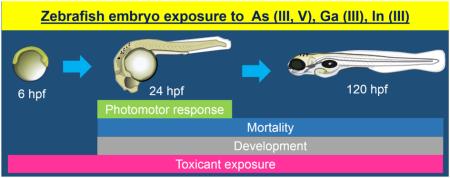
Abstract
Gallium arsenide (GaAs), indium gallium arsenide (InGaAs) and other III/V materials are finding increasing application in microelectronic components. The rising demand for III/V-based products is leading to increasing generation of effluents containing ionic species of gallium, indium, and arsenic. The ecotoxicological hazard potential of these streams is unknown. While the toxicology of arsenic is comprehensive, much less is known about the effects of In(III) and Ga(III). The embryonic zebrafish was evaluated for mortality, developmental abnormalities, and photomotor response (PMR) behavior changes associated with exposure to As(III), As(V), Ga(III), and In(III). The As(III) lowest observable effect level (LOEL) for mortality was 500 μM at 24 and 120 hours post-fertilization (hpf). As(V) exposure was associated with significant mortality at 63 μM. The Ga(III)-citrate LOEL was 113 μM at 24 and 120 hpf. There was no association of significant mortality over the tested range of In(III)-citrate (56-900 μM) or sodium citrate (213-3400 μM) exposures. Only As(V) resulted in significant developmental abnormalities with LOEL of 500 μM. Removal of the chorion prior to As(III) and As(V) exposure was associated with increased incidence of mortality and developmental abnormality suggesting that the chorion may normally attenuate mass uptake of these metals by the embryo. Finally, As(III), As(V), and In(III) caused PMR hypoactivity (49-69% of control PMR) at 900-1,000 μM. Overall, our results represent the first characterization of multidimensional toxicity effects of III/V ions in zebrafish embryos helping to fill a significant knowledge gap, particularly in Ga(III) and In(III) toxicology.
Keywords: III/V materials, arsenic, gallium, indium, ecotoxicity, zebrafish
Graphical abstract
1. Introduction
Gallium arsenide (GaAs) and indium gallium arsenide (InGaAs) are semiconductor materials containing elements from the groups III and V in the periodic table. GaAs, InGaAs, and alloys of other III/V materials are finding increasing application in semiconductor manufacturing due to their unique photonic properties (Jadvar et al., 1991; Yang and Chen, 2003; Torrance and Kennan, 2009). GaAs, the most widely used III/V material, has higher electronic speeds compared to silicon and is used in supercomputers and satellite communication systems (Flora and Dwivedi, 2012). GaAs also has superior properties as a photon emitter and is extensively used in the manufacture of light-emitting diodes (LEDs), laser diodes, photo detectors, and solar cells. The global demand for gallium and indium has increased many-fold during the past 30 years (USGS, 2013a, b), and it is expected to remain high as demand for III/V-based products continues to increase (White and Hemond, 2012). In 2013, the world production of Ga and In was 348 and 799 metric tons, respectively (USGS, 2013a, b).
The semiconductor industry shift towards the use of III/V materials is expected to lead to the generation of large amounts of slurries and waste streams containing dissolved III/V species, such as arsenite (As (III)), arsenate (As (V)), Ga(III), and In(III). The semiconductor industry treats their waste streams prior to discharge to municipal sewers or surface water to comply with federal regulations limiting the concentration of arsenic and other pollutants (Swartzbaugh and Sturgill, 1998; USEPA, 2015), but Ga and In are currently not regulated. Alkaline precipitation using calcium, magnesium or ferric hydroxides is often applied to remove these contaminants from semiconductor effluents but other methods have also been considered at the laboratory scale (Sturgill et al., 2000; Baranov et al., 2005).
Whereas arsenic toxicity has been widely studied, the ecotoxicity of III/V ions including Ga and In is still poorly characterized. There is a lack of studies reporting the toxicity of Ga(III) and In(III) in in vivo assays with aquatic organisms. Recently, some studies have looked at ecotoxicity of Ga(III) in marine and brackish fish (Onikura et al., 2005), and Ga(III) and In(III) in swamp shrimp (Yang, 2014), but in general the toxicants were in mixtures such as indium nitrite and indium tin oxide (Brun et al., 2014) or the studies were carried out in vitro (Zurita et al., 2007; Brun et al., 2014) .
Zebrafish (Danio rerio) embryos can function as water quality biosensors, and are emerging as a powerful tool to guide decision making around the development of environmentally safer consumer products (Lin and Hwang, 1998; Kim and Tanguay, 2013). A growing database of reference toxicant effects on a wide array of zebrafish developmental endpoints, and a wealth of comprehensive transcriptome data are enabling rapid pursuit of the mechanisms that underlie toxicant effects in this model (Howe et al., 2013; Kim and Tanguay, 2013; Dai et al., 2014; Truong et al., 2014).
In this study, we have evaluated developmental effects of As(III), As(V), In(III), and Ga(III) in zebrafish embryos using a 22-endpoint protocol with assessment at 24 and 120 hours post-fertilization (Truong et al., 2014). The results are expected to improve our understanding of the potential ecotoxicity implications of III/V ion contamination. The toxicity data can be used to support future development of appropriate effluent discharge standards for In(III) and Ga(III), for which there are no existing regulatory requirements.
2. Materials and Methods
2.1 Chemicals and solutions tested
The compounds tested in this study were: sodium arsenate heptahydrate (CAS# 10048-95-0, 99.99% purity, JT Baker, Phillipsburg, NJ), sodium arsenite (CAS# 7784-46-5, 99.99%, Fisher Scientific, Waltham, MA), indium chloride tetrahydrate (CAS# 10025-82-8, 99.99%, Strem Chemicals, Newburyport, MA), gallium chloride (CAS# 13450-90-3, 99.99%, Acros Organics-Fisher Scientific), and sodium citrate (CAS# 6132-04-3, 99.95%, Fisher Scientific). To avoid precipitation due to the low solubility of In(III)- and Ga(III) at circumneutral pH (Wood and Samson, 2006), the stock solutions of In and Ga were supplied with citrate at a molar ratio 1:3.75, metal:citrate.. Stock solutions of III/V ions (56-1,000 μM) were prepared in embryo medium (EM), which contained (in mg L−1): NaCl (875.0), KCl (37.5), CaCl2•H2O (145.0), KH2PO4 (20.5), Na2HPO4 (7.1), and MgSO4•7H2O (4.9). The pH value of these solutions was adjusted with NaOH to be within the range of 6-8 to avoid embryo toxicity effects caused by low pH levels.
2.2 Zebrafish embryo assays
The protocol outlined by Truong et al. (2014) was used to test the III/V ions. Briefly, 4 hour post-fertilization (hpf) embryos were dechorionated enzymatically using pronase as described previously (Truong et al., 2014). The zebrafish embryos were exposed to the tested solutions upon 6 hpf in 96-well plates in embryo medium. A total of 32 embryos were exposed to each concentration of chemical used and to the embryo medium controls. The plates were incubated at 28°C in the dark. Upon 24 hpf, morphological assessment and photomotor response behavior using a custom photomotor response activity tool (PRAT) based on tail movement were performed (Truong et al., 2014). The plates were placed back in the incubator until 120 hpf, when a second morphological assessment was performed. Table 1 shows the endpoints assessed at 24 and 120 hpf. Endpoint scoring and statistical analyses were performed in R software as described previously (Truong et al., 2014).
Table 1.
Morphology endpoints assessed at 24 and 120 hours post fertilization (hpf).
| Endpoint | 24 hpf | 120 hpf |
|---|---|---|
| Mortality | ||
| Developmental delay | ||
| Spontaneous movement | ||
| Notochord | ||
| Yolk sac edema | ||
| Body axis | ||
| Eye defect | ||
| Snout | ||
| Jaw | ||
| Otic vesicle | ||
| Pericardial edema | ||
| Brain | ||
| Somite | ||
| Pectoral fin | ||
| Caudal fin | ||
| Pigment | ||
| Circulation | ||
| Truncated body | ||
| Touch response |
The toxicity effects of As(III) and As(V) was also tested in embryos whose chorions were not removed in order to determine if there were any permeability limitations with these ions. Citrate complexation with In(III) and Ga(III) could cause further complications with chorion permeability and therefore these two ions were not testes with zebrafish embryos with chorions. Otherwise, the methodology used in the toxicity assays was similar to the one described before.
3. Results and Discussion
Mortality, developmental, and behavioral effects of arsenite (As(III)), arsenate (As(V)), indium (III), and gallium (III) to zebrafish embryos were evaluated using a 22-endpoint protocol with assessment at 24 and 120 hours post-fertilization (hpf) with an exposure range of 56-1000 μM. The range chosen includes environmentally relevant levels of the toxicants, which have been reported as low as 0.08 μM for indium and 0.45 μM of arsenic in groundwater next to a semiconductor industry park (Flora and Dwivedi, 2012), and up to 270 mM for gallium and 130 mM for arsenic based on direct waste solutions from GaAs semiconductor etching (Baranov et al., 2005). In the United States, arsenic limitations for point source discharges of effluents from facilities manufacturing electrical and electronic components are 2.09 mg L−1 (1-day maximum) and 0.83 mg L−1 (30-day average) (USEPA, 2015).
3.1 Mortality
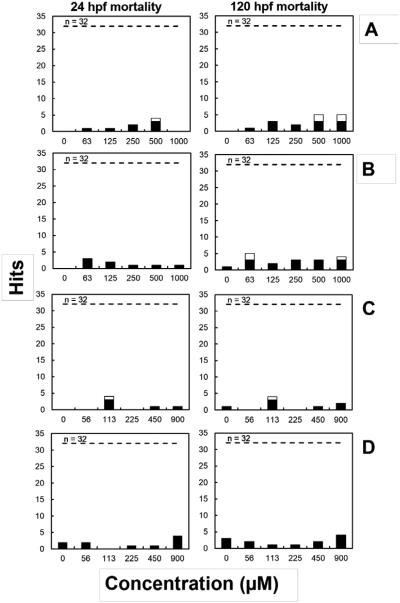
Mortality was the main endpoint significantly associated with III/V ion exposures (Fig. 1). On a molar basis, As(III) exposure was associated with the highest rate of mortality, with a LOEL (12.5-15.6% mortality) at 24 and 120 hpf of 500 μM. The mortality LOEL for As(III) is in agreement with findings from a similar study (Li et al., 2009) reporting no significant zebrafish embryo mortality at concentrations lower than 0.5 mM. As(V) exposure was associated with low but significant mortality at 63 μM (15.6%) at 120 hpf. Mortality was not associated with the intermediate concentrations tested. Zebrafish have been reported to be more tolerant to As(V) toxicity compared to other fish species. A study with zebrafish larvae determined a 96-h LC50 of 1.43 mM for As(V) and noted that zebrafish are less sensitive to As(V) than fathead minnows (Pimephales promelas), rainbow trout (Oncorhynchus mykiss), and Colorado squawfish (Ptychocheilus lucius), whose LC50 range from 450-580 μM (Liu et al., 2005).
Fig. 1.
Mortality incidence in zebrafish embryos (out of 32 replicates) after 24 and 120 hpf. Rows: As(III) (A), As(V) (B), Ga(III)-citrate (C), In(III)-citrate (D). White bars indicate hits above the statistically significant threshold (p ≤ 0.05).
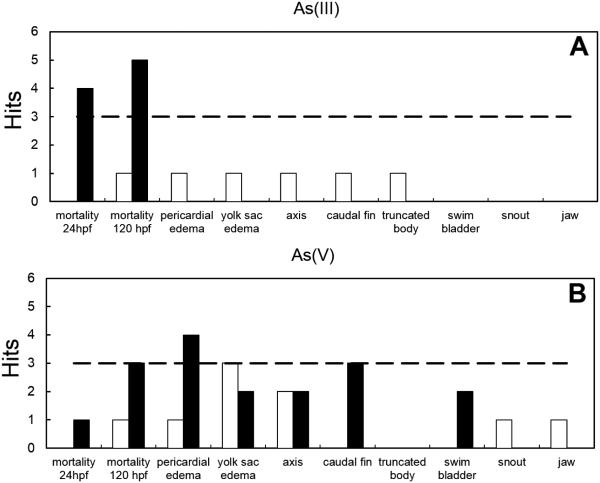
Mortality endpoints following exposure to 500 μM As(III) and As(V) with and without chorions was compared to determine the effects of the presence of the chorion in the metalloid uptake and impacts to toxicity (Fig. 2). In general, there was a higher incidence of mortality for dechorionated embryos without chorions exposed to As(III) at 24 and 120 hpf. These results suggested that the presence of the chorion could represent a barrier to uptake of As(III) and As (V) that might affect mortality endpoints.
Fig. 2.
Comparison of active endpoints for 500 μM As(III) (Panel A) and As(V) (Panel B) exposure to zebrafish embryos with chorions (white bars) and without chorions (black bars). The dashed line is the statistical significance threshold (p ≤ 0.05) for endpoints with total hits above the threshold.
Ga(III) exposure was associated with low but significant mortality (12.5%) at 113 μM for 24 and 120 hpf, but not at higher concentrations up to 900 μM. It is possible that the single occurrence for statistical significance mortality at 113 μM but not at higher doses might have been due to an increased number of non-viable embryos for that concentration at the beginning of the assay, since there was a mortality incidence higher than the statistical significance threshold as early as 24 hpf. We are not aware of any previous study reporting on lethality of Ga(III) or In(III) in zebrafish embryos. Nonetheless Ga(III) has been shown to display some inhibitory effects on fish and other aquatic species at moderate concentrations. The 96-h LC50 value of Ga2(SO4)3 for common carp (Cyprinus carpio) was reported as 0.28 mM (Yang and Chen, 2003). Moreover, the 24-h LC50 of Ga(Cl)3 for two crustacean species, Americamysis bahia and Artemia salina, were 0.33 and 0.78 mM, respectively (Onikura et al., 2005). These results suggest that zebrafish embryos are less sensitive to Ga(III) than other aquatic species.
In(III) citrate exposures were not associated with significant mortality over the 56 - 900 μM test range). Likewise, sodium citrate did not result in significant mortality rates (Fig. S-1).
3.2 Morphology/developmental endpoints
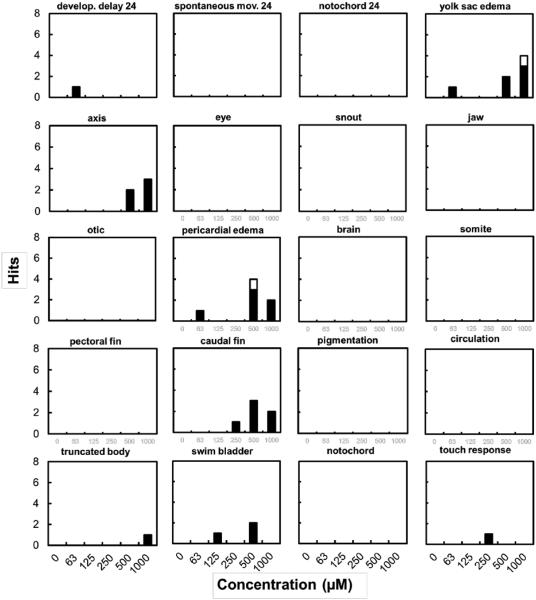
3.2.1 As (III) and As(V)
Overall, developmental exposure to the III/V metal ions was associated with very modest developmental toxicity over a wide range of concentrations (56 - 1,000 μM). Other than mortality, most developmental endpoints were unaffected (Supplementary Material Figs. S-2 through S-5). At 120 hpf, the high range As(V) exposures were associated with yolk sac edema (LOEL= 1,000 μM) and pericardial edema (LOEL= 500 μM) (Fig. 3). Malformations were not associated with As(III) exposures up to 1 mM. We note that exposure of zebrafish embryos while still in the chorion to very high As(III) concentrations (5 mM or 375 mg L−1) has been previously reported to lead to 97% incidence of developmental abnormalities (Li et al., 2009).
Fig. 3.
Activity of developmental endpoints in zebrafish embryo assay for As(V). White bars indicate hits above the statistically significant threshold (p ≤ 0.05). In all treatments and controls, the total number of embryos assayed was 32.
Developmental abnormalities were also assessed in embryos with and without chorions following 500 μM exposure to As(III) and As(V). While overall the significant hits were low, there was a higher incidence for abnormalities in dechorionated embryos, similar to the results found for mortality endpoints. Statistical significant hits were obtained in the pericardial edema endpoint for As(V) exposure (Fig. 2), compared to non-significant hits obtained in the embryo with chorion for the same endpoint.
Based on the increased incidence of endpoint hits in the dechorionated embryos, it is possible that there is a diffusion problem across the chorion even for small inorganic molecules, such as As(III) and As(V) ions. The zebrafish chorion has been reported to reduce the permeability of organic molecules (Brox et al., 2014) ; however, for heavy metals it could also represent a barrier since these could accumulate at the surface or inside the chorion (Henn and Braunbeck, 2011). Moreover, in the case of arsenic, low bioaccumulation factors have been reported for As2O3 in zebrafish larvae, which may also affect its bioavailability (López-Serrano Oliver et al., 2011). Taken these points into consideration, our results suggest that the chorion could be a barrier for the transport of As(III, V) and that dechorionated embryos would be more sensitive to negative effects since the toxicant would be more bioavailable. Similar trends have been observed in studies with zebrafish embryos exposed to silver nanoparticles (Kim and Tanguay, 2014). The authors attributed the higher sensitivity of dechorionated embryos to the protective role of the chorion in limiting embryonic permeability by serving as a barrier for agglomerated nanoparticles (Kim and Tanguay, 2014).
3.2.2 Ga (III)-citrate and In(III)-citrate
Developmental exposure to Ga(III) and In(III) citrate complexes was not associated with abnormalities in any of the endpoints examined. An in vitro study reported that the no observable adverse effect level (NOAEL) for the fish cell line PLHC-1 exposed to In(III), was 1.4 mM, and that morphological changes occurred at higher concentrations. Moreover, cell death occurred at 3 mM for an exposure period of 24 h (Zurita et al., 2007). Sodium citrate was also not active in any of the endpoints tested (Fig. S-5).
3.3 Photomotor response (PMR) behavioral assay
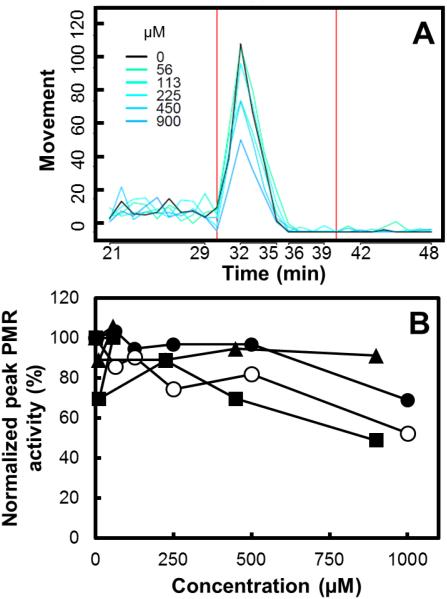
Embryonic tail flexions of the photomotor response (PMR) at 24 hpf are an involuntary neuro-motor phenomenon important for normal trunk muscle, central nervous system (CNS) and cardiovascular development in zebrafish (Bruni et al., 2014). Stimulation of a burst of tail flexions in the PMR with bright white light is a highly sensitive assay for which abnormal responses are highly predictive of later adverse developmental outcomes (Reif et al., 2015). An example of the 24 hpf zebrafish activity recorded in the PMR for In(III)-citrate is shown in Fig. 4A Hypoactivity at the excitation phase after the initial light pulse in the PMR was associated with exposure to As(III), As(V), and In(III)-citrate, at the highest concentrations tested (900-1,000 μM), resulting in 31-51% reduction of the control movement index (Fig. 4, Fig. S-6). Ga-citrate and sodium citrate were not associated with changes in photomotor activity across the concentrations tested; 56-900 and 213-3,400 μM, respectively (Fig. S-7).
Fig. 4.
Photomotor response (PMR) behavioral assay. Panel A – Average movement in 24 hpf embryos exposed to In(III)-citrate. Red line indicates a light pulse. Panel B - Normalized peak movement index upon initial light pulse for As(III) (●), As(V) (○), In(III)-citrate (■), and Ga(III)-citrate (▲).
Metals may act as neurotoxicants (Rodríguez et al., 2003; Lee and Freeman, 2014). In particular, As(III) has been reported to alter neural function in zebrafish embryos (Li et al., 2009; Lee and Freeman, 2014) at relatively high concentrations (2 mM) based on reduced photo-induced reflexive motion and immunostaining results for axon tracts of nerve cells (Li et al., 2009). As(V) at 0.75 mM has been reported to impair long term memory in avoidance training behavioral tasks in zebrafish due to neurotransmitter and antioxidant system alterations (de Castro et al., 2009). Finally, while hypoactivity was also detected at the highest concentration tested for In(III)-citrate (900 μM), no literature to our knowledge is available on potential neurotoxicity effects and mechanisms for this toxicant and more studies are needed.
Although the embryonic zebrafish model is a powerful tool for understanding how cells in vertebrates, including humans, respond to environmental chemicals, the fact that the embryos are not capable of independent feeding during the duration of the bioassay (120 hpf) (Straehle et al., 2012) precludes testing of exposure to chemicals through food. Therefore, additional testing using juvenile- and adult-life stages of zebrafish and/or other relevant aquatic species is required to understand the health risks of chemicals to aquatic organisms and assess the impact of chemical bioavailability. Dietary exposure can have a significant contribution to the overall bioaccumulation and toxicity of some metals and metalloids in aquatic environments (Handy, 2009; Wang, 2013).
4. Conclusions
The III/V materials tested, As(III), As(V), Ga(III), and In(III) caused modest mortality, developmental, and PMR behavioral effects in assays with the zebrafish embryos at concentrations up to 1,000 μM. These results taken globally indicate that polluted streams with these materials pose limited toxicological effects to zebrafish embryos and other related vertebrate species. However, this study represents the first characterization of toxicological comprehensive in vivo vertebrate work for In(III) and Ga(III) as individual ionic species. We hope that the insights provided by this work will help start to fill the knowledge gaps on toxicology literature on III/V ions, and particularly on In(III) and Ga(III).
Supplementary Material
Highlights.
As(III), As(V), Ga(III) caused significant but moderate mortality (500-1,000 μM).
Only As(V) caused developmental abnormalities (LOEL 500 μM).
Zebrafish embryo dechorionation increased endpoint incidence for As(III) and As(V).
As(III), As(V), In (III) (0.9-1 mM) significantly reduced photomotor response activity.
Acknowledgments
This work was supported by the Semiconductor Research Corporation (SRC) Engineering Research Center for Environmentally Benign Semiconductor Manufacturing and the NIEHS Superfund Research Program (P42 ES04940). We would like to thank the Sinnhuber Research Laboratory/Tanguay lab members and screening team (Greg Gonnerman). CIO was supported in part by the Mexican National Council for Science and Technology (CONACyT) and the University of Arizona Water Sustainability Program. His research stay at the Dr. Tanguay’s lab was supported by a NIEHS KC Donnelly Externship 2014 award.
5. References
- Baranov S, Cinic B, Redwing J, Stavila V. Gallium arsenate removal from waste waters. Moldavian J. Phys. Sci. 2005;4:222–230. [Google Scholar]
- Brox S, Ritter AP, Kuster E, Reemtsma T. Influence of the perivitelline space on the quantification of internal concentrations of chemicals in eggs of zebrafish embryos (Danio rerio) Aquat. Toxicol. 2014;157:134–140. doi: 10.1016/j.aquatox.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Brun NR, Christen V, Furrer G, Fent K. Indium and indium tin oxide induce endoplasmic reticulum stress and oxidative stress in zebrafish (Danio rerio) Environ. Sci. Technol. 2014;48:11679–11687. doi: 10.1021/es5034876. [DOI] [PubMed] [Google Scholar]
- Bruni G, Lakhani P, Kokel D. Discovering novel neuroactive drugs through high-throughput behavior-based chemical screening in the zebrafish. Front. Pharmacol. 2014;5:1–7. doi: 10.3389/fphar.2014.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y-J, Jia Y-F, Chen N, Bian W-P, Li Q-K, Ma Y-B, Chen Y-L, Pei D-S. Zebrafish as a model system to study toxicology. Environ. Toxicol. Chem. 2014;33:11–17. doi: 10.1002/etc.2406. [DOI] [PubMed] [Google Scholar]
- de Castro MR, Lima JV, de Freitas DP, Valente Rde S, Dummer NS, de Aguiar RB, dos Santos LC, Marins LF, Geracitano LA, Monserrat JM, Barros DM. Behavioral and neurotoxic effects of arsenic exposure in zebrafish (Danio rerio, Teleostei: Cyprinidae) Comp. Biochem. Phys. C. 2009;150:337–342. doi: 10.1016/j.cbpc.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Flora SJS, Dwivedi N. A toxicochemical review of gallium arsenide. Defence Sci. J. 2012;62:95–104. [Google Scholar]
- Handy RD. Dietary exposure to toxic metals in fish. In: Taylor EW, editor. Toxicology of aquatic pollution: physiological, molecular and cellular approaches. Cambridge University Press; Cambridge, MA.: 2009. pp. 29–60. [Google Scholar]
- Henn K, Braunbeck T. Dechorionation as a tool to improve the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) Comp. Biochem. Phys. C. 2011;153:91–98. doi: 10.1016/j.cbpc.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL, Begum S, Lloyd C, Lanz C, Raddatz G, Schuster SC. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadvar R, McCoy BJ, Ford B, Galt J. Recovery of gallium and arsenic from GaAs wafer manufacturing slurries. Environ. Prog. 1991;10:278–281. [Google Scholar]
- Kim K-T, Tanguay RL. Integrating zebrafish toxicology and nanoscience for safer product development. Green Chem. 2013;15:872–880. doi: 10.1039/C3GC36806H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KT, Tanguay RL. The role of chorion on toxicity of silver nanoparticles in the embryonic zebrafish assay. Environ. Health Toxicol. 2014;29:1–6. doi: 10.5620/eht.e2014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Freeman J. Zebrafish as a model for developmental neurotoxicity assessment: The application of the zebrafish in defining the effects of arsenic, methylmercury, or lead on early neurodevelopment. Toxics. 2014;2:464–495. [Google Scholar]
- Li D, Lu C, Wang J, Hu W, Cao Z, Sun D, Xia H, Ma X. Developmental mechanisms of arsenite toxicity in zebrafish (Danio rerio) embryos. Aquat. Toxicol. 2009;91:229–237. doi: 10.1016/j.aquatox.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Lin HC, Hwang PP. Acute and chronic effects of gallium chloride (GaCl3) on tilapia (Oreochromis mossambicus) larvae. B. Environ. Contam. Tox. 1998;60:931–935. doi: 10.1007/s001289900717. [DOI] [PubMed] [Google Scholar]
- Liu F, Kendall RJ, Theodorakis CW. Joint toxicity of sodium arsenate and sodium perchlorate to zebrafish Danio rerio larvae. Environ. Toxicol. Chem. 2005;24:1505–1507. doi: 10.1897/04-313r.1. [DOI] [PubMed] [Google Scholar]
- López-Serrano Oliver A, Sanz-Landaluze J, Muñoz-Olivas R, Guinea J, Cámara C. Zebrafish larvae as a model for the evaluation of inorganic arsenic and tributyltin bioconcentration. Water Res. 2011;45:6515–6524. doi: 10.1016/j.watres.2011.09.052. [DOI] [PubMed] [Google Scholar]
- Onikura N, Nakamura A, Kishi K. Acute toxicity of gallium and effects of salinity on gallium toxicity to brackish and marine organisms. B. Environ. Contam. Tox. 2005;75:356–360. doi: 10.1007/s00128-005-0761-5. [DOI] [PubMed] [Google Scholar]
- Reif DM, Truong L, Mandrell D, Marvel S, Zhang G, Tanguay RL. High-throughput characterization of chemical-associated embryonic behavioral changes predicts teratogenic outcomes. Arch. Toxicol. 2015 doi: 10.1007/s00204-015-1554-1. DOI 10.1007/s00204-015-1554-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez VM, Jiménez-Capdeville ME, Giordano M. The effects of arsenic exposure on the nervous system. Toxicology Lett. 2003;145:1–18. doi: 10.1016/s0378-4274(03)00262-5. [DOI] [PubMed] [Google Scholar]
- Straehle U, Scholz S, Geisler R, Greiner P, Hollert H, Rastegar S, Schumacher A, Selderslaghs I, Weiss C, Witters H, Braunbeck T. Zebrafish embryos as an alternative to animal experiments-A commentary on the definition of the onset of protected life stages in animal welfare regulations. Reprod. Toxicol. 2012;33:128–132. doi: 10.1016/j.reprotox.2011.06.121. [DOI] [PubMed] [Google Scholar]
- Sturgill JA, Swartzbaugh JT, Randall PM. Pollution prevention in the semiconductor industry through recovery and recycling of gallium and arsenic from GaAs polishing wastes. Clean Techn. Environ. Policy. 2000;2:0018–0027. [Google Scholar]
- Swartzbaugh JT, Sturgill JA. Reduction of arsenic wastes in the semiconductory industry. University of Dayton Research Institute - Environmental Science and Engineering Group; Dayton, OH, USA: 1998. [Google Scholar]
- Torrance K, Kennan HE. Characterization of arsenic-rich waste slurries generated during gallium arsenide wafer lapping and polishing. 2009 International Conference on Compound Semiconductor Manufacturing Technology; Tampa, Florida, USA. 2009. [Google Scholar]
- Truong L, Reif DM, St Mary L, Geier MC, Truong HD, Tanguay RL. Multidimensional in vivo hazard assessment using zebrafish. Toxicol. Sci. 2014;137:212–233. doi: 10.1093/toxsci/kft235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USEPA 2015. Effluent guidelines and standards. Part 469. Electrical and electronic components point source category. Effluent limitations representing the degree of effluent reduction attainable by the application of the best practicable control technology currently available (BPT). Title 40 e-C.F.R. § 469.24.
- USGS Kelly TD, Matos GR, editors. U.S. Geological Survey. Gallium statistics [through 2013; last modified January 30, 2015] Historical statistics for mineral and material commodities in the United States (2015 version): U.S. Geological Survey Data Series 140. 2013a.
- USGS Kelly TD, Matos GR, editors. U.S. Geological Survey. Indium statistics [through 2013; last modified January 30, 2015] Historical statistics for mineral and material commodities in the United States (2015 version): U.S. Geological Survey Data Series 140. 2013b.
- Wang W-X. Dietary toxicity of metals in aquatic animals: Recent studies and perspectives. Chinese Sci. Bull. 2013;58:203–213. [Google Scholar]
- White SJO, Hemond HF. The anthrobiogeochemical cycle of indium: A review of the natural and anthropogenic cycling of indium in the environment. Crit. Rev. Env. Sci. Tec. 2012;42:155–186. [Google Scholar]
- Wood SA, Samson IM. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006;28:57–102. [Google Scholar]
- Yang J-L. Comparative acute toxicity of gallium(III), antimony(III), indium(III), cadmium(II), and copper(II) on freshwater swamp shrimp (Macrobrachium nipponense) Biol. Res. 2014;47:1–4. doi: 10.1186/0717-6287-47-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-L, Chen H-C. Effects of gallium on common carp (Cyprinus carpio): acute test, serum biochemistry, and erythrocyte morphology. Chemosphere. 2003;53:877–882. doi: 10.1016/S0045-6535(03)00657-X. [DOI] [PubMed] [Google Scholar]
- Zurita JL, Jos A, del Peso A, Salguero M, Cameán AM, López-Artíguez M, Repetto G. Toxicological assessment of indium nitrate on aquatic organisms and investigation of the effects on the PLHC-1 fish cell line. Sci. Total. Environ. 2007;387:155–165. doi: 10.1016/j.scitotenv.2007.07.057. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.