Abstract
Background
Preterm (PT) children show early cognitive and language deficits and display altered cortical connectivity for language compared to term (T) children. Developmentally, functional connectivity networks become more segregated and integrated, through the weakening of short-range and strengthening of long-range connections.
Methods
Longitudinal intrinsic connectivity distribution (ICD) values were assessed in PT (n = 13) compared to T children (n = 12) at ages 8 vs. 16 using a Linear Mixed Effects model. Connectivity values in regions generated by the group × age interaction analysis were then correlated to scores on full IQ (FSIQ), verbal IQ (VIQ), verbal comprehension IQ (VCIQ), performance IQ (PIQ), Peabody picture vocabulary test—revised (PPVTR), and Rapid Naming Composite (RDRL_Cmp).
Results
Nine regions were generated by the group × age interaction analysis. PT connectivity significantly increased over time in all but two regions, and they ultimately displayed greater relative connectivity at age 16 than Ts in all areas except the left occipito-temporal cortex (OTC). PTs underwent significant connectivity reductions in the left OTC, which corresponded with worse performance on FSIQ, VIQ, and PIQ. These findings differed from Ts, who did not undergo any significant changes in connectivity over time.
Conclusions
These findings suggest that the developmental alterations in connectivity in PT children at adolescence are both pervasive and widespread. The persistent and worsening cognitive and language deficits noted in the PT subjects may be attributed to the loss of connections in the left OTC.
Abbreviations: BA, Brodmann area; FSIQ, full scale IQ; OTC, occipito-temporal cortex; PIQ, performance IQ; PPVT, Peabody picture vocabulary test; PT, preterm; RDRL_Cmp, Rapid Naming Composite; ROI, region of interest; RSC, resting state connectivity; RSN, resting state network; T, term; VCIQ, verbal comprehension IQ; VIQ, verbal IQ; VWFA, visual word form area
Keywords: Intrinsic connectivity distribution, Functional connectivity, Resting state, Language, Preterm, Development
Highlights
-
•
From ages 8 to 16, PTs tend to undergo significant expansion of relative functional connectivity over time, while Ts do not.
-
•
PTs show a paradoxical decrease in left OTC connectivity, while T connectivity is essentially unchanged.
-
•
Left OTC connectivity is positively correlated with VIQ, PIQ, and FSIQ.
-
•
Altered PT connections may be underscoring cognitive and language impairment.
1. Introduction
1.1. Cognitive impairment in PTs
Preterm (PT) birth is a major global health burden, with up to 11% of all live born infants worldwide being born at less than 37 weeks gestation (Blencowe et al., 2012, Beck et al., 2010), and as many as one-third of prematurely born infants suffering from significant cognitive impairments during early childhood (Blencowe et al., 2013, Neubauer et al., 2013, Robertson et al., 2009, Saigal and Doyle, 2008). While Saigal et al. (2006) demonstrated that, by adulthood, PTs are comparable to their term (T) peers in educational attainment and functional independence, several studies have shown that PTs persistently display global impairment in cognition, language, and motor function (Botting et al., 1998, Bowen et al., 2002, Hack, 2009).
Even in early childhood, language deficits are evident. At age 2.5, PT had lower scores on tasks of cognition, receptive and expressive communication, with over 10% of PT children showing moderate–severe delay in these areas (Mansson and Stjernqvist, 2014). Similarly, at age 6, PTs had significantly poorer reading, vocabulary and comprehension than Ts (Pritchard et al., 2009).
Promisingly, there is some evidence to suggest that these early language deficits in PTs may improve with age. Luu et al. (2009) found that from ages 3 to 12, PTs had poorer receptive vocabulary compared to controls, but they improved over time and nearly approached the normative values by age 12. These “catch-up gains” in receptive vocabulary were also seen at age 16, although they continued to show impairment in phonology (Luu et al., 2011).
1.2. Altered neural structures in PTs
PT birth substantially alters neurodevelopment, with the brains of PT, school-age children being 5–6% smaller than those of matched T controls (Nosarti et al., 2002, Peterson et al., 2000). During childhood and adolescence, PT brains fail to undergo similar white matter expansion and gray matter pruning in temporal and frontal lobes (Ment et al., 2009), resulting in significant decreases in left frontal and bilateral temporal white matter volumes (Schafer et al., 2009). At young adulthood, PTs display alterations in both regional volume and microstructural connectivity in language areas, including the left frontal language regions, temporal and parietal cortices, and both cerebellar hemispheres (Boardman et al., 2010, Haldipur et al., 2011, Lind et al., 2011, Nosarti et al., 2011, Tam et al., 2009).
Several studies have noted that these structural alterations mediate cognitive deficits. Nosarti et al. (2008) observed that PTs had diffuse decreases in regional volumes compared to Ts, which correlated with greater cognitive impairment. Parker et al. (2008) also showed that PTs had reduced cerebellar volumes compared to Ts, and although initial cognitive measures showed a positive correlation with cerebellar volume, this did not persist after controlling for white matter volume.
Notably, Schafer et al. (2009) found that, despite PTs having significant differences in functional connectivity to language areas as well as a reduction in left frontal and bilateral temporal white matter, PT subjects performed comparably on semantic language tasks to normal term controls. Lubsen et al. (2011) echoed these findings, demonstrating that despite PTs having lower fractional anisotropy values, a marker of microstructural connectivity, in several language regions, they performed comparably to Ts.
1.3. Development of resting state functional connectivity networks
Recently, resting state functional connectivity MRI has come into focus as a method for identifying functional neural networks. It is based on the finding that distinct neural regions that display temporally related spontaneous BOLD fluctuations at rest reflect a functionally connected network (Cohen et al., 2008).
Few studies have investigated resting state connectivity (RSC) in children or across development. Of these studies, it has been reported that the functional organization of the brain in children is significantly different than that of adults. Children display more local, short-range connections between adjacent brain regions, which eventually shift to more long-range, distributed connections in adults (Dosenbach et al., 2010). This developmental trend encompasses “segregation” of neural networks via the weakening of short-range connections and concurrent “integration” of distant regions into functional networks via the strengthening of long-range connections (Fair et al., 2009).
The evolving functional architecture over development appears to correspond to maturing behavioral and cognitive abilities. Neural regions that subserve higher level functioning exhibit stronger functional activation or deactivation over time, enhancing cognitive maturity (Rubia, 2013). A synchrony of stronger activation within networks and enhanced deactivation of antagonistic networks has been shown to correlate with more mature performance on tasks of higher order executive function, such as attention, working memory, and regulatory control (Barber et al., 2013, Marsh et al., 2006).
While this trend of “segregation and integration” seems to characterize maturation in a number of neural networks, little is known about the development and refinement of language network connectivity, or how this development is affected by PT birth. At adolescence, PTs display globally stronger intra- and inter-hemispheric connectivity to the superior temporal lobes than Ts, but functional connectivity between these language regions and overall network efficiency are reduced in PTs (Wilke et al., 2014). PTs continue to demonstrate greater connectivity at age 20 in hypothesized language processing areas, including left temporal–parietal areas, left and right inferior temporal lobes, and the medial frontal lobes (Scheinost et al., 2012). Although previous studies have demonstrated significant connectivity differences between PTs and Ts (Wilke et al., 2014, Scheinost et al., 2012), it is unclear whether these connections are present at birth and are not pruned through the course of development or if they develop over time.
Furthermore, the use of resting state functional MRI data has several limitations. For one, it relies on pre-selected regions of interest (ROI) to be investigated and thus may overlook non-selected areas that similarly display differential connectivity. Also, it relies on arbitrarily defined correlation thresholds to describe functional connectivity differences. To overcome these limitations, Scheinost et al. (2012) utilized intrinsic connectivity distribution (ICD). ICD allows for the characterization of all connections via whole-brain survey, without requiring a priori defined ROIs or connectivity thresholds. It measures the connectivity of each voxel to all other neural voxels and allows for elaboration of a specific voxel's degree of connectivity throughout the brain without being limited to connectivity within a pre-defined network (Scheinost et al., 2015).
In this longitudinal study, we investigate how intrinsic functional connectivity is altered from childhood through adolescence in PTs compared to Ts, as well as how these changes in connectivity relate to cognitive, semantic, and phonologic testing scores. We hypothesize that, when compared to Ts, PTs will display altered connectivity trajectories between childhood and adolescence. Functional connectivity will be correlated with performance on language tasks.
2. Materials and methods
This study was performed at the Yale University School of Medicine, New Haven, CT and Brown Medical School, Providence, RI. The protocols were reviewed and approved by institutional review boards at each location. Children provided written assent; parent(s) provided written consent for the study. All scans were obtained at Yale University and were analyzed at Yale University.
2.1. Subjects
The PT cohort consisted of children who were enrolled in the follow-up MRI component of the Multicenter Randomized Indomethacin Intraventricular Hemorrhage Prevention Trial (Ment et al., 1994). Only those PT children without evidence of intraventricular hemorrhage, periventricular leukomalacia and/or low-pressure ventriculomegaly and who lived within 200 miles of the Yale study center were included. T control children were recruited from the local communities of the study children. They were group-matched to the PT children for age, sex, and minority status. Minority status was defined as being of non-Caucasian race and was reported by parents at the time of the assessment. Only PTs and Ts with data collected at both 8 years and 16 years of age were included.
2.2. Neurodevelopmental assessments
Serial standardized neuropsychological assessments were performed by testers blinded to the randomization status of the subjects in the IVH prevention study. Intellectual ability was measured using the Weschler Intelligence Scale for Children, Third Edition (WISC-III), from which the verbal IQ (VIQ), performance IQ (PIQ), verbal comprehension IQ (VCIQ) and full-scale IQs (FSIQ) were obtained. Specific language skills were assessed with the Peabody picture vocabulary test—revised (PPVT-R) and the Rapid Naming Composite (RDRL_Cmp). The PPVT tests receptive vocabulary, while the RDRL_Cmp measures phonologic awareness and memory, and rapid naming.
2.3. Residual data between task paradigms during fMRI scanning
For the 16-year-old subjects, each subject performed an event-related cue-target identity task that required a match/mismatch judgment between pictures and words that were presented acoustically and/or in printed form on each trial. Responses were made via a button press. Between 8 and 10 runs were completed per subject. This task is described in detail in Frost et al. (2009). For the 8-year-old subjects, each subject passively listened to the Ugly Duckling story presented either normally, with words scrambled, or with a low pass filter applied. This task is described in detail in Ment et al. (2006). For both tasks, the task-based data were analyzed using a general linear model described in each of the respective papers. Then the model fit was subtracted from the raw data to create a residual data set, which was used as the input to the connectivity analysis.
The use of residuals has been described previously by Finn et al. (2014). The effect of task was regressed out to leave residual fluctuations that we believe are more closely representative of intrinsic, spontaneous neural activity. By avoiding task-based data, we prevent our results from being dominated by activation coupled to the onset and processing of each stimulus and instead are able to examine spontaneous fluctuations. Furthermore, the use of purely continuous resting-state scan data may introduce confounding “tasks”, such as mind-wandering, which are likely inhibited in task-based studies. Thus, we believe that the use of residual data during a task-based study may more accurately reflect spontaneous neural changes and better enhance underlying functional organization between the groups.
2.4. Preprocessing
All data were converted from Digital Imaging and Communication in Medicine (DICOM) format to Analyze format using XMedCon (http://xmedcon.sourcefroge.net/). During the conversion process, the first four images at the beginning of each of the ten functional series were discarded to enable the signal to achieve steady state, leaving 209 measurements for analysis. Images were first slice time corrected using sinc interpolation and then motion corrected using SPM5 (http://www.fil.ion.ucl.ac.uk/spm/software/spm5/). Runs with linear motion in excess of 1.5 mm or rotation greater than 2° were discarded. All voxels with signal less than 5% of the maximum were set to zero, and drift removal (up to 3rd order) and temporal Gaussian smoothing (standard deviation = 1) were then performed on the time-course of each voxel. Finally, the global time-course was regressed out. As group differences in motion have been shown to confound functional connectivity results as seen in Van Dijk et al. (2012), average frame-to-frame displacement was calculated for each run and compared between the four groups: 8 year PT, 8 year T, 16 year PT and 16 year T. To level the mean displacement across groups to 0.054 mm, runs with displacement greater than 0.09 mm for the 8 year PT (58 of 150 runs), 0.08 mm for the 8 year T (29 of 62 runs), and 0.12 mm for the 16 year PT (55 of 288 runs) were additionally discarded. No runs were additionally discarded from the 16 year T subjects (349 runs).
2.5. Residual functional connectivity maps
All remaining residual data runs for each subject were concatenated, and the functional connectivity of each voxel, as measured by the intrinsic connectivity distribution (ICD), was calculated as described in Scheinost et al. (2012). Similar to most voxel-based functional connectivity measures, ICD involves correlating the time course for any given gray-matter voxel with the time course of every other gray-matter voxel in the brain, and then summarizing these correlations with a network theory metric. Specifically, ICD models the entire distribution of the network measure of degree, therefore eliminating the need to specify a connection threshold. A histogram of these positive correlations was constructed to estimate the distribution of connections to the voxel in question. This distribution of connections was converted to a survival function and the survival function was fitted with a stretched exponential with unknown variance, α. As alpha controls the spread of the distribution of connections, a larger alpha indicates a greater number of high correlation connections. Finally, this process is repeated for all voxels in the gray matter resulting in a parametric image of the alpha parameter for each subject, which was used in all between group and correlational analyses. Each subject's ICD map was then normalized by subtracting that subject's mean and dividing by the standard deviation across all voxels. This normalization process removes the large global connectivity differences to better investigate the more subtle relative connectivity differences (Mitchell et al., 2013). Finally, a 6 mm Gaussian filter was applied to each normalized ICD map.
2.6. Registration to a common reference space
To take individual subject data into a common reference space, three registrations were calculated within the Yale BioImage Suite software package (http://www.bioimagesuite.org) (Duncan et al., 2004) and then concatenated and applied as one registration. The first was a linear registration between the individual subject's raw functional data and the subject's T1 anatomical image collected at the same slice locations. The second linear registration was between the individual's T1 anatomical image and the individual's 1 mm isotropic MP-Rage anatomical image. Finally, a non-linear registration was computed between the individuals' MP-Rage anatomical image and the Colin27 Brain (Holmes et al., 1998) in order to transform data into the standardized space defined by the Montreal Neurological Institute (MNI). The inverse transformation from the MNI space to the individual functional space was also computed.
2.7. Group comparison LME
To compare the longitudinal changes of intrinsic connectivity across the groups, a Linear Mixed Effects model (LME) using age (8 years vs. 16 years) and group (PT vs. T) as factors was computed using AFNI. The age by group interaction was investigated using a threshold significance of p < 0.05 with a conjoint cluster of 184 voxels corresponding to a p < 0.05 family-wise error (FWE) correction as determined by AFNI's AlphaSim program.
2.8. Other statistical analyses
Demographic data were analyzed using Fisher's exact test for categorical variables and t test for continuous variables. Mixed model repeated measures analysis was performed to compare the longitudinal changes in both resting state functional connectivity and neurocognitive scores between PTs and Ts, with covariate adjustment for gender, race and maternal education status and inclusion of time by group interaction. Linear contrasts were performed to examine the changes in both connectivity and cognitive scores from age 8 to 16 for each group, compare groups at each age, and compare these connectivity and cognitive score changes between groups. Pearson correlation analysis was performed to examine the correlations between all regional connectivity and cognitive scores at age 16. The correlation analysis was also stratified by PTs and Ts. The significance level was p < 0.05, two-sided.
3. Results
3.1. Subjects
Thirteen PT children and twelve T children were included. Demographic data for all subjects are reported in Table 1. Perinatal data for the PT subjects are also shown in Table 1. There were no significant differences between the two groups in the number of males, handedness, race, or years of maternal education. Both groups were comparable at age of scan, although PTs were slightly older than Ts at age 16, which trended toward significance (16.31 vs. 16.12 years, p = 0.059).
Table 1.
Demographic data for the study children (mean ± SD).
| Preterm | Term | p | |
|---|---|---|---|
| Number | 13 | 12 | – |
| Males | 9 (69%) | 6 (50%) | 0.428 |
| Right-handed | 13 (100%) | 12 (100%) | – |
| Minority status | 5 (38%) | 4 (33%) | 1.000 |
| Age at 8 year old scan | 8.89 ± 0.49 | 8.80 ± 0.49 | 0.654 |
| Age at 16 year old scan | 16.31 ± 0.29 | 16.12 ± 0.18 | 0.059 |
| Mat ed < HS | 0 | 0 | – |
| Birthweight (grams) | 948.46 ± 188.05 | – | – |
| Gestational age (weeks) | 27.31 ± 2.43 | – | – |
As shown in Table 2, there were no significant differences between PTs and Ts in cognitive scores at age 8, but PIQ and FSIQ trended toward significance (p = 0.06 and p = 0.07), with PTs having poorer performance. Similarly at age 16, PTs scored lower than Ts in VIQ, PIQ, FSIQ (p = 0.04, p = 0.006, and p = 0.006, respectively).
Table 2.
Cognitive data adjusted for gender, race, and maternal education. Presented as least squares means (95% confidence interval) and p values.
| Outcomes | Pre-term | Term | Group difference | Interaction (slope difference) | |
|---|---|---|---|---|---|
| VIQ | 8 years | 104.1 (96.7, 111.5) | 109.8 (102.3, 117.4) | − 5.7 (− 16.3, 4.9) p = 0.27 | |
| 16 years | 98.3 (90.8, 105.8) | 109.6 (101.6, 117.6) | − 11.4 (− 22.4, − 0.3) p = 0.04 | ||
| Changes from 8 to 16 years | − 5.9 (− 12.6, 0.9) p = 0.09 | − 0.2 (− 7.6, 7.2) p = 0.95 | − 5.6 (− 15.8, 4.5) p = 0.26 | ||
| PIQ | 8 years | 97.2 (87.8, 106.7) | 110.0 (100.5, 119.5) | − 12.8 (− 26.2, 0.7) p = 0.06 | |
| 16 years | 94.0 (84.1, 103.9) | 114.9 (104.7, 125.1) | − 20.9 (− 35.1, − 6.6) p = 0.006 | ||
| Changes from 8 to 16 years | − 3.2 (− 8.0, 1.6) p = 0.18 | 4.9 (− 0.3, 10.1) p = 0.06 | − 8.1 (− 15.2, − 1.0) p = 0.03 | ||
| FSIQ | 8 years | 100.6 (92.2, 108.9) | 111.3 (102.9, 119.7) | − 10.7 (− 22.6, 1.2) p = 0.07 | |
| 16 years | 95.8 (87.5, 104.1) | 113.5 (104.9, 122.0) | − 17.7 (− 29.6, − 5.7) p = 0.006 | ||
| Changes from 8 to 16 years | − 4.8 (− 9.0, − 0.5) p = 0.03 | 2.2 (− 2.5, 6.8) p = 0.34 | − 6.9 (− 13.2, − 0.7) p = 0.03 | ||
| VCOMPIQ | 8 years | 106.5 (98.7, 114.2) | 109.2 (101.3, 117.1) | − 2.8 (− 13.9, 8.4) p = 0.61 | |
| 16 years | 100.5 (93.1, 108.0) | 108.2 (100.2, 116.1) | − 7.6 (− 18.6, 3.3) p = 0.16 | ||
| Changes from 8 to 16 years | − 5.9 (− 13.6, 1.7) p = 0.12 | − 1.0 (− 9.3, 7.2) p = 0.79 | − 4.9 (− 16.1, 6.3) p = 0.38 | ||
| PPVT | 8 years | 101.0 (90.3, 111.6) | 113.6 (102.8, 124.4) | − 12.7 (− 27.9, 2.6) p = 0.10 | |
| 16 years | 104.0 (93.5, 114.5) | 117.5(106.8, 128.1) | − 13.5 (− 28.5, 1.6) p = 0.08 | ||
| Changes from 8 to 16 years | 3.0 (− 4.1, 10.1) p = 0.39 | 3.8 (− 3.6, 11.3) p = 0.30 | − 0.8 (− 11.1, 9.5) p = 0.87 | ||
| RDRL_Cmp | 8 years | – | – | – | |
| 16 years | 98.8 (82.1, 115.5) | 100 (88.3, 111.7) | − 1.2 (− 13.2, 10.9) p = 0.84 | ||
| Changes from 8 to 16 years | – | – | – |
The bolded values are the p-values that are significant (< 0.05).
Comparing changes in testing scores from age 8 to 16 by group, PT scores worsened with increasing age, while T scores improved, with PIQ and FSIQ reaching significance (p = 0.03 and p = 0.03). PTs also performed significantly worse on FSIQ at age 16 than age 8 (p = 0.03).
3.2. Generated regions
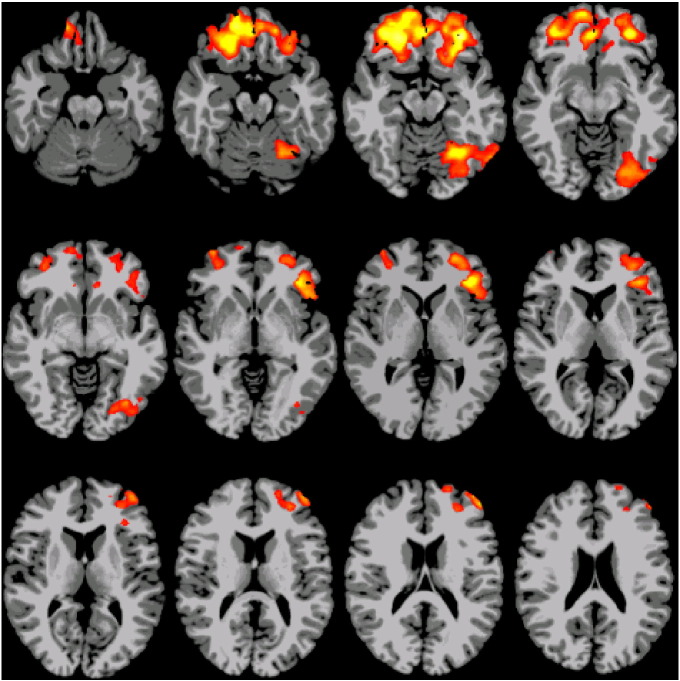
The group × age interaction analysis revealed several regions with significant differences in longitudinal changes in connectivity, including left fusiform-BA18-BA19 (occipito-temporal cortex), bilateral BA47-BA11-BA10-left BA45 (inferior frontal gyri, orbitofrontal and anterior prefrontal cortices) for PT compared to T controls (Fig. 1). This included some areas that had been both previously published in the literature and identified in our previous studies (Gozzo et al., 2009, Myers et al., 2010, Schafer et al., 2009).
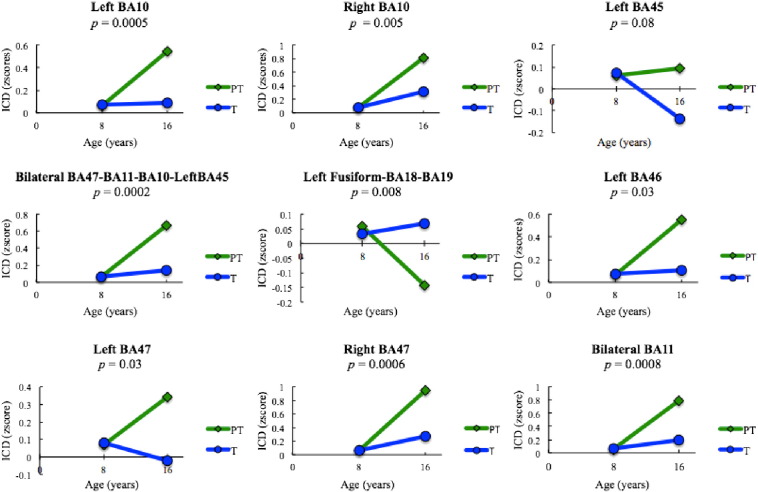
Fig. 1.
Interaction result from Linear Mixed Effects model using age (8 years vs. 16 years) and group (preterm vs. term), with p < 0.05 threshold significance. Regions were selected from this analysis.
Additional normalized ICD maps were generated comparing groups at age 8 and at age 16 (Supplemental Figs. 1 & 2). Some connectivity differences were observed between groups at age 8. However, these areas did not undergo significant longitudinal changes in connectivity, and thus did not persist in the group × age interaction map. The interaction map at age 16 showed similar regions of connectivity differences as the group × age interaction map.
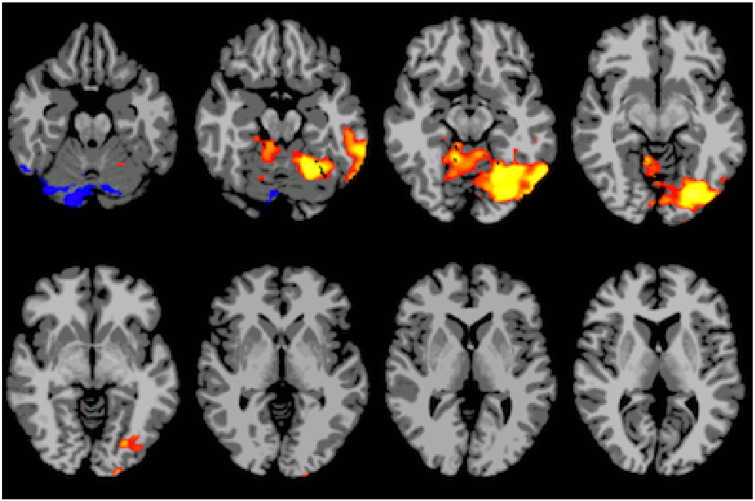
Supplemental Fig. 1.
Interaction result showing group differences at age 8, with p < 0.05 threshold significance. These areas did not undergo significant longitudinal connectivity changes.
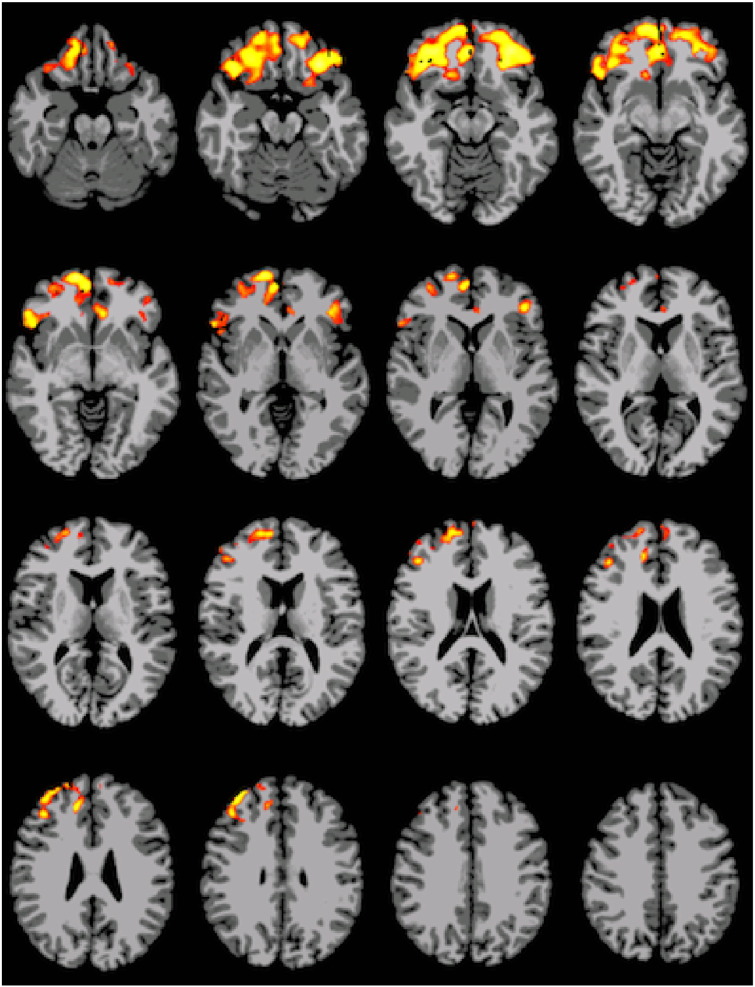
Supplemental Fig. 2.
Interaction result showing group differences at age 16, with p < 0.05 threshold significance.
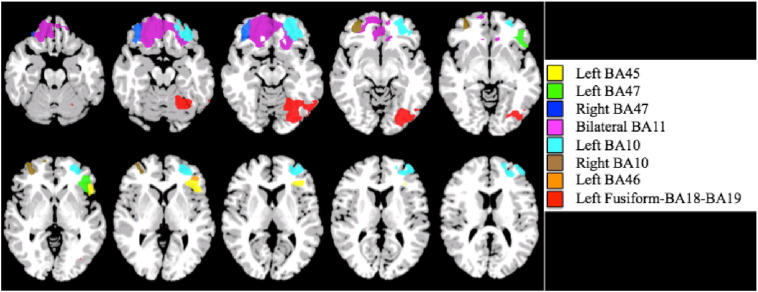
The larger frontal region was separated into seven individual regions based on Brodmann areas (BA) for further investigation (Fig. 2). All regions were defined in reference space, with the center of mass MNI coordinates listed in Table 3, as defined in the Yale Bioimagesuite Software (Bioimagesuite.org). For all regions, the inverse transformation from reference space was used to warp each region back to individual functional space.
Fig. 2.
Regions generated from group by age interaction analysis.
Table 3.
Montreal Neurological Institute (MNI) coordinates of generated regions.
| Brodmann's areas (BA) | Center of Mass MNI (x, y, z) |
|---|---|
| Bilateral BA47-BA11-BA10-Left BA45 | − 6, 43, − 9 |
| Left BA 45 | − 43, 28, 2 |
| Left BA 47 | − 43, 31, − 4 |
| Right BA 47 | 36, 41, − 15 |
| Bilateral BA 11 | 8, 43, − 16 |
| Left BA 10 | − 30, 48, − 3 |
| Right BA 10 | 33, 52, − 4 |
| Left BA 46 | − 44, 40, 1 |
| Left fusiform-BA18-BA19 | − 33, − 71, − 13 |
3.3. Intrinsic connectivity over time
Least squares means (LSM) for the resting state intrinsic connectivity data for each generated region are shown in Fig. 3 and supplemental Table 1; these were adjusted for gender, race, and maternal education.
Fig. 3.
ICD plots and p values for group by age interaction. At age 8, PTs and Ts displayed similar connectivity in regions. PT but not T underwent significant increases in connectivity by age 16 in all areas except left fusiform-BA18-BA19 & left BA45.
PTs demonstrated significant increases in connectivity from age 8 to age 16 in bilateral BA47-BA11-BA10-Left BA45 (LSM 0.61, p < 0.0001), left and right BA 47 (0.27, p = 0.02 and 0.89, p < .0001, respectively), bilateral BA 11 (0.74, p < 0.0001), left and right BA 10 (0.48, p < 0.0001 and 0.74, p < 0.0001), and left BA 46 (0.49, p = 0.002). PTs also displayed significant decreases in connectivity in left fusiform-BA18-BA19 over time (− 0.20, p = 0.002). Terms did not undergo significant alterations in connectivity over time in any regions, although the increase observed in right BA 10 trended toward significance (0.23, p = 0.06).
At age 8, none of the regions showed different connectivity between two groups. At age 16, the majority of the interrogated regions displayed significantly different connectivity in PTs compared to Ts. PTs had greater connectivity than Ts at age 16 in the following regions: bilateral BA47-BA11-BA10-Left BA45 (0.53, p = 0.0002), left and right BA 47 (0.37, p = 0.03 and 0.68, p = 0.0007), bilateral BA11 (0.60, p = 0.0009), left and right BA 10 (0.46, p = 0.0006 and 0.51, p = 0.005), and left BA 46 (0.46, p = 0.03). Conversely, Ts had greater connectivity than PTs in left fusiform-BA18-BA19 (− 0.21, p = 0.04).
Finally, in comparing the changes in connectivity over time in PTs versus Ts, significant differences were again seen in bilateral BA47-BA11-BA10-Left BA45 (0.53, p = 0.0002), left fusiform-BA18-BA19 (− 0.24, p = 0.008), left and right BA 47 (0.38, p = 0.03 and 0.68, p = 0.0006), bilateral BA 11 (0.60, p = 0.0008), left and right BA 10 (0.46, p = 0.0005 and 0.51, p = 0.005), and left BA 46 (0.46, p = 0.03).
3.4. Intrinsic connectivity & language scores
Exploratory analysis using Pearson correlations were performed to correlate connectivity in generated regions with cognitive measures of all subjects at age 16; results are listed in Table 4. In analyses, significant correlations were only seen between connectivity of the left fusiform-BA18-BA19 and VIQ (r = 0.467, p = 0.021), PIQ (r = 0.419, p = 0.041), and FSIQ (r = 0.491, p = 0.015). Scores on the other cognitive tasks were not significantly associated with connectivity in the other regions. Furthermore, in comparing PTs and Ts, there were no statistically significant differences between these groups in the correlations of generated regions' connectivity to cognitive scores at age 16 (Supplemental Table 2). In correlating changes in connectivity from ages 8 to 16 and overall cognitive outcome measures (ages 8 and 16), again only the left fusiform-BA18-BA19 was significantly associated with scores on VIQ (r = 0.466, p = 0.022), PIQ (r = 0.431, p = 0.036), and FSIQ (r = 0.498, p = 0.013). Comparisons of the association of connectivity changes from ages 8 to 16 and cognitive scores at age 16 revealed no significant group differences between PTs and Ts. Furthermore, no significant group differences between PTs and Ts were seen when comparing connectivity at age 16 to changes in cognitive scores from ages 8 to 16.
Table 4.
Unadjusted Pearson correlations between generated regions' connectivity and score at age 16.
| VERBIQ | VCIQ | PERFIQ | FULLIQ | PPVT | RDRL_Cmp | ||
|---|---|---|---|---|---|---|---|
| Bilateral BA47_BA11_BA10_LeftBA45 | Pearson correlation coefficients | − 0.0750 | − 0.05056 | − 0.27232 | − 0.20537 | 0.15723 | 0.23503 |
| p | 0.7266 | 0.8145 | 0.1980 | 0.3357 | 0.4529 | 0.2581 | |
| Left Fusiform_BA18_BA19 | Pearson correlation coefficients | 0.46707 | 0.36750 | 0.41928 | 0.49088 | 0.24065 | 0.10950 |
| p | 0.0214 | 0.0773 | 0.0414 | 0.0149 | 0.2466 | 0.6023 | |
| Left BA45 | Pearson correlation coefficients | − 0.07473 | − 0.05031 | − 0.10132 | − 0.09214 | 0.04644 | 0.17545 |
| p | 0.7286 | 0.8154 | 0.6376 | 0.6685 | 0.8255 | 0.4015 | |
| Left BA47 | Pearson correlation coefficients | − 0.17325 | − 0.14823 | − 0.18649 | − 0.19468 | − 0.00563 | 0.11965 |
| p | 0.4182 | 0.4894 | 0.3829 | 0.3620 | 0.9787 | 0.5689 | |
| Right BA47 | Pearson correlation coefficients | − 0.03099 | − 0.06313 | − 0.26783 | − 0.18179 | − 0.15021 | 0.31215 |
| p | 0.8857 | 0.7695 | 0.2058 | 0.3952 | 0.4736 | 0.1287 | |
| Bilateral BA11 | Pearson correlation coefficients | − 0.01667 | 0.02428 | − 0.23584 | − 0.15659 | − 0.16158 | 0.18251 |
| p | 0.9384 | 0.9103 | 0.2672 | 0.4649 | 0.4403 | 0.3826 | |
| Left BA10 | Pearson correlation coefficients | − 0.10211 | − 0.12229 | − 0.30733 | − 0.23721 | − 0.12313 | 0.31724 |
| p | 0.6350 | 0.5692 | 0.1441 | 0.2644 | 0.5576 | 0.1223 | |
| Right BA10 | Pearson correlation coefficients | − 0.30007 | − 0.18210 | − 0.13887 | − 0.23281 | − 0.28134 | − 0.01274 |
| p | 0.1543 | 0.3944 | 0.5175 | 0.2736 | 0.1731 | 0.9518 | |
| Left BA46 | Pearson correlation coefficients | − 0.24017 | − 0.24532 | − 0.26274 | − 0.27499 | − 0.06541 | − 0.07850 |
| p | 0.2583 | 0.2479 | 0.2148 | 0.1934 | 0.7561 | 0.7092 |
The bolded values are the p-values that are significant (< 0.05).
4. Discussion
Developmental changes in connectivity differ in prematurely-born subjects compared to healthy term controls during that critical period of late childhood through adolescence. To the best of our knowledge, this is the first report of longitudinal changes in intrinsic connectivity in PTs and Ts from 8 to 16 years. At age 8, PTs and Ts displayed grossly equivalent connectivity in the interrogated regions but then underwent dramatic alterations through age 16. PT but not T children demonstrated significant increases in connectivity in the generated regions over time, especially in left and right BA47, bilateral BA11, left and right BA10, and left BA46. Ultimately, PTs showed greater connectivity than Ts at age 16 in all areas of interest except the left fusiform-BA18-BA19, in which PT connectivity had significantly decreased over time. Finally, in both PTs and Ts, left fusiform-BA18-BA19 connectivity was significantly and positively associated with scores on the FSIQ, PIQ, and VIQ.
4.1. Altered development of resting state connectivity
Several studies have investigated the development of PT RSC in infancy and early childhood. Seed-based correlation analysis has shown that PTs, when studied at term in the neonatal period, demonstrate RSC networks that closely, topographically resemble those of T controls (Doria et al., 2010). However, ROI studies show that PT RSNs have weaker connectivity and complexity, especially in those networks subserving higher-order functions, e.g. language, frontoparietal control, and default mode networks (Smyser et al., 2014). By age 4, ROI studies demonstrate that the strong, predominantly local RSN connections at birth eventually shift to increased inter-hemispheric connectivity (Lee et al., 2013).
Although our study did not attempt to map out the geography of these connections, we found that at age 8, both PTs and Ts displayed largely, quantitatively similar connectivity in the interrogated regions. When solely comparing relative connectivity between PTs and Ts at age 8, we did observe significant group differences. This is in keeping with previous findings of connectivity and volumetric differences at age 8 (Gozzo et al., 2009, Ment et al., 2009). However, these areas of contrast in our 8 year old interaction map did not undergo significant longitudinal changes, and thus were not generated by our group × age interaction map, which was the variable of interest in this study.
ROI studies show that at several time points in childhood and adolescence, functional connectivity to various language regions differs significantly between PTs and Ts (Gozzo et al., 2009, Myers et al., 2010, Schafer et al., 2009). Furthermore, these structural alterations confer cognitive, functional implications. These findings are similarly confirmed by microstructural analyses. In PT adolescents, for example, the microstructural integrity of white matter tracts within ventral and dorsal language pathways, which were significantly altered compared to Ts, positively correlated with performance on semantic and phonological tasks, respectively (Mullen et al., 2011).
Our present findings are of particular importance because they suggest that differences in PT and T neural connectivity are not simply reflections of early and preserved perturbations, but rather are the result of PTs more rapidly altering the number of connections to these areas over the course of adolescence.
4.2. Maturation of the visual word form area
Interestingly, we found only a few regions that underwent reductions in RSC from childhood through adolescence, with only left fusiform-BA18-BA19 reaching significance in PTs. This area of left occipito-temporal cortex encompasses the visual word form area (VWFA) (Yeatman et al., 2013). RSC studies have shown that the involvement of VWFA in different RSNs changes across development. In children, for example, VWFA participates in the visual RSN and then transitions to the fronto-parietal network at adolescence (Vogel et al., 2013). A longitudinal fMRI study of left occipital–temporal sulcus found that the size of the VWFA region activated to visual word stimuli increased from ages 8 to 12, then decreased from ages 13 to 15, and remained largely stable into adulthood (Ben-Shachar et al., 2011).
Studies of anatomic connections from the VWFA show predominate extension to hypothesized language areas, including left superior temporal gyrus, posterior medial temporal gyrus, Broca's area, and within the left occipito-temporal sulcus (Bouhali et al., 2014, Yeatman et al., 2013). The reduction in VWFA functional connectivity observed in our PTs may represent either a global decrease in these connections to VWFA or a loss of connections from specific neural regions. Furthermore, connectivity loss may be the result of a steady decrease in RSC or an initial expansion and later pruning, as observed in Ben-Shachar et al. (2011).
4.3. Association between VWFA connectivity and cognition
Notably, despite such remarkable and widespread connectivity changes over time, only left VWFA connectivity correlated to scores on VIQ, PIQ, and FSIQ before correction for multiple comparisons. The involvement of the VWFA in reading has long been established. Early lesion studies demonstrating pure alexia (Greenblatt, 1973) have since been corroborated by task-based fMRI studies showing VWFA activation during reading tasks (Ben-Shachar et al., 2011, Wandell, 2011, Wandell et al., 2012). Additionally, DTI studies have shown that VWFA receives white matter input from visual cortex and projects fiber tracts to cortical language areas, further speaking to its role in visual language processing (Bouhali et al., 2014, Yeatman et al., 2013).
Although its name implies specific involvement in visual word processing, recent studies have argued against this specificity. For one, although RSC correlations have been shown between purported “reading regions” (Koyama et al., 2010), RSC whole brain analyses have not demonstrated a designated “reading network” (Power et al., 2011, Vogel et al., 2013, Yeo et al., 2011). Furthermore, RSC studies demonstrate that VWFA is only weakly correlated with hypothesized reading regions and is most strongly correlated with the dorsal attention network (Vogel et al., 2012a). This suggests that, while the VWFA plays a pivotal role in visual word processing, it is not exclusively dedicated to this function. Rather, it is likely more generally involved in the processing of complex visual stimuli (Vogel et al., 2012b, Vogel et al., 2014).
In light of the positive correlation we found between left VWFA connectivity and cognitive and verbal performance, the decrease in connectivity for PTs is paradoxical. It indicates that they are not effectively or adaptively recruiting connections in these regions. Furthermore, this active loss of connections does not reflect an enhanced, “mature” brain (Fair et al., 2009, Stevens et al., 2009), but rather is associated with inferior cognitive performance. PTs' worsening scores on VIQ, PIQ, and FSIQ from ages 8 to 16 can be attributed in part to this decrease in connectivity. Interestingly, we did not observe a correlation between VWFA connectivity and rapid naming scores (RDRL_Cmp). This differs from expected given that several studies have found the VWFA to be involved in visual letter and word recognition (Price et al., 1996, Cohen et al., 2002, Ben-Shachar et al., 2011, Wandell, 2011, Wandell et al., 2012). As we did not adjust for multiple comparisons, the significance of these correlations in this exploratory analysis should be interpreted with caution (Bender and Lange, 2001).
4.4. Limitations
One limitation of this study is the use of interleaved residual fMRI data to approximate resting state connectivity. Although Fair et al. (2007) have described that interleaved residual data are both quantitatively and qualitatively similar to continuous resting data and may be an adequate alternative for RSC analyses, it is possible that these approximations are not an accurate measure of true RSC. Other limitations include the relatively small sample size, unadjusted Pearson correlations, and analyzing longitudinal data from only two time points. Additionally, we cannot interpret the changes in connectivity as being attributed solely to time or age differences due to the possibility of confounding from magnet effects. Regardless, this does not minimize the observation that PT connectivity changes are significantly different than T connectivity changes.
To the best of our knowledge, this is the first longitudinal study evaluating the development of intrinsic functional connectivity and language in PT children through adolescence. The children who participated in this study are part of a well-studied cohort with neuroimaging available from early in the neonatal period and extending through young adulthood.
Future longitudinal studies should assess connectivity at several time points over the course of childhood through adolescence, ideally with larger numbers of PT and T children. It may be pertinent to correlate changes in cortical thickness to RSC as a metric for brain maturation.
5. Conclusions
PTs undergo significant expansion of RSC over time, which differs markedly from Ts. Most notably, PTs showed paradoxical decreases in RSC in the left occipito-temporal cortex, which was significantly correlated with verbal and IQ measures. These data suggest that the development of RSC in PTs does not reflect compensatory alterations in connectivity, but rather may underscore and perpetuate impairment in language and cognitive processing.
The following are the supplementary data related to this article.
Generated region outcomes adjusted for gender, race, and maternal education. Presented as LS means (95% confidence interval) and p values.
Group effect (difference in the correlations of regional connectivity and score between preterm and term at age 16), presented as p values. There is no significant group effect in any correlation between connectivity and scores.
Acknowledgments
This work was supported by funding from NIH R01 NS 27116 and DHHS grant # 2T35HL00764926.
Footnotes
Ethics: This work has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Conflict of interest: The authors have no conflicts of interest, including any financial, personal, or other relationships that could inappropriately influence or be perceived to influence this work.
Submission verification: This work has not been published previously and is not being considered for publication elsewhere.
References
- Barber A.D., Caffo B.S., Pekar J.J. Developmental changes in within- and between-network connectivity between late childhood and adulthood. Neuropsychologia. 2013;51(1):156–167. doi: 10.1016/j.neuropsychologia.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Wojdyla D., Say L. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull. World Health Organ. 2010;88(1):31–38. doi: 10.2471/BLT.08.062554. (Jan 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender R., Lange S. Adjusting for multiple testing — when and how? J. Clin. Epidemiol. 2001;54(4):343–349. doi: 10.1016/s0895-4356(00)00314-0. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar M., Dougherty R.F., Deutsch G.K. The development of cortical sensitivity to visual word forms. J. Cogn. Neurosci. 2011;23(9):2387–2399. doi: 10.1162/jocn.2011.21615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe H., Cousens S., Oestergaard M.Z. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- Blencowe H., Lee A.C., Cousens S. Preterm birth-associated neurodevelopmental impairment estimates at regional and global levels for 2010. Pediatr. Res. 2013;74(Suppl. 1):17–34. doi: 10.1038/pr.2013.204. (Dec 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boardman J.P., Craven C., Valappil S. A common neonatal image phenotype predicts adverse neurodevelopmental outcome in children born preterm. NeuroImage. 2010;52(2):409–414. doi: 10.1016/j.neuroimage.2010.04.261. (Aug 15 2010) [DOI] [PubMed] [Google Scholar]
- Botting N., Powls A., Cooke R.W.I. Cognitive and educational outcome of very-low-birthweight children in early adolescence. Dev. Med. Child Neurol. 1998;40(10):652–660. doi: 10.1111/j.1469-8749.1998.tb12324.x. [DOI] [PubMed] [Google Scholar]
- Bouhali F., Thiebaut de Schotten M., Pinel P. Anatomical connections of the visual word from area. J. Neurosci. 2014;34(46):15402–15414. doi: 10.1523/JNEUROSCI.4918-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen J.R., Gibson F.L., Hand P.J. Educational outcome at 8 years for children who were born extremely prematurely: a controlled study. J. Paediatr. Child Health. 2002;38(5):438–444. doi: 10.1046/j.1440-1754.2002.00039.x. [DOI] [PubMed] [Google Scholar]
- Cohen L., Lehéricy S., Chochon F. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain. 2002;125(5):1054–1069. doi: 10.1093/brain/awf094. [DOI] [PubMed] [Google Scholar]
- Cohen A.L., Fair D.A., Dosenbach N.U. Defining functional areas in individual human brains using resting functional connectivity MRI. NeuroImage. 2008;41(1):45–57. doi: 10.1016/j.neuroimage.2008.01.066. (May 15 2008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doria V., Beckmann C.F., Arichi T. Emergence of resting state networks in the preterm human brain. Proc. Natl. Acad. Sci. U.S.A. 2010;107(46):20015–20020. doi: 10.1073/pnas.1007921107. (Nov 16 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach N.U., Nardos B., Cohen A.L. Prediction of individual brain maturity using fMRI. Science. 2010;329(5997):1358–1361. doi: 10.1126/science.1194144. (Sep 10 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J.S., Papademetris X., Yang J. Geometric strategies for neuroanatomic analysis from MRI. NeuroImage. 2004;23(Suppl. 1):S34–S45. doi: 10.1016/j.neuroimage.2004.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Schlaggar B.L., Cohen A.L. A method for using blocked and event-related fMRI data to study “resting state” functional connectivity. NeuroImage. 2007;35(1):396–405. doi: 10.1016/j.neuroimage.2006.11.051. (Mar 2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair D.A., Cohen A.L., Power J.D. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009;5(5) doi: 10.1371/journal.pcbi.1000381. (May 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Holahan J.M. Disruption of functional networks in dyslexia: a whole-brain, data-driven analysis of connectivity. Biol. Psychiatry. 2014;76(Suppl. 1):S4–S5. doi: 10.1016/j.biopsych.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost S.J., Landi N., Mencl W.E. Phonological awareness predicts activation patterns for print and speech. Ann. Dyslexia. 2009;59(1):78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozzo Y., Vohr B., Lacadie C. Alterations in neural connectivity in preterm children at school age. NeuroImage. 2009;48(2):458–463. doi: 10.1016/j.neuroimage.2009.06.046. (Nov 1 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenblatt S.H. Alexia without agraphia or hemianopsia. Anatomical analysis of an autopsied case. Brain. 1973;96(2):307–316. doi: 10.1093/brain/96.2.307. [DOI] [PubMed] [Google Scholar]
- Hack M. Adult outcomes of preterm children. J. Dev. Behav. Pediatr. 2009;30(5):460–470. doi: 10.1097/DBP.0b013e3181ba0fba. [DOI] [PubMed] [Google Scholar]
- Haldipur P., Bharti U., Alberti C. Preterm delivery disrupts the developmental program of the cerebellum. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C.J., Hoge R., Collins L. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. (Mar-Apr 1998) [DOI] [PubMed] [Google Scholar]
- Koyama M.S., Kelly C., Shehzad Z. Reading networks at rest. Cereb. Cortex. 2010;11:2549–2559. doi: 10.1093/cercor/bhq005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W., Morgan B.R., Shroff M.M., Sled J.G., Taylor M.J. The development of regional functional connectivity in preterm infants into early childhood. Neuroradiology. 2013;55(Suppl. 2):105–111. doi: 10.1007/s00234-013-1232-z. (Sep 2013) [DOI] [PubMed] [Google Scholar]
- Lind A., Parkkola R., Lehtonen L. Associations between regional brain volumes at term-equivalent age and development at 2 years of age in preterm children. Pediatr. Radiol. 2011;41(8):953–961. doi: 10.1007/s00247-011-2071-x. (Aug 2011) [DOI] [PubMed] [Google Scholar]
- Lubsen J., Vohr B., Myers E. Microstructural and functional connectivity in the developing preterm brain. Semin. Perinatol. 2011;35(1):34–43. doi: 10.1053/j.semperi.2010.10.006. (Feb 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu T.M., Vohr B.R., Schneider K.C. Trajectories of receptive language development from 3 to 12 years of age for very preterm children. Pediatrics. 2009;124(1):333–341. doi: 10.1542/peds.2008-2587. (Jul 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu T.M., Vohr B.R., Allan W. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128(2):313–322. doi: 10.1542/peds.2010-2655. (Aug 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansson J., Stjernqvist K. Children born extremely preterm show significant lower cognitive, language and motor function levels compared with children born at term, as measured by the Bayley-III at 2.5 years. Acta Paediatr. 2014;103(5):504–511. doi: 10.1111/apa.12585. [DOI] [PubMed] [Google Scholar]
- Marsh R., Zhu H., Schultz R.T. A developmental fMRI study of self-regulatory control. Hum. Brain Mapp. 2006;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment L.R., Oh W., Ehrenkranz R.A. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J. Pediatr. 1994;124(6):951–955. doi: 10.1016/s0022-3476(05)83191-9. [DOI] [PubMed] [Google Scholar]
- Ment L.R., Peterson B.S., Meltzer J.A. A functional magnetic resonance imaging study of the long-term influences of early indomethacin exposure on language processing in the brains of prematurely born children. Pediatrics. 2006;118(3):961–970. doi: 10.1542/peds.2005-2870. (Sep 2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ment L.R., Kesler S., Vohr B. Longitudinal brain volume changes in preterm and term control subjects during late childhood and adolescence. Pediatrics. 2009;123(2):503–511. doi: 10.1542/peds.2008-0025. (Feb 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.R., Balodis I.M., DeVito E.E. A preliminary investigation of Stroop-related intrinsic connectivity in cocaine dependence: associations with treatment outcomes. Am. J. Drug Alcohol Abuse. 2013;39(6):392–402. doi: 10.3109/00952990.2013.841711. (Nov 2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen K.M., Vohr B.R., Katz K.H. Preterm birth results in alterations in neural connectivity at age 16 years. NeuroImage. 2011;54(4):2563–2570. doi: 10.1016/j.neuroimage.2010.11.019. (Feb 14 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E.H., Hampson M., Vohr B. Functional connectivity to a right hemisphere language center in prematurely born adolescents. NeuroImage. 2010;51(4):1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. (Jul 15 2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer V., Griesmaier E., Pehbock-Walser N. Poor postnatal head growth in very preterm infants is associated with impaired neurodevelopment outcome. Acta Paediatr. 2013;102(9):883–888. doi: 10.1111/apa.12319. (Sep 2013) [DOI] [PubMed] [Google Scholar]
- Nosarti C., Al-Asady M.H.S., Frangou S. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125(7):1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- Nosarti C., Giouroukou E., Healy E. Grey and white matter distribution in very preterm adolescents mediates neurodevelopmental outcome. Brain. 2008;131(Pt 1):205–217. doi: 10.1093/brain/awm282. (Jan 2008) [DOI] [PubMed] [Google Scholar]
- Nosarti C., Mechelli A., Herrera A. Structural covariance in the cortex of very preterm adolescents: a voxel-based morphometry study. Hum. Brain Mapp. 2011;32(10):1615–1625. doi: 10.1002/hbm.21133. (Oct 2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker J., Mitchell A., Kalpakidou A. Cerebellar growth and behavioural & neuropsychological outcome in preterm adolescents. Brain. 2008;131(Pt 5):1344–1351. doi: 10.1093/brain/awn062. (May 2008) [DOI] [PubMed] [Google Scholar]
- Peterson B.S., Vohr B., Staib L.H. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. J. Am. Med. Assoc. 2000;284(15):1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J., Wise R.J., Frackowiak R.S. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb. Cortex. 1996;6(1):62–70. doi: 10.1093/cercor/6.1.62. [DOI] [PubMed] [Google Scholar]
- Pritchard V.E., Clark C.A., Liberty K. Early school-based learning difficulties in children born very preterm. Early Hum. Dev. 2009;85(4):215–224. doi: 10.1016/j.earlhumdev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Robertson C.M., Watt M.J., Dinu I.A. Outcomes for the extremely premature infant: what is new? And where are we going? Pediatr. Neurol. 2009;40(3):189–196. doi: 10.1016/j.pediatrneurol.2008.09.017. (Mar 2009) [DOI] [PubMed] [Google Scholar]
- Rubia K. Functional brain imaging across development. Eur. Child Adolesc. Psychiatry. 2013;22(12):719–731. doi: 10.1007/s00787-012-0291-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S., Doyle L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371(9608):261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Saigal S., Stoskopf B., Streiner D. Transition of extremely low-birth-weight infants from adolescence to young adulthood. J. Am. Med. Assoc. 2006;295(6):667–675. doi: 10.1001/jama.295.6.667. [DOI] [PubMed] [Google Scholar]
- Schafer R.J., Lacadie C., Vohr B. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132(Pt 3):661–670. doi: 10.1093/brain/awn353. (Mar 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Benjamin J., Lacadie C.M. The intrinsic connectivity distribution: a novel contrast measure reflecting voxel level functional connectivity. NeuroImage. 2012;62(3):1510–1519. doi: 10.1016/j.neuroimage.2012.05.073. (Sep 2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheinost D., Finn E.S., Tokoglu F. Sex differences in normal age trajectories of functional brain networks. Hum. Brain Mapp. 2015;36(4):1524–1535. doi: 10.1002/hbm.22720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyser C.D., Snyder A.Z., Shimony J.S. Resting-state network complexity and magnitude are reduced in prematurely born infants. Cereb. Cortex. 2014 doi: 10.1093/cercor/bhu251. (Oct 2014 [Epub ahead of print]) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens M.C., Pearlson G.D., Calhoun V.D. Changes in the interaction of resting-state neural networks from adolescence to adulthood. Hum. Brain Mapp. 2009;30(8):2356–2366. doi: 10.1002/hbm.20673. (Aug 2009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam E.W., Ferriero D.M., Xu D. Cerebellar development in the preterm neonate: effect of supratentorial brain injury. Pediatr. Res. 2009;66(1):102–106. doi: 10.1203/PDR.0b013e3181a1fb3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59(1):431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.C., Miezin F.M., Peterson S.E. The putative visual word from area is functionally connected to the dorsal attention network. Cereb. Cortex. 2012;22:537–549. doi: 10.1093/cercor/bhr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.C., Petersen S.E., Schlaggar B.L. The left occipito-temporal cortex does not show preferential activity for words. Cereb. Cortex. 2012;22:2715–2732. doi: 10.1093/cercor/bhr295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.C., Church J.A., Power J.D. Functional network architecture of reading-related regions across development. Brain Lang. 2013;125:231–243. doi: 10.1016/j.bandl.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel A.C., Peterson S.E., Schlaggar B.L. The VWFA: it's not just for words anymore. Front. Hum. Neurosci. 2014;8(88):1–10. doi: 10.3389/fnhum.2014.00088. (Mar 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell B.A. The neurobiological basis of seeing words. Ann. N. Y. Acad. Sci. 2011;1224:63–80. doi: 10.1111/j.1749-6632.2010.05954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandell B.A., Rauschecker A.M., Yeatman J.D. Learning to see words. Annu. Rev. Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M., Hauser T.K., Krageloh-Mann I. Specific impairment of functional connectivity between language regions in former early preterms. Hum. Brain Mapp. 2014;35(7):3372–3384. doi: 10.1002/hbm.22408. (Jul 2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Rauschecker A.M., Wandell B.A. Anatomy of the visual word form area: adjacent cortical circuits and long-range white matter connections. Brain Lang. 2013;125(2):146–155. doi: 10.1016/j.bandl.2012.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T., Krienen F.M., Sepulcre J. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generated region outcomes adjusted for gender, race, and maternal education. Presented as LS means (95% confidence interval) and p values.
Group effect (difference in the correlations of regional connectivity and score between preterm and term at age 16), presented as p values. There is no significant group effect in any correlation between connectivity and scores.