Abstract
Purpose
To elucidate the dynamic accommodative movements of the lens capsule, posterior lens and the strand that attaches to the posterior vitreous zonule insertion zone and posterior lens equator (PVZ INS-LE), and their age-related changes.
Methods
Twelve human subjects (ages 19–65 years) and twelve rhesus monkeys (ages 6–27 years) were studied. Accommodation was induced pharmacologically (humans) or by central electrical stimulation (monkeys). Ultrasound biomicroscopy was used to image intraocular structures in both species. Surgical procedures and contrast agents were utilized in the monkey eyes to elucidate function and allow visualization of the intraocular accommodative structures.
Results
Human: The posterior pole of the lens moves posteriorly during accommodation in proportion to accommodative amplitude and ciliary muscle movement. Monkey: Similar accommodative movements of the posterior lens pole were seen in the monkey eyes. Following extracapsular lens extraction (ECLE), the central capsule bows backward during accommodation in proportion to accommodative amplitude and ciliary muscle movement, while the peripheral capsule moves forward. During accommodation the ciliary muscle moved forward by ~1.0 mm, pulling forward the vitreous zonule and the PVZ INS-LE structure. During the accommodative response the PVZ INS-LE structure moved forward when the lens was intact and when the lens substance and capsule were removed. In both the monkey and the human eyes these movements declined with age.
Conclusions
The accommodative shape change of the central capsule may be due to the elastic properties of the capsule itself. For these capsule/lens accommodative posterior movements to occur, the vitreous face must either allow for it or facilitate it. The PVZ INS-LE structure may act as a “strut” to the posterior lens equator (pushing the lens equator forward) and thereby facilitate accommodative forward lens equator movement and lens thickening. The age-related posterior restriction of the ciliary muscle, vitreous zonule and the PVZ-INS LE structure dampens the accommodative lens shape change. Future descriptions of the accommodative mechanism, and approaches to presbyopia therapy, may need to incorporate these findings.
INTRODUCTION
The process of accommodation that allows a person to focus on objects at close range involves contraction of the ciliary muscle, which is attached to the lens capsule via the anterior zonules. As the muscle moves forward and inward during contraction, these zonules relax. This relaxation allows the lens to thicken and move forward, increasing its refractive power. Every human loses the ability to accommodate (presbyopia) throughout life, so that by the age of 45–50 years emmetropic and hyperopic eyes require corrective lenses for reading, while most myopic individuals must remove their distance glasses.
The classic Helmholtz theory of accommodation posits that the ciliary muscle moves forward and inward, releasing tension on the anterior zonula and thus allowing the lens equator to move away from the sclera.1 The lens equatorial diameter decreases and the lens anterior-posterior (A-P) thickness increases.1 Fincham 2 postulated that the capsule moulds the lens into an accommodated state once the anterior zonula begin to relax during muscle contraction. We refer to the above (Helmholtz theory and Fincham capsular theory) as the “basic” mechanism of accommodation hereafter in this paper.
The loss of lens deformability is clearly the optical reason for presbyopia. However, two thirds of the eye’s accommodative ability is lost before the lens begins to harden. It is unknown how the age-related loss in ciliary muscle mobility might contribute to presbyopia, or how this loss in mobility might limit the effect of accommodating intraocular lenses (IOLs). Some evidence, including findings from research by our group, suggests that this loss of mobility may be due to decreased elasticity of the tissues that attach the ciliary muscle to the back of the eye (i.e., posterior ciliary muscle tendons, choroid, and/or vitreous zonule insertion zone). It is the elasticity of these tissues that allows the ciliary muscle to move forward and inward during contraction. In addition to having a structurally and functionally similar accommodative apparatus and developing presbyopia on a similar timescale relative to lifespan, the human and rhesus monkey eyes behave similarly with regard to age-related effects on ciliary muscle movement and its restriction. Ciliary muscle mobility continues to exist in the aged eyes of both species but is markedly reduced. Contrast agents and surgical procedures can be utilized in the monkey eye to elucidate structural and functional relationships that are relevant to the human eye.
There is some dispute as to whether and in what direction the posterior lens pole moves during accommodation. In addition, there are reports that demonstrate the moulding effect of the capsule on the lens.3 While Fincham2, and Glasser and Campbell3 attribute the accommodative lens shape change to capsular moulding, Coleman has reported that the capsule exerts insufficient force to change the shape of the lens substance. Coleman postulates that the accommodative lens shape change is induced by vitreous pressure (hydraulic forces) on the posterior lens (catenary theory). 4, 5 However, previous research by our group demonstrated for the first time that the peripheral anterior hyaloid bows backward, i.e., away from the lens, during accommodation, in contradiction to Coleman’s theory.6 But the question regarding what is happening in the eye at the lens posterior pole (central lens posterior surface) and the central anterior vitreous remains. Further, if the capsule moulds the lens substance during accommodation, then the capsule should exhibit a similar or enhanced shape change absent the lens substance and absent an IOL. Indeed, in the absence of the lens substance and an IOL the centripetal accommodative movement of the equatorial capsule was enhanced. 7 However, the normal axial shape change (i.e. anterior/posterior movements) of the central and peripheral capsule during accommodation has not been reported in absence of the lens substance or an IOL. There are many reports of the lens movements in the normal phakic eye 8,9–11 and capsule movements with an IOL 12 or force gauge present13 inside or outside the capsular bag following ECLE, but the presence of these devices would influence the normal capsular dimensions and movements.
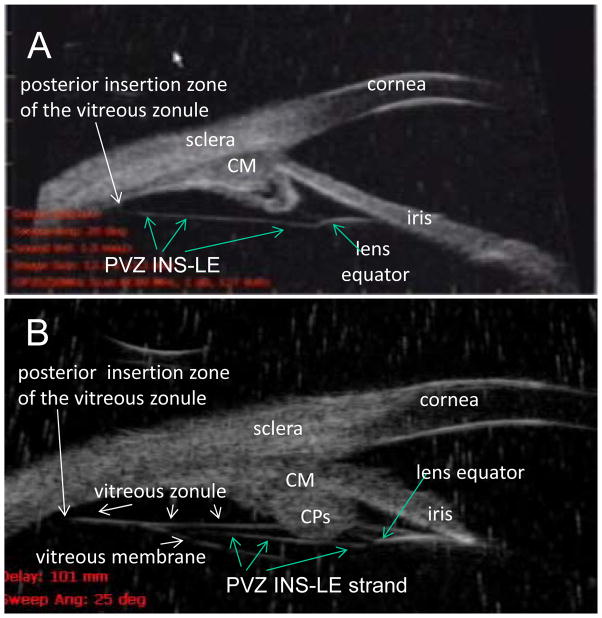
Recent studies have shown a new structure that extends from the posterior insertion zone of the vitreous zonule in a straight course directly to the posterior lens equator, without passing in proximity to the zonular plexus that originates from the walls of the valleys between the ciliary processes. This strand which extends from the posterior vitreous zonule insertion zone to the lens equator is termed the PVZ INS-LE structure (Fig. 1) and is referred to as such hereafter in this paper. The PVZ INS-LE is distinct from and not to be confused with the vitreous zonule that extends from the posterior insertion zone and attaches to the plexus at the walls of the ciliary processes and then changes direction to become the anterior zonule that attach to the lens near the equator, as described by Rohen 14 and as described by Coleman et al. as “accessory zonules.”15 The PVZ INS-LE courses in a straight line from the posterior insertion zone to the posterior lens equator and attaches to it - without attaching to the plexus at the ciliary processes. We first noticed the PVZ INS-LE structure in the human subjects that were imaged by ultrasound biomicroscopy (UBM). 16, 17 Since the posterior insertion zone of the vitreous zonule undergoes an age-related decline in accommodative movement,18 it is not surprising that the PVZ INS-LE structure is associated with accommodative amplitude and lens thickening16, 17: the vitreous zonule, the insertion zone and the PVZ INS-LE structure all move forward during accommodation, pulled by the ciliary muscle in proportion to accommodative amplitude, and these movements decline with age.17, 18 However, the PVZ INS-LE structure (Fig. 1) requires further study to elucidate its function. Accordingly, we utilized various techniques to visualize the PVZ INS-LE, capsule and lens, to determine the accommodative movements and function of these structures in young and old human and monkey eyes. Specifically, we determined the accommodative movements of the posterior lens pole in live, comparably aged human and monkey eyes, and we examined accommodative movements of the lens capsule and the PVZ INS-LE in live monkeys after extracapsular lens extraction (ECLE) or intracapsular lens extraction (ICLE).
Figure 1.
(Panel A) Ultrasound biomicroscopy (UBM) images (50 mHz) in the nasal quadrant of a 19-year-old male human. There was always a prominent continuous strand that extended from the posterior insertion zone of the vitreous zonule directly toward the posterior aspect of the lens equator and attached to it, seen in all 12 human subjects and termed PVZ INS-LE strand (which stands for posterior vitreous zonule insertion to lens equator strand). (Panel B) UBM image in a 65-year-old human subject. Note that a portion of the PVZ INS-LE strand is visible that extends between the posterior insertion zone of the vitreous zonule and the posterior lens equator, as in (A) with the strand passing immediately internal to the CPs. The PVZ INS-LE strand lies between the vitreous zonule and vitreous membrane. Note: Panel B shows both the vitreous zonule and the PVZ INS-LE strand. During accommodation the muscle pulls forward the vitreous zonule and the PVZ INS-LE strand. The PVZ INS-LE strand does not relax during accommodation. [Croft, Kaufman and Lütjen-Drecoll: ARVO 2010 IOVS 2013] CM = ciliary muscle. Reprinted with permission from: Croft et al. Accommodative Movements of the Vitreous Membrane, Choroid, and Sclera in Young and Presbyopic Human and Nonhuman Primate Eyes. Invest Ophthalmol Vis Sci 2013;54:5049–5058; copyright Association for Research in Vision and Ophthalmology (ARVO).
MATERIALS AND METHODS
Human Subjects
The same recorded images for twelve human subjects, as reported in Croft and Kaufman et al 201316, 17, were used. Briefly, subjects (5 males and 7 females) age 19 years to 65 years with normal eyes were recruited, and informed consent was obtained. Potential subjects received a complete eye examination by an ophthalmologist. Preliminary data collection included refraction measurement, slit-lamp biomicroscopy, direct ophthalmoscopy, and external and ocular motility examination. Potential subjects with any ocular abnormalities, or a refractive correction greater than 2.0 dioptres from emmetropia, were excluded. The research adhered to the tenets of the Declaration of Helsinki.
Monkeys
Twelve rhesus monkeys (Macaca mulatta) of either sex, aged 6–27 years and weighing 5.8–14.5 kg, with normal eyes were obtained, housed and eventually euthanized at the end of the study, as previously described.19 The monkeys were bilaterally iridectomised and a bipolar electrode was placed in the Edinger-Westphal nucleus to stimulate accommodation.20, 21 The animals were randomly selected and the sample size was determined based on the information from previous studies of the average ciliary muscle and lens movement amplitudes and the variability of the data.7, 19, 22, 23 All procedures conformed to the ARVO Statement for the Use of Animals in Research and were in accordance with institutionally approved animal protocols.
Age-Matched Groups
Based on the time course of presbyopia in rhesus monkeys and humans, the monkeys and humans were grouped, age-matched (2.5:1; monkeys to humans), and the adult age groups’ classifications (e.g., “young,” “mature,” “older”) were adjusted according to the relative lifespans of the two species as follows:
Accommodation Stimulation and Response Measurements
Refractive error was measured in the resting and accommodated states to determine accommodative amplitude.
Refractometry
Total refractive power of the subjects’ eyes was measured with the Hartinger coincidence refractometer during disaccommodation and accommodation in both species. Accommodative amplitude was the difference in refractive power between the two accommodative states.
Human Subjects
Disaccommodation was induced by one drop of 5% clinical grade homatropine hydrobromide in both eyes, and refractions were repeated every 15 min until they no longer changed (~ 45–60 min), to ensure that the ciliary muscle was in the relaxed state. One week to three months later, maximum accommodation24–26 was induced by two drops of 4% clinical grade pilocarpine hydrochloride separated by a 5-min interval, and refractions were again repeated every 15 min until they no longer changed (~ 45–60 min).
Monkeys
Accommodation was induced by midbrain electrical stimulation and measured by Hartinger coincidence refractometry.21 Stimulus settings were chosen that induced maximum forward ciliary body movement and maximum dioptric accommodation, allowing comparisons between age groups.
Ultrasound Biomicroscopy (UBM)
Instrumentation
Several high-resolution contact imaging techniques (e.g., dynamic goniovideography and UBM) can be performed during the dynamic accommodation response in anesthetized monkeys implanted with a midbrain stimulating electrode,21, 27–30 but this setup is not possible in humans. We imaged the human eye in the unaccommodated and then the maximally accommodated states (see Human Subjects section below). While magnetic resonance imaging (MRI) has proved useful in the human eye, 31–34 MRI resolution is limited and cannot consistently image the vitreous zonule.
The UBM-H (Humphrey Instruments, Model # 840, 80 MHz, 50 μm lateral resolution, 37 μm axial resolution, (Paradigm-medical.com/ubms.html) has higher resolution, but its field of view is limited to 5.0 mm, and thus it was used to image the anterior portion of the ciliary body, the vitreous zonule, the PVZ INS-LE and the lens equator. The UBM-ER (50 MHz resolution ((Model # MHF-1 Ultraview System Model P60, Reichert.com); or E-Technologies, Etechultrasound.net/) has lower resolution than the UBM-H, but it has a wider field of view (i.e., 13 mm) and thus was used to image the entire sagittal extent of the ciliary body from the region of the ora serrata to the cornea, the vitreous zonule, the PVZ INS-LE, the lens equator, and the anterior and posterior lens surfaces (Figs. 1, 2). All images were recorded to videotape. The UBM techniques used have been reported previously, peer reviewed and published. 21, 23, 30 Some of the results from these 12 subjects were reported previously. 17,16 The current study reports new data from the same eyes. Images were accepted that provided the best definition of the accommodative apparatus, showing clearly defined edges of the ciliary body, lens equator, anterior and posterior lens surfaces, vitreous zonule, and cornea.
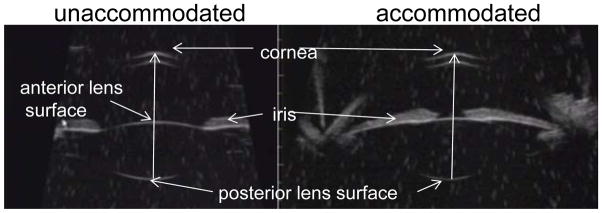
Figure 2.
Ultrasound biomicroscopy (UBM) images taken in a human eye. UBM allows visualization of the anterior chamber and the anterior/posterior lens surfaces. The distance between the posterior lens pole and the corneal epithelium was measured in the resting and accommodated states.
Human Subjects
UBM was carried out in the human subjects as described by Pavlin.35 Each subject was seated in the examination chair and was then placed in the supine position.35 Each eye received a short-acting topical anaesthetic (proparacaine hydrochloride 0.5%; Bausch & Lomb Inc., Tampa, FL), before the obligatory scleral cup was positioned on the eye. Goniosol (hydroxypropyl methylcellulose ophthalmic solution, USP 2.5%; HUB Pharmaceuticals, LLC, Rancho Cucamonga, CA 91730) was used as the acoustic couplant. The subject was asked to rotate the eye and maintain it in the desired position to facilitate the proper orientation for obtaining anterior segment images (including the anterior and posterior lens surfaces) in both the nasal and the temporal quadrants. The UBM probe was stabilized manually by the operator. Images were collected in the unaccommodated and maximally accommodated states.
Monkeys
UBM in the rhesus monkey has been described in detail elsewhere.21, 27, 30 Briefly, each anesthetized (pentobarbital Na (10–15 mg/kg IV, supplemented with 0.5–10 mg/kg IV, as needed) monkey was placed supine with the head stabilized facing upward in a head holder, and a saline fluid-well was placed around the eye.30, 35 The eye was rotated using a suture passed beneath the lateral rectus muscle.30 During UBM imaging, the eye was stabilized with extraocular muscle sutures, so that during accommodation there was minimal convergent eye movement, if any. The stabilizing arm of the ultrasound instrument held the transducer in place. Thus, there was very little, if any, change in angle of the transducer to the eye during accommodation. Dynamic UBM images were obtained during central stimulation of accommodation and then recorded to videotape.21
For both the monkeys and the human subjects, analogous images were collected, with the anterior/posterior lens surfaces and cornea symmetrically oriented in a horizontal direction within all images.21 The probe was oscillated back and forth to ensure that the location of the UBM scan was in the mid-region of the corneal apex and anterior and posterior lens or capsule surfaces.
Measurement of Various Intraocular Distances
The axial distances between the central corneal epithelium and (a) the posterior lens pole (Fig. 2) and (b) the central posterior capsule following ECLE (Fig. 3) were measured. Further, the axial distance between the central cornea and the axial plane of the peripheral capsule was measured as defined in Figure 3. Measurements were taken of the various intraocular distances within the UBM images (Figs. 2, 3), and comparisons were made between the images in the unaccommodated and accommodated states.
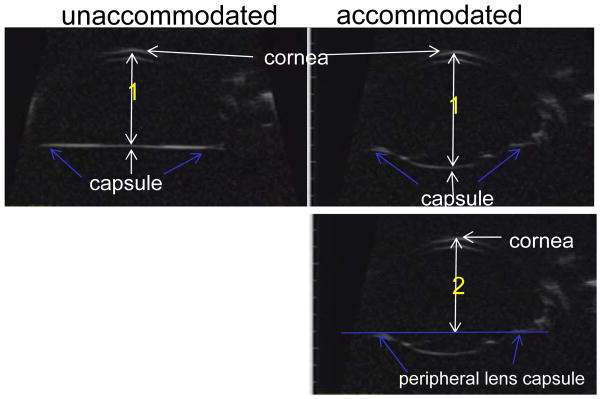
Figure 3.
Ultrasound biomicroscopy (UBM) images taken in a monkey eye following extracapsular lens extraction (ECLE). UBM allows visualization of the anterior chamber and capsule following ECLE. The following distances were measured: Distance 1: The axial distance from the posterior central capsule to the corneal epithelium was measured in the resting and accommodated states. Distance 2: The axial distance from the peripheral lens capsule (blue arrows) to the corneal epithelium was measured in the resting and accommodated states.
Optical Coherence Tomography (OCT)
Following ECLE in the rhesus monkey eye a Heidelberg Spectralis OCT instrument (Heidelbergengineering.com/us/products/spectralis-models/; Heidelberg Engineering, Inc.) was used to image the mid region of the cornea and capsule through the mid region of the capsulhorrhexis during accommodation. A smart phone (Samsung S III, Los Angeles, CA) was used to capture the dynamic images from the OCT monitor during the accommodative response. The monkeys were placed in the prone position, the monkey’s head placed in a headholder with the head held upright facing forward. to collect the images.
Monkey Surgical Procedures
Three rhesus monkeys (ages 6–24 years) underwent a specialized intracapsular lens extraction (ICLE: removal of the lens nucleus and cortex within the intact capsule). The α-chymotrypsin was allowed to remain in the eye for <30 seconds to lyse the connection to the zonula before fluid rinsing and removal of the lens with a cryoprobe. The reduced exposure time to the α-chymotrypsin enzyme minimized the disruption to the anterior zonula. Wieger’s ligament and the PVZ INS-LE remained largely intact in these eyes post-ICLE, and mechanical vitrectomy was not required. Four other monkeys (ages 8–22 years) underwent extracapsular lens extraction (ECLE: ~4 mm anterior capsulorrhexis, removal of the lens nucleus and cortex). Both surgical procedures were performed using standard clinical techniques as described elsewhere 7, with the minor technical variations noted above.
Statistical Analysis
A two-tailed paired t-test was used to detect significant differences between various intraocular measurements taken in the resting and accommodated states (e.g., posterior lens pole A/P position with respect to the corneal epithelium). A p-value ≤0.05 was considered significant; 0.05 ≤ p ≤ 0.10 was considered to indicate a trend, given the small number of monkeys or humans. Simple linear regression (i.e., posterior lens pole position with respect to the cornea versus age) was undertaken to determine if there was a significant relationship between variables.
RESULTS
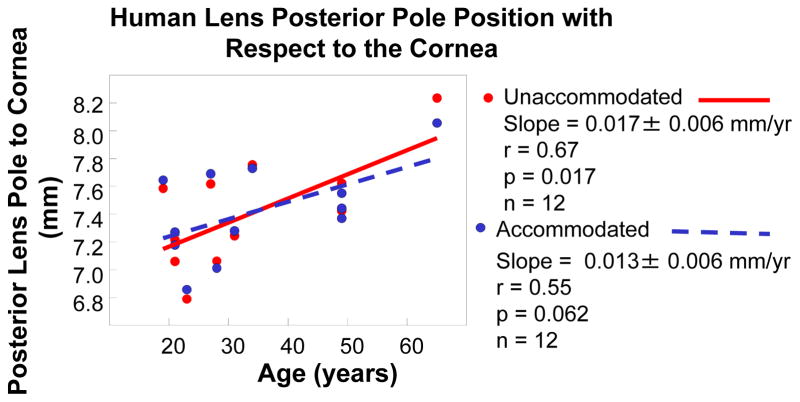
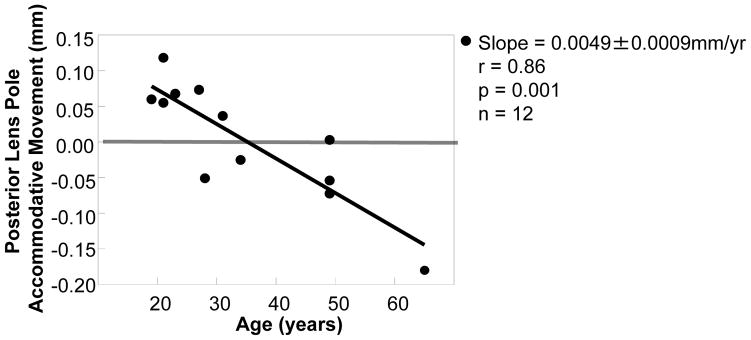
The posterior pole of the human lens in the resting state became more posteriorly positioned with age by 0.017 ± 0.006 mm/yr (r = 0.67, p = 0.017, n = 12; Fig. 4). During accommodation in the young human eyes the posterior lens pole moved posteriorly by 0.08 ± 0.01 mm (p=0.01), and this movement declined with age by 0.0049 ± 0.00089 mm/yr (Fig. 5). The posterior lens movement was lost by mature adult age (0.01 ± 0.03 mm; Table 2), and during accommodation in the older subjects, the posterior lens pole tended to move anteriorly, but not significantly (0.08 ± 0.04; p=0.14); Table 2, Fig. 5). Similar results were seen in the monkey eye and dynamic accommodative movements are shown in Video Clip #1.
Figure 4.
The data points represent the human lens posterior pole position with respect to the central cornea (Fig. 2). The posterior pole of the lens in the resting eye becomes more posteriorly positioned with age. Similar results were seen in the monkey eye.
Figure 5.
Data points represent the amount of posterior accommodative movement of the posterior lens pole. In the human eye the accommodative posterior movement of the lens posterior pole is lost with age. Similar results were seen in the monkey eye. The amount of posterior lens pole movement was obtained for each eye by calculating the distance change between the posterior lens pole and the central cornea (Fig. 2) in the accommodated state minus unaccommodated state.
Table 2.
Data are mean ± s.e.m. accommodative posterior movement of the posterior lens pole for each age group in human eyes. The amount of posterior lens pole movement was obtained for each eye by calculating the distance change between the posterior lens pole and the central cornea (Fig. 2) in the accommodated state minus unaccommodated state.
| Human Lens Accommodative Posterior Pole Movement | ||||
|---|---|---|---|---|
| Accommodation* (Dioptres) | Mean Posterior | |||
| Adult Age | Movement ± s.e.m. (mm) | n | ||
| 12.2 ±1.2* | Young (19–23 yr) | 0.08 ± 0.01 | 4 | p = 0.02 |
| 8.5 ±0.9* | Mature (27–31 yr) | 0.01 ± 0.03 | 4 | p = 0.78 |
| 1.0 ±0.5* | Older (49–65 yr) | −0.08 ± 0.04 | 4 | p = 0.14 |
Reprinted with permission from: Croft et al. Extralenticular and Lenticular Aspects of Accommodation and Presbyopia in Human versus Monkey Eyes. Invest Ophthalmol Vis Sci 2013;54:5035–5048; copyright Association for Research in Vision and Ophthalmology (ARVO).
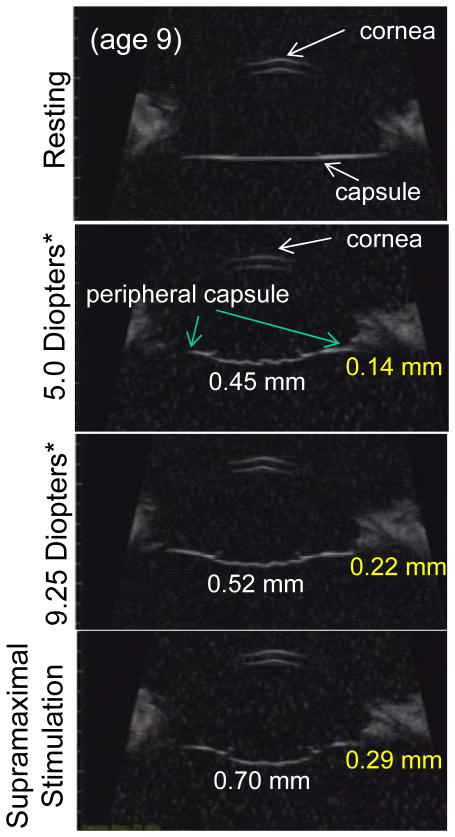
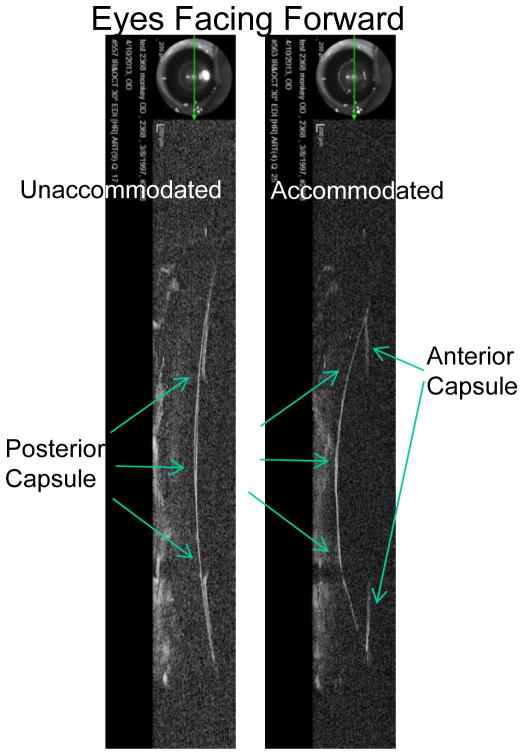
In the monkey eye following ECLE, the central capsule bowed backward in relation to the cornea in a stimulation/accommodation dose-dependent manner (Fig. 6). The higher the accommodative amplitude/stimulation the more pronounced the accommodative backward bowing of the capsule (Fig. 6). The capsule bowed backward whether the monkey was in the supine (eyes facing the ceiling) or prone (eyes facing forward) position (Figs. 6, 7, Video clips 2–5). While there was pronounced backward bowing of both the posterior and anterior capsule surfaces, backward bowing of the anterior capsule was not as rapid as the backward bowing of the posterior capsule (Video Clip #5). Nonetheless, at the end of the 2.2 sec stimulus train, the anterior and posterior capsule surfaces were for the most part adjacent to each other, with the exception that the capsular ring immediately encircling the capsulorrhexis was slightly anterior to the central posterior capsule (Fig. 6). The accommodative capsule backward bowing was diminished with age from 0.70 ± 0.03 mm in the young eye to 0.26 ± 0.03 mm in the older eye (Fig. 8, Video clip 2, 3, Table 3). During accommodation in the older eye, there was slightly more pronounced separation of the anterior and posterior capsule than in the young eye. While the central capsule bowed backward, the peripheral capsule A/P position moved slightly forward during accommodation and was diminished with age from by 0.29 ± 0.02 mm in the young eye to 0.10 ± 0.02 mm in the older eye (Table 3, Fig. 6).
Figure 6.
Ultrasound biomicroscopy (UBM) images showing the anterior segment and capsule two weeks after ECLE in a young (aged 9) rhesus monkey eye. During accommodation the central capsule bowed backward while the peripheral capsule moved forward in relation to the cornea in a stimulation/accommodation dose-dependent manner.
White numbers in the panels represent the amount of backward bowing of the central capsule in relation to the cornea and yellow numbers represent the amount of forward movement of the peripheral capsule in relation to the cornea. The peripheral capsule A/P position moves slightly forward (~0.29 mm) during accommodation.
*Accommodation values were taken prior to lens extraction.
Figure 7.
Heidelberg Spectralis OCT images collected in a rhesus monkey eye (age 17) two weeks after ECLE. The images show that the capsule also bows backward if the monkey is prone (eyes facing forward). The OCT instrument was provided courtesy of Steve Eastman, Cedarburg WI.
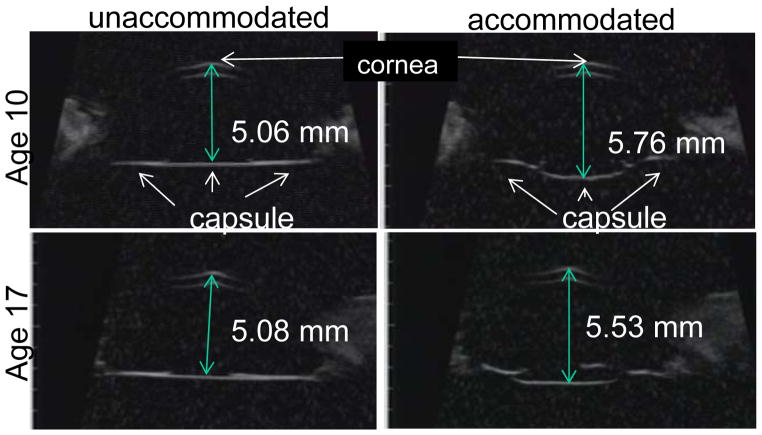
Figure 8.
Ultrasound biomicroscopy (UBM) images obtained in rhesus monkey eyes two weeks after ECLE in the resting and maximally accommodated states. Numbers represent the axial distance of the central capsule from the cornea. During accommodation the central capsule bows backward, but not the peripheral capsule. The accommodative backward bowing is diminished with age.
Table 3.
Data are age and mean ± s.e.m. (mm) backward bowing of the central capsule or forward movement of the peripheral capsule (Fig. 3) during maximally stimulated accommodation in rhesus monkey eyes two weeks after ECLE.
| Rhesus Monkey Capsule Movements | |||
|---|---|---|---|
| Accommodation* (Diopters) | Age (years) | Central Capsule Posterior Bowing | Peripheral Capsule Forward Movement |
| 9.25 | 8 | 0.70 ± 0.03 | 0.29 ± 0.02 |
| 6.50 | 17 | 0.45 ± 0.02 | 0.15 ± 0.04 |
| 1.50 | 22 | 0.26 ± 0.03 | 0.10 ± 0.02 |
Accommodation values were taken prior to lens extraction.
Thus, during accommodation the lens equator moved forward and the PVZ INS-LE remained straight (i.e., it did not relax).17, 18 In the rhesus monkey during accommodation, following ECLE the peripheral capsule (equator) moved forward in the region where the anterior end of the PVZ INS-LE structure attaches to the posterior peripheral capsule. Following removal of the lens and capsule (ICLE), the PVZ INS-LE still moved forward during accommodation (Video Clip #6).
DISCUSSION
During accommodation, the vitreous allows or facilitates a small amount of posterior movement of the posterior pole of the lens and allows or facilitates pronounced backward movement of the capsule following ECLE, but this posterior accommodative movement declines with age.
In the young eye, both the posterior and anterior capsule bow backward significantly. The enhanced backward bowing of the young posterior capsule in absence of the lens substance might be expected given that the posterior capsule “moulds” the posterior lens surface into the accommodated form. Given that the anterior lens surface undergoes greater accommodative movement than the posterior lens surface, one would expect a more pronounced forward movement of the young anterior capsule without the lens substance. We observed backward bowing of the anterior capsule in all 4 young monkeys. Although this could be due to the presence of a 4 mm capsulorrhexis, the opening is small and in the central anterior capsule where the capsule is thinnest, leaving intact the more peripheral region of the anterior capsule where it is thickest. 2, 36 The anterior capsule overall is thicker than the posterior capsule and there is no anterior chamber shallowing or accommodative pressure increase due to a thickening lens, since the lens is absent. With all of the above observations, one would expect that the anterior capsule might have at the very least maintained its anterior/posterior position within the eye. This suggests that the capsule moulding the lens into the accommodated state may not be the only structure/process facilitating accommodative lens shape change. Whether one agrees or disagrees with this thought, the ECLE surgical procedure performed in the monkey eye was a standard clinical procedure (except for the slightly smaller capsulorrhexis) that every human eye receives during cataract surgery. The age-related decrease of the accommodative backward bowing of the capsule could be related to a number of things: age-related changes in capsule elasticity, 37,38, 39 age-related ciliary muscle immobility, 21, 22, 40, 41,7, 17, 23 age-related increase in central vitreous liquefaction 42–44 and/or build-up of peripheral vitreous aggregates, 16 and/or age-related ocular geometric changes. 16 The point is that even in the older 22 year old monkey eye (equivalent to a 53 year old human) the capsule bowed backward by 0.26 mm. Therefore, designs of accommodating IOLs placed inside or outside the capsular bag need to take into account that fact that both the anterior and posterior capsule bow backward during the accommodative response post-ECLE. For example, there was a counterproductive accommodative backward shift of the AT 45 Crystalens accommodating IOL, perhaps explaining why this IOL induced so little accommodation. 45
The backward bowing of the central posterior capsule occurs in an accommodative dose-dependent manner (i.e., the higher the accommodation stimulus, the more pronounced the backward bowing of the central capsule) and occurs whether the monkey is prone (facing forward) or supine (facing the ceiling). With the pronounced accommodative backward bowing of the central capsule, how is it that the peripheral capsule near the equator moves forward during accommodation? The answer may found in examining the accommodative movements of the PVZ INS-LE (see below). The PVZ INS-LE (which extends from the posterior insertion zone of the vitreous zonule to the posterior peripheral lens/capsule) is pulled forward by the ciliary muscle during accommodation and remains straight during the accommodative response (i.e., it does not relax). The lens equator also moves forward during the accommodative response. This suggests that the PVZ INS-LE may act as a semi-rigid “strut” to the posterior lens equator (pushing the lens equator forward) and facilitate its forward movement and thereby facilitate lens thickening. Alternatively, the PVZ INS-LE may also act to resist longitudinal compression. In the older eye, with decreased accommodative forward muscle movement, the PVZ INS-LE would not only supply less “forward push” or support to the posterior lens equator, but may also provide a direct drag against its forward movement and thereby against lens thickening during accommodation.17
Further evidence that the PVZ INS-LE structure acts as a strut during accommodation can be found in monkey eyes following ECLE or ICLE. After ECLE, in the region where the anterior end of the PVZ INS-LE structure attaches to the posterior capsule the peripheral capsule moves forward during accommodation. After the specialized ICLE, in which the lens and capsule are removed intact after connections to the anterior zonule are enzymatically weakened/lysed/severed in a gentler than normal manner, leaving much of the PVZ INS-LE intact, the anterior end of the PVZ INS-LE still moves forward (Video Clip #6). Thus, the data suggest that in the presence or absence of the lens/capsule, the anterior end of the PVZ INS-LE moved forward during accommodation. This finding might support of Coleman’s theory (i.e., vitreous support to the posterior lens periphery during accommodation), if one considers the PVZ INS-LE part of the vitreous. The vitreous is partially composed of collagen type materials 46,43, 44, 47 and the PVZ INS-LE may be “stiff” thereby supplying forward push to the lens equator as the muscle and insertion zone move forward during accommodation. However, Coleman’s theory is not consistent with the accommodative movements of the anterior hyaloid membrane bowing backward16 and not consistent with the accommodative backward movement of the posterior lens/capsule found in the current study.
The hydraulic theory posits that during accommodation, contraction of the ciliary muscle exerts pressure upon the aqueous in the posterior chamber, where it is confined by the iris as the pupil constricts onto the lens surface, compressing the lens equator and causing the anterior lens surface to bulge. 48,49,50 Fincham discounted this theory, due to the fact that accommodation is unimpaired by iridectomy or aniridia.” 2, 51
The accommodative function of the capsule/lens (“basic mechanism” of accommodation”) may be facilitated by the forward movement of the PVZ INS-LE and backward movement of the central vitreous and anterior hyaloid. The question remains as to how far back the accommodative movement of the central vitreous extends. Recent findings have shown that the accommodative posterior movement of the central vitreous extends all of the way back to the optic nerve region; the reverse is true during disaccommodation. [Croft, Kaufman et al, ARVO IOVS 2013, 2015; Croft, Kaufman et al, ESCRS 2014] One might argue that the posterior bowing of the central capsule and anterior hyaloid suggests that the volume of the vitreous decreases. However, while the central vitreous moves posteriorly, the peripheral vitreous neighbouring the vitreous zonule in the region of the ora serrata is pulled forward by the vitreous zonule during accommodative ciliary muscle contraction. 16 This may suggest that the vitreous undergoes redistribution of fluid during the accommodative response and not necessarily a volume change. Similarly, with the change in shape of the lens during accommodation - the lens mass is redistributed- the lens thickens and the equator moves away from the sclera, and the aqueous is redistributed around the lens equator toward the anterior hyaloid.
Before the discovery that the anterior hyaloid bowed backward, it was demonstrated that as the ciliary muscle contracted, the lens thickened, the anterior chamber shallowed and aqueous left the anterior chamber via the trabecular meshwork and Schlemm’s canal at a higher rate as demonstrated by an increase in outflow facility following pilocarpine induced accommodation. 52, 53,54,25 Thus, we know that, during accommodation, at least some of the displaced aqueous leaves the anterior chamber via the trabecular meshwork.
Much new relevant information has been added that is beyond the basic mechanism of accommodation, such as the accommodative forward movement of the vitreous zonule insertion zone, the accommodative backward bowing of the anterior hyaloid and now the function of the PVZ INS-LE strand. These new findings/observations do not contradict the basic mechanism of accommodation and a role for the capsule in reshaping the lens, nor do the findings eliminate a role for the vitreous in accommodation. It just may be that the system is more complex than previously thought.
These findings may have implications for the mechanism of accommodation, pathophysiology of presbyopia and function of accommodating IOLs. Age-related posterior restriction of the ciliary muscle limits the accommodative movements of the vitreous zonule, the PVZ INS-LE and capsule and thereby limits accommodation. Eliminating such restrictions could restore muscle mobility and facilitate the function of accommodating IOLs. Identification and study of all the accommodative intraocular structures will increase our understanding of how the eye accommodates earlier in life, and perhaps change how we understand and treat presbyopia. Given their similarities, the monkey remains the best predictive model for human accommodation and presbyopia.
Supplementary Material
Table 1.
Age group classifications for rhesus monkeys and humans.
| Age Group Classifications | ||
|---|---|---|
| Age (Years) | ||
| Adult Age Group | Monkey | Human |
| Young (pre-presbyopic) | 6–9 yr; n=5 | 19–23 yr; n=4 |
| Mature | 12–13 yr; n=3 | 27–31 yr; n=4 |
| Older (presbyopic) | 19–27 yr; n=3 | 49–65 yr; n=4 |
Acknowledgments
Support:
This work was funded in part by NEI grants RO1 EY10213, R21 EY018370-01A2, and R21 EY018370-01A2S1 to PLK; the Ocular Physiology Research & Education Foundation; the Wisconsin National Primate Research Center, University of Wisconsin-Madison base grant # 5P51 RR 000167; the Core Grant for Vision Research grant # P30 EY016665; Research to Prevent Blindness unrestricted Departmental Challenge Grant (all to PLK).
Footnotes
Portions of these findings have been presented at the annual Association for Research in Vision and Ophthalmology (ARVO) 2013 and 2015 meetings and the International Society of Presbyopia (ISOP) 2013 meeting.
Conflict of Interest: For MAC and PLK: Alcon: Financial Support; Z-Lens LLC: Financial Support; Refocus Group: Consultant, Recipient; Lens AR: Financial Support; Vista Ocular: Financial Support; Bridge Labs: Recipient; Aleyegn Inc.: Consultant. MAC and PLK engage in various consultant work and projects to test various devices. We do not own stock in any company.
References
- 1.von Helmholtz H. Helmholtz’s treatise on physiological optics. In: Southall JPC, editor; Southall JPCT, translator. Handbuch der Physiologischen Optik. I & II. New York: Dover Publications; 1909. pp. 143–172. Mechanism of accommodation. [Google Scholar]
- 2.Fincham E. The mechanism of accommodation. British Journal of Ophthalmology. 1937;8:7–80. [Google Scholar]
- 3.Glasser A, Campbell MCW. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vis Res. 1999;39:1991–2015. doi: 10.1016/s0042-6989(98)00283-1. [DOI] [PubMed] [Google Scholar]
- 4.Coleman DJ. On the hydraulic suspension theory of accommodation. Transactions of the American Ophthalmological Society. 1986;84:846–868. [PMC free article] [PubMed] [Google Scholar]
- 5.Coleman DJ, SKF Presbyopia, accommodation, and the mature catenary. Ophthalmology. 2001;108(9):1544–1551. doi: 10.1016/s0161-6420(01)00691-1. [DOI] [PubMed] [Google Scholar]
- 6.Coleman DJ. Unified model for accommodative mechanism. Am J Ophthalmol. 1970;69:1063–1079. doi: 10.1016/0002-9394(70)91057-3. [DOI] [PubMed] [Google Scholar]
- 7.Croft M, McDonald JP, James RJ, Heatley GA, Lin T-L, Lütjen-Drecoll E, Kaufman PL. Surgical intervention and accommodative responses: I. Centripetal ciliary body, capsule, and lens movement in rhesus monkeys of various ages. Invest Ophthalmol Vis Sci. 2008;49:5484–5494. doi: 10.1167/iovs.08-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koretz JF, Cook CA, Kaufman PL. Accommodation and presbyopia in the human eye: Changes in the anterior segment and crystalline lens with focus. Investigative Ophthalmology and Visual Science. 1997;38:569–578. [PubMed] [Google Scholar]
- 9.Koretz JF, Cook CA, Kuszak JR. The zones of discontinuity in the human lens: Development and distribution with age. Vision Research. 1994;34:2955–2962. doi: 10.1016/0042-6989(94)90267-4. [DOI] [PubMed] [Google Scholar]
- 10.Koretz JF, Neider MW, Kaufman PL, Bertasso AM, DeRousseau CJ, Bito LZ. Slit-lamp studies of the rhesus monkey eye: I. Survey of the anterior segment. Exp Eye Res. 1987;44:307–318. doi: 10.1016/s0014-4835(87)80014-3. [DOI] [PubMed] [Google Scholar]
- 11.Koretz JF, Strenk SA, Strenk LM, Semmlow JL. Scheimpflug and high-resolution manetic resonance imaging of the anterior segment: a comparative study. Journal of the Optical Society of America. 2004;21(3):346–354. doi: 10.1364/josaa.21.000346. [DOI] [PubMed] [Google Scholar]
- 12.Modesti M, Pasqualitto G, Appolloni R, Pecorella I, Sourdille P. Preoperative and postoperative size and movements of the lens capsular bag:ultrasound biomicroscopy analysis. J Cataract Refract Surg. 2011;37:1775–1784. doi: 10.1016/j.jcrs.2011.04.035. [DOI] [PubMed] [Google Scholar]
- 13.Alió JL, Ben-Nun J. Study of the force dynamics at the capsular interface related to ciliary body stimulation in a primate model. J Cataract Refract Surg. 2015;31(2):124–128. doi: 10.3928/1081597X-20150122-08. [DOI] [PubMed] [Google Scholar]
- 14.Rohen JW. Scanning electron microscopic studies of the zonular apparatus in human and monkey eyes. Investigative Ophthalmology and Visual Science. 1979;18:133–144. [PubMed] [Google Scholar]
- 15.Coleman DJ, Silverman RH, Lloyd H. Physiology of accommodation and the role of the vitreous body. New York, NY: Springer Science+Business Media; 2014. Chapter IV.D. [Google Scholar]
- 16.Croft MA, Nork TM, McDonald JP, Katz A, Lütjen-Drecoll E, Kaufman PL. Accommodative movements of the vitreous membrane, choroid and sclera in young and presbyopic human and nonhuman primate eyes. Invest Ophthalmol Vis Sci. 2013;54:5049–5058. doi: 10.1167/iovs.12-10847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Croft MA, McDonald JP, Katz A, Lin TL, Lütjen-Drecoll E, Kaufman PL. Extralenticular and lenticular aspects of accommodation and presbyopia in human versus monkey eyes. Invest Ophthalmol Vis Sci. 2013;54:5035–5048. doi: 10.1167/iovs.12-10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Croft MA, Heatley G, Nork TM, et al. Accommodative movements of the lens/capsule in relation to the vitreous face and aging. ARVO Abstract #386. 2013 [Google Scholar]
- 19.Lütjen-Drecoll E, Kaufman P, Wasielewski R, Ting-Li L, Croft M. Morphology and accommodative function of the vitreous zonule in human and monkey eyes. Invest Ophthalmol Vis Sci. 2010;51(3):1554–1564. doi: 10.1167/iovs.09-4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford K, Terasawa E, Kaufman PL. Reproducible stimulation of ciliary muscle contraction in the cynomolgus monkey via a permanent indwelling midbrain electrode. Brain Research. 1989;503:265–272. doi: 10.1016/0006-8993(89)91673-9. [DOI] [PubMed] [Google Scholar]
- 21.Croft M, Glasser A, Heatley G, McDonald J, Ebbert T, Kaufman PL. Accommodative ciliary body and lens function in rhesus monkeys. I. Normal lens, zonule and ciliary process configuration in the iridectomized eye. Invest Ophthalmol Vis Sci. 2006;47(3):1076–1086. doi: 10.1167/iovs.04-1523. [DOI] [PubMed] [Google Scholar]
- 22.Wasielewski R, McDonald JPHG, Lütjen-Drecoll E, Kaufman PL, Croft MA. Surgical intervention and accommodative responses, II. Forward ciliary body accommodative movement is facilitated by zonular attachments to the lens capsule. Invest Ophthalmol Vis Sci. 2008;49:5495–5502. doi: 10.1167/iovs.08-1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Croft M, McDonald JP, Nadkarni NV, Lin TL, Kaufman PL. Age-related changes in centripetal ciliary body movement relative to centripetal lens movement in monkeys. Exp Eye Res. 2009;89:824–832. doi: 10.1016/j.exer.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman PL. Anticholinesterase-induced cholinergic subsensitivity in primate accommodative mechanism. American Journal of Ophthalmology. 1978;85:622–631. doi: 10.1016/s0002-9394(14)77094-1. [DOI] [PubMed] [Google Scholar]
- 25.Croft MA, Oyen MJ, Gange SJ, Fischer MR, Kaufman PL. Aging effects on accommodation and outflow facility responses to pilocarpine in humans. Arch Ophthalmol. 1996;114:586–592. doi: 10.1001/archopht.1996.01100130578015. [DOI] [PubMed] [Google Scholar]
- 26.Koretz JF, Kaufman PL, Neider MW, Goeckner PA. Accommodation and presbyopia in the human eye. II. Aging of the anterior segment. Vis Res. 1989;29:1685–1692. doi: 10.1016/0042-6989(89)90150-8. [DOI] [PubMed] [Google Scholar]
- 27.Croft MA, Glasser A, Heatley G, et al. The zonula, lens, and circumlental space in the normal iridectomized rhesus monkey eye. Invest Ophthalmol Vis Sci. 2006;47:1087–1095. doi: 10.1167/iovs.04-1524. [DOI] [PubMed] [Google Scholar]
- 28.Croft MA, Kaufman PL, Crawford KS, Neider MW, Glasser A, Bito LZ. Accommodation dynamics in aging rhesus monkeys. American Journal of Physiology - Regulatory Integrative and Comparative Physiology. 1998;44:R1885–R1897. doi: 10.1152/ajpregu.1998.275.6.R1885. [DOI] [PubMed] [Google Scholar]
- 29.Glasser A, Kaufman PL. The mechanism of accommodation in primates. Ophthalmology. 1999;106:863–872. doi: 10.1016/S0161-6420(99)00502-3. [DOI] [PubMed] [Google Scholar]
- 30.Glasser A, Croft MA, Brumback L, Kaufman PL. Ultrasound biomicroscopy of the aging rhesus monkey ciliary region. Optom Vis Sci. 2001;78:417–424. doi: 10.1097/00006324-200106000-00014. [DOI] [PubMed] [Google Scholar]
- 31.Strenk S, Strenk LM, Guo S. Magnetic resonance imaging of aging, accommodating, phakic, and pseudophakic ciliary muscle diameters. Journal of Cataract and Refractive Surgery. 2006;32:1792–1798. doi: 10.1016/j.jcrs.2006.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Strenk SA, Semmlow JL, Mezrich RS. Magnetic resonance imaging of the ciliary muscle and the lens in presbyopes. Investigative Ophthalmology and Visual Science. 1992;33(ARVO Suppl):1193. [Google Scholar]
- 33.Strenk SA, Semmlow JL, Strenk IM, Munoz P, Gronlund-Jacob J, DeMarco JK. Age-related changes in human ciliary muscle and lens: a magnetic resonance imaging study. Investigative Ophthalmology and Visual Science. 1999;40:1162–1169. [PubMed] [Google Scholar]
- 34.Strenk SA, Strenk LM, Guo S. Magnetic resonance imaging of the anteroposterior position and thickness of the aging, accommodating, phakic, and pseudophakic ciliary muscle. Journal of Cataract and Refractive Surgery. 2010:235–241. doi: 10.1016/j.jcrs.2009.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pavlin CJ, Foster FS. Ultrasound biomicroscopy of the eye. New York: Springer Verlag; 1995. Examination techniques; pp. 30–46. [Google Scholar]
- 36.Barraquer RI, Michael R, Abreu R, Lamarca J, Tresserra F. Human lens capsule thickness as a function of age and location along the sagittal lens perimeter. Invest Ophthalmol Vis Sci. 2006;47(5):2053–2060. doi: 10.1167/iovs.05-1002. [DOI] [PubMed] [Google Scholar]
- 37.Fisher RF. Elastic constants of the human lens capsule. Journal of Physiology (London) 1969;201:1–19. doi: 10.1113/jphysiol.1969.sp008739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krag S, Olsen T, Andreassen TT. Biomechanical characteristics of the human anterior lens capsule in relation to age. Investigative Ophthalmology and Visual Science. 1997;38:357–363. [PubMed] [Google Scholar]
- 39.Krag S, Andreassen TT. Mechanical properties of the human lens capsule. Progress in Retinal and Eye Research. 2003;22(6):749–767. doi: 10.1016/s1350-9462(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 40.Tamm E, Croft MA, Jungkunz W, Lütjen-Drecoll E, Kaufman PL. Age-related loss of ciliary muscle mobility in the rhesus monkey: role of the choroid. Arch Ophthalmol. 1992;110:871–876. doi: 10.1001/archopht.1992.01080180143043. [DOI] [PubMed] [Google Scholar]
- 41.Lütjen-Drecoll E, Tamm E, Kaufman PL. Age-related loss of morphologic responses to pilocarpine in rhesus monkey ciliary muscle. Archives of Ophthalmology. 1988;106:1591–1598. doi: 10.1001/archopht.1988.01060140759051. [DOI] [PubMed] [Google Scholar]
- 42.Balazs EA, Denlinger JL. Aging changes in the vitreous. In: Liss AR, editor. Aging and the human visual function. New York: 1982. [Google Scholar]
- 43.Sebag J. The vitreous. In: hart WM, editor. Adler’s Physiology of the Eye. 9. St. Louis, MO: G.F. Stamathis; 1992. pp. 268–347. [Google Scholar]
- 44.Sebag J. Vitreous: from biochemistry to clinical relevance. In: Tasman W, Jaeger EA, editors. Duane’s Foundations of Clinical Ophthalmology. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 45.Koeppl C, Findl O, Menapace R, et al. Pilocarpine-induced shift of an accommodating intraocular lens: AT-45 Crystalens. J Cataract Refract Surg. 2005;31:1290–1297. doi: 10.1016/j.jcrs.2005.03.055. [DOI] [PubMed] [Google Scholar]
- 46.Kaczerowski MI. The surface of the vitreous. Am J Ophth. 1967;3:419. doi: 10.1016/0002-9394(67)90238-3. [DOI] [PubMed] [Google Scholar]
- 47.Sebag J, Balazs EA. Human Vitreous Fibers and Vitreoretinal Disease. Trans Ophthalmol Soc U K. 1985;104:123–128. [PubMed] [Google Scholar]
- 48.Hill L. Hunterian Oration on blood vessels and pressure. Lancet. 1920;195:5033, 359–366. [Google Scholar]
- 49.Johnson L. Une Nouvelle Theorie De L’Accommodation. Arch Ophthalmol d’Ophtal. 1924;41:746–750. [Google Scholar]
- 50.Noiszewski Le Mecanisme Hydraulique De L’Accommodation. Arch d’Ophtal. 1925;41:477–480. [Google Scholar]
- 51.Fisher RF. Is the Vitreous Necessary for accommodation in man. British Journal of Ophthalmology. 1983;67:206. doi: 10.1136/bjo.67.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gabelt BT, Crawford K, Kaufman PL. Outflow facility and its response to pilocarpine decline in aging rhesus monkeys. Archives of Ophthalmology. 1991;109:879–882. doi: 10.1001/archopht.1991.01080060143044. [DOI] [PubMed] [Google Scholar]
- 53.Gabelt BT, Kaufman PL. Aqueous humor hydrodynamics. In: Kaufman P, Alm A, editors. Adler’s Physiology of the Eye, Clinical Applications. 10. Vol. 6. St. Louis: Mosby; 2002. pp. 237–289. Chapter 8. [Google Scholar]
- 54.Kaufman PL, Gabelt BT. Aging, accommodation and outflow facility. In: Lütjen-Drecoll E, editor. Basic aspects of glaucoma research III. Stuttgart: Schattauer; 1993. pp. 257–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.