Summary
In commissural neurons of Drosophila, the conserved Frazzled (Fra)/Deleted in Colorectal Cancer (DCC) receptor promotes midline axon crossing by signaling locally in response to Netrin and by inducing transcription of commissureless (comm), an antagonist of Slit-Roundabout midline repulsion, through an unknown mechanism. Here, we show that Fra is cleaved to release its intracellular domain (ICD), which shuttles between the cytoplasm and the nucleus, where it functions as a transcriptional activator. Rescue and gain-of-function experiments demonstrate that the Fra ICD is sufficient to regulate comm expression and that both γ-secretase proteolysis of Fra and Fra's function as a transcriptional activator are required for its ability to regulate comm in vivo. Our data uncover an unexpected role for the Fra ICD as a transcription factor whose activity regulates the responsiveness of commissural axons at the midline and raise the possibility that nuclear signaling may be a common output of axon guidance receptors.
Introduction
During the development of the nervous system, chemotropic cues serve as navigational signals for growing axons. These cues signal through axon guidance receptors, which are expressed on axonal growth cones. In the canonical view of axon guidance receptor signaling, ligand binding recruits protein complexes to receptor cytoplasmic domains to locally remodel the growth cone plasma membrane and underlying cytoskeleton. In this way, guidance receptors are thought to transduce gradients of cues into asymmetrical structural changes in growth cones, to steer them toward sources of attractants and away from sources of repellents (reviewed in O’Donnell et al., 2009). The observation that isolated growth cones that have been physically severed from their cell bodies remain capable of responding to guidance cues provides a particularly dramatic demonstration that local signaling is sufficient to execute some chemotropic responses (Campbell and Holt, 2001).
Growing axons must also modulate their responsiveness to guidance cues in order to navigate intermediate targets on the way to their final synaptic partners. One of the best-studied examples of this phenomenon is the growth of commissural axons across the ventral midline of the embryonic central nervous system in bilaterally symmetric animals (reviewed in Neuhaus-Follini and Bashaw, 2015). Throughout the period of time when commissural axons are crossing the midline, cells at the midline produce a host of chemotropic cues, including both attractants and repellents. In both insects and vertebrates, these include Netrins, which signal attraction through Frazzled (Fra)/Deleted in Colorectal Cancer (DCC) receptors (Serafini et al., 1994; Kennedy et al., 1994; Harris et al., 1996; Keino-Masu et al., 1996; Kolodziej et al., 1996; Mitchell et al., 1996; Serafini et al., 1996; Fazeli et al., 1997; Brankatschk and Dickson, 2006), and Slits, which signal repulsion through Roundabout (Robo) receptors (Seeger et al., 1993; Holmes et al., 1998; Kidd et al., 1998a; Brose et al., 1999; Kidd et al., 1999; Li et al., 1999; Rajagopalan et al., 2000; Simpson et al., 2000; Long et al., 2004; Jaworski et al., 2010). As commissural neurons are growing toward the midline, their responsiveness to midline-derived repellents, including Slits, is suppressed. Once these axons have crossed the midline, they become responsive to Slits and other midline repellents, which facilitates midline exit and prevents re-crossing (Seeger et al., 1993; Kidd et al., 1998a; Kidd et al., 1998b; Zou et al., 2000; Keleman et al., 2002; Sabatier et al., 2004; Keleman et al., 2005; Chen et al., 2008; Nawabi et al., 2010; Parra and Zou, 2010; Charoy et al., 2012; Phillipp et al., 2012; Yam et al., 2012).
In Drosophila, while commissural axons are crossing the midline, the endosomal protein Commissureless (Comm) reduces sensitivity to Slit by inhibiting the trafficking of Robo to the growth cone plasma membrane (Keleman et al., 2002; Keleman et al., 2005). Expression of comm mRNA is tightly spatially and temporally regulated such that commissural neurons transiently express comm while their axons are crossing the midline, but not before or after. Ipsilateral neurons, whose axons do not normally cross the midline, rarely express comm (Keleman et al., 2002). Previously, we found that in addition to its canonical role in signaling Netrin-dependent outgrowth and/or chemoattraction, Fra has a second way of promoting midline axon crossing: independent of Netrins, Fra induces comm mRNA expression in commissural neurons (Yang et al., 2009). However, the mechanism(s) by which Fra regulates gene expression remain unknown.
The intracellular domains (ICDs) of Fra and its orthologs contain three small, conserved sequence motifs – P1, P2, and P3 (Kolodziej et al., 1996) – which have been implicated in a variety of protein-protein interactions and receptor signaling outputs. We were particularly intrigued by a pair of in vitro reports that the ICDs of Fra's vertebrate orthologs, DCC and Neogenin (Neo), are capable of translocating to the nucleus and functioning as transcriptional activators in reporter assays following γ-secretase-dependent receptor proteolysis (Taniguchi et al., 2003; Goldschneider et al., 2008). However, whether the ICDs of these receptors function as transcription factors in vivo and what, if any, is the biological significance of their transcriptional outputs is unknown.
Here, we report that Fra is cleaved by γ-secretase, releasing its ICD, which shuttles between the cytoplasm and the nucleus. This proteolysis is required for Fra's ability to regulate comm expression. In rescue and gain-of-function assays in vivo, the Fra ICD is sufficient to induce comm expression and midline crossing. In addition, the P3 motif in the Fra ICD encodes a transcriptional activation domain. A point mutant variant of Fra that is specifically deficient for transcriptional activation, but is intact for other P3-dependent functions, cannot regulate comm expression in vivo. Moreover, comm-regulatory function can be restored to this receptor with a heterologous transcriptional activation domain, providing strong in vivo evidence that Fra's transcriptional activation function is required. Thus, Fra acts in two different cellular compartments to control midline crossing: at the growth cone, Fra regulates local membrane and cytoskeletal dynamics in response to its canonical Netrin ligands and, in the nucleus, Fra functions as a transcription factor to modulate growth cone sensitivity to Slit-Robo repulsion.
Results
Fra is cleaved by γ-secretase
Fra's vertebrate orthologs, DCC and Neogenin (Neo) are substrates for metalloprotease-dependent ectodomain shedding and subsequent γ-secretase-dependent intramembrane proteolysis (Galko and Tessier-Lavigne, 2000; Taniguchi et al., 2003; Parent et al., 2005; Goldschneider et al., 2008; Bai et al., 2011, Okamura et al., 2011), prompting us to examine whether Fra also undergoes proteolytic processing. We panneurally expressed C-terminally-tagged UAS-Fra-Myc with elav-Gal4 and probed embryo lysates with an antibody against Myc (Figure 1A). We detected a ~200 kDa band corresponding to the full-length receptor, as well as smaller C-terminal fragments of approximately 50 kDa, 35 kDa and 25 kDa (ICD A, B, and C, respectively). We made a transgenic line that allowed us to express the ICD of Fra, without any extracellular or transmembrane residues, under Gal4/UAS control. When we expressed UAS-Fra ICDMyc with elav-Gal4, we detected a doublet that corresponds in size to the largest of these C-terminal fragments, as well as the smaller C-terminal species (Figure 1A). To determine whether these C-terminal fragments are specific cleavage products of the Fra cytoplasmic domain, we replaced the Myc epitope with a smaller HA epitope and again examined the sizes of Fra ICD fragments. Consistent with our observations using the Myc-tagged receptor, we detected three C-terminal fragments in lysates from embryos pan-neurally expressing Fra-HA (Figure 1B). All three of these fragments are shifted to lower molecular weights (~45 kDa, 30 kDa and 20 kDa), commensurate with the decrease in the size of the epitope tag. We also examined lysates from embryos expressing a truncated, C-terminally HA-tagged Fra receptor that is missing its entire cytoplasmic domain (FraΔC-HA) and did not detect Fra ICD fragments (Figure 1B). Together these observations indicate that the Fra receptor can be processed to generate distinct C-terminal fragments.
Figure 1. Fra is cleaved by γ-secretase.
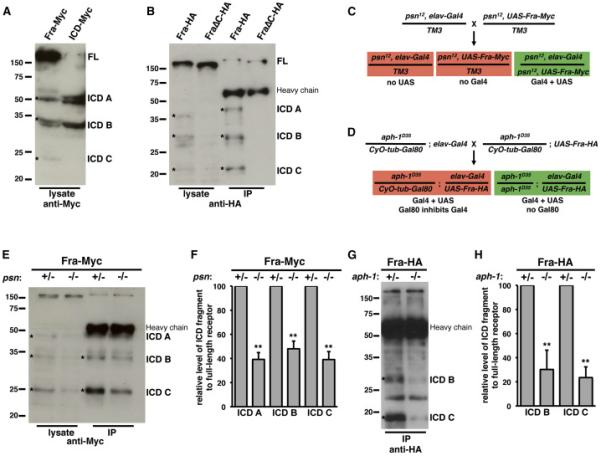
A) Protein extracts were made from embryos pan-neurally expressing either a full-length Fra receptor with a C-terminal 6× Myc tag (first lane) or an equivalently tagged Fra ICD (second lane). Proteins were resolved by SDS-PAGE and Western blots were performed with anti-Myc antibody. We detect full-length receptor at approximately 200 kDa (FL) and several C-terminal fragments, including species at approximately 50 kDa, 35 kDa, and 25 kDa (ICD A, ICD B, and ICD C; indicated by asterisks).
B) Protein extracts were made from embryos pan-neurally expressing either a full-length Fra receptor with a C-terminal 3× HA tag (first lane) or a Fra receptor missing its cytoplasmic domain (second lane) and HA-tagged proteins were immunoprecipitated from these extracts with anti-HA antibody (third and fourth lanes). Proteins were resolved by SDS-PAGE and Western blots were performed with anti-HA antibody. The 3× HA tag is smaller than the 6× Myc tag and, accordingly, ICD A, ICD B, and ICD C are shifted to smaller sizes of approximately 45 kDa, 30 kDa, and 20 kDa in both total protein extracts and immunoprecipitates (first and third lanes, indicated by asterisks). These species are not detected in extracts or immunoprecipitates from embryos expressing FraΔC (second and fourth lanes). The position of the IgG heavy chain in the lanes that contain immunoprecipitates is indicated.
C) Schematic of strategy used to express UAS-Fra-Myc with elav-Gal4 specifically in psn mutants.
D) Schematic of strategy used to express UAS-Fra-HA with elav-Gal4 specifically in aph-1 mutants.
E) Protein extracts from embryos pan-neurally expressing Fra-Myc in psn12 mutants were resolved by SDS-PAGE and Western blots were performed with anti-Myc antibody. All three C-terminal fragments (indicated by asterisks) are reduced in abundance relative to full-length receptor in the total lysates (compare first and second lanes) and the two smaller fragments are reduced in abundance in immunoprecipitates (compare third and fourth lanes). ICD A is obscured in immunoprecipitates by the IgG heavy chain.
F) Quantification of Fra ICD fragments in total lysates relative to full-length receptor in psn12/+ compared to psn12/psn12. Data were analyzed by Student's t-test. ** indicates p<0.005. Error bars indicate standard deviation. Data are from six independent experiments.
G) Protein extracts from embryos pan-neurally expressing Fra-HA in aph-1D35 mutants were made and HA-tagged proteins were immunoprecipitated with anti-HA antibody. Proteins were resolved by SDS-PAGE and Western blots were performed with anti-HA antibody. The two smaller fragments (indicated by asterisks) are reduced in abundance in immunoprecipitates. The largest fragment is obscured by the IgG heavy chain.
H) Quantification of Fra ICD fragments relative to full-length receptor in aph-1D35/+ compared to aph-1D35/ aph-1D35. Data were analyzed by Student's t-test. ** indicates p<0.005. Error bars indicate standard deviation. Data are from four independent experiments.
γ-secretase cleaves its substrates in the membrane, releasing their ICDs, which can signal intracellularly in a variety of ways (reviewed in Haapasalo and Kovacs, 2011). The largest C-terminal peptide generated by proteolysis of Fra is approximately the size of the Fra ICD, suggesting that this fragment might be a product of γ-secretase proteolysis. To investigate whether Fra is cleaved by γ-secretase, we examined lysates from embryos in which γ-secretase function was reduced. Presenilin (Psn) is the catalytic subunit of γ-secretase (Wolfe et al., 1999), a multi-protein complex that also includes Aph-1, Nicastrin, and Pen-2 (Yu et al., 2000; Francis et al., 2002; Goutte et al., 2002; Edbauer et al., 2003; Fraering et al., 2004). We analyzed lysates from genetically heterogeneous populations of embryos in which C-terminally epitope-tagged UAS-Fra transgene expression was pan-neurally driven by elav-Gal4 only in psn or aph-1 mutant embryos. To restrict UAS-Fra expression to mutant embryos, we used flies in which the Gal4 and UAS elements were recombined onto mutant chromosomes or we used flies in which the chromosomes bearing the mutations were maintained as heterozygotes with balancer chromosomes ubiquitously expressing the Gal4 repressor Gal80 (see Figure 1C-1D for details). As γ-secretase components are maternally deposited (Ye et al., 1999; Hu et al., 2002), we analyzed late stage 17 embryos (20-24 hours) in order to minimize the amount of Psn or Aph-1 present. In these embryos, Psn or Aph-1 function is likely strongly reduced, but not absent. In either lysates or immunoprecipitates from psn12 or aph-1D35 mutant embryos, the abundance of both the Fra ICD and the smaller C-terminal fragments of Fra is reduced (Figure 1E-1H), suggesting that the Fra ICD is a product of γsecretase proteolysis. In addition, these experiments suggest that even though the smaller fragments are not likely to be directly generated by γ-secretase proteolysis, subsequent processing of the ICD depends on γ-secretase cleavage. DCC and Neo are cleaved approximately in the middle of their ICDs by caspases and this proteolysis is required for the abilities of these receptors to induce apoptosis (Mehlen et al., 1998; Matsunaga et al., 2004). The caspase cleavage site in DCC and Neo is not conserved in Fra, but there are several aspartate residues in the Fra ICD that are candidate caspase cleavage sites.
The Fra ICD is sufficient to induce comm expression
Fra promotes midline crossing of commissural axons both by signaling outgrowth and/or chemoattraction in response to Netrins and by promoting comm transcription, independent of Netrins, to inhibit Slit-Robo midline repulsion. We reasoned that if the Fra ICD regulates comm by acting as a transcription factor, it should be sufficient to perform the aspects of Fra's function that are due to its regulation of comm, but not the aspects that are due to its ability to transduce Netrin signals. To test this idea, we examined the ability of the Fra ICD to rescue fra loss-of-function phenotypes. The eg-Gal4 element is expressed in a subset of neurons in the embryo, including three commissural EW interneurons per abdominal hemisegment (Dittrich et al., 1997). We used eg-Gal4 to drive the expression of UAS-Tau-Myc-GFP, a marker that labels the axons and cell bodies of the EW neurons and facilitates quantitative evaluation of axonal trajectories. We combined this labeling with fluorescent in situ hybridization, using a probe that recognizes comm mRNA, so that we could score comm expression in each individual EW neuron. In embryos that are wild type for fra or heterozygous for fra3, axons of the EW neurons have reached the midline by stage 14, the time when these neurons express maximal amounts of comm mRNA (Figure 2A-2C; Keleman et al., 2002; Yang et al., 2009). In fra3 mutants, these neurons often fail to express comm and their axons fail to cross the midline at the appropriate time (Figure 2A-2C; Yang et al., 2009). These midline crossing and comm expression defects can be rescued by expression of a full-length UAS-Fra transgene with eg-Gal4 (Figure 2B-2C; Yang et al., 2009). In addition, expression of UAS-Fra ICD with eg-Gal4 partially rescues midline crossing defects and fully rescues comm expression in the EW neurons of fra3 mutants (Figure 2A-2C).
Figure 2. The Fra ICD is sufficient to fully rescue comm expression and partially rescue midline crossing defects in commissural neurons of fra mutants.
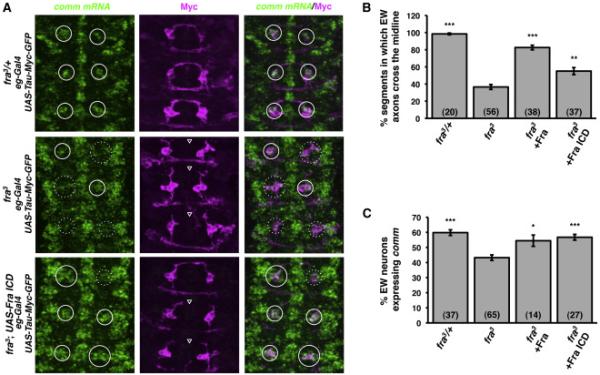
A) Fluorescent in situ hybridization for comm mRNA (green) in stage 14 embryos. Anterior is up. The cell bodies and axons of EW neurons are labeled with eg-Gal4 driving expression of UAS-Tau-Myc-GFP. Anti-Myc immunostaining is shown in magenta. White circles indicate the positions of EW neuron cell bodies. Solid circles indicate EW neurons that express comm and dotted circles indicate EW neurons that do not express comm. Open arrowheads indicate segments in which EW axons fail to cross the midline.
B) Quantification of EW axon crossing in stage 14 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to fra3 mutants. ** indicates p=0.0002, compared to fra3 mutants. Error bars indicate SEM. Number in parentheses indicates number of embryos scored. Note that the data for fra3 heterozygotes and mutants are also shown in Figures 6A and S3.
C) Quantification of comm expression in EW neurons in stage 14 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to fra3 mutants. * indicates p<0.01, compared to fra mutants. Error bars indicate SEM. Number in parentheses indicates number of embryos scored. Note that the data for fra3 heterozygotes and mutants are also shown in Figures 6B and S2.
We also examined the Fra ICD's ability to regulate comm expression in a subset of ipsilateral neurons, using a similar approach. The ap-Gal4 element is expressed in three ipsilateral interneurons per abdominal hemisegment (the ap neurons; O’Keefe et al., 1998), which stochastically express comm at stages 16-17 (Figure 3A-3C; Keleman et al., 2002; Yang et al., 2009). Expression of either full-length UAS-Fra or UAS-Fra ICD with ap-Gal4 induces ectopic midline crossing of ap axons and ectopic expression of comm in the dorsal ap neuron (Figure 3A-3C; Yang et al., 2009). Together, these rescue and gain-of-function genetic data support the idea that the Fra ICD is sufficient to carry out the transcriptional regulatory component of Fra's activity, but not the local, Netrin-dependent component.
Figure 3. γ-secretase proteolysis of Fra is required for Fra's ability to regulate comm expression.
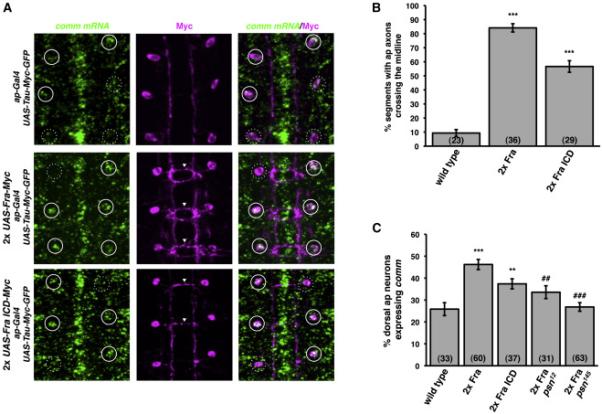
A) Fluorescent in situ hybridization for comm mRNA (green) in stage 17 embryos. Anterior is up. The cell bodies and axons of ap neurons are labeled with ap-Gal4 driving expression of UAS-Tau-Myc-GFP. Anti-Myc immunostaining is shown in magenta. White circles indicate the positions of dorsal apterous neuron cell bodies. Solid circles indicate ap neurons that express comm and dotted circles indicate ap neurons that do not express comm. Arrowheads indicate segments in which ap axons ectopically cross the midline.
B) Quantification of ap axon crossing in stage 16-17 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to wild type embryos. Error bars indicate SEM. Number in parentheses indicates number of embryos scored. Note that the data for wild type embryos are also shown in Figure 5D.
C) Quantification of comm expression in dorsal ap neurons in stage 16-17 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to wild type embryos. ** indicates p<0.005, compared to wild type embryos. ### indicates p<0.0001, compared to wild type embryos expressing two copies of Fra. ## indicates p<0.002, compared to wild type embryos expressing two copies of Fra. Error bars indicate SEM. Number in parentheses indicates number of embryos scored.
γ-secretase proteolysis of Fra is required for Fra to regulate comm expression
We used this gain-of-function assay to test whether Fra's ability to regulate comm expression depends on its proteolysis by γ-secretase. When we analyzed embryos in which UAS-Fra was misexpressed with ap-Gal4 in psn mutants, we found that Fra's ability to induce comm expression is reduced in two different psn mutant backgrounds (Figure 3C), suggesting that γ-secretase proteolysis of Fra is required for Fra's ability to regulate comm. Fra-induced ectopic midline crossing is not suppressed in psn mutants (data not shown), but interpretation of midline crossing phenotypes in these experiments is confounded by several factors, including reports that proteolysis of DCC can antagonize canonical Netrin-DCC signaling (Galko and Tessier-Lavigne, 2000; Bai et al., 2011); the observation that Robo activity, which plays a key role in preventing the ap neurons from crossing the midline, is regulated by metalloprotease-dependent ectodomain shedding (Coleman et al., 2010), an event which is typically followed by γsecretase proteolysis; and the likelihood that ectopic crossing events induced by full-length Fra are primarily a consequence of Netrin-dependent attraction (Figure 5D; O’Donnell and Bashaw, 2013).
Figure 5. The Fra ICD encodes a transcriptional activation domain.
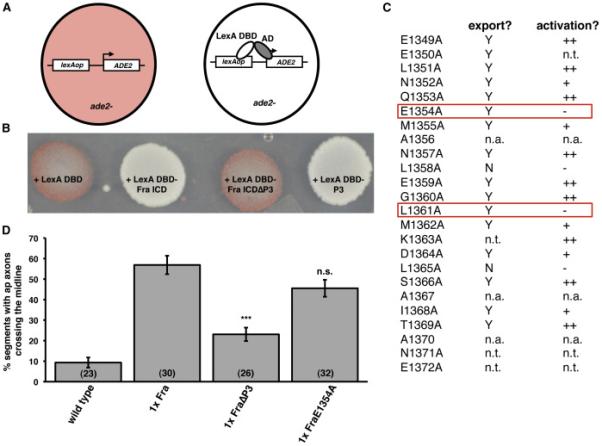
A) Schematic of yeast activation assay.
B) Yeast were transformed with plasmids encoding LexA DBD and the indicated forms of the Fra ICD. Note that P3 is necessary and sufficient for activation.
C) Summary of an alanine mutagenesis scan to identify point mutations within P3 that specifically disrupt transcriptional activation. Data in the export column indicate whether the mutant ICD was exported from the nucleus in S2R+ cells. Y indicates that the ICD did not accumulate in the nucleus in the absence of Leptomycin B. N indicates that the ICD accumulated in the nucleus in the absence of Leptomycin B. Data in the activation column indicate whether the mutant ICD functioned as transcriptional activator in the yeast assay. ++ indicates that the yeast appeared white; + indicates that the yeast appeared light pink; - indicates that the yeast appeared dark pink. n.t. indicates that the mutant was not tested. n.a. indicates alanine residues within P3. The mutants enclosed in the red boxes appear functional for nuclear export, but non-functional for transcriptional activation.
D) Quantification of ap axon crossing in stage 16-17 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to embryos expressing Fra. n.s. indicates p>0.05, compared to embryos expressing Fra. Error bars indicate SEM. Number in parentheses indicates number of embryos scored. Note that the data for wild type embryos are also shown in Figure 3B.
See also Figure S2.
The Fra ICD shuttles between the cytoplasm and the nucleus
If Fra regulates comm expression by functioning as a transcription factor, its ICD should be localized in nuclei. We initially investigated the subcellular localization of the Fra ICD in Drosophila S2R+ cells expressing a C-terminally epitope-tagged Fra ICD. In these experiments, we labeled nuclei by staining cells with an antibody against nuclear lamin, a component of the nuclear envelope. Under control conditions, the Fra ICD appears to be excluded from the nucleus. However, when nuclear export is blocked, either pharmacologically, with Leptomycin B, an inhibitor of CRM1-dependent nuclear export, or genetically, by deleting the P3 motif, which encodes Fra's nuclear export signal (NES), the Fra ICD accumulates in the nucleus (Figure 4A), suggesting that the Fra ICD normally shuttles between the nucleus and the cytoplasm. To examine the subcellular localization of the Fra ICD in vivo, we expressed UAS-Fra ICD-Myc with the restricted drivers eg-Gal4 and ap-Gal4, which allows for single cell resolution of nuclear localization. We observed some cells in which the Fra ICD is enriched in the nucleus and others in which the Fra ICD is mostly cytoplasmic (Figure 4B-C). When we expressed UAS-Fra ICDΔP3-Myc with either eg-Gal4 or ap-Gal4, we detected its expression in the nucleus in every cell we examined, suggesting that the Fra ICD shuttles between the nucleus and cytoplasm in vivo and indicating that the NES we mapped in vitro appears to have the same activity in vivo (Figure 4D and data not shown).
Figure 4. The Fra ICD shuttles between the cytoplasm and the nucleus.
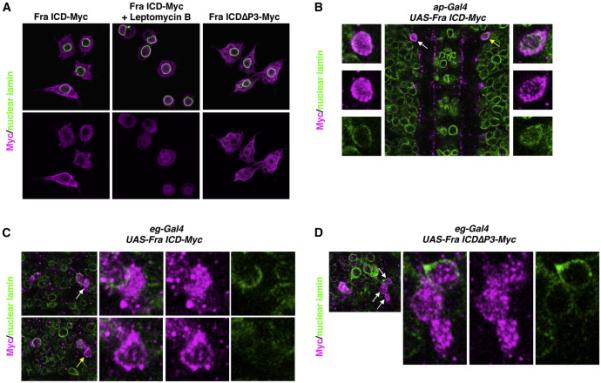
A) S2R+ cells were transfected with the indicated Myc-tagged constructs and treated with Leptomycin B or vehicle, as indicated. Cells were immunostained with antibodies against Myc (magenta) and nuclear lamin (green). For each condition, a single optical plane is shown.
B) Stage 16 embryo in which ap-Gal4 is driving expression of UAS-Fra ICD-Myc. Anterior is up. A single optical plane is shown. The embryo is stained with antibodies against Myc (magenta) and nuclear lamin (green). White arrow indicates a cell in which the Fra ICD is enriched in the nucleus (enlarged in the panels on the left). Yellow arrow indicates a cell in which the Fra ICD is largely excluded from the nucleus (enlarged in the panels on the right).
C) One segment of a stage 14 embryo in which eg-Gal4 is driving expression of UASFra ICD-Myc. Anterior is up. Two different single optical planes are shown. The embryo is stained with antibodies against Myc (magenta) and nuclear lamin (green). In the top row, the white arrow indicates a cell in which the Fra ICD is enriched in the nucleus (enlarged in the panels on the right). In the bottom row, the yellow arrow indicates a cell in which the Fra ICD is largely excluded from the nucleus (enlarged in the panels on the right).
D) One segment of a stage 14 embryo in which eg-Gal4 is driving expression of UASFra ICDΔP3-Myc. Anterior is up. A single optical plane is shown. The embryo is stained with antibodies against Myc (magenta) and nuclear lamin (green). White arrows indicate three cells in which the Fra ICD is enriched in the nucleus (enlarged in the panels on the right).
See also Figure S1.
When we expressed full-length Fra in S2R+ cells or in embryos, we could not detect its C-terminus in the nucleus, even when nuclear export was blocked (data not shown), suggesting that the amount of nuclear ICD generated from full-length receptor is too low at any given time for us to detect. Our inability to detect the C-terminus of full-length Fra in nuclei is reminiscent of reports that the C-terminus of full-length Notch cannot be detected in the nucleus by conventional immunostaining (Fehon et al., 1991; Lieber et al., 1993; Rebay et al., 1993), despite the finding that nuclear localization of the Notch ICD is necessary for its function (Struhl and Adachi, 1998).
We attempted to make a variant of the Fra ICD that lacks the ability to enter the nucleus, in order to test whether nuclear localization of the Fra ICD is required for its ability to regulate comm. We made serial deletions across the entire Fra ICD and tested the localization of these variants in S2R+ cells. Using this assay, we did not identify a sequence that is required for nuclear localization (Figure S1A). We also used a reporter assay in yeast to test which sequences within the Fra ICD are sufficient to confer nuclear localization. We used a strain of yeast in which a lexAop insertion upstream of the ADE2 gene disrupts endogenous ADE2 expression, causing the cells to accumulate a red pigment. In this strain, ADE2 is under the control of lexAop, so the expression of a transcriptional activator with a LexA DBD causes the yeast to turn white (Figure S1B). We fused a series of sequences spanning the entire Fra ICD to a transcription factor consisting of a DNA-binding domain from the bacterial transcription factor LexA (mutLexA DBD) and an activation domain from the yeast transcription factor Gal4 (Gal4 AD). The mutLexA DBD that we used in these experiments has mutations that abolish its intrinsic ability to enter the nucleus (Rhee et al., 2000; Marshall et al., 2007) and the Gal4 AD does not localize to the nucleus (Silver et al., 1988). We expressed these fusion proteins in ADE2 reporter yeast and identified three different regions of the Fra ICD that are sufficient to confer nuclear localization (Figure S1C). This redundancy prevented us from generating a Fra ICD variant that is defective for nuclear localization.
The Fra ICD encodes a transcriptional activation domain
The ICDs of DCC and Neo have been shown to function as transcriptional activators in reporter assays in vitro (Taniguchi et al., 2003; Goldschneider et al., 2008), suggesting a potentially direct mechanism through which Fra could regulate comm expression and prompting us to examine whether the Fra ICD shares this property with its vertebrate orthologs. To determine whether the Fra ICD, like its vertebrate orthologs, contains an activation domain, we returned to the ADE2 reporter yeast strain. For these experiments, we took advantage of the fact that expression of a transcription factor consisting of a LexA DBD (fused, in this case, to a strong nuclear localization signal (NLS)) and any activation domain drives expression of ADE2, causing the yeast to turn white (Figure 5A). Expression of a LexA DBD-Fra ICD fusion produces white yeast, indicating that the Fra ICD can function as a transcriptional activator (Figure 5B). A fusion between a LexA DBD and a Fra ICD lacking the conserved P3 motif (Fra ICDΔP3) fails to drive reporter expression, while a fusion between LexA DBD and P3 functions as a transcriptional activator, indicating that P3 is necessary and sufficient for Fra's transcriptional activation function (Figure 5B).
Fra regulates midline axon crossing and comm expression by functioning as a transcriptional activator
To determine whether Fra's ability to regulate commissural axon guidance and comm expression depends on its function as a transcriptional activator, we examined whether FraΔP3, which lacks Fra's activation domain, could rescue fra loss-of-function phenotypes. Expression of UAS-FraΔP3 with eg-Gal4 fails to rescue comm expression in the EW neurons of fra3 mutants (Figure S2). To more directly test whether this lack of rescue is a consequence of the loss of Fra's activation domain or reflects other defects in the receptor, we performed a domain replacement experiment using the VP16 AD. Expression of UAS-FraΔP3-VP16AD with eg-Gal4 does not rescue comm expression infra3 mutants (Figure S2).
This result could either mean that Fra's function as a transcriptional activator is not required for its ability to regulate comm or that P3 has an additional function in Fra's comm-regulatory pathway besides its function as an activation domain. To distinguish between these possibilities, we attempted to make mutations in Fra that specifically abrogate its transcriptional function, while leaving P3, which forms an alpha helix (Hirano et al., 2011; Wei et al., 2011), structurally intact. We performed an alanine mutagenesis scan across P3 and determined whether each point mutant had a functional activation domain and NES (Figure 5C). We used the presence of a functional NES as a proxy for the structural integrity of P3, as leucine-rich NESs, such as the one in P3, are alpha helices and this structure, rather than primary sequence, is the basis of their recognition by the nuclear export karyopherin CRM1 (Dong et al., 2009). Therefore, we reasoned that mutant ICDs that lacked functional activation domains, but retained functional NESs were good candidates to have specific deficits in transcriptional activation without deficits in other P3-dependent functions. To determine whether a point mutant ICD had a functional activation domain, we fused it to LexA DBD and expressed it in ADE2 reporter yeast. To determine whether a point mutant ICD had a functional NES, we fused it to a C-terminal epitope tag and examined its localization in S2R+ cells. Using this approach, we identified two point mutants, L1351A and E1354A, that are deficient for transcriptional activation, but are normally exported from the nucleus (Figure 5C) and we selected E1354A for further study. When we misexpressed UASFraE1354A with ap-Gal4, we found that it induces ectopic midline crossing almost as effectively as wild type Fra, suggesting that this mutant is able to carry out canonical Netrin signaling (Figure 5D). In contrast, expression of UAS-FraΔP3 with ap-Gal4 causes a much weaker ectopic crossing phenotype (Figure 5D).
Having defined specific mutations that disrupt transcriptional activation without disrupting other P3-dependent activities of the receptor, we next tested whether FraE1354A is able to rescue Fra's midline guidance and transcriptional regulatory activities. Expression of UAS-FraE1354A with eg-Gal4 fails to rescue the loss of comm expression in EW neurons of fra3 mutants, strongly suggesting that Fra's transcriptional activation function is required for this activity (Figure 6B). We were surprised to find that FraE1354A provides no rescue of midline crossing (Figure 6A), even though this receptor is likely intact for Netrin-dependent signal transduction (Figure 5D). In fact, we found that expression of FraE1354A antagonizes midline crossing in embryos heterozygous for fra3 (Figure S3), suggesting that FraE1354A acts as a dominant negative with respect to midline crossing. To rigorously test whether FraE1354A's inability to rescue midline crossing and comm expression stems from the disruption of Fra's activation domain, we generated a UAS-FraE1354A transgene with the VP16 AD fused to its C-terminus and evaluated its ability to rescue midline crossing and comm expression in fra3 mutants. Strikingly, we found that addition of a heterologous VP16 AD to the FraE1354A receptor restores its ability to rescue both midline crossing and comm expression, providing compelling in vivo evidence that Fra's function as a transcriptional activator is required for its ability to promote midline crossing and regulate comm (Figure 6A-6B).
Figure 6. Fra's transcriptional activation function is required for its ability to regulate midline crossing and comm expression.
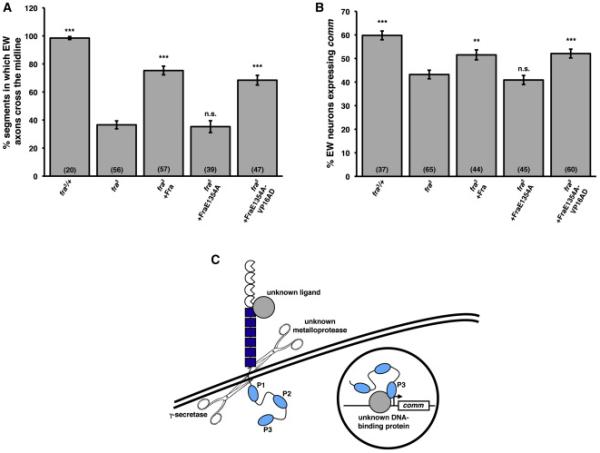
A) Quantification of EW axon crossing in stage 14 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to fra mutants. n.s. indicates p>0.05, compared to fra mutants. Error bars indicate SEM. Number in parentheses indicates number of embryos scored. Note that the data for fra3 heterozygotes and fra3 mutants are also shown in Figures 2B and S3.
B) Quantification of comm expression in EW neurons in stage 14 embryos. Data were analyzed by ANOVA, followed by Student's t-test. *** indicates p<0.0001, compared to fra mutants. ** indicates p<0.005, compared to fra mutants. n.s. indicates p>0.05, compared to fra mutants. Error bars indicate SEM. Number in parentheses indicates number of embryos scored. Note that the data for fra3 heterozygotes and fra3 mutants are also shown in Figures 2C and S2 and that the data for fra3 mutants rescued with wild type Fra are also shown in Figure S2.
C) A model for Fra-dependent comm expression. Full-length Fra is cleaved by γ-secretase, likely in response to an unknown ligand, which stimulates metalloprotease cleavage. The soluble ICD then translocates to the nucleus, where it functions as a transcriptional activator to induce comm expression, either directly or indirectly. The Fra ICD likely associates with DNA by interacting with one or more unknown DNA-binding proteins. P3 functions as a transcriptional activation domain.
See also Figure S3.
Discussion
In this study, we identify the Fra ICD as a transcription factor that regulates the expression of comm, a key modulator of axonal responsiveness at the midline. γ-secretase proteolysis of Fra releases its ICD, which is capable of nuclear translocation and is sufficient to promote midline crossing and regulate comm expression in rescue and gain-of-function assays in vivo. The conserved P3 motif within the Fra ICD functions as a transcriptional activation domain and this activity is required for Fra's regulation of comm expression. Thus, in addition to its canonical role signaling locally to regulate growth cone dynamics, Fra functions as a transcription factor to regulate axonal responsiveness at the midline.
Regulation of Fra's function as a transcription factor
comm is expressed in commissural neurons with exquisite temporal specificity (Keleman et al., 2002). How might the transcriptional activity of the Fra ICD be regulated to contribute to comm's expression pattern? γ-secretase proteolysis is typically the second cleavage event in a proteolytic cascade, preceded by ectodomain shedding. Indeed, pharmacological experiments suggest that DCC's ectodomain is shed as a result of metalloprotease cleavage and that this proteolytic event is required for subsequent γsecretase-dependent processing (Galko and Tessier-Lavigne, 2000; Bai et al., 2010). Metalloprotease-dependent ectodomain shedding is often ligand-dependent, while subsequent γ-secretase processing depends on the shape of the membrane-tethered metalloprotease cleavage product. For example, metalloprotease-dependent shedding of the Notch ectodomain is stimulated by the binding of Notch ligands (Brou et al., 2000; Mumm et al., 2000), and the subsequent γ-secretase cleavage of the membrane-tethered ICD is constitutive (Struhl and Adachi, 2000). As Fra regulates comm independent of Netrins (Yang et al., 2009), Fra ectodomain shedding may occur in response to the binding of a different ligand. Alternative ligands for DCC have been identified, including the vertebrate-specific proteins Draxin (Ahmed et al., 2011) and Cerebellin 4 (Haddick et al., 2014). In addition, the secreted protein MADD-4 physically associates with the C. elegans ortholog of Fra/DCC, UNC-40, and guides sensory neurons and muscle arms in an UNC-40-dependent manner (Seetharaman et al., 2011; Chan et al., 2014). The function of the Drosophila ortholog of MADD-4, Nolo, has not been investigated, nor has its ability to bind to Fra.
It seems unlikely that the transcriptional activity of the Fra ICD is controlled at the level of nuclear localization. When we express Fra ICDΔP3 (lacking a NES) in the commissural EW neurons in vivo, it accumulates in the nucleus at the earliest developmental stages we can observe (data not shown), suggesting that the Fra ICD is constitutively imported into the nucleus. We observe nuclear accumulation of full-length Fra ICD (with a NES) only occasionally (Figure 4B-4C), implying that after the Fra ICD translocates to the nucleus, it is rapidly exported. The fact that Fra's NES and activation domain are both encoded by P3 raises the possibility that when Fra is engaged in transcriptional activation, the association of co-activators with P3 might prevent it from associating with nuclear export machinery, coupling Fra's nuclear activity to its nuclear retention.
Mechanism of Fra's function as a transcription factor
Our finding that Fra's ability to regulate comm expression depends on its function as a transcriptional activator seems to imply that the Fra ICD can associate with chromatin, but the Fra ICD does not contain an obvious DNA-binding domain. A Neo DNA-binding domain has not been identified either, but chromatin immunoprecipitation experiments have demonstrated that the Neo ICD associates with chromatin in vitro (Goldschneider et al., 2008). The Fra ICD's DNA-binding activity and specificity likely arise from associations between the Fra ICD and DNA-binding partners, as is the case with Notch. The Notch ICD has no DNA-binding activity of its own and associates with DNA as part of a complex including an obligate CSL (CBF1/RBPjκ, Su(H), Lag-1) DNA-binding partner (Nam et al., 2006; Wilson and Kovall, 2006). If the Fra ICD can associate with multiple DNA-binding proteins, it might allow the Fra ICD to regulate the expression of many different target genes, depending on which of its DNA-binding partners are expressed in particular cell types or developmental contexts.
The observation that a structurally intact P3 is required for Fra-dependent transcription (Figure S2) suggests that P3 plays another role in Fra's transcriptional output besides its function as an activation domain. One possibility is that P3 is required for Fra's association with chromatin, perhaps by functioning as a binding interface for Fra's DNA-binding co-factors. This idea is supported by our observation that FraE1354A antagonizes midline crossing in both fra mutants and heterozygotes, while FraΔP3 has only a mild effect (Figures 6A-6B and S3). Perhaps the ICD of FraE1354A inhibits midline crossing by occupying chromatin sites that are normally targets of both Fra and other transcriptional activators that act in a parallel pathway; the ICD of FraΔP3 would not have this effect if P3 is required for Fra's association with chromatin. FraE1354A is not likely to be inhibiting endogenous Fra in our rescue experiments, as fra3 is either a strong hypomorphic or null allele (Kolodziej et al., 1996; Yang et al., 2009). This model predicts that Fra has other transcriptional targets in EW neurons that are relevant for commissural axon guidance. It will be informative to identify additional transcriptional targets of Fra both in embryonic commissural neurons and in other cell types. In the retina, R8 photoreceptor axons have targeting defects that are much milder in Netrin mutants than in fra mutants (Timofeev et al., 2012), raising the possibility that the Netrin-independent output of Fra signaling in this system might be through the transcriptional pathway we have identified.
Proteolytic regulation of axon guidance receptor signaling
Cleavage of axon guidance receptors has been shown to regulate the activities of these receptors in a number of different ways. Degradation of axon guidance receptors can provide temporal control of axonal sensitivity to guidance cues. In vertebrates, this mode of regulation controls axonal responsiveness to members of the class 3 family of secreted Semaphorins (Sema3s), which signal repulsion through Neuropilin (Nrp)/Plexin (Plex) co-receptors. Calpain proteolysis of PlexA1 in pre-crossing spinal commissural neurons reduces their sensitivity to Sema3B, which is expressed in the ventral spinal cord as these axons are growing toward the ventral midline (Nawabi et al., 2010). ADAM metalloprotease cleavage of Nrp1 reduces the sensitivity of proprioceptive sensory axons to Sema3A allowing them to terminate in the ventral spinal cord, where Sema3A expression is high (Romi et al., 2014). In addition, γ-secretase proteolysis of DCC in vertebrate motor neurons inhibits their responsiveness to midline-derived Netrin, preventing them from ectopically projecting toward the midline (Bai et al., 2011).
Proteolytic processing has also been implicated as a requisite step in local repulsive Robo signaling in Drosophila (Coleman et al., 2010). The Robo ectodomain is cleaved by the ADAM metalloprotease Kuzbanian and this proteolytic event is required for Robo's ability to transduce repulsive signals in vivo and for Slit-dependent recruitment of effectors of local Robo signaling in vitro. As γ-secretase-dependent intramembrane proteolysis is typically constitutive following ectodomain shedding, and occurs subsequent to metalloprotease processing of the human Robo1 receptor (Seki et al., 2010), it is likely that Drosophila Robo is cleaved to produce a soluble ICD. The observation that Robo proteolysis is required for local Slit-Robo signaling does not exclude the possibility that the Robo ICD may also have a nuclear function that contributes to axon guidance, but this possibility has not yet been explored.
Proteolysis has also been identified as a regulator of contact-mediated axonal repulsion. Eph receptors signal repulsion in response to their transmembrane ephrin ligands; ephrins can also function as receptors, signaling repulsion in response to Eph binding. Metalloprotease and subsequent γ-secretase cleavage of both Ephs and ephrins have been demonstrated, providing a mechanism through which adhesive interactions can be broken to allow for repulsive signaling (Hattori et al., 2000; Janes et al., 2005; Tomita et al., 2006; Litterst et al., 2007; Lin et al., 2008; Gatto et al., 2014). The importance of this mode of regulation for axon targeting has not yet been established in vivo and a recent study using an EphA4 variant that is insensitive to metalloprotease cleavage suggests that EphA4 proteolysis is not required for EphA4-dependent motor axon targeting (Gatto et al., 2014).
Here, we have identified a new way in which axon guidance receptor proteolysis can influence axon responsiveness to guidance cues. γ-secretase-dependent processing of Fra releases its ICD, which translocates to the nucleus, where it functions as a transcription factor to regulate the guidance of commissural axons (Figure 6C). We propose that the ability to signal from the nucleus may be a common property of axon guidance receptors and may serve as a general mechanism through which axon guidance receptors regulate their own activities or the activities of other proteins. Human Robo1 is processed by sequential metalloprotease and γ-secretase cleavage and its ICD localizes to the nucleus in vitro (Seki et al., 2010). It remains to be seen whether the ICDs of Ephs and ephrins, which are cleaved by γ-secretase, and of Plexins, which are proteolytically processed, but have not yet been identified as γ-secretase substrates, translocate to the nucleus as well. It will also be interesting to determine whether the ICDs of Fra and other axon guidance receptors signal from the nucleus to regulate aspects of neuronal morphogenesis and function besides axon pathfinding. Finally, recent work indicating that the cleaved C-terminus of the Drosophila Wnt receptor Frizzled translocates to the nucleus and contributes to the establishment of postsynaptic structures by regulating RNA export (Mathew et al., 2005; Mosca and Schwarz, 2010; Speese et al., 2012) serves as a reminder that the trafficking of cell surface receptor fragments to the nucleus may allow these fragments to signal not only by regulating transcription, but in other ways as well.
Experimental Procedures
Molecular biology
See Supplemental Experimental Procedures for details about plasmid construction.
Genetics
See Supplemental Experimental Procedures for details about mutant alleles and transgenic lines used in this study.
Immunostaining of embryos
Embryo fixation and staining were performed as described (Kidd et al., 1998a). See Supplemental Experimental Procedures for details.
Fluorescent
in situ hybridization
Fluorescent in situ hybridization was performed as previously described (Labrador et al., 2005) and antisense, digoxigenin-labeled comm probes were generated as previously described (Yang et al., 2009).
Cell culture and immunostaining
Drosophila S2R+ cells were cultured and stained as previously described (Evans et al., 2015). See Supplemental Experimental Procedures for details.
Imaging and phenotypic analysis
Images of embryos and S2R+ cells were acquired using a spinning disk confocal system (Perkin Elmer) built on a Nikon Ti-U inverted microscope using a Nikon OFN25 60× objective with a Hamamatsu C10600-10B CCD camera and Yokogawa CSU-10 scanner head with Volocity imaging software. Images were processed using ImageJ. When scoring EW crossing, a segment was considered to have a crossing defect if one or both bundles of EW axons failed to reach the midline. When scoring ap crossing, a segment was considered to have an ectopic cross if it contained at least one continuous projection that extended across the midline and reached the lateral bundle of ap axons on the contralateral side. comm expression was scored using Volocity imaging software. Embryos expressed UAS-Tau-Myc-GFP and EW or ap neurons were identified by anti-Myc immunostaining. If the cell body of a neuron could be detected by the in situ signal, that neuron was scored as positive. Crossing and comm expression were scored in EW neurons at stage 14 and in ap neurons at stages 16-17. For all analyses, segments A1-A7 were scored. Midline crossing phenotypes and comm mRNA expression were scored blind to genotype whenever possible.
Biochemistry
Embryo lysates were generated and immunoprecipitations and western blots were performed as previously described (Evans et al., 2015). See Supplemental Experimental Procedures for details.
Yeast transformations
The yeast strain used for both nuclear localization and activation reporter assays was Y860 [α his3-11, 15 leu2-3, 112 trp1-1 ade2-1 can1-100 ura3-1::URA3:lexAop-ADE2] (a gift from Erfei Bi). Yeast were grown overnight at 30°C in liquid YPD media until log phase (OD600 = 0.4-0.6) and transformed using the PEG/lithium acetate method (Ito et al., 1983). Yeast were then plated onto solid SD media lacking histidine and grown at 30°C for 2-3 days.
Supplementary Material
Highlights.
-γ-secretase cleaves Fra to release an ICD that shuttles between nucleus and cytoplasm
-The Fra ICD is sufficient to regulate comm expression and midline axon crossing
-The P3 motif in the Fra ICD encodes a transcriptional activation domain
-Fra's transcriptional activation function is required to regulate comm expression
Acknowledgements
We thank Mike O’Donnell and Celine Santiago for thoughtful suggestions throughout the course of this work and for comments on the manuscript and Patrick Mehlen for sharing unpublished data. We thank Joe Mymryk and Jonathan Raper for plasmids and Erfei Bi and Aaron Gitler for yeast strains and reagents. A.N.-F. was supported by NIH grant T32GM008216. This work was supported by NIH grant R01NS054739, NSF grant IOS-1355181, and March of Dimes grant #1-FY12-445 to G.J.B.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed G, Shinmyo Y, Ohta K, Islam SM, Hossain M, Naser IB, Riyadh MA, Su Y, Zhang S, Tessier-Lavigne M, et al. Draxin inhibits axonal outgrowth through the netrin receptor DCC. J Neurosci. 2011;31:14018–14023. doi: 10.1523/JNEUROSCI.0943-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai G, Chivatakarn O, Bonanomi D, Lettieri K, Franco L, Xia C, Stein E, Ma L, Lewcock JW, Pfaff SL. Presenilin-dependent receptor processing is required for axon guidance. Cell. 2011;144:106–118. doi: 10.1016/j.cell.2010.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9:188–194. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, Tessier-Lavigne M, Kidd T. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell. 1999;96:795–806. doi: 10.1016/s0092-8674(00)80590-5. [DOI] [PubMed] [Google Scholar]
- Brou C, Logeat F, Gupta N, Bessia C, LeBail O, Doedens JR, Cumano A, Roux P, Black RA, Israël A. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- Chan KK, Seetharaman A, Bagg R, Selman G, Zhang Y, Kim J, Roy PJ. EVA-1 functions as an UNC-40 co-receptor to enhance attraction to the MADD-4 guidance cue in Caenorhabditis elegans. PLoS Genet. 2014;10:e1004521. doi: 10.1371/journal.pgen.1004521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoy C, Nawabi H, Reynaud F, Derrington E, Bozon M, Wright K, Falk J, Helmbacher F, Kindbeiter K, Castellani V. gdnf activates midline repulsion by Semaphorin3B via NCAM during commissural axon guidance. Neuron. 2012;75:1051–1066. doi: 10.1016/j.neuron.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Chen Z, Gore BB, Long H, Ma L, Tessier-Lavigne M. Alternative splicing of the Robo3 axon guidance receptor governs the midline switch from attraction to repulsion. Neuron. 2008;58:325–332. doi: 10.1016/j.neuron.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Coleman HA, Labrador JP, Chance RK, Bashaw GJ. The Adam family metalloprotease Kuzbanian regulates the cleavage of the roundabout receptor to control axon repulsion at the midline. Development. 2010;137:2417–2426. doi: 10.1242/dev.047993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittrich R, Bossing T, Gould AP, Technau GM, Urban J. The differentiation of the serotonergic neurons in the Drosophila ventral nerve cord depends on the combined function of the zinc finger proteins Eagle and Huckebein. Development. 1997;124:2515–2525. doi: 10.1242/dev.124.13.2515. [DOI] [PubMed] [Google Scholar]
- Dong X, Biswas A, Süel KE, Jackson LK, Martinez R, Gu H, Chook YM. Structural basis for leucine-rich nuclear export signal recognition by CRM1. Nature. 2009;458:1136–1141. doi: 10.1038/nature07975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of γ-secretase activity. Nat Cell Biol. 2003;5:486–488. doi: 10.1038/ncb960. [DOI] [PubMed] [Google Scholar]
- Evans TA, Santiago C, Arbeille E, Bashaw GJ. Robo2 acts in trans to inhibit Slit- Robo1 repulsion in pre-crossing commissural axons. Elife. 2015 doi: 10.7554/eLife.08407. doi: 10.7554/eLife.08407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazeli A, Dickinson SL, Hermiston ML, Tighe RV, Steen RG, Small CG, Stoeckli ET, Keino-Masu K, Masu M, Rayburn H, et al. Phenotype of mice lacking functional Deleted in colorectal cancer (Dcc) gene. Nature. 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Johansen K, Rebay I, Artavanis-Tsakonas S. Complex cellular and subcellular regulation of notch expression during embryonic and imaginal development of Drosophila: implications for Notch function. J Cell Biol. 1991;113:657–669. doi: 10.1083/jcb.113.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, et al. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of ßAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- Fraering PC, Ye W, Strub JM, Dolios G, LaVoie MJ, Ostaszewski BL, van Dorsselaer A, Wang R, Selkoe DJ, Wolfe MS. Purification and characterization of the human γ secretase complex. Biochemistry. 2004;43:9774–9789. doi: 10.1021/bi0494976. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Tessier-Lavigne M. Function of an axonal chemoattractant modulated by metalloprotease activity. Science. 2000;289:1365–1367. doi: 10.1126/science.289.5483.1365. [DOI] [PubMed] [Google Scholar]
- Gatto G, Morales D, Kania A, Klein R. EphA4 receptor shedding regulates spinal motor axon guidance. Curr Biol. 2014;24:2355–2365. doi: 10.1016/j.cub.2014.08.028. [DOI] [PubMed] [Google Scholar]
- Goldschneider D, Rama N, Guix C, Mehlen P. The neogenin intracellular domain regulates gene transcription via nuclear translocation. Mol Cell Biol. 2008;28:4068–4079. doi: 10.1128/MCB.02114-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci USA. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo A, Kovacs DM. The many substrates of presenilin/γ-secretase. J Alzheimers Dis. 2011;25:3–28. doi: 10.3233/JAD-2011-101065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddick PC, Tom I, Luis E, Quiñones G, Wranik BJ, Ramani SR, Stephan JP, Tessier-Lavigne M, Gonzalez LC. Defining the ligand specificity of the deleted in colorectal cancer (DCC) receptor. PLoS One. 2014;9:e84823. doi: 10.1371/journal.pone.0084823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila Netrin/UNC-6 homologs. Neuron. 1996;17:217–228. doi: 10.1016/s0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- Hattori M, Osterfield M, Flanagan JG. Regulated cleavage of a contact-mediated axon repellent. Science. 2000;289:1360–1365. doi: 10.1126/science.289.5483.1360. [DOI] [PubMed] [Google Scholar]
- Hirano Y, Hatano T, Takahashi A, Toriyama M, Inagaki N, Hakoshima T. Structural basis of cargo recognition by the myosin-X MyTH4-FERM domain. EMBO J. 2011;30:2734–2747. doi: 10.1038/emboj.2011.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes GP, Negus K, Burridge L, Raman S, Algar E, Yamada T, Little MH. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech Dev. 1998;79:57–72. doi: 10.1016/s0925-4773(98)00174-9. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ye Y, Fortini ME. Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2:69–78. doi: 10.1016/s1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes PW, Saha N, Barton WA, Kolev MV, Wimmer-Kleikamp SH, Nievergall E, Blobel CP, Himanen JP, Lackmann M, Nikolov DB. Adam meets Eph: an ADAM substrate recognition module acts as a molecular switch for ephrin cleavage in trans. Cell. 2005;123:291–304. doi: 10.1016/j.cell.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Jaworski A, Long H, Tessier-Lavigne M. Collaborative and specialized functions of Robo1 and Robo2 in spinal commissural axon guidance. J Neurosci. 2010;30:9445–9453. doi: 10.1523/JNEUROSCI.6290-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keino-Masu K, Masu M, Hinck L, Leonardo ED, Chan SS, Culotti JG, Tessier-Lavigne M. Deleted in Colorectal Cancer (DCC) encodes a netrin receptor. Cell. 1996;87:175–185. doi: 10.1016/s0092-8674(00)81336-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Rajagopalan S, Cleppien D, Teis D, Paiha K, Huber LA, Technau GM, Dickson BJ. Comm sorts robo to control axon guidance at the Drosophila midline. Cell. 2002;110:415–427. doi: 10.1016/s0092-8674(02)00901-7. [DOI] [PubMed] [Google Scholar]
- Keleman K, Ribiero C, Dickson BJ. Comm function in commissural axon guidance: cell autonomous sorting of Robo in vivo. Nat Neurosci. 2005;8:156–163. doi: 10.1038/nn1388. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ, Fetter RD, Tessier-Lavigne M, Goodman CS, Tear G. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionarily conserved guidance receptors. Cell. 1998a;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998b;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, Jan YN. frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87:197–204. doi: 10.1016/s0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- Labrador JP, O'Keefe D, Yoshikawa S, McKinnon RD, Thomas JB, Bashaw GJ. The homeobox transcription factor even-skipped regulates Netrin-receptor expression to control dorsal motor-axon projections in Drosophila. Curr Biol. 2005;15:1413–1419. doi: 10.1016/j.cub.2005.06.058. [DOI] [PubMed] [Google Scholar]
- Li HS, Chen JH, Wu W, Fagaly T, Zhou L, Yuan W, Dupuis S, Jiang ZH, Nash W, Gick C, et al. Vertebrate slit, a secreted ligand for the transmembrane protein roundabout, is a repellent for olfactory bulb axons. Cell. 1999;96:807–818. doi: 10.1016/s0092-8674(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Lieber T, Kidd S, Alcamo E, Corbin V, Young MW. Antineurogenic phenotypes induced by truncated Notch proteins indicate a role in signal transduction and may point to a novel function for Notch in nuclei. Genes Dev. 1993;7:1949–1965. doi: 10.1101/gad.7.10.1949. [DOI] [PubMed] [Google Scholar]
- Lin KT, Sloniowski S, Ethell DW, Ethell IM. Ephrin-B2-induced cleavage of EphB2 receptor is mediated by matrix metalloproteinases to trigger cell repulsion. J Biol Chem. 2008;283:28969–28979. doi: 10.1074/jbc.M804401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litterst C, Georgakopoulos A, Shioi J, Ghersi E, Wisniewski T, Wang R, Ludwig A, Robakis NK. Ligand binding and calcium influx induce distinct ectodomain/γ secretase-processing pathways of the EphB2 receptor. J Biol Chem. 2007;282:16155–16163. doi: 10.1074/jbc.M611449200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L, Plump A, Yuan W, Ornitz DM, Tamada A, Murakami F, Goodman CS, Tessier-Lavigne M. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Marshall KS, Zhang Z, Curran J, Derbyshire S, Mymryk JS. An improved genetic system for detection and analysis of protein nuclear import signals. BMC Mol Biol. 2007;8:6. doi: 10.1186/1471-2199-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Ataman B, Chen J, Zhang Y, Cumberledge S, Budnik V. Wingless signaling at synapses is through cleavage and nuclear import of receptor DFrizzled2. Science. 2005;310:1344–1347. doi: 10.1126/science.1117051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E, Tauzig-Delamasure S, Monnier PP, Mueller BK, Strittmatter SM, Mehlen P, Chédotal A. RGM and its receptor neogenin regulate neuronal survival. Nat Cell Biol. 2004;6:749–755. doi: 10.1038/ncb1157. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Rabizadeh S, Snipas SJ, Assa-Munt N, Salvesen GS, Bredesen DE. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 1998;395:801–804. doi: 10.1038/27441. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, Dickson BJ. Genetic analysis of Netrin genes in Drosophila: Netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17:203–215. doi: 10.1016/s0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- Mosca TJ, Schwarz TL. The nuclear import of Frizzled2-C by Importins-β11 and [.alpha]2 promotes postsynaptic development. Nat Neurosci. 2010;13:935–943. doi: 10.1038/nn.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, Ray WJ, Kopan R. A ligand-induced extracellular cleavage regulates γ-secretase-like proteolytic activation of Notch1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- Nam Y, Sliz P, Song L, Aster JC, Blacklow SC. Structural basis for cooperativity in recruitment of MAML coactivators to Notch transcription complexes. Cell. 2006;124:973–983. doi: 10.1016/j.cell.2005.12.037. [DOI] [PubMed] [Google Scholar]
- Nawabi H, Briançon-Marjollet A, Clark C, Sanyas I, Takamatsu H, Okuno T, Kumanogoh A, Bozon M, Takeshima K, Yoshida Y, et al. A midline switch of receptor processing regulates commissural axon guidance in vertebrates. Genes Dev. 2010;24:396–410. doi: 10.1101/gad.542510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhaus-Follini A, Bashaw GJ. Crossing the embryonic midline: molecular mechanisms regulating axon responsiveness at an intermediate target. Wiley Interdiscip Rev Dev Biol. 2015;4:377–389. doi: 10.1002/wdev.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell MP, Bashaw GJ. Src inhibits midline axon crossing independent of Frazzled/Deleted in Colorectal Carcinoma (DCC) receptor tyrosine phosphorylation. J Neurosci. 2013;33:305–314. doi: 10.1523/JNEUROSCI.2756-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura Y, Kohmura E, Yamashita T. TACE cleaves neogenin to desensitize cortical neurons to the repulsive guidance molecule. Neurosci Res. 2011;71:63–70. doi: 10.1016/j.neures.2011.05.012. [DOI] [PubMed] [Google Scholar]
- O'Keefe DD, Thor S, Thomas JB. Function and specificity of LIM domains in Drosophila nervous system and wing development. Development. 1998;125:3915–3923. doi: 10.1242/dev.125.19.3915. [DOI] [PubMed] [Google Scholar]
- Parent AT, Barnes NY, Taniguchi Y, Thinakaran G, Sisodia SS. Presenilin attenuates receptor-mediated signaling and synaptic function. J Neurosci. 2005;25:1540–1549. doi: 10.1523/JNEUROSCI.3850-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra LM, Zou Y. Sonic hedgehog induces response of commissural axons to Semaphorin repulsion during midline crossing. Nat Neurosci. 2010;13:29–35. doi: 10.1038/nn.2457. [DOI] [PubMed] [Google Scholar]
- Philipp M, Niederkofler V, Debrunner M, Alther T, Kunz B, Stoeckli ET. RabGDI controls axonal midline crossing by regulating Robo1 surface expression. Neural Dev. 2012;7:36. doi: 10.1186/1749-8104-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S, Nicolas E, Vivancos V, Berger J, Dickson BJ. Crossing the midline: roles and regulation of Robo receptors. Neuron. 2000;28:767–777. doi: 10.1016/s0896-6273(00)00152-5. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fehon RG, Artavanis-Tsakonas Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993;74:319–329. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- Rhee Y, Gurel F, Gafni Y, Dingwall C, Citovsky V. A genetic system for detection of protein nuclear import and export. Nat Biotechnol. 2000;18:433–437. doi: 10.1038/74500. [DOI] [PubMed] [Google Scholar]
- Romi E, Gokhman I, Wong E, Antonovsky N, Ludwig A, Sagi I, Saftig P, Tessier-Lavigne M, Yaron A. ADAM metalloproteases promote a developmental switch in responsiveness to the axonal repellant Sema3A. Nat Commun. 2014;5:4058. doi: 10.1038/ncomms5058. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump AS, Ma L, Brose K, Tamada A, Murakami F, Lee EY, Tessier-Lavigne M. The divergent Robo family protein rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing by commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Seeger M, Tear G, Ferres-Marco D, Goodman CS. Mutations affecting growth cone guidance in Drosophila: genes necessary for guidance toward or away from the midline. Neuron. 1993;10:409–426. doi: 10.1016/0896-6273(93)90330-t. [DOI] [PubMed] [Google Scholar]
- Seetharaman A, Selman G, Puckrin R, Barbier L, Wong E, D'Souza SA, Roy PJ. MADD-4 is a secreted cue required for midline-oriented guidance in Caenorhabditis elegans. Dev Cell. 2011;21:669–680. doi: 10.1016/j.devcel.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Seki M, Watanabe A, Enomoto S, Kawamura T, Ito H, Kodama T, Hamakubo T, Aburatani H. Human ROBO1 is cleaved by metalloproteinases and γ-secratase and migrates to the nucleus in cancer cells. FEBS Lett. 2010;584:2909–2915. doi: 10.1016/j.febslet.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, Tessier-Lavigne M. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- Silver PA, Chiang A, Sadler I. Mutations that alter both localization and production of a yeast nuclear protein. Genes Dev. 1988;2:707–717. doi: 10.1101/gad.2.6.707. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short-range and long-range guidance by Slit and its Robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Speese SD, Ashley J, Jokhi V, Nunnari J, Barria R, Li Y, Ataman B, Koon A, Chang YT, Li Q, et al. Nuclear envelope budding enables large ribonuclear particle export during synaptic Wnt signaling. Cell. 2012;149:832–846. doi: 10.1016/j.cell.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Nuclear access and action of notch in vivo. Cell. 1998;93:649–660. doi: 10.1016/s0092-8674(00)81193-9. [DOI] [PubMed] [Google Scholar]
- Struhl G, Adachi A. Requirements for presenilin-dependent cleavage of notch and other transmembrane proteins. Mol Cell. 2000;6:625–636. doi: 10.1016/s1097-2765(00)00061-7. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC). J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita T, Tanaka S, Morohashi Y, Iwatsubo T. Presenilin-dependent intramembrane cleavage of ephrin-B1. Mol Neurodegener. 2006;1:2. doi: 10.1186/1750-1326-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z, Yan J, Lu Q, Pan L, Zhang M. Cargo recognition mechanism of myosin X revealed by the structure of its tail MyTH4-FERM tandem in complex with the DCC P3 domain. Proc Natl Acad Sci USA. 2011;108:3572–3577. doi: 10.1073/pnas.1016567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JJ, Kovall RA. Crystal structure of the CSL-Notch-Mastermind ternary complex bound to DNA. Cell. 2006;124:985–996. doi: 10.1016/j.cell.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Wolfe MS, Xia W, Ostaszeweski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- Yam PT, Kent CB, Morin S, Farmer WT, Alchini R, Lepelletier L, Colman DR, Tessier- Lavigne M, Fournier AE, Charron F. 14-3-3 proteins regulate a cell-intrinsic switch from sonic hedgehog-mediated commissural axon attraction to repulsion after midline crossing. Neuron. 2012;76:735–749. doi: 10.1016/j.neuron.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Yang L, Garbe DS, Bashaw GJ. A frazzled/DCC-dependent transcriptional switch regulates midline axon guidance. Science. 2009;2009;324:944–947. doi: 10.1126/science.1171320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Lukinova N, Fortini ME. Neurogenic phenotypes and altered Notch processing in Drosophila Presenilin mutants. Nature. 1999;398:525–529. doi: 10.1038/19096. [DOI] [PubMed] [Google Scholar]
- Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, et al. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and ßAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- Zou Y, Stoeckli E, Chen H, Tessier-Lavigne M. Squeezing axons out of the gray matter: a role for slit and semaphorin proteins from midline and ventral spinal cord. Cell. 2000;102:363–375. doi: 10.1016/s0092-8674(00)00041-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


