Abstract
This review covers history underlying the discovery of the molecular mediators of nicotine's effects in the brain and the diversity of the nicotinic acetylcholine receptor (nAChR) subtypes. Models are presented for both their structure and their function as mediators of signal transduction, with special consideration of the differences between the two main subtypes: heteromeric receptors, which are specialized for rapid electrochemical signal transduction, and homomeric α7 receptors, which have come to be implicated in both ionotropic and metabotropic signaling. The review presents perspectives on the pharmacology and therapeutic targeting of nAChRs for the treatment of nicotine dependence or disease.
Graphical Abstract
The diversity of nicotinic acetylcholine receptors
The pioneering work of Langley on the "receptive substances" in tissues such as smooth and striated muscle led to the discovery of the two classes of molecular receptors of signals generated from the central nervous system. Based on their sensitivity to the plant alkaloids muscarine and nicotine, the receptors in smooth and striated muscle were classified as muscarinic and nicotinic, respectively. Langley observed that the receptive elements on ganglionic nerve cells were more sensitive to nicotine than the corresponding elements on striated muscle, but that, in both tissues, although nicotine produced a brief period of stimulation, the continued presence of nicotine prevented the natural transmission of the stimuli originating from the central nervous system [1]. It was more than two decades later that Otto Loewi confirmed that a natural neurotransmitter, the substance of the vagus (vagusstoff), was mimicked by both muscarine and nicotine [2]. Vagusstoff was to subsequently confirmed by Henry Dale to be acetylcholine [3], a stimulator of the receptive substances in tissues.
After the discovery of acetylcholine (ACh) as the signaling molecule, the challenge remained to discover the way in which the receptors postulated by Langley functioned to stimulate the tissues. It was known that in muscle there was a wave of electrical excitation, similar to that recorded in nerves preceding contraction. Bernard Katz and his co-workers [4] were among the first to describe minute electrical responses arising from the activations of nicotine receptors by acetylcholine. Our current appreciation for the molecular targets of nicotine and ACh has been enlarged by the methods of modern molecular biology, which revealed the rich diversity of related receptors in muscle cells, autonomic ganglia, and in the brain.
The neuromuscular junction was one lamppost that illuminated our first steps to understanding nicotine's receptors and their effects; a second lamppost was the discovery that the electric organ of the Torpedo ray relies on high concentrations of muscle-type nicotinic acetylcholine receptors (nAChR) to generate large noxious electrical currents. The nAChRs of the fish electroplaque organ are so densely concentrated that biochemical isolation of the proteins was possible, aided by snake toxins that bound the proteins with high affinity [5]. The isolation of the fish receptor proteins led to the molecular cloning of the Torpedo receptor subunits [6] and mammalian muscle subunits [7]. Once the sequences of muscle-type receptor subunits were known, the cloning of the nAChRs expressed in nerve cells became possible [8]. It was subsequently appreciated that nAChRs are part of a superfamily of ligand-gated ion channels which include receptors for the inhibitory transmitters GABA and glycine and one type of serotonin receptor. Several structural features are conserved in all members of this gene family, most notably a disulfide-linked sequence of fifteen amino acids that constitutes what has been called the "signature Cys-loop", so that the whole family is referred to as the "Cys-loop superfamily" of ligand-gated ion channels [9].
The first biochemical characterizations of the Torpedo receptor revealed that each receptor was composed of five subunits, arranged like staves of a barrel around a central axis through the membrane, that upon the binding of ACh could form a water permeable ion channel. Torpedo receptors are made up of four different proteins, classified as alpha (α), beta (β), gamma (γ), and delta (δ) based on their sizes determined in gel separation, α being the smallest but with two α subunits in each complex. Snake toxins, such as α-cobra toxin and α-bungarotoxin, competitive antagonists of the receptors, bound only to the α-type subunit in the isolated preparations. Based on these data, the hypothesis was established that the key element for agonist binding was located on the alpha subunit. We now appreciate that the agonist binding sites are at the interface between subunits, in which the alpha subunits provide a primary surface and adjacent subunits provide a complementary surface.
In addition to homologs of the four subunits of Torpedo receptors, it was discovered that muscle nAChRs sometimes contained an alternative subunit, epsilon (ε), which substituted for γ at mature neuromuscular junctions. The alpha subunits of Torpedo and muscle-type receptors contain a pair of vicinal (adjacent) cysteines which are disulfide linked, and reduction of that disulfide bond strongly impairs receptor function. As the family of identified putative nAChR subunits was enlarged, the presence of homologous vicinal cysteines on some subunits was used to classify the newly discovered candidate proteins as alpha subunits.
The agonist binding sites of muscle-type receptors are at the interfaces between the α (α1 in current nomenclature) subunits and the δ and either γ or ε subunits. The β1 subunit does not participate in agonist binding. Subunits filling this fifth position in a pentamer have come to be known as "structural" or "accessory" subunits [9].
The sequence of muscle-type receptor subunits permitted the homology cloning of rat neuronal nAChRs [10]. As noted above, neuronal subunits that contained vicinal cysteines in the ligand-binding domain were classified as alpha-type subunits, but, based on homology alone, it was difficult to classify other neuronal subunits. When it was discovered that some of the newly cloned non-alpha subunits could be co-expressed with mixtures of muscle subunits and substitute for the β1 (but not δ or γ), the neuronal nonalpha subunits were classified as β subunit homologs. Note that, at the time, it was not yet appreciated that the muscle β1 subunit did not participate in agonist binding sites. A total of nine additional alpha-type subunits (α2–α10) and three additional beta subunits (β2−β4) have been cloned from neuronal tissue, although α8 has not yet been reported to be expressed in mammals (Table 1).
Table 1.
Nicotinic acetylcholine receptor subunits
| Heteromeric receptors | ||||
| Muscle type | ||||
| Ligand binding dimers | Structural | |||
| α1δ | α1γ (embryonic) |
α1ε (adult) |
β1 | |
| Neuronal type | ||||
| Ligand-binding alphas | Ligand-binding betas | Structural | ||
| α2 α3 α4 α6 | β2 β4 | α5 β3 | ||
| Homomeric receptors | ||||
| α7 (α8)* α9 (α10)† | ||||
α8 found only in chick
α10 may form heteromeric receptors with a9
The neuronal α2, α3, and α4 nAChR subunits formed functional heteromeric receptors when co-expressed in Xenopus oocytes with either β2 or β4 [10], each pair of subunits forming receptors with distinct pharmacological [11] and single-channel properties [12]. Two subunits, α5 and β3, did not form functional receptors in pairwise co-expression experiments but were later shown be functional homologs of the muscle β1 subunits, able to co-assemble with other subunits as structural subunits. Although structural subunits do not contribute to the primary agonist binding sites, they nonetheless have important impact on the function and pharmacology of the receptor subunit complexes [9].
A second perspective on the potential sites for nicotine's action in the brain came from autoradiographic characterization of nicotine binding sites in brain [13], which showed a correspondence between the binding sites for nicotine and ACh and the expression pattern for α4 and β2 subunits [14]. Heteromeric receptors containing α4 and β2 subunits are now known to constitute the most abundant high-affinity nicotine receptors in rodent brain.
Interestingly, the sites in the brain which bound nicotine with high affinity did not bind α-bungarotoxin, a snake toxin which had proven useful in isolating the muscle nAChR. There was, however, a second class of sites in rodent brain, distinct from those which bound ACh and nicotine with high affinity, which did bind α-bungarotoxin. It remained a mystery for a number of years whether these α-bungarotoxin binding sites represented another class of AChR or some entirely different type of protein. The mystery was only solved by the discovery of a second family of nAChR subunits, α7 – α10 (Table 1), which could function as homomeric, or sometimes heteromeric, complexes without requiring co-assembly with β subunits [15, 16]. Numerous important functional roles have been ascribed to homomeric α7 nAChRs, which are now known to account for the once-mysterious α-bungarotoxin binding sites in brain. Unique properties of these homomeric receptors will be the focus of later sections of this review.
Nicotinic receptor structure
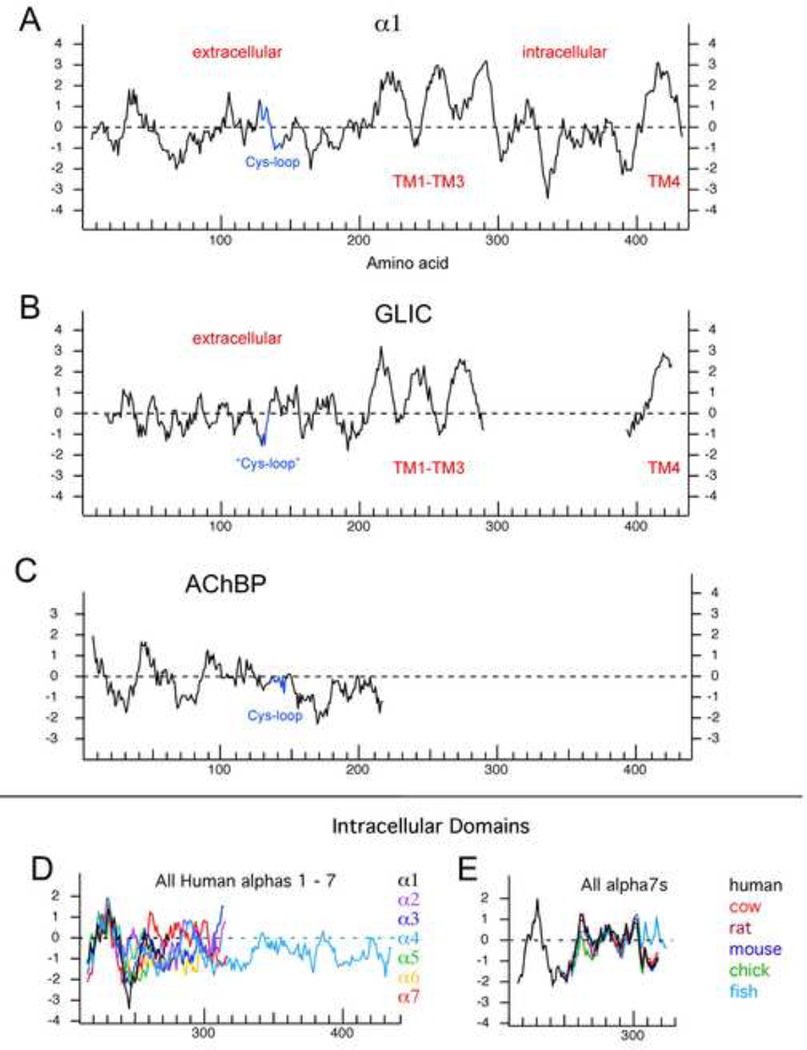
Information from numerous complementary approaches allows us to have a maturing view of the structure of nAChRs and their emergent and diverse functional properties. The nAChR subunit proteins vary in length with a well-conserved transmembrane topology, illustrated in the hydrophobicity plot of the human α1 subunit in Figure 1A. There is a relatively hydrophilic extracellular domain of about 200 amino acids wherein the Cys-loop is embedded. There follow three transmembrane domains, the second of which lines the ion channel [17]. There is an intracellular domain, which varies significantly from one subunit to another, and a fourth transmembrane domain, so that both the amino and carboxy terminals are extracellular. Several ancestral homologs to the Cys-loop receptors have been identified. The hydrophobicity profile of a subunit of the proton-gated receptor, GLIC from Gloeobacter violaceus, is shown in Figure 1B aligned with the α1 sequence. The GLIC protein was crystallized [18], and the X-ray structure shows that it forms homomeric pentamers. GLIC contains a sequence believed to be homologous to the Cys-loop family of vertebrate receptors, although the defining disulfide-linked cysteines are not present. The most outstanding feature of the comparison between the vertebrate protein and the putative bacterial homolog is that neither GLIC nor other putative ancestral bacterial homologs have any intracellular domains. If we are to entertain the hypothesis that the eukaryote receptors evolved from ancestral forms of these bacterial homologs, then one or more gene-linking events must have occurred to combine sequence for intracellular domains from other evolutionary sources.
Figure 1.
Structural elements of nAChR subunits and analogs represented with hydrophobicity profiles. The Kyte-Doolittle plots were generated in DNA Strider (CEA, France). A) Human α1 subunit. B) Bacterial homolog of Cys-loop ligand-gated ion channels, GLIC. Note the absence of any intracellular domain. C) The snail AChBP which has been used for homology modeling of nAChRs. D) The fingerprinting of human nAChR alpha subunits based on their diverse intracellular domains. E) The alignment of α7 intracellular domains from different species. The numbers on the X-axis are based on the amino acid sequence of the α1 subunit in panel A and the respective alignments in the other panels.
As noted above, with the exception of a small (8 amino acid) segment of sequence in the extracellular domain known as the main immunogenic region (MIR) [19], the most diverse element in the nAChR subunit family is the intracellular domain. For example, the intracellular domain of the α4 subunit is 160 amino acids longer than that of the α1 subunit illustrated and more than 100 amino acids longer than any of the other nAChR we know. Figure 1C illustrates the intracellular domains of a variety of alpha subunits.
The swap of the very divergent MIR sequences between β2 and β4 had no detectible physiological or pharmacological effects (Papke and Heinemann, unpublished) aside from antigenicity. In contrast, it is certainly the case that the large intracellular domains of the nAChR subunits play important and diverse functional roles, most of which remain to be elucidated. The intracellular domains are known to contain sites for functionally important phosphorylation [20] and control of differential trafficking of receptors and surface clustering [21].
It is interesting to note that, although the intracellular domains vary from one subunit to another, a phylogenetic analysis of the intracellular domains of nAChR subunits from fish to humans shows that these domains are well conserved for each specific subunit (Figure 1C). That is, the intracellular domain of any human nAChR subunit is more like that of the corresponding subunit from zebrafish than it is to the intracellular domain of any other human receptor. Sadly, for as important as the intracellular domains of nAChRs are likely to be, their absence from the crystallized bacterial proteins and their inability to form ordered structure in high resolution electromicroscopic images of Torpedo receptors [22] means there is essentially no structure information for any of the nAChR intracellular domains.
Although there is not yet any true crystal structure for a nAChR, or for even just the extracellular domain, an interesting method for synaptic modulation evolved in mollusks and other invertebrates which has provided models of nAChR extracellular domains in the form of a crystal structure for an acetylcholine binding protein (AChBP) secreted by glial cells [23]. This protein can function as a neurotransmitter buffer and is homologous to the extracellular domain of homomeric nAChRs, even to the degree that it forms a pentamer with a central vestibule like that of a true nAChR. The hydrophobicity profile of the AChBP is shown in Figure 1D. Although there is a disulfide-defined Cysloop, the AChBP lacks the ability to function as an ion channel activator when spliced onto the appropriate sequence of a homomeric Cys-loop (5HT3) receptor unless sequence changes are made in the Cys-loop and other sub-domains of the AChBP [24]. However, numerous insights of predictive value in terms of the ligand binding domain itself have been gained by studies of AChBP structure and homology models based on that structure [25, 26]. The models that emerged from these structures have largely confirmed hypotheses previously proposed based on studies using site-directed mutants [27].
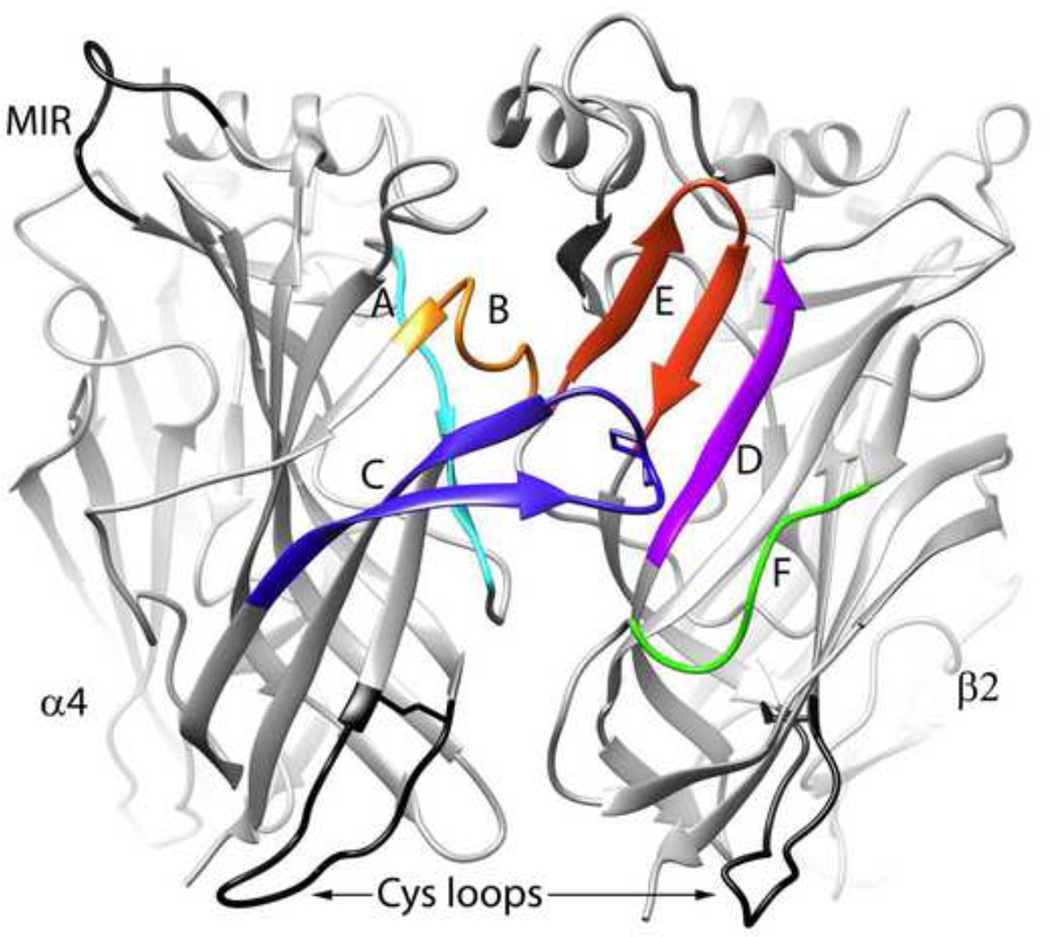
As noted earlier, the AChR ligand binding domains can be configured at the interface between alpha subunits (all but α5) and select non-alpha subunits. The primary surface of the binding domain has three important subdomains, identified as the "A", "B", and "C" loops (Figure 2). The alpha subunit-defining vicinal cysteines are at the apex of the C loop. Three subdomains can also be identified on the complementary surface, designated as the "D", "E", and "F" loops. Since the AChBP forms a homomeric pentamer, its closest analog among the nAChRs is the homomeric α7 receptor. In both the AChBP and the α7 receptor there are five hypothetically identical binding sites. However, each binding site is at the interface between two alpha subunits, so the complementary surface of these binding domains may lack the specializations which evolved in the non-alpha subunits of heteromeric receptors. This is most especially likely to be true for the α7 binding sites, perhaps explaining why these receptors do not bind ACh or nicotine with high affinity and were undetectable in early radioligand binding studies.
Figure 2.
Homology model [106] of the interface between α4 and β2 subunits highlighting the subdomains important for agonist binding that are located on the primary (Loops A, B, and C in the α4 subunit) or complementary surface (Loops D, E, and F in the β2 subunit) of the ligand binding domain. Only the contours of the backbone are shown except for the disulfide-linked vicinal cysteines on the C-loop of α4. The figure was prepared in UCSF Chimera by Dr. Nicole Horenstein.
As noted above, the significance of these subdomains for receptor function was confirmed by site-directed mutagenesis, and these same domains determine the unique pharmacological properties of specific receptor subtypes [25, 28, 29], allowing for the development of subtype selective ligands [30, 31, 32, 33].
Nicotinic receptor function
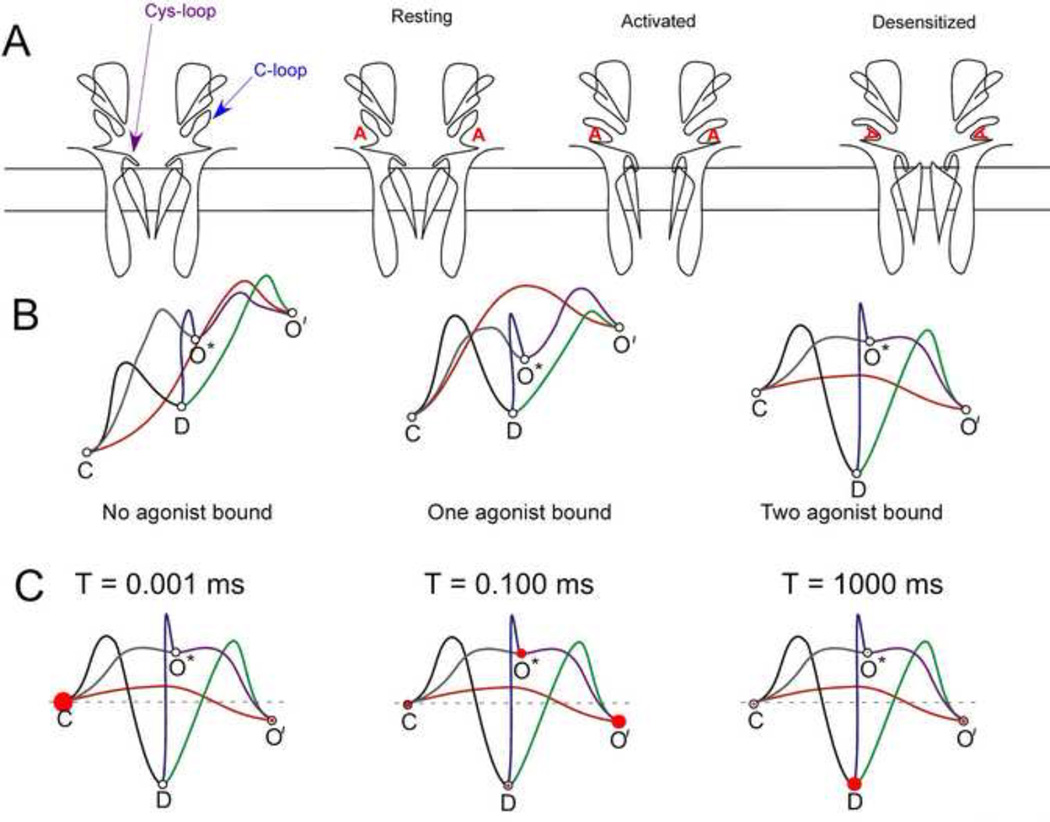
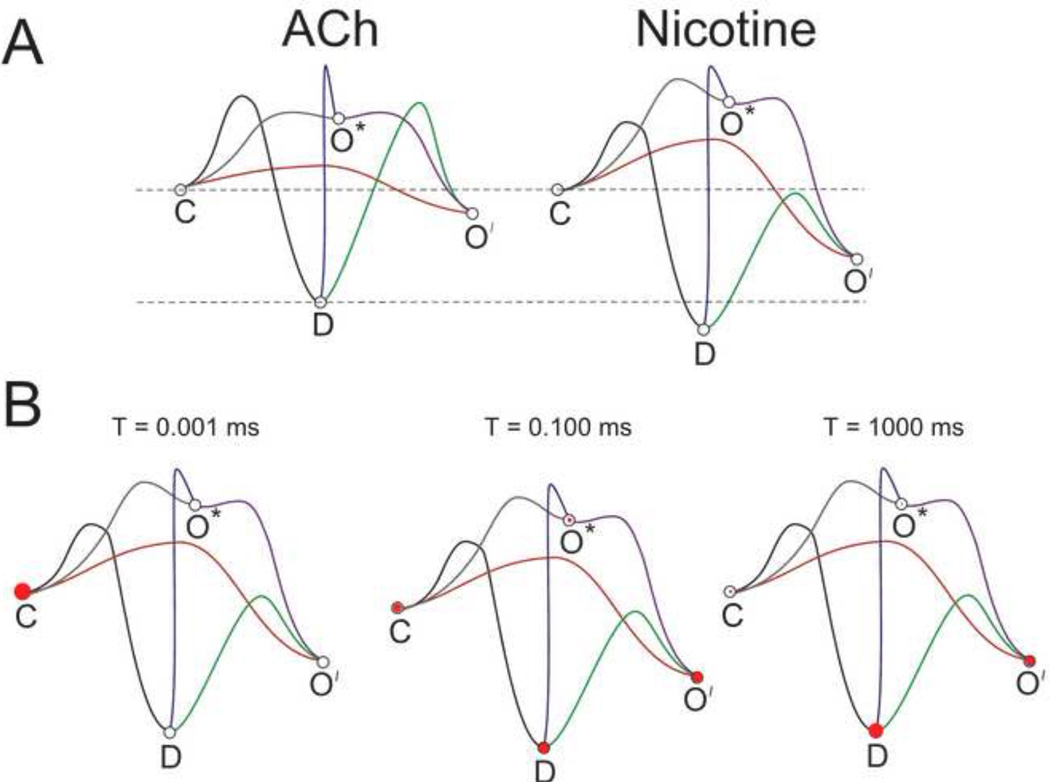
Nicotinic acetylcholine receptors are allosteric proteins that have multiple conformational states, with the equilibria among these states regulated by ligand binding. Just as muscle-type nAChRs provided the early basis for the study of synaptic transmission, they also were among the very first receptors to be studied at the level of single-channel currents and kinetics. The simplest models allow for the existence of four distinct states, illustrated in the cartoons of Figure 3A. In the unbound receptor (resting state) the C-loop of each subunit, which is located over the agonist binding site, extends away from the surface in the "Apo-conformation" [34], and the Cys-loop is in proximity to the short extracellular loop (ECL) between transmembrane domains TM2 and TM3. When agonist binds, a series of conformational changes are energetically favored that lead to the C-loop closing in on the ligand and the dissolution of point-to-point amino acid interactions that are stable in the resting state [25]. This begins a wave of conformational change that is transmitted through the protein promoting an effect at the Cys-loop-ECL interface, leading to the formation of the ion channel and resulting in the activated state. However, the activated state of the receptor is intrinsically unstable and further conformational changes are likely to occur, generating a nonconducting desensitized state. Some conformational changes are retained in the ligand binding domain of desensitized receptors, so that, at least for heteromeric receptors, agonists are bound with high affinity to desensitized receptors.
Figure 3.
Models for the activation and desensitization of heteromeric nAChRs. A) Cartoons of the conformational states of nAChRs highlighting the transmembrane topology and key elements associated with ligand binding and conformational change. B) Theoretical energy landscapes for heteromeric nAChRs with different levels of agonist occupancy. C) Time-dependent changes in the occupation of conformational states by a population of receptors following an instantaneous jump in agonist concentration. The diameter of the red circle represents the proportion of channels in each state at different times.
Shown in Figure 3B are hypothetical energy landscapes for conformation transitions and the absolute free energy of the various states with different levels of agonist occupancy. Heteromeric nAChRs have two agonist binding sites, and the conformational equilibria among the states will vary with the level of agonist binding. Single-channel studies of muscle [35] and neuronal nAChRs [12] revealed the existence of at least two open states which are distinguished by their durations. When agonist concentrations are low, most events recorded are brief (O*), while longer events (O') are observed when concentrations are higher. It was once proposed that the brief events were specifically associated with singly liganded receptors [35]; however, binding site knockout experiments have suggested that receptors with agonist bound to a single activatible site can promote both brief and long-lived events [36].
Two sorts of information are conveyed by the energy landscapes in Figure 3B. Assuming the channel is in any given state, the transition to connecting states will be associated with rate constants inversely proportional to the log of the respective energy barriers. A state which is connected to other states by pathways with shallow energy barriers will be occupied for only brief durations of time. The barriers are therefore indicative of both rate constants and time constants. The second feature reflected in these landscapes is that the absolute height of the states predicts the relative occupancy of the various states if the system is allowed to achieve conformational equilibrium.
For unliganded receptors, the resting closed state is most stable, with very low probability for spontaneous openings, and at equilibrium perhaps one in one hundred receptors would be in the desensitized state [37] and the remainder in the resting closed state. For receptors with agonists bound, the barriers are reduced for the transition from the resting state to the open states, and the equilibrium energies favor the desensitized state, especially when two agonist molecules are bound.
However, the concept of equilibrium among the nAChR conformational states has little relevance to the function of nAChRs as mediators of synaptic transmission. That is because the rapid jump in agonist concentration associated with the vesicular release of acetylcholine will rapidly move a large population of receptors from the condition associated with the left-most landscape (no agonist bound), to the condition reflected in the right-most landscape (both agonist sites bound) [38]. Therefore, when studied as a population, synaptic receptors manifest non-stationary rather than equilibrium kinetic properties. Since, en masse, they have moved from the unbound resting state to the fully bound resting state, they will initially show synchronized behavior, as illustrated in Figure 3C, and many channels will be open all at once for a brief period of time. Subsequent to an initial period, when many channels will be open with high probability, channels will begin to accumulate in the desensitized state and remain there with greatest probability until levels of agonist occupancy are reduced, following the diffusion or metabolism of transmitter. The nAChRs directly under a release site at the neuromuscular junction have been estimated to have a 70–80% probability of opening in the first few milliseconds after transmitter release [38], although in the prolonged presence of agonist, only a small percentage of receptors will be open at any given time as they progress back and forth between activatible and desensitized states [35]. It should be noted though, that at the neuromuscular junction there is a co-localization of nAChRs with high levels of acetylcholine-esterase. This enzyme, which breaks down ACh to its precursors acetate and choline, is embedded throughout the basal lamina, a fibrous matrix that is interposed between pre- and post-synaptic membranes. There is approximately one esterase molecule for every ten receptors, and the breakdown of ACh is so rapid that it has been estimated that those molecules of ACh which diffuse to the postsynaptic surface (≈ 80% of the total released) are unlikely to bind to a receptor more than once [38].
When studied with methods designed to emulate the rapid jumps in ACh concentration that occur at the neuromuscular junction, it is observed that heteromeric neuronal nAChRs can also generate large synchronous currents of short duration [39]. This observation is consistent with the efficient functioning of α3-containing receptors in the synapses of autonomic ganglia, but is of little relevance for the ACh activation of heteromeric nAChRs in the brain, and of even less relevance for understanding the effects of nicotine. Not only is the presentation of nicotine to receptors in the brain different from that of ACh, but the conformational dynamics of receptors bound by nicotine are different from receptors bound by ACh, as can be observed from the macroscopic responses of α4β2 receptors expressed in oocytes [40]. While nicotine is less effective than ACh at producing a large transient response, allowing it to be classified on that basis as a partial agonist, nicotine-evoked responses are more sustained. Although nicotine is very effective at stabilizing α4β2 desensitization, low concentrations of nicotine also produce measurable amounts of steady-state current [41], a phenomenon characterized as "smoldering" [42]. Figure 4 shows a hypothetical model for nicotine compared to that proposed for ACh (Figure 3). Due to a higher initial activation barrier between the resting closed state and the open states, nicotine would initially produce less activation. However, a lower barrier between the D state and the O' state would allow for the steady state current observed experimentally.
Figure 4.
A) Hypothetical energy landscapes for the activation of α4β2 nAChRs by either ACh (Figure 3) or nicotine. Note that nicotine more effectively stabilizes the desensitized state than does ACh. B) Hypothetical time-dependent changes in the occupation of conformational states by a population of receptors following an instantaneous jump in nicotine concentration. Note that although there is initially less synchronous activation of channels than predicted for ACh (see Figure 3C), there is more steady-state current after equilibration is achieved.
As discussed above, the synaptic delivery of acetylcholine to nAChRs at sites like autonomic ganglia or neuromuscular junctions promotes a rapid and transient change in membrane ionic conductance, resulting in the generation of action potentials. The transient nature of these signals is due to both the subsequent rapid metabolism of ACh and the induction of relatively stable nonconducting (i.e. desensitized) conformational states. However, the concept of nAChRs as mediators of fast synaptic transmission cannot be applied to the activity of nicotine, or even ACh, on nAChRs in the brain. The nAChRs of the brain are primarily localized at presynaptic, perisynaptic, or somatic sites rather than concentrated at synapses. In the brain ACh is released in a relatively diffuse manner and functions primarily as a modulator of neuronal excitability, subsequently modulating the release of various neurotransmitters, including glutamate, GABA, norepinephrine, ACh itself, and dopamine (DA) [43].
Even though the cholinergic signals in brain are slower and more diffuse than those at cholinergic synapses such as the neuromuscular junction, the presentation of nicotine to the receptors is even more diffuse, slower still by several orders of magnitude, and not rapidly reversed by metabolism. Additionally, nicotine will affect all of the many different nAChR subtypes in brain to varying degrees. Such varying effects of nicotine have been characterized using both ex vivo preparations of native rodent receptors [44] and pure populations of heterologously expressed human nAChRs [45] in vitro. However, the conclusions derived from the majority of these preclinical studies are compromised because of a mismatch between how nicotine reaches the receptors in a typical experimental protocol and how much more slowly it reaches receptors in the brain [41]. Specifically, when a potentially activating molecule like nicotine is delivered slowly, as it would be with systemic administration via a patch or pill, there is relatively little synchronized receptor activation, and, instead, receptors equilibrate amongst multiple conformational states [46]. This equilibrium predominantly favors desensitization and therefore also blunts receptor-mediated responses to endogenous cholinergic stimuli. Of course, some forms of nicotine delivery, whether from tobacco products or nicotine replacement therapies used for smoking abatement, will provide faster delivery of the drugs to receptors in the brain than other forms. With the relatively rapid delivery achieved with cigarettes, the onset of equilibrium desensitization will be preceded by a phase of direct receptor activation. Although it remains something of a topic for debate [47], it is generally believed that the transient phase of receptor activation achieved with cigarette delivery of nicotine is important for the immediate reinforcing properties of the drug and that this reinforcement is associated with a transient increase in mesolimbic DA release. While it is generally believed that the transient release of DA is essential for drug reinforcement, the neuropharmacology of nicotine dependence is more complicated. The chronic use of the drug changes the expression levels and the molecular character of the brain nAChRs themselves, so that even endogenous cholinergic signals are affected [48].
Studies of genetically modified (knockout) mice have been especially useful for identifying the specific receptor subtypes associated with nicotine reward and dependence [49]. Animals lacking either α4 or β2 subunits are unlikely to become nicotine dependent through self-administration. The α4β2* receptors (containing two α4β2 agonist binding dimers and a fifth subunit, most often α4, β2, or α5) have been shown to be directly affected by chronic exposure to nicotine. They increase in number, and also, at least in some tissues and cell types, the subunit composition of the receptors changes from ones containing three α4 and two β2 subunits to receptors with the reverse ratio, due to a preferential chaperoning of α4(2)β2(3) receptors by the nicotine. Illustrating the functional importance of structural subunits, the α4(2)β2(3) receptors induced by nicotine exposure are referred to as a high sensitivity (HS) subtype since they respond to relatively low concentrations of ACh and nicotine compared to receptors with the reverse stoichiometry, α4(3)β2(2), low sensitivity (LS) α4β2 receptors [50]. A second class of nAChRs associated with nicotine reward, if not dependence, are those containing α6 subunits, often in combination with α4, β2 and β3. These α6* receptors are found on dopaminergic neuron cell bodies and terminals and contribute to the increase in mesolimbic DA release transiently stimulated by nicotine [51]. The β3 subunit, which, as noted above, functions only as a structural element, is of special importance for the function of some α6-containing receptors [52].
The inclusion of the other obligatory structural subunit (i.e. not known to be able to form ligand binding sites), α5, has also been shown to have important functional effects, imparting high sensitivity to agonists when co-expressed with α4 and β2 or α2 and β2 [53]. It has also been shown to be required for animals to manifest aversive effects to high nicotine doses. This latter effect is especially associated with α5 expression in the medial habenula, a brain nucleus highly enriched in nAChR subunit expression [14], where α5 is believed to co-assemble with α3 and β4 subunits [54]. Receptors containing α3, β4, and α5 are also one of the subtypes in autonomic ganglia, along with other α3-containing receptors [55]. These three subunits are part of the same gene cluster, and polymorphisms in α5 have been associated with heavy smoking and increased cancer risk [56].
Homomeric α7 nAChRs
Alpha7 nAChRs are distinguished from other nAChRs by a number of unique physiological and pharmacological properties, including a high permeability to calcium (PCa:PNa of ≥10) and rapid and reversible desensitization [15, 57, 58]. The α7 subunit is expressed at high levels in the hippocampus and hypothalamus [15], and α7 nAChRs have also been shown to have functionally important expression in non-neuronal tissues such as cells of the immune system [59]. Phylogenetic data indicate that the α7 gene represents a sort of ancestral nAChR, a protein that may have evolved in organisms that did not rely on fast chemical neurotransmission. This is consistent with the presence of α7 in numerous non-neuronal cell types [60, 61] and the fact that α7 receptors are not strictly receptors for acetylcholine but respond also to choline [62], the ubiquitous precursor to ACh. This feature alone would suggest that they are not adapted for rapid synaptic transmission, as occurs at the neuromuscular junction.
The functional properties of α7 receptors have been studied in Xenopus oocytes [58, 63], cultured hippocampal neurons [64], mammalian cell lines [57], and native neuronal tissues [65]. One consistent finding is that the kinetic properties of α7 receptors cannot be adequately described by the models of heteromeric nAChRs discussed above.
For a quiescent population of heteromeric nAChRs in the absence of agonist, the rapid application of a concentration of ACh sufficiently high to saturate the agonist binding sites produces maximal synchronous transient activation. When a similar application of agonist is made to a population of α7 receptors, the maximal synchronous transient activation occurs when only a fraction of the agonist binding sites are occupied [36, 57, 58, 63, 66]. This observation suggests that, as with the case of heteromeric receptors, the allosteric effects of binding to just one or two of the five possible binding sites may promote channel opening [36, 67], but that at higher levels of binding, the receptor is most likely to adopt nonconducting conformations. This concentration-dependent form of desensitization, described in detail elsewhere [58], is unique to α7, which are sensitive to a class of highly selective positive allosteric modulators (PAMs) that can destabilize that desensitized state that is unique to α7 and thereby greatly increase the probability of channel opening.
Heteromeric nAChRs are well adapted to function as mediators of synaptic signaling, so that when a population of, for example, α4β2-containing receptors are stimulated by a rapid increase in ACh concentration, as occurs at a synapse, each activatible receptor has an 80% probability of opening during a large synchronized current [39] that then decays due to receptor desensitization and the rapid hydrolysis of ACh. In contrast, under similar conditions, homomeric α7 receptors have only an 0.3% probability of opening, and under steady-state conditions the probability of any single α7 receptor being open is less than one in a million [57]. While the average open times of heteromeric neuronal nAChRs are several milliseconds and often occur in bursts, for α7 receptors openings are typically less than 100 µs and usually occur in isolation.
Extremely efficacious α7 PAMs, such as PNU-120596, can promote bursts of single channel openings lasting for several seconds, accounting for a hundred thousand-fold increase in a single channel's current. It is interesting to note, however, that the same PAM produces only a 200–500-fold increase in whole-cell currents, so the basic mechanism of the potentiation relies on large effects on a small fraction of channels in the PAM-sensitive desensitized state (Ds), while the majority of the channels remain in a desensitized condition that is insensitive to the PAM (Di) [58].
The desensitized states of α7 receptors can also be further distinguished from those of heteromeric nAChRs because they do not show significantly increased affinity for agonists compared to the resting state of the receptor. Consequently, while the prolonged presence of a low concentration of ACh, or a drug like nicotine, will induce high levels of heteromeric receptor desensitization, α7 receptors will remain responsive to fluctuating stimuli. The functional significance of this difference has been highlighted in studies of the dopaminergic outflow from the ventral tegmentum, where α7 and non-α7 receptors play contrasting neuromodulatory roles [68].
The ancestral character of α7 receptors, their sensitivity to choline, intrinsically low ion-channel activity, and their presence in cell types where receptor-mediated ion currents have not been reported [69], all suggest that these receptors have functions beyond what can be ascribed to ion channel activation [70]. Even though the ionic currents of α7 receptors are very small, it is generally assumed by those who study signaling in neuronal cells, that even receptor-independent changes in intracellular calcium are nonetheless initiated by α7 nAChR-mediated ion current, either producing sufficient depolarization to activate voltage-dependent channels or enough calcium to stimulate calcium-dependent calcium release [71]. However, this remains unproven and is especially unlikely to be true for non-neuronal cells, where no α7-mediated ionic currents can be detected [69].
The key function of α7 nAChRs in non-neuronal tissues [72] appears to be metabotropic rather than ionotropic [69, 73]. Moreover, α7-mediated signal transduction can be effectively stimulated by very weak partial agonists such as GTS-21 [74] or alternative ligands that produce essentially no ion channel activation but induce the conformational state associated with ion channel desensitization [75]. These data suggest that there may be multiple forms of α7-mediated signaling. Some α7-mediated effects, such as positive results in cognitive tests, may depend on ion channel activation [76], while other effects may be independent of ion-channel currents. Proteomic analysis has shown that α7 nAChRs interact with many intracellular signal transducing proteins [70]. These interactions rely on the intracellular domain, which is unique to the α7 proteins. The many studies indicating α7-mediated ion channel-independent signal transduction make it reasonable to hypothesize that even while the ion channel may be desensitized, ligand binding can communicate to the intracellular domain and effect metabotropic signaling.
Embracing the complexity of nAChR function in the brain
Several studies have described the diversity of native nAChR subtypes in different regions of the brain, and even within a single tissue such as the hippocampus or the VTA, different nAChR subtypes show unique patterns of cellular or subcellular localization [9] and function [77]. Optogenetics and in vivo microdialysis studies [78, 79] are beginning to shed light on the nature of the endogenous cholinergic signaling. The questions remain though: to what degree does nicotine replace the natural activation of receptors by ACh, augment it, subvert it, or shift the balance among the effects of different nAChR subtypes? The answer is probably, "all of the above", but it depends on whether it follows from the first puff of the today’s cigarette or the lingering effects of another cigarette late in the day.
Our best tools for addressing questions in whole animal experiments are drugs which have the ability to be probative of the activation or inhibition of specific nAChR subtypes in vivo. However, the use of these drugs will also rely on meaningful interpretation of context, how they reach the receptors in the brain, and how their effects will segue from the effects of either nicotine or ACh. Some drugs need help to cross the blood brain barrier [80]; others are most probative only if delivered to specific target nuclei within the brain. For example, while nicotine-evoked dopamine release from synaptosomes prepared from the nucleus accumbens can be blocked in vitro by the nAChR antagonist mecamylamine, the release of dopamine in the nucleus accumbens stimulated by the systemic delivery of nicotine and measured in vivo by microdialysis is not blocked by delivery of mecamylamine directly into the nucleus accumbens but rather by delivery of mecamylamine into the ventral tegmentum [81], showing that the nAChR responsible for nicotine-evoked release in the nucleus accumbens in vivo are not the presynaptic receptors on dopaminergic terminals in the nucleus accumbens, but rather the ones on glutamatergic or dopaminergic neurons in the ventral tegmentum.
The challenge we face is to assemble all of the little pieces revealed by the available approaches into a coherent picture that will allow us to understand how a drug like nicotine can have so many effects on behavior, some profound, and others amazingly subtle and almost insidious.
Nicotinic receptor pharmacology
Drugs affecting nAChRs can be broadly classified as agonists, antagonists, or modulators, and each of these categories can be further subdivided. Based on their relative efficacies compared to a reference agonist (usually ACh), activating molecules may be classified as full or partial agonists, or occasionally as super or hyper agonists if they activate receptors more effectively than ACh. Antagonists may be classified as either competitive or noncompetitive, based on whether they target (i.e. compete at) the same site as agonists. Competitive antagonism can be overcome by increasing the concentration of the agonist, while noncompetitive antagonism is not surmountable.
Modulators can be either direct- or indirect-acting, depending on whether they bind to the receptor itself or to other proteins that control the metabolism or uptake of agonists. Inhibitors of acetylcholinesterase are indirect modulators of nAChRs, but only in regard to activation by ACh, since they do not affect activation by nicotine or other drugs. By binding to the receptor itself, direct-acting modulators are likely to affect the activation by any agonist. Direct-acting modulators change the energy landscape for conversion between the conformational states of the receptor by altering energy barriers and/or the equilibrium distribution of receptors among the conformational states [82], making them allosteric modulators. However, from the original sense of nAChRs as allosteric proteins [83], agonists are also allosteric modulators. The current trend is to identify agonists as "orthosteric" ligands and their binding site as the "orthosteric site", as distinguished from modulators which produce no activation on their own but through binding to distinct "allosteric" sites alter the energy landscape for conformational changes that occur when agonists bind to the orthosteric site.
For all of these types of drugs, a key factor is whether they have selectivity for one type or class of nAChRs compared to others, in which case they become potentially useful as experimental tools. Additionally, if we want to move toward therapeutic targeting of nAChRs, the second key factor is whether or not the drugs will reach sites in the brain if given systemically. Depending on the indication, drugs may only be useful if they enter the brain, or, alternatively, brain penetration may lead to undesired side effects if the therapeutic target is peripheral. For example, hexamethonium and mecamylamine are both relatively non-selective neuronal nAChR antagonists and have some activity as antihypertensives due to their ability to block transmission through autonomic ganglia. Mecamylamine enters the brain while hexamethonium cannot, so mecamylamine has been investigated for other indications such as Tourette's, smoking cessation, and depression [84]. In contrast, physostigmine and neostigmine are both acetylcholinesterase inhibitors, but neostigmine is the preferred treatment for the muscle weakness in myasthenia gravis since it will not enter the brain and produce a wide spectrum of cholinergic side effects.
The concepts of selectivity and partial agonism impart additional levels of complexity to the simple concepts of agonists and antagonists. An agent which selectively activates one receptor subtype may bind to other receptors as well and act as a competitive antagonist [85] or desensitizing agent [86] at those receptors. Likewise, there is a complicated range of effects that may occur based on interactions between full and partial agonists, such that, depending on relative concentrations and modes of delivery, a partial agonist may augment or inhibit the effects of a full agonist, and in the absence of the full agonist, it may provide activation on its own [41]. Nicotinic drugs in use or proposed for indications such as smoking cessation [87], Alzheimer's disease [88], depression [89], and schizophrenia [90] are partial agonists. They will have varying degrees of intrinsic activity and will also strongly modulate intrinsic cholinergic activity as well as the effects of nicotine and other drugs. While nicotine can readily penetrate the blood-brain barrier, it is an agonist with varying efficacy for all nAChR subtypes. Although it binds to certain heteromeric nAChRs with high affinity, such as α4- and α2-containing receptors, it activates them with potency much lower than the binding affinity [40]. The progression of nicotine's effects in the brain will also follow the dynamics illustrated in Figure 3C. If rapidly delivered to receptors in their resting states, as with the first puff of the first cigarette of the day, it will produce synchronous activation of receptors. However, with the accumulation of nicotine through a smoker's day, or with slower delivery of nicotine as from a patch, receptors will be moved toward the equilibrium condition favoring desensitization and reduced responses to endogenous ACh.
Drugs selective for α7 nAChRs
Selective antagonists are extremely valuable tools for teasing apart the roles played by nAChR subtypes in vivo or in complex ex vivo preparations such as brain slices [91] or synaptosomal preparations [92]. The usual α7-selective antagonist of choice is methyllycaconitine (MLA). While in acute co-application experiments MLA is neither particularly potent nor selective for α7 receptors [93] due to the slow reversibility of the α7 block, pre-applications of MLA block α7 receptors effectively at concentrations of 10–30 nM with little effect on other receptors at concentrations < 100 nM. Although normally used for in vitro or ex vivo experiments, MLA has also been used in vivo with systemic delivery and apparent effects in the CNS [94]. Other α7-selective antagonists have come from the characterization and refinement of conotoxins, for example alpha-conotoxin ArIB [V11L,V16D] [95], which when infused locally into the nucleus accumbens was used to indicate that decreased α7 nAChR function in this area increased motivation of animals to work for nicotine infusions.
There has been a great deal of drug development in the area of α7-selective agonists and partial agonists. The classic pharmacophore for a nicotinic agonist, developed from studies of the neuromuscular receptor [96], suggested that a precise spatial arrangement of two elements, a cationic nitrogen and a hydrogen-bond acceptor were required for an effective nicotinic agonist. Subsequent analysis of nAChRs in a heterologous expression system [62] showed that the only element required for full activation of neuronal nAChRs was the quaternary nitrogen, as in the minimal structure of tetramethylammonium. Elaboration of, or extensions to, this minimal core element has provided the basis for the development of several lines of selective agents, since the ligand binding domains of the various receptor subtypes provide different locks accepting diverse keys. While both heteromeric neuronal and α7 nAChRs are readily activated by both tetramethylammonium and the somewhat larger ethyltrimethylammonium, the additional hydroxyl group present in choline largely precludes the activation of heteromeric nAChRs but not α7. Presumably the presence of the hydroxyl in the specialized binding site of heteromeric receptors prevents the quaternary nitrogen of choline from orienting in such a way so as to lower the energy barriers for activation, or the hydroxyl itself participates in point-to-point interactions which favor non-conducting states. It cannot be because choline is simply larger than ethyltrimethylammonium, since the esterification of choline with the larger acetate group results in ACh, a key that fits the locks of all nAChRs.
While we can identify tetramethylammonium as the minimal pharmacophore for neuronal nAChRs, several other more elaborate core agonist structures have been identified which have been the basis for many drug development programs. Nicotine is one such molecule, along with others such as anabaseine, cytisine, and quinuclidine, which activate multiple types of nAChRs. Interestingly, α7-selective agonists can be derived from any of these core structures based on any one of several α7-selectivity motifs [31]. Just as the addition of a hydroxyl to ethyltrimethylammonium leads to α7 selectivity of choline, addition of a hydroxyl to the nonselective agonist quinuclidine results in the α7-selective quinuclidinol. Two other α7-selectivity motifs are based on the addition of either large or small hydrophobic groups, either distant or adjacent to the cationic center, respectively. The first class of α7-selective agonists to be identified were benzylidene anabaseines [97], which have α7-selectivity associated with the addition of a large benzene ring to the non-selective core agonist anabaseine. The same modification to quinuclidine also results in α7-selectivity [31]. In addition to being able to generate α7-selectivity to ethyltrimethylammonium through the addition of a hydroxyl, the addition of a single methyl to the other pole of ethyltrimethylammonium results in diethyldimethylammonium, which is also an α7 selective agonist. Likewise, the same motif can be applied to quinuclidine to generate another α7-selective quinuclidine derivative based on what has been identified as the tropane motif [31].
Many of the α7-selective agonists that have been identified are partial agonists, some with efficacy quite a bit lower than that of ACh. Interestingly, although as discussed above, the α7 ion channels are never very effectively opened, even by the most efficacious α7 agonist, α7 partial agonists with relatively low efficacy have been shown to have good functional activity in assays of signal transduction both in vivo and in vitro. For example, one of the first α7-selective partial agonists to be used in any human trials, GTS-21 (3-(2,4-dimethoxybenzylidene)anabaseine, also DMXB-A), has very low efficacy for human α7 compared to rat yet had positive effects in several human trials for different indications [98, 99]. It has been proposed that GTS-21 may function as a prodrug in humans since its primary metabolite 4OH-GTS-21 (3-(4-hydroxy-2-methoxybenzylidene) shows significantly greater efficacy for human α7 receptors [100]. However, GTS-21 has also been effective in cell-based assays for signal transduction where metabolism to 4OH-GTS-21 would not have occurred [74]. This highlights another interesting aspect of GTS-21 and certain other α7-selective agonists, which challenges the dogmatic thinking that all signaling by a ligand-gated ion channel receptor has to rely strictly on ion channel activation. Although relatively ineffective at activating the ion channel of α7, GTS-21 has the effect of stabilizing non-conducting (desensitized) conformations, which may, in fact, be capable of mediating channel-independent signal transduction. Alpha7 receptors are expressed in many non-excitable cells such as macrophages and microglia [74, 101], and stimulation of those receptors with α7-selective drugs such as GTS-21 modulates signal transduction under conditions where no ion channel currents could be recorded [69]. Further support for the hypothesis that α7 receptors may mediate two qualitatively different forms of signal transduction comes from drugs such as NS6740, an α7-selective partial agonist with efficacy so low as to be essentially zero. NS6740, a drug which might be considered a "silent agonist" [102], has been compared to efficacious analogs in two different paradigms. NS6740 was ineffective when tested for activity in an assay of cognitive performance compared to the more efficacious drugs A-582941 and NS6784 [76]. However, when tested for their activity for suppressing a pro-inflammatory response by microglia, both NS6740 and GTS-21 were effective, while the more efficacious agonists SSR180711 and A-582941were ineffective.
In recent years a rich new area for drug development and experimental investigation has come from the discovery of α7-selective positive allosteric modulators (PAMs, reviewed in [82]). PAMs of other Cys-loop receptors have been in clinical usage for decades in the form of benzodiazepines such as diazepam (valium), which increases the effectiveness of the inhibitory transmitter GABA at its receptors. While a few PAMs have been identified for heteromeric nAChRs [82], their activity is relatively low compared to new drugs that been have developed targeting α7. The most efficacious of the α7 PAMs, such as PNU-120596, seem to be able to reverse or destabilize the unique desensitized states of α7, producing large increases in amplitude and duration of whole cell currents [58]. These PAMs have been classified as type II modulators. Other PAMs such as 5-hydroxy indole, which increase the amplitude of α7-mediated transient currents but not their durations, are classified as type I PAMs [82]. In the context of the energy landscapes for the conformational states of the receptors, type I PAMs appear to primarily lower the energy barriers into the open state, while type II PAMs may also lower the overall free energy of the conducting states, as well as promoting novel conformations which are also able to conduct ions [103]. Also, although it has the effect of destabilizing some desensitized states, the receptors ultimately achieve an equilibrium favoring states which are insensitive to the channel activating effects of the drug.
The ion channel of the α7 receptor, when open, is highly permeable to calcium [15], and so it has been the conventional thinking to equate all α7 signaling not only to channel activation but more specifically to channel-mediated calcium flux. This thinking prevails, even though most cell-based calcium fluorescence assays of α7 in the absence of PAMs have been shown to report not channel-mediated increases in calcium but calcium release from intracellular stores or calcium flux through voltage-dependent calcium channels [104, 105]. As we continue to investigate the broad range of effects that extremely efficacious PAMs like PNU-120596 can have, the trend to associate the effects of the PAM with channel-mediated calcium flux will undoubtedly continue. However, this may not always be the true effect. For example, we have reported that PNU-120596 in combination with choline can have MLA-sensitive cytotoxic effects on an α7-expressing cell line, an effect that was assumed to be due to calcium-mediated excitotoxicity [57]. However, in subsequent studies with non-competitive antagonists, it was discovered that the most protective antagonists were those which had mechanisms least consistent with simple channel block, and, moreover, the cytotoxic effects of PNU-120596 plus choline did not require extracellular calcium and were consistent with apoptotic rather than excitotoxic cell death [103].
With highly selective antagonists, agonists, and modulators available, α7 nAChRs are approachable targets, both experimentally and therapeutically. However, the challenge remains to appreciate that these receptors have evolved on a path quite different from that of muscle-type nAChRs and other subtypes more specialized for synaptic transmission or high-probability transient activation by ACh.
Summary and conclusion
Tremendous progress has been achieved from the observation of Galvani in the mid 1700's that electrical stimulation of a nerve fiber connected to a muscle could lead to muscle contraction. In this review we have focused on a single molecular mediator in such a chain of events, the nicotinic acetylcholine receptor. At the time of Galvani's experiments, people in European cultures had already gathered over a hundred years of empirical experience on the effects of nicotine in the form of tobacco products on human brain. As a culture we are still trying to deal with the enduring effects of those experiments.
We can now appreciate that, while our understanding of the nicotinic receptor of the neuromuscular junction was a valuable starting point for understanding the natural function of nicotinic receptors in the brain, we must also reconsider those lessons in order to conceive of therapeutically targeting neuronal nicotinic receptors for the management of disease or nicotine dependence. Moreover, in the case of functions served by α7 nAChRs, we must take that challenge to think differently even further. The old perspective that the importance of a ligand-gated ion channel decreases with the square of the distance from the membrane's electric field must be discarded if we are to ever understand the cascading effects of stimulating such an ancient receptor.
Acknowledgments
RLP is supported by [RO1 GM57481]. I thank Dr. Nicole Horenstein for helpful comments and preparation of Figure 2.
Abbreviations
- ACh
acetylcholine
- nAChR
nicotinic acetylcholine receptor
- PAMs
Positive allosteric modulators
- Ds
PNU-120596-sensitive desensitization
- Di
PNU-120596-insensitive desensitization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Langley JN. On the reaction of cells and nerve-endings to certain poisons, chiefly as regards the reaction of striated muscle to nicotine and to curari. J Physiol. 1905;33:374–413. doi: 10.1113/jphysiol.1905.sp001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loewi O. On the background of the discovery of neurochemical transmission. J Mt Sinai Hosp N Y. 1957;24:1014–1016. [PubMed] [Google Scholar]
- 3.Dale H. Transmission of Nervous Effects by Acetylcholine: Harvey Lecture, May 20, 1937. Bulletin of the New York Academy of Medicine. 1937;13:379–396. [PMC free article] [PubMed] [Google Scholar]
- 4.Fatt P, Katz B. An analysis of the endplate potential recorded with an intracellular electrode. Journal of Physiology (London) 1951;115:320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlin A, Cowburn DA. The affinity-labeling of partially purified acetylcholine receptor from electric tissue of electrophorus. Proc Natl Acad Sci USA. 1973;70:3636–3640. doi: 10.1073/pnas.70.12.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noda M, Furutani Y, Takahashi H, Toyosato M, Tanabe T, Shimizu S, et al. Cloning and sequence analysis of calf cDNA and human genomic DNA encoding alpha-subunit precursor of muscle acetylcholine receptor subunits. Nature. 1983;302:818–823. doi: 10.1038/305818a0. [DOI] [PubMed] [Google Scholar]
- 7.Tanabe T, Noda M, Furutani Y, Takai T, Takahashi H, Tanaka K-I, et al. Primary structure of alpha subunit precursor of calf muscle acetylcholine receptor deduced from cDNA sequence. Eur J Biochem. 1984;144:11–17. doi: 10.1111/j.1432-1033.1984.tb08424.x. [DOI] [PubMed] [Google Scholar]
- 8.Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neural nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci USA. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78:703–711. doi: 10.1016/j.bcp.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 10.Heinemann S, Boulter J, Deneris E, Conolly J, Duvoisin R, Papke R, et al. The brain nicotinic acetylcholine receptor gene family. Prog Brain Res. 1990;86:195–203. doi: 10.1016/s0079-6123(08)63177-5. [DOI] [PubMed] [Google Scholar]
- 11.Luetje CW, Patrick J. Both α- and β-subunits contribute to the agonist sensitivity of neuronal nicotinic acetylcholine receptors. J Neurosci. 1991;11:837–845. doi: 10.1523/JNEUROSCI.11-03-00837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Papke RL. The kinetic properties of neuronal nicotinic receptors: genetic basis of functional diversity. Prog in Neurobio. 1993;41:509–531. doi: 10.1016/0301-0082(93)90028-q. [DOI] [PubMed] [Google Scholar]
- 13.Clarke PBS, Schwartz RD, Paul SM, Pert CB, Pert A. Nicotinic binding in rat brain: autoradiographic comparison of [3H] acetylcholine [3H] nicotine and [125I]-alpha-bungarotoxin. J Neurosci. 1985;5:1307–1315. doi: 10.1523/JNEUROSCI.05-05-01307.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wada E, Wada K, Boulter J, Deneris E, Heinemann S, Patrick J, et al. Distribution of alpha2, alpha3, alpha4, and beta2 neuronal nicotinic receptor subunit mRNAs in the central nervous system: a hybridization histochemical study in the rat. J Comp Neurol. 1989;284:314–335. doi: 10.1002/cne.902840212. [DOI] [PubMed] [Google Scholar]
- 15.Seguela P, Wadiche J, Dinely-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13(2):596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgoyhen AB, Vetter DE, Katz E, Rothlin CV, Heinemann SF, Boulter J. alpha10: a determinant of nicotinic cholinergic receptor function in mammalian vestibular and cochlear mechanosensory hair cells. Proc Natl Acad Sci U S A. 2001;98:3501–3506. doi: 10.1073/pnas.051622798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hucho F, Oberthuer W, Lottspeich F. The ion channel of the nicotinic acetylcholine receptor is formed by the homologous helices M II of the receptor subunits. FEBS Lett. 1986;205:137–142. doi: 10.1016/0014-5793(86)80881-x. [DOI] [PubMed] [Google Scholar]
- 18.Bocquet N, Nury H, Baaden M, Le Poupon C, Changeux JP, Delarue M, et al. X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature. 2009;457:111–114. doi: 10.1038/nature07462. [DOI] [PubMed] [Google Scholar]
- 19.Tzartos SJ, Kokla A, Walgrave SL, Conti-Tronconi BM. Localization of the main immunogenic region of human muscle acetylcholine receptor to residues 67-76 of the α subunit. Proc Natl Acad Sci USA. 1988;85:2899–2903. doi: 10.1073/pnas.85.9.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollock VV, Pastoor T, Katnik C, Cuevas J, Wecker L. Cyclic AMP-dependent protein kinase A and protein kinase C phosphorylate alpha4beta2 nicotinic receptor subunits at distinct stages of receptor formation and maturation. Neuroscience. 2009;158:1311–1325. doi: 10.1016/j.neuroscience.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kracun S, Harkness PC, Gibb AJ, Millar NS. Influence of the M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br J Pharmacol. 2008;153:1474–1484. doi: 10.1038/sj.bjp.0707676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005;346:967–989. doi: 10.1016/j.jmb.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 23.Smit AB, Syed NI, Schaap D, van Minnen J, Klumperman J, Kits KS, et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature. 2001;411:261–268. doi: 10.1038/35077000. [DOI] [PubMed] [Google Scholar]
- 24.Bouzat C, Gumilar F, Spitzmaul G, Wang HL, Rayes D, Hansen SB, et al. Coupling of agonist binding to channel gating in an ACh-binding protein linked to an ion channel. Nature. 2004;430:896–900. doi: 10.1038/nature02753. [DOI] [PubMed] [Google Scholar]
- 25.Mukhtasimova N, Free C, Sine SM. Initial coupling of binding to gating mediated by conserved residues in the muscle nicotinic receptor. J Gen Physiol. 2005;126:23–39. doi: 10.1085/jgp.200509283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isaacson MD, Horenstein NA, Stokes C, Kem WR, Papke RL. Point-to-point ligand-receptor interactions across the subunit interface modulate the induction and stabilization of conformational states of alpha7 nAChR by benzylidene anabaseines. Biochem Pharmacol. 2013;85:817–828. doi: 10.1016/j.bcp.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corringer PJ, Le Novere N, Changeux JP. Nicotinic receptors at the amino acid level. Annu Rev Pharmacol Toxicol. 2000;40:431–458. doi: 10.1146/annurev.pharmtox.40.1.431. [DOI] [PubMed] [Google Scholar]
- 28.Williams DK, Stokes C, Horenstein NA, Papke RL. Differential regulation of receptor activation and agonist selectivity by highly conserved tryptophans in the nicotinic acetylcholine receptor binding site. J Pharmacol Exp Ther. 2009;330:40–53. doi: 10.1124/jpet.109.151225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horenstein NA, McCormack TJ, Stokes C, Ren K, Papke RL. Reversal of agonist selectivity by mutations of conserved amino acids in the binding site of nicotinic acetylcholine receptors. J Biol Chem. 2007;282:5899–5909. doi: 10.1074/jbc.M609202200. [DOI] [PubMed] [Google Scholar]
- 30.Gotti C, Riganti L, Vailati S, Clementi F. Brain neuronal nicotinic receptors as new targets for drug discovery. Curr Pharm Des. 2006;12:407–428. doi: 10.2174/138161206775474486. [DOI] [PubMed] [Google Scholar]
- 31.Horenstein NA, Leonik FM, Papke RL. Multiple pharmacophores for the selective activation of nicotinic alpha7-type acetylcholine receptors. Mol Pharmacol. 2008;74:1496–1511. doi: 10.1124/mol.108.048892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glennon RA, Dukat M, Liao L. Musings on alpha4beta2 Nicotinic Acetylcholine (nACh) Receptor Pharma-cophore Models. Curr Top Med Chem. 2004;4:631–644. doi: 10.2174/1568026043451122. [DOI] [PubMed] [Google Scholar]
- 33.Tonder JE, Olesen PH, Hansen JB, Begtrup M, Pettersson I. An improved nicotinic pharmacophore and a stereoselective CoMFA-model for nicotinic agonists acting at the central nicotinic acetylcholine receptors labelled by. J Comput Aided Mol Des. 2001;15:247–258. doi: 10.1023/a:1008140021426. [DOI] [PubMed] [Google Scholar]
- 34.Hansen SB, Sulzenbacher G, Huxford T, Marchot P, Taylor P, Bourne Y. Structures of Aplysia AChBP complexes with nicotinic agonists and antagonists reveal distinctive binding interfaces and conformations. Embo J. 2005;24:3635–3646. doi: 10.1038/sj.emboj.7600828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colquhoun D, Sakmann B. Fast events in single-channel currents activated by acetylcholine and its analogues at the frog muscle end-plate. Journal of Physiology (London) 1985;369:501–557. doi: 10.1113/jphysiol.1985.sp015912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams DK, Stokes C, Horenstein NA, Papke RL. The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol. 2011;137:369–384. doi: 10.1085/jgp.201010587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Changeux J-P. The acetylcholine receptor: an "allosteric" membrane protein. New York: Academic Press Inc; 1981. [PubMed] [Google Scholar]
- 38.Land BR, Harris WV, Salpeter EE, Salpeter MM. Diffusion and binding constants for acetylcholine derived from the falling phase of miniature endplate currents. Proc Natl Acad Sci U S A. 1984;81:1594–1598. doi: 10.1073/pnas.81.5.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li P, Steinbach JH. The neuronal nicotinic alpha4beta2 receptor has a high maximal probability of being open. Br J Pharm. 2010;160:1906–1915. doi: 10.1111/j.1476-5381.2010.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Papke RL, Dwoskin LP, Crooks PA. The pharmacological activity of nicotine and nornicotine on nAChRs subtypes: relevance to nicotine dependence and drug discovery. J Neurochem. 2007;101:160–167. doi: 10.1111/j.1471-4159.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- 41.Papke RL, Trocme-Thibierge C, Guendisch D, Abbas Al Rubaiy SA, Bloom SA. Electrophysiological perspectives on the therapeutic use of nicotinic acetylcholine receptor partial agonists. J Pharmacol Exp Ther. 2011;337:367–379. doi: 10.1124/jpet.110.177485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campling BG, Kuryatov A, Lindstrom J. Acute activation, desensitization and smoldering activation of human acetylcholine receptors. PLoS One. 2013;8:e79653. doi: 10.1371/journal.pone.0079653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wonnacott S, Irons J, Rapier C, Thorne B, Lunt GG. Presynaptic modulation of transmitter release by nicotinic receptors. Prog Brain Res. 1989;79:157–163. doi: 10.1016/s0079-6123(08)62475-9. [DOI] [PubMed] [Google Scholar]
- 44.Collins AC, Salminen O, Marks MJ, Whiteaker P, Grady SR. The road to discovery of neuronal nicotinic cholinergic receptor subtypes. Handb Exp Pharmacol. 2009:85–112. doi: 10.1007/978-3-540-69248-5_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuryatov A, Onksen J, Lindstrom J. Roles of accessory subunits in alpha4beta2(*) nicotinic receptors. Mol Pharmacol. 2008;74:132–143. doi: 10.1124/mol.108.046789. [DOI] [PubMed] [Google Scholar]
- 46.Jones MV, Westbrook GL. The impact of receptor desensitization on fast synaptic transmission. Trends Neurosci. 1996;19:96–101. doi: 10.1016/s0166-2236(96)80037-3. [DOI] [PubMed] [Google Scholar]
- 47.Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not "either/or": activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol. 2008;84:329–342. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breese CR, Marks MJ, Logel J, Adams CE, Sullivan B, Collins AC, et al. Effect of smoking history on [3H]nicotine binding in human postmortem brain. J Pharmacol Exp Ther. 1997;282:7–13. [PubMed] [Google Scholar]
- 49.Picciotto MR, Caldarone BJ, Brunzell DH, Zachariou V, Stevens TR, King SL. Neuronal nicotinic acetylcholine receptor subunit knockout mice: physiological and behavioral phenotypes and possible clinical implications. Pharmacol Ther. 2001;92:89–108. doi: 10.1016/s0163-7258(01)00161-9. [DOI] [PubMed] [Google Scholar]
- 50.Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol. 2003;63:332–341. doi: 10.1124/mol.63.2.332. [DOI] [PubMed] [Google Scholar]
- 51.Grady SR, Salminen O, Laverty DC, Whiteaker P, McIntosh JM, Collins AC, et al. The subtypes of nicotinic acetylcholine receptors on dopaminergic terminals of mouse striatum. Biochem Pharmacol. 2007;74:1235–1246. doi: 10.1016/j.bcp.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salminen O, Drapeau JA, McIntosh JM, Collins AC, Marks MJ, Grady SR. Pharmacology of alpha-conotoxin MII-sensitive subtypes of nicotinic acetylcholine receptors isolated by breeding of null mutant mice. Mol Pharmacol. 2007;71:1563–1571. doi: 10.1124/mol.106.031492. [DOI] [PubMed] [Google Scholar]
- 53.Papke RL, Stokes C, Muldoon P, Imad Damaj M. Similar activity of mecamylamine stereoisomers in vitro and in vivo. Eur J Pharmacol. 2013;720:264–275. doi: 10.1016/j.ejphar.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frahm S, Slimak MA, Ferrarese L, Santos-Torres J, Antolin-Fontes B, Auer S, et al. Aversion to nicotine is regulated by the balanced activity of beta4 and alpha5 nicotinic receptor subunits in the medial habenula. Neuron. 2011;70:522–535. doi: 10.1016/j.neuron.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 55.David R, Ciuraszkiewicz A, Simeone X, Orr-Urtreger A, Papke RL, McIntosh JM, et al. Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes. Eur J Neurosci. 2010;31:978–993. doi: 10.1111/j.1460-9568.2010.07133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen X, Chen J, Williamson VS, An SS, Hettema JM, Aggen SH, et al. Variants in nicotinic acetylcholine receptors alpha5 and alpha3 increase risks to nicotine dependence. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:926–933. doi: 10.1002/ajmg.b.30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams DK, Peng C, Kimbrell MR, Papke RL. The Intrinsically Low Open Probability of alpha7 nAChR Can be Overcome by Positive Allosteric Modulation and Serum Factors Leading to the Generation of Excitotoxic Currents at Physiological Temperatures. Mol Pharmacol. 2012;82:746–759. doi: 10.1124/mol.112.080317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams DK, Wang J, Papke RL. Investigation of the Molecular Mechanism of the Alpha7 nAChR Positive Allosteric Modulator PNU-120596 Provides Evidence for Two Distinct Desensitized States. Mol Pharmacol. 2011;80:1013–1032. doi: 10.1124/mol.111.074302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Yu M, Ochani M, Amella CA, Tanovic M, Susarla S, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 60.Sharma G, Vijayaraghavan S. Nicotinic receptor signaling in nonexcitable cells. J Neurobio. 2002;53:524–534. doi: 10.1002/neu.10114. [DOI] [PubMed] [Google Scholar]
- 61.Wessler I, Kirkpatrick CJ. Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br J Pharmacol. 2008;154:1558–1571. doi: 10.1038/bjp.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papke RL, Bencherif M, Lippiello P. An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the α7 subtype. Neurosci Lett. 1996;213:201–204. doi: 10.1016/0304-3940(96)12889-5. [DOI] [PubMed] [Google Scholar]
- 63.Papke RL, Papke JKP. Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br J of Pharm. 2002;137:49–461. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. [PubMed] [Google Scholar]
- 65.Uteshev VV, Meyer EM, Papke RL. Activation and inhibition of native neuronal alpha-bungarotoxin-sensitive nicotinic ACh receptors. Brain Res. 2002;948:33–46. doi: 10.1016/s0006-8993(02)02946-3. [DOI] [PubMed] [Google Scholar]
- 66.Papke RL, Meyer E, Nutter T, Uteshev VV. Alpha7-selective agonists and modes of alpha7 receptor activation. Eur J Pharmacol. 2000;393:179–195. doi: 10.1016/s0014-2999(00)00009-1. [DOI] [PubMed] [Google Scholar]
- 67.Andersen N, Corradi J, Sine SM, Bouzat C. Stoichiometry for activation of neuronal alpha7 nicotinic receptors. Proc Natl Acad Sci U S A. 2013 doi: 10.1073/pnas.1315775110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–919. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 69.de Jonge WJ, Ulloa L. The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br J Pharmacol. 2007;151:915–929. doi: 10.1038/sj.bjp.0707264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kabbani N, Nordman JC, Corgiat BA, Veltri DP, Shehu A, Seymour VA, et al. Are nicotinic acetylcholine receptors coupled to G proteins? Bioessays. 2013 doi: 10.1002/bies.201300082. [DOI] [PubMed] [Google Scholar]
- 71.Gilbert D, Lecchi M, Arnaudeau S, Bertrand D, Demaurex N. Local and global calcium signals associated with the opening of neuronal alpha7 nicotinic acetylcholine receptors. Cell Calcium. 2009;45:198–207. doi: 10.1016/j.ceca.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Egleton RD, Brown KC, Dasgupta P. Nicotinic acetylcholine receptors in cancer: multiple roles in proliferation and inhibition of apoptosis. Trends Pharmacol Sci. 2008;29:151–158. doi: 10.1016/j.tips.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 73.Arredondo J, Chernyavsky AI, Jolkovsky DL, Pinkerton KE, Grando SA. Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 2006;20:2093–2101. doi: 10.1096/fj.06-6191com. [DOI] [PubMed] [Google Scholar]
- 74.Rosas-Ballina M, Goldstein RS, Gallowitsch-Puerta M, Yang L, Valdes-Ferrer SI, Patel NB, et al. The selective alpha7 agonist GTS-21 attenuates cytokine production in human whole blood and human monocytes activated by ligands for TLR2, TLR3, TLR4, TLR9, and RAGE. Mol Med. 2009;15:195–202. doi: 10.2119/molmed.2009.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thomsen MS, Mikkelsen JD. The alpha7 nicotinic acetylcholine receptor ligands methyllycaconitine, NS6740 and GTS-21 reduce lipopolysaccharide-induced TNF-alpha release from microglia. J Neuroimmunol. 2012;251:65–72. doi: 10.1016/j.jneuroim.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 76.Briggs CA, Gronlien JH, Curzon P, Timmermann DB, Ween H, Thorin-Hagene K, et al. Role of channel activation in cognitive enhancement mediated by alpha7 nicotinic acetylcholine receptors. Br J Pharmacol. 2009;158:1486–1494. doi: 10.1111/j.1476-5381.2009.00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frazier CJ, Strowbridge BW, Papke RL. Nicotinic acetylcholine receptors on local circuit neurons in the dentate gyrus: a potential role in the regulation of granule cell excitability. J Neurophysiol. 2003;89:3018–3028. doi: 10.1152/jn.01036.2002. [DOI] [PubMed] [Google Scholar]
- 78.Gu Z, Yakel JL. Timing-dependent septal cholinergic induction of dynamic hippocampal synaptic plasticity. Neuron. 2011;71:155–165. doi: 10.1016/j.neuron.2011.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vinson PN, Justice JB., Jr Effect of neostigmine on concentration and extraction fraction of acetylcholine using quantitative microdialysis. J Neurosci Methods. 1997;73:61–67. doi: 10.1016/s0165-0270(96)02213-3. [DOI] [PubMed] [Google Scholar]
- 80.Mineur YS, Eibl C, Young G, Kochevar C, Papke RL, Gundisch D, et al. Cytisine-based nicotinic partial agonists as novel antidepressant compounds. J Pharmacol Exp Ther. 2009;329:377–386. doi: 10.1124/jpet.108.149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rahman S, Zhang Z, Papke RL, Crooks PA, Dwoskin LP, Bardo MT. Regionspecific effects of N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide on nicotine-induced increase in extracellular dopamine in vivo. Br J Pharmacol. 2007 doi: 10.1038/sj.bjp.0707612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Williams DK, Wang J, Papke RL. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem Pharmacol. 2011;82:915–930. doi: 10.1016/j.bcp.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Changeux J-P, Devillers-Thiery A, Chemouilli P. Acetylcholine receptor: An allosteric protein. Science. 1984;225:1335–1345. doi: 10.1126/science.6382611. [DOI] [PubMed] [Google Scholar]
- 84.Shytle R, Sheehan D, Sanberg PR, Arias HR. Neuronal nicotinic receptors as therapeutic targets for mood disorders. In: Arias HR, editor. Pharmacology of Nicotinic Acetylcholine Receptors from the Basic and Therapeutic Perspectives. India: Research Signpost; 2011. pp. 187–198. [Google Scholar]
- 85.Papke RL, Schiff HC, Jack BA, Horenstein NA. Molecular dissection of tropisetron, an alpha7 nicotinic acetylcholine receptor-selective partial agonist. Neurosci let. 2005;378:140–144. doi: 10.1016/j.neulet.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 86.Zwart R, Carbone AL, Moroni M, Bermudez I, Mogg AJ, Folly EA, et al. Sazetidine-A is a potent and selective agonist at native and recombinant alpha 4 beta 2 nicotinic acetylcholine receptors. Mol Pharmacol. 2008;73:1838–1843. doi: 10.1124/mol.108.045104. [DOI] [PubMed] [Google Scholar]
- 87.Garrison GD, Dugan SE. Varenicline: a first-line treatment option for smoking cessation. Clinical therapeutics. 2009;31:463–491. doi: 10.1016/j.clinthera.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 88.Wallace TL, Ballard TM, Pouzet B, Riedel WJ, Wettstein JG. Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacol Biochem Behav. 2011;99:130–145. doi: 10.1016/j.pbb.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 89.Mineur YS, Picciotto MR. Nicotine receptors and depression: revisiting and revising the cholinergic hypothesis. Trends Pharmacol Sci. 2010;31:580–586. doi: 10.1016/j.tips.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacology (Berl) 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. [DOI] [PubMed] [Google Scholar]
- 91.Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- 92.Salminen O, Murphy KL, McIntosh JM, Drago J, Marks MJ, Collins AC, et al. Subunit composition and pharmacology of two classes of striatal presynaptic nicotinic acetylcholine receptors mediating dopamine release in mice. Mol Pharmacol. 2004;65:1526–1535. doi: 10.1124/mol.65.6.1526. [DOI] [PubMed] [Google Scholar]
- 93.Lopez-Hernandez GY, Thinschmidt JS, Zheng G, Zhang Z, Crooks PA, Dwoskin LP, et al. Selective inhibition of acetylcholine-evoked responses of alpha7 neuronal nicotinic acetylcholine receptors by novel tris- and tetrakis-azaaromatic quaternary ammonium antagonists. Mol Pharmacol. 2009;76:652–666. doi: 10.1124/mol.109.056176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Escubedo E, Chipana C, Perez-Sanchez M, Camarasa J, Pubill D. Methyllycaconitine prevents methamphetamine-induced effects in mouse striatum: involvement of alpha7 nicotinic receptors. J Pharmacol Exp Ther. 2005;315:658–667. doi: 10.1124/jpet.105.089748. [DOI] [PubMed] [Google Scholar]
- 95.Brunzell DH, McIntosh JM. Alpha7 nicotinic acetylcholine receptors modulate motivation to self-administer nicotine: implications for smoking and schizophrenia. Neuropsychopharmacology. 2012;37:1134–1143. doi: 10.1038/npp.2011.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beers WH, Reich E. Structure and activity of acetylcholine. Nature. 1970;228:917–922. doi: 10.1038/228917a0. [DOI] [PubMed] [Google Scholar]
- 97.de Fiebre CM, Meyer EM, Zoltewicz J, Henry JC, Muraskin S, Kem WR, et al. Characterization of a family of anabaseine-derived compounds reveals that the 3-4-dimethylaminocinnamylidine derivative (DMAC) is a selective agonist at neuronal nicotinic α7/[125I]α-bungarotoxin receptor subtypes. Mol Pharm. 1995;47:164–171. [PubMed] [Google Scholar]
- 98.Kox M, van Velzen JF, Pompe JC, Hoedemaekers CW, van der Hoeven JG, Pickkers P. GTS-21 inhibits pro-inflammatory cytokine release independent of the Toll-like receptor stimulated via a transcriptional mechanism involving JAK2 activation. Biochem Pharmacol. 2009;78:863–872. doi: 10.1016/j.bcp.2009.06.096. [DOI] [PubMed] [Google Scholar]
- 99.Tregellas JR, Olincy A, Johnson L, Tanabe J, Shatti S, Martin LF, et al. Functional Magnetic Resonance Imaging of Effects of a Nicotinic Agonist in Schizophrenia. Neuropsychopharmacology. 2010;35:938–942. doi: 10.1038/npp.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meyer E, Kuryatov A, Gerzanich V, Lindstrom J, Papke RL. Analysis of 40H-GTS-21 selectivity and activity at human and rat a7 nicotinic receptors. J Pharmacol Exp Ther. 1998;287:918–925. [PubMed] [Google Scholar]
- 101.Shytle RD, Mori T, Townsend K, Vendrame M, Sun N, Zeng J, et al. Cholinergic modulation of microglial activation by alpha 7 nicotinic receptors. J Neurochem. 2004;89:337–343. doi: 10.1046/j.1471-4159.2004.02347.x. [DOI] [PubMed] [Google Scholar]
- 102.Chojnacka K, Papke RL, Horenstein NA. Synthesis and evaluation of a conditionally-silent agonist for the alpha7 nicotinic acetylcholine receptor. Bioorg Med Chem Lett. 2013;23:4145–4149. doi: 10.1016/j.bmcl.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peng C, Kimbrell MR, Tian C, Pack TF, Crooks PA, Fifer EK, et al. Multiple modes of alpha7 nAChR non-competitive antagonism of control agonist-evoked and allosterically enhanced currents. Mol Pharmacol. 2013;84:459–475. doi: 10.1124/mol.113.086462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ren K, Puig V, Papke RL, Itoh Y, Hughes JA, Meyer EM. Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J Neurochem. 2005;94:926–933. doi: 10.1111/j.1471-4159.2005.03223.x. [DOI] [PubMed] [Google Scholar]
- 105.Feuerbach D, Lingenhohl K, Dobbins P, Mosbacher J, Corbett N, Nozulak J, et al. Coupling of human nicotinic acetylcholine receptors alpha 7 to calcium channels in GH3 cells. Neuropharmacology. 2005;48:215–227. doi: 10.1016/j.neuropharm.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 106.Le Novere N, Grutter T, Changeux JP. Models of the extracellular domain of the nicotinic receptors and of agonist- and Ca2+-binding sites. Proc Natl Acad Sci U S A. 2002;99:3210–3215. doi: 10.1073/pnas.042699699. [DOI] [PMC free article] [PubMed] [Google Scholar]