Abstract
Parkinson’s disease (PD) is a progressive neurodegenerative disease affecting the nigrostriatal dopaminergic pathway. Several epidemiological studies have demonstrated an association between pesticide exposure and the incidence of PD. Studies from our laboratory and others have demonstrated that certain pesticides increase levels of the dopamine transporter (DAT), an integral component of dopaminergic neurotransmission and a gateway for dopaminergic neurotoxins. Here, we report that repeated exposure (3 injections over 2 weeks) of mice to two commonly used pyrethroid pesticides, deltamethrin (3 mg/kg) and permethrin (0.8 mg/kg), increases DAT-mediated dopamine uptake by 31 and 28%, respectively. Using cells stably expressing DAT, we determined that exposure (10 min) to deltamethrin and permethrin (1 nM–100 μM) had no effect on DAT-mediated dopamine uptake. Extending exposures to both pesticides for 30 min (10 μM) or 24 h (1, 5, and 10 μM) resulted in significant decrease in dopamine uptake. This reduction was not the result of competitive inhibition, loss of DAT protein, or cytotoxicity. However, there was an increase in DNA fragmentation, an index of apoptosis, in cells exhibiting reduced uptake at 30 min and 24 h. These data suggest that up-regulation of DAT by in vivo pyrethroid exposure is an indirect effect and that longer-term exposure of cells results in apoptosis. Since DAT can greatly affect the vulnerability of dopamine neurons to neurotoxicants, up-regulation of DAT by deltamethrin and permethrin may increase the susceptibility of dopamine neurons to toxic insult, which may provide insight into the association between pesticide exposure and PD.
Keywords: Deltamethrin, Permethrin, Pyrethroid, Dopamine transporter, Parkinson’s disease
Parkinson’s disease (PD) is a disabling neurodegenerative disorder characterized by the loss of nigrostriatal dopamine neurons and the formation of intraneuronal inclusions termed Lewy bodies (Olanow and Tatton, 1999). Although the exact etiology of PD is unknown, both genetic and environmental factors are thought to contribute to the pathogenesis of PD. While there are rare instances of genetically-linked PD, data from a recent comprehensive twin study found no significant contribution of genetics to late-onset PD (Tanner et al., 1999). This finding suggests that environmental factors or gene–environment interactions play a pivotal role in the development of sporadic PD.
Several epidemiological studies have identified pesticide exposure as a significant risk factor for Parkinson’s disease (Tanner and Langston, 1990; Gorell et al., 1998; Le Couteur et al., 1999; Priyadarshi et al., 2001). Other studies have demonstrated that drinking well-water and living in a rural setting, both of which may increase exposure to agricultural pesticides, increase the risk of developing PD (Rajput et al., 1986; Barbeau et al., 1987; Rajput et al., 1987; Golbe et al., 1990; Semchuk et al., 1991). In addition, exposure to pesticides used in the home has been linked to PD (Stephenson, 2000). However, the majority of studies have not identified specific pesticides or the mechanism by which pesticides damage the dopaminergic system and increase the risk of PD.
Studies by our laboratory and others have demonstrated that exposure of mice to the organochlorine insecticide heptachlor increases the expression of the plasma membrane dopamine transporter (DAT; Miller et al., 1999a; Kirby et al., 2001) at dosage levels that elicit no overt toxicity. DAT is an integral component of normal dopamine function and is responsible for terminating dopamine neurotransmission by rapid reuptake of dopamine into the presynaptic terminal (Shimada et al., 1991; Giros and Caron, 1993; Miller et al., 1999b). Several studies have demonstrated that alterations in the expression of DAT can greatly affect the vulnerability of the dopamine neuron to neurotoxins such as MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine). Gainetdinov and colleagues demonstrated the requirement of DAT for the toxicity of MPTP (Gainetdinov et al., 1997), while Donovan et al. (1999) have shown that overexpression of DAT in transgenic mice results in greater loss of dopamine neurons following MPTP exposure. Therefore, exposure to pesticides that increase DAT may increase the susceptibility of dopamine neurons to endogenous neurotoxic dopamine metabolites or exogenous neurotoxicants by increasing their uptake by DAT.
In addition to heptachlor, exposure of mice to the pyrethroid pesticides deltamethrin and permethrin has been demonstrated to increase DAT-mediated dopamine uptake (Kirby et al., 1999; Karen et al., 2001; Gillette and Bloomquist, 2003). Pesticides in the pyrethroid class are widely used in household and agricultural applications and are popular because of their low mammalian toxicity. Although pyrethroids are often considered environmentally labile, they readily cross the blood–brain barrier and can achieve considerable concentrations in the brain (Anadon et al., 1996). Acute toxicity of pyrethroids is primarily mediated through interaction with sodium channels, leading to prolonged depolarization and hyperexcitation of the nervous system (Narahashi, 1982; Tabarean and Narahashi, 2001). Pyrethroids have also been shown to be potent releasers of neurotransmitters, including dopamine (Eells and Dubocovich, 1988; Kirby et al., 1999). However, the mechanism by which pyrethroids are capable of increasing DAT-mediated dopamine uptake is not clear.
Here, we report that in vivo exposure to deltamethrin and permethrin not only causes functional up-regulation of dopamine uptake, but increased levels of DAT protein as well. In addition, acute exposure of SK-N-MC cells stably expressing DAT to these pyrethroids has no effect on dopamine uptake, indicating that deltamethrin and permethrin do not directly interact with DAT. Finally, we found that longer-term exposure to deltamethrin and permethrin reduces dopamine uptake in these cells, and that this effect is most likely the result of an ongoing apoptotic process. Taken together, our results suggest that the effects of pyrethroids on DAT are indirect and that longer-term exposures may be capable of damaging cells through an apoptotic mechanism.
Materials and methods
Materials
Analytical grade (purity ≥98%) deltamethrin and permethrin were obtained from ChemService Inc. (West Chester, PA). 3H-dopamine (58 Ci/mmol) and 3H-WIN 35,428 (85 Ci/mmol) were purchased from Perkin-Elmer Life Sciences (Boston, MA). The rat monoclonal antibody to DAT was purchased from Chemicon (Temecula, CA) and the monoclonal anti-mouse α-tubulin was purchased from Sigma (St. Louis, MO). The goat anti-rat secondary antibody was purchased from ICN (Costa Mesa, CA) and the goat anti-mouse secondary antibody was from Bio-Rad (Hercules, CA). Super Signal West substrate and stripping buffer were obtained from Pierce (Rockford, IL). Cell culture media and supplements were obtained from Meditech (Herndon, VA). All other reagents were obtained from Sigma or Fisher Scientific (Pittsburgh, PA).
Animals and treatments
Male C57BL/6j mice (8 weeks of age) were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were group housed (6 per cage) under a 12:12 light–dark cycle and acclimatized for 1 week prior to initiation of experiments. Standard rodent chow and tap water was available ad libitum. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and previously approved by the Institutional Animal Care and Use Committee at Emory University.
A total of 24 mice were used for these experiments. Control mice were injected intraperitoneally with vehicle (methoxytriglycol; n = 12) and treated mice were injected with deltamethrin (3 mg/kg; n = 6) or permethrin (0.8 mg/ kg; n = 6) three times over a 2-week period (Days 1, 8, and 15) as described previously (Kirby et al., 1999; Miller et al., 1999b; Gillette and Bloomquist, 2003). One day following the last treatment, striatal tissue was dissected out and prepared for assay as described below.
Synaptosomal dopamine uptake, 3H-WIN 35,428 binding, and Western blot analysis
Dopamine uptake studies were performed as described previously (Miller et al., 1999a). Briefly, crude synaptosomes were prepared from fresh striatal tissue and incubated in assay buffer (4 mM Tris, 6.25 HEPES, 120 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 0.6 mM ascorbic acid, 5.5 mM glucose, 10 μM pargyline; pH 7.4) containing a saturating concentration of dopamine (1 μM final concentration) and a tracer amount of 3H-dopamine (20 nM). A single saturating concentration of dopamine was chosen to assess effects of pyrethroids on the Vmax of DAT, since previous studies using the same dosing paradigm have demonstrated no significant effect on Km (Kirby et al., 1999; Karen et al., 2001). Uptake was allowed to proceed for 3 min at 37 °C, and then terminated by the addition of ice-cold buffer and rapid vacuum filtration over GF/B filter paper using a Brandel harvester. Filters were washed twice more with buffer, allowed to air dry, and placed in scintillation vials containing 8 ml of Econoscint (Fisher Scientific, Pittsburgh, PA) for scintillation counting. Uptake rates were calculated as specific uptake (total uptake–non-specific uptake), with non-specific uptake defined by the inclusion of 10 μM nomifensine. Following determination of synaptosomal protein concentration (Bradford, 1976), uptake rates were calculated as pmol/min-mg protein and expressed as percentage of control values.
Determination of 3H-WIN 35,428 binding to DAT was performed essentially as described by Coffey and Reith (1994) with modifications to reduce the total volume to 200 μl, for assay in 96-well microtiter plates. Preliminary kinetic studies indicated that the binding of 3H-WIN 35,428 to striatal synaptosomes was best fit to a one-site model determined by non-linear curve fitting techniques (Graph-Pad Prism 3.0) with a Kd of 6.58 nM and a Bmax of 1.08 pmol/mg protein. Therefore, binding studies with crude synaptosomes were conducted with a single concentration (10 nM) of 3H-WIN 35,428 in 25 mM sodium phosphate buffer (125 mM NaCl, 5 mM KCl; pH 7.4) for 1 h at 4 °C in 96-well plates. Incubations were terminated by rapid vacuum filtration onto GF/B filter plates and radioactivity was determined by liquid scintillation counting. Non-specific binding was determined by the inclusion of 10 μM nomifensine and specific binding was calculated as the total binding (incubated without 10 μM nomifensine) minus non-specific binding (incubated with nomifensine). Data were calculated as pmol/mg protein and expressed as percentage of control values.
Western blots were performed as previously described (Richardson and Miller, 2004). Briefly, samples (20 μg) were subjected to SDS PAGE on 10% precast NuPage gels (InVitrogen, Carlsbad, CA). Samples were electrophoretically transferred to a polyvinylidene difluoride membrane, and non-specific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline (135 mM NaCl, 2.5 mM KCl, 50 mM Tris, and 0.1% Tween 20, pH 7.4). Membranes were then incubated in a monoclonal antibody (Chemicon, Temecula, CA) to the N-terminus of DAT (Miller et al., 1997). Antibody binding was detected using a goat anti-rat horse-radish peroxidase secondary antibody (ICN, Costa Mesa, CA) and enhanced chemiluminescence. The chemiluminescent signal was captured on an Alpha Innotech Fluorchem 8800 (San Leandro, CA) imaging system and stored as a digital image. Densitometric analysis was performed and calibrated to co-blotted dilutional standards of pooled cells from all control samples. Membranes were then stripped for 15 min at 25 °C with Pierce Stripping Buffer and reprobed with a monoclonal α-tubulin antibody to ensure equal protein loading across samples.
Cell culture
SK-N-MC (human neuroblastoma) cells stably expressing human DAT (SK-DAT; Stephans et al., 2002) were maintained in minimum essential medium (MEM) supplemented with Earle’s salts, 10% heat-inactivated fetal bovine serum, 50 U/ml penicillin, 50 μg/ml streptomycin, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1 mM non-essential amino acids (Mediatech, Herndon, VA), and incubated under a humidified atmosphere of 5% CO2 in air at 37 °C. For pyrethroid exposure, deltamethrin and permethrin were dissolved in dimethylsulfoxide (DMSO) at a concentration of 10 mM. Further dilutions were made in uptake buffer for experiments with dopamine uptake and in serum-free media for experiments to determine cytotoxicity and DNA fragmentation. The final concentration of DMSO was ≤0.1% for all experiments and had no effect on any of the parameters studied. Control experiments were performed in the presence of DMSO in a concentration similar to that used in the pyrethroid-treated cells.
3H-Dopamine uptake and Western blot studies in cells
Dopamine uptake by SK-DAT cells was performed as described elsewhere (Pifl et al., 1996). Briefly, cells were plated in 24-well plates and incubated for 48 h in the above MEM medium. Cells were washed once with the uptake buffer (4 mM Tris, 6.25 HEPES, 120 mM NaCl, 5 mM KCl, 1.2 mM CaCl2, 1.2 mM MgSO4, 0.6 mM ascorbic acid, 5.5mM glucose; pH 7.4). For acute studies (10 and 30 min), cells were incubated with various concentrations of either deltamethrin or permethrin (1 nM–10 μM). For longer-term studies (24 h), cells were exposed to the pyrethroids in serum-free media for 24 h and then washed once in uptake buffer. Following the wash step, cells were incubated for 5 min at 37 °C with uptake buffer containing unlabeled DA (2.5 μM) and a tracer amount (20 nM) of 3H-dopamine. Pargyline (10 μM) was included during all the uptake periods to inhibit monoamine oxidase and non-specific uptake was defined in the presence of 10 μM GBR-12935. After the incubation period, the buffer was quickly aspirated off and cells were washed twice with ice-cold buffer. Cells were then dissolved in 0.5 ml of 0.1 M NaOH and the solubilized cellular contents were transferred to liquid scintillation vials containing 8 ml of liquid scintillation cocktail. The radioactivity was measured by scintillation counting and an aliquot of the solubilized cells was used for protein determination using bovine serum albumin as standard (Lowry et al., 1951). Uptake rates were calculated as specific uptake (total uptake–non-specific uptake) and expressed as percentage of control values.
To determine the effects of pyrethroids on the Km and Vmax of dopamine uptake in SK-DAT cells, cells were incubated with pyrethroids for 10 min or 24 h and dopamine uptake was determined as described above using increasing concentrations (0.5–40 μM) of dopamine. Km and Vmax were determined by non-linear regression using GraphPad Prism 3.0 (GraphPad Software, San Diego, CA).
Western blots were performed as previously described (Miller et al., 1997). Briefly, cells were scraped from culture plates and sonicated at 4 °C in a buffer containing 300 mM sucrose, 10 mM HEPES, and 1 μg/ml of leupeptin, aprotinin, and pepstatin. Samples (20 μg) were subjected to SDS PAGE on 10% precast NuPage gels (InVitrogen, Carlsbad, CA). Samples were electrophoretically transferred to a polyvinylidene difluoride membrane, and non-specific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline (135 mM NaCl, 2.5 mM KCl, 50 mM Tris, and 0.1% Tween 20, pH 7.4). Membranes were then incubated in a monoclonal antibody (Chemicon, Temecula, CA) to the N-terminus of DAT (Miller et al., 1997). Antibody binding was detected using a goat anti-rat horseradish peroxidase secondary antibody (ICN, Costa Mesa, CA) and enhanced chemiluminescence. The chemiluminescent signal was captured on an Alpha Innotech Fluorchem 8800 (San Leandro, CA) imaging system and stored as a digital image. Densitometric analysis was performed and calibrated to co-blotted dilutional standards of pooled cells from all control samples. Membranes were then stripped for 15 min at 25 °C with Pierce Stripping Buffer and reprobed with a monoclonal α-tubulin antibody to ensure equal protein loading across samples.
Cytotoxicity and DNA fragmentation assays
The possible cytotoxic effects of pyrethroid exposure on SK-DAT cells was evaluated by measuring lactate dehydrogenase (LDH) leakage into the extracellular fluid using a cytotoxicity detection kit (Roche Applied Science, Indianapolis, IN). Briefly, cells (1 × 104 cells/well) were incubated with different concentrations of pyrethroids (1, 5, or 10 μM) for 24 h in serum-free MEM and the incubation medium was collected and centrifuged. The cell-free supernatant (100 μl) was then mixed with 100 μl of the catalyst-dye mix (included in kit) in a 96-well microtiter plate. LDH activity in the media was determined spectrophotometrically at 490 nm by monitoring the increase in absorbance over a 30-min period. To determine the total amount of LDH in each sample, the original cells and media were lysed in 1% Triton X-100 for 30 min and LDH activity was determined as described above. The LDH release for each sample was defined as the LDH activity in the incubation media divided by the total amount of LDH activity following Triton-lysis and data expressed presented as percentage LDH leakage.
To determine whether pyrethroid exposure resulted in apoptosis in SK-DAT cells, we used the Cell Death Detection ELISA Plus Assay kit (Roche Applied Science, Indianapolis, IN), which provides an index of DNA fragmentation. This kit measures amount of histone-associated low molecular weight DNA, which is indicative of histone-associated DNA fragments which have been cleaved by endonuclease, in the cytoplasm of cells and has been used as a measure of apoptosis in cells exposed to other toxicants (Anantharam et al., 2002; Kitazawa et al., 2002). Briefly, cells were seeded in microplate wells (1 × 104 cells/well) and treated for 24 h in serum-free MEM with either deltamethrin or permethrin. After treatment, cells were pelleted and washed once with phosphate-buffered saline. Cells were then incubated with lysis buffer (supplied with the kit) at room temperature for 30 min and centrifuged. Aliquots of supernatant (20 μl) were dispensed into a streptavidin-coated 96-well microtiter plate (supplied with the kit) and incubated with 80 μl of antibody cocktail for 2 h at room temperature with shaking. The antibody cocktail consisted of a mixture of anti-histone biotin and anti-DNA-HRP, which binds to both single-stranded DNA and double-stranded DNA, which are major constituents of nucleosomes. After incubation, plates were washed with incubation buffer and determination of the amount of nucleosomes retained by anti-DNA-HRP was determined spectrophotometrically with 2,2′-azino-di[3-ethoxybenzyl thiazoline sulfonate] as an HRP substrate (supplied with the kit). Measurements were made at 405 nm using a Spectramax Plus microplate reader (Molecular Devices). Non-specific signal was determined by subtraction of a reagent blank and data were expressed as mU (defined as absorbance × 10−3) cytoplasmic oligonucleosomes.
Statistical analysis
Results were expressed as the mean ± SEM. In instances where data were presented as percentage of control, all statistical procedures were performed on the raw numbers. Data were analyzed by Student’s t test or one-way analysis of variance (ANOVA). If a significant F was determined by ANOVA, post hoc analysis was performed with Dunnett’s test. Statistical significance is reported at the P ≤ 0.05 level.
Results
No overt signs of toxicity, defined as tremor, choreoathetosis, and salivation, were observed following administration of either deltamethrin or permethrin. There were also no significant changes in weight in any of the treated animals (data not shown).
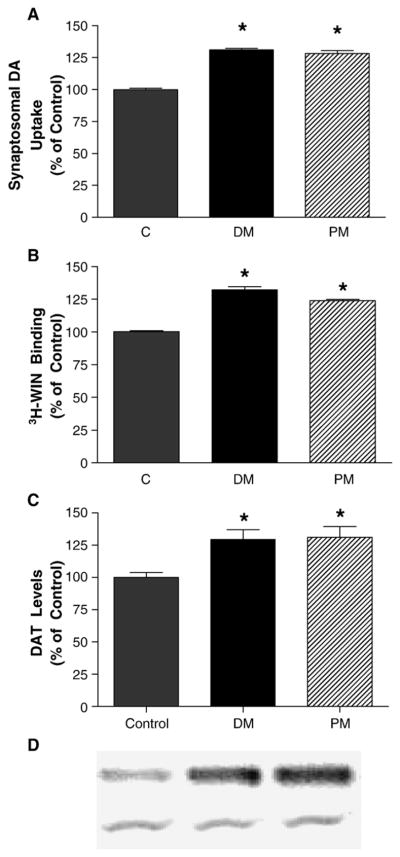
Based upon previous studies demonstrating increased dopamine uptake following deltamethrin or permethrin exposure (Kirby et al., 1999; Gillette and Bloomquist, 2003), we administered deltamethrin (3 mg/kg) or permethrin (0.8 mg/kg) three times over 2 weeks to determine the effects of these compounds on DAT-mediated dopamine uptake and the number of DAT-binding sites. Deltamethrin exposure increased DAT-mediated dopamine uptake in striatal synaptosomes by 31% ( P < 0.01) 1 day following the last treatment (Fig. 1A). At this same time, permethrin exposure increased dopamine uptake by 28% ( P < 0.01). The increases in dopamine uptake observed were accompanied by increases in DAT-binding sites as determined by 3H-WIN 35,428 binding in striatal synaptosomes (Fig. 1B). Deltamethrin resulted in a 32% increase ( P < 0.01), while permethrin exposure increased DAT-binding sites by 24% ( P < 0.01). The increase in DAT-binding sites and uptake by exposure to deltamethrin and permethrin were accompanied by similar increases (31.2% and 29.3%, P < 0.01) in total DAT protein as measured by Western immunoblotting (Fig. 1C).
Fig. 1.
Repeated administration of deltamethrin (DM; 3 mg/kg) or permethrin (PM; 0.8 mg/kg) to C57 mice increases (A) 3H-dopamine uptake in striatal synaptosomes, (B) DAT levels in striatal synaptosomes as determined by 3H-WIN 35,428 binding, and (C) DAT levels in striatal synaptosomes as determined by Western immunoblotting. Data are presented as percentage of control values and represent mean ± SEM (n = 5–6 animals per treatment for pyrethroids and 12 animals for control). *Groups are significantly different from control values ( P ≤ 0.01) using the untransformed data as determined by ANOVA followed by Dunnett’s test. (D) Representative Western blots of DAT (top) and α-tubulin (bottom) from control, deltamethrin-, and permethrin-treated animals.
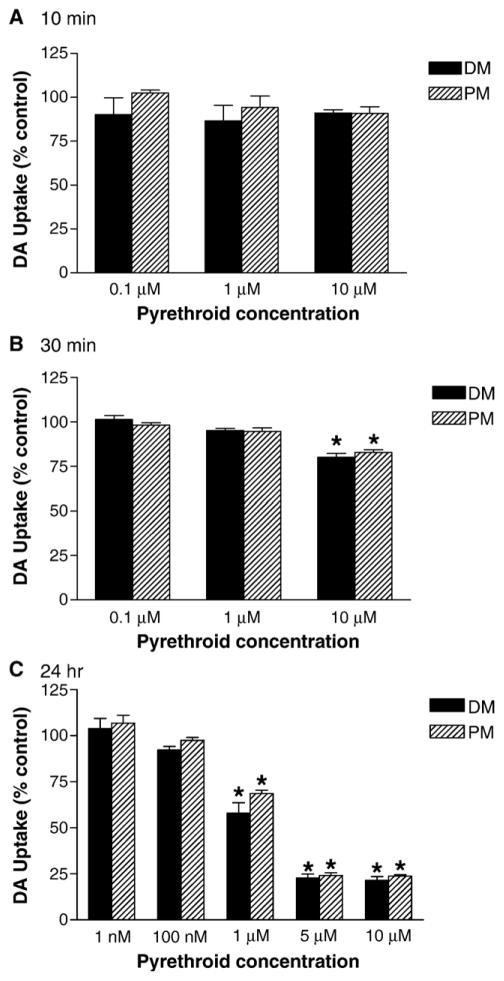
Since we observed significant up-regulation of DAT following in vivo exposure to deltamethrin and permethrin, we sought to determine whether these effects were the result of direct action of the pyrethroids on DAT. To accomplish this, we exposed SK-N-MC neuroblastoma cells stably expressing DAT (SK-DAT) to various concentrations of pyrethroids for 10 min, 30 min, or 24 h. Exposure of SK-DAT cells for 10 min with either deltamethrin or permethrin (1 μM to 10 μM) had no significant effect on DAT-mediated dopamine uptake (Fig. 2A). Extending the incubation time to 30 min resulted in a significant decrease in dopamine uptake by both deltamethrin (20%; P < 0.01) and permethrin (18%; P < 0.01) only at a concentration of 10 μM (Fig. 2B). Further extending the incubation time to 24 h resulted in a greater decrease of dopamine uptake, as both permethrin ( P < 0.01) and deltamethrin ( P < 0.01) decreased dopamine uptake by 32 to 42% at 1 μM and 75% at concentrations of 5 and 10 μM (Fig. 2C). To determine the nature of the inhibition of DAT-mediated uptake by deltamethrin and permethrin, we performed kinetic analysis of dopamine uptake in SK-DAT cells exposed to 10 μM of either compound for 24 h. Both pyrethroids showed significant alterations in Vmax, with variable effects on Km, suggesting that the decreased uptake may be the result of non-competitive inhibition (Figs. 3A and B). Similar results were observed following 30-min incubations with both compounds (data not shown).
Fig. 2.
Effects of deltamethrin (DM) and permethrin (PM) on dopamine uptake in SK-N-MC neuroblastoma cells stably expressing the human DAT. Cells were incubated with various concentrations of DM or PM for 10 min (A), 30 min (B), or 24 h (C) and dopamine uptake was determined as described in Materials and methods. Data are presented as percentage of control values and represent mean ± SEM (n = 3). *Groups are significantly different from control values ( P ≤ 0.01) using the untransformed data as determined by ANOVA followed by Dunnett’s test.
Fig. 3.
Effects of (A) deltamethrin (DM; 10 μM) or (B) permethrin (PM; 10 μM) treatment for 24 h on the kinetics of dopamine uptake in SK-N-MC neuroblastoma cells stably expressing the human DAT. Cells were incubated with DM or PM for 24 h and the kinetics of dopamine uptake were determined by using varying concentrations of dopamine as described in Materials and methods. Data represent mean ± SEM (n = 3) and absence of error bars indicates that the standard error resides within the size of the symbol. (C) Total DAT levels in cells treated with DM or PM for 24 h as determined by Western immunoblotting. Data represent mean ± SEM (n = 3).
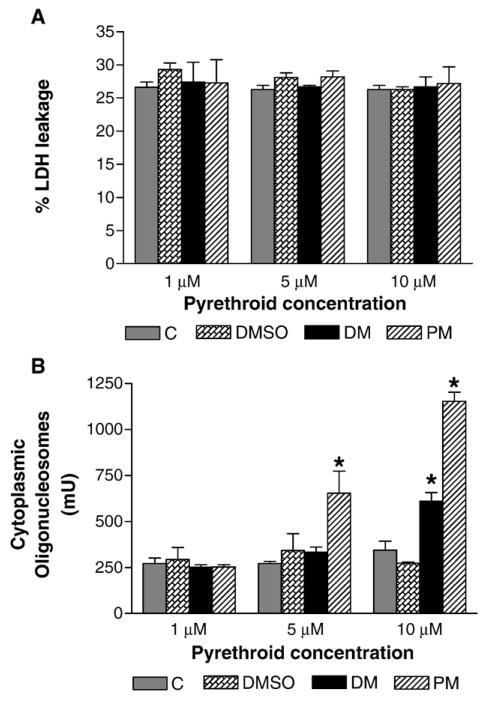
Based upon the time and concentrations required for deltamethrin and permethrin to cause decreased dopamine uptake, we considered that the decreased uptake may be the result of loss of DAT protein. Exposure of cells to 10 μM of deltamethrin or permethrin was without effect on the total levels of DAT as determined by Western immunoblotting (Fig. 3C). We next examined whether exposure to deltamethrin or permethrin resulted in cytotoxicity by assessing LDH leakage from the cells into the incubation medium. Treatment of SK-DAT cells with 1 to 10 μM of deltamethrin or permethrin for 24 h did not produce any significant change in LDH leakage (Fig. 4A), effectively ruling out overt cytotoxicity as a mechanism for the decreased dopamine uptake. However, exposure of SK-DAT cells to 5 or 10 μM of permethrin or 10 μM deltamethrin for 24 h significantly increased DNA fragmentation, an indication of an active apoptotic process (Fig. 4B). Exposure to 5 μM permethrin increased the amount of fragmentation by 191% ( P < 0.05) and exposure to 10 μM increased fragmentation by 422% ( P < 0.01). Deltamethrin increased fragmentation by 223% ( P < 0.05) only at 10 μM. Similarly, increased DNA fragmentation was observed following 30 min of exposure to 10 μM of deltamethrin (35%; P < 0.05) or permethrin (65%; P < 0.05). No significant effects were observed with lower concentrations (data not shown).
Fig. 4.
Effects of deltamethrin or permethrin on (A) LDH leakage and (B) DNA fragmentation. SK-N-MC cells stably expressing the human DAT were treated with media (C), vehicle (DMSO), deltamethrin (DM; 1–10 μM), or permethrin (PM; 1–10 μM) for 24 h. After exposure, cell-free media samples were collected and assayed for LDH levels by spectrophotometry. DNA fragmentation in cells following 24 h of exposure was determined as described in Materials and methods. Data for LDH are presented as percentage of LDH leakage and represent mean ± SEM (n = 3). *Groups are significantly different from control values ( P ≤ 0.05) using the untransformed data as determined by ANOVA followed by Dunnett’s test.
Discussion
Previous studies have demonstrated that repeated exposure of mice to the pyrethroid pesticides, deltamethrin and permethrin, results in increased synaptosomal dopamine uptake (Kirby et al., 1999; Karen et al., 2001; Gillette and Bloomquist, 2003). In this study, we confirm these observations and extend them by demonstrating that the functional up-regulation is accompanied by increases in DAT-binding sites. In addition, we demonstrate that permethrin and deltamethrin have no direct effect on DAT and that longer-term in vitro exposure of cells stably expressing DAT results in decreased DAT-mediated dop-amine uptake and DNA fragmentation.
Deltamethrin and permethrin are members of the pyrethroid class of pesticides which are synthetic derivatives of the naturally occurring pyrethrum from chrysanthemum flowers. These compounds exert their toxicity primarily through binding to sodium channels and prolonging the opening of the channel (Narahashi, 1996; Soderlund et al., 2002). However, recent data suggest that these compounds may specifically target the dopaminergic system. It has been demonstrated that exposure of mice to deltamethrin or permethrin results in an increase in dopamine uptake in striatal synaptosomes, possibly indicative of an up-regulation of DAT (Karen et al., 2001; Kirby et al., 1999). In addition, up-regulation of dopamine uptake following deltamethrin exposure was accompanied by increased binding of 3H-GBR 12935 (Gillette and Bloomquist, 2003). In this study, we found significant increases in DAT-binding sites as measured with 3H-WIN 35,428 that mirrored the increase in DAT-mediated dopamine uptake. While no specific mechanism has been identified for the increase of DAT by these compounds, chemicals known to cause dopamine release, like amantidine and the organo-chlorine pesticide heptachlor, can increase DAT expression (Gordon et al., 1996; Miller et al., 1999a, 1999b; Page et al., 2000; Kirby et al., 2002). If this were to be sustained over time, one would expect that the elevated extracellular dopamine would increase the expression of the dopamine transporter in an attempt to clear and recycle dopamine. Indeed, deltamethrin has been demonstrated to cause dopamine release from pre-loaded synaptosomes (Kirby et al., 1999; Bloomquist et al., 2002). Another possibility is up-regulation of DAT at the transcriptional level. The transcription factor Nurr1 is critical for the development of the dopaminergic phenotype and has been shown to directly enhance transcription of DAT (Sacchetti et al., 2001; Hermanson et al., 2003). Since Nurr1 transcription is enhanced by neuronal activity and membrane depolarization (Brosenitsch and Katz, 2001), dopamine release and/or blockade of sodium channels by pyrethroids may cause up-regulation of Nurr1, ultimately leading to increased expression of DAT.
In contrast to the in vivo data, our in vitro results show that short-term (10 min) incubation of SK-DAT cells with deltamethrin or permethrin had no effect on DAT-mediated dopamine uptake. However, if the incubation time was extended to 30 min or 24 h, significant decreases in dopamine uptake were observed. Thus, the reduction of dopamine uptake in SK-DAT cells by deltamethrin and permethrin, is both time and concentration dependent. The lack of a significant effect on dopamine uptake after the 10-min incubation time suggests that both compounds are devoid of any direct effect on dopamine uptake. In addition, the decreases we observed with the longer exposure times were associated with significant changes in Vmax and little effect on Km, providing further evidence that pyrethroids do not directly interact with DAT. We also found that these effects were not the result of loss of DAT protein. Since a direct (cocaine-like) action on DAT is excluded by our results, other mechanism(s) appear to be involved in the reduction of dopamine uptake observed here. One possibility is that long-term higher-level exposure to pyrethroids leads to prolonged depolarization (Narahashi, 1982; Tabarean and Narahashi, 2001). Indeed, in vitro exposure of rat striatal synaptosomes to high concentrations (10–50 μM) of veratridine, a sodium channel activator, resulted in decreased dopamine uptake (Holz and Coyle, 1974). In addition, pyrethroids have been demonstrated to inhibit respiratory chain function in isolated mitochondria at submicromolar levels, which could lead to down-regulation of DAT function (Gassner et al., 1997; Maragos et al., 2002; Braguini et al., 2004). Although there are few studies reporting pyrethroid concentrations in the brain following systemic administration, Sheets et al. (1994) reported that brain levels of deltamethrin were 0.023 μg/g and 0.145 μg/g following a single oral exposure to 4 or 80 mg/kg in adult rats. These brain concentrations are roughly equivalent to 45 and 287 nM, which are in the range of the concentrations used in our in vitro studies that were without effect on the DAT, but lower than those demonstrated to effect mitochondrial function (EC50 793 nM for permethrin and >200 nM for deltamethrin; Gassner et al., 1997; Braguini et al., 2004). Thus, it is likely that the concentrations employed in our in vitro studies are similar to the higher doses of permethrin (25–200 mg/kg) demonstrated to decrease mitochondrial function, dopamine uptake, and DAT immunoreactivity in mice (Karen et al., 2001; Bloomquist et al., 2002; Gillette and Bloomquist, 2003; Pittman et al., 2003).
Since we found that neither deltamethrin nor permethrin has a direct effect on DAT and we estimated that the concentrations employed were similar to those that decrease mitochondrial function, we sought to determine whether the reduction in dopamine uptake was the result of pyrethroid-induced cytotoxicity. Following 30 min or 24 h exposure to 10 μM of deltamethrin or permethrin, there was no significant cytotoxic effect as determined by LDH assay. This is in agreement with the observation that exposure to pyrethroids for 24 h did not produce any significant effect on LDH release from mouse cerebellar granule cells (Imamura et al., 2000), and indicates that the decreased dopamine uptake we observed is not due to cytotoxicity. Since we observed no overt cytotoxicity at any of the concentrations or times tested, we tested the possibility that deltamethrin and permethrin were causing apoptosis. Our findings reveal that both deltamethrin and permethrin induce apoptosis, as indicated by increased DNA fragmentation, following 30-min and 24-h incubations at the highest concentration used (10 μM), whereas at lower concentrations (5 μM), only permethrin induced apoptosis. These data suggest that apoptosis may explain, in part, some of the observed decrease in dopamine uptake in these cells at the higher concentrations used. There is evidence indicating that apoptosis might play a crucial role in the toxic actions of pyrethroids by induction of apoptosis and altering the expression of p53, Bax, and Bcl-2 genes (Wu and Liu, 2000a, 2000b), although the doses used were greater than 4-fold higher than we used in our in vivo studies. Taken together with our results, these studies suggest that higher-level exposure to pyrethroids may result in apoptosis, similar to that seen with our in vitro studies.
The alteration of DAT function and expression by pyrethroid exposure is of particular interest when taken in context of the role of DAT in Parkinson’s disease (PD). Several studies have identified pesticide exposure as a risk factor for PD (Priyadarshi et al., 2001). However, the mechanism by which pesticides enhance the risk of PD is not known. Previously, we and others have demonstrated that alterations of DAT expression can greatly affect the vulnerability of the dopamine neuron to neurotoxins such as MPTP or methamphetamine (Gainetdinov et al., 1997; Donovan et al., 1999; Fumagalli et al., 1998). In addition, the brain regions most vulnerable to parkinsonism-inducing toxin MPTP and those most affected by PD display the highest levels of DAT expression (Miller et al., 1999b; Uhl, 1998). Supporting the observation in humans, animals overexpressing DAT (Donovan et al., 1999) are more susceptible to MPTP toxicity. Therefore, enhanced DAT levels and function by pesticides may increase the susceptibility of dopamine neurons to endogenous neurotoxic dopamine metabolites or exogenous toxicants by increasing uptake through DAT. Additionally, the decreased dopamine uptake and increased DNA fragmentation, suggestive of an ongoing apoptotic process, following in vitro pyrethroid exposure may be relevant to PD as well. Positron emission topographic imaging with 11C-WIN 35,428 has revealed early reductions in DAT levels in mild cases of PD (Frost et al., 1993), suggesting that reductions in DAT levels may be an early indicator of clinical PD in humans. Additionally, activated caspase-3, a primary apoptotic effector, has been demonstrated to precede apoptotic death in human PD brain (Hartmann et al., 2000). Therefore, lower level exposure to pyrethroids may contribute to PD through up-regulation of DAT and increased uptake of endogenous and exogenous neurotoxicants while increased levels result in apoptotic cell death. However, the exact mechanism of pesticides, including pyrethroids, in the etiology of PD remains to be established.
In conclusion, the present study clearly demonstrates that deltamethrin and permethrin increase DAT and DAT-mediated dopamine uptake in striatal synaptosomes following in vivo exposure. However, in vitro experiments revealed that the in vivo effects are likely indirect as acute in vitro exposure of cells stably expressing DAT had no effect on dopamine uptake. We also found that prolonged higher-level exposure decreases dopamine uptake in SK-DAT cells, which may be due in part to induction of apoptosis. These results may shed light on the mechanisms underlying pyrethroids-induced neurotoxicity and might implicate pyrethroids as environmental risk factors leading to the development of PD.
Acknowledgments
This work was supported by the US Army Medical Research and Materiel Command under Award No. 00267036 (GWM), and in part by NIH U54 ES012068 (GWM) and F32 ES013457 (JRR), and an EPA STAR Fellowship (TSG). Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the U.S. Army, NIH, or EPA.
References
- Anadon A, Martinez-Larranaga MR, Fernandez-Cruz ML, Diaz MJ, Fernandez MC, Martinez MA. Toxicokinetics of deltamethrin and its 4′-HO-metabolite in the rat. Toxicol Appl Pharmacol. 1996;141:8–16. doi: 10.1006/taap.1996.0254. [DOI] [PubMed] [Google Scholar]
- Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3 dependent proteolytic cleavage of protein kinase C delta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22:1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbeau A, Roy M, Cloutier T, Plasse L, Paris S. Environmental and genetic factors in the etiology of Parkinson’s disease. Adv Neurol. 1987;45:299–306. [PubMed] [Google Scholar]
- Bloomquist JR, Barlow RL, Gillette JS, Li W, Kirby ML. Selective effects of insecticides on nigrostriatal dopaminergic nerve pathways. Neurotoxicology. 2002;23:537–544. doi: 10.1016/s0161-813x(02)00031-1. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for detecting microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Braguini WL, Cadena SM, Carnieri EG, Rocha ME, de Oliveira MB. Effects of deltamethrin on functions of rat liver mitochondria and on native and synthetic model membranes. Toxicol Lett. 2004;152:191–202. doi: 10.1016/j.toxlet.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci. 2001;21:2571–2579. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey LL, Reith ME. [3H]WIN 35,428 binding to the dopamine uptake carrier: I. Effect of tonicity and buffer composition. J Neurosci Methods. 1994;51:23–30. doi: 10.1016/0165-0270(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, Kostic V, Philpot RM, Kirstein CL, Rothman RB, Schindler CW, Uhl GR. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter over-expression. Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Eells JT, Dubocovich ML. Pyrethroid insecticides evoke neurotransmitter release from rabbit striatal slices. J Pharmacol Exp Ther. 1988;246:514–521. [PubMed] [Google Scholar]
- Frost JJ, Rosier AJ, Reich SG, Smith JS, Ehlers MD, Snyder SH, Ravert HT, Dannals RF. Positron emission tomographic imaging of the dopamine transporter with 11C-WIN 35,428 reveals marked declines in mild Parkinson’s disease. Ann Neurol. 1993;34:423–431. doi: 10.1002/ana.410340331. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzano KJ, Caron MG. Role of dopamine transporter in methamphetamine-induced neurotoxicity: evidence from mice lacking the transporter. J Neurosci. 1998;18:4861–4869. doi: 10.1523/JNEUROSCI.18-13-04861.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gassner B, Wuthrich A, Scholtysik G, Solioz M. The pyrethroids permethrin and cyhalothrin are potent inhibitors of the mitochondrial complex I. J Pharmacol Exp Ther. 1997;281:855–860. [PubMed] [Google Scholar]
- Gillette JS, Bloomquist JR. Differential up-regulation of striatal dopamine transporter and alpha-synuclein by the pyrethroid insecticide permethrin. Toxicol Appl Pharmacol. 2003;192:287–293. doi: 10.1016/s0041-008x(03)00326-0. [DOI] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–49. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Golbe LI, Farrell TM, Davis PH. Follow-up study of early-life protective and risk factors in Parkinson’s disease. Mov Disord. 1990;5:66–70. doi: 10.1002/mds.870050116. [DOI] [PubMed] [Google Scholar]
- Gordon I, Weizman R, Rehavi M. Modulatory effect of agents active in the presynaptic dopaminergic system on the striatal dopamine transporter. Eur J Pharmacol. 1996;298:27–30. doi: 10.1016/0014-2999(95)00770-9. [DOI] [PubMed] [Google Scholar]
- Gorell JM, Johnson CC, Rybicki BA, Peterson EL, Richardson RJ. The risk of Parkinson’s disease with exposure to pesticides, farming, well water, and rural living. Neurology. 1998;50:1346–1350. doi: 10.1212/wnl.50.5.1346. [DOI] [PubMed] [Google Scholar]
- Grosse G, Thiele T, Heuckendorf E, Schopp E, Merder S, Pickert G, Ahnert-Hilger G. Deltamethrin differentially affects neuronal subtypes in hippocampal primary culture. Neuroscience. 2002;112:233–241. doi: 10.1016/s0306-4522(01)00573-5. [DOI] [PubMed] [Google Scholar]
- Hartmann A, Hunot S, Michel PP, Muriel MP, Vyas S, Faucheux BA, Mouatt-Prigent A, Turmel H, Srinivasan A, Ruberg M, Evan GI, Agid Y, Hirsch EC. Caspase-3: a vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci USA. 2000;97:2875–2880. doi: 10.1073/pnas.040556597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson E, Joseph B, Castro D, Lindqvist E, Aarnisalo P, Wallen A, Benoit G, Hengerer B, Olson L, Perlmann T. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp Cell Res. 2003;288:324–334. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- Holz RW, Coyle JT. The effect of various salts, temperature, and the alkaloids veratridine and batrachotoxin on the uptake of [3H]dopamine into synaptosomes from rat striatum. Mol Pharmacol. 1974;10:746–758. [Google Scholar]
- Imamura L, Hasegawa H, Kurashina K, Hamanishi A, Tabuchi A, Tsuda M. Repression of activity-dependent c-fos and brain-derived neurotrophic factor mRNA expression by pyrethroid insecticides accompanying a decrease in Ca(2+) influx into neurons. J Pharmacol Exp Ther. 2000;295:1175–1182. [PubMed] [Google Scholar]
- Karen DJ, Li W, Harp PR, Gillette JS, Bloomquist JR. Striatal dopaminergic pathways as a target for the insecticides permethrin and chlorpyrifos. Neurotoxicology. 2001;22:811–817. doi: 10.1016/s0161-813x(01)00063-8. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Castagnoli K, Bloomquist JR. In vivo effects of deltamethrin on dopamine neurochemistry and the role of augmented neurotransmitter release. Pestic Biochem Physiol. 1999;65:160–168. [Google Scholar]
- Kirby ML, Barlow RL, Bloomquist JR. Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol Sci. 2001;61:100–106. doi: 10.1093/toxsci/61.1.100. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Barlow RL, Bloomquist JR. Selective effects of cyclodiene insecticides on dopamine release in mammalian synaptosomes. Toxicol Appl Pharmacol. 2002;181:89–92. doi: 10.1006/taap.2002.9405. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Wagner JR, Kirby ML, Anantharam A, Kanthasamy AG. Oxidative stress and mitochondrial-mediated apoptosis in dopaminergic cells exposed to methylcyclopentadienyl manganes tricarbonyl. J Pharmacol Exp Ther. 2002;302:26–35. doi: 10.1124/jpet.302.1.26. [DOI] [PubMed] [Google Scholar]
- Le Couteur DG, McLean AJ, Taylor MC, Woodham BL, Board PG. Pesticides and Parkinson’s disease. Biomed Pharmacother. 1999;53:122–130. doi: 10.1016/S0753-3322(99)80077-8. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with Folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Maragos WF, Zhu J, Chesnut MD, Dwoskin LP. Mitochondrial toxin inhibition of [(3H)] dopamine uptake into rat striatal synaptosomes. Biochem Pharmacol. 2002;63:1499–1505. doi: 10.1016/s0006-2952(02)00910-3. [DOI] [PubMed] [Google Scholar]
- Miller GW, Staley JK, Heilman CJ, Perez JT, Mash DC, Rye DB, Levey AI. Immunochemical analysis of dopamine transporter protein in Parkinson’s disease. Ann Neurol. 1997;41:530–539. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Miller GW, Kirby ML, Levey AI, Bloomquist JR. Heptachlor alters expression and function of dopamine transporters. Neurotoxicology. 1999a;20:631–637. [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999b;20:424–429. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Cellular and molecular mechanisms of action of insecticides: neurophysiological approach. Neurobehav Toxicol Teratol. 1982;4:753–758. [PubMed] [Google Scholar]
- Narahashi T. Neuronal ion channels as targets of insecticides. Pharmacol Toxicol. 1996;79:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogensis of Parkinson’s disease. Ann Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Page G, Peeters M, Maloteaux JM, Hermans E. Increased dopamine uptake in striatal synaptosomes after treatment of rats with amantadine. Eur J Pharmacol. 2000;403:75–80. doi: 10.1016/s0014-2999(00)00573-2. [DOI] [PubMed] [Google Scholar]
- Pifl C, Giros B, Caron MG. The dopamine transporter. the cloned target site of parkinsonism-inducing toxins and of drugs of abuse. Adv Neurol. 1996;69:235–238. [PubMed] [Google Scholar]
- Pittman JT, Dodd CA, Klein BG. Immunohistochemical changes in the mouse striatum induced by the pyrethroid insecticide permethrin. Int J Toxicol. 2003;22:359–370. doi: 10.1177/109158180302200504. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and Parkinson’s disease: a metaanalysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Uitti RJ, Stern W, Laverty W. Early onset Parkinson’s disease in Saskatchewan—Environmental considerations for etiology. Can J Neurol Sci. 1986;13:312–316. doi: 10.1017/s0317167100036635. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Uitti RJ, Stern W, Laverty W, O’Donnell K, O’Donnell D, Yuen WK, Dua A. Geography, drinking water chemistry, pesticides and herbicides and the etiology of Parkinson’s disease. Can J Neurol Sci. 1987;14:414–418. doi: 10.1017/s0317167100037823. [DOI] [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to arochlor 1016 or 1260 differentially affects dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ. Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem. 2001;76:1565–1572. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to rural environmental factors: a population based case-control study. Can J Neurol Sci. 1991;18:279–286. doi: 10.1017/s0317167100031826. [DOI] [PubMed] [Google Scholar]
- Sheets LP, Doherty JD, Law MW, Reiter LW, Crofton KM. Age-dependent differences in the susceptibility of rats to deltamethrin. Toxicol Appl Pharmacol. 1994;126:186–190. doi: 10.1006/taap.1994.1106. [DOI] [PubMed] [Google Scholar]
- Shimada S, Kitayama S, Lin CL, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl G. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Miller GW, Levey AI, Greenamyre JT. Acute mitochondrial and chronic toxicological effects of 1-methyl-4-phenyl-pyridinium in human neuroblastoma cells. Neurotoxicology. 2002;23:569–580. doi: 10.1016/s0161-813x(02)00060-8. [DOI] [PubMed] [Google Scholar]
- Stephenson J. Exposure to home pesticides linked to Parkinson’s disease. JAMA. 2000;283:3055–3056. doi: 10.1001/jama.283.23.3055. [DOI] [PubMed] [Google Scholar]
- Tabarean IV, Narahashi T. Kinetics of modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by tetramethrin and deltamethrin. J Pharmacol Exp Ther. 2001;299:988–997. [PubMed] [Google Scholar]
- Tanner CM, Langston JW. Do environmental toxins cause Parkinson’s disease: a critical review. Neurology. 1990;40:S17–S30. [PubMed] [Google Scholar]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, Mayeux R, Langston JW. Parkinson’s disease in twins: an etiologic study. JAMA. 1999;281:341–346. doi: 10.1001/jama.281.4.341. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Hypothesis: the role of dopaminergic transporters in selective vulnerability of cells in Parkinson’s disease. Ann Neurol. 1998;43:555–560. doi: 10.1002/ana.410430503. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y. Apoptotic cell death in rat brain following deltamethrin treatment. Neurosci Lett. 2000a;279:85–88. doi: 10.1016/s0304-3940(99)00973-8. [DOI] [PubMed] [Google Scholar]
- Wu A, Liu Y. Deltamethrin induces delayed apoptosis and altered expression of p53 and bax in rat brain. Environ Toxicol Pharmacol. 2000b;8:183–189. doi: 10.1016/s1382-6689(00)00039-9. [DOI] [PubMed] [Google Scholar]