Abstract
Dietary deficiency of docosahexaenoic acid (C22: 6n-3; DHA) is linked to the neuropathology of several cognitive disorders, including anxiety. DHA, which is essential for brain development and protection, is primarily obtained through the diet or synthesized from dietary precursors, however the conversion efficiency is low. Curcumin (diferuloylmethane), which is a principal component of the spice turmeric, complements the action of DHA in the brain, and this study was performed to determine molecular mechanisms involved. We report that curcumin enhances the synthesis of DHA from its precursor, α-linolenic acid (C18: 3n-3; ALA) and elevates levels of enzymes involved in the synthesis of DHA such as FADS2 and elongase 2 in both liver and brain tissue. Furthermore, in vivo treatment with curcumin and ALA reduced anxiety-like behavior in rodents. Taken together, these data suggest that curcumin enhances DHA synthesis, resulting in elevated brain DHA content. These findings have important implications for human health and the prevention of cognitive disease, particularly for populations eating a plant-based diet or who do not consume fish, a primary source of DHA, since DHA is essential for brain function and its deficiency is implicated in many types of neurological disorders.
Keywords: DHA synthesis, Curcumin, ALA, DPA, omega 3 fatty acids, docosahexaenoic acid
Introduction
Docosahexaenoic acid (DHA, C22: 6n-30) is the most prevalent omega 3 (n-3) fatty acid in brain tissue, and its deficiency is linked to several neurocognitive disorders such as anxiety-like behavior [1, 2], Alzheimer’s disease [3], major depressive disorder [4], schizophrenia [5] with psychosis [6] and impaired attention [7, 8]. Extensive reports using rodent models have identified that deficiency of DHA during growth and development causes significant learning and memory impairments [2, 9–12]. In addition to being critical for brain development [13–17] dietary DHA is particularly important during challenging situations such as aging [18–21], or brain injury [22, 23]. Notably, low levels of DHA are associated with generalized anxiety [4] and supplementation with DHA has been shown to have anxiolytic effects [24–27]. Thus, n-3 fatty acids play a critical role in brain health and the overall prevention of cognitive disease.
Omega-3 fatty acids require n-3 fatty acid precursors for de novo synthesis in mammals and therefore n-3 fatty acids must be obtained through the diet. The n-3 fatty acids DHA and eicosapentaenoic acid EPA (C20: 5n-3) can be obtained directly from animal sources or can be synthesized from plant derived n-3 fatty acid precursors. Primary sources of DHA are fish and seafood. Sources of the precursor for DHA and EPA: α-linolenic acid (C18:3 n-3; ALA) include sunflower and soybean oil (> 50% of the fat) [28]. DHA synthesis from its precursors ALA, EPA and docosapentaenoic acid (C22:5 n-3; DPA) mainly takes place in the liver since the synthesis of DHA in the brain is very limited [29]. In general, the conversion efficiency of DHA synthesis from ALA is quite low [30–32]. Vegetarians and vegans thus have reduced plasma DHA compared to omnivores [33–36], yet many populations thrive on an entirely plant based diet and are able to obtain adequate levels of DHA to support cognitive development and plasticity. This raises the question as to whether other food components commonly obtained in the vegetarian diet might enhance the conversion of DHA from n-3 precursors. There is a discrepancy between animal studies showing cognitive impairment associated with DHA deficiency and human data reporting vegetarians have normal cognitive abilities. We conducted an investigation asking whether components commonly consumed in traditional vegetarian diets could enhance DHA content in the brain and the synthesis of DHA from plant-based sources.
Turmeric (Curcuma Longa) is native to Southeast India, and the yellow curry spice derived from the plant is a staple in Indian cooking. Ironically, DHA is poorly consumed in India based on the vegetarian prevalence in the population. Turmeric contains the polyphenolic secondary metabolite curcumin, which has been implicated to improve brain health including reducing degeneration in Alzheimer’s disease [19, 37–39], ischemia [40] and traumatic brain injury [22, 41–43]. In addition to having profound antioxidant and anti-inflammatory effects in various tissues [44][45][46][47][48][49][50], we recently showed that curcumin prevents reduced DHA content in the brain following brain trauma, benefiting brain plasticity as well as reducing oxidative damage [22]. Additionally, while curcumin + DHA had additive beneficial effects on plasticity, behavior and brain DHA content, the combined supplementation with curcumin + DHA reduced brain content of the DHA precursor n-3 DPA, raising the question as to whether curcumin stimulates the synthesis of DHA [22]. Therefore, the standing question is what are the molecular mechanisms by which curcumin would support the action of DHA on brain function and plasticity under homeostatic conditions. Here we explore the possibility that curcumin increases the production of DHA by influencing precursors such ALA and DPA.
Experimental Procedures
Animals
Male Sprague–Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) weighing between 200 and 240 g were singly housed and maintained in environmentally controlled rooms (22–24 °C) on a 12 h light/dark cycle. All experiments were performed in accordance with the United States National Institute of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of California at Los Angeles Chancellor’s Animal Research Committee.
Feeding study
After 1 week acclimatization on standard chow, the rats (n=5–6/group) were fed control diet (CTL), ALA diet (ALA; 2.7% of fat), curcumin diet (CUR, 500ppm), or ALA + CUR diet (ALA, 2.7% of fat; CUR, 500 ppm). The diet components, including fatty acid profiles are shown in Table 1. Diets were custom made (Dyets Inc., Bethlehem, PA) and provided to animals in powder ad libitum. After 3.5 weeks of feeding of the respective diet, rats were sacrificed by decapitation, and brains were rapidly dissected out, frozen on dry ice, and stored at −70°C until use for biochemical analyses. One half of each brain was used for immunoblotting and the other half for lipids analysis.
Table 1.
Composition of experimental diets
| Ingredient | Amount (g/100g diet) | ||
|---|---|---|---|
|
| |||
| CTL | ALA Diet | ALA+Cur Diet | |
| Alacid 710, acid casein | 20 | 20 | 20 |
| Cornstarch | 15 | 15 | 15 |
| Sucrose | 10 | 10 | 10 |
| Dextrose | 19.9 | 19.9 | 19.9 |
| Maltose-dextrin | 15 | 15 | 15 |
| Cellulose | 5 | 5 | 5 |
| Salt-mineral mix | 3.5 | 3.5 | 3.5 |
| Vitamin mix | 1 | 1 | 1 |
| L-cystine | 0.3 | 0.3 | 0.3 |
| Choline bitartrate | 0.25 | 0.25 | 0.25 |
| TBHQ | 0.002 | 0.002 | 0.002 |
| Fat sources: | |||
| Hydrogenated coconut oil | 8.1 | 7.45 | 7.45 |
| Safflower oil | 1.9 | 1.77 | 1.77 |
| Flaxseed oil | --- | 0.48 | 0.48 |
| Curcumin | -- | -- | 500 or 250 ppm |
| Fatty acid composition as a percentage of total fatty acids | |||
| Total saturated | 82 | 79 | 79 |
| Total monounsaturated | 3 | 3 | 3 |
| Linoleic acid (n-6) | 15 | 15 | 15 |
| α linoleic acid (n-3) | --- | 2.8 | 2.8 |
Elevated Plus Maze
The elevated plus maze (EPM) test was performed as described by Walf and Frye [51]. Briefly, the EPM apparatus is made of laminated wood consisting of 2 opposing open arms (10×50 cm) and 2 opposing closed arms (10×50 cm with 30 cm high walls). The maze was placed 60 cm above the floor and enclosed with curtains. An overhead video camera was used to record behavior over a period of 5 min. Each rat was placed in the middle of the maze pointed toward the open arm that faced away from the experimenter. The time spent and the number of entries into each arm was recorded using AnyMaze video tracking software (San Diego Instruments, San Diego, CA). Data are reported as time spent in the open arm (seconds).
Immunoblotting
The total proteins from hippocampus were extracted. Protein samples were separated by electrophoresis on an 8% polyacrylamide gel and electrotransferred to a nitrocellulose membrane. Non-specific binding sites were blocked in TBS, overnight at 4°C, with 2% BSA and 0.1% Tween-20. Membranes were rinsed for 10 min in buffer (0.1% Tween-20 in TBS) and then incubated with anti-FADS2, anti-Elov2 (elongase 2), anti-4HNE and anti-β-actin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), followed by anti-goat or anti-rabbit IgG horseradish peroxidase-conjugate (Santa Cruz Biotechnology, Santa Cruz, CA). Immunocomplexes were visualized by chemiluminescence using an ECL kit (Amersham Pharmacia Biotech Inc., Piscataway, NJ) according to the manufacturer’s instructions. The film signals were digitally scanned and then quantified using NIH Image software.
Lipids analysis
Total lipids were extracted from cerebral tissues or cultured cells with lysis solution of chloroform-methanol (2:1, vol:vol) containing 0.005% Butylated hydroxytoluene in a ratio of sample to lysis solution of 1:20 (i.e. 100 mg tissue + 2ml lysis solution). Samples were homogenized and extracted overnight at 4°C, then centrifuged for 5 min at 1200×g and the supernatant was aspirated and transferred to a new tube. 1 mL of lysis solution was added to the pellet and the centrifugation was repeated and supernatant collected. To the combined supernatant solution, 0.2 mL 0.9 % sodium chloride solution was added and the liquid centrifuged at 2170×g for 15 min at 4°C. The chloroform layer was transferred to a new 15 ml tube, and then mixed with an additional 0.2 mL 0.9% saline. After centrifugation again for 10 min at 2170×g at 4°C, the chloroform layer was transferred to another 15 ml tube and evaporated under nitrogen gas. The sample of total lipids was dissolved in 1 mL hexane and methylated by 14% boron trif luoride–methanol reagent (1 mL) at 90°C for 1 hour. Oxygen was displaced using nitrogen gas. After cooling to 21°C 1 mL of distilled deionized water was added and samples were vortexed and allowed to sit for 30 min to separate the water from the organic layer. The organic layer was transferred to a new tube and evaporated using nitrogen gas. Fatty acid profiles were determined by using gas chromatography (GC). The system consisted of model Clarus 500 gas chromatograph (PerkinElmer) with a built-in Autosampler. An Elite-WAX column (60m, 0.32-mm internal diameter, PerkinElmer) was used, with hydrogen as the carrier gas. GC oven temperature was initially held at 140 °C for 2 min and raised with a gradient of 5 °C min−1 until 250 °C and held for 10 min. The total run time is 34 min. The injector and detector were maintained at 250 and 300 °C, respectively. A 1μl sample of fatty acid methyl esters (FAME) was injected in split injection mode with a 100:1 split ratio. Peaks of resolved fatty acid methyl esters were identified and quantified by comparison with standards (Supelco 37-component FAME Mix).
In vitro study with cell culture
HepG2 liver cells were grown in DMEM (Life Technologies) with 10% fetal bovine serum (Life Technologies) and 100 units/mL penicillin, 100 μg/mL streptomycin (Life Technologies) for 2 days. Cells were then treated with vehicle (0.5% ethanol in distilled deionized water), DPA (Nu-Chek-Prep, Elysian, MN) (50μM), or DPA+ CUR (Sigma-Aldrich, Saint Louis, MO) (10, 20, or 40μM) for 48 hours. A separate set of cells were treated with FADS2 (Δ6-desaturase) inhibitor SC-26196 (Sigma-Aldrich, Saint Louis, MO) (2μM) 30min before treatment with DPA (50μM) + Cur (20μM). At the end of each experiment, the cells were harvested for immunoblotting or lipid analysis.
Statistical analysis
For DHA, DPA (n-3), and protein levels, the values were converted to percent of control and represented as the mean ± SEM. The data were analyzed by one-way ANOVA followed by Tukey’s post hoc test to adjust for multiple comparisons. Correlations were performed using linear regression. Statistical differences were considered significant at p <0.05. All statistical analyses were performed using Prism version 6.0 (Graphpad software Inc.).
Curcumin increases DHA in the brain when consumed with the n-3 precursor ALA
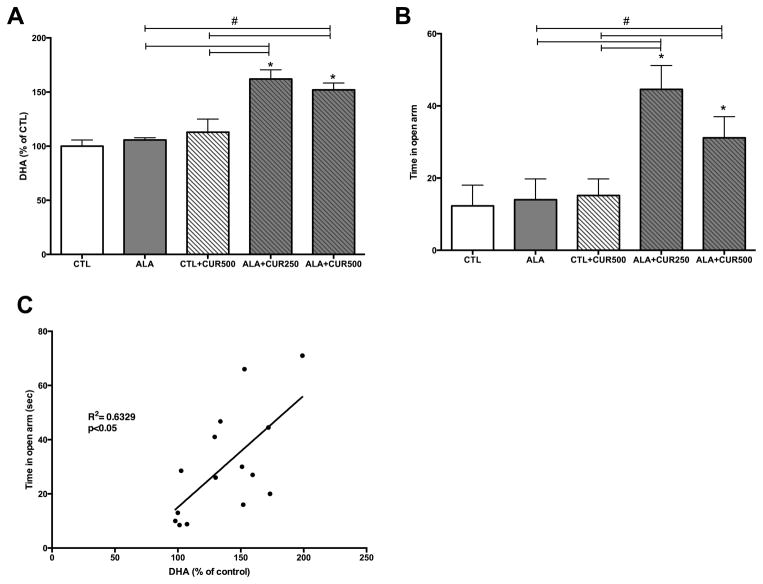
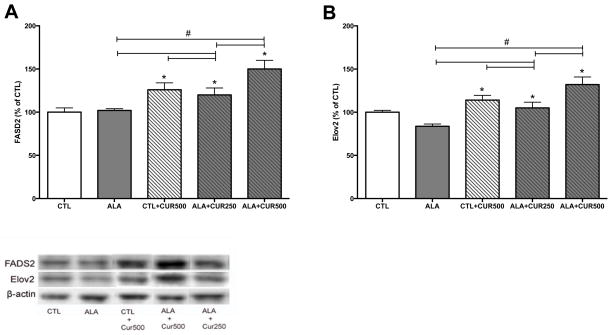
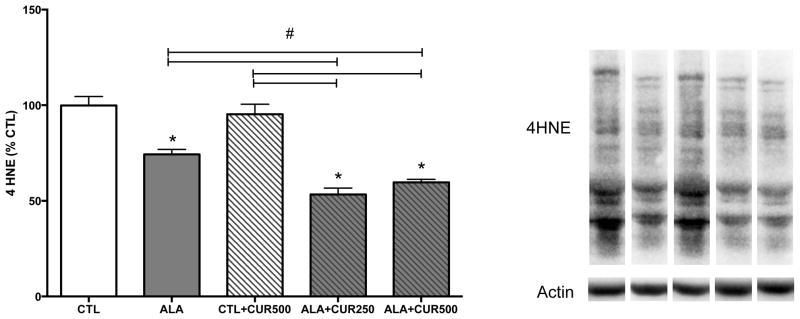
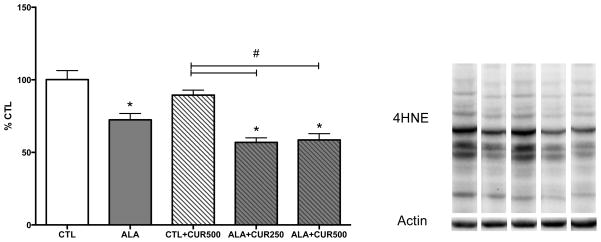
Animals were maintained on a diet supplemented with ALA (2.7% of total fatty acids) or ALA+CUR (250 or 500 ppm). ALA alone or CUR alone did not increase DHA content in the hippocampus compared to control group (p>0.05; Fig. 1A). However, when rats were fed ALA+CUR, DHA content was significantly higher (162 or 152% of CTL for 250 or 500 ppm curcumin-fed animals, respectively) in ALA+CUR than ALA alone group (p<0.05; Fig. 1A). These findings suggest that CUR may enhance the conversion of ALA to DHA in vivo and elevate DHA content in brain. We assessed the relevance of the dietary intervention on cognitive function using the elevated-plus maze, a measure of anxiety-like behavior. We found that the time spent in the open arm was significantly higher in ALA+CUR groups compared with ALA or CUR alone or CTL (p<0.05; Fig. 1B). We regressed DHA% of CTL measured in the hippocampus against elevated plus maze scores and found a significant positive correlation between brain DHA levels and time spent in the open arms, indicating that DHA in the brain is associated with reduced anxiety-like behavior (p<0.05; Fig. 1C). We quantified enzymes involved in the synthesis of DHA from n-3 precursors in brain tissue using immunoblotting. The enzyme FADS2 is involved in the desaturation process during the conversion of precursor ALA to DHA. Elov2 is involved in the elongation steps during DHA synthesis. In the brain we found that CUR alone increased levels of FADS2 (126% of control, p<0.05; Fig. 2A), and the presence of ALA in the diet increased the effects of CUR at 500 ppm on FADS2 (150% of control, p<0.05; Fig. 2A), whereas ALA alone did not affect FADS2 levels. Though significantly elevated compared to CTL, CUR at a dose of 250ppm with ALA led to lower FADS2 (120% of CTL, p<0.05) compared to the CUR 500ppm + ALA treatment group (Fig. 2A). CUR (500ppm) also significantly elevated the levels of Elov2 when provided alone (114% of control, p<0.05) or in combination with ALA (132% of control, p<0.05; Fig. 2B). CUR at dose of 250ppm + ALA led to lower Elov2 (105% of CTL, p<0.05) compared to ALA + CUR at a dose of 500 ppm (p<0.05; Fig. 2B). The enzymes FASD2 and Elov2 are involved in synthesis of n-6 fatty acids as well as n-3 fatty acids. Table 2 shows levels of n-3 and n-6 fatty acids quantified in brain tissue. The intermediates 18:2 n-6, 20:3 n-6 and 20:4 n-6 were elevated by CUR + ALA at the 250 ppm dose relative to CTL. Notably, 20:4n-6 was elevated at both doses of CUR + ALA relative to controls, however the n-6 end product 22:5n-6 was not elevated by curcumin treatment whereas 22:6 n-3 was elevated in both groups. The n-3 EPA and DPA are not pictured because levels were too low for detection using our methods. Curcumin has known antioxidant and anti-inflammatory activities, thus it is possible that the effects of CUR + ALA are due to neuroprotective effects of CUR rather than a direct product of enhanced DHA synthesis. We sought to determine whether curcumin in the diet affects markers of lipid peroxidation using as a marker the lipid degradation product 4 hydroxynonenol (4-HNE). Curcumin alone did not affect levels of 4-HNE, however there was a significant reduction in 4-HNE in animals fed ALA, which was further elevated with the addition of CUR to the diet (Fig 3.) Taken together these findings suggest that dietary CUR, when combined with the ALA precursor, increases DHA in the brain, reduces anxiety-like behavior and that CUR increases the quantity of DHA processing enzymes in the hippocampus.
Figure 1. Curcumin combined with ALA increases DHA in the brain, which correlates with reduced anxiety-like behavior.
ALA alone and CUR alone did not increase DHA in the hippocampus (A). A1LA plus CUR significantly increased DHA levels at both 250 and 500 ppm doses of CUR. The time in open arm was significantly higher in ALA+CUR groups compared with CTL or ALA alone (B). DHA in the brain was positively correlated with time spent in the open arm (C). CTL: control, ALA: α-linolenic acid, CUR: curcumin. *compared with control (p<0.05); # compared with indicated p<0.05 (n=5–6)
Figure 2. Curcumin increases FADS2 and Elov2 in the brain.
Curcumin significantly increased the levels of FADS2 (A) and Elov2 (B) with or without ALA as detected by immunoblotting. Combined, ALA + CUR 500 ppm increased both enzymes more than 500pm CUR alone or ALA +250 ppm (A, B). FADS2: delta 6 desaturase, Elov2: elongase, HIP: hippocampus, CTL: control, ALA: α-linolenic acid, CUR: curcumin. *compared with control (p<0.05); # compared with indicated p<0.05 (n=5–6).
Table 2.
Composition of fatty acids in the hippocampus
| CTL | ALA | CTL+CUR | ALA+CUR250 | ALA+CUR500 | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| C18:2n-6 | 0.1335 | 0.0147 | 0.1354 | 0.0058 | 0.128 | 0.01430 | 0.2106abc | 0.0210 | 0.1756 | 0.0082 | <0.01 |
| C20:3n-6 | 0.1672 | 0.0158 | 0.1871 | 0.0139 | 0.1768 | 0.0178 | 0.2452a | 0.0190 | 0.2343 | 0.0153 | <0.01 |
| C20:4n-6 | 4.9686 | 0.4334 | 4.7316 | 0.1831 | 5.4403 | 0.4383 | 7.474abc | 0.5900 | 6.8812ab | 0.4099 | <0.001 |
| C22:5n-6(DPA) | 0.6036 | 0.0896 | 0.3749 | 0.0170 | 0.52 | 0.0467 | 0.5485 | 0.0570 | 0.4618 | 0.0709 | ns |
| C22:6n-3(DHA) | 5.6981 | 0.3692 | 6.1008 | 0.0930 | 6.3589 | 0.6617 | 9.249abc | 0.5481 | 8.662abc | 0.3948 | <0.001 |
CTL: control, ALA: α-linolenic acid, CUR: curcumin, CTL+CUR: control + curcumin (500 pmol).
compared with CTL;
compared with ALA;
compared with CTL+CUR.
Figure 3. The n-3 precursor ALA supports the antioxidant action of curcumin in the brain.
In combination with dietary ALA, however, CUR significantly reduces the lipid peroxidation product 4 hydroxynonenol (4-HNE). Representative bands from Western blot are shown. CTL: control, ALA: α-linolenic acid, CUR: curcumin *compared with control (p<0.05); # compared with indicated p<0.05 (n=5–6).
Curcumin increases DHA synthesis in the liver
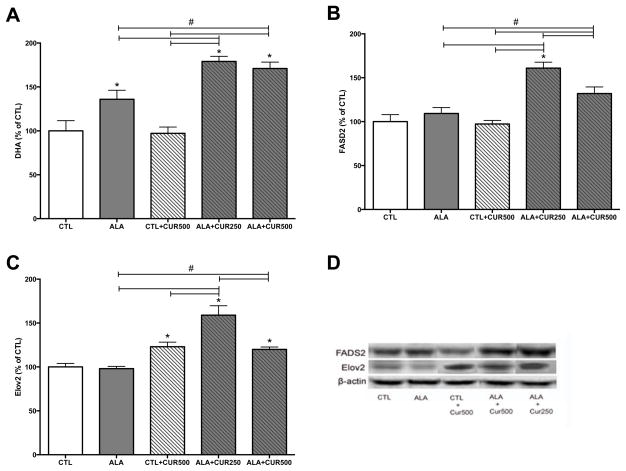
Since DHA is mainly synthesized in the liver, we sought to determine the effects of CUR on hepatic DHA. Compared with CTL, ALA alone increased DHA in the liver (136% of control, p>0.05; Fig. 4A). CUR alone did not affect DHA content in the liver (p>0.05; Fig. 4A), however hepatic DHA was significantly increased when CUR was combined with ALA (171% of CTL for 500 the ppm dose, 179% of CTL for the 250 ppm dose, p<0.05; Fig. 4A). We measured levels of the enzymes FADS2 and Elov2 in liver to evaluate effects of CUR on enzymes involved in hepatic DHA synthesis. CUR 500ppm + ALA significantly increased the levels of FADS2 (132% of CTL, p<0.05; Fig. 4B, D), whereas CUR + 250ppm further elevated FADS2 (161% of CTL) significantly more than the CUR 500ppm group (p<0.05; Fig. 3B, D). CUR alone or in combination with ALA significantly elevated the levels of Elov2 (123% or 120% of control, respectively; p<0.05; Fig. 4C–D). Similar to FADS2, CUR 250ppm had greater Elov2 (159% of CTL) compared to CUR 500ppm (p<0.05; Fig. 4C–D). Together these findings suggest that CUR may enhance the conversion of ALA to DHA in the liver. CUR alone did not reduce levels of the lipid peroxidation product 4 HNE, however in combination with ALA levels of 4 HNE were significantly reduced compared with CTL and CTL+CUR. ALA alone also reduced levels of 4-HNE compared with CTL and there was a trend toward further reduction when ALA was combined with CUR 250 or CUR 500 (p=0.1 and 0.2, respectively; Fig. 5). Levels of n-3 and n-6 fatty acids were quantified in liver and are presented in Table 3. In the liver, ALA, CUR and ALA+CUR 250ppm did not significantly affect C18:2 n-6 or C20:3n-6 but significantly reduced levels of 20:4n-6. Both CUR and ALA reduced C22:5n-6 relative to CTL, whereas levels of C22:5n-3 were increased by ALA and in both ALA+CUR groups, though CUR+CTL had no effect. Compared with CTL, ALA and CTL+CUR 22:6 n-3 was significantly increased in both CUR+ALA groups. We sought next to corroborate the action of these enzymes on the effects of CUR in isolated liver cells in culture.
Figure 4. Curcumin and ALA combined increases DHA and DHA-synthesis-related enzymes in the liver.
ALA alone increase DHA in the liver, which is further elevated when ALA is combined with CUR at both 250 and 500 ppm doses, whereas CUR alone did not elevate liver DHA content (A). FADS2 was significantly increased in both ALA+CUR groups compared to other groups (B). Elov2 was higher in all CUR groups compared to other groups (C). Representative bands for FADS2 and Elov2 (D). FADS2: delta 6 desaturase, Elov2: elongase, CTL: control, ALA: α-linolenic acid, CUR: curcumin. *compared with control (p<0.05); # compared with indicated p<0.05 (n=5–6).
Figure 5. The DHA precursor ALA supports the antioxidant action of curcumin in the liver.
In combination with dietary ALA, CUR significantly reduces the lipid peroxidation product 4 hydroxynonenol (4-HNE). Representative bands from Western blot are shown. CTL: control, ALA: α-linolenic acid, CUR: curcumin *compared with control (p<0.05); # compared with indicated p<0.05 (n=5–6).
Table 3.
Composition of fatty acids in the liver
| CTL | ALA | CTL+CUR | ALA+CUR500 | ALA+CUR250 | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | ||
| C18:2n-6 | 4.4466 | 0.3013 | 3.5083 | 0.1161 | 3.2111 | 0.1403 | 4.1782 | 0.1213 | 3.8729 | 0.9998 | ns |
| C20:3n-6 | 0.4571 | 0.0602 | 0.3944 | 0.0218 | 0.3679 | 0.0383 | 0.4509 | 0.0149 | 0.4669 | 0.0429 | ns |
| C20:4n-6(AA) | 9.1706bc | 0.9311 | 5.9512a | 0.1372 | 7.0566a | 0.2734 | 6.9291a | 0.1977 | 7.6216 | 0.2276 | 0.001 |
| C22:5n6(DPA) | 1.0274bc | 0.0412 | 0.255ac | 0.0389 | 0.7852ab | 0.0646 | 0.2363ac | 0.0143 | 0.1807ac | 0.0383 | <0.0001 |
| C22:5n3(DPA) | 0.0982 | 0.0075 | 0.2437c | 0.0150 | 0.0674b | 0.0057 | 0.3208ac | 0.0347 | 0.3224ac | 0.0670 | <0.0001 |
| C22:6n3(DHA) | 1.6033b | 0.1886 | 2.1874ac | 0.1527 | 1.4885b | 0.1217 | 2.7713abc | 0.1016 | 2.8746abc | 0.0960 | <0.0001 |
CTL: control, ALA: α-linolenic acid, CUR: curcumin, CTL+CUR: control + curcumin (500 pmol).
compared with CTL;
compared with ALA;
compared with CTL+CUR.
Curcumin enhances FADS2-dependent DHA synthesis in cultured HepG2 cells
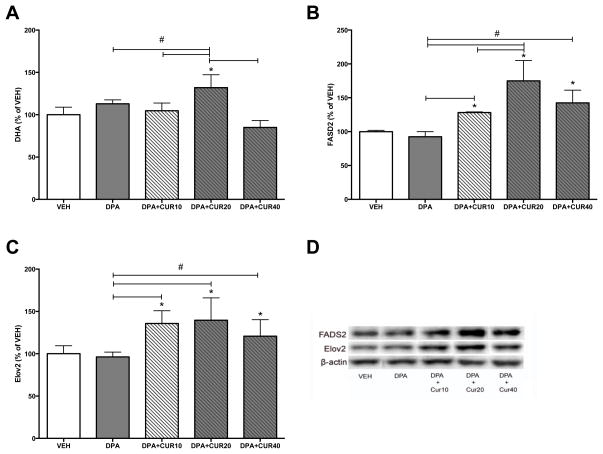
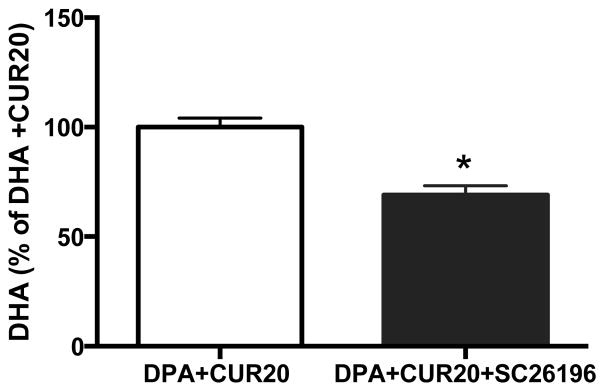
We treated cultured liver cells HepG2 with the DHA precursor n-3 DPA (50 μM) or DPA plus three different doses of CUR (10, 20, or 40μM). In an initial experiment we treated the cells with the precursor ALA + curcumin, however we found that in cultured liver cells ALA + curcumin did not increase synthesis of DHA (data not shown). Similar findings were previously reported indicating that treating cultured HepG2 cells with ALA does not increase DHA synthesis [52]. The lack of existing fatty acid precursors such as DPA, which are present in an in vivo study, cause FADS2 to preferentially bind to ALA, thus levels of EPA and DPA accumulate while the final conversion from DPA → DHA is negligible [52]. The conversion of DPA → DHA by FADS2 is the rate-limiting step in DHA synthesis from ALA, therefore for our in vitro study cells were treated with DPA. The combination of DPA + CUR (20μM) significantly increased DHA levels (132% of VEH) compared to DPA treatment alone (p<0.05; Fig. 6A). Lower doses of CUR at doses of 10 μm did not have effects on DHA synthesis while the higher dose tended to reduce DHA levels to 85% of VEH and significantly reduced DHA synthesis compared to the 20μM dose. These results suggest that CUR has a dose specific effect on the conversion of n-3 DPA to DHA. Protein levels of FADS2 and Elov2 were elevated by CUR + DPA at all measured doses (Fig 6B–D). Similar to the DHA content, the 20 μM dose caused a large increase in FADS2 (175% of VEH; p<0.05; Fig. 6B) which was significantly greater than the 10 μM dose (p<0.05), and tended to be higher than the 40 μM dose. All doses of CUR caused a greater increase in FADS2 compared with VEH or DPA alone (Fig 6B, D). Similarly, DPA + CUR increased the levels of Elov2 at all three doses (p<0.05; Fig. 6C–D). Finally, we sought to determine the role of FADS2 on the process underlying CUR-enhanced DHA synthesis. Cultured HepG2 cells were pre-treated with the FADS2 inhibitor SC-26196 (2 μM) prior to adding DPA (50 μM) + CUR (20 μm) to the culture media. The FADS2 inhibitor SC-26196 significantly reduced DHA levels in cells treated with DPA plus CUR (69% of DPA + CUR control group p<0.05; Fig. 7). Together these findings suggest that CUR promotes hepatic DHA synthesis, in which the desaturase FADS2 plays a critical role.
Figure 6. DHA and DHA-synthesis-related enzymes are elevated by curcumin in cultured liver cells (HepG2).
The combination of DPA (50 μM) and CUR (20μM) significantly increased DHA levels compared to DPA treatment alone (A). CUR significantly increased the enzyme FADS2 (B) and Elov2 (C) at all doses compared with DPA (50 μM) alone. DPA plus CUR at dose of 20 μM significantly increase FADS2 (B) and Elov2 (C) compared to other doses of curcumin treatment. Representative bands for FADS2 and Elov2 (D). FADS2: delta 6 desaturase, Elov2: elongase, CTL: control, DPA: Docosapentaenoic acid, CUR: curcumin. *compared with control (p<0.05); # compared with indicated p<0.05.
Figure 7. FADS2 is essential to curcumin effects on DHA synthesis in hepatic HepG2 cells.
SC-26196, an inhibitor of FADS2, significantly reduced the DHA levels in cells treated with DPA plus curcumin compared to the cells with similar treatment except inhibitor SC-26196. * p<0.05
Discussion
We report novel data showing that curcumin elevates DHA synthesis from n-3 precursors in liver cells, and that in combination with dietary ALA, curcumin increases DHA content in vivo in both the liver and brain. Enzymes involved in the synthesis of DHA, FADS2 and elongase 2, were concurrently elevated, suggesting that curcumin may increase DHA content, in part, by increasing the pool of enzymes available to facilitate the conversion from either ALA or DPA. In the liver, dietary ALA elevated the DHA precursor n-3 DPA, whereas curcumin did not increase levels of this precursor (Table 3). In support of this, we found that inhibition of the FASD2 enzyme in liver cells attenuated increases in DHA content associated with the combined treatment with DPA and curcumin. In this study, we also report that elevated DHA content in the brain associated with curcumin and ALA in the diet correlates with reduced anxiety-like behavior. These findings have important implications for human health and the prevention of cognitive disease, particularly for populations eating a plant-based diet or who do not consume fish, a primary source of DHA, since DHA is essential for brain function and its deficiency is implicated in many types of neurological disorders.
Structurally, DHA accounts for roughly one third of the fatty acids in the gray matter of the brain and is essential to normal healthy brain development [13–17]. Dietary deficiency of n-3 fatty acids during developmental years has detrimental effects on cognitive abilities [10, 11, 53–55], but cognitive performance can be improved by increasing brain DHA content [55]. Animal studies strongly suggest that dietary deficiency of DHA increases the risk for neurocognitive disorders [56, 57], and that diets enriched with DHA fosters learning and memory [18, 58–62] and are protective against cognitive decline during aging [18, 20]. In humans, circulating DHA is significantly related to cognitive abilities during aging [63] and inversely associated with cognitive decline [64].
While DHA can be obtained through animal sources in the diet, vegetarians and vegans may face challenges getting adequate dietary DHA [35]. Increasingly, vegetarianism and veganism is being adopted in the Western world [65]. Circulating omega 3 fatty acids are lower in vegetarians and non-fish eaters than in people who consume fish [66]. Furthermore, changes in farming practices and food consumption have taken place over the last century such that the dietary n-3 fatty content may be declining [67]. For example, aquaculture currently accounts for nearly one half of the seafood supply for human consumption in the world, which is large increase compared with 1980’s, where aquaculture contributed only 9% or even 2000 where fisheries made up 34% [68]. Terrestrial oilseeds [69] and maize [70] are commercially used in fish feed, which can increase the ratio of n-6:n-3 in seafood [71–74]. Other dietary sources of DHA, such as beef [75] and eggs [76] have reduced DHA content when using the most prevalent farming practices of grain corn feeding vs pasture-feeding. Thus, even for omnivores, obtaining DHA through the diet may be increasingly difficult in the current modern food climate. Paradoxically, in Asia, vegetarianism has been practiced for centuries without high incidence of cognitive impairment. No ill effects were reported in pregnancy outcomes of South Asian vegetarians vs North London omnivores [77] and Seventh Day Adventists residing in the Southwestern United States reported significantly less negative emotion compared with omnivores, despite reduced levels of DHA [78].
Due to the apparent discrepancy between animal studies showing clear cognitive impairment associated with reduced DHA in the brain and human data showing improved mental health in vegetarians, we sought to investigate whether other components commonly consumed in traditional vegetarian diets could enhance DHA content in the brain and the synthesis of DHA from vegetarian sources. In India 31% of the population are lacto-vegetarian or vegan [79]. Turmeric is a household staple in India, with reported average daily consumption ranging from 0.24 g/person/day to as high as 4g/person/day [80, 81]. Curcumin, which is a naturally polyphenolic compound found in turmeric has multifaceted medicinal properties and has been implicated in helping to treat several types of neurological disorders including Alzheimer’s disease [39, 82, 83], retinal diseases [84, 85], Parkinson’s disease [86, 87], stroke [88], brain injury [22, 41, 42] and psychiatric disorders [89–91]. We recently reported that curcumin supplementation promoted increased DHA content in the brain following TBI, and that this effect was associated with elevated levels of enzymes involved in DHA synthesis [22]. We therefore investigated whether curcumin enhances DHA synthesis from vegetarian precursors. Here we report that, when combined with dietary ALA, curcumin increases the DHA content in both liver and brain tissues. Furthermore, we found that the elevated DHA content was associated with elevated levels of the enzymes FADS2 and elongase 2, which are important for DHA synthesis.
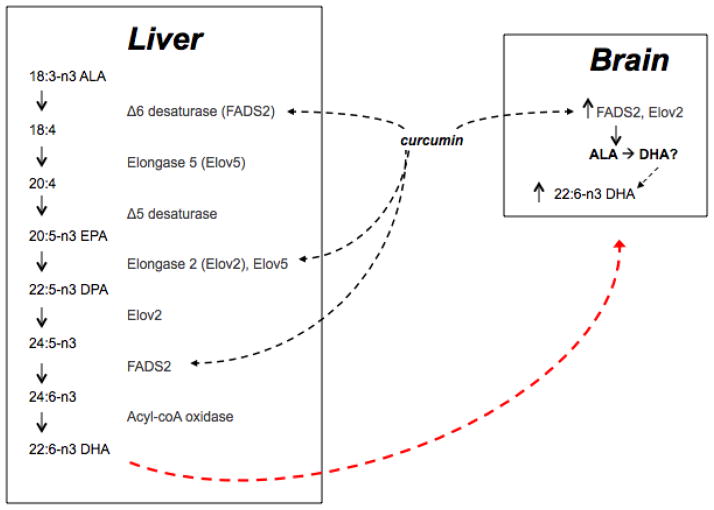
Emerging evidence indicates that hepatic DHA synthesis from ALA increases the DHA supply to the brain, but the rate of DHA conversion from its precursors is low [28, 92]. This may be partially due to the fact that the plant-derived omega 6 and omega 3 fatty acids linoleic acid (18:2 n-6;LA) and ALA, respectively, compete for enzymes to catalyze the desaturation and elongation reactions in order to generate longer chain fatty acids. There are two stages involved in the process of DHA synthesis from ALA. The first stage includes a series of elongation by elongases such as Elov5 and desaturation by desaturases such as FADS1 (Δ5-desaturase) and FADS2 (Δ6-desaturase) (Fig. 8). This results in the conversion of ALA to eicosapentaenoic acid (C20:5 n-3; EPA). The second stage involves the conversion from EPA to docosapentaenoic acid (C22:5 n-3; DPA) and ultimately to DHA. In mammals, this process involves elongation (by elongases), desaturation (FADS2), and β-oxidation (by Acox1 and 17β-HSD4) [93]. In particular, the intermediate C22:5 n-3 is converted to C24:6 n-3, which is then retroconverted to C22:6 n-3 (DHA) [94, 95]. It has been reported that in order to achieve similar amounts of long chain omega 3 fatty acids in the brain on an ALA enriched, vs a preformed EPA + DHA enriched diet, ALA needed to be consumed at 33.5 times the amount [96]. The action of FADS2 and Elov2 are important control points (enzymatic reaction-limitation) during the synthesis of DHA. Excessive ALA consumption may inhibit the activity of enzymes involved in DHA synthesis. In particular FADS2 is subject to competitive inhibition between substrates. For example, administration of ALA to HepG2 cells results in increased accumulation of EPA, but not DHA, consistent with competitive inhibition of FADS2 by DPA [52]. We found that, when combined with the vegetarian precursor ALA, dietary curcumin increased levels of FADS2 and elongase 2 in both liver and brain tissues. Accordingly, our results showed that curcumin produced greater hepatic and central DHA increases compared to animals fed ALA alone. Thus, our data support that by increasing the level of these key enzymes curcumin may enhance the conversion of ALA to DHA, even when ALA levels are increased in the diet. Accordingly, our in vitro data suggest that the effects of curcumin to enhance DHA synthesis are dependent on the effective functioning of FASD2 enzyme, suggesting that curcumin effects are partially at the level of DHA synthesis.
Figure 8. Schematic of the mammalian DHA synthesis pathway and the influence of curcumin.
Curcumin elevates the enzymes FASD2 and Elov2 in both liver and brain tissues and increases DHA levels in these tissues when fed in combination with ALA. In cultured liver cells, curcumin + DPA increases DHA production, an action which is prevented by inhibiting the FASD2 enzyme. Thus we propose that curcumin enhances DHA synthesis by increasing levels of DHA processing enzymes in the liver and brain, though we cannot say for sure whether the increased DHA content in the brain is a product of elevated DHA synthesis in the brain tissue or whether DHA produced in the liver is transported to the brain. FADS2: delta 6 desaturase, Elov2: elongase, ALA: α-linolenic acid DPA: Docosapentaenoic acid, DHA: Docosahexaenoic acid.
We report that curcumin significantly increased the synthesis of DHA from DPA in cultured liver cells. These data strongly suggest that curcumin increases the hepatic synthesis of DHA from its precursors. Since the liver is the primary site for most of the DHA synthesis in the body, this raises the question as to whether some of the health effects of curcumin can be attributed to the synthesis of DHA. For example, deficient liver biosynthesis of DHA was linked to cognitive impairment in Alzheimer’s patients who showed reduced expression of enzymes involved in DHA synthesis [3]. We report that feeding animals a combination of curcumin and ALA elevated DHA content in both the liver and the brain. Furthermore, elevations in brain DHA were closely associated with the reduced anxiety-like behavior tested by EPM. These results are in agreement with previous studies describing an association between DHA dietary deficiency and anxiety-like behavior [2].
Herein we report that curcumin elevates levels of DHA in the brain, and that these effects required the presence of ALA in the diet. Curcumin has been reported to cross the blood brain barrier [97, 98], and to be neuroprotective [99][41, 100, 101]. It is known that DHA is an essential component of nerve cell membranes [102, 103], but the synthesis of DHA is very limited in the brain [29]. Sources of DHA for the brain include dietary sources such as fish and DHA synthesized by the liver from precursors such as ALA (C18:3n-3), DPA (C22:5n-3), EPA (C20:5n-3), and tetracosahexaenoic acid (C24:6n-3) [104–107]. Our current study provides new evidence indicating that curcumin increases levels of enzymes FADS2 and elongase 2, which are involved in DHA synthesis, in brain tissue. Additionally, we found that brain levels of DHA and DHA synthesis enzymes were enriched in animals fed curcumin and ALA together, but were unchanged when rats were fed the ALA-enriched diet without curcumin. Though the rate of synthesis is low, the brain seems to have the capacity to synthesis DHA from ALA [29]. Thus it is possible that curcumin elevates DHA in the brain, in part, through de novo synthesis in brain tissue. This possibility warrants further investigation, though liver synthesis seems the more likely contributor to increased brain pools of DHA, since curcumin is highly metabolized in the liver [108]. From our current dataset, we cannot exclude the possibility that observed elevations in brain DHA comes from the liver, since curcumin also promotes the DHA synthesis in the liver.
Curcumin has known antioxidant, anti-inflammatory and antiapoptotic properties (for review see [109]), thus we cannot exclude the possibility that elevated levels of DHA measured in brain and liver are the indirect result of reduced oxidative stress or anti-inflammatory properties of curcumin. For example, oxidative stress is inversely related to liver FASD2 and Δ5 desaturase activities and it has been hypothesized that reduction in plasma antioxidant activity may promote the direct inactivation or reduced expression of liver FASD2 [110]. Thus, the effect of curcumin on levels of FASD2 may be indirectly related to its antioxidant properties. During oxidative stress free radicals generate lipid degradation products, such as 4-HNE. We measured levels of 4-HNE in brain and liver tissues as a biomarker of oxidative stress. Surprisingly, curcumin alone did not reduce 4-HNE in brain or liver, suggesting that curcumin alone in the diet did not reduce lipid peroxidation under homeostatic conditions. In these terms, it is likely that the antioxidant action of curcumin may be more prevalent under challenging conditions such as brain injury. Treatment with ALA either alone or in combination with curcumin significantly reduced levels of 4-HNE. In brain tissue, the combined treatment with ALA and curcumin further reduced lipid peroxidation, whereas in liver it did not. Interestingly, ALA alone was associated with elevated DHA in the liver, but not in the brain. Therefore, if levels of DHA contributed to the reduced lipid peroxidation, we would expect the differences between the ALA group and the ALA + CUR groups to be greater in the brain compared with the liver, which is what the data show. Herein we report that curcumin + ALA elevates levels of enzymes related to DHA synthesis, elevates levels of DHA in brain and liver, as well as reduces n-6 precursors in the liver, suggesting that it is likely that curcumin increases the synthesis of DHA from the ALA precursor in vivo, further studies are warranted to elucidate whether affecting synthesis is the mechanism by which curcumin enhances DHA levels in the liver and brain.
Conclusions
The n-3 fatty acids DHA and EPA are essential for human health that are obtained through dietary animal sources or synthesized from precursors. We provide evidence that curcumin enhances the biosynthesis of hepatic DHA from n-3 precursors and enhances DHA accretion in the brain. These data provide a novel insight into a potential mechanism by which curcumin ameliorates neurocognitive disease.
Acknowledgments
This study was supported by NIH award NS50465.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen HF, Su HM. Exposure to a maternal n-3 fatty acid-deficient diet during brain development provokes excessive hypothalamic-pituitary-adrenal axis responses to stress and behavioral indices of depression and anxiety in male rat offspring later in life. The Journal of nutritional biochemistry. 2013;24:70–80. doi: 10.1016/j.jnutbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia HS, Agrawal R, Sharma S, Huo YX, Ying Z, Gomez-Pinilla F. Omega-3 fatty acid deficiency during brain maturation reduces neuronal and behavioral plasticity in adulthood. PloS one. 2011;6:e28451. doi: 10.1371/journal.pone.0028451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, Piomelli D. Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PloS one. 2010;5:e12538. doi: 10.1371/journal.pone.0012538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu JJ, Galfalvy HC, Cooper TB, Oquendo MA, Grunebaum MF, Mann JJ, Sublette ME. Omega-3 polyunsaturated fatty acid (PUFA) status in major depressive disorder with comorbid anxiety disorders. The Journal of clinical psychiatry. 2013;74:732–738. doi: 10.4088/JCP.12m07970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McNamara RK, Jandacek R, Rider T, Tso P, Dwivedi Y, Pandey GN. Adult medication-free schizophrenic patients exhibit long-chain omega-3 Fatty Acid deficiency: implications for cardiovascular disease risk. Cardiovascular psychiatry and neurology. 2013;2013:796462. doi: 10.1155/2013/796462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sethom MM, Fares S, Bouaziz N, Melki W, Jemaa R, Feki M, Hechmi Z, Kaabachi N. Polyunsaturated fatty acids deficits are associated with psychotic state and negative symptoms in patients with schizophrenia. Prostaglandins, leukotrienes, and essential fatty acids. 2010;83:131–136. doi: 10.1016/j.plefa.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Colombo J, Kannass KN, Shaddy DJ, Kundurthi S, Maikranz JM, Anderson CJ, Blaga OM, Carlson SE. Maternal DHA and the development of attention in infancy and toddlerhood. Child development. 2004;75:1254–1267. doi: 10.1111/j.1467-8624.2004.00737.x. [DOI] [PubMed] [Google Scholar]
- 8.Kannass KN, Colombo J, Carlson SE. Maternal DHA levels and toddler free-play attention. Developmental neuropsychology. 2009;34:159–174. doi: 10.1080/87565640802646734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Y, Wang L, Xu RJ, Chen ZY. DHA depletion in rat brain is associated with impairment on spatial learning and memory. Biomedical and environmental sciences : BES. 2006;19:474–480. [PubMed] [Google Scholar]
- 10.Fedorova I, Hussein N, Baumann MH, Di Martino C, Salem N., Jr An n-3 fatty acid deficiency impairs rat spatial learning in the Barnes maze. Behavioral neuroscience. 2009;123:196–205. doi: 10.1037/a0013801. [DOI] [PubMed] [Google Scholar]
- 11.Fedorova I, Hussein N, Di Martino C, Moriguchi T, Hoshiba J, Majchrzak S, Salem N., Jr An n-3 fatty acid deficient diet affects mouse spatial learning in the Barnes circular maze. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77:269–277. doi: 10.1016/j.plefa.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bach SA, de Siqueira LV, Muller AP, Oses JP, Quatrim A, Emanuelli T, Vinade L, Souza DO, Moreira JD. Dietary omega-3 deficiency reduces BDNF content and activation NMDA receptor and Fyn in dorsal hippocampus: implications on persistence of long-term memory in rats. Nutritional neuroscience. 2014;17:186–192. doi: 10.1179/1476830513Y.0000000087. [DOI] [PubMed] [Google Scholar]
- 13.Anderson GJ, Connor WE, Corliss JD. Docosahexaenoic acid is the preferred dietary n-3 fatty acid for the development of the brain and retina. Pediatric research. 1990;27:89–97. doi: 10.1203/00006450-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Neuringer M, Anderson GJ, Connor WE. The essentiality of n-3 fatty acids for the development and function of the retina and brain. Annual review of nutrition. 1988;8:517–541. doi: 10.1146/annurev.nu.08.070188.002505. [DOI] [PubMed] [Google Scholar]
- 15.O’Brien JS, Sampson EL. Fatty acid and fatty aldehyde composition of the major brain lipids in normal human gray matter, white matter, and myelin. Journal of lipid research. 1965;6:545–551. [PubMed] [Google Scholar]
- 16.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. Journal of lipid research. 1968;9:570–579. [PubMed] [Google Scholar]
- 17.Brenna JT, Carlson SE. Docosahexaenoic acid and human brain development: Evidence that a dietary supply is needed for optimal development. Journal of human evolution. 2014 doi: 10.1016/j.jhevol.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 18.Gamoh S, Hashimoto M, Hossain S, Masumura S. Chronic administration of docosahexaenoic acid improves the performance of radial arm maze task in aged rats. Clinical and experimental pharmacology & physiology. 2001;28:266–270. doi: 10.1046/j.1440-1681.2001.03437.x. [DOI] [PubMed] [Google Scholar]
- 19.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugimoto Y, Taga C, Nishiga M, Fujiwara M, Konishi F, Tanaka K, Kamei C. Effect of docosahexaenoic acid-fortified Chlorella vulgaris strain CK22 on the radial maze performance in aged mice. Biological & pharmaceutical bulletin. 2002;25:1090–1092. doi: 10.1248/bpb.25.1090. [DOI] [PubMed] [Google Scholar]
- 21.Whalley LJ, Starr JM, Deary IJ. Diet and dementia. The journal of the British Menopause Society. 2004;10:113–117. doi: 10.1258/1362180043654575. [DOI] [PubMed] [Google Scholar]
- 22.Wu A, Ying Z, Gomez-Pinilla F. Dietary strategy to repair plasma membrane after brain trauma: implications for plasticity and cognition. Neurorehabilitation and neural repair. 2014;28:75–84. doi: 10.1177/1545968313498650. [DOI] [PubMed] [Google Scholar]
- 23.Desai A, Kevala K, Kim HY. Depletion of brain docosahexaenoic acid impairs recovery from traumatic brain injury. PloS one. 2014;9:e86472. doi: 10.1371/journal.pone.0086472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez MA, Terreros G, Dagnino-Subiabre A. Long-term omega-3 fatty acid supplementation induces anti-stress effects and improves learning in rats. Behavioral and brain functions : BBF. 2013;9:25. doi: 10.1186/1744-9081-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buydens-Branchey L, Branchey M. n-3 polyunsaturated fatty acids decrease anxiety feelings in a population of substance abusers. Journal of clinical psychopharmacology. 2006;26:661–665. doi: 10.1097/01.jcp.0000246214.49271.f1. [DOI] [PubMed] [Google Scholar]
- 26.Buydens-Branchey L, Branchey M, Hibbeln JR. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:568–575. doi: 10.1016/j.pnpbp.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen HF, Su HM. Fish oil supplementation of maternal rats on an n-3 fatty acid-deficient diet prevents depletion of maternal brain regional docosahexaenoic acid levels and has a postpartum anxiolytic effect. The Journal of nutritional biochemistry. 2012;23:299–305. doi: 10.1016/j.jnutbio.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 28.Smink W, Gerrits WJ, Gloaguen M, Ruiter A, van Baal J. Linoleic and alpha-linolenic acid as precursor and inhibitor for the synthesis of long-chain polyunsaturated fatty acids in liver and brain of growing pigs. Animal : an international journal of animal bioscience. 2012;6:262–270. doi: 10.1017/S1751731111001479. [DOI] [PubMed] [Google Scholar]
- 29.Igarashi M, DeMar JC, Jr, Ma K, Chang L, Bell JM, Rapoport SI. Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation. Journal of lipid research. 2007;48:1150–1158. doi: 10.1194/jlr.M600549-JLR200. [DOI] [PubMed] [Google Scholar]
- 30.Kidd PM. Omega-3 DHA and EPA for cognition, behavior, and mood: clinical findings and structural-functional synergies with cell membrane phospholipids. Alternative medicine review : a journal of clinical therapeutic. 2007;12:207–227. [PubMed] [Google Scholar]
- 31.Plourde M, Cunnane SC. Extremely limited synthesis of long chain polyunsaturates in adults: implications for their dietary essentiality and use as supplements. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme. 2007;32:619–634. doi: 10.1139/H07-034. [DOI] [PubMed] [Google Scholar]
- 32.Brenna JT, Salem N, Jr, Sinclair AJ, Cunnane SC A. International Society for the Study of Fatty, I. Lipids. alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins, leukotrienes, and essential fatty acids. 2009;80:85–91. doi: 10.1016/j.plefa.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 33.Kornsteiner M, Singer I, Elmadfa I. Very low n-3 long-chain polyunsaturated fatty acid status in Austrian vegetarians and vegans. Annals of nutrition & metabolism. 2008;52:37–47. doi: 10.1159/000118629. [DOI] [PubMed] [Google Scholar]
- 34.Mann N, Pirotta Y, O’Connell S, Li D, Kelly F, Sinclair A. Fatty acid composition of habitual omnivore and vegetarian diets. Lipids. 2006;41:637–646. doi: 10.1007/s11745-006-5014-9. [DOI] [PubMed] [Google Scholar]
- 35.Rosell MS, Lloyd-Wright Z, Appleby PN, Sanders TA, Allen NE, Key TJ. Long-chain n-3 polyunsaturated fatty acids in plasma in British meat-eating, vegetarian, and vegan men. The American journal of clinical nutrition. 2005;82:327–334. doi: 10.1093/ajcn.82.2.327. [DOI] [PubMed] [Google Scholar]
- 36.Sanders TA. DHA status of vegetarians. Prostaglandins, leukotrienes, and essential fatty acids. 2009;81:137–141. doi: 10.1016/j.plefa.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 37.Begum AN, Jones MR, Lim GP, Morihara T, Kim P, Heath DD, Rock CL, Pruitt MA, Yang F, Hudspeth B, Hu S, Faull KF, Teter B, Cole GM, Frautschy SA. Curcumin structure-function, bioavailability, and efficacy in models of neuroinflammation and Alzheimer’s disease. The Journal of pharmacology and experimental therapeutics. 2008;326:196–208. doi: 10.1124/jpet.108.137455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang F, Lim GP, Begum AN, Ubeda OJ, Simmons MR, Ambegaokar SS, Chen PP, Kayed R, Glabe CG, Frautschy SA, Cole GM. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. The Journal of biological chemistry. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y, Yin H, Wang L, Shuboy A, Lou J, Han B, Zhang X, Li J. Curcumin as a potential treatment for Alzheimer’s disease: a study of the effects of curcumin on hippocampal expression of glial fibrillary acidic protein. The American journal of Chinese medicine. 2013;41:59–70. doi: 10.1142/S0192415X13500055. [DOI] [PubMed] [Google Scholar]
- 40.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life sciences. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 41.Wu A, Ying Z, Gomez-Pinilla F. Dietary curcumin counteracts the outcome of traumatic brain injury on oxidative stress, synaptic plasticity, and cognition. Experimental neurology. 2006;197:309–317. doi: 10.1016/j.expneurol.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Sharma S, Zhuang Y, Ying Z, Wu A, Gomez-Pinilla F. Dietary curcumin supplementation counteracts reduction in levels of molecules involved in energy homeostasis after brain trauma. Neuroscience. 2009;161:1037–1044. doi: 10.1016/j.neuroscience.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Samini F, Samarghandian S, Borji A, Mohammadi G, bakaian M. Curcumin pretreatment attenuates brain lesion size and improves neurological function following traumatic brain injury in the rat. Pharmacology, biochemistry, and behavior. 2013;110:238–244. doi: 10.1016/j.pbb.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2011;27:123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 45.Ueki M, Ueno M, Morishita J, Maekawa N. Curcumin ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in mice. Journal of bioscience and bioengineering. 2013;115:547–551. doi: 10.1016/j.jbiosc.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 46.Kawanishi N, Kato K, Takahashi M, Mizokami T, Otsuka Y, Imaizumi A, Shiva D, Yano H, Suzuki K. Curcumin attenuates oxidative stress following downhill running-induced muscle damage. Biochemical and biophysical research communications. 2013;441:573–578. doi: 10.1016/j.bbrc.2013.10.119. [DOI] [PubMed] [Google Scholar]
- 47.Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, Yang CX. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neuroscience letters. 2014;560:81–85. doi: 10.1016/j.neulet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 48.Soetikno V, Sari FR, Lakshmanan AP, Arumugam S, Harima M, Suzuki K, Kawachi H, Watanabe K. Curcumin alleviates oxidative stress, inflammation, and renal fibrosis in remnant kidney through the Nrf2-keap1 pathway. Molecular nutrition & food research. 2013;57:1649–1659. doi: 10.1002/mnfr.201200540. [DOI] [PubMed] [Google Scholar]
- 49.Gao S, Duan X, Wang X, Dong D, Liu D, Li X, Sun G, Li B. Curcumin attenuates arsenic-induced hepatic injuries and oxidative stress in experimental mice through activation of Nrf2 pathway, promotion of arsenic methylation and urinary excretion. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. 2013;59:739–747. doi: 10.1016/j.fct.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 50.Mei XT, Xu DH, Xu SK, Zheng YP, Xu SB. Zinc(II)-curcumin accelerates the healing of acetic acid-induced chronic gastric ulcers in rats by decreasing oxidative stress and downregulation of matrix metalloproteinase-9. Food and chemical toxicology: an international journal published for the British Industrial Biological Research Association. 2013;60:448–454. doi: 10.1016/j.fct.2013.07.075. [DOI] [PubMed] [Google Scholar]
- 51.Walf AA, Frye CA. The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nature protocols. 2007;2:322–328. doi: 10.1038/nprot.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Portolesi R, Powell BC, Gibson RA. Competition between 24:5n-3 and ALA for Delta 6 desaturase may limit the accumulation of DHA in HepG2 cell membranes. Journal of lipid research. 2007;48:1592–1598. doi: 10.1194/jlr.M700081-JLR200. [DOI] [PubMed] [Google Scholar]
- 53.Garcia-Calatayud S, Redondo C, Martin E, Ruiz JI, Garcia-Fuentes M, Sanjurjo P. Brain docosahexaenoic acid status and learning in young rats submitted to dietary long-chain polyunsaturated fatty acid deficiency and supplementation limited to lactation. Pediatric research. 2005;57:719–723. doi: 10.1203/01.PDR.0000156506.03057.AD. [DOI] [PubMed] [Google Scholar]
- 54.Lim SY, Hoshiba J, Moriguchi T, Salem N., Jr N-3 fatty acid deficiency induced by a modified artificial rearing method leads to poorer performance in spatial learning tasks. Pediatric research. 2005;58:741–748. doi: 10.1203/01.PDR.0000180547.46725.CC. [DOI] [PubMed] [Google Scholar]
- 55.Moriguchi T, Salem N., Jr Recovery of brain docosahexaenoate leads to recovery of spatial task performance. Journal of neurochemistry. 2003;87:297–309. doi: 10.1046/j.1471-4159.2003.01966.x. [DOI] [PubMed] [Google Scholar]
- 56.Greiner RS, Moriguchi T, Slotnick BM, Hutton A, Salem N. Olfactory discrimination deficits in n-3 fatty acid-deficient rats. Physiology & behavior. 2001;72:379–385. doi: 10.1016/s0031-9384(00)00437-6. [DOI] [PubMed] [Google Scholar]
- 57.Moriguchi T, Greiner RS, Salem N., Jr Behavioral deficits associated with dietary induction of decreased brain docosahexaenoic acid concentration. Journal of neurochemistry. 2000;75:2563–2573. doi: 10.1046/j.1471-4159.2000.0752563.x. [DOI] [PubMed] [Google Scholar]
- 58.Lim SY, Suzuki H. Effect of dietary docosahexaenoic acid and phosphatidylcholine on maze behavior and fatty acid composition of plasma and brain lipids in mice. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Journal international de vitaminologie et de nutrition. 2000;70:251–259. doi: 10.1024/0300-9831.70.5.251. [DOI] [PubMed] [Google Scholar]
- 59.Lim SY, Suzuki H. Intakes of dietary docosahexaenoic acid ethyl ester and egg phosphatidylcholine improve maze-learning ability in young and old mice. The Journal of nutrition. 2000;130:1629–1632. doi: 10.1093/jn/130.6.1629. [DOI] [PubMed] [Google Scholar]
- 60.Minami M, Kimura S, Endo T, Hamaue N, Hirafuji M, Togashi H, Matsumoto M, Yoshioka M, Saito H, Watanabe S, Kobayashi T, Okuyama H. Dietary docosahexaenoic acid increases cerebral acetylcholine levels and improves passive avoidance performance in stroke-prone spontaneously hypertensive rats. Pharmacology, biochemistry, and behavior. 1997;58:1123–1129. doi: 10.1016/s0091-3057(97)00300-6. [DOI] [PubMed] [Google Scholar]
- 61.Chytrova G, Ying Z, Gomez-Pinilla F. Exercise contributes to the effects of DHA dietary supplementation by acting on membrane-related synaptic systems. Brain research. 2010;1341:32–40. doi: 10.1016/j.brainres.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gamoh S, Hashimoto M, Sugioka K, Shahdat Hossain M, Hata N, Misawa Y, Masumura S. Chronic administration of docosahexaenoic acid improves reference memory-related learning ability in young rats. Neuroscience. 1999;93:237–241. doi: 10.1016/s0306-4522(99)00107-4. [DOI] [PubMed] [Google Scholar]
- 63.Whalley LJ, Fox HC, Wahle KW, Starr JM, Deary IJ. Cognitive aging, childhood intelligence, and the use of food supplements: possible involvement of n-3 fatty acids. The American journal of clinical nutrition. 2004;80:1650–1657. doi: 10.1093/ajcn/80.6.1650. [DOI] [PubMed] [Google Scholar]
- 64.Heude B, Ducimetiere P, Berr C, Study EVA. Cognitive decline and fatty acid composition of erythrocyte membranes--The EVA Study. The American journal of clinical nutrition. 2003;77:803–808. doi: 10.1093/ajcn/77.4.803. [DOI] [PubMed] [Google Scholar]
- 65.Key TJ, Appleby PN, Rosell MS. Health effects of vegetarian and vegan diets. The Proceedings of the Nutrition Society. 2006;65:35–41. doi: 10.1079/pns2005481. [DOI] [PubMed] [Google Scholar]
- 66.Welch AA, Shakya-Shrestha S, Lentjes MA, Wareham NJ, Khaw KT. Dietary intake and status of n-3 polyunsaturated fatty acids in a population of fish-eating and non-fish-eating meat-eaters, vegetarians, and vegans and the product-precursor ratio [corrected] of alpha-linolenic acid to long-chain n-3 polyunsaturated fatty acids: results from the EPIC-Norfolk cohort. The American journal of clinical nutrition. 2010;92:1040–1051. doi: 10.3945/ajcn.2010.29457. [DOI] [PubMed] [Google Scholar]
- 67.Blasbalg TL, Hibbeln JR, Ramsden CE, Majchrzak SF, Rawlings RR. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. The American journal of clinical nutrition. 2011;93:950–962. doi: 10.3945/ajcn.110.006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.FAO. The State of World Fisheries and Aquaculture. Rome: 2012. p. 209. [Google Scholar]
- 69.Crampton VO, Nanton DA, Ruohonen K, Skjervold PO, El-Mowafi A. Demonstration of salmon farming as a net producer of fish protein and oil. Aquacult Nutr. 2010;16:437–446. [Google Scholar]
- 70.Gu JN, Krogdahl A, Sissener NH, Kortner TM, Gelencser E, Hemre GI, Bakke AM. Effects of oral Bt-maize (MON810) exposure on growth and health parameters in normal and sensitised Atlantic salmon, Salmo salar L. Brit J Nutr. 2013;109:1408–1423. doi: 10.1017/S000711451200325X. [DOI] [PubMed] [Google Scholar]
- 71.Turchini GM, Francis DS. Fatty acid metabolism (desaturation, elongation and beta-oxidation) in rainbow trout fed fish oil- or linseed oil-based diets. The British journal of nutrition. 2009;102:69–81. doi: 10.1017/S0007114508137874. [DOI] [PubMed] [Google Scholar]
- 72.Bell JG, Tocher DR, Henderson RJ, Dick JR, Crampton VO. Altered fatty acid compositions in atlantic salmon (Salmo salar) fed diets containing linseed and rapeseed oils can be partially restored by a subsequent fish oil finishing diet. The Journal of nutrition. 2003;133:2793–2801. doi: 10.1093/jn/133.9.2793. [DOI] [PubMed] [Google Scholar]
- 73.Menoyo D, Lopez-Bote CJ, Obach A, Bautista JM. Effect of dietary fish oil substitution with linseed oil on the performance, tissue fatty acid profile, metabolism, and oxidative stability of Atlantic salmon. Journal of animal science. 2005;83:2853–2862. doi: 10.2527/2005.83122853x. [DOI] [PubMed] [Google Scholar]
- 74.Hixson SM, Parrish CC, Anderson DM. Changes in tissue lipid and fatty acid composition of farmed rainbow trout in response to dietary camelina oil as a replacement of fish oil. Lipids. 2014;49:97–111. doi: 10.1007/s11745-013-3862-7. [DOI] [PubMed] [Google Scholar]
- 75.Daley CA, Abbott A, Doyle PS, Nader GA, Larson S. A review of fatty acid profiles and antioxidant content in grass-fed and grain-fed beef. Nutrition journal. 2010;9:10. doi: 10.1186/1475-2891-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torde RG, Therrien AJ, Shortreed MR, Smith LM, Lamos SM. Multiplexed analysis of cage and cage free chicken egg fatty acids using stable isotope labeling and mass spectrometry. Molecules. 2013;18:14977–14988. doi: 10.3390/molecules181214977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Reddy S, Sanders TA, Obeid O. The influence of maternal vegetarian diet on essential fatty acid status of the newborn. European journal of clinical nutrition. 1994;48:358–368. [PubMed] [Google Scholar]
- 78.Beezhold BL, Johnston CS, Daigle DR. Vegetarian diets are associated with healthy mood states: a cross-sectional study in seventh day adventist adults. Nutrition journal. 2010;9:26. doi: 10.1186/1475-2891-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yadav YaKS. The Hindu. 2006. The food habits of a nation. [Google Scholar]
- 80.Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, Roodenrys S, Keogh JB, Clifton PM, Williams PG, Fazio VA, Inge KE. Health benefits of herbs and spices: the past, the present, the future. The Medical journal of Australia. 2006;185:S4–24. doi: 10.5694/j.1326-5377.2006.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 81.Hutchins-Wolfbrandt A, Mistry AM. Dietary turmeric potentially reduces the risk of cancer. Asian Pacific journal of cancer prevention : APJCP. 2011;12:3169–3173. [PubMed] [Google Scholar]
- 82.Cheng KK, Yeung CF, Ho SW, Chow SF, Chow AH, Baum L. Highly stabilized curcumin nanoparticles tested in an in vitro blood-brain barrier model and in Alzheimer’s disease Tg2576 mice. The AAPS journal. 2013;15:324–336. doi: 10.1208/s12248-012-9444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lazar AN, Mourtas S, Youssef I, Parizot C, Dauphin A, Delatour B, Antimisiaris SG, Duyckaerts C. Curcumin-conjugated nanoliposomes with high affinity for Abeta deposits: possible applications to Alzheimer disease. Nanomedicine : nanotechnology, biology, and medicine. 2013;9:712–721. doi: 10.1016/j.nano.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 84.Wang LL, Sun Y, Huang K, Zheng L. Curcumin, a potential therapeutic candidate for retinal diseases. Molecular nutrition & food research. 2013;57:1557–1568. doi: 10.1002/mnfr.201200718. [DOI] [PubMed] [Google Scholar]
- 85.Mirza M, Volz C, Karlstetter M, Langiu M, Somogyi A, Ruonala MO, Tamm ER, Jagle H, Langmann T. Progressive retinal degeneration and glial activation in the CLN6 (nclf) mouse model of neuronal ceroid lipofuscinosis: a beneficial effect of DHA and curcumin supplementation. PloS one. 2013;8:e75963. doi: 10.1371/journal.pone.0075963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mythri RB, Bharath MM. Curcumin: a potential neuroprotective agent in Parkinson’s disease. Current pharmaceutical design. 2012;18:91–99. doi: 10.2174/138161212798918995. [DOI] [PubMed] [Google Scholar]
- 87.Jiang TF, Zhang YJ, Zhou HY, Wang HM, Tian LP, Liu J, Ding JQ, Chen SD. Curcumin ameliorates the neurodegenerative pathology in A53T alpha-synuclein cell model of Parkinson’s disease through the downregulation of mTOR/p70S6K signaling and the recovery of macroautophagy. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2013;8:356–369. doi: 10.1007/s11481-012-9431-7. [DOI] [PubMed] [Google Scholar]
- 88.Lapchak PA. Neuroprotective and neurotrophic curcuminoids to treat stroke: a translational perspective. Expert opinion on investigational drugs. 2011;20:13–22. doi: 10.1517/13543784.2011.542410. [DOI] [PubMed] [Google Scholar]
- 89.Sanmukhani J, Anovadiya A, Tripathi CB. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: an acute and chronic study. Acta poloniae pharmaceutica. 2011;68:769–775. [PubMed] [Google Scholar]
- 90.Sanmukhani J, Satodia V, Trivedi J, Patel T, Tiwari D, Panchal B, Goel A, Tripathi CB. Efficacy and safety of curcumin in major depressive disorder: a randomized controlled trial. Phytotherapy research : PTR. 2014;28:579–585. doi: 10.1002/ptr.5025. [DOI] [PubMed] [Google Scholar]
- 91.Gomez-Pinilla F, Nguyen TT. Natural mood foods: the actions of polyphenols against psychiatric and cognitive disorders. Nutritional neuroscience. 2012;15:127–133. doi: 10.1179/1476830511Y.0000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kaur G, Begg DP, Barr D, Garg M, Cameron-Smith D, Sinclair AJ. Short-term docosapentaenoic acid (22:5 n-3) supplementation increases tissue docosapentaenoic acid, DHA and EPA concentrations in rats. The British journal of nutrition. 2010;103:32–37. doi: 10.1017/S0007114509991334. [DOI] [PubMed] [Google Scholar]
- 93.Qiu X. Biosynthesis of docosahexaenoic acid (DHA, 22:6–4, 7,10,13,16,19): two distinct pathways. Prostaglandins, leukotrienes, and essential fatty acids. 2003;68:181–186. doi: 10.1016/s0952-3278(02)00268-5. [DOI] [PubMed] [Google Scholar]
- 94.Su HM, Moser AB, Moser HW, Watkins PA. Peroxisomal straight-chain Acyl-CoA oxidase and D-bifunctional protein are essential for the retroconversion step in docosahexaenoic acid synthesis. The Journal of biological chemistry. 2001;276:38115–38120. doi: 10.1074/jbc.M106326200. [DOI] [PubMed] [Google Scholar]
- 95.Ferdinandusse S, Denis S, Mooijer PA, Zhang Z, Reddy JK, Spector AA, Wanders RJ. Identification of the peroxisomal beta-oxidation enzymes involved in the biosynthesis of docosahexaenoic acid. Journal of lipid research. 2001;42:1987–1995. [PubMed] [Google Scholar]
- 96.Talahalli RR, Vallikannan B, Sambaiah K, Lokesh BR. Lower efficacy in the utilization of dietary ALA as compared to preformed EPA + DHA on long chain n-3 PUFA levels in rats. Lipids. 2010;45:799–808. doi: 10.1007/s11745-010-3464-6. [DOI] [PubMed] [Google Scholar]
- 97.Chiu SS, Lui E, Majeed M, Vishwanatha JK, Ranjan AP, Maitra A, Pramanik D, Smith JA, Helson L. Differential distribution of intravenous curcumin formulations in the rat brain. Anticancer research. 2011;31:907–911. [PMC free article] [PubMed] [Google Scholar]
- 98.Kakkar V, Mishra AK, Chuttani K, Kaur IP. Proof of concept studies to confirm the delivery of curcumin loaded solid lipid nanoparticles (C-SLNs) to brain. International journal of pharmaceutics. 2013;448:354–359. doi: 10.1016/j.ijpharm.2013.03.046. [DOI] [PubMed] [Google Scholar]
- 99.Gazal M, Valente MR, Acosta BA, Kaufmann FN, Braganhol E, Lencina CL, Stefanello FM, Ghisleni G, Kaster MP. Neuroprotective and antioxidant effects of curcumin in a ketamine-induced model of mania in rats. European journal of pharmacology. 2014;724:132–139. doi: 10.1016/j.ejphar.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 100.Rajasekar N, Dwivedi S, Tota SK, Kamat PK, Hanif K, Nath C, Shukla R. Neuroprotective effect of curcumin on okadaic acid induced memory impairment in mice. European journal of pharmacology. 2013;715:381–394. doi: 10.1016/j.ejphar.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 101.Wu A, Ying Z, Schubert D, Gomez-Pinilla F. Brain and spinal cord interaction: a dietary curcumin derivative counteracts locomotor and cognitive deficits after brain trauma. Neurorehabilitation and neural repair. 2011;25:332–342. doi: 10.1177/1545968310397706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marszalek JR, Lodish HF. Docosahexaenoic acid, fatty acid-interacting proteins, and neuronal function: breastmilk and fish are good for you. Annual review of cell and developmental biology. 2005;21:633–657. doi: 10.1146/annurev.cellbio.21.122303.120624. [DOI] [PubMed] [Google Scholar]
- 103.Crawford MA, Bazinet RP, Sinclair AJ. Fat intake and CNS functioning: ageing and disease. Annals of nutrition & metabolism. 2009;55:202–228. doi: 10.1159/000229003. [DOI] [PubMed] [Google Scholar]
- 104.Burdge GC, Calder PC. Conversion of alpha-linolenic acid to longer-chain polyunsaturated fatty acids in human adults. Reproduction, nutrition, development. 2005;45:581–597. doi: 10.1051/rnd:2005047. [DOI] [PubMed] [Google Scholar]
- 105.Rapoport SI, Rao JS, Igarashi M. Brain metabolism of nutritionally essential polyunsaturated fatty acids depends on both the diet and the liver. Prostaglandins, leukotrienes, and essential fatty acids. 2007;77:251–261. doi: 10.1016/j.plefa.2007.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim HY. Novel metabolism of docosahexaenoic acid in neural cells. The Journal of biological chemistry. 2007;282:18661–18665. doi: 10.1074/jbc.R700015200. [DOI] [PubMed] [Google Scholar]
- 107.Rapoport SI, Igarashi M. Can the rat liver maintain normal brain DHA metabolism in the absence of dietary DHA? Prostaglandins, leukotrienes, and essential fatty acids. 2009;81:119–123. doi: 10.1016/j.plefa.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Molecular pharmaceutics. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 109.Aggarwal BB, Sung B. Pharmacological basis for the role of curcumin in chronic diseases: an age-old spice with modern targets. Trends in pharmacological sciences. 2009;30:85–94. doi: 10.1016/j.tips.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 110.Araya J, Rodrigo R, Pettinelli P, Araya AV, Poniachik J, Videla LA. Decreased liver fatty acid delta-6 and delta-5 desaturase activity in obese patients. Obesity. 2010;18:1460–1463. doi: 10.1038/oby.2009.379. [DOI] [PubMed] [Google Scholar]