Abstract
Lesion-deficit studies support the hypothesis that the left anterior temporal lobe (ATL) plays a critical role in retrieving names of concrete entities. They further suggest that different regions of the left ATL process different conceptual categories. Here we test the specificity of these relationships and whether the anatomical segregation is related to the underlying organization of white matter connections. We reanalyzed data from a previous lesion study of naming and recognition across five categories of concrete entities. In voxelwise logistic regressions of lesion-deficit associations, we formally incorporated measures of disconnection of long-range association fiber tracts (FTs) and covaried for recognition and non-category specific naming deficits. We also performed fiber tractwise analyses to assess whether damage to specific FTs was preferentially associated with category-selective naming deficits. Damage to the basolateral ATL was associated with naming deficits for both unique (famous faces) and non-unique entities, whereas the damage to the temporal pole was associated with naming deficits for unique entities only. This segregation pattern remained after accounting for comorbid recognition deficits or naming deficits in other categories. The tractwise analyses showed that damage to the uncinate fasciculus was associated with naming impairments for unique entities, while damage to the inferior longitudinal fasciculus was associated with naming impairments for non-unique entities. Covarying for FT transection in voxelwise analyses rendered the cortical association for unique entities more focal. These results are consistent with the partial segregation of brain system support for name retrieval of unique and non-unique entities at both the level of cortical components and underlying white matter fiber bundles. Our study reconciles theoretic accounts of the functional organization of the left ATL by revealing both category-related processing and semantic hub sectors.
Keywords: naming, object recognition, anterior temporal cortex, fiber tract disconnection, lesiondeficit relationship, lexical retrieval
1. Introduction
A growing body of literature supports the hypothesis that the anterior temporal lobes (ATLs) play a critical role in the retrieval of semantic knowledge. Accessing semantic knowledge entails activating meaning-based mental representations of entities (e.g. 'rabbit') that are abstracted from specific autobiographical experiences (Tulving, 1972).
Evidence that the ATLs are critical for accessing semantic knowledge has come primarily from two lines of inquiry: 1) lesion-deficit association studies of patients with stable circumscribed brain damage (Damasio et al, 1996, 2004; Drane et al 2008, 2013) and 2) analysis and computational modeling of cognitive impairments associated with semantic dementia (Hodges et al., 1992; Lambon Ralph et al., 2001; Rogers et al., 2004, 2006; Patterson et al., 2007). While both lines of research document a systematic relationship between ATL damage and semantic retrieval impairments, they have reached different conclusions regarding the functional organization of the ATLs with respect to semantic processing. Lesion studies have supported the hypothesis that partially segregated systems within the ATLs support access to different types of semantic knowledge, with the left ATL specifically supporting access to verbal stores. By contrast, computational models designed to explain semantic dementia symptoms have supported the hypothesis that the ATLs function as an integrated semantic system. Central to these differing interpretations are two longstanding topics of debate in neuropsychology: the organization of the brain in integrated versus modality-specific, and/or category-related semantic knowledge store(s); and the interfacing of language with conceptual knowledge.
Lesion-deficit reports have emphasized that semantic deficits can be selective in nature, and that the selectivity is related to the topography of brain damage within and beyond the ATLs. In their original and subsequent studies, Damasio and colleagues investigated the neural correlates of selective deficits by separately operationalizing and assessing recognition and naming performance for several categories of concrete entities in a large group of lesion participants (Damasio et al., 1996, 2004; Tranel et al., 1997). The results revealed a systematic relationship between the anatomic location of brain damage and the presence of impairments pertaining to specific conceptual categories of knowledge [e.g. unique ('Judy Garland') vs. non-unique ('rabbit')]. Moreover, the pattern of findings supported the hypothesis that the neural substrates of name retrieval are distinct from those of concept retrieval (those that are sufficient for recognition but not naming), and that there is partial functional segregation of different sectors of the left ATL in supporting the retrieval of names from different semantic categories (Damasio et al., 1996, 2004). These results also highlighted the partial dissociation of the left temporal pole (LTP) and left inferotemporal cortex in the retrieval of names of unique entities (foremost famous persons) and non-unique entities, respectively, as well as the roles of the right temporal pole and posterior association regions in the recognition of unique versus non-unique entities (Damasio et al., 1996, 2004; Tranel et al., 1997, 2008).
Damasio (1989) proposed that an architecture based on convergent (and reciprocally divergent) structural connectivity could explain the observed anatomical dissociations of semantic retrieval deficits in the brain (Damasio et al., 1996; 2004). According to this framework, conceptual knowledge is grounded in the binding pattern of multimodal “images” that are represented in early sensory and motor cortices. These cortices project onto higher-order association regions functioning as convergence-divergence systems that implement the binding pattern and provide feedback modulating the activity of sensory and motor cortices (Damasio, 1989; Mesulam, 2001). This arrangement, which is in part hierarchical, and involves bidirectional information flow, provides a functional architecture to support semantic knowledge as follows. 1) Cortical areas that process information about sensorimotor, interoceptive and/or emotional attributes support images that are the phenomenological primitives for the development of conceptual knowledge. 2) Sensorimotor neural patterns encoded in these areas successively converge on higher-order regions that integrate and abstract information as the basis for semantic representations of entities. 3) These latent representations may be retrieved through reactivation of bound patterns in early sensorimotor regions using the same hierarchy of convergence-divergence regions. 4) Category-related functional segregation effects emerge due to differences in the sensorimotor modalities and contingencies associated with different conceptual representations of entities (Warrington & Shallice, 1984; Warrington & McCarthy, 1987; Farah & McClelland, 1991). These requirements dictate the cortical location of the corresponding convergence-divergence regions. 5) Lexical retrieval is supported by regions that mediate the link between conceptual and word-form knowledge. As such, category-related anatomic effects propagate to convergence-divergence regions supporting retrieval of entity names, which are organized anatomically to link the convergence-divergence regions supporting their recognition and left perisylvian convergence-divergence regions supporting access to phonology and production.
An alternative account of the functional organization of semantic processing in the brain has come from research on semantic dementia (SD), a disease characterized by progressive atrophy of the ATLs. Studies of SD have emphasized the longitudinal correlation between ATL atrophy and domain-general semantic knowledge impairments. Unlike patients with stable focal lesions, SD patients have semantic retrieval impairments that may not be specific to any category and can manifest at both lexical and conceptual levels of processing. The symptoms of SD have been used to support explanatory computational models in which the left and right ATLs correspond to a “unitary” semantic hub (Rogers et al., 2004). According to this view, lexical information is on par with other modalities of information (e.g. visual) that comprise semantic representations of entities.
The pattern of ATL atrophy in SD has typically been characterized as anatomically undifferentiated, limiting inferences about the finer functional organization of the ATLs. While atrophy in SD eventually affects both ATLs entirely, it is characteristically right-left asymmetric in early stages of the disease, and sometimes markedly so. In some cases of left-predominant polar atrophy, the initial symptom is anomia (Graham et al., 1995; Czarnecki et al., 2008), which reflects a pure failure to activate phonology, then a bi-directional inability to link word-form and meaning appears, and finally a frank nonverbal semantic loss develops as atrophy becomes more extensive and bilateral. In other cases of right-predominant atrophy, a selective failure to recognize faces of persons has been documented (Simons et al., 2001; Snowden et al., 2012). These SD findings provide some evidence for modality-specific anatomic segregation of function in agreement with the aforementioned lesion work. The phenomena of SD thus provide incomplete support for a “unitary” system if that notion is taken in the strongest sense to mean a distributed semantic store encompassing all conceptual categories, levels of specificity, and verbal or nonverbal modalities.
Over the years, other experimental approaches, including PET, fMRI, MEG, repetitive transcranial magnetic stimulation, and intraoperative cortical and fiber tract stimulation have emerged and been used to investigate the neural support for semantic knowledge (cf. Wong and Gallete, 2012). While these approaches provide support for a role for the ATLs in semantic retrieval (but see Visser et al., 2010), they have not been able to adjudicate between the differing accounts of this role. Conclusions have varied as to whether the ATLs act in concert in a unitary semantic system (Bright et al., 2004; Rogers et al., 2006; Lambon Ralph et al., 2009), whether lexical aspects are left lateralized (Mummery et al., 1999; Grabowski et al., 2001; Noppeney and Price, 2002; Thompson-Schill, 2003; Spitsyna et al., 2006), or whether distinct subregions within the ATLs support different types of semantic knowledge (Binney et al., 2010; Damasio et al., 1996; Devlin et al., 2002; Grabowski et al., 2001; Simmons et al., 2010; Skipper et al., 2011). Nevertheless this work has complemented neuropsychological studies by situating the left ATL in the context of a larger dynamic system involved in semantic processing. While lesion-deficit studies focus on identifying critical regions whose damage impairs the normal semantic process, functional imaging studies identify the extended functional network(s). Results from these studies have shown that occipito-temporal, frontal, and parietal regions (outside the classical language areas) are also recruited during semantic processing (cf. Binder et al., 2009). Studies using EEG and MEG have identified the timing of ATL involvement within the network, revealing recurrent interactions between the left ATL, a putative integrative hub, and more posterior regions involved in perceptual processes (Clarke et al., 2011; van Ackeren MJ and Rueschemeyer SA; Clarke et al., 2014). It is also informative that stimulation of long-range fiber tracts connecting the ATL and posterior and frontal regions has been shown to disrupt semantic knowledge retrieval (Shinoura et al., 2010; Papagno et al., 2011; Von der Heide, 2013).
Recent cytoarchitectonic and functional connectivity studies provide a functional anatomic basis for reconciling unitary and modality-related views of left ATL support of semantic knowledge retrieval (Ding et al., 2009; Fan et al., 2014 Binney et al., 2012; Pascual et al., 2013) by showing how both are plausible and likely. These studies reveal that the left ATL is comprised of a mosaic of cortical sectors with distinct histological and connectivity profiles. There are several architecturally distinct subregions within the temporal pole in addition to area TG (corresponding to classical Brodmann area 38; Von Economo, 1929). These areas extend outside the temporal pole proper, and include anterolaterally situated areas at the apex of the ventral visual stream (TE), superiorly situated high-order auditory and polymodal association areas (TAr, TAp), inferomedially situated olfactory/insular regions (TI), and the rostral extension of entorhinal cortex and other medial temporal regions (areas 35 and 36) (Ding et al., 2009). Since the ATL appears segregated according to patterns of anatomical connectivity, and such patterns within cortical sectors are thought to contribute critically to their functional specialization (Passingham et al., 2002; Knösche and Tittgemeyer, 2011), we can surmise that the different subregions within the ATL support different aspects of semantic knowledge retrieval. Of relevance are two patterns of connectivity that distinguish between ATL subregions. The first is found in the rostral ATL including the temporal pole, and consists of long-range association fiber tract terminations (Catani et al., 2003; Kier et al., 2004; Martino et al., 2010; Martino et al., 2011; Axer et al., 2013). Two relevant tracts are the inferior longitudinal fasciculus, with connections to posterior occipito-temporal visual association cortices; and the uncinate fasciculus, with connections to orbital and polar frontal cortices (Shinoura et al., 2010; Martino et al., 2011; Dick and Tremblay, 2012; Von der Heide, 2013; Lam et al., 2014). Both tracts have been implicated in name retrieval, with some evidence for their differential involvement depending on conceptual category and/or level of specificity (Shinoura et al., 2010; Papagno et al., 2011; Von der Heide, 2013). ATL subdivisions targeted by the inferior longitudinal fasciculus and the uncinate fasciculus can reasonably be hypothesized to support semantic access in which visual perceptual properties and social/emotional properties, respectively, are key constituents. The second pattern of connectivity is found in a basolateral temporal region, caudal to the temporal pole, where connections are predominantly intra- versus extra-temporal (Binney et al., 2012). This second-order pattern of connectivity (i.e. interconnection of regions that have different profiles of connections to modal association regions) is compatible with a function of transmodal integration to support general semantic knowledge access (Binney et al., 2012).
The above anatomical and theoretical considerations led us to an overarching hypothesis that connectivity plays a critical role in the functional organization of the left ATL with respect to semantic knowledge retrieval. In other words, certain cortical sectors and association fiber tracts are inter-related components of large-scale systems supporting semantic retrieval and jointly critical for normal performance. Thus we hypothesized that damage to rostral ATL sectors would be associated with category-selective naming impairments and damage to the left basolateral ATL would be associated with category-general naming impairments. We also hypothesized that there would be parallel associations between category-selective naming deficits and damage to specific long-range association fiber tracts with terminations in the left ATL, consistent with the nature of the information they carry, e.g. visual perceptual versus emotional.
To test these hypotheses, we reanalyzed the data from a previous lesion study of the neural basis for naming versus recognition deficits across five conceptual categories (Damasio et al., 2004). We leveraged interim advances in fiber tract anatomy and extended lesion methods developed to provide an analysis framework to formally address the functional specificity of damage to cortical regions, and/or white matter fiber tracts (Rudrauf et al., 2008b; Thiebaut de Schotten et al., 2008; Philippi et al., 2009; Galantucci et al., 2011; Turken and Dronkers, 2011; Han et al., 2013).
2. Materials and methods
2.1. Dataset
We reanalyzed the data from the Damasio 2004 study. The participants, materials, and methods (e.g., experimental protocol, MRI acquisition, lesion delineation) were described extensively in that previous publication (Damasio et al., 2004) and are briefly summarized below.
2.1.1. Participants
Data came from 129 participants with unilateral circumscribed, stable brain lesions; those same participants were described in Rudrauf et al. (2008a). Participants were selected from the Patient Registry of the University of Iowa's Division of Behavioral Neurology and Cognitive Neuroscience, and had given informed consent in accordance with University and Federal requirements. Patients with unilateral circumscribed lesions were included regardless of their lesion location. (See Fig. 1 for lesion coverage). Thus the study included patients with lesions restricted to left ATL as well as patients with lesions outside the left ATL or lesions extending beyond the left ATL boundary. The lesions were caused by stroke, herpes simplex encephalitis, focal intracerebral hemorrhage due to head trauma, or surgical resection (predominantly anterior temporal lobectomy for epilepsy). All the lobectomy cases had documented left hemisphere language dominance. All head trauma subjects had frank, solitary, intraparenchymal hematomas after head trauma, necessitating surgical resections, and were included on the basis of these focal resections (visible as a CSF cavity on the scan in the chronic epoch). Each of these subjects was reviewed with reference to the medical information, and it was determined that their clinical data and deficit profiles were not consistent with more global brain damage. See Supplementary Table S1 for more details.
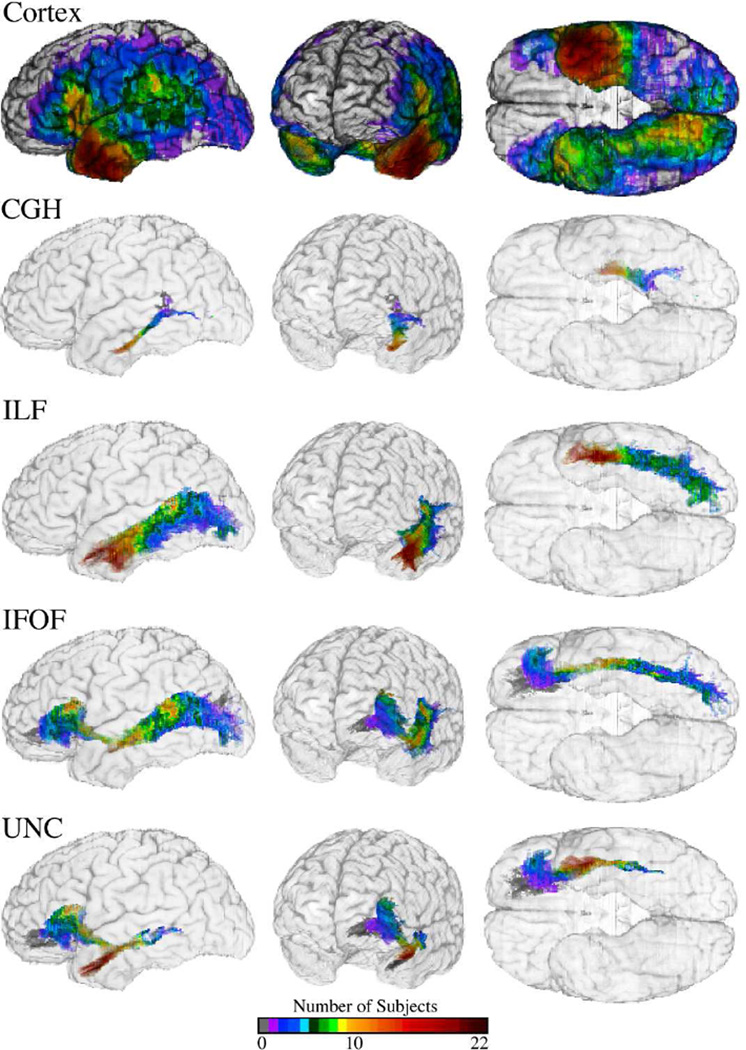
Figure 1.
Lesion coverage in the cortex and along the fiber tracts. The color maps shows the number of participants with lesions along the cortex (top row) and along the long-range association fiber tracts with connections to the left anterior temporal lobe (ATL) (bottom four rows). Abbreviations for the tracts are as follows: CGH (cingulum hippocampal process), ILF (inferior longitudinal fasciculus), IFOF (inferior fronto-cccipital fasciculus), and UNC (uncinate fasciculus). The coverage reflects the subjects used for analysis of naming deficits for Faces (N = 123).
All participants had normal intelligence (as measured by the WAIS-R or WAIS-III) and an average of 12 years of formal education. No participant had difficulty attending to and perceiving visual stimuli. While some participants in the sample were recovered aphasics, none had residual aphasia of such severity so as to preclude the production of scorable responses. We did not explicitly exclude participants on the basis of neuropsychological tests of language function. All participants were characterized neuropsychologically and neuroanatomically in the chronic epoch (at least three months post onset of lesion), according to the standard protocols of the Benton Neuropsychology Laboratory (Tranel, 2009 and the Laboratory of Human Neuroanatomy and Neuroimaging (Damasio and Damasio, 1989; Damasio, 1995; Frank et al., 1997).
2.1.2. Stimuli and data acquisition procedure
The stimuli used for the naming and recognition tests were pictures of both unique and nonunique concrete entities corresponding to five different conceptual categories: (1) famous faces (n=133; hereafter Faces), (2) animals (n=90), (3) tools and utensils (n=104; hereafter Tools), (4) fruits and vegetables (n=67), and (5) musical instruments (n=16).
Naming and recognition performance was assessed by serially presenting participants with visual stimuli and asking them to name the entities. If participants could not produce the name of an entity, they were asked to describe it. The entity was considered as ‘correctly named’ when participants produced the expected name, and as ‘correctly recognized’ if participants either produced the expected name, or when two independent raters were able to identify the specific entity from the transcribed verbal descriptions provided by the participants (if unable to produce the name). If the entity could not be identified from the description by both raters, it was scored as incorrectly recognized and not used in calculating the naming scores. Thus we operationalized separate criteria for assessing naming and recognition performance.
We calculated recognition and naming scores for each category of concrete entities, for each participant. Recognition scores were computed as the percentage of correctly recognized items based on the total number of presented items. By contrast, the naming scores were computed as the percentage of correctly named items based on the number of correctly recognized items. As in our previous studies (Damasio et al., 2004; Rudrauf et al., 2008a), participants were only included in the naming analysis for a given category if they were able to recognize at least 50% of entities in that category. We used this minimum criterion of at least 50% successfully recognized items to ensure a sufficient number of items on which to assess naming performance. Normal performance was defined by naming and recognition scores of 55 healthy normal individuals, demographically matched to the brain-damaged participants on age, education and gender.
We treated the recognition and naming data dichotomously. Such measures are appropriately treated dichotomously when the hypothesis is that a region is critical i.e. necessary for normal function, as evidenced by the association of local damage with abnormal functioning in the chronic epoch. In our previous study (Damasio et al., 2004), we assessed the appropriateness of dichotomizing performance of our study sample. Analyses revealed that naming and recognition scores were better fit with two Gaussian distributions versus one, in particular if participants in the “gray zone” [1.5 – 2 standard deviations] were excluded. (Refer to Damasio et al., 2004, pages 184 –186 for details.) The bimodal distribution of performance among participants suggests distinct impaired and intact subgroups. Thus we were led to classify a participant as having (a) no-deficit if the score was within 1.5 standard deviations or above of the mean defined by a normal comparison group, (b) deficit if the score was two or more standard deviations below the mean defined by the comparison group, or (c) indeterminate and to be excluded from analysis if the scores fell below between 1.5 and 2 standard deviations from the mean defined by the comparison group. These criteria resulted in the inclusion of different numbers of participants in the analysis for each category for each task (see Table 1).
Table 1.
| a) The scores (percent correct) for recognition and naming tests for normal comparison participants. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAMING | RECOGNITION | |||||||||
| Faces | Animals | Tools | Fruits & Vegetables |
Musical Instruments |
Faces | Animals | Tools | Fruits & Vegetables |
Musical Instruments |
|
| Means | 92.3 | 95.7 | 97.2 | 94.3 | 96.9 | 75.7 | 91.9 | 96.2 | 92.6 | 96.3 |
| Standard Diviation | 6.2 | 3.1 | 3.9 | 3.7 | 4.5 | 6.7 | 2.8 | 3.3 | 3.9 | 3.4 |
| b) The numbers of participants included the naming and recognition analyses (out of the possible 129 study participants) for each category. The numbers in the row, “Excluded”, indicate how many participants were excluded in the analyses, and the numbers in the row, “Included” indicate how many participants remained in each category. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NAMING | RECOGNITION | |||||||||
| Faces | Animals | Tools | Fruits & Vegetables |
Musical Instruments |
Faces | Animals | Tools | Fruits & Vegetables |
Musical Instruments |
|
| Included | 115 | 115 | 123 | 123 | 123 | 125 | 128 | 121 | 126 | 129 |
| With Deficit | (35) | (28) | (27) | (23) | (38) | (22) | (43) | (13) | (58) | (65) |
| Without Deficit | (80) | (87) | (96) | (100) | (85) | (103) | (85) | (108) | (68) | (64) |
| Excluded | 14 | 14 | 6 | 6 | 6 | 4 | 1 | 8 | 3 | 0 |
2.1.3. Neuroanatomical data, registration and lesion delineation
Brain lesions were assessed using T1-weighted MRI scans obtained with a 1.5-T General Electric Signa scanner with a 3D SPGR sequence yielding 1.5–1.7 mm contiguous coronal slices and reconstructed in 3D with Brainvox. In participants with MRI contraindications, brain images were acquired with computerized axial tomography (CT). Relatively few participants had CT scans (14 out of 129). Of the 14 participants with CT scans, only seven had lesions in the left hemisphere, of which only three included at least partial coverage (10 – 40%) of the left ATL. Participants with CT scans were equally distributed within the deficit/no deficit condition across all five categories (for both naming and recognition).
Lesion delineation and transfer onto a reference brain was performed interactively by an expert anatomist (HD) using the MAP3 approach (Frank et al., 1997; Fiez et al., 2000; Damasio et al., 2004). MAP3 has been used to generate lesion overlap maps across a variety of cognitive domains (Tranel et al., 1997, 2001, 2003; Adolphs et al., 2000, 2002; Barrash et al., 2000; Damasio et al., 2004).
2.2. Estimation of the likelihood of fiber tract disconnections
While the potential for damage to fibers of passage, notably long-range association tracts, in causing language-related deficits has long been appreciated, methods to directly assess the impact of fiber tract damage on cognition and behavior are a recent development and still under refinement. In this study, we extended the standard voxelwise lesion-deficit approach to incorporate measures of cerebral disconnection, building upon the framework introduced and validated in Rudrauf et al. (2008b). The availability of a probabilistic tract atlas from diffusion MRI made it possible to estimate the amount of damage to particular fiber tracts for each lesion participant, and, perhaps more informatively, estimate whether or not a particular tract was transected.
We used a freely available probabilistic fiber tract atlas developed by Johns Hopkins University (http://lbam.med.jhmi.edu/) to estimate the probability of tract damage by a lesion. The atlas was probabilistic in that it was derived from diffusion MRI data from a population of 20 normal participants (as of August 2011), with the tracts defined using FACT (a deterministic fiber tracking algorithm) and normalized to the JHU-MNI-SS Atlas template (Zhang et al., 2010). Image dimensions for the tract atlas were 181 × 217 × 181, 1 mm isotropic voxels. Given our hypotheses, we focused on the (eight) long-range association fiber tracts available as part of the atlas: the Cingulum (hippocampal process) (CGH), the Inferior Fronto-Occipital Fasciculus (IFOF), the Inferior Longitudinal Fasciculus (ILF), the Uncinate Fasciculus (UNC), Cingulum cingulum (CGC), and three branches of the Superior Longitudinal Fasciculus (aka arcuate fasciculus) connecting fronto-parietal (SLF-FP), fronto-temporal (SLF-FT), and parietaltemporal (SLF-PT) cortices.
Using a combined volumetric and surface registration technique (Postelnicu et al., 2009), we registered the T1-weighted template brain of the fiber tract atlas to our MAP3 reference brain. This registration method has been shown to optimally align cortical and subcortical structures. We then applied the derived deformation fields to bring the eight long-range association fiber tracts into register with MAP3 reference brain. The registration approach preserved each tract as a single spatially contiguous cluster of voxels in the MAP3 space.
The spatially normalized fiber tracts were masked to the white matter of the reference brain and thresholded at lower-bound fiber tract probability of 10 percent. Thresholding was performed to exclude components where there was less than a 10 percent probability of the presence of a tract among the subject population used to derive the atlas. This criterion prevented inclusion of tract components idiosyncratic to a single subject when calculating the disconnection measures.
To characterize fiber tract (FT) damage, we calculated two metrics of disconnection: 1) FT damage defined as the proportion of damage to the tract, weighted by the voxelwise probability values of the tract (similar to our approach in Philippi et al., 2009); and 2) FT transection defined as a binary measure of transection (see below) weighted by the computed FT damage. (See Fig. 2 for illustrations clarifying the two measures.) For both measures, we exploit the idea that disconnection of critical tracts, in particular along the core of the tracts, is likely to cause consistent deficits irrespective of the location of damage along the tract (Rudrauf et al., 2008b).
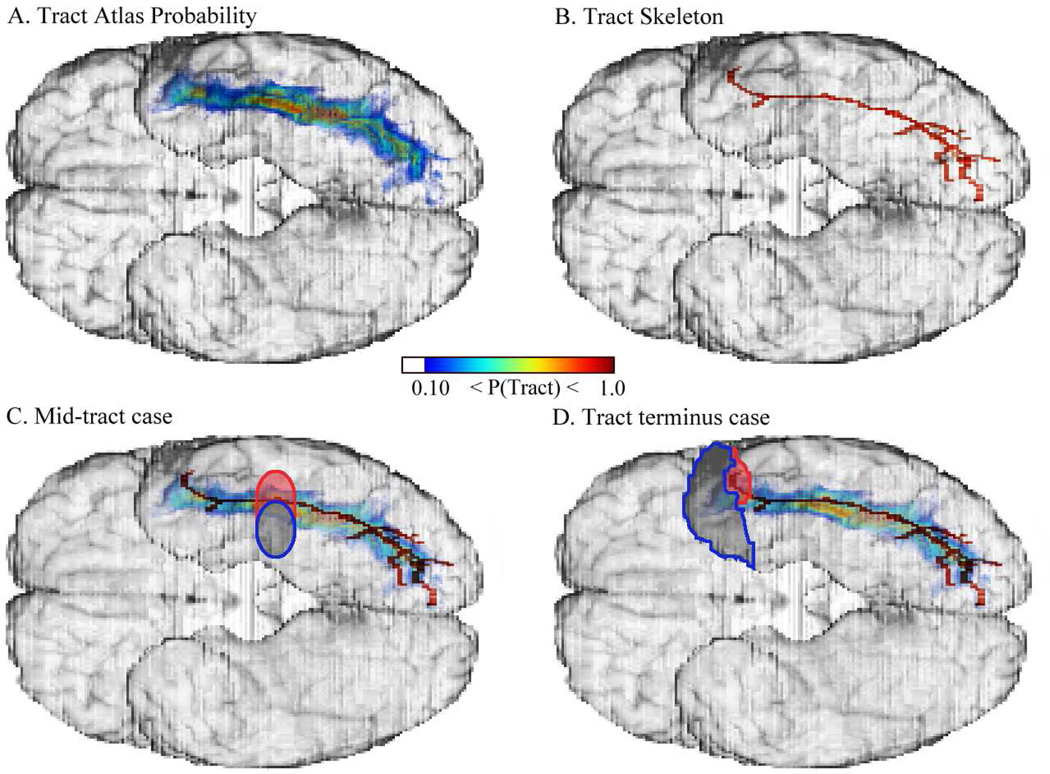
Figure 2.
Fiber tract (FT) measures of disconnection. The figure illustrates the difference between the 'FT damage' and 'FT transection' measures, using the left inferior longitudinal fasciculus (ILF), viewed from the ventral aspect of the brain. A. Tract Atlas Probability: The color palette for the ILF indicates the probability of the tract being present at a given location among the subject population used to derive the atlas. Values are thresholded at a lower-bound fiber tract probability of 0.10 (10 percent). 'FT damage' is calculated by summing the number of voxels encompassed by the lesion and weighting them by the tract atlas probability values. B. Tract Skeleton: The lower-bound thresholded ILF in A was skeletonized with the subvoxel skeletonization method of van Uitert & Bitter (2007), and overlaid on the brain. Panels C and D illustrate the use of the tract skeleton to differentiate 'FT transection' from 'FT damage'. C. Mid-tract damage vs. transection: The lesion (indicated by the oval outlined in blue) partially overlaps the ILF and would produce a non-zero 'FT damage' value but a zero 'FT transection' value, as it does not overlap the tract skeleton nor segment the tract into at least two separate components that include the skeleton. However, if the lesion also included the partial oval outlined in red, it would meet the mid-tract transection criteria and produce a non-zero 'FT transection' value. D. Tract terminus damage vs. transection: The anterior temporal pole lesion, indicated by the blue outline, would produce a non-zero 'FT damage' value but a zero 'FT transection' value as it reflects damage confined to the tract boundary and not to the skeleton. If the lesion was augmented by the area outlined in red, it would meet the tract terminus transection criteria and produce a non-zero 'FT transection' value.
For the first measure, that we designated “FT damage”, we calculated the number of tract voxels encompassed by a lesion (weighted by the tract atlas probability values) divided by the total number of tract voxels (weighted by the tract atlas probability values). We weighted the tract damage by the atlas probability values to assign greater weight to the tract core. By performing this weighting, the measure better indexed the likelihood of substantial disconnection (versus damage per se) than if we had simply used the proportion of damage to the tract.
For the second measure, that we designated “FT transection”, we exploited the fact that each atlas tract comprised a single cluster of spatially contiguous voxels, and operationalized transection with respect to segmentation of that cluster by the superimposed lesion. To determine transection, we first skeletonized the tracts using a precise subvoxel skeletonization method (van Uitert & Bitter, 2007). The skeletonization procedure determined the center silhouette of the tract equidistant to its surface boundaries, thereby allowing us to algorithmically identify the core of the tract. Using the identified tract core, we then assessed whether a lesion severed connectivity along it. We were thus able to define a tract as “transected” by a lesion if any one of the following criteria were met: (a) Mid-tract case: The deletion of tract voxels coinciding with the lesion removed 1cm of the tract skeleton and separated the fiber tract image into at least 2 segments of at least 100 voxels, each including 10 voxels of the tract skeleton (b) Tract terminus case: the lesion included at least 400 voxels along the boundary of the fiber tract and at least 25 voxels of the skeleton.
The FT transection metric was novel to this study and operationalized so that non-zero values required complete severing of the tract by the lesion. In addition, the measure enforced a binary distinction between cortical damage that encompasses the tract terminus and substantive damage to the tract itself in an attempt to disentangle the effects of cortical and tract damage. We note that the criteria used to achieve these objectives reflect arbitrary cut-off decisions, with optimal parameterization the subject of future work. We considered the analyses with the FT transection metric to be secondary, following up the analyses with the FT damage metric.
2.3. Analysis framework and logistic regression models
We performed a series of logistic regression analyses with three aims: (a) To identify category-specific naming deficit associations with left ATL cortical subregions, covarying for concurrent recognition deficits within the same category or naming deficits in other categories, respectively. (b) To assess whether fiber tract damage of particular tracts was associated with category-specific naming deficits. (c) To determine unique contribution of cortical and fiber tract damage to naming deficits. The analysis approaches and the logistic regression models are described below.
2.3.1. Lesion-deficit analysis approaches
We adapted both a generalized voxelwise (Bates et al., 2003; Damasio et al., 2004; Rorden & Karnath, 2004; Rudrauf et al., 2008b) and tractwise (Rudrauf et al., 2008b; Philippi et al., 2009) lesion-deficit analyses. We used simple and multiple logistic regressions to model the relationships between a dichotomous outcome variable (e.g., deficit or not) and one or more independent variable(s) (e.g. cortical/fiber tract damage). Analyses were performed in Matlab (MathWorks, Inc., Natick, MA), which invoked an R package (http://www.r-project.org) to perform the logistic regression (Heinze and Schemper, 2002; Heinze and Ploner, 2004) using the Firth’s penalized likelihood estimation (Firth, 1993).
2.3.2. Cortical-deficit associations within the left ATL (voxelwise analyses)
Based on our hypotheses, we limited our voxelwise analyses to the left ATL cortex. As the ATL is not defined by a clear set of anatomical boundaries and there is disagreement regarding its posterior extent (Wong and Gallete, 2012), we defined the region for the purpose of the current study, as encompassing the temporal pole and the anterior portions of the superior, middle, inferior, and fusiform temporal, and parahippocampal gyri. The anatomical delineation of the left ATL we used is shown in Fig. 3A.
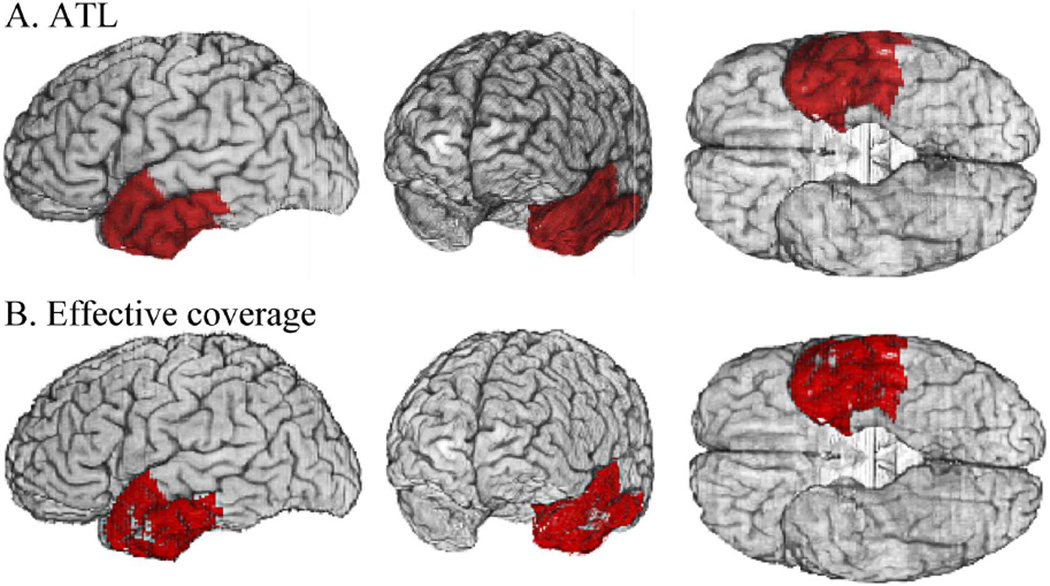
Figure 3.
The left anterior temporal lobe (ATL) region of interest. A: The ATL (red) was defined by a voxel mask in the MAP3 reference brain space that includes the temporal pole and anterior aspects of the inferior temporal, middle temporal, superior temporal, fusiform, and parahippocampal gyri. The parcellation is based on Desikan et al. (2006), and was mapped onto the anatomy of the reference brain by Hanna Damasio and Joel Bruss. B: Within the ATL, effective coverage (a proxy for voxelwise statistical power; Rudrauf et al 2008a) was calculated, indicating where the observed lesion coverage was sufficient to test the hypothesis that damage was necessarily associated with impairment. The red shaded voxels represent the intersection of effective coverage maps for the voxelwise analyses across the 5 conceptual category analyses. Small sectors of the left anterior superior and middle temporal gyri did not have effective coverage. All of our cortical analyses were restricted to the left ATL cortex within this refined ATL mask.
To facilitate interpretation of the spatial distribution of results, we further restricted the voxelwise analyses within the left ATL to areas delineated by the intersection of effective coverage maps for naming in each of the five categories (see Fig. 3B). Effective coverage maps (Rudrauf et al., 2008a) indicate where significant effects can be detected at a given significance threshold, for the maximal lesion-deficit relationships permitted by the lesion sample, and serve as a proxy for the statistical power afforded by the study sample. The effective coverage maps, thresholded at p < 0.05, indicated the ability to detect effects in most of the left ATL, with the exception of a small medial and lateral section, for all categories. Hereafter, we refer to this restricted left ATL region, with the additional effective coverage criteria, as the left ATL mask. For the naming analyses there were at least two participants with deficits who had damage at each left ATL mask voxel. For voxelwise analyses, we used false discovery rate (Benjamini & Hochberg, 1995) to correct for multiple spatial comparisons (q ≤ .05) within the left ATL mask. We considered some marginal effects when interpreting the spatial topography of category-related effects so as to balance Type I and Type II errors. Type II errors are of particular concern in lesion-deficit studies due to their impact on the interpretation of specificity and to low and spatially heterogeneous statistical power of lesion studies (see discussion in Rudrauf et al., 2008a).
We performed voxelwise logistic regression analyses with three models to examine the relationship between cortical-damage and deficit: 1) the standard lesion-deficit analysis to determine whether lesion damage (a dichotomized measure) predicted deficit (a dichotomous measure), 2) the standard analysis augmented with a binary covariate indicating recognition deficits within the same category (a dichotomous measure), and 3) the standard analysis augmented with a covariate for deficit selectivity (a dichotomous measure indicating whether or not there were also naming deficits in one or more other categories). To aid in the interpretation of the second analysis (covarying for recognition), we also performed a separate analysis of recognition deficits (across the full spectrum of recognition deficits, including participants with less 50% recognition item success.)
2.3.3. Fiber tract damage-deficit associations (tractwise analyses)
We performed tractwise analyses to examine the relationship between left FT damage and a deficit. The purpose of the analysis was to assess whether deficits were predicted by any of the four long-range association tracts with putative terminations in, or passage through, the ATL (uncinate fasciculus, inferior longitudinal fasciculus, inferior fronto-occipital fasciculus, cingulum), after covarying for other long-range FTs available as part of the atlas. The three branches of the superior longitudinal fasciculus, considered part of the dorsal language pathway but not terminating or passing through ATL were also available as part of the atlas. Given their role in language production and phonological access these were included in our model covariates of no interest. We were obliged to exclude Cingulum cingulum (posterior cingulum tract) from our analyses due to insufficient lesion coverage. These analyses were performed for the two fiber tract damage measures described above. We assessed collinearity among the fiber tracts of interest to determine if there was sufficient unique variance to allow detection of an effect. For the tracts of interest, multicollinearity was not an issue (variance inflation factor [VIF] < 5 for FT damage measure and < 2.5 for the FT transection measure). To correct for multiple tractwise comparisons (among the four tracts of interest), we adjusted the p values using a Bonferroni correction (p < 0.0125, corrected p < 0.05), for the tractwise results.
2.3.4. Separating cortical and fiber tract damage contributions (combined analyses)
To determine unique contribution of cortical and fiber tract damage to naming deficits, we performed two additional and complementary analyses: 1) voxelwise cortical analysis covarying for concurrent tract damage, and 2) tractwise analysis covarying for concurrent left ATL cortical damage (based on a continuous measure of the amount of left ATL cortical damage). Analyses were performed using both FT disconnection measures as described above. When the cortical ATL covariable was added to the regression model, there was a high degree of multicollinearity for some of the tracts as well as the cortical ATL covariable when the first disconnection measure (FT damage) was used to quantify fiber tract damage. When the second disconnection measure (FT transection) was employed, multicollinearity was alleviated (generalized variance inflation factor (VIF) < 2.5) for the covariates of interest.
2.3.5. Analysis of possible confounding effects of heterogeneous etiology
Our study included participants with four lesion etiology types: surgical resection (primarily for epilepsy, but also vascular malformations), stroke, encephalitis, and focal contusion from head trauma. As these different etiologies have the potential to systematically affect the neuroanatomical site of damage, lesion size, relative amount of damage to the cortex versus fiber tracts, neuropsychological deficit profile, and possibility of recovery, we analyzed our data within etiology sub groups to assess whether the results differed and were therefore impacted by lesion etiology. Descriptions of these analyses and their result are included as supplemental material.
3. Results
3.1. Lesion coverage
Fig. 1. illustrates the lesion coverage for the cortex (top row) and the four long-range fiber tracts of interest with terminal connections in the ATL (bottom four rows) (for Faces, N=123). The coverage represents the number of participants with a lesion at each voxel in the reference space. Due to the participant inclusion criteria, lesion coverage varied slightly per category.
3.2. Cortical-deficit associations within the left ATL (voxelwise analyses)
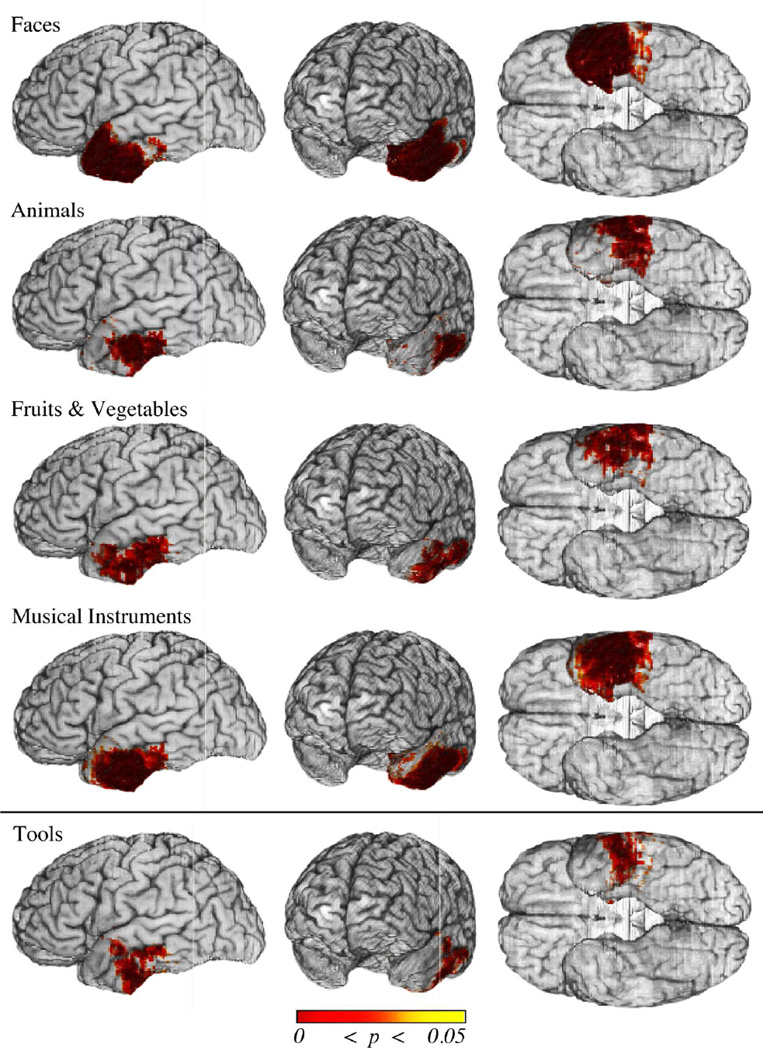
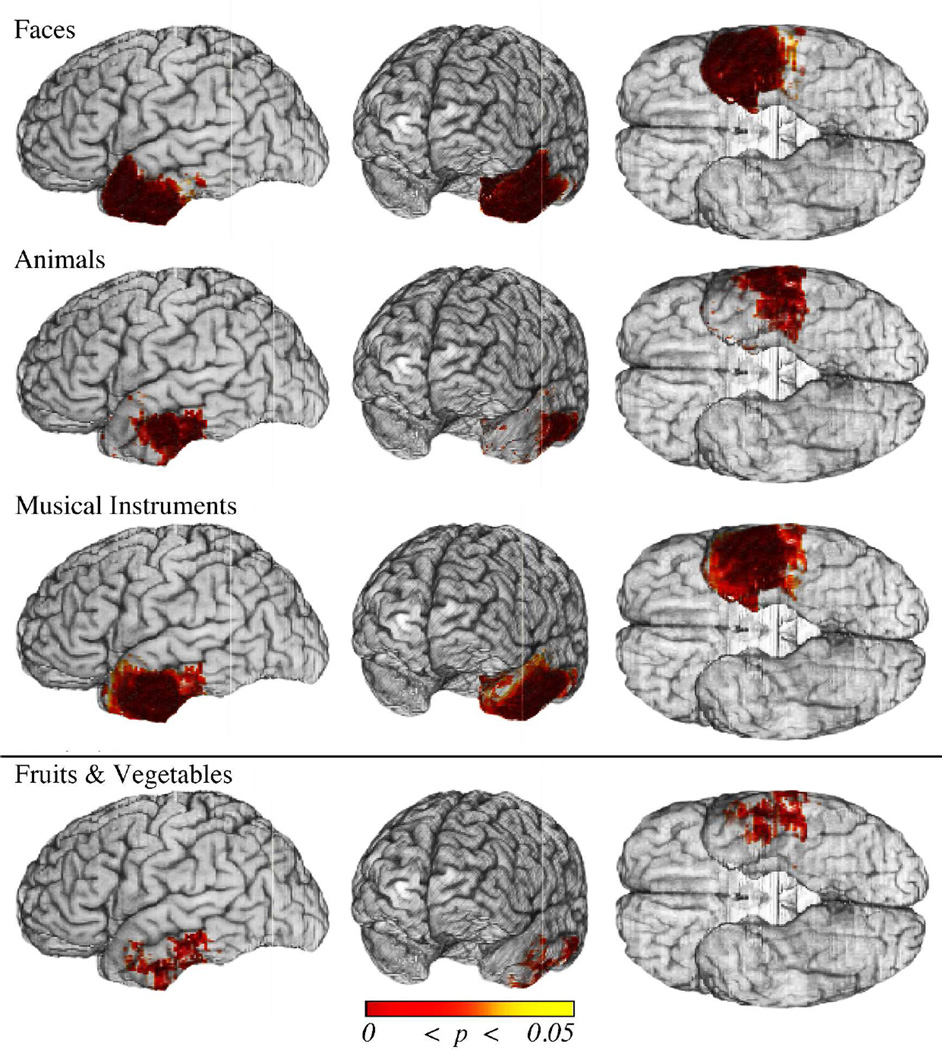
The voxelwise analyses (without inclusion of covariates) provided a baseline for the left cortical ATL damage association with each category of naming deficit. The FDR-corrected results are displayed in Fig. 4. The results revealed a significant association between left ATL cortical damage and naming deficits for Faces, Animals, Fruits & Vegetables, and Musical Instruments (q = 0.05). There was also a trend for an association between naming deficits for Tools and damage to the ATL (q = 0.10). Naming deficits in all five categories were associated with damage to the basolateral ATL (posterior to the temporal pole). In addition, naming deficits for Faces were associated with cortical damage to the temporal pole. Thus cortical sectors were identified that appeared to be critical for a select category, as well as common across categories.
Figure 4.
Cortical damage in the left ATL associated with naming deficits. Shown are thresholded results (p values) from the voxelwise logistic regression analyses of naming deficits for each category in the space of the MAP3 reference brain. Statistical results were thresholded using FDR (q ≤ .05, top four rows; q ≤ .10, bottom row). Significant results are observed for four out of the five categories (Faces, Animals, Fruits & Vegetables, and Musical Instruments). There is a trend for significance for Tools (bottom row). Only Faces are associated with cortical damage to the entire temporal pole (i.e. including the anterior and superior aspects). By contrast, all categories are associated with damage to the posterior ventrolateral ATL. These results serve as a baseline for subsequent voxelwise analyses in which potential confounds are taken into account.
To ascertain the specificity of these findings for naming, we performed voxelwise analyses in which we covaried for recognition deficits in the same category. The FDR-corrected results are displayed in upper panel of Fig. 5. Significant results (q = 0.05) were similar in topography to those obtained in the baseline analyses (described above) for Faces, Animals, and Musical Instruments. For Fruits & Vegetables, the extent of implicated cortex was reduced (though still included the basolateral ATL) and only marginally significant (q = 0.10) (lower panel of Fig. 5). No association was observed between naming deficits for Tools and damage to the ATL. Finally and importantly, no significant lesion-deficit associations were observed for recognition deficits in the left ATL (for the separate analyses of recognition).
Figure 5.
Cortical damage in the left ATL associated with naming deficits when accounting for recognition deficits. Shown are FDR-thresholded results (p values) from the voxelwise logistic regression analyses of naming deficits when accounting for recognition deficits in the same category. Significant results are observed for three out of the five categories (Faces, Animals, and Musical Instruments). These results are shown in the upper three rows, thresholded using FDR (q ≤ .05). There is a trend for Fruits & Vegetables (bottom row, thresholded using FDR q<0.10), but no significant result for Tools. Only Faces are associated with cortical damage to the entire temporal pole (that includes the anterior and superior aspects). By contrast, the three other categories are associated with damage to the posterior ventrolateral ATL. Compared to the baseline analysis (Figure 4), the extent of cortical damage in the ATL associated with Fruits & Vegetables is reduced (restricted primarily to the posterior ventrolateral ATL).
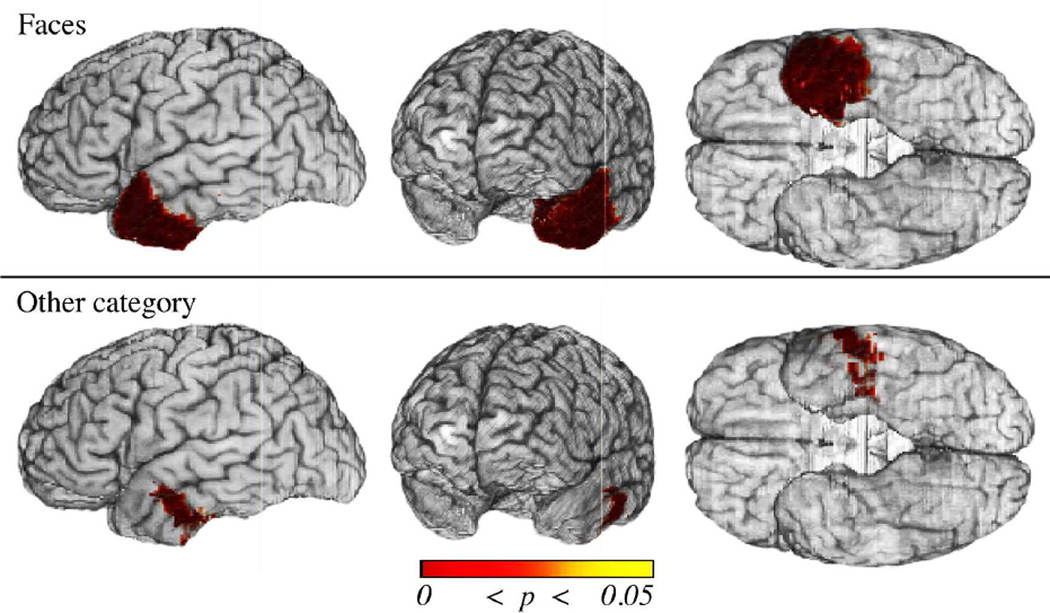
To ascertain category specificity of these effects, we performed additional voxelwise analyses covarying for naming deficit selectivity (i.e. naming deficits in the other categories). The FDR-corrected results are displayed in Fig. 6. There was a significant association between naming deficits for Faces and damage to the left temporal pole (Fig. 6, top row). By contrast, damage to more posterior regions (i.e. basolateral ATL) was not specific to deficits for Faces, but was rather associated with naming deficits in one or more of the other categories (Fig. 6, bottom row). There was no significant association between naming deficits selective to any of the other categories and left ATL damage, with the exception of Tools, for which a small sector in the posterior superior ATL was implicated (results not shown).
Figure 6.
Cortical damage in the left ATL mask associated with naming deficits when controlling for category-nonspecific effects (i.e. the presence of a naming deficit in one or more other of the categories). Shown are thresholded results (p values) from the voxelwise logistic regression analyses of naming deficits when covarying for naming deficits in another category overlaid. Statistical results were thresholded using FDR (q ≤ .05, top three rows; q ≤ .10, bottom row). Significant results are observed for Faces (top row) and Tools (small sector in the superior temporal sector, not shown). Significant results associated with damage to at least one category other than Faces are observed in the ventrolateral ATL (bottom row). These results show the dissociation of cortical damage associated with impairments for unique and non-unique entities, and the specificity of the temporal pole for the former.
3.3. Fiber tract damage-deficit associations (tractwise analyses)
Using the FT damage measure, specific tractwise analyses (covarying for damage to the other tracts) showed significant associations between naming deficits and damage to long-range association fiber tracts that terminate in the left ATL. The implicated tracts varied depending on category, and there was a clear dissociation between the tracts associated with naming deficits for unique entities (Faces) and those for non-unique entities. Damage to the uncinate fasciculus was associated with impaired naming for Faces (χ2 =10.4; corrected p < 0.006), while damage to the inferior longitudinal fasciculus was associated with impaired naming for Animals (χ2 = 6.4; corrected p < 0.05), Fruits & Vegetables (χ2 = 11.6; corrected p < 0.003), and Musical Instruments (χ2 =6.8; corrected p < 0.04). Damage to the inferior longitudinal fasciculus was also associated with impaired naming for Tools, though the results were only marginally significant (χ2 = 5.2; corrected p < 0.09). No significant relationship was observed between damage to any of the left hemisphere tracts of interest and impaired recognition. Tractwise analysis results using the FT transection measure replicated the above findings, though the association of naming deficits for Tools and ILF damage was also significant.
3.4. Separating cortical and fiber tract damage contributions (combined analyses)
In order to investigate the relative contributions of cortical and fiber tract damage associated with naming deficits, we performed analyses in which we covaried for tract damage (voxelwise analyses) or amount of left ATL damage (tractwise analysis). When using the FT damage measure, no significant findings were observed for either the voxelwise or tractwise analyses. Given the significant findings from the independent cortical and tractwise analyses, these results are consistent with a high degree of collinearity between cortical damage and the probability of fiber tract damage. Such collinearity of effects is expected to the extent that cortical areas and fiber tracts are part of the same large-scale system.
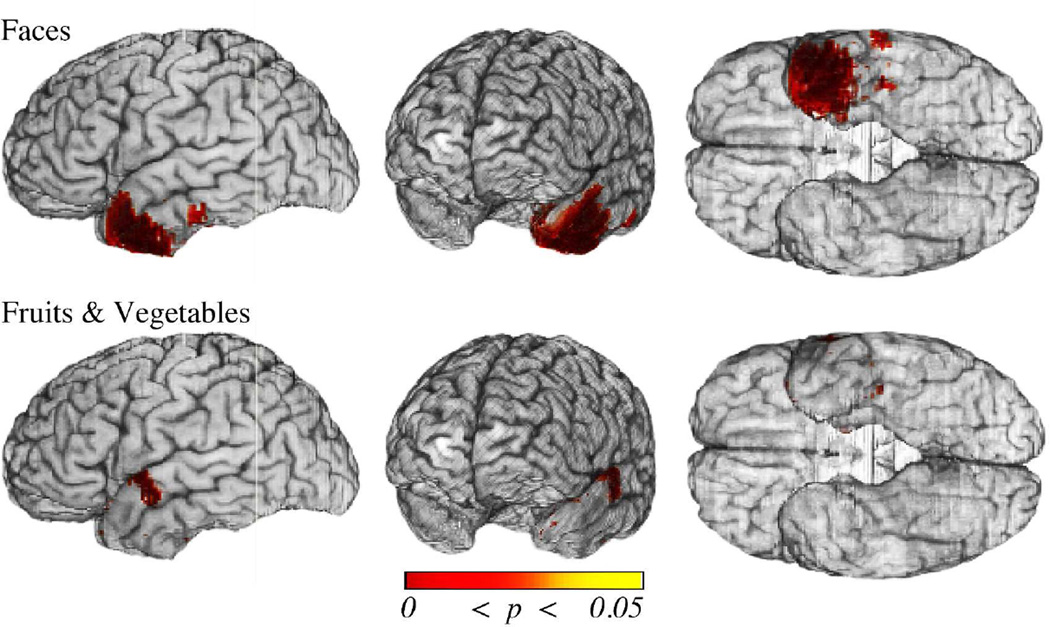
In an attempt to overcome the collinearity of cortical and adjacent white matter damage, we turned to the second, more stringent measure for fiber tract damage that explicitly operationalized tract transection (as described in the Methods section). These analyses revealed an association between impaired naming for Faces and damage to the temporopolar cortex (beyond effects due tract damage). Compared to the baseline analysis results for Faces (section 3.2), the cortex implicated was more restricted, no longer including the superior aspect of the ATL, but still included a small sector in the anterior inferolateral temporal cortex. The voxelwise results also revealed an association between impaired naming for Fruits and Vegetables and damage to a small area in the basolateral ATL as well as an area in the superior temporal gyrus. (See Fig. 7.)
Figure 7.
Cortical damage in the left ATL associated with naming deficits when controlling for fiber tract damage (FT transection). Shown are thresholded results (p values) from the voxelwise logistic regression analyses of naming deficits when covarying for long-range association fiber tract disconnection/damage. Statistical results were thresholded using FDR (q ≤ .05). Cortical regions remain significant for Faces and Fruits & Vegetables but are reduced in their extent. Sparse regions in the posterior ventral cortex are implicated for deficits in both categories, as is an additional ventrolateral sector for Faces. For Faces, much of the temporal pole remains implicated except notably for the superior aspect of the superior temporal gyrus.
Tractwise analysis using the extent of fiber tract damage, and covarying for amount of ATL damage revealed that damage to the ILF was still significantly associated with impaired naming for Animals (χ2 =7.1; corrected p < 0.03) and Fruits and Vegetables (χ2 =9.1; corrected p < 0.01). The extent of ATL damage itself was not significantly associated with impaired naming for any category. The parallel analysis using the FT transection measure showed that the extent of ATL damage was associated with impaired naming for Faces (χ2 =7.4; corrected p < 0.03). No other significant results were observed.
4. Discussion
Lesion studies have elucidated neural systems supporting retrieval of semantic knowledge, and revealed how selective deficits may arise from a brain architecture organized around the convergence of modality-related systems (Damasio et al., 1996, 2004; Tranel et al., 1997). These studies demonstrate that the left ATL is a critical component of systems supporting name retrieval, and suggest category-related functional segregation within the region. Although damage to fibers of passage, especially long-range association tracts, has long been understood to underlie language deficits, lesion methods to isolate these effects are a recent development (Rudrauf et al., 2008b; Thiebaut de Schotten et al., 2008; Philippi et al., 2009; Galantucci et al., 2011; Kuceyeski et al., 2011; Turken and Dronkers, 2011; Han et al., 2013).
In this study, we performed voxelwise lesion- and fiber tract disconnection-deficit analyses to assess the functional importance of the left ATL and its connections in name retrieval. Our overarching hypothesis was that category-related naming deficits are associated with damage to different functional subdivisions, and that these subdivisions are related to differences in corticocortical connectivity. By explicitly controlling for confounding factors including concurrent recognition deficits, category-selectivity of naming deficits, and cortical versus underlying white matter fiber tract damage, we identified cortical subregions and long-range fiber tract connections critical for retrieving names for categories of unique (famous Faces) and non-unique (Animals, Tools, Fruits and Vegetables, and Musical Instruments) concrete entities.
4.1. Lexical specificity and the role of the left ATL
Consistent with previous reports (Damasio et al., 1996, 2004; Drane et al., 2008, 2013), we demonstrate that damage to the left ATL is associated with lexical (as assessed by naming) but not concept (as assessed by recognition) retrieval deficits for all categories (though only as a trend for Tools). We explicitly assessed the lexical-specificity of these naming deficit associations by controlling for concurrent recognition deficits in the same category. Collectively, these results suggest that the critical role of the left ATL in name retrieval is independent of concept retrieval across categories of unique and non-unique entities.
Our results support a lexical role for the left ATL in semantic retrieval, but the exact nature of the role remains unclear. Part of this uncertainty reflects the debate over the distinction between lexical semantic knowledge (‘words’ as symbolic representations) and concept knowledge (non verbal representations) and the purported relationship to the left and right ATLs for their retrieval. Some investigators maintain verbal descriptors, including names, are features of a unitary bilateral ATL semantic system (e.g. Rogers et al., 2004; Lambon Ralph et al., 2008, 2010), while others consider names as ‘containers’ distinct from the conceptual information they encapsulate and bind (Westermann & Mareschal, 2014). The latter view corresponds more or less to the idea that the lexical concept is a non-decomposed level of knowledge of an entity (Indefrey & Levelt, 2004), different, and possibly with a finer-grained structure than a nonlexically bounded store (Mesulam et al., 2013). This perspective is compatible with the view that the left ATL is a “mediation” zone linking areas supporting specific concepts with left perisylvian areas supporting word-form knowledge (Damasio et al., 1996). In a 'mediation' zone framework, however, the sparse and essentially arbitrary links between phonological word forms and conceptual information raise the possibility that what appears to be a selective name retrieval deficit could still reflect a degraded unitary semantic store (cf. Lambon Ralph et al., 2001).
4.2 The role of left anterior temporal lobe cortical subdivisions in lexical retrieval
The specific subregions associated with naming impairments within the left ATL varied according to conceptual category and/or level of specificity, with both category-general and category-selective regions identified. Damage to the left basolateral ATL was associated with naming impairments in all categories for both unique and non-unique entities (again only a trend for Tools). This subregion coincides with the ‘semantic hub’, an area implicated in accessing semantic representations across a variety of semantic tasks and experimental modalities (Binney et al., 2010; Rogers et al., 2006; Patterson et al., 2007). This ATL subregion is dominated by intra-temporal connectivity (Binney et al., 2012), consistent with an integrative role among different temporal lobe subregions, including more rostral regions, as would be expected if the region supports domain general semantic representations during naming.
While left basolateral ATL damage was associated with name retrieval deficits for all categories studied, the association for Tools was not statistically significant. Other work (Drane et al., 2013) has reported less frequent or consistent naming impairment for this category after ATL damage. Tools may be more visually and/or semantically distinct, reducing requirements for iterative processing and reliance on semantic stores to resolve their lexical-semantic identity.
By contrast, there was a dissociation of cortical sectors implicated in naming Faces (a category of unique entities) versus the four categories of non-unique entities. Damage to the left temporal pole was associated with impaired naming of Faces specifically, consistent with studies using a variety of approaches (Damasio et al., 1996; Damasio et al., 2004; Tranel et al., 2009; Simmons et al., 2010) and stimulus materials (Belfi et al., 2014; Waldron et al., 2014). The requirement of additional anatomic substrate(s) for normal retrieval of proper names of faces is consistent with proposals that their retrieval entails additional process(es) (cf. Semenza et al., 2011), such as resolution of visual and conceptual ambiguity (Grabowski et al., 2001), differences in token vs. type reference for unique and non-unique entity retrieval (Semenza et al., 2011), and/ or processes specifically related to social and affective associations (Zahn et al., 2007; Ross & Olson, 2010; Simmons et al., 2010).
The findings support the notion of convergence-divergence regions and suggest that category-selective and -general ATL components arise from modality inputs and their integration. While not completely consistent with separate sensory modal stores, the results suggest that modal channels may drive segregation within the ATL, manifesting as category-related differences.
4.3. The role of long-range association tracts and connections in lexical retrieval
The fiber tractwise analyses assessed the importance of four long-range association fiber tracts in the left ATL for name retrieval for each category. These tracts belong to the ventral language pathway (involved with mapping meaning to sound). As we covaried for effects of other tracts, including those from the dorsal language pathway, the results indicate specific contributions per tract. The results revealed segregated effects for unique Faces versus non-unique entities, and highlight a specific role for the uncinate fasciculus (UNC). The uncinate fasciculus interconnects anterior temporal cortical and subcortical limbic structures with frontal regions, especially the lateral orbitofrontal and ventral medial prefrontal (VMPF) cortex. These regions are associated with reward, motivation, affective and social processing and autobiographical memory. Thus the UNC role may reflect activation of emotion-feeling associations (Papagno et al., 2011) and/or greater demand for semantic control (Harvey et al., 2013) in proper name selection.
In contrast, our results identified a critical role for the inferior longitudinal fasciculus (ILF) in naming nonunique entities. The ILF interconnects posterior occipito-temporal regions with anterior temporal lobe structures (Mandonnet et al., 2007). The ILF finding is consistent with a top-down component to semantic retrieval facilitated by long-range association projections interconnecting early visual and visual association regions with the ATL (Coutanche et al., 2014; Tranel et al., 2005); and with previous studies showing that damage to the ILF is associated with impaired object naming (Agosta et al., 2013; Shinoura et al., 2010). The ILF was not found to be critical for Faces, perhaps because Faces place more demands on right hemisphere visual processing, and as such it may be that right ILF (Tavor et al., 2014), or commissural fibers crossing more anteriorly, play a major functional role in this case (Di Virgilio, 1999).
Our findings confirm an essential role for long-range association fiber tracts in naming and are in line with explanations of visual object recognition (Bar, 2003; Bar et al., 2006; Kersten et al., 2004; DiCarlo et al., 2012; Yvert et al., 2012) that emphasize rapid interactions between the emotional semantics of visual scenes, emotion and visual attention (Rudrauf et al., 2008c) that include anterior elements of the ventral visual stream, limbic structures, and long-range parallel pathways between posterior and anterior cortices. They also are in line with Bayesian accounts of resolution of ambiguous sensory input through the influence of context (see Friston et al., 2006). Engineered top-down dynamic and Bayesian systems typically require iteration and their feasibility and efficiency require rapid communication. Thus an additional requirement for a semantic system may be the ability to rapidly coordinate brain activity across sensory and central semantic nodes, enabled by long-range fiber tracts. This view is consistent with early activation of the ATL during naming in MEG and microelectrode recordings (Chan et al., 2011; Clarke, et al., 2011, 2013; Bar et al., 2003); and with dynamic causal modeling studies showing reliance on feedback connections from the ATL to posterior regions (Rudrauf et al., 2008c Campo et al., 2013) and from frontal regions to the ATL (Campo et al., 2013).
Analyses of the relative contributions of tract and cortical damage within the left ATL confirmed a high degree of collinearity between the two. When tract effects using FT damage were estimated first, no significant variance associated with naming in any category remained explained by cortical damage. Similarly, when the amount of ATL damage was estimated first, there remained only an association between damage to the ILF and naming deficits for two categories of non-unique entities (Animals and Fruits & Vegetables). These predominately null findings, when controlling for cortical or FT damage, are expected to the extent that particular cortical sectors and white matter tracts are components of the same system. Although the analyses using the FT transection measure also eliminated the majority of cortical and tract findings, these analyses led to spatial refinement of the cortical sectors implicated in naming Faces and Fruits & Vegetables. Notably, the association between damage to inferior (but not superior) aspects of left temporal pole (LTP) and naming deficits for Faces remained after covarying tract disconnection. Thus the effects of inferior LTP damage appear to be independent of the uncinate fasciculus and the connectivity it affords. The same independence was not obtained for the superior LTP. These results show that the proposed measure of fiber tract transection is a useful approach for modeling fiber tract damage in the study of lesion-deficit relationships, for understanding the importance of long-range (vs. local) connectivity, and for gaining insight into its relationship to cortical support of semantic knowledge retrieval.
4.4 Limitations of the study
The study has important limitations. First, it relied on a heterogeneous lesion sample and assumes that lesion location, not mechanism, is the dominant explanatory factor of a deficit. Lesions of different etiologies may affect tissue differently, and isolating damaged tissue based on brain scans has limitations. We employed only etiologies giving rise to circumscribed tissue destruction, and only defined such regions as “lesion”. This conservative approach aims to identify areas where damage is consistently associated with abnormal function. Ideally these common areas reflect the conjunction of lesion-deficit correlations observed across mechanisms. Although we did not have sufficient statistical power (ascertained by effective coverage maps) to assess lesion-deficit correlations per etiology, we demonstrated that lesion mechanism did not affect the probability of deficit for the left ATL subregions that were identified as critical for naming in our analyses. Like all studies, our findings will be strengthened by replication in an independent sample, and by convergence with other approaches.
Second, our approach for modeling fiber tract 'disconnection' has limitations. The measures of FT damage and FT transection were operationalized on theoretical grounds and are not claimed to be optimal. We assumed deficits associated with tract damage would be independent of their location along the tract. This oversimplification does not consider the fact that tracts are comprised of multiple branches with different patterns of interconnectivity, and hence support different functions (Tavor et al., 2014). Moreover, the measures are estimated with reference to information from co-registered normal brains, not diffusion data from the specific brains with lesions (which were unavailable). Nonetheless, in a previous validation study (Rudrauf et al., 2008b), we demonstrated the ability of our atlas-based approach to correctly separate gray and white matter damage contributions to visual deficits in the geniculo-calcarine system.
Third, since clinical lesions tend to be large and involve both gray and white matter, fiber tract damage is highly collinear with cortical damage. While our approach advances the means to address this confound, more refined methods are needed. Simulations may be useful to validate disconnection measures and to assess the impact of their inclusion on the ability to detect cortical lesion-deficit correlations. At some level, and especially near critical cortical components, the dependency of connectivity and cortical function is likely to be irresolvable.
Fourth, our study was limited by the tracts we included. When more than one fiber tract is involved, e.g. IFOF and ILF, neither may be highlighted as having a specific contribution. A similar issue occurs if pathways are partially redundant, as hypothesized for the ventral language pathways of a direct (IFOF) and indirect (ILF + UNC) pathway. Thus our results cannot be taken to mean that impaired naming of unique entities is only or solely associated with left UNC damage, or that impaired naming of non-unique entities is only or solely caused by damage of the left ILF. Another more basic limitation is that the ontology of long-range tracts has controversial aspects. Some groups include the UNC as part of the ventral extreme capsule/IFOF pathway (cf. Axer et al., 2013). Finally, at all levels of the ATL, cortical regions communicate with contralateral regions, and especially their homologues, via white matter commissural fibers. While we focused our analysis of disconnection on within-hemisphere long-distance projection tracts, between-hemisphere connectivity is also relevant. Nevertheless, our results support the importance of within-hemisphere connectivity, which may dominate function in heteromodal association regions such as the ATL (Hurley et al., 2014).
Lastly, we only studied one category of unique entities (faces of specific persons). More recent work has suggested that the left temporal pole is also important for retrieval of other categories of unique entities, such as famous landmarks, melody names, or names associated with famous voices (Tranel, 2006; Belfi and Tranel, 2014; Waldron et al., 2014; Abel et al., 2015). Analyzing other unique categories in a disconnection framework is a direction for future work.
5. Conclusions
In this study, we extended the standard voxelwise lesion-deficit analysis framework using analysis methods that formally incorporate information about connectivity and disconnection. We reanalyzed a previous lesion study of impaired naming and recognition across five categories of concrete entities using a voxelwise logistic regression approach that also analyzed the impact of the disconnection of long-range association fiber tracts. Our overarching goal was to investigate the hypothesis that category-related naming deficits are associated with damage to different functional subdivisions of the left ATL, in a manner that is consistent with a connectivity-based architecture of convergence-divergence regions.
The results demonstrate that the neural systems for naming of unique and non-unique concrete entities include segregated components at both the cortical and fiber tract levels. The results reconcile category-related and semantic hub accounts of the left ATL through considering a finer granularity of organization that may support both. We find a left ATL region critical for name retrieval across concrete categories, and another specifically for retrieval of names of famous faces. The critical support for name retrieval is shared between these regions and the inferior longitudinal fasciculus and uncinate fasciculus, respectively. The left temporal polar subregions may support lexical semantic information for unique and/or social entities such as specific persons, while the laterobasal region may integrate a sensory amodal and category general store. The results are most compatible with a specific role of the left ATL in storing lexical conceptual information, with partial segregation of this store according to category.
Supplementary Material
Acknowledgements
Research support was provided by NINDS R01 NS058658, by NINDS P50 NS019632, and by an NRSA to S.M (F31 NS090855).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abel TJ, Rhone AE, Nourski KV, Kawasaki H, Oya H, Griffiths TD, Howard MA, Tranel D. Direct physiologic evidence of a heteromodal convergence region for proper naming in human anterior temporal lobe. Journal of Neuroscience. 2015;35(4):1513–1520. doi: 10.1523/JNEUROSCI.3387-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R. Neural systems for recognizing emotion. Curr Opin Neurobiol. 2002;12(2):169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D, Cooper G, Damasio AR. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J Neurosci. 2000;20(7):2683–2690. doi: 10.1523/JNEUROSCI.20-07-02683.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axer H, Klingner CM, Prescher A. Fiber anatomy of dorsal and ventral language streams. Brain Lang. 2013;127(2):192–204. doi: 10.1016/j.bandl.2012.04.015. [DOI] [PubMed] [Google Scholar]
- Bar M. A cortical mechanism for triggering top-down facilitation in visual object recognition. J Cogn Neurosci. 2003;15(4):600–609. doi: 10.1162/089892903321662976. [DOI] [PubMed] [Google Scholar]
- Bar M, Kassam KS, Ghuman AS, Boshyan J, Schmid AM, Dale AM, Halgren E. Top-down facilitation of visual recognition. Proc Natl Acad Sci U S A. 2006;103(2):449–454. doi: 10.1073/pnas.0507062103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Damasio H, Adolphs R, Tranel D. The neuroanatomical correlates of route learning impairment. Neuropsychologia. 2000;38(6):820–836. doi: 10.1016/s0028-3932(99)00131-1. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Belfi AM, Tranel D. Impaired naming of famous musical melodies is associated with left temporal polar damage. Neuropsychology. 2014;28(3):429–435. doi: 10.1037/neu0000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B. 1995;57(1):289–300. [Google Scholar]
- Benjamini Y. Discovering the false discovery rate. Journal of the Royal Statistical Society: Series B (Statistical Methodology) 2010;72(4):405–416. doi: 10.1111/j.1467-9868.2010.00746.x. [DOI] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends Cogn Sci. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binney RJ, Embleton KV, Jefferies E, Parker GJ, Ralph MA. The ventral and inferolateral aspects of the anterior temporal lobe are crucial in semantic memory: evidence from a novel direct comparison of distortion-corrected fMRI, rTMS, and semantic dementia. Cereb Cortex. 2010;20(11):2728–2738. doi: 10.1093/cercor/bhq019. [DOI] [PubMed] [Google Scholar]
- Binney RJ, Parker GJ, Lambon Ralph MA. Convergent connectivity and graded specialization in the rostral human temporal lobe as revealed by diffusion-weighted imaging probabilistic tractography. J Cogn Neurosci. 2012;24(10):1998–2014. doi: 10.1162/jocn_a_00263. [DOI] [PubMed] [Google Scholar]
- Bright P, Moss H, Tyler LK. Unitary vs multiple semantics: PET studies of word and picture processing. Brain and Language. 2004;89(3):417–432. doi: 10.1016/j.bandl.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Campo P, Poch C, Toledano R, Igoa JM, Belinchon M, Garcia-Morales I, Gil-Nagel A. Anterobasal temporal lobe lesions alter recurrent functional connectivity within the ventral pathway during naming. J Neurosci. 2013;33(31):12679–12688. doi: 10.1523/JNEUROSCI.0645-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Jones DK, Donato R, Ffytche DH. Occipito-temporal connections in the human brain. Brain. 2003;126(Pt 9):2093–2107. doi: 10.1093/brain/awg203. [DOI] [PubMed] [Google Scholar]
- Chan AM, Baker JM, Eskandar E, Schomer D, Ulbert I, Marinkovic K, Halgren E. First-pass selectivity for semantic categories in human anteroventral temporal lobe. J Neurosci. 2011;31(49):18119–18129. doi: 10.1523/JNEUROSCI.3122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Taylor KI, Devereux B, Randall B, Tyler LK. From perception to conception: how meaningful objects are processed over time. Cereb Cortex. 2013;23(1):187–197. doi: 10.1093/cercor/bhs002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke A, Taylor KI, Tyler LK. The evolution of meaning: spatio-temporal dynamics of visual object recognition. J Cogn Neurosci. 2011;23(8):1887–1899. doi: 10.1162/jocn.2010.21544. [DOI] [PubMed] [Google Scholar]
- Clarke A, Devereux BJ, Randall B, Tyler LK. Predicting the Time Course of Individual Objects with MEG. Cereb Cortex. 2014 Sep 9; doi: 10.1093/cercor/bhu203. pii: bhu203. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutanche MN, Thompson-Schill SL. Creating Concepts from Converging Features in Human Cortex. Cereb Cortex. 2014 doi: 10.1093/cercor/bhu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnecki K, Duffy JR, Nehl CR, Cross SA, Molano JR, Jack CR, Jr, Boeve BF. Very early semantic dementia with progressive temporal lobe atrophy: an 8-year longitudinal study. Arch Neurol. 2008;65(12):1659–1663. doi: 10.1001/archneurol.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio H, Damasio A. Lesion analysis in neuropsychology. New York: Oxford University Press; 1989. [Google Scholar]
- Damasio AR. Time-locked multiregional retroactivation: a systems-level proposal for the neural substrates of recall and recognition. Cognition. 1989;33(1–2):25–62. doi: 10.1016/0010-0277(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Damasio H. Human brain anatomy in computerized images. New York: Oxford University Press; 1995. [Google Scholar]
- Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996;380(6574):499–505. doi: 10.1038/380499a0. [DOI] [PubMed] [Google Scholar]
- Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92(1–2):179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006 Jul 1;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devlin JT, Russell RP, Davis MH, Price CJ, Moss HE, Fadili MJ, Tyler LK. Is there an anatomical basis for category-specificity? Semantic memory studies in PET and fMRI. Neuropsychologia. 2002;40:54–75. doi: 10.1016/s0028-3932(01)00066-5. [DOI] [PubMed] [Google Scholar]
- Di Virgilio G, Clarke S, Pizzolato G, Schaffner T. Cortical regions contributing to the anterior commissure in man. Exp Brain Res. 1999;124(1):1–7. doi: 10.1007/s002210050593. [DOI] [PubMed] [Google Scholar]
- DiCarlo JJ, Zoccolan D, Rust NC. How does the brain solve visual object recognition? Neuron. 2012;73(3):415–434. doi: 10.1016/j.neuron.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Bernal B, Tremblay P. The language connectome: new pathways, new concepts. Neuroscientist. 2014;20(5):453–467. doi: 10.1177/1073858413513502. [DOI] [PubMed] [Google Scholar]
- Dick AS, Tremblay P. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language. Brain. 2012;135(Pt 12):3529–3550. doi: 10.1093/brain/aws222. [DOI] [PubMed] [Google Scholar]
- Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol. 2009;514(6):595–623. doi: 10.1002/cne.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann GA, Aylward E, Ojemann JG, Johnson LC, Silbergeld DL, Tranel D. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsychologia. 2008;46(5):1242–1255. doi: 10.1016/j.neuropsychologia.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drane DL, Ojemann JG, Phatak V, Loring DW, Gross RE, Hebb AO, Tranel D. Famous face identification in temporal lobe epilepsy: support for a multimodal integration model of semantic memory. Cortex. 2013;49(6):1648–1667. doi: 10.1016/j.cortex.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Wang J, Zhang Y, Han W, Yu C, Jiang T. Connectivity-based parcellation of the human temporal pole using diffusion tensor imaging. Cereb Cortex. 2014;24(12):3365–3378. doi: 10.1093/cercor/bht196. [DOI] [PubMed] [Google Scholar]
- Farah MJ, McClelland JL. A computational model of semantic memory impairment: modality specificity and emergent category specificity. J Exp Psychol Gen. 1991;120(4):339–357. [PubMed] [Google Scholar]
- Fiez JA, Damasio H, Grabowski TJ. Lesion segmentation and manual warping to a reference brain: intra- and interobserver reliability. Hum Brain Mapp. 2000;9(4):192–211. doi: 10.1002/(SICI)1097-0193(200004)9:4<192::AID-HBM2>3.0.CO;2-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- Foster T, Gillespie K, McClelland R, Patterson C. Risk factors for suicide independent of DSM-III-R Axis I disorder. Case-control psychological autopsy study in Northern Ireland. Br J Psychiatry. 1999;175:175–179. doi: 10.1192/bjp.175.2.175. [DOI] [PubMed] [Google Scholar]
- Frank RJ, Damasio H, Grabowski TJ. Brainvox: an interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Friston K, Kilner J, Harrison L. A free energy principle for the brain. J Physiol Paris. 2006;100(1–3):70–87. doi: 10.1016/j.jphysparis.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Galantucci S1, Tartaglia MC, Wilson SM, Henry ML, Filippi M, Agosta F, Dronkers NF, Henry RG, Ogar JM, Miller BL, Gorno-Tempini ML. White matter damage in primary progressive aphasias: a diffusion tensor tractography study. Brain. 2011 Oct;134(Pt 10):3011–3029. doi: 10.1093/brain/awr099. Epub 2011 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88(2):237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Bauer MD, Foreman D, Mehta S, Eaton BL, Graves WW, Bolinger L. Adaptive pacing of visual stimulation for fMRI studies involving overt speech. Neuroimage. 2006;29(3):1023–1030. doi: 10.1016/j.neuroimage.2005.08.064. [DOI] [PubMed] [Google Scholar]
- Grabowski TJ, Damasio H, Tranel D, Ponto LL, Hichwa RD, Damasio AR. A role for left temporal pole in the retrieval of words for unique entities. Hum Brain Mapp. 2001;13(4):199–212. doi: 10.1002/hbm.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham KA, Patterson K, Hodges JR. Progressive pure anomia: insufficient activation of phonology by meaning. NeuroCase. 1995;1:25–38. (1995) [Google Scholar]
- Han Z, Ma Y, Gong G, He Y, Caramazza A, Bi Y. White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain : A Journal of Neurology. 2013;136(Pt 10):2952–2965. doi: 10.1093/brain/awt205. [DOI] [PubMed] [Google Scholar]
- Harvey DY, Wei T, Ellmore TM, Hamilton AC, Schnur TT. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia. 2013;51(5):789–801. doi: 10.1016/j.neuropsychologia.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Statistics in Medicine. 2002;21:2409–2419. doi: 10.1002/sim.1047. 2002. [DOI] [PubMed] [Google Scholar]
- Heinze G, Ploner M. Technical Report 2/2004, Section for Clinical Biometrics. CeMSIIS, Medical University of Vienna; 2004. A SAS macro, S-PLUS library and R package to perform logistic regression without convergence problems. [Google Scholar]
- Hodges JR, Patterson K, Oxbury S, Funnell E. Semantic dementia: Progressive fluent aphasia with temporal lobe atrophy. Brain. 1992;115:1783–1806. doi: 10.1093/brain/115.6.1783. [DOI] [PubMed] [Google Scholar]
- Hurley RS, Bonakdarpour B, Wang X, Mesulam MM. Asymmetric Connectivity between the Anterior Temporal Lobe and the Language Network. J Cogn Neurosci. 2014:1–10. doi: 10.1162/jocn_a_00722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten D, Mamassian P, Yuille A. Object perception as Bayesian inference. Annu Rev Psychol. 2004;55:271–304. doi: 10.1146/annurev.psych.55.090902.142005. [DOI] [PubMed] [Google Scholar]
- Kier EL, Staib LH, Davis LM, Bronen RA. MR imaging of the temporal stem: anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer's loop of the optic radiation. AJNR Am J Neuroradiol. 2004;25(5):677–691. [PMC free article] [PubMed] [Google Scholar]
- Knösche TR, Tittgemeyer M. The role of long-range connectivity for the characterization of the functional-anatomical organization of the cortex. Front Syst Neurosci. 2011;5:58. doi: 10.3389/fnsys.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuceyeski A1, Maruta J, Niogi SN, Ghajar J, Raj A. The generation and validation of white matter connectivity importance maps. Neuroimage. 2011 Sep 1;58(1):109–121. doi: 10.1016/j.neuroimage.2011.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BY, Halliday GM, Irish M, Hodges JR, Piguet O. Longitudinal white matter changes in frontotemporal dementia subtypes. Hum Brain Mapp. 2014;35(7):3547–3557. doi: 10.1002/hbm.22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, McClelland JL, Patterson K, Galton CJ, Hodges JR. No right to speak? The relationship between object naming and semantic impairment: neuropsychological evidence and a computational model. J Cogn Neurosci. 2001;13(3):341–356. doi: 10.1162/08989290151137395. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Patterson K. Generalization and differentiation in semantic memory: insights from semantic dementia. Ann N Y Acad Sci. 2008;1124:61–76. doi: 10.1196/annals.1440.006. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA1, Pobric G, Jefferies E. Conceptual knowledge is underpinned by the temporal pole bilaterally: convergent evidence from rTMS. Cereb Cortex. 2009 Apr;19(4):832–838. doi: 10.1093/cercor/bhn131. [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Jones RW, Mayberry EJ. Coherent concepts are computed in the anterior temporal lobes. Proc Natl Acad Sci U S A. 2010;107(6):2717–2722. doi: 10.1073/pnas.0907307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambon Ralph MA, Sage K, Roberts J. Classical anomia: a neuropsychological perspective on speech production. Neuropsychologia. 2000;38(2):186–202. doi: 10.1016/s0028-3932(99)00056-1. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92(1–2):101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Mandonnet E, Nouet A, Gatignol P, Capelle L, Duffau H. Does the left inferior longitudinal fasciculus play a role in language? A brain stimulation study. Brain : A Journal of Neurology. 2007;130(Pt 3):623–629. doi: 10.1093/brain/awl361. [DOI] [PubMed] [Google Scholar]
- Martino J, Brogna C, Robles SG, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46(5):691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Martino J, De Witt Hamer PC, Vergani F, Brogna C, de Lucas EM, Vazquez-Barquero A, Duffau H. Cortex-sparing fiber dissection: an improved method for the study of white matter anatomy in the human brain. J Anat. 2011;219(4):531–541. doi: 10.1111/j.1469-7580.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J, Vergani F, Robles SG, Duffau H. New insights into the anatomic dissection of the temporal stem with special emphasis on the inferior fronto-occipital fasciculus: implications in surgical approach to left mesiotemporal and temporoinsular structures. Neurosurgery. 2010;66(3 Suppl Operative):4–12. doi: 10.1227/01.NEU.0000348564.28415.FA. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Primary progressive aphasia. Ann Neurol. 2001;49(4):425–432. [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Hurley R, Rademaker A, Thompson CK, Weintraub S, Rogalski EJ. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain. 2013;136(Pt 2):601–618. doi: 10.1093/brain/aws336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mummery CJ, Shallice T, Price CJ. Dual-process model in semantic priming: a functional imaging perspective. Neuroimage. 1999;9(5):516–525. doi: 10.1006/nimg.1999.0434. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ. Retrieval of visual, auditory, and abstract semantics. Neuroimage. 2002;15(4):917–926. doi: 10.1006/nimg.2001.1016. [DOI] [PubMed] [Google Scholar]
- Papagno C. Naming and the role of the uncinate fasciculus in language function. Curr Neurol Neurosci Rep. 2011;11(6):553–559. doi: 10.1007/s11910-011-0219-6. [DOI] [PubMed] [Google Scholar]
- Papagno C, Miracapillo C, Casarotti A, Romero Lauro LJ, Castellano A, Falini A, Bello L. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134(Pt 2):405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Pascual B, Masdeu JC, Hollenbeck M, Makris N, Insausti R, Ding SL, Dickerson BC. Large-Scale Brain Networks of the Human Left Temporal Pole: A Functional Connectivity MRI Study. Cereb Cortex. 2013 doi: 10.1093/cercor/bht260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passingham RE, Stephan KE, Kotter R. The anatomical basis of functional localization in the cortex. Nat Rev Neurosci. 2002;3(8):606–616. doi: 10.1038/nrn893. [DOI] [PubMed] [Google Scholar]
- Patterson RL, Teigen JR. Conditioning and post-hospital generalization of nondelusional responses in a chronic psychotic patient. J Appl Behav Anal. 1973;6(1):65–70. doi: 10.1901/jaba.1973.6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K1, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nat Rev Neurosci. 2007 Dec;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Philippi CL, Mehta S, Grabowski T, Adolphs R, Rudrauf D. Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci. 2009;29(48):15089–15099. doi: 10.1523/JNEUROSCI.0796-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postelnicu G, Zollei L, Fischl B. Combined volumetric and surface registration. IEEE Trans Med Imaging. 2009;28(4):508–522. doi: 10.1109/TMI.2008.2004426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, Mechelli A, Gorno-Tempini ML, Patterson K, Price CJ. Anterior temporal cortex and semantic memory: reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 2006;6(3):201–213. doi: 10.3758/cabn.6.3.201. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Lambon Ralph MA, Garrard P, Bozeat S, McClelland JL, Hodges JR, Patterson K. Structure and deterioration of semantic memory: a neuropsychological and computational investigation. Psychol Rev. 2004;111(1):205–235. doi: 10.1037/0033-295X.111.1.205. [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO. Using human brain lesions to infer function: a relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5(10):813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010;49(4):3452–3462. doi: 10.1016/j.neuroimage.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Bruss J, Tranel D, Damasio H, Grabowski TJ. Thresholding lesion overlap difference maps: application to category-related naming and recognition deficits. Neuroimage. 2008a;41(3):970–984. doi: 10.1016/j.neuroimage.2007.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudrauf D, Mehta S, Grabowski TJ. Disconnection's renaissance takes shape: Formal incorporation in group-level lesion studies. Cortex. 2008b;44(8):1084–1096. doi: 10.1016/j.cortex.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Rudrauf D, David O, Lachaux JP, Kovach CK, Martinerie J, Renault B, Damasio A. Rapid interactions between the ventral visual stream and emotion-related structures rely on a two-pathway architecture. J Neurosci. 2008c;28(11):2793–2803. doi: 10.1523/JNEUROSCI.3476-07.2008. 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MF, Kimberg DY, Walker GM, Faseyitan O, Brecher A, Dell GS, Coslett HB. Anterior temporal involvement in semantic word retrieval: voxel-based lesionsymptom mapping evidence from aphasia. Brain. 2009;132(Pt 12):3411–3427. doi: 10.1093/brain/awp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza C. Retrieval pathways for common and proper names. Cortex. 2006;42(6):884–891. doi: 10.1016/s0010-9452(08)70432-5. [DOI] [PubMed] [Google Scholar]
- Semenza C. Naming with proper names: the left temporal pole theory. Behav Neurol. 2011;24(4):277–284. doi: 10.3233/BEN-2011-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoura N, Suzuki Y, Tsukada M, Yoshida M, Yamada R, Tabei Y, Yagi K. Deficits in the left inferior longitudinal fasciculus results in impairments in object naming. Neurocase. 2010;16(2):135–139. doi: 10.1080/13554790903329174. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Reddish M, Bellgowan PS, Martin A. The selectivity and functional connectivity of the anterior temporal lobes. Cereb Cortex. 2010;20(4):813–825. doi: 10.1093/cercor/bhp149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Graham KS, Galton CJ, Patterson K, Hodges JR. Semantic knowledge and episodic memory for faces in semantic dementia. Neuropsychology. 2001;15(1):101–114. [PubMed] [Google Scholar]
- Skipper LM, Ross LA, Olson IR. Sensory and semantic category subdivisions within the anterior temporal lobes. Neuropsychologia. 2011;49(12):3419–3429. doi: 10.1016/j.neuropsychologia.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Neary D. Famous people knowledge and the right and left temporal lobes. Behav Neurol. 2012;25(1):35–44. doi: 10.3233/BEN-2012-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsyna G, Warren JE, Scott SK, Turkheimer FE, Wise RJS. Converging language streams in the human temporal lobe. J Neurosci. 2006;26:7328–7336. doi: 10.1523/JNEUROSCI.0559-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavor I, Yablonski M, Mezer A, Rom S, Assaf Y, Yovel G. Separate parts of occipito-temporal white matter fibers are associated with recognition of faces and places. Neuroimage. 2014;86:123–130. doi: 10.1016/j.neuroimage.2013.07.085. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Kinkingnéhun S, Delmaire C, Lehéricy S, Duffau H, Thivard L, Volle E, Levy R, Dubois B, Bartolomeo P. Visualization of disconnection syndromes in humans. Cortex. 2008 Sep;44(8):1097–1103. doi: 10.1016/j.cortex.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: Inferring “how” from “where”. Neuropsychologia. 2003;41:280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Tranel D. Impaired naming of unique landmarks is associated with left temporal polar damage. Neuropsychology. 2006;20(1):1–10. doi: 10.1037/0894-4105.20.1.1. [DOI] [PubMed] [Google Scholar]
- Tranel D. The Iowa-Benton school of neuropsychological assessment. In: Grant I, Adams KM, editors. Neuropsychological assessment of neuropsychiatric disorders. 3rd. New York: Oxford University Press; 2009. pp. 66–83. [Google Scholar]
- Tranel D, Damasio H, Damasio AR. A neural basis for the retrieval of conceptual knowledge. Neuropsychologia. 1997;35(10):1319–1327. doi: 10.1016/s0028-3932(97)00085-7. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Eichhorn GR, Grabowski T, Ponto LL, Hichwa RD. Neural correlates of naming animals from their characteristic sounds. Neuropsychologia. 2003;41(7):847–854. doi: 10.1016/s0028-3932(02)00223-3. [DOI] [PubMed] [Google Scholar]
- Tranel D, Feinstein J, Manzel K. Further lesion evidence for the neural basis of conceptual knowledge for persons and other concrete entities. J Neuropsychol. 2008;2(Pt 1):301–320. doi: 10.1348/174866407x227033. [DOI] [PubMed] [Google Scholar]
- Tranel D, Grabowski TJ, Lyon J, Damasio H. Naming the same entities from visual or from auditory stimulation engages similar regions of left inferotemporal cortices. J Cogn Neurosci. 2005;17(8):1293–1305. doi: 10.1162/0898929055002508. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of Memory. New York: Academic Press; 1972. p. 381e403. [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011 Feb;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ackeren MJ, Rueschemeyer SA. Cross-modal integration of lexical-semantic features during word processing: evidence from oscillatory dynamics during EEG. PLoS One. 2014 Jul 9;9(7):e101042. doi: 10.1371/journal.pone.0101042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uitert R, Bitter I. Subvoxel precise skeletons of volumetric data based on fast marching methods. Med Phys. 2007;34(2):627–638. doi: 10.1118/1.2409238. [DOI] [PubMed] [Google Scholar]
- Visser M, Jefferies E, Lambon Ralph Ma. Semantic processing in the anterior temporal lobes: a meta-analysis of the functional neuroimaging literature. Journal of Cognitive Neuroscience. 2010;22(6):1083–1094. doi: 10.1162/jocn.2009.21309. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013;136(Pt 6):1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Economo C. The cytoarchitectonics of the human cerebral cortex. London: Oxford University Press; 1929. [Google Scholar]
- Waldron EJ, Manzel K, Tranel D. The left temporal pole is a heteromodal hub for retrieving proper names. Front Biosci (Schol Ed) 2014;6:50–57. doi: 10.2741/s413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrington EK, McCarthy RA. Categories of knowledge. Further fractionations and an attempted integration. Brain. 1987;110(Pt 5):1273–1296. doi: 10.1093/brain/110.5.1273. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Shallice T. Category specific semantic impairments. Brain. 1984;107(Pt 3):829–854. doi: 10.1093/brain/107.3.829. [DOI] [PubMed] [Google Scholar]
- Westermann G, Mareschal D. From perceptual to language-mediated categorization. Philos Trans R Soc Lond B Biol Sci. 2014;369(1634):20120391. doi: 10.1098/rstb.2012.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C, Gallate J. The function of the anterior temporal lobe: a review of the empirical evidence. Brain Res. 2012;1449:94–116. doi: 10.1016/j.brainres.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Yvert G, Perrone-Bertolotti M, Baciu M, David O. Dynamic causal modeling of spatiotemporal integration of phonological and semantic processes: an electroencephalographic study. J Neurosci. 2012;32(12):4297–4306. doi: 10.1523/JNEUROSCI.6434-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahn R, Moll J, Krueger F, Huey ED, Garrido G, Grafman J. Social concepts are represented in the superior anterior temporal cortex. Proc Natl Acad Sci U S A. 2007;104(15):6430–6435. doi: 10.1073/pnas.0607061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Zhang J, Oishi K, Faria AV, Jiang H, Li X. Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy. Neuroimage. 2010;52(4):1289–1301. doi: 10.1016/j.neuroimage.2010.05.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.