Abstract
BACKGROUND
The anticipated clinical outcome of the standard/control arm is an important parameter in the design of randomized phase 3 (RP3) trials to properly calculate sample size, power, and study duration. Changing patterns of care or variation in the study population enrolled may lead to a deviation from the initially anticipated outcome. The authors hypothesized that recent changes in patterns of care in epithelial ovarian cancer (EOC) have led to challenges in correctly estimating the outcome of control groups.
METHODS
A systematic review of the literature was conducted for RP3 trials of EOC published between January 2000 and December 2010. The expected outcome of the control arm as well as the actual outcome achieved by this cohort was collected and a ratio (actual‐over‐expected ratio) was calculated. The estimation of outcome was deemed accurate if the outcome of the control arm was between 0.75 to 1.25 times the anticipated outcome.
RESULTS
A total of 35 trials were eligible for analysis. Fifteen trials had survival as the primary endpoint whereas 20 had a progression‐based primary endpoint. In total, 12 of 15 trials with a survival‐based endpoint significantly underestimated the outcome of the control arm, whereas only 4 of 20 trials with a progression‐based endpoint did. Studies with a survival endpoint underestimated outcome more frequently than those with a progression endpoint (P<.001).
CONCLUSIONS
Survival of the control arm has frequently been underestimated in recent EOC RP3 trials. This underestimation means that the initial statistical assumptions of these trials may have been inaccurate. Underestimating the outcome of the control arm may result in trials being underpowered to demonstrate the absolute benefit they were designed to show. Cancer 2015;121:413–422. © 2014 American Cancer Society.
Keywords: randomized, phase 3 trials, epithelial ovarian cancer, endpoints, survival, statistical design
Short abstract
The anticipated clinical outcome of the standard/control arm is an important parameter in the design of randomized phase 3 trials for the accurate calculation of sample size, power, and study duration but is often underestimated in ovarian cancer trials. Changing patterns of care and variations in enrolled study populations may result in a deviation from the anticipated outcome and subsequent inaccurate statistical assumptions.
INTRODUCTION
When designing a randomized phase 3 (RP3) trial, an appropriate sample size calculation is necessary to ensure adequate study power to demonstrate a statistically significant difference between the experimental and standard arms. The majority of trial designs estimate the required sample size by incorporating 2 variables: the expected outcome for the standard/control arm and the size effect hypothesized for the experimental treatment or, in other words, the magnitude of the benefit the experimental arm is expected to confer relative to the standard arm.1 Thus, accurate estimation of the control arm outcome is necessary in order for initial sample size calculations to be precise and reliable.
The basic concepts of trial design evolve from the initial selection of the null hypothesis and the alternative hypothesis. The consequent treatment effect is intimately related and integral to the calculation of the sample size to achieve adequate statistical power.2 Type I (α) errors and type II (β) errors are central concepts in this process.2 A type I error is the probability of rejecting the null hypothesis when in fact it is true: a false‐positive result.2 This in general is set at a low value (conventionally 0.05).2 A type II error is the probability of accepting the null hypothesis when in fact it is false: a false‐negative result.2 The power of the study reflects the probability of correctly rejecting the null hypothesis (1‐β).2 Sample size calculation is an exercise in determining the number of participants required to simultaneously achieve both the desired power and type I error.3 Although this is a simplistic explanation of the concepts of trial design and neglects many of the important intricacies, it emphasizes that inaccurate estimation of any of these components potentially compromises the ultimate results of the trial, even before it has started.
The expected outcome in the control arm is generally inferred from completed clinical trials, using historical data from comparable patient populations treated with similar therapies. However, it is recognized that correctly estimating the outcome of a contemporaneous population is difficult, because variations in patient population, changes in treatment patterns, and random error can result in a significant deviation from even the most robust historical data.4
Estimating the outcome of women treated for advanced‐stage epithelial ovarian cancer (EOC) may be particularly challenging given the surgical and therapeutic advances made over the last decades, which have significantly influenced patient outcomes. Up until the early 1980s, standard‐of‐care chemotherapy for patients with advanced‐stage EOC was cyclophosphamide and doxorubicin. In <30 years, platinum agents, taxanes, and several other chemotherapeutic drugs have been incorporated into the routine care of patients with EOC. This coupled with improved access to standard therapy and surgery has resulted in survival gains both in the first‐line setting and among patients with recurrent disease. In contrast to trials performed in the early 1980s, in which the median survival in phase 2 and 3 trials ranged from 15 to 24 months,5, 6 the median survival times published in the last decade have ranged from 36 to 40 months.7, 8
We hypothesized that this improvement in reported survival in conjunction with changes in treatment patterns and standard of care has led to challenges when estimating the expected control arm outcome in RP3 trials reported within the past decade. To test this hypothesis, a systematic literature review was conducted to assess the accuracy of control arm outcome predictions in RP3 trials of patients with advanced‐stage EOC that were published or reported from 2000 through 2010.
MATERIALS AND METHODS
Search Strategy
A search in MEDLINE and EMBASE was conducted for studies published in the English language between January 2000 and December 2010. Medical Subject Headings (MeSH) terms were “random allocation” AND “ovarian neoplasm.” Keywords were (“ovarian” AND “neoplasm”) OR “ovarian neoplasm” OR (“ovarian” and “cancer”) OR “ovarian cancer” AND (“random” AND “allocation”) OR “random allocation” OR “randomized.”
Citation lists of relevant publications were also reviewed for articles that might have been missed with the search strategy. Finally, abstracts from the annual meetings of the American Society of Clinical Oncology (ASCO) and the European Society for Medical Oncology (ESMO) from 2008 through 2010 were reviewed to include reported yet unpublished trials.
Study Selection
Trials with the following characteristics were included: RP3 clinical trials performed among patients with EOC comparing 1 systemic treatment (experimental arm) over another (control arm). Only trials in which the primary outcome was overall survival (OS), progression‐free survival (PFS), or time to disease progression (TTP) were included. Trials reporting different primary endpoints (eg, response rate, quality of life) were excluded. Trials had to state explicitly the expected outcome of the control arm used for sample size calculation (either in the publication or in an appendix) and the actual or estimated outcome of the control arm once the study was finished. Trials with a non‐inferiority design were excluded.
Data Analysis
Trials were screened for eligibility and data were collected using standardized collection forms. Data retrieved included publication details, methodological components, and trial characteristics such as sample size, interventions, and outcome measures. The expected control arm outcome used for sample size calculation, hypothesized experimental arm outcome, and finally the actual or estimated result achieved by the control arm in the trial was recorded. To allow analysis, whenever the expected or actual result was stated as a percentage of patients at a given time point, the result was transformed to a median using an exponential model. If the expected outcome of the control arm and sample size were modified at an interim analysis because of significant imprecision, the parameters used at the time of the initial trial design were used for the primary analysis. However, the revised interim parameters were collected for a separate analysis.
Statistical Analysis
A simple ratio of the actual (A) outcome of the control arm for the primary endpoint divided by the expected (E) outcome used for sample size calculation was calculated (the A/E ratio). When the expected outcome was stated as a median, a ratio of the A/E median was calculated. When the expected outcome was stated as a percentage of patients at a precise time point, this was transformed to a median result assuming an exponential distribution. For example, if the 5‐year survival rate was reported to be 40%, this was transformed to a median using the following calculation: median = (log (0.5)/log(0.4))*5 = 3.8. In this scenario, the median survival would have been reported to be 3.8 years. This allowed for the calculation of a median when only 1 survival rate was known at a certain time point. Once the median value was calculated, it was used to determine the A/E ratio.
An A/E ratio of 0.75 to 1.25 was defined as reflecting an accurate prediction. This was based on the premise that a 25% difference in the actual versus expected outcome was likely to be clinically and statistically relevant. It was believed that this degree of imprecision was sufficient to cause inaccurate initial power calculations. Thus, a ratio of >1.25 was considered as underestimation whereas a ratio of <0.75 was termed an overestimation. For analysis, studies were stratified by whether the primary endpoint was survival‐based (OS) or progression‐based (PFS or TTP).
A Wilcoxon 2‐sample test was used to compare the A/E ratios of survival‐based trials with those of progression‐based trials to assess whether one of the 2 strata tended to be more inaccurate than the other. The significance level was set at an alpha error of ≤.05.
RESULTS
Included Studies
With the described search strategy, a total of 61 RP3 trials of systemic therapy in patients with EOC were identified. Twenty‐nine trials were excluded: 19 did not explicitly state the expected control arm outcome in their methods, 4 used response rate as a primary endpoint, 3 trials had a non‐inferiority design, and 3 trials did not clearly report the primary endpoint result.9, 10, 11 A total of 32 trials met all prerequired criteria and were included in the current analysis. In addition, 3 additional unpublished trials met all eligibility criteria: 2 from the ESMO annual meeting abstracts and 1 from the ASCO annual meeting abstracts.12, 13, 14 A total of 35 trials met all prespecified criteria and were included in the analysis (Fig. 1). Of these 35 trials, 15 had OS as a primary endpoint and 20 had either PFS or TTP as a primary endpoint.
Figure 1.
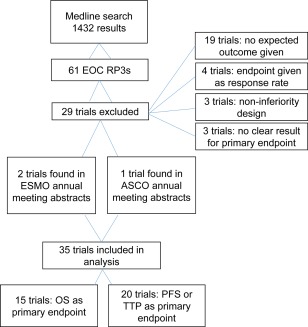
Search results for eligible phase 3 trials of epithelial ovarian cancer (EOC) performed between January 2000 and December 2010 are shown. RP3s indicates randomized phase 3 trials; ESMO, European Society for Medical Oncology; ASCO, American Society of Clinical Oncology; OS, overall survival; PFS, progression‐free survival; TTP, time to disease progression.
For 10 trials, the outcomes for the expected and/or actual primary endpoint were transformed from a percentage of patients alive at a fixed time to a median survival assuming an exponential distribution.15, 16, 17, 18, 19, 20, 21, 22, 23, 24 This was then used to calculate the A/E ratio.
Trials With a Survival‐Based Primary Endpoint
Fifteen trials with OS as a primary endpoint met all criteria (Table 1).13, 15, 16, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29 Twelve trials underestimated the actual OS as defined by an A/E ratio >1.25, whereas 3 trials were accurate in predicting OS as defined by an A/E ratio of 0.75 to 1.25. None of the trials overestimated the actual OS. The range of A/E calculated was from 1.0 to 4.7, with no trial having an A/E ratio of <1 (Fig. 2). The mean and median of all ratios was 2.0 and 1.5, respectively.
Table 1.
Actual‐Over‐Expected Ratio for Trials Using OS as a Primary Endpoint
| Trial No. | Reference | Setting | Control Arm | Experimental Arm | Expected OS for Control Arm | Actual OS | Sample Size of Control Arm | Size Effect Studied | A/E Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 1 | du Bois 200625 | First‐line | Paclitaxel and carboplatin | Paclitaxel, carboplatin, and epirubicin | 36 mo (median) | 41 mo | 635 | 1.2 | 1.1 |
| 2 | Spriggs 200726 | First‐line | Cisplatin and paclitaxel (24 h) | Cisplatin and paclitaxel (96 h) | 27 mo (median) | 29.9 mo | 140 | 1.3 | 1.1 |
| 3 | ICON Group 200220 | First‐line | Carboplatin or cyclophosphamide, doxorubicin, and cisplatin | Paclitaxel and carboplatin | 50% (2‐y OS) | 63% (2‐y OS) | 1364 | 1.1 | 1.5 |
| 4 | Bolis 201019 | First‐line | Paclitaxel and carboplatin | Paclitaxel, carboplatin, and topotecan | 20% (3‐y OS) | 53% (3‐y OS) | 172 | 1.8 | 2.5 |
| 5 | Bolis 200418 | First‐line | Cisplatin and paclitaxel (175 mg/m2) | Cisplatin and paclitaxel (225 mg/m2) | 30% (4‐y OS) | 46% (4‐y OS) | 207 | 1.3 | 1.6 |
| 6 | Ray‐Coquard 200723 | First‐line | Cyclophosphamide (500 mg/m2), epirubicin, and cisplatin | Cyclophosphamide (1800 mg/m2), epirubicin, cisplatin, and G‐CSF | 50% (2‐y OS) | 66% (2‐y OS) | 85 | 1.3 | 1.7 |
| 7 | Pfisterer 200622 | First‐line maintenance | Observation | Topotecan maintenance | 50% (3‐y OS) | 58% (3‐y OS) | 650 | 1.2 | 1.3 |
| 8 | Rustin 201024 | Recurrence | Delayed treatmenta | Early treatmenta | 5% (2‐y OS) | 53% (2‐y OS) | 264 | 3.0 | 4.7 |
| 9 | Colombo 201013 | Recurrence | Pegylated liposomal doxorubicin | Patupilone | 8.9 mo (median) | 12.7 mo | 416 | 1.3 | 1.4 |
| 10 | Vergote 200927 | Recurrence | Pegylated liposomal doxorubicin or topotecan | Canfosfamide | 6 mo (median) | 13.5 mo | 229 | 1.4 | 2.3 |
| 11 | Hall 200428 | Recurrence | Observation | Interferon | 15 mo (median) | 33 mo | 151 | 1.5 | 2.2 |
| 12 | Meier 200916 | Recurrence | Treosulfan | Topotecan | 55% at 6 mo (median, 6.7 mo) | 9.5 mo | 119 | 1.3 | 1.4 |
| 13 | Parmar 200321 | Recurrence | Any platinum‐based therapy | Paclitaxel and carboplatin | 5% (2‐y OS) | 50% (2‐y OS) | 392 | 1.2 | 4.3 |
| 14 | du Bois 200215 | Recurrence | Leuprolide | Treosulfan | 40% (6‐mo OS) (median, 4.8 mo) | 6.9 mo | 39 | 1.5 | 1.5 |
| 15 | Alberts 200829 | Recurrence | Carboplatin | Carboplatin and pegylated liposomal doxorubicin | 18 mo (median) | 18 mo | 30 | 1.3 | 1.0 |
Abbreviations: A/E, actual‐over‐expected ratio; G‐CSF, granulocyte‐colony‐stimulating factor; ICON, International Collaborative Ovarian Neoplasm Group; OS, overall survival.
Detailed is expected survival of the control arm used for statistical calculations and actual survival achieved by this arm as well as size effect expected from the experimental treatment. Finally, the actual‐over‐expected ratio for survival of the control arm is given. A/E ratios were rounded to one decimal place.
This trial was designed to determine if there was a survival benefit with early treatment of relapse based on an elevated CA125 concentration alone.
Figure 2.
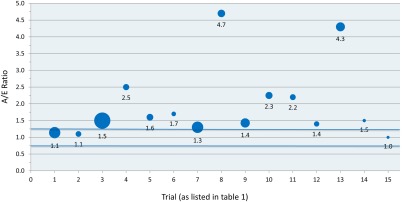
Actual‐over‐expected ratios (A/E) for trials with overall survival as a primary endpoint are shown. The size of the bubble is proportional to the sample size of the control cohort. Blue lines delineate the region between 0.75 and 1.25, termed as being an accurate estimation.
In 2 of these 15 trials, survival was severely underestimated, as reflected by A/E ratios of 4.3 and 4.7, respectively.21, 24 In both of these trials, the expected survival in the control arm was revised in interim protocol amendments. This resulted in more precise estimations (A/E ratio of 1.1 and 1.0, respectively). When using these revised parameters, the mean and median of all ratios still remained elevated at 1.5 and 1.4, respectively.
Of the 15 trials, 10 underestimated the control arm outcome by such a margin that the control arm did better than hypothesis for the experimental arm. This highlights the degree of the underestimation.
Trials With a Progression‐Based Primary Endpoint
Twenty trials met all criteria with either PFS or TTP as a primary endpoint. Four trials underestimated the actual outcome (A/E ratio of >1.25), Eleven trials were accurate (A/E ratio of 0.75‐1.25), and 5 trials overestimated the outcome (A/E ratio of 0.75) (Table 2).12, 14, 17, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 The A/E ratios ranged from 0.5 to 1.6, with a mean and median ratio of 1.0 and 1.0, respectively (Fig. 3).
Table 2.
Actual‐Over‐Expected Ratio for Trials Using PFS or TTP as a Primary Endpoint
| Trial No. | Reference | Setting | Control Arm | Experimental Arm | Expected PFS in Control Arm) | Actual PFS | Sample Size in Control Arm | Size Effect | A/E Ratio |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Katsumata 200930 | First‐line | Paclitaxel and carboplatin | Dose‐dense paclitaxel and carboplatin | 16 mo (median) | 17.2 mo | 320 | 1.4 | 1.1 |
| 2 | Markman 200931 | First‐line maintenance | Paclitaxel management (3 cycles) | Paclitaxel maintenance (12 cycles) | 20 mo (median) | 14 mo | 128 | 1.3 | 0.7 |
| 3 | Burger 201012 | First‐line | Paclitaxel and carboplatin | Paclitaxel, carboplatin, and bevacizumab | 14 mo (median) | 10 mo | 625 | 1.3 | 0.7 |
| 4 | Perren 201014 | First‐line | Paclitaxel and carboplatin | Paclitaxel, carboplatin, and bevacizumab | 18 mo (median) | 16 mo | 764 | 1.3 | 0.9 |
| 5 | Pfisterer 200632 | Recurrence | Carboplatin | Carboplatin and gemcitabine | 6 mo (median) | 5.8 mo | 178 | 1.4 | 1.0 |
| 6 | Monk 201033 | Recurrence | Pegylated liposomal doxorubicin | Pegylated liposomal doxorubicin and trabectedin | 3.7 mo (median) | 5.8 mo | 335 | 1.3 | 1.6 |
| 7 | Neijt 200034 | First‐line | Paclitaxel and cisplatin | Paclitaxel and carboplatin | 12 mo (median) | 16 mo | 108 | 1.7 | 1.3 |
| 8 | Vasey 200435 | First‐line | Paclitaxel and carboplatin | Docetaxel and carboplatin | 17 mo (median) | 14.8 mo | 538 | 1.25 | 0.9 |
| 9 | Papadimitriou 200836 | First‐line | No further treatment | High‐dose melphalan | 18 mo (median)a | 18 moa | 43 | 2.0 | 1.0 |
| 10 | Bookman 200937 | First‐line | Paclitaxel and carboplatin | Paclitaxel, carboplatin and a 3rd agentb | 15 mo (median) | 16 mo | 864 | 1.25 | 1.1 |
| 11 | Pecorelli 200938 | First‐line maintenance | Paclitaxel and carboplatin | Paclitaxel, carboplatin and paclitaxel management | 50% (2‐y PFS) | 54% (2‐y PFS) | 99 | 1.3 | 1.1 |
| 12 | Hoskins 201039 | First‐line | Paclitaxel and carboplatin | Sequential cisplatin and topotecan and paclitaxel and carboplatin | 16 mo (median) | 16.2 mo | 410 | 1.25 | 1.0 |
| 13 | Lhomme 200840 | First‐line | Paclitaxel and carboplatin | Paclitaxel, carboplatin, and valspodar | 18 mo (median)a | 13.5 moa | 377 | 1.3 | 0.8 |
| 14 | Hirte 200641 | First‐line maintenance | Observation | Tanomastat | 20 mo (median) | 9.2 mo | 121 | 1.4 | 0.5 |
| 15 | De Placido 200442 | First‐line maintenance | Observation | Topotecan | 18 mo (median) | 28 mo | 93 | 1.5 | 1.6 |
| 16 | Ferrandina 200843 | Recurrence | Pegylated liposomal doxorubicin | Gemcitabine | 2.8 mo (median)a | 3.7 moa | 76 | 1.6 | 1.3 |
| 17 | Berek 200444 | First‐line maintenance | Observation | Oregovomab | 18 mo (median)c | 10.8 moc | 72 | 1.5 | 0.6 |
| 18 | Mobus 200717 | First‐line | Paclitaxel and carboplatin ± etoposide | High‐dose chemotherapyd |
0.35 (2‐y PFS) 16.8 mo (median) |
20.5 mo | 89 | 1.4 | 1.1 |
| 19 | Vergote 201045 | Recurrence | Pegylated liposomal dxorubicin | Canfosfamide and pegylated liposomal doxorubicin | 3.5 mo | 3.7 mo | 60 | 1.5 | 1.1 |
| 20 | Reed 200646 | First‐line | Treosulfan | Carboplatin | 9.2 mo | 5.0 mo | 102 | 1.5 | 0.5 |
Abbreviations: A/E, actual‐over‐expected ratio; PFS, progression‐free survival; TTP, time to disease progression.
Detailed is expected PFS/TTP of the control arm used for statistical calculations, actual PFS/TTP achieved by this arm as well as size effect expected from the experimental treatment. Finally A/E ratio for PFS/TTP of the control arm is given. A/E ratios were rounded to one decimal place.
Used TTP as progression measure.
Third chemotherapy agent included gemcitabine, pegylated liposomal doxorubicin, or topotecan.
Used time to disease recurrence as a progression measure.
High‐dose chemotherapy includes 2 cycles of fortnightly paclitaxel and cyclophosphamide with peripheral blood stem cell harvest followed by 3 cycles of carboplatin and paclitaxel with melphalan included in the final cycle. Both arms have the option of etoposide and up to 4 cycles of maintenance topotecan.
Figure 3.
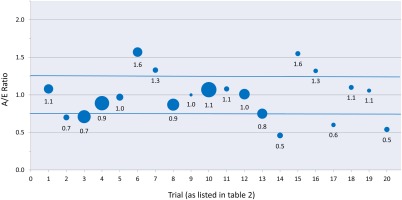
Actual‐over‐expected ratios (A/E) for trials using progression‐free survival and time to disease progression as a primary endpoint are shown. The size of the bubble is proportional to the sample size of the control cohort. Blue lines delineate the region between 0.75 and 1.25, termed as being an accurate estimation of the endpoint.
Comparison of the Accuracy of Predictions of Survival‐Based And Progression‐Based Endpoints
When compared with the 20 trials with a progression‐based primary outcome, the 15 trials with OS as a primary endpoint were found to be significantly more likely to underestimate the control arm outcome (Wilcoxon 2‐sample test, P<.001).
To account for potential random error introduced by the inclusion of trials with small sample sizes, the 2 groups were compared again after excluding those in which the control group sample size was <100 patients. A total of 3 and 7 studies were excluded from the OS15, 23, 29 and PFS17, 36, 38, 42, 43, 44, 45 analyses, respectively. This confirmed a statistically significant difference (P = .001). A secondary analysis was performed to account for the 2 trials in which the expected survival was updated at an interim analysis.21, 24 Even with the revised expected survival, there was statistically more underestimation noted among survival‐based trials than progression‐based trials (P = .002).
DISCUSSION
The results of the current study demonstrate that for EOC trials published over the past decade there has been significant imprecision when estimating the control arm outcome in RP3 trials. This imprecision is present in trials with both progression‐based and survival‐based primary endpoints. However the data presented herein indicate that trials with an OS endpoint were significantly more likely to underestimate the outcome of the control arm than those using PFS or TTP as the endpoint.
A limitation of the current review is that relatively few RP3 trials met all eligibility criteria. Approximately one‐half of the EOC RP3 trials were excluded, in most instances because of missing information regarding sample size calculations. This finding is perhaps not surprising because it has previously been reported that information regarding sample size calculation is frequently missing in clinical trial publications.47 A further limitation is that some of the trials included had a small sample size, sometimes as a result of poor accrual, and as such are subject to random error. To minimize the potential impact of this confounding factor, a sensitivity analysis was performed excluding those trials with a small sample size. This demonstrated similar results to those observed in the analysis of all trials.
It is important to consider why OS was significantly underestimated when designing systemic therapy trials in EOC. It is possible that changing patterns of care and improvements in survival for patients with EOC during the years these trials were designed and conducted may have rendered historical data obsolete, causing investigators to underestimate the outcome anticipated with standard therapy. Improved surgical techniques and consequent stage migration may also have had an impact on OS estimations.
The time between the initial trial design and the completion of accrual typically spans many years. After the completion of accrual, more time elapses until enough events have occurred to analyze survival. Thus, even when factoring in differences in patterns of care between reported historical data and available care at the moment of study design, it is possible that evolving therapy during the conduct of the trial may further confound these estimations.
It should be noted that since 1990, paclitaxel, gemcitabine, and pegylated liposomal doxorubicin have demonstrated efficacy in patients with EOC and have been approved for treatment.32, 39, 48 Most recently, antiangiogenic agents have demonstrated effectiveness in EOC clinical trials.7, 8 The progressive introduction of these agents in trials and in routine care during the conduct of the majority of the EOC trials published within the last decade may well have caused actual survival to deviate from historical controls.
Moreover, for many of the clinical trials included in the current analysis, the experimental agents studied (eg, topotecan, anthracyclines, and gemcitabine) were commercially available either during or after the study was conducted, thereby raising the potential for off‐trial crossover with the experimental agent in a percentage of patients, further confounding, and possibly increasing observed OS in control arms.
As a consequence of the small sample size, our ability to delineate whether underestimation of OS is becoming more problematic with time is hindered, but one would expect this to be the case, reflecting increasing therapeutic options after disease progression and a longer time to accrue patients to trials. This issue is likely to be clinically relevant in both the first‐line and recurrent setting and should be considered in the design of future research.
It is perhaps not surprising that such a prominent underestimation of outcome is not observed when trials use a progression‐based endpoint. In contrast to PFS, OS is a composite of both PFS and survival after disease progression.49 Time from treatment initiation until either disease recurrence or progression is not influenced by post‐trial treatment nor crossover, making historical publications regarding the efficacy of a single treatment more reliable than estimates of survival that reflect a sequence of treatments that dynamically evolve as new drugs become available.
When patients with metastatic disease such as ovarian cancer develop disease progression, there are several potential interventions available including: 1) crossover; 2) treatment with an alternative agent; 3) continuation with the same agent if there is symptomatic benefit; or 4) no further therapy.49 The heterogeneity of these options makes it difficult to assess the influence (if any) of the initial randomized therapy on OS due to the confounding and diluting effect from each subsequent intervention.49 Fewer variables come into play when estimating TTP or PFS than when estimating time to death. Moreover, the time to the event is shorter, thereby leading to more predictable estimates.
The finding that OS has been underestimated when designing EOC trials has potentially important implications both for interpreting recently published trials and for designing future trials. A significant underestimation of the anticipated control arm outcome means that the observed event rate will be lower than anticipated during the time the trial is being conducted. Because sample size is proportional to the square power of the difference in the event rate between the control and experimental arms, the trials become underpowered to detect a difference of the magnitude they were designed for. As the event rate decreases, the sample size will need to increase exponentially to demonstrate the same absolute difference in survival. Table 3 illustrates how relatively small changes in observed outcome and relative size effect studied affect the sample size required to maintain adequate statistical power to demonstrate the same benefit.
Table 3.
Theoretical Scenarios to Illustrate How Variations in Survival of the Control Cohort Impact on the Sample Size Required to Maintain Statistical Power
| Scenario | A/E Ratio | Expected Outcome of Control Arm | Size Effect Studied | Absolute Benefit in Survival to Demonstrate the Same Benefit | Expected Outcome of Experimental Arm | Sample Size Required |
|---|---|---|---|---|---|---|
| Initial trial design | Not applicable | 24 mo | 1.25 | 6 mo | 30 mo | 455 patients per arm |
| No. of patients to demonstrate same maintenance of absolute benefit | 1.5 | 36 mo | 1.166 | 6 mo | 40 mo | 1685 patients per arm |
| No. of patients to demonstrate maintenance of effect size | 1.5 | 36 mo | 1.25 | 9 mo | 45 mo | 495 patients per arm |
Abbreviation: A/E ratio, actual‐over‐expected ratio.
Calculations were made assuming an accrual rate of 20 patients per month and a follow‐up time of 24 months for the first scenario and 36 months for the latter 2 scenarios.
Discrepancies between the expected and actual outcome of the control arm can potentially result in clinically relevant survival differences being missed because of a lack of sufficient statistical power. When the result of an endpoint is underestimated owing to lower event rates, the trial duration is likely to be longer and more expensive than anticipated. Consequently, it is concerning that EOC trials published within the past decade were potentially underpowered to demonstrate the magnitude of absolute survival benefit they were designed for despite being longer and potentially more costly.
The difficulty in predicting and detecting OS improvements has significant ramifications for regulatory agencies that rely on these trials and the accurate determination of the size of the benefit to make funding decisions. Furthermore, the underestimation of OS in most EOC trials published to date highlights the challenges in adequately designing trials that are powered to demonstrate differences in survival due to the number of variables requiring consideration. These challenges may be more problematic in malignancies with a longer survival and many available treatment options, such as EOC, low‐grade lymphomas, or breast cancer, compared with those with limited treatment options and short survival times such as metastatic pancreatic or lung cancer. In contrast, among diseases in which the median survival after disease progression has been classically shorter (ie, <12 months), such as advanced colorectal cancer and non‐small lung cancer, a stronger correlation between PFS and OS has been demonstrated.49, 50, 51 This may mean that OS is easier to predict in these diseases. Data regarding the accuracy of trial survival predictions in other disease sites need to be collected to formerly test this hypothesis. However, this report highlights that when designing a trial that is properly powered to address differences in OS in cancers with many treatment options, correctly estimating the control arm outcome can be challenging.
The data from the current study highlight the difficulty inherent in estimating the actual outcome of a cohort of patients. In EOC, this has led to an almost routine underestimation of expected survival in recently reported RP3 trials. These challenges should be addressed when designing future phase 3 trials in EOC as well as other malignancies. Severely underestimating the control arm outcome can lead to a trial being more complex and expensive to conduct than initially planned but remaining statistically underpowered to demonstrate the clinically meaningful survival difference it was designed to detect.
FUNDING SUPPORT
No specific funding was disclosed.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
See editorial on pages 335–8, this issue.
REFERENCES
- 1. Schulz KF, Grimes DA. Sample size calculations in randomised trials: mandatory and mystical. Lancet. 2005;365:1348–1353. [DOI] [PubMed] [Google Scholar]
- 2. Green SB. Hypothesis testing in clinical trials. Hematol Oncol Clin North Am. 2000;14:785‐795, vii‐viii. [DOI] [PubMed] [Google Scholar]
- 3. Wittes J. Sample size calculations for randomized controlled trials. Epidemiol Rev. 2002;24:39‐53. [DOI] [PubMed] [Google Scholar]
- 4. Lee CK, Lord SJ, Stockler MR, et al. Historical cross‐trial comparisons for competing treatments in advanced breast cancer–an empirical analysis of bias. Eur J Cancer. 2010;46:541‐548. [DOI] [PubMed] [Google Scholar]
- 5. Omura GA, Morrow CP, Blessing JA, et al. A randomized comparison of melphalan versus melphalan plus hexamethylmelamine versus adriamycin plus cyclophosphamide in ovarian carcinoma. Cancer. 1983;51:783‐789. [DOI] [PubMed] [Google Scholar]
- 6. Omura G, Blessing JA, Ehrlich CE, et al. A randomized trial of cyclophosphamide and doxorubicin with or without cisplatin in advanced ovarian carcinoma. A Gynecologic Oncology Group Study. Cancer. 1986;57:1725‐1730. [DOI] [PubMed] [Google Scholar]
- 7. Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365:2473‐2483. [DOI] [PubMed] [Google Scholar]
- 8. Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. 2011;365:2484‐2496. [DOI] [PubMed] [Google Scholar]
- 9. Alberts DS, Hannigan EV, Liu PY, et al. Randomized trial of adjuvant intraperitoneal alpha‐interferon in stage III ovarian cancer patients who have no evidence of disease after primary surgery and chemotherapy: an intergroup study. Gynecol Oncol. 2006;100:133‐138. [DOI] [PubMed] [Google Scholar]
- 10. Alberts DS, Marth C, Alvarez RD, et al; GRACES Clinical Trial Consortium . Randomized phase 3 trial of interferon gamma‐1b plus standard carboplatin/paclitaxel versus carboplatin/paclitaxel alone for first‐line treatment of advanced ovarian and primary peritoneal carcinomas: results from a prospectively designed analysis of progression‐free survival. Gynecol Oncol. 2008;109:174‐181. [DOI] [PubMed] [Google Scholar]
- 11. Piccart MJ, Floquet A, Scarfone G, et al. Intraperitoneal cisplatin versus no further treatment: 8‐year results of EORTC 55875, a randomized phase III study in ovarian cancer patients with a pathologically complete remission after platinum‐based intravenous chemotherapy. Int J Gynecol Cancer. 2003;13(suppl 2):196‐203. [DOI] [PubMed] [Google Scholar]
- 12. Burger RA, Brady MF, Bookman MA, et al. Phase III trial of bevacizumab (BEV) in the primary treatment of advanced epithelial ovarian cancer (EOC), primary peritoneal cancer (PPC), or fallopian tube cancer (FTC): a Gynecologic Oncology Group study. Presented at the American Society of Clinical Oncology Annual Meeting; June 4–8, 2010; Chicago, IL.
- 13. Colombo N, Schwartz P, Bamias A, et al. Results of a randomized, open‐label, phase III trial of patupilone (P) versus pegylated liposomal doxorubicin (PLD) in taxane/platinum refractory/resistant patients with recurrent ovarian, fallopian, or peritoneal cancer. Presented at the European Society of Medical Oncology Annual Meeting; October 8–12, 2010; Milan, Italy.
- 14. Perren TJ, Swart AM, Pfisterer J, et al. ICON7: A phase III randomised gynaecologic cancer intergroup trial of concurrent bevacizumab and chemotherapy followed by maintenance bevacizumab, versus chemotherapy alone in women with newly diagnosed epithelial ovarian, primary peritoneal or fallopian tube cancer. Presented at the European Society of Medical Oncology Annual Meeting; October 8–12, 2010; Milan, Italy.
- 15. du Bois A, Meier W, Luck HJ, et al. Chemotherapy versus hormonal treatment in platinum‐ and paclitaxel‐refractory ovarian cancer: a randomised trial of the German Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) Study Group Ovarian Cancer. Ann Oncol. 2002;13:251‐257. [DOI] [PubMed] [Google Scholar]
- 16. Meier W, du Bois A, Reuss A, et al. Topotecan versus treosulfan, an alkylating agent, in patients with epithelial ovarian cancer and relapse within 12 months following 1st‐line platinum/paclitaxel chemotherapy. A prospectively randomized phase III trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group (AGO‐OVAR). Gynecol Oncol. 2009;114:199‐205. [DOI] [PubMed] [Google Scholar]
- 17. Mobus V, Wandt H, Frickhofen N, et al; AGO‐Ovar/AIO ; EBMT . Phase III trial of high‐dose sequential chemotherapy with peripheral blood stem cell support compared with standard dose chemotherapy for first‐line treatment of advanced ovarian cancer: intergroup trial of the AGO‐Ovar/AIO and EBMT. J Clin Oncol. 2007;25:4187‐4193. [DOI] [PubMed] [Google Scholar]
- 18. Bolis G, Scarfone G, Polverino G, et al. Paclitaxel 175 or 225 mg per meters squared with carboplatin in advanced ovarian cancer: a randomized trial. J Clin Oncol. 2004;22:686‐690. [DOI] [PubMed] [Google Scholar]
- 19. Bolis G, Scarfone G, Raspagliesi F, et al. Paclitaxel/carboplatin versus topotecan/paclitaxel/carboplatin in patients with FIGO suboptimally resected stage III‐IV epithelial ovarian cancer a multicenter, randomized study. Eur J Cancer. 2010;46:2905‐2912. [DOI] [PubMed] [Google Scholar]
- 20. International Collaborative Ovarian Neoplasm Group . Paclitaxel plus carboplatin versus standard chemotherapy with either single‐agent carboplatin or cyclophosphamide, doxorubicin, and cisplatin in women with ovarian cancer: the ICON3 randomised trial. Lancet. 2002;360:505‐515. [DOI] [PubMed] [Google Scholar]
- 21. Parmar MK, Ledermann JA, Colombo N, et al. Paclitaxel plus platinum‐based chemotherapy versus conventional platinum‐based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO‐OVAR‐2.2 trial. Lancet. 2003;361:2099‐2106. [DOI] [PubMed] [Google Scholar]
- 22. Pfisterer J, Weber B, Reuss A, et al; AGO‐OVAR ; GINECO . Randomized phase III trial of topotecan following carboplatin and paclitaxel in first‐line treatment of advanced ovarian cancer: a gynecologic cancer intergroup trial of the AGO‐OVAR and GINECO. J Natl Cancer Inst. 2006;98:1036‐1045. [DOI] [PubMed] [Google Scholar]
- 23. Ray‐Coquard I, Paraiso D, Guastalla JP, et al. Intensified dose of cyclophosphamide with G‐CSF support versus standard dose combined with platinum in first‐line treatment of advanced ovarian cancer a randomised study from the GINECO group. Br J Cancer. 2007;97:1200‐1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rustin GJ, van der Burg ME, Griffin CL, et al. Early versus delayed treatment of relapsed ovarian cancer (MRC OV05/EORTC 55955): a randomised trial. Lancet. 2010;376:1155‐1163. [DOI] [PubMed] [Google Scholar]
- 25. du Bois A, Weber B, Rochon J, et al; Arbeitsgemeinschaft Gynaekologische Onkologie ; Ovarian Cancer Study Group ; Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens . Addition of epirubicin as a third drug to carboplatin‐paclitaxel in first‐line treatment of advanced ovarian cancer: a prospectively randomized gynecologic cancer intergroup trial by the Arbeitsgemeinschaft Gynaekologische Onkologie Ovarian Cancer Study Group and the Groupe d'Investigateurs Nationaux pour l'Etude des Cancers Ovariens. J Clin Oncol. 2006;24:1127‐1135. [DOI] [PubMed] [Google Scholar]
- 26. Spriggs DR, Brady MF, Vaccarello L, et al. Phase III randomized trial of intravenous cisplatin plus a 24‐ or 96‐hour infusion of paclitaxel in epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:4466‐4471. [DOI] [PubMed] [Google Scholar]
- 27. Vergote I, Finkler N, del Campo J, et al. Phase 3 randomised study of canfosfamide (Telcyta, TLK286) versus pegylated liposomal doxorubicin or topotecan as third‐line therapy in patients with platinum‐refractory or ‐resistant ovarian cancer. Eur J Cancer. 2009;45:2324‐2332. [DOI] [PubMed] [Google Scholar]
- 28. Hall GD, Brown JM, Coleman RE, et al. Maintenance treatment with interferon for advanced ovarian cancer: results of the Northern and Yorkshire gynaecology group randomised phase III study. Br J Cancer. 2004;91:621‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alberts DS, Liu PY, Wilczynski SP, et al; Southwest Oncology Group . Randomized trial of pegylated liposomal doxorubicin (PLD) plus carboplatin versus carboplatin in platinum‐sensitive (PS) patients with recurrent epithelial ovarian or peritoneal carcinoma after failure of initial platinum‐based chemotherapy (Southwest Oncology Group Protocol S0200). Gynecol Oncol. 2008;108:90‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Katsumata N, Yasuda M, Takahashi F, et al. Dose‐dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open‐label, randomised controlled trial. Lancet. 2009;374:1331‐1338. [DOI] [PubMed] [Google Scholar]
- 31. Markman M, Liu PY, Moon J, et al. Impact on survival of 12 versus 3 monthly cycles of paclitaxel (175 mg/m2) administered to patients with advanced ovarian cancer who attained a complete response to primary platinum‐paclitaxel: follow‐up of a Southwest Oncology Group and Gynecologic Oncology Group phase 3 trial. Gynecol Oncol. 2009;114:195‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pfisterer J, Plante M, Vergote I, et al; AGO‐OVAR ; NCIC CTG ; EORTC GCG . Gemcitabine plus carboplatin compared with carboplatin in patients with platinum‐sensitive recurrent ovarian cancer: an intergroup trial of the AGO‐OVAR, the NCIC CTG, and the EORTC GCG. J Clin Oncol. 2006;24:4699‐4707. [DOI] [PubMed] [Google Scholar]
- 33. Monk BJ, Herzog TJ, Kaye SB, et al. Trabectedin plus pegylated liposomal doxorubicin in recurrent ovarian cancer. J Clin Oncol. 2010;28:3107‐3114. [DOI] [PubMed] [Google Scholar]
- 34. Neijt JP, Engelholm SA, Tuxen MK, et al. Exploratory phase III study of paclitaxel and cisplatin versus paclitaxel and carboplatin in advanced ovarian cancer. J Clin Oncol. 2000;18:3084‐3092. [DOI] [PubMed] [Google Scholar]
- 35. Vasey PA, Jayson GC, Gordon A, et al. Phase III randomized trial of docetaxel‐carboplatin versus paclitaxel‐carboplatin as first‐line chemotherapy for ovarian carcinoma. J Natl Cancer Inst. 2004;96:1682‐1691. [DOI] [PubMed] [Google Scholar]
- 36. Papadimitriou C, Dafni U, Anagnostopoulos A, et al. High‐dose melphalan and autologous stem cell transplantation as consolidation treatment in patients with chemosensitive ovarian cancer: results of a single‐institution randomized trial. Bone Marrow Transplant. 2008;41:547‐554. [DOI] [PubMed] [Google Scholar]
- 37. Bookman MA, Brady MF, McGuire WP, et al. Evaluation of new platinum‐based treatment regimens in advanced‐stage ovarian cancer: a Phase III Trial of the Gynecologic Cancer Intergroup. J Clin Oncol. 2009;27:1419‐1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pecorelli S, Favalli G, Gadducci A, et al; After 6 Italian Cooperative Group . Phase III trial of observation versus 6 courses of paclitaxel in patients with advanced epithelial ovarian cancer in complete response after 6 courses of paclitaxel/platinum‐based chemotherapy: final results of the After‐6 protocol 1. J Clin Oncol. 2009;27:4642‐4648. [DOI] [PubMed] [Google Scholar]
- 39. Hoskins P, Vergote I, Cervantes A, et al. Advanced ovarian cancer: phase III randomized study of sequential cisplatin‐topotecan and carboplatin‐paclitaxel vs carboplatin‐paclitaxel. J Natl Cancer Inst. 2010;102:1547‐1556. [DOI] [PubMed] [Google Scholar]
- 40. Lhomme C, Joly F, Walker JL, et al. Phase III study of valspodar (PSC 833) combined with paclitaxel and carboplatin compared with paclitaxel and carboplatin alone in patients with stage IV or suboptimally debulked stage III epithelial ovarian cancer or primary peritoneal cancer. J Clin Oncol. 2008;26:2674‐2682. [DOI] [PubMed] [Google Scholar]
- 41. Hirte H, Vergote IB, Jeffrey JR, et al. A phase III randomized trial of BAY 12‐9566 (tanomastat) as maintenance therapy in patients with advanced ovarian cancer responsive to primary surgery and paclitaxel/platinum containing chemotherapy: a National Cancer Institute of Canada Clinical Trials Group Study. Gynecol Oncol. 2006;102:300‐308. [DOI] [PubMed] [Google Scholar]
- 42. De Placido S, Scambia G, Di Vagno G, et al. Topotecan compared with no therapy after response to surgery and carboplatin/paclitaxel in patients with ovarian cancer: Multicenter Italian Trials in Ovarian Cancer (MITO‐1) randomized study. J Clin Oncol. 2004;22:2635‐2642. [DOI] [PubMed] [Google Scholar]
- 43. Ferrandina G, Ludovisi M, Lorusso D, et al. Phase III trial of gemcitabine compared with pegylated liposomal doxorubicin in progressive or recurrent ovarian cancer. J Clin Oncol. 2008;26:890‐896. [DOI] [PubMed] [Google Scholar]
- 44. Berek JS, Taylor PT, Gordon A, et al. Randomized, placebo‐controlled study of oregovomab for consolidation of clinical remission in patients with advanced ovarian cancer. J Clin Oncol. 2004;22:3507‐3516. [DOI] [PubMed] [Google Scholar]
- 45. Vergote I, Finkler NJ, Hall JB, et al. Randomized phase III study of canfosfamide in combination with pegylated liposomal doxorubicin compared with pegylated liposomal doxorubicin alone in platinum‐resistant ovarian cancer. Int J Gynecol Cancer. 2010;20:772‐780. [DOI] [PubMed] [Google Scholar]
- 46. Reed NS, Poole CJ, Coleman R, et al. A randomised comparison of treosulfan and carboplatin in patients with ovarian cancer: a study by the Scottish Gynaecological Cancer Trials Group (SGCTG). Eur J Cancer. 2006;42:179‐185. [DOI] [PubMed] [Google Scholar]
- 47. Charles P, Giraudeau B, Dechartres A, Baron G, Ravaud P. Reporting of sample size calculation in randomised controlled trials: review. BMJ. 2009;338:b1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gordon AN, Tonda M, Sun S, et al. Long‐term survival advantage for women treated with pegylated liposomal doxorubicin compared with topotecan in a phase 3 randomized study of recurrent and refractory epithelial ovarian cancer. Gynecol Oncol. 2004;95:1‐8. [DOI] [PubMed] [Google Scholar]
- 49. Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression‐free survival. J Natl Cancer Inst. 2009;101:1642‐1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Foster NR, Qi Y, Shi Q, et al. Tumor response and progression‐free survival as potential surrogate endpoints for overall survival in extensive stage small‐cell lung cancer: findings on the basis of North Central Cancer Treatment Group trials. Cancer. 2011;117:1262‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buyse M, Burzykowski T, Carroll K, et al. Progression‐free survival is a surrogate for survival in advanced colorectal cancer. J Clin Oncol. 2007;25:5218‐5224. [DOI] [PubMed] [Google Scholar]


