Abstract
Although banned in the 1970s, significant levels of the organochlorine pesticide heptachlor are still present in the environment raising concern over potential human exposure. In particular, organochlorine pesticides have been linked to an increased risk of Parkinson's disease. Studies from our laboratory and others have demonstrated that exposure of laboratory animals to heptachlor alters the levels and function of the dopamine transporter (DAT), an integral component of dopaminergic neurotransmission and a gateway for the dopaminergic neurotoxin MPTP. In this study, we examined the effects of developmental exposure to heptachlor on DAT, and other key components of the dopaminergic system, including the vesicular monoamine transporter 2 (VMAT2), tyrosine hydroxylase (TH), and aromatic amino acid decarboxylase (AADC). Female C57BL/6J mice received 0 or 3 mg/kg heptachlor in peanut butter every 3 days for 2 weeks prior to breeding and throughout gestation and lactation until the offspring were weaned on postnatal day (PND) 21. On postnatal day 28, DAT, VMAT2, and TH levels were increased by 100, 70, and 30%, respectively, with no change in AADC levels or total dopamine levels. The ratio of DAT:VMAT2 was increased 29%. Since an increase in the DAT:VMAT2 ratio appears to predict susceptibility of brain regions to Parkinson's disease (PD) and results in increased toxicity of MPTP, these results suggest that alterations of the dopaminergic system by developmental heptachlor exposure may increase the susceptibility of dopamine neurons to toxic insult.
Keywords: Heptachlor, Developmental neurotoxicity, Dopamine transporter, Vesicular monoamine transporter 2, Parkinson's disease, Tyrosine hydroxylase
INTRODUCTION
Heptachlor is an organochlorine insecticide that was used extensively throughout the 1960s and 1970s in both household and agricultural settings. In the early 1980s residents of Hawaii were exposed to high levels of heptachlor in their milk supply after dairy cattle were fed pineapple green chop that had been contaminated with heptachlor (Baker et al., 1991). This raised concern for the developmental consequences of this exposure in the offspring of mothers, who were exposed in utero and during breast-feeding (i.e. perinatal exposure), and in children who consumed the contaminated milk. Although the use of heptachlor and other cyclodienes has been discontinued in the United States for over 20 years, its chemical and physical characteristics have allowed residues of the insecticide to persist in the environment (Fendick et al., 1990). A report by the United States Geological Society found elevated levels of heptachlor residues in the Mississippi River Basin, confirming the continued presence and possibility of exposure of heptachlor from the environment (Schmitt, 2002). In addition, a recent study of pesticide levels in food found up to 29% of certain food samples contained detectable levels of heptachlor epoxide, the most stable metabolite of heptachlor (USDA, 1997; Fendick et al., 1990). These data suggest that the potential for further developmental exposure to heptachlor and its deleterious neurological effects are still of concern.
Heptachlor is converted in vivo to heptachlor epoxide, which exerts its insecticidal effects through blockade of the GABAA receptor. This blockade results in inhibition of the chloride ion flux through the GABAA receptor and subsequent hyperexcitation of the nervous system (Narahashi, 1996). While the effects of heptachlor on GABAA receptors are well known, our laboratory and others have demonstrated that heptachlor increases the expression of the plasma membrane dopamine transporter (DAT; Kirby et al., 2001; Miller et al., 1999c) at dosage levels that do not cause overt toxicity. We also found that heptachlor increases the protein levels of VMAT2, but at the same time directly inhibits transport function (Miller et al., 1999c). DAT is a crucial component of normal dopamine function and is responsible for terminating dopamine neurotransmission by rapid reuptake of dopamine into the presynaptic terminal (Giros and Caron, 1993; Miller et al., 1999b). Following reuptake by DAT or synthesis through aromatic amino acid decarboxylase (AADC) and tyrosine hydroxylase (TH), dopamine present in the cytoplasm of the presynaptic nerve terminal is quickly transported into small synaptic and dense core vesicles by VMAT2 (Erickson et al., 1992). Sequestration of dopamine by VMAT2 is critical, as cytosolic dopamine can be oxidized and damage cells (Hastings et al., 1996). Therefore, regulation of dopamine levels, both in the synapse and within the cell, is accomplished through the coordinate actions of DAT and VMAT2 and alteration of these transporters may result in enhanced susceptibility of dopamine neurons to toxic insult from compounds that are transported by DAT and sequestered by VMAT2, such as dopamine and MPTP (Miller et al., 1999b).
As mentioned above, we have found that administration of heptachlor to adult mice alters DAT levels and function (Miller et al., 1999c). More recently, it has been shown that developmental exposure of rats to heptachlor persistently increases DAT levels. In this study, we report that perinatal exposure of mice to lower levels of heptachlor than we have previously used in adult mice (Miller et al., 1999c) and lower levels than used in developmental studies with rats not only results in increased DAT, but also increased VMAT2 and TH. No change was observed with AADC, but the increased levels of DAT and VMAT2 significantly increased the DAT:VMAT2 ratio by 29%. Because the DAT:VMAT2 ratio predicts susceptibility of dopaminergic neurons to damage, these results suggest that perinatal exposure to heptachlor may increase the vulnerability of dopamine neurons to toxic insult.
MATERIALS AND METHODS
Chemicals
Heptachlor (purity ≥98%) was obtained from Chem Service Inc. (West Chester, PA). Monoclonal anti-rat DAT, polyclonal anti-rabbit AADC, polyclonal anti-rabbit TH, and polyclonal anti-rabbit VMAT2 were purchased from Chemicon (Temecula, CA) and monoclonal anti-mouse α-tubulin was purchased from Sigma (St. Louis, MO). Secondary antibodies coupled to horseradish peroxidase were purchased from ICN (anti-rat; Costa Mesa, CA) and Bio-Rad (anti-rabbit and anti-mouse; Hercules, CA). SuperSignal West Dura Extended duration substrate and stripping buffer were obtained from Pierce (Rockford, IL). Monoamine standards for dopamine (DA), 3,4-dihydroxyphenylacetic acid (DOPAC), and homovanillic acid (HVA) were obtained from Sigma (St. Louis, MO). Mobile phase was obtained from ESA Inc. (Chelmsford, MA). 3H-mazindol, was obtained from Perkin-Elmer and nomifensine was purchased from Sigma (St. Louis, MO).
Animals and Treatment
Eight week-old female and male C57BL/6J mice purchased from Jackson Laboratory (Bar Harbor, ME) were used for developmental studies. Mice were maintained on a 12:12 light/dark cycle and food and water were available ad libitum. All procedures were conducted in accordance with the Guide for Care and Use of Laboratory Animals (National Institutes of Health) and previously approved by the Institutional Animal Care and Use Committee at Emory University.
Female mice were administered 3 mg/kg heptachlor dissolved in corn oil vehicle and mixed with peanut butter every 3 days for 2 weeks prior to introduction of male mice for breeding. Control mice received an equivalent amount of corn oil vehicle in peanut butter. Mice were monitored to ensure total consumption of the treatment, which generally occurred within 10 min. Oral exposures were chosen since the most likely route of exposure to organochlorines in the human population is through the ingestion of contaminated food. The dosage chosen (3 mg/kg) is greater than 20-fold less than the acute oral LD50 in mice and is lower than the medium dosage (4.2 mg/kg/day) used in similar developmental studies with rats (Purkerson-Parker et al., 2001). The use of peanut butter as a vehicle was chosen to reduce stress to the dam during gestation, since stress from repeated injections during gestation has been shown to alter GABAA subunit development (Liu et al., 1997). Dosing continued on the same schedule throughout gestation and lactation ending upon weaning of the pups on postnatal day (PND) 21. Offspring were sacrificed on PND 28 and tissue was collected for neurochemical analysis. Tissue from one pup from each litter was used for Western and HPLC determinations and tissue from an additional pup was used for binding studies. Only data from male offspring are presented in this study because our previous studies have focused on male mice. PND 28 was chosen as the primary endpoint based on previous studies identifying PND 28 as the age at which the dopaminergic system reaches maturity (Giorgi et al., 1987).
Western Blot Analysis
Western blots were used to quantify the amount of DAT, TH, VMAT2, AADC, and α-tubulin present in samples of striatal tissue from treated and control mice. Analysis was performed as previously described by Richardson and Miller (2004). Briefly, right striata samples were homogenized in buffer (320 mM sucrose and 5 mM HEPES) with protease inhibitors (1 μg/ml each of aprotinin, leupeptin, and pepstatin). Homogenized samples were centrifuged at 2000 × g for 5 min and the supernatant was centrifuged at 30,000 × g for 30 min. The final pellet was resuspended in homogenization buffer and total protein concentrations were determined by a Bradford protein assay (Bradford, 1976). We have previously used this preparation to examine the dose-related effects of MPTP on the dopamine system and found excellent correlations between reductions of DAT, VMAT2, and TH and loss of striatal dopamine (Tillerson et al., 2002). Samples (15 μg protein) were subjected to polyacrylamide gel electrophoresis on 10% precast NuPage gels (Invitrogen, Carlsbad, CA). Samples were electrophoretically transferred to a polyvinylidene difluoride membrane, and non-specific sites were blocked in 7.5% nonfat dry milk in Tris-buffered saline (135 mM NaCl, 2.5 mM KCl, 50 mM Tris, and 0.1% Tween 20, pH 7.4). Membranes were then incubated overnight in a monoclonal antibody to the N-terminus of DAT (Miller et al., 1997). DATantibody binding was detected using a goat anti-rat horseradish peroxidase secondary antibody and enhanced chemiluminescence. The luminescence signal was captured on an Alpha Innotech Fluorochem imaging system and stored as a digital image. Densitometric analysis was performed and calibrated to co-blotted dilutional standards of pooled striata from all control samples. Membranes were stripped for 15 min at room temperature with Pierce Stripping Buffer and sequentially reprobed with polyclonal VMAT2, TH, and AADC antibody and a monoclonal α-tubulin antibody. α-Tubulin blots were used to ensure equal protein loading across samples.
HPLC-EC Determination of Dopamine and its Metabolites
HPLC-EC analysis of neurochemistry was performed as previously described by Richardson and Miller (2004). Briefly, dissected left striata were sonicated in 0.1 M perchloric acid containing 347 μM sodium bisulfite and 134 μM EDTA. Homogenates were centrifuged at 15,000 × g for 20 min at 4 °C, the supernatant removed, and filtered through a 0.22 μm filter by centrifugation at 15,000 × g for 20 min. The supernatants were then analyzed for levels of DA, DOPAC, and HVA. Levels were measured using HPLC with an eight-channel coulometric electrode array (ESA Coularray, Chelmsford, MA). Quantification was made by reference to calibration curves made with individual monoamine standards.
3H-Mazindol Binding to the Dopamine Transporter
For determination of dopamine transporter (DAT) binding sites, 3H-mazindol binding was determined. Briefly, samples of striatum homogenized in 50 mM Tris–HCl containing 300 mM NaCl and 5 mM KCl with a glass mortar using a Wheaton motorized tissue grinder and a Teflon pestle. Homogenates were centrifuged at 48,000 × g for 10 min and the supernatant discarded. The pellet was resuspended in the same buffer by homogenization and washed twice more by centrifugation to yield crude membrane preparations.
DAT levels were determined in crude striatal membranes by binding of the specific antagonist 3H-mazindol according to the methods of Javitch and coworkers (1984), with modifications to reduce the assay volume to 200 μl. Preliminary kinetic studies indicated that the binding of 3H-mazindol to striatal membranes was best fit to a one-site model determined by non-linear curve fitting techniques (GraphPad Prism 3.0) with a Kd of 5.8 ± 1.3 nM and a Bmax of 1.4 ± 0.08 pmol/mg. Therefore, binding studies with crude striatal membranes were conducted with a single concentration (10 nM) of 3H-mazindol for 1 h at 4 °C in 96-well plates. A single concentration was selected based on observations that the Kd for these ligands does not vary during development (Shimizu and Prasad, 1991) and we and others have not observed changes in Kd in adult mice exposed to heptachlor (Kirby et al., 2001; Miller et al., 1999c). Incubations were terminated by rapid vacuum filtration onto GF/B filter plates and radioactivity was determined by liquid scintillation counting. Non-specific binding was determined by the inclusion of 10 μM nomifensine and specific binding was calculated as the total binding (incubated without 10 μM nomifensine) minus non-specific binding (incubated with nomifensine), and expressed as pmol/mg protein.
Statistical Analysis
Each litter was treated as an individual experimental unit. Data are presented as mean ± S.E.M. For statistical analysis, data were log-transformed and analyzed by Student's t-test with significance reported at the p ≤ 0.05 level.
RESULTS
Administration of 3 mg/kg of heptachlor to female C57BL/6J mice prior to breeding, during gestation and lactation and continuing through PND 21 resulted in no overt toxicity in the dam or offspring as evidenced by no change in weight gain of the dams or pups (data not shown). Additionally, there were no apparent differences in litter size or sex distribution of pups between control and treated dams.
Western blot analysis was used to determine the effects of developmental exposure to heptachlor on DAT, VMAT2, TH, and AADC levels on postnatal day 28. DAT was detected as a single band between 80 and 85 kDA (Miller et al., 1997) and VMAT2 was detected as three bands at 75, 55, and 45 kDA, representing the glycosylated, native, and truncated form (Miller et al., 1999a). For VMAT2 quantitation, only the functional 75 kDA band was quantified. AADC and TH were detected as single bands at 50 and 60 kDA, respectively. Densitometry of α-tubulin revealed a single band at 55 kDA and no differences between any of the groups, ensuring equal loading of sample. Representative samples are illustrated under each graph.
DAT levels in the striatum were increased by 100% (t = 5.973, p = 0.004) in the offspring of mice exposed to heptachlor compared to control (Fig. 1A). To confirm these changes we determined the levels of 3H-mazindol binding to DAT and observed a 40% increase (t = 3.83, p = 0.019) of binding sites in the striatum of heptachlor-exposed offspring (Fig. 1B). VMAT2 levels were increased by 70% (t = 5.700, p = 0.0013; Fig. 2A) and TH levels by 30% (t = 6.19, p = 0.0008; Fig. 2B) in synaptosomes prepared from the striatum of heptachlor-exposed off-spring. There was no difference in AADC levels between control animals and those exposed to heptachlor (Fig. 3). Using data obtained from the DAT and VMAT2 Westerns, the DAT:VMAT2 ratio was significantly increased by 29% (t = 4.542, p = 0.01) in the striatum of heptachlor-exposed offspring (Fig. 4).
Fig. 1.
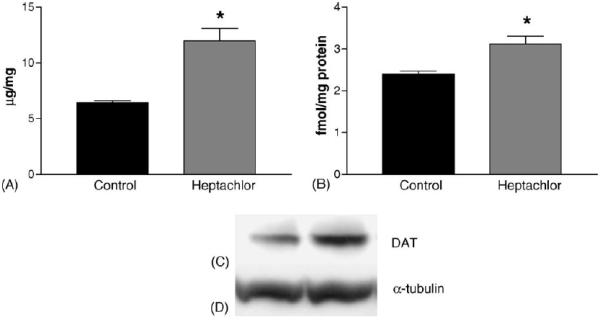
Striatal DAT levels as measured by Western immunoblotting (A) and 3H-mazindol binding (B) in the offspring of mice exposed to 3 mg/kg heptachlor throughout gestation and lactation. Representative Western blots of DAT (C) and α-tubulin (D) to ensure equal protein loading. Data represent mean ± S.E.M. (n = 3–4 animals per treatment group). (*) Indicates that the groups are significantly different from each other (p ≤ 0.05) by Student's t-test.
Fig. 2.
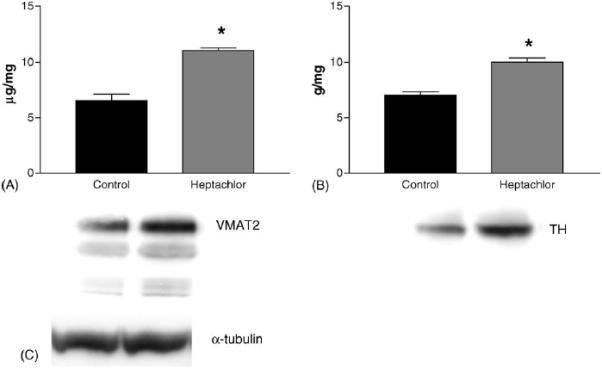
Striatal VMAT2 (A), and TH (B) levels as measured by Western immunoblotting in the offspring of mice exposed to 3 mg/kg heptachlor throughout gestation and lactation. Representative Western blots of VMAT2 and TH are represented below each graph. (C) Representative Western blot of α-tubulin to ensure equal protein loading. Data represent mean ± S.E.M. (n = 3–4 animals per treatment group). (*) Indicates that the groups are significantly different from each other (p ≤ 0.05) by Student's t-test.
Fig. 3.
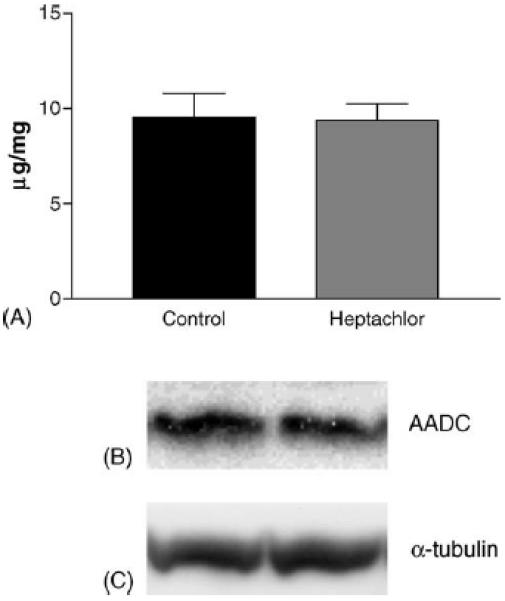
Striatal AADC (A) as measured by Western immunoblotting in the offspring of mice exposed to 3 mg/kg heptachlor throughout gestation and lactation. Representative Western blots of AADC (B) and α-tubulin (C) to ensure equal protein loading.
Fig. 4.
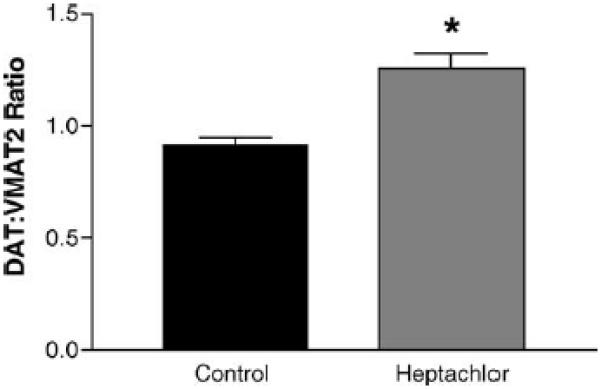
Striatal DAT:VMAT2 ratio in the offspring of mice exposed to 3 mg/kg heptachlor throughout gestation and lactation. Data were from Western blot quantitation presented in Figs. 1 and 2 and represent mean ± S.E.M. (n = 3–4 animals per treatment group). () Indicates that the groups are significantly different from each other (p ≤ 0.05) by Student's t-test.
Results for striatal dopamine and metabolite levels are summarized in Table 1. Developmental heptachlor exposure did not affect total tissue dopamine levels or the levels of the dopamine metabolites, DOPAC, and HVA.
Table 1.
Mean striatal monoamine levels in control and heptachlor-exposed mice
| Control | Heptachlor | |
|---|---|---|
| Dopamine | 19.84 ± 0.34 | 19.51 ± 1.51 |
| DOPAC | 2.02 ± 0.11 | 2.22 ± 0.07 |
| HVA | 1.81 ± 0.08 | 1.79 ± 0.07 |
Monoamine levels expressed as ng/mg tissue. Data expressed as mean ± S.E.M. (n = 3–4).
DISCUSSION
Previously, we have reported that exposure of adult mice to heptachlor results in up-regulation of the dopamine transporter and the vesicular monoamine transporter 2 (VMAT2; Miller et al., 1999c). In this study, we demonstrate that lower-level developmental exposure of mice to heptachlor, which is devoid of overt toxic effects, increases DAT, VMAT2 levels, and TH levels in the offspring.
Heptachlor is a member of the cyclodiene class of insecticides that exerts acute toxicity through the binding to the chloride channel of GABAA receptors and decreasing chloride flux, resulting in hyperstimulation of the nervous system (Narahashi, 1996). At first glance, it may not be apparent how blockade of GABAA receptors by heptachlor would result in alterations of the dopaminergic system observed in this study. However, GABA is one of the earliest neurotransmitters to appear during neuronal development and serves as a trophic signal in the development of other neurotransmitter systems, including the dopaminergic system (Lauder et al., 1998; Liu et al., 1998). Indeed, studies by Liu et al. (1997, 1998) have shown that blockade of the GABAA receptors by bicuculline and the cyclodiene insecticide dieldrin, resulted in alteration of monoamine neuron formation. However, the precise mechanism by which cyclodienes alter the development of the dopaminergic system has yet to be determined.
The most robust effect observed in this study was the up-regulation of DAT by perinatal heptachlor exposure as determined by Western immunoblotting and mazindol binding. Studies from our laboratory (Miller et al., 1999c) and others (Kirby et al., 2001) have consistently observed similar effects on DAT-mediated dopamine uptake in adult mice given higher dosages of heptachlor. Similar to the results obtained in this study, perinatal exposure of rats to 4.2 and 8.4 mg/kg/day of heptachlor have been demonstrated to increase DAT levels as measured by mazindol binding (Purkerson-Parker et al., 2001). Therefore, it appears that DAT is a particularly sensitive target of heptachlor exposure, but the mechanism(s) by which heptachlor alters DAT is not known.
One potential mechanism for the increase in DAT levels and activity observed is to compensate for enhanced dopamine release. Heptachlor has been shown to release dopamine from pre-loaded synaptosomes in vitro (Kirby et al., 2002) and picrotoxin, which binds to the same site as heptachlor on GABAA receptors has been demonstrated to cause dopamine release in vivo (Gong et al., 1998). Additionally, inhibition of GABAA receptors has been shown to increase burst firing in dopamine neurons (Celeda et al., 1999; Paladini and Tepper, 1999). However, there are currently no data on the ability of heptachlor or heptachlor epoxide to cause dopamine release in vivo. Another possibility is up-regulation of DAT at the transcriptional level. The transcription factor NURR1 has been shown to directly enhance transcription of DAT (Sacchetti et al., 2001; Hermanson et al., 2003). In addition to DAT, NURR1 can regulate expression of VMAT2 and TH, but not aromatic amino acid decarboxylase (AADC; Hermanson et al., 2003; Smits et al., 2003). Since NURR1 transcription is enhanced by neuronal activity (Brosenitsch and Katz, 2001), blockade of GABAA receptors by heptachlor epoxide and the resultant increased neuronal activity may cause up-regulation of NURR1, ultimately leading to increased expression of DAT, VMAT2, and TH. Because we observed effects on DAT, TH, and VMAT2 but not AADC in this study, transcriptional activation by NURR1 may represent a plausible mechanism for the effects observed.
Although we observed increased levels of synaptosomal DAT, VMAT2, and TH, there were no changes in the levels of striatal dopamine or its metabolites, DOPAC and HVA. This lack of effect on total dopamine levels has also been observed in adult mice repeatedly exposed to heptachlor and exhibiting increased DAT levels (Kirby et al., 2001). We suggest that this observation may be the result of feedback inhibition of TH activity resulting from increased dopamine uptake through DAT. Feedback inhibition of TH by dopamine is well established (Kumer and Vrana, 1996) and is suggested to be primarily the result of dopamine newly taken up through DAT. Therefore, increased dopamine uptake by the elevated DAT levels observed in this study and others (Miller et al., 1999c; Kirby et al., 2001; Purkerson-Parker et al., 2001), may result in inhibition of TH activity to maintain cellular homeostasis. Indeed, DAT appears to influence the regulation of TH, as mice lacking DAT have reduced TH protein levels, which are not the result of loss of dopamine neurons, and increased TH activity (Jones et al., 1999; Jaber et al., 1999). However, we did not measure TH activity in this study and the potential role of feedback inhibition of TH in the maintenance of dopamine levels in the offspring of heptachlor-exposed animals remains to be determined.
The alteration of DAT and VMAT2 levels by developmental heptachlor exposure is of particular interest when taken in context of the role of DAT in Parkinson's disease (PD). Several studies have identified pesticide exposure as a risk factor for Parkinson's disease (reviewed by Priyadarshi et al., 2000) and elevated levels of organochlorine pesticides have been found in post-mortem PD brain (Fleming et al., 1994; Corrigan et al., 2000). However, the mechanism by which pesticides, in particular organochlorines, enhance the risk of PD is not known.
Previously, we and others have demonstrated that alterations in the ratio of DAT and VMAT2 can greatly affect the vulnerability of the dopamine neuron to neurotoxins such as MPTP or methamphetamine (Donovan et al., 1999; Fumagalli et al., 1999; Gainetdinov et al., 1997, 1998; Uhl et al., 2000). Specifically, as the ratio of DAT to VMAT2 increases, the vulnerability of the brain region to MPTP and Parkinson's disease increases (Miller et al., 1999b; Uhl, 1998). For example, the putamen is more affected in PD than the caudate and has a two-fold higher DAT:VMAT2 protein ratio (Miller et al., 1999b). This increased protein ratio is mirrored by differences in mRNA expression in the cell bodies from which these terminals originate (Uhl, 1998). Supporting the observation in humans, animals with genetic reduction of VMAT2 (Gainetdinov et al., 1998; Takahashi et al., 1997) and animals overexpressing DAT (Donovan et al., 1999) are more susceptible to MPTP toxicity. Therefore, alteration of the ratio of DAT:VMAT2 by developmental exposure to heptachlor, as observed in this study, may increase the susceptibility of dopamine neurons to endogenous neurotoxic dopamine metabolites or exogenous toxicants by increasing uptake through DAT. However, the potential consequences of this altered ratio on dopaminergic neurons remain to be established.
In summary, we have demonstrated that exposure to low levels of the organochlorine pesticide heptachlor during development results in alteration of the dopaminergic system through increased levels of synaptosomal DAT, VMAT2, and TH. These results suggest that exposure to heptachlor during critical time points of nervous system development alters the function of the dopamine system. In turn, these alterations could increase the susceptibility of DA neurons to damage from future exogenous or endogenous insult, providing a possible mechanism for the increased risk of PD linked to organochlorine pesticide exposure.
ACKNOWLEDGEMENTS
This work was supported by NIH U54 ES01268 and R21 ES012315 (GWM) and T32-NS07480 (JRR).
REFERENCES
- Baker DB, Loo S, Barker J. Evaluation of human exposure to the heptachlor epoxide contamination of milk in Hawaii. Hawaii Med J. 1991;50:108–12. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci. 2001;21:2571–9. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celeda P, Paladini CA, Tepper JM. GABAergic control of rat substantia nigra dopaminergic neurons: role of the globus pallidus and substantia nigra pars reticulata. Neuroscience. 1999;89:813–25. doi: 10.1016/s0306-4522(98)00356-x. [DOI] [PubMed] [Google Scholar]
- Corrigan FM, Wienburg CL, Shore RF, Daniel SE, Mann D. Organochlorine insecticides in substantia nigra in Parkinson's disease. J Toxicol Environ Health. 2000;59:229–34. doi: 10.1080/009841000156907. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Miner LL, Perry MP, Revay RS, Sharpe LG, Przedborski S, et al. Cocaine reward and MPTP toxicity: alteration by regional variant dopamine transporter overexpression. Mol Brain Res. 1999;73:37–49. doi: 10.1016/s0169-328x(99)00235-1. [DOI] [PubMed] [Google Scholar]
- Erickson JD, Eiden LE, Hoffman BJ. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci USA. 1992;89:10993–7. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendick EA, Mather-Mihaich E, Houck KA, St. Clair MB, Faust JB, Rockwell CH, et al. Ecological toxicology and human health effects of heptachlor. Rev Environ Contam Toxicol. 1990;111:61–142. doi: 10.1007/978-1-4612-3340-4_2. [DOI] [PubMed] [Google Scholar]
- Fleming L, Mann JB, Bean J, Briggle T, Sanchez-Ramos JR. Parkinson's disease and brain levels of organochlorine pesticides. Ann Neurol. 1994;36:100–3. doi: 10.1002/ana.410360119. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Valenzan K, Wang YM, Miller GW, Caron MG. Increased methamphetamine toxicity in heterozygote VMAT2 knockout mice. J Neurosci. 1999;19:2424–31. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–4. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Wang Y, Jones SR, Miller GW, Caron MG. Increased sensitivity to MPTP in heterozygote VMAT2 knockout mice. J Neurochem. 1998;70:1973–8. doi: 10.1046/j.1471-4159.1998.70051973.x. [DOI] [PubMed] [Google Scholar]
- Giorgi O, De Montis G, Porceddu ML, Mele S, Calderini G, Toffano G, et al. Developmental and age-related changes in D1-dopamine receptors and dopamine content in the rat striatum. Brain Res. 1987;432:283–90. doi: 10.1016/0165-3806(87)90053-8. [DOI] [PubMed] [Google Scholar]
- Giros B, Caron MG. Molecular characterization of the dopamine transporter. Trends Pharmacol Sci. 1993;14:43–9. doi: 10.1016/0165-6147(93)90029-j. [DOI] [PubMed] [Google Scholar]
- Gong W, Neill DB, Justice JB., Jr. GABAergic modulation of ventral pallidal dopamine release studied by in vivo microdialysis in the freely moving rat. Synapse. 1998;29:406–12. doi: 10.1002/(SICI)1098-2396(199808)29:4<406::AID-SYN12>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Hastings TG, Lewis DA, Zigmond MJ. Role of oxidation in the neurotoxic effects of intrastriatal dopamine injections. Proc Natl Acad Sci USA. 1996;93:1956–61. doi: 10.1073/pnas.93.5.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson E, Joseph B, Castro D, Lindqvist E, Aarnisalo P, Wallen A, et al. Nurr1 regulates dopamine synthesis and storage in MN9D dopamine cells. Exp Cell Res. 2003;288:324–34. doi: 10.1016/s0014-4827(03)00216-7. [DOI] [PubMed] [Google Scholar]
- Jaber M, Dumartin B, Sagne C, Haycock JW, Roubert C, Giros B, et al. Differential regulation of tyrosine hydroxylase in the basal ganglia of mice lacking the dopamine transporter. Eur J Neurosci. 1999;11:3499–511. doi: 10.1046/j.1460-9568.1999.00764.x. [DOI] [PubMed] [Google Scholar]
- Javitch JA, Blaustein RO, Snyder SH. [3H] mazindol binding associated with dopamine and norepinephrine uptake sites. Mol Pharm. 1984;26:35–44. [PubMed] [Google Scholar]
- Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1999;95:4029–34. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML, Barlow RL, Bloomquist JR. Neurotoxicity of the organochlorine insecticide heptachlor to murine striatal dopaminergic pathways. Toxicol Sci. 2001;61:100–6. doi: 10.1093/toxsci/61.1.100. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Barlow RL, Bloomquist JR. Selective effects of cyclodiene insecticides on dopamine release in mammalian synaptosomes. Toxicol Appl Pharm. 2002;181:89–92. doi: 10.1006/taap.2002.9405. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67:433–62. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Lauder JM, Liu J, Devaud L, Morrow AL. GABA as a trophic factor for developing monoamine neurons. Perspect Dev Neurobiol. 1998;5:247–59. [PubMed] [Google Scholar]
- Liu J, Brannen KC, Grayson DR, Morrow AL, Devaud LL, Lauder JM. Prenatal exposure to the pesticide dieldrin or the GABA(A) receptor antagonist bicuculline differentially alters the expression of GABA(A) receptor subunit mRNAs in fetal rat brainstem. Dev Neurosci. 1998;20:83–92. doi: 10.1159/000017302. [DOI] [PubMed] [Google Scholar]
- Liu J, Morrow AL, Devaud LL, Grayson DR, Lauder JM. Regulation of GABA(A) receptor subunit mRNA expression by the pesticide dieldrin in embryonic brainstem cultures: a quantitative, competitive reverse transcription-polymerase chain reaction study. J Neurosci Res. 1997;49:553–645. doi: 10.1002/(SICI)1097-4547(19970901)49:5<645::AID-JNR15>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Miller GW, Heilman CJ, Perez JT, Staley JK, Mash D, Rye DB, et al. Immunohistochemical analysis of dopamine transporter protein in Parkinson's disease. Ann Neurol. 1997;41:530–9. doi: 10.1002/ana.410410417. [DOI] [PubMed] [Google Scholar]
- Miller GW, Erickson JD, Perez JT, Penland SN, Mash DC, Rye DB, et al. Immunochemical analysis of vesicular monoamine transporter protein in Parkinson's disease. Exp Neurol. 1999;156:138–48. doi: 10.1006/exnr.1998.7008. [DOI] [PubMed] [Google Scholar]
- Miller GW, Gainetdinov RR, Levey AI, Caron MG. Dopamine transporters and neuronal injury. Trends Pharmacol Sci. 1999;20:424–9. doi: 10.1016/s0165-6147(99)01379-6. [DOI] [PubMed] [Google Scholar]
- Miller GW, Kirby ML, Levey AI, Bloomquist JR. Heptachlor alters expression and function of dopamine transporters. Neurotoxicology. 1999;20:631–8. [PubMed] [Google Scholar]
- Narahashi T. Neuronal ion channels as the target sites for insecticides. Pharmacol Toxicol. 1996;79:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Tepper JM. GABA(A) and GABA(B) antagonists differentially affect the firing pattern of substantia nigra dopaminergic neurons in vivo. Synapse. 1999;32:165–76. doi: 10.1002/(SICI)1098-2396(19990601)32:3<165::AID-SYN3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Purkerson-Parker S, McDaniel KL, Moser VC. Dopamine transporter binding in the rat striatum is increased by gestational, perinatal, and adolescent exposure to heptachlor. Toxicol Sci. 2001;64:216–23. doi: 10.1093/toxsci/64.2.216. [DOI] [PubMed] [Google Scholar]
- Priyadarshi A, Kuder SA, Schaub EA, Shrivastava S. A meta-analysis of Parkinson's disease and exposure to pesticides. Neurotoxicology. 2000;21:435–40. [PubMed] [Google Scholar]
- Richardson JR, Miller GW. Acute exposure to Aroclor 1016 and 1260 differentially affects the dopamine transporter and vesicular monoamine transporter 2 levels. Toxicol Lett. 2004;148:29–40. doi: 10.1016/j.toxlet.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Sacchetti P, Mitchell TR, Granneman JG, Bannon MJ. Nurr1 enhances transcription of the human dopamine transporter gene through a novel mechanism. J Neurochem. 2001;76:1565–72. doi: 10.1046/j.1471-4159.2001.00181.x. [DOI] [PubMed] [Google Scholar]
- Schmitt CJ. Organochlorine chemical residues in fish from the Mississippi River Basin, 1995. Arch Environ Contam Toxicol. 2002;43:81–97. doi: 10.1007/s00244-002-1127-1. [DOI] [PubMed] [Google Scholar]
- Shimizu I, Prasad C. Relationship between [3H] mazindol binding to dopamine uptake sites and [3H] dopamine uptake during aging. J Neurochem. 1991;56:575–9. doi: 10.1111/j.1471-4159.1991.tb08188.x. [DOI] [PubMed] [Google Scholar]
- Smits SM, Ponnio T, Conneely OM, Burbach JP, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003;18:1731–8. doi: 10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Miner LL, Sora I, Ujike H, Revay RS, Kostic V, et al. VMAT2 knockout mice: heterozygotes display reduced amphetamine-conditioned reward, enhanced amphetamine locomotion and enhanced MPTP toxicity. Proc Natl Acad Sci USA. 1997;94:9938–43. doi: 10.1073/pnas.94.18.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Reveron ME, Miller GW. Detection of behavioral impairments correlated to neurochemical deficits in mice treated with moderate doses of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Exp Neurol. 2002;178:80–90. doi: 10.1006/exnr.2002.8021. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Hypothesis: the role of dopaminergic transporters in selective vulnerability of cells in Parkinson's disease. Ann Neurol. 1998;43:555–60. doi: 10.1002/ana.410430503. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Li S, Takahashi N, Itokawa K, Lin Z, Hazama M, et al. The VMAT2 gene in mice and humans: amphetamine responses, locomotion, cardiac arrhythmias, aging, and vulnerability to dopaminergic toxins. FASEB J. 2000;14:2459–65. doi: 10.1096/fj.00-0205rev. [DOI] [PubMed] [Google Scholar]
- US Department of AgriculturePesticide data program annual summary calendar. Agricultural Marketing Service, USDA; Washington, DC: 1997. [Google Scholar]


