Abstract
In this short review, I introduce an integrated vision of human hair follicle behavior and describe opposing influences that control hair follicle homeostasis, from morphogenesis to hair cycling. The interdependence and complementary roles of these influences allow us to propose that the hair follicle is a true paradigm of a “Yin Yang” type, that is a cold/slow-hot/fast duality. Moreover, a new promising field is emerging, suggesting that glycans are key elements of hair follicle growth control.
Keywords: Glyco-biology, hair follicle biology, alopecia, hair cycling, glycan
Introduction
The hair follicle is a true paradigm of mesenchymal-epithelial interaction. From early morphogenesis to a fully formed organ, the hair follicle life-cycle is controlled by a dialog between mesenchymal and epithelial compartments 1. However, this dialog relies on a delicate balance between conflicting and/or opposing influences.
With respect to hair follicle morphogenesis, the reaction-diffusion model explains how slowly diffusing inducers and rapidly diffusing inhibitors orchestrate, through local activation and at distance inhibition, the hair follicle patterned formation. Indeed, the seminal work of A. Turing 2 has been recently confirmed through a formal identification of morphogen activator-inhibitor couples, such as Wnt/DKK1 3 ( Figure 1) and EDAR/BMP 4.
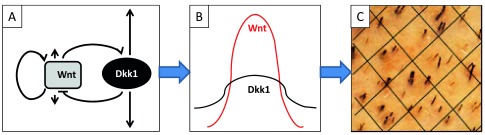
Figure 1. From reaction-diffusion to hair follicle patterning.
( A) Wnt morphogen stimulates its own synthesis as well as that of Dkk1, its inhibitor. Wnt diffuses slowly while Dkk1 diffuses rapidly. ( B) As a result, in a periodic way, Wnt concentration is higher than that of DKK1, and a hair placode can develop. ( C) The reaction-diffusion process thus explains the patterned distribution of hair follicles at the surface of the scalp.
Considering its dual mesenchymal and epithelial origin, the hair follicle can be considered a composite organ, with a concentric structure. Dermal and epithelial compartments interact with each other and are characterized by specific differentiation programs. Opposing signaling pathways concur to control the unique behavior of human hair follicle and maintain its unique intrinsic homeostasis. As the activity of diffusible factors, such as growth factors and morphogens, can be modulated by glycans, their possible role in hair growth control must be taken into account.
Hair follicle behavior
The hair follicle is the only organ in mammals that sequentially and repeatedly transits from a phase of active fiber production (anagen) to a resting phase (telogen), through rapid phases of tissue regression (catagen) and regeneration (neogen). A recently published comprehensive guide describes most of the morphological and immunohistological markers that characterize the different stages of the human hair follicle cycle and the intense tissue remodeling events which take place 5. Of note, hair follicle regeneration relies on the cyclical activation of stem cells 6. In the human hair follicle, these stem cells are harbored within two distinct reservoirs 7, 8, one of them bathing in a hypoxic environment 9. Instead of a cyclical behavior with an intrinsic automaton, the human hair follicle exhibits a stochastic behavior, the probability of duration of each phase fitting with a lognormal equation 10. A new concept ( Figure 2) postulates the existence of a bi-stable equilibrium 11 which controls human hair follicle dynamics, including an active steady state (the anagen stage) and a resting steady state (the telogen stage), the transition between these two steady states involving either a degradation phase (the catagen phase) or a neo-morphogenesis phase (the neogen phase). It is now believed that mesenchymal and epithelial oscillators control the stochastic autonomous switching between these two steady states 12, 13. The transition phases are both controlled by a complex and dynamic network of interacting activators and inhibitors, diffusible morphogens, and growth factors of opposite influences 14. Of note, however, extrapolating from results only obtained in rodents must be approached with caution, since major differences exist between human and mouse hair follicles in terms of phase duration, synchronicity, tissue remodeling, stem cell reservoirs, and so on.
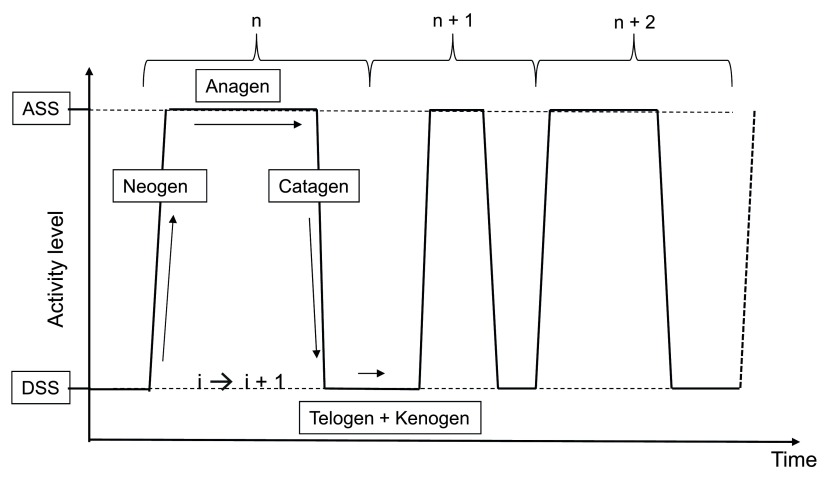
Figure 2. New representation of hair follicle behavior.
An active steady state (ASS) of fiber production (anagen) and a dormant steady state (DSS) (telogen/kenogen) are interspaced by short-lasting phases of regression (catagen) and neomorphogenesis (neogen).
During the active steady state, hair fiber production results from a finely, timely, and spatially tuned choreography of gene expression, which is highly sensitive to stimulatory and inhibitory signals. A number of signaling pathways 15, cytokines 16, 17, neuropeptides 18, hormones 19– 22, prostaglandins 23, and growth factors 24 are known to modulate the duration of the active steady state of the hair follicle ( Figure 3). For example, while insulin-like growth factor (IGF)-1 is required for anagen maintenance 25, 26, fibroblast growth factor (FGF)-5 appears to be a crucial regulator of hair length in humans 27, as a strong inducer of the catagen phase. Moreover, the human hair follicle is endowed with an autonomous androgen metabolism 28, a strict dependence on arginine 29, polyamines 30, and glucose 31 for growth, and a specific immunological response 32. The hair follicle is also endowed with a full prostaglandin metabolism and a complex network of prostaglandin (PG) receptors 33, 34. Recent data suggest that a delicate equilibrium between PGE2/PGF2a on the one hand and PGD2 on the other hand controls the duration of the active steady state. PGE2/PGF2a promotes hair growth maintenance, while PGD2 inhibits it and triggers anagen to catagen transition 35. Finally, re-evaluating the mechanisms by which agents such as cyclosporine A 36 or JAK-STAT inhibitors 37 promote human hair growth might help to identify new key genes and pathways involved in the control of hair growth.
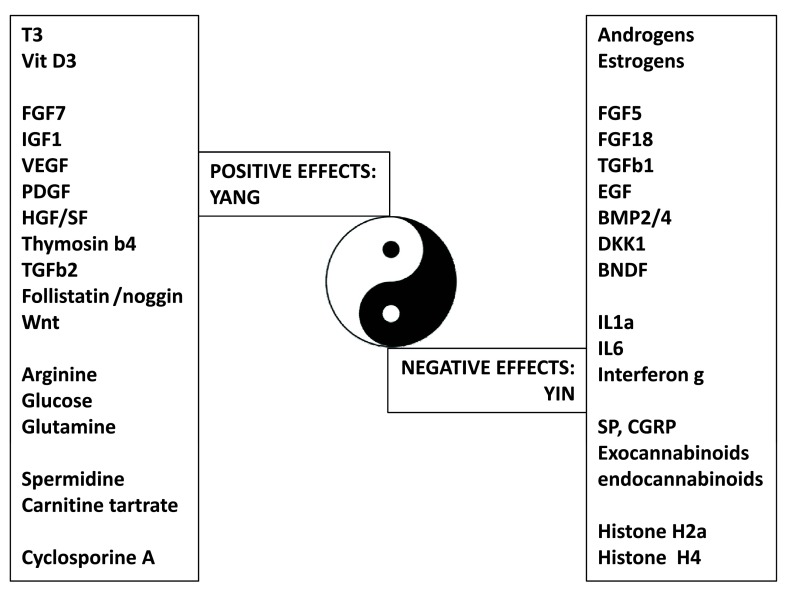
Figure 3. The Yin Yang of the human hair follicle.
Summary of diffusible factors having positive (Yang) or negative (Yin) effects on hair growth and cycling.
Besides the active steady state, new data demonstrate that the resting steady state is not as quiescent as suspected and can be divided into a refractory period and a permissive period. Indeed, during the telogen phase, the follicle is under the influence of factors that would repress the onset of the neogen phase and factors that would trigger it. Specifically, a strong expression of bone morphogenetic protein (BMP) and FGF-18 defines the refractory period, during which the neogen onset is prevented. The progressive increase in the production of BMP antagonist noggin, Wnt/Fzz/b-catenin pathway activators, and transforming growth factor (TGF)-β2 then reaches a critical threshold that shifts the telogen follicle to a competency status, receptive to FGF-7, secreted by the nearby dermal papilla, and, ultimately, triggers the onset of the neogen phase 38.
Glyco-biology of the human hair follicle
It is clear from the above that the complex and rhythmic behavior of the human hair follicle is under the control of multiple, intricate pathways with opposing influences. In this respect, the interdependence and complementary roles of these influences allow us to propose that the hair follicle is a true paradigm of a “Yin Yang” type duality and harmony. However, in our opinion, the fine tuning of these influences cannot solely rely on the timely and spatially controlled gene expression, but also on glycans, “the third revolution in evolution” 39. Glycans are endowed with such a huge molecular diversity that they can be considered the third language of life, after DNA and proteins.
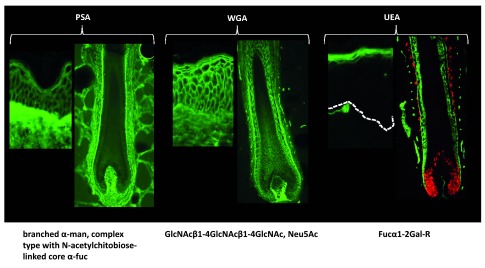
Linear or branched oligosaccharides can be attached to a protein backbone via O-(serine/threonine) or N-(asparagine) linkages. They form the large class of N-Complex type glycans. Glycosaminoglycans are linear copolymers of 6-O-sulfated disaccharide units which define them as chondroitin, dermatan, keratin, or heparin sulfates. Proteoglycans have one or more glycosaminoglycan side chains attached to a core protein. Glycosaminoglycans, proteoglycans, and glycan moieties of glycoproteins have long been known to play important roles in the maintenance of protein conformation and solubility, protection against proteolytic degradation, mediation of biological activity, intracellular sorting and externalization, and embryonic development and differentiation 40– 45. The distribution of proteoglycans in the human hair follicle was originally described in the early 1990s, namely for chondroitin sulfate, dermatan sulfate, and heparin sulfate proteoglycans 46, for syndecan 1, perlecan and decorin 47, and for versican 48. Thanks to the availability of new immunological tools, the distribution of proteoglycans in the human hair follicle has been further refined 49 ( Figure 4), highlighting a complex, dynamic, and regionalized network of proteoglycans. With respect to cell surface complex type N-glycans, the use of specific fluorescently labeled lectins (saccharide-binding proteins) revealed a differential N-glycan composition among the different hair follicle compartments 50– 52 ( Figure 5).
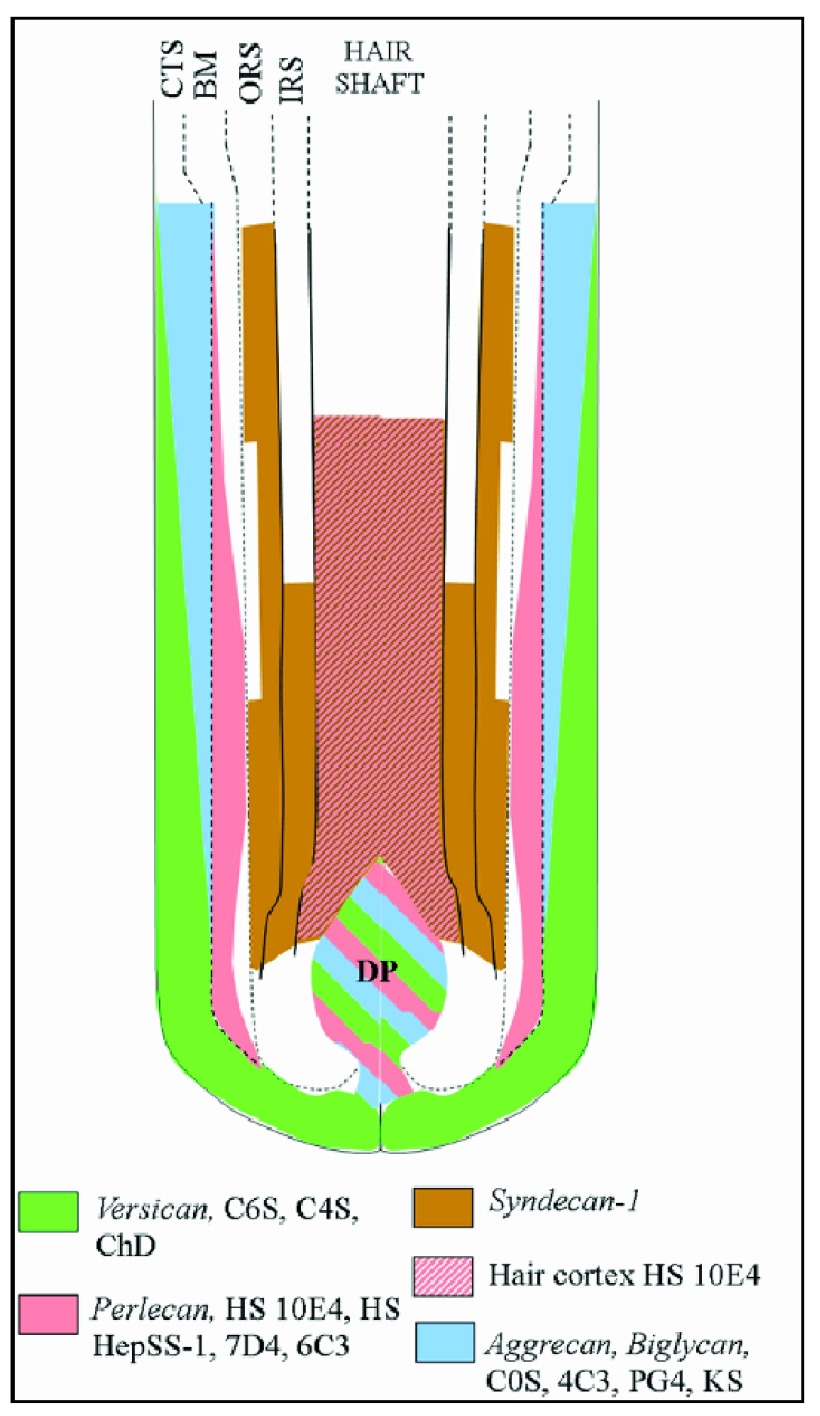
Figure 4. Diagram of proteoglycan expression in the human hair follicle.
Diagram shows the distribution of versican, perlecan, syndecan 1, aggrecan, biglycan, and heparan sulfate proteoglycans in the different hair follicle compartments. BM, basement membrane; CTS, connective tissue sheath; IRS, inner root sheath; ORS, outer root sheath.
Figure 5. Diagram of proteoglycan expression in the human hair follicle.
Distribution of N-glycans identified by their reactivity with fluorescently labelled Pisum sativum agglutinin (PSA), wheat germ agglutinin (WGA) and Ulex europeus agglutinin (UEA) in both skin and hair follicles. PSA mainly decorates the dermal compartments of skin and hair follicles, while WGA decorates both dermal and epithelial compartments. UEA only decorates the epidermis stratum granulosum and the hair follicle IRS.
What could be the role of these glycans? It has been known for quite a long time that growth factor activation could be regulated by proteoglycans 53, 54 and that heparan sulfate proteoglycans were involved in fine-tuning mammalian physiology 55 and in cell signaling during development 56. With respect to key regulators of hair follicle growth and cycling, syndecans modulate Wnt signaling cascades 57, the glycosaminoglycan chains of proteoglycans shape Hedgehog gradients and signal transduction 58, and O-linked glycosylation controls Notch1 interaction with its cognate Delta-like 4 receptor 59. Decorin, a small leucine-rich proteoglycan, directly modulates TGF-β, epidermal growth factor (EGF), IGF-1 and hepatocyte growth factor (HGF) signaling, all known actors of hair follicle cycling 60, and appears to act as an anagen inducer 61. Altogether, these recent results designate glycans as long time ignored key players in hair growth control. But, on top of that, enzymes can further modulate the biological activity of these glycans. For example, fucosyl transferase is absolutely required for Notch activity, and disruption of fucosyl transferase expression in murine hair follicle lineages results in aberrant telogen morphology, a decrease of bulge stem cell markers, a delay in anagen re-entry, and dysregulation of proliferation and apoptosis during the hair cycle transition 62. With respect to proteoglycans, heparanase (an endoglycosidase that cleaves heparin sulfate) was found expressed in the outer root sheath of murine hair follicles and identified as an important regulator of hair growth through its ability to release heparin-bound growth factors 63. In the human hair follicle, however, heparanase was found located in the inner root sheath. Its inhibition provoked an immediate transition from anagen to catagen 64. In this case, the HPSG/heparanase network appears to be a key controller of internal hair follicle homeostasis.
Finally, extracellular sulfatases appear to be critical regulators of heparin sulfate activities. Sulf1 and Sulf2, by removing glucosamine-6S groups from specific regions of heparan sulfate chain, modulate (a) Wnt interaction with its cognate receptor Frizzled, (b) BMP signaling by releasing BMP antagonist Noggin, and (c) FGF-2 ability to form the functional FGF-2-HS-FGFR ternary complex 65, 66. Of note, TGF-β1, by inducing Sulf1 expression 67, might indirectly modulate Wnt, BMP, and FGF-2 activities, which could explain its inhibitory effect on hair growth. From a clinical point of view, alterations of glycosaminoglycan degradation provoke mucopolysaccharidoses and abnormalities in hair morphology 68, which can be reversed by appropriate enzyme replacement therapy 69.
Conclusion
The hair follicle is clearly endowed with a unique behavior. Its bi-stability and the intense remodeling processes that it provokes rely on the permanent dialog between opposing and complementary influences, impacting all follicle compartments. From this interdependent duality, one can easily understand that an optimal way to describe the complex equilibrium which controls hair follicle homeostasis is the concept of “Yin Yang”. Until recently, the understanding of hair growth mainly relied on deciphering the patterns of gene expression within the different hair follicle compartments throughout the hair cycle 70, 71. From now on, the fine-tuning of the activities of growth factors and morphogens by the modulating effects of glycans will also have to be taken into consideration.
From a prospective point of view, it is likely that a better understanding of hair diseases, and more specifically the role of inflammation and immune response in the development of alopecia areata 72 and androgenetic alopecia 73, will likely provide further insights into the role of the so-called immune privilege 74 in hair growth control. Moreover, with the advent of mature metabolomics technologies 75 coupled with in vitro human hair growth technology 76, one can predict that this integrative approach will permit us to identify these key metabolic pathways sustaining normal hair growth.
Acknowledgements
I thank Ms E. Debecker (L’Oréal R&I) for her expert assistance in lectin labeling experiments.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Rodney Sinclair, Epworth Dermatology, Victoria, Australia
Gill Westgate, Centre for Skin Sciences, University of Bradford, Bradford, BD7 1DP, UK
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Sennett R, Rendl M: Mesenchymal-epithelial interactions during hair follicle morphogenesis and cycling. Semin Cell Dev Biol. 2012;23(8):917–927. 10.1016/j.semcdb.2012.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 2. Turing A: The chemical basis of morphogenesis. Philos Trans R Soc Lond B Biol Sci. 1952;237:37–72. 10.1098/rstb.1952.0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schlake T, Sick S: Canonical WNT signalling controls hair follicle spacing. Cell Adh Migr. 2007;1(3):149–151. 10.4161/cam.1.3.5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mou C, Jackson B, Schneider P, et al. : Generation of the primary hair follicle pattern. Proc Natl Acad Sci U S A. 2006;103(24):9075–9080. 10.1073/pnas.0600825103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Oh JW, Kloepper J, Langan EA, et al. : A guide to Studying Human Hair Follicle Cycling In Vivo. J Invest Dermatol. 2015;136(1):34–44. 10.1038/jid.2015.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alonso L, Fuchs E: The hair cycle. J Cell Sci. 2006;119(Pt 3):391–393. [DOI] [PubMed] [Google Scholar]
- 7. Commo S, Gaillard O, Bernard BA: The human hair follicle contains two distinct K19 positive compartments in the outer root sheath: a unifying hypothesis for stem cell reservoir? Differentiation. 2000;66(4–5):157–164. 10.1046/j.1432-0436.2000.660401.x [DOI] [PubMed] [Google Scholar]
- 8. Purba TS, Haslam IS, Poblet E, et al. : Human epithelial hair follicle stem cells and their progeny: current state of knowledge, the widening gap in translational research and future challenges. Bioessays. 2014;36(5):513–525. 10.1002/bies.201300166 [DOI] [PubMed] [Google Scholar]
- 9. Rathman-Josserand M, Genty G, Lecardonnel J, et al. : Human hair follicle stem/progenitor cells express hypoxia markers. J Invest Dermatol. 2013;133(8):2094–2097. 10.1038/jid.2013.113 [DOI] [PubMed] [Google Scholar]
- 10. Halloy J, Bernard BA, Loussouarn G, et al. : Modeling the dynamics of human hair cycles by a follicular automaton. Proc Natl Acad Sci U S A. 2000;97(15):8328–8333. 10.1073/pnas.97.15.8328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bernard BA: The human hair follicle, a bistable organ? Exp Dermatol. 2012;6(8):401–403. 10.1111/j.1600-0625.2012.01457.x [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. Al-Nuaimi Y, Goodfellow M, Paus R, et al. : A prototypic mathematical model of the human hair cycle. J Theor Biol. 2012;310:143–159. 10.1016/j.jtbi.2012.05.027 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 13. Tasseff R, Bheda-Malge A, DiColandrea T, et al. : Mouse hair cycle expression dynamics modeled as coupled mesenchymal and epithelial oscillators. PLoS Comput Biol. 2014;11(8):e1003914. 10.1371/journal.pcbi.1003914 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Murray PJ, Maini PK, Plikus MV, et al. : Modelling hair follicle growth dynamics as an excitable medium. PLoS Comput Biol. 2012;8(12):e1002804. 10.1371/journal.pcbi.1002804 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 15. Lee J, Tumbar T: Hairy tale of signaling in hair follicle development and cycling. Semin Cell Dev Biol. 2012;23(8):906–916. 10.1016/j.semcdb.2012.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahé YF, Buan B, Billoni N, et al. : Pro-inflammatory cytokine cascade in human plucked hair. Skin Pharmacol. 1996;9(6):366–375. 10.1159/000211447 [DOI] [PubMed] [Google Scholar]
- 17. Kwack MH, Ahn JS, Kim MK, et al. : Dihydrotestosterone-inducible IL-6 inhibits elongation of human hair shafts by suppressing matrix cell proliferation and promotes regression of hair follicles in mice. J Invest Dermatol. 2012;132(1):43–49. 10.1038/jid.2011.274 [DOI] [PubMed] [Google Scholar]
- 18. Samuelov L, Kinori M, Bertolini M, et al. : Neural controls of human hair growth: calcitonin gene-related peptide (CGRP) induces catagen. J Dermatol Sci. 2012;67(2):153–155. 10.1016/j.jdermsci.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 19. Billoni N, Buan B, Gautier B, et al. : Thyroid hormone receptor beta1 is expressed in the human hair follicle. Br J Dermatol. 2000;142(4):645–652. 10.1046/j.1365-2133.2000.03408.x [DOI] [PubMed] [Google Scholar]
- 20. Meier N, Langan D, Hilbig H, et al. : Thymic peptides differentially modulate human hair follicle growth. J Invest Dermatol. 2012;132(5):1516–1519. 10.1038/jid.2012.2 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 21. Inui S, Itami S: Molecular basis of androgenetic alopecia: From androgen to paracrine mediators through dermal papilla. J Dermatol Sci. 2011;61(1):1–6. 10.1016/j.jdermsci.2010.10.015 [DOI] [PubMed] [Google Scholar]
- 22. Hu HM, Zhang SB, Lei XH, et al. : Estrogen leads to reversible hair cycle retardation through inducing premature catagen and maintaining telogen. PLoS One. 2012;7(7):e40124. 10.1371/journal.pone.0040124 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 23. Khidhir KG, Woodward DF, Farjo NP, et al. : The prostamide-related glaucoma therapy, bimatoprost, offers a novel approach for treating scalp alopecias. FASEB J. 2013;27(2):557–567. 10.1096/fj.12-218156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imamura T: Physiological functions and underlying mechanisms of fibroblast growth factor (FGF) family members: recent findings and implications for their pharmacological application. Biol Pharm Bull. 2014;37(7):1081–1089. 10.1248/bpb.b14-00265 [DOI] [PubMed] [Google Scholar]
- 25. Philpott MP, Sanders DA, Kealey T: Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol. 1994;102(6):857–861. [DOI] [PubMed] [Google Scholar]
- 26. Ahn SY, Pi LQ, Hwang ST, et al. : Effect of IGF-I on Hair Growth Is Related to the Anti-Apoptotic Effect of IGF-I and Up-Regulation of PDGF-A and PDGF-B. Ann Dermatol. 2012;24(1):26–31. 10.5021/ad.2012.24.1.26 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Higgins CA, Petukhova L, Harel S, et al. : FGF5 is a crucial regulator of hair length in humans. Proc Natl Acad Sci U S A. 2014;111(29):10648–10653. 10.1073/pnas.1402862111 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Gerst C, Dalko M, Pichaud P, et al. : Type-1 steroid 5 alpha-reductase is functionally active in the hair follicle as evidenced by new selective inhibitors of either type-1 or type-2 human steroid 5 alpha-reductase. Exp Dermatol. 2002;11(1):52–58. 10.1034/j.1600-0625.2002.110106.x [DOI] [PubMed] [Google Scholar]
- 29. Michelet JF, Bernard BA, Juchaux F, et al. : Importance of L-Arginine for human hair growth. 28th IFSCC Meeting Proceedings. 2014;1123–1128. [Google Scholar]
- 30. Ramot Y, Marzani B, Pinto D, et al. : N 1-methylspermidine, a stable spermidine analog, prolongs anagen and regulates epithelial stem cell functions in human hair follicles. Arch Dermatol Res. 2015;307(9):841–847. 10.1007/s00403-015-1592-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 31. Williams R, Philpott MP, Kealey T: Metabolism of freshly isolated human hair follicles capable of hair elongation: a glutaminolytic, aerobic glycolytic tissue. J Invest Dermatol. 1993;100(6):834–840. [DOI] [PubMed] [Google Scholar]
- 32. Paus R, Nickoloff BJ, Ito T: A 'hairy' privilege. Trends Immunol. 2005;26(1):32–40. 10.1016/j.it.2004.09.014 [DOI] [PubMed] [Google Scholar]
- 33. Colombe L, Vindrios A, Michelet JF, et al. : Prostaglandin metabolism in human hair follicle. Exp Dermatol. 2007;16(9):762–769. 10.1111/j.1600-0625.2007.00586.x [DOI] [PubMed] [Google Scholar]
- 34. Colombe L, Michelet JF, Bernard BA: Prostanoid receptors in anagen human hair follicles. Exp Dermatol. 2008;17(1):63–72. 10.1111/j.1600-0625.2007.00639.x [DOI] [PubMed] [Google Scholar]
- 35. Garza LA, Liu Y, Yang Z, et al. : Prostaglandin D 2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Sci Transl Med. 2012;4(126):126ra34. 10.1126/scitranslmed.3003122 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 36. Hawkshaw NJ, Haslam IS, Ansell DM, et al. : Re-Evaluating Cyclosporine A as a Hair Growth-Promoting Agent in Human Scalp Hair Follicles. J Invest Dermatol. 2015;135(8):2129–2132. 10.1038/jid.2015.121 [DOI] [PubMed] [Google Scholar]
- 37. Harel S, Higgins CA, Cerise JE, et al. : Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci Adv. 2015;1(9):e1500973. 10.1126/sciadv.1500973 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Geyfman M, Plikus MV, Treffeisen E, et al. : Resting no more: re-defining telogen, the maintenance stage of the hair growth cycle. Biol Rev Camb Philos Soc. 2015;90(4):1179–1196. 10.1111/brv.12151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lauc G, Krištić J, Zoldoš V: Glycans - the third revolution in evolution. Front Genet. 2014;5:145. 10.3389/fgene.2014.00145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boucaut JC, Bernard B, Aubery M, et al. : Concanavalin A binding to amphibian embryo and effect on morphogenesis. J Embryol Exp Morphol. 1979;51:63–72. [PubMed] [Google Scholar]
- 41. Bernard BA, Yamada KM, Olden K: Carbohydrates selectively protect a specific domain of fibronectin against proteases. J Biol Chem. 1982;257(14):8549–8554. [PubMed] [Google Scholar]
- 42. Codogno P, Bernard B, Font J, et al. : Changes in protein glycosylation during chick embryo development. Biochim Biophys Acta. 1983;763(3):265–275. 10.1016/0167-4889(83)90134-9 [DOI] [PubMed] [Google Scholar]
- 43. Bernard BA, Newton SA, Olden K: Effect of size and location of the oligosaccharide chain on protease degradation of bovine pancreatic ribonuclease. J Biol Chem. 1983;258(20):12198–12202. [PubMed] [Google Scholar]
- 44. Olden K, Bernard BA, Humphries M, et al. : Function of glycoprotein glycans. TIBS. 1985;10(2):78–82. 10.1016/0968-0004(85)90238-5 [DOI] [Google Scholar]
- 45. Wang H, Zhou T, Peng J, et al. : Distinct roles of N-glycosylation at different sites of corin in cell membrane targeting and ectodomain shedding. J Biol Chem. 2015;290(3):1654–1663. 10.1074/jbc.M114.606442 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Westgate GE, Messenger AG, Watson LP, et al. : Distribution of proteoglycans during the hair growth cycle in human skin. J Invest Dermatol. 1991;96(2):191–195. [DOI] [PubMed] [Google Scholar]
- 47. Couchman JR: Hair follicle proteoglycans. J Invest Dermatol. 1993;101(1 Suppl):60S-64. 10.1111/1523-1747.ep12362642 [DOI] [PubMed] [Google Scholar]
- 48. du Cros DL, LeBaron RG, Couchman JR: Association of versican with dermal matrices and its potential role in hair follicle development and cycling. J Invest Dermatol. 1995;105(3):426–431. 10.1111/1523-1747.ep12321131 [DOI] [PubMed] [Google Scholar]
- 49. Malgouries S, Thibaut S, Bernard BA: Proteoglycan expression patterns in human hair follicle. Br J Dermatol. 2008;158(2):234–242. 10.1111/j.1365-2133.2007.08339.x [DOI] [PubMed] [Google Scholar]
- 50. Ohno J, Fukuyama K, Epstein WL: Glycoconjugate expression of cells of human anagen hair follicles during keratinization. Anat Rec. 1990;228(1):1–6. 10.1002/ar.1092280102 [DOI] [PubMed] [Google Scholar]
- 51. Tezuka M, Ito M, Ito K, et al. : Differential analysis of the human anagen hair apparatus using lectin binding histochemistry. Arch Dermatol Res. 1991;283(3):180–185. 10.1007/BF00372059 [DOI] [PubMed] [Google Scholar]
- 52. Heng MC, Levine S, Fine H, et al. : Expression of the L-fucose moiety on infrainfundibular follicular keratinocytes of terminal follicles, its decreased expression on vellus and indeterminate follicles of androgenetic alopecia, and re-expression in drug-induced hair regrowth. J Invest Dermatol. 1992;98(1):73–78. [DOI] [PubMed] [Google Scholar]
- 53. Schlessinger J, Lax I, Lemmon M: Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell. 1995;83(3):357–360. 10.1016/0092-8674(95)90112-4 [DOI] [PubMed] [Google Scholar]
- 54. Kresse H, Schönherr E: Proteoglycans of the extracellular matrix and growth control. J Cell Physiol. 2001;189(3):266–274. 10.1002/jcp.10030 [DOI] [PubMed] [Google Scholar]
- 55. Bishop JR, Schuksz M, Esko JD: Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 2007;446(7139):1030–1037. 10.1038/nature05817 [DOI] [PubMed] [Google Scholar]
- 56. Lin X: Functions of heparan sulfate proteoglycans in cell signaling during development. Development. 2004;131(24):6009–6021. 10.1242/dev.01522 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 57. Pataki CA, Couchman JR, Brábek J: Wnt Signaling Cascades and the Roles of Syndecan Proteoglycans. J Histochem Cytochem. 2015;63(7):465–480. 10.1369/0022155415586961 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 58. Whalen DM, Malinauskas T, Gilbert RJ, et al. : Structural insights into proteoglycan-shaped Hedgehog signaling. Proc Natl Acad Sci U S A. 2013;110(41):16420–16425. 10.1073/pnas.1310097110 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 59. Luca VC, Jude KM, Pierce NW, et al. : Structural biology. Structural basis for Notch1 engagement of Delta-like 4. Science. 2015;347(6224):847–853. 10.1126/science.1261093 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 60. Inui S, Itami S: A newly discovered linkage between proteoglycans and hair biology: decorin acts as an anagen inducer. Exp Dermatol. 2014;23(8):547–548. 10.1111/exd.12471 [DOI] [PubMed] [Google Scholar]
- 61. Jing J, Wu XJ, Li YL, et al. : Expression of decorin throughout the murine hair follicle cycle: hair cycle dependence and anagen phase prolongation. Exp Dermatol. 2014;23(7):486–491. 10.1111/exd.12441 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 62. Lin HY, Kao CH, Lin KM, et al. : Notch signaling regulates late-stage epidermal differentiation and maintains postnatal hair cycle homeostasis. PLoS One. 2011;6(1):e15842. 10.1371/journal.pone.0015842 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 63. Zcharia E, Philp D, Edovitsky E, et al. : Heparanase regulates murine hair growth. Am J Pathol. 2005;166(4):999–1008. 10.1016/S0002-9440(10)62321-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 64. Malgouries S, Donovan M, Thibaut S, et al. : Heparanase 1: a key participant of inner root sheath differentiation program and hair follicle homeostasis. Exp Dermatol. 2008;17(12):1017–1023. 10.1111/j.1600-0625.2008.00739.x [DOI] [PubMed] [Google Scholar]
- 65. Lamanna WC, Kalus I, Padva M, et al. : The heparanome--the enigma of encoding and decoding heparan sulfate sulfation. J Biotechnol. 2007;129(2):290–307. 10.1016/j.jbiotec.2007.01.022 [DOI] [PubMed] [Google Scholar]
- 66. Seffouh A, Milz F, Przybylski C, et al. : HSulf sulfatases catalyze processive and oriented 6- O-desulfation of heparan sulfate that differentially regulates fibroblast growth factor activity. FASEB J. 2013;27(6):2431–2439. 10.1096/fj.12-226373 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 67. Yue X, Li X, Nguyen HT, et al. : Transforming growth factor-beta1 induces heparan sulfate 6- O-endosulfatase 1 expression in vitro and in vivo. J Biol Chem. 2008;283(29):20397–20407. 10.1074/jbc.M802850200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Malinowska M, Jakóbkiewicz-Banecka J, Kloska A, et al. : Abnormalities in the hair morphology of patients with some but not all types of mucopolysaccharidoses. Eur J Pediatr. 2008;167(2):203–209. 10.1007/s00431-007-0462-7 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 69. Kloska A, Bohdanowicz J, Konopa G, et al. : Changes in hair morphology of mucopolysaccharidosis I patients treated with recombinant human alpha-L-iduronidase (laronidase, Aldurazyme). Am J Med Genet A. 2005;139(3):199–203. 10.1002/ajmg.a.31021 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 70. Ohyama M, Kobayashi T, Sasaki T, et al. : Restoration of the intrinsic properties of human dermal papilla in vitro. J Cell Sci. 2012;125(Pt 17):4114–4125. 10.1242/jcs.105700 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 71. Sennett R, Wang Z, Rezza A, et al. : An Integrated Transcriptome Atlas of Embryonic Hair Follicle Progenitors, Their Niche, and the Developing Skin. Dev Cell. 2015;34(5):577–591. 10.1016/j.devcel.2015.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 72. Kang H, Wu WY, Lo BK, et al. : Hair follicles from alopecia areata patients exhibit alterations in immune privilege-associated gene expression in advance of hair loss. J Invest Dermatol. 2010;130(11):2677–2680. 10.1038/jid.2010.180 [DOI] [PubMed] [Google Scholar]
- 73. Mahé YF, Michelet JF, Billoni N, et al. : Androgenetic alopecia and microinflammation. Int J Dermatol. 2000;39(8):576–584. 10.1046/j.1365-4362.2000.00612.x [DOI] [PubMed] [Google Scholar]
- 74. Christoph T, Müller-Röver S, Audring H, et al. : The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. 2000;142(5):862–873. 10.1046/j.1365-2133.2000.03464.x [DOI] [PubMed] [Google Scholar]
- 75. Menni C, Kastenmüller G, Petersen AK, et al. : Metabolomic markers reveal novel pathways of ageing and early development in human populations. Int J Epidemiol. 2013;42(4):1111–1119. 10.1093/ije/dyt094 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 76. Langan EA, Philpott MP, Kloepper JE, et al. : Human hair follicle organ culture: theory, application and perspectives. Exp Dermatol. 2015;24(12):903–911. 10.1111/exd.12836 [DOI] [PubMed] [Google Scholar]