Abstract
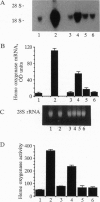
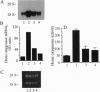
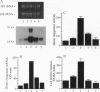
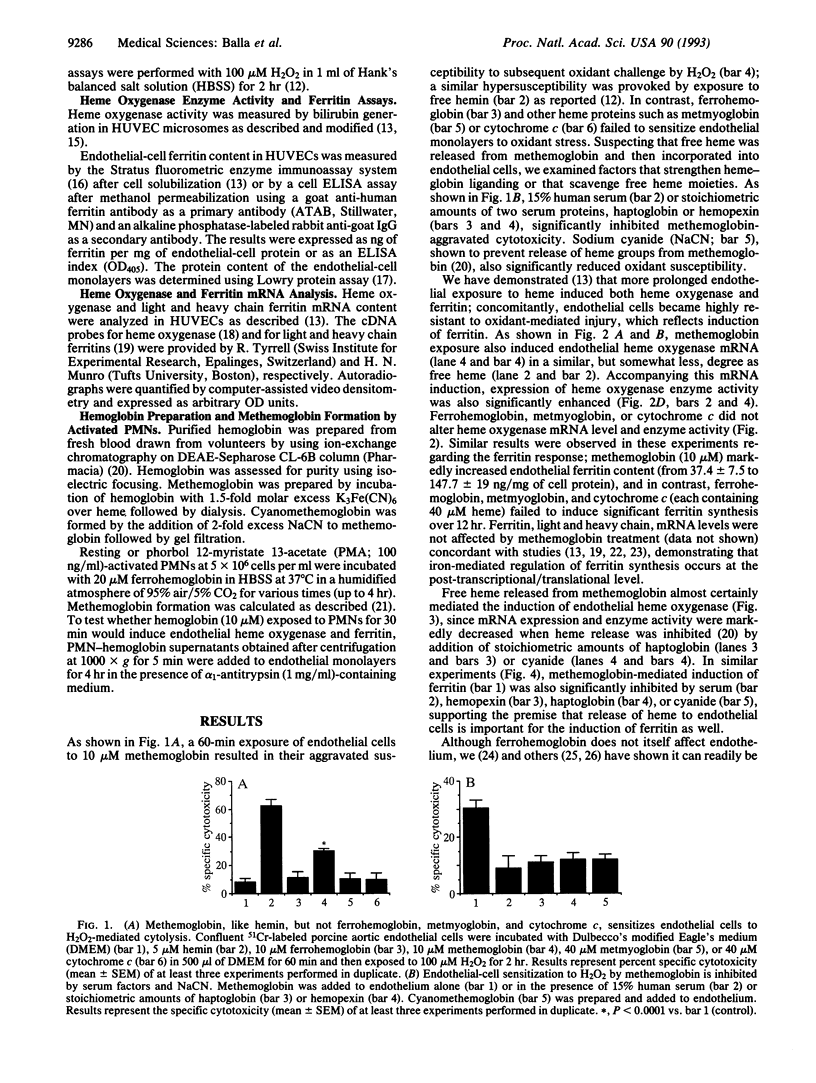
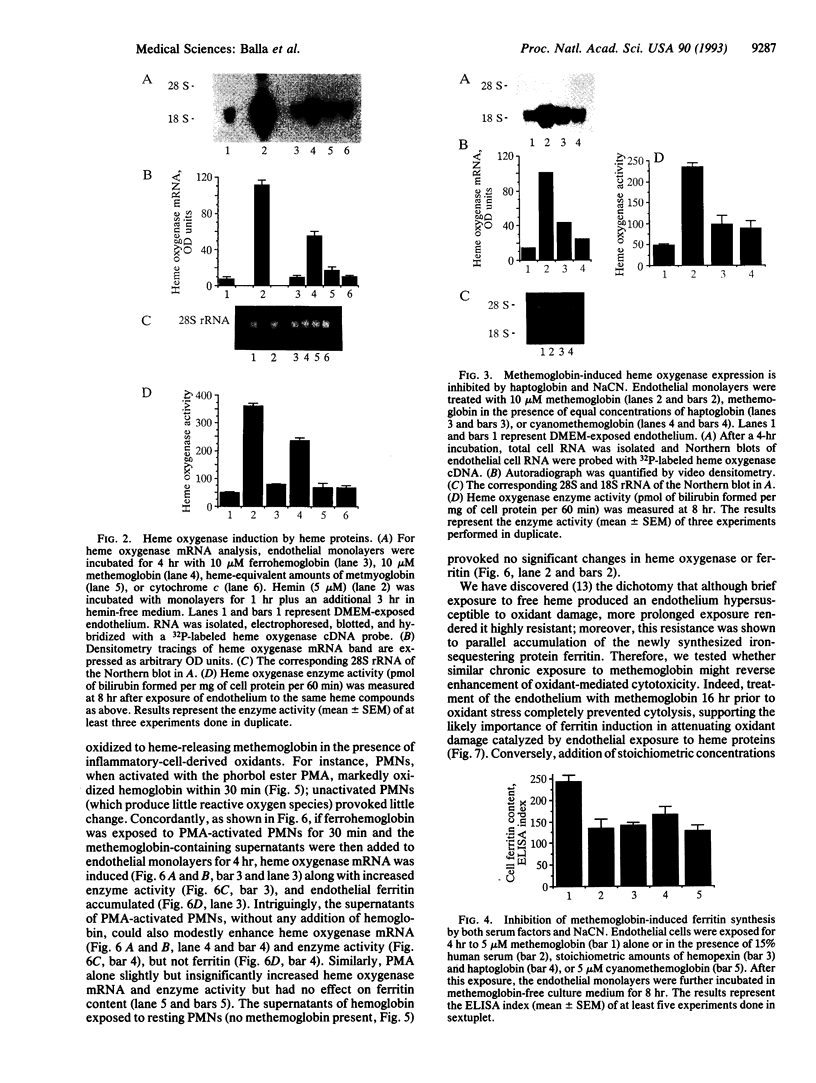
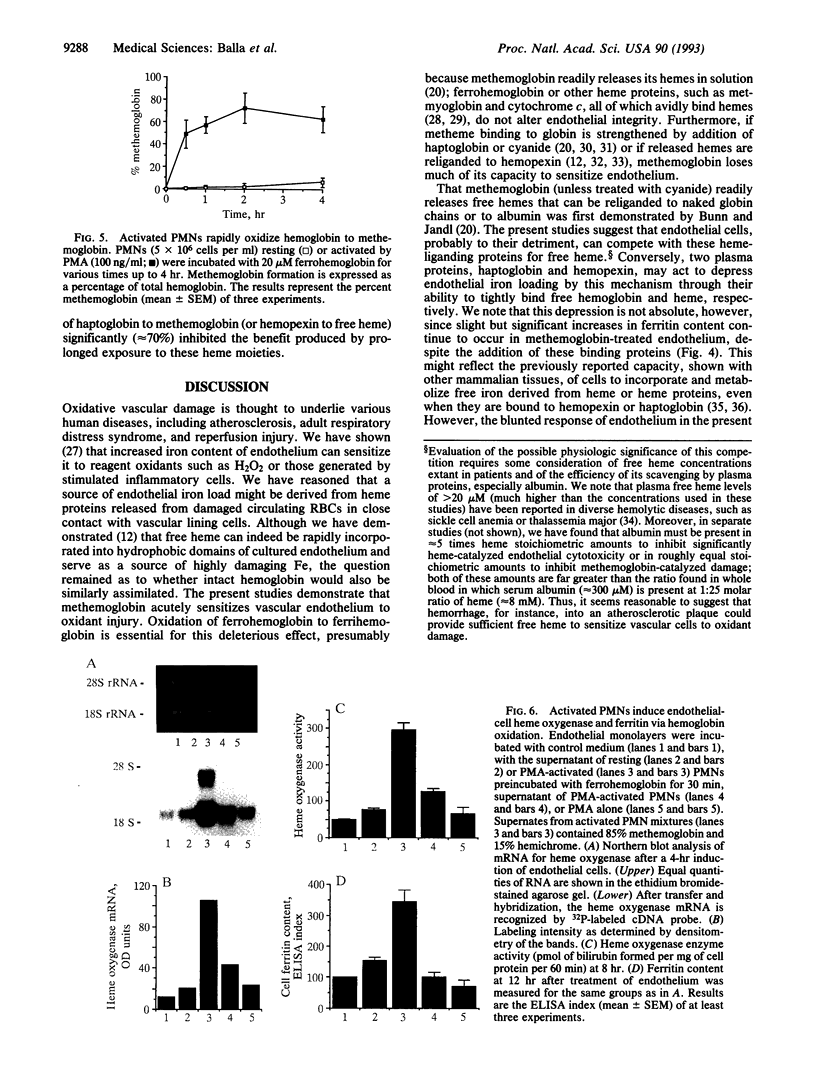
Iron-derived reactive oxygen species are implicated in the pathogenesis of various vascular disorders including atherosclerosis, vasculitis, and reperfusion injury. The present studies examine whether heme, when liganded to physiologically relevant proteins as in hemoglobin, can provide potentially damaging iron to intact endothelium. We demonstrate that reduced ferrohemoglobin, while relatively innocuous to cultured endothelial cells, when oxidized to ferrihemoglobin (methemoglobin), greatly amplifies oxidant (H2O2)-mediated endothelial-cell injury. Drawing upon our previous observation that free heme similarly primes endothelium for oxidant damage, we posited that methemoglobin, but not ferrohemoglobin, releases its hemes that can then be incorporated into endothelial cells. In support, cultured endothelial cells exposed to methemoglobin--in contrast to exposure to ferrohemoglobin, cytochrome c, or metmyoglobin--rapidly increased their heme oxygenase mRNA and enzyme activity, thereby supporting heme uptake; ferritin production was also markedly increased after such exposure, thus attesting to eventual incorporation of Fe. These cellular methemoglobin effects were inhibited by the heme-scavenging protein hemopexin and by haptoglobin or cyanide, agents that strengthen the liganding between heme and globin. If the endothelium is exposed to methemoglobin for a more prolonged period (16 hr), it accumulates large amounts of ferritin; concomitantly, and presumably associated with iron sequestration by this protein, the endothelium converts from hypersusceptible to hyperresistant to oxidative damage. We conclude that when oxidation of hemoglobin facilitates release of its heme groups, catalytically active iron is provided to neighboring tissue environments. The effect of this relinquished heme on the vasculature is determined both by extracellular factors--i.e., plasma proteins, such as haptoglobin and hemopexin--as well as intracellular factors, including heme oxygenase and ferritin. Acutely, if both extra- and intracellular defenses are overwhelmed, cellular toxicity arises; chronically, when ferritin is induced, resistance to oxidative injury may supervene.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam J., Smith A. Heme-hemopexin-mediated induction of metallothionein gene expression. J Biol Chem. 1992 Aug 15;267(23):16379–16384. [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Balla J., Rosenberg M., Nath K., Apple F., Eaton J. W., Vercellotti G. M. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992 Sep 5;267(25):18148–18153. [PubMed] [Google Scholar]
- Balla G., Jacob H. S., Eaton J. W., Belcher J. D., Vercellotti G. M. Hemin: a possible physiological mediator of low density lipoprotein oxidation and endothelial injury. Arterioscler Thromb. 1991 Nov-Dec;11(6):1700–1711. doi: 10.1161/01.atv.11.6.1700. [DOI] [PubMed] [Google Scholar]
- Balla G., Vercellotti G. M., Eaton J. W., Jacob H. S. Iron loading of endothelial cells augments oxidant damage. J Lab Clin Med. 1990 Oct;116(4):546–554. [PubMed] [Google Scholar]
- Balla G., Vercellotti G. M., Muller-Eberhard U., Eaton J., Jacob H. S. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991 May;64(5):648–655. [PubMed] [Google Scholar]
- Bunn H. F., Jandl J. H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J Biol Chem. 1968 Feb 10;243(3):465–475. [PubMed] [Google Scholar]
- Dallegri F., Ballestrero A., Frumento G., Patrone F. Augmentation of neutrophil-mediated erythrocyte lysis by cells derived in vitro from human monocytes. Blood. 1987 Dec;70(6):1743–1749. [PubMed] [Google Scholar]
- Eisenstein R. S., Garcia-Mayol D., Pettingell W., Munro H. N. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Gebicki J., Puhl H., Jürgens G. The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med. 1992 Oct;13(4):341–390. doi: 10.1016/0891-5849(92)90181-f. [DOI] [PubMed] [Google Scholar]
- Giegel J. L., Brotherton M. M., Cronin P., D'Aquino M., Evans S., Heller Z. H., Knight W. S., Krishnan K., Sheiman M. Radial partition immunoassay. Clin Chem. 1982 Sep;28(9):1894–1898. [PubMed] [Google Scholar]
- Glagov S., Zarins C., Giddens D. P., Ku D. N. Hemodynamics and atherosclerosis. Insights and perspectives gained from studies of human arteries. Arch Pathol Lab Med. 1988 Oct;112(10):1018–1031. [PubMed] [Google Scholar]
- Gutteridge J. M., Smith A. Antioxidant protection by haemopexin of haem-stimulated lipid peroxidation. Biochem J. 1988 Dec 15;256(3):861–865. doi: 10.1042/bj2560861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter G. C., Dubick M. A., Keen C. L., Eskelson C. D. Effects of hypertension on aortic antioxidant status in human abdominal aneurysmal and occlusive disease. Proc Soc Exp Biol Med. 1991 Mar;196(3):273–279. doi: 10.3181/00379727-196-43188. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. J., Daniels-McQueen S., Patino M. M., Gaffield L., Walden W. E., Thach R. E. Derepression of ferritin messenger RNA translation by hemin in vitro. Science. 1990 Jan 5;247(4938):74–77. doi: 10.1126/science.2294594. [DOI] [PubMed] [Google Scholar]
- Muller-Eberhard U., Javid J., Liem H. H., Hanstein A., Hanna M. Plasma concentrations of hemopexin, haptoglobin and heme in patients with various hemolytic diseases. Blood. 1968 Nov;32(5):811–815. [PubMed] [Google Scholar]
- Okuda M., Tokunaga R., Taketani S. Expression of haptoglobin receptors in human hepatoma cells. Biochim Biophys Acta. 1992 Aug 12;1136(2):143–149. doi: 10.1016/0167-4889(92)90249-b. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh S. M., Graf E., Panter S. S., Hallaway P. E., Eaton J. W. Hemoglobin. A biologic fenton reagent. J Biol Chem. 1984 Dec 10;259(23):14354–14356. [PubMed] [Google Scholar]
- Salonen J. T., Nyyssönen K., Korpela H., Tuomilehto J., Seppänen R., Salonen R. High stored iron levels are associated with excess risk of myocardial infarction in eastern Finnish men. Circulation. 1992 Sep;86(3):803–811. doi: 10.1161/01.cir.86.3.803. [DOI] [PubMed] [Google Scholar]
- Sato M., Ishizawa S., Yoshida T., Shibahara S. Interaction of upstream stimulatory factor with the human heme oxygenase gene promoter. Eur J Biochem. 1990 Mar 10;188(2):231–237. doi: 10.1111/j.1432-1033.1990.tb15394.x. [DOI] [PubMed] [Google Scholar]
- Smith C., Mitchinson M. J., Aruoma O. I., Halliwell B. Stimulation of lipid peroxidation and hydroxyl-radical generation by the contents of human atherosclerotic lesions. Biochem J. 1992 Sep 15;286(Pt 3):901–905. doi: 10.1042/bj2860901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Paul J., Ohlsson P. I., Hjortsberg K., Paul K. G. Heme-protein fission under nondenaturing conditions. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):882–886. doi: 10.1073/pnas.88.3.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Theil E. C. Regulation of ferritin and transferrin receptor mRNAs. J Biol Chem. 1990 Mar 25;265(9):4771–4774. [PubMed] [Google Scholar]
- Varani J., Fligiel S. E., Till G. O., Kunkel R. G., Ryan U. S., Ward P. A. Pulmonary endothelial cell killing by human neutrophils. Possible involvement of hydroxyl radical. Lab Invest. 1985 Dec;53(6):656–663. [PubMed] [Google Scholar]
- Vercellotti G. M., van Asbeck B. S., Jacob H. S. Oxygen radical-induced erythrocyte hemolysis by neutrophils. Critical role of iron and lactoferrin. J Clin Invest. 1985 Sep;76(3):956–962. doi: 10.1172/JCI112095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S. H., Grady R. W., Shaklai N., Snider J. M., Muller-Eberhard U. The influence of heme-binding proteins in heme-catalyzed oxidations. Arch Biochem Biophys. 1988 Sep;265(2):539–550. doi: 10.1016/0003-9861(88)90159-2. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Neutrophil-mediated methemoglobin formation in the erythrocyte. The role of superoxide and hydrogen peroxide. J Biol Chem. 1982 Mar 25;257(6):2947–2953. [PubMed] [Google Scholar]