. doi: 10.1007/s10142-013-0342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Neill S., O’Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 2015;16:1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 54.Pan D.A., Storlien L.H. Dietary lipid profile is a determinant of tissue phospholipid fatty acid composition and rate of weight gain in rats. J. Nutr. 1993;123:512–519. doi: 10.1093/jn/123.3.512. [DOI] [PubMed] [Google Scholar]
- 55.Panchal S.K., Brown L. Rodent models for metabolic syndrome research. J. Biomed. Biotechnol. 2011;2011:351982. doi: 10.1155/2011/351982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poussin C., Ibberson M., Hall D., Ding J., Soto J. Oxidative phosphorylation flexibility in the liver of mice resistant to high-fat diet-induced hepatic steatosis. Diabetes. 2011;60:2216–2224. doi: 10.2337/db11-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raffaella C., Francesca B., Italia F., Marina P., Giovanna L., Susanna I. Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity. 2008;16:958–964. doi: 10.1038/oby.2008.10. [DOI] [PubMed] [Google Scholar]
- 58.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 59.Reuter T.Y. Diet-induced models for obesity and type 2 diabetes. Drug. Discov. Today: Dis. Model. 2007;4:3–8. [Google Scholar]
- 60.M. Ritskes-Hoitinga, Chapter 28-Nutrition of Laboratory Mice, in: The Laboratory Mouse, ed. PHJHG Bullock:463-79, Academic Press, London, 2004, pp. 463–79.
- 61.Rolo A.P., Teodoro J.S., Palmeira C.M. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic. Biol. Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Ronchi J.A., Figueira T.R., Ravagnani F.G., Oliveira H.C., Vercesi A.E., Castilho R.F. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic. Biol. Med. 2013;63:446–456. doi: 10.1016/j.freeradbiomed.2013.05.049. [DOI] [PubMed] [Google Scholar]
- 63.Satapati S., Sunny N.E., Kucejova B., Fu X., He T.T. Elevated TCA cycle function in the pathology of diet-induced hepatic insulin resistance and fatty liver. J. Lipid Res. 2012;53:1080–1092. doi: 10.1194/jlr.M023382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schaffer J.E. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Scorletti E., Byrne C.D. Omega-3 fatty acids, hepatic lipid metabolism, and nonalcoholic fatty liver disease. Annu. Rev. Nutr. 2013;33:231–248. doi: 10.1146/annurev-nutr-071812-161230. [DOI] [PubMed] [Google Scholar]
- 66.Serviddio G., Sastre J., Bellanti F., Vina J., Vendemiale G., Altomare E. Mitochondrial involvement in non-alcoholic steatohepatitis. Mol. Asp. Med. 2008;29:22–35. doi: 10.1016/j.mam.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 67.Tahara E.B., Navarete F.D., Kowaltowski A.J. Tissue-, substrate-, and site-specific characteristics of mitochondrial reactive oxygen species generation. Free Radic. Biol. Med. 2009;46:1283–1297. doi: 10.1016/j.freeradbiomed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 68.Takeuchi H., Matsuo T., Tokuyama K., Shimomura Y., Suzuki M. Diet-induced thermogenesis is lower in rats fed a lard diet than in those fed a high oleic acid safflower oil diet, a safflower oil diet or a linseed oil diet. J. Nutr. 1995;125:920–925. doi: 10.1093/jn/125.4.920. [DOI] [PubMed] [Google Scholar]
- 69.Than N.N., Newsome P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Tiniakos D.G., Vos M.B., Brunt E.M. Nonalcoholic Fatty Liver Disease: Pathology and Pathogenesis. Annu. Rev. Pathol.: Mech. Dis. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 71.Varga O., Harangi M., Olsson I.A., Hansen A.K. Contribution of animal models to the understanding of the metabolic syndrome: a systematic overview. Obes. Rev. 2010;11:792–807. doi: 10.1111/j.1467-789X.2009.00667.x. [DOI] [PubMed] [Google Scholar]
- 72.Vial G., Dubouchaud H., Couturier K., Cottet-Rousselle C., Taleux N. Effects of a high-fat diet on energy metabolism and ROS production in rat liver. J. Hepatol. 2011;54:348–356. doi: 10.1016/j.jhep.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 73.Watmough N.J., Frerman F.E. The electron transfer flavoprotein: ubiquinone oxidoreductases. Biochim. Biophys. Acta. 2010;1797:1910–1916. doi: 10.1016/j.bbabio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 74.White P.J., Marette A. Potential role of omega-3-derived resolution mediators in metabolic inflammation. Immunol. Cell Biol. 2014;92:324–330. doi: 10.1038/icb.2013.112. [DOI] [PubMed] [Google Scholar]
- 75.Yuzefovych L.V., Musiyenko S.I., Wilson G.L., Rachek L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS One. 2013;8:e54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]