Abstract
BACKGROUND
The existence of stem/progenitor cells in the endometrium was postulated many years ago, but the first functional evidence was only published in 2004. The identification of rare epithelial and stromal populations of clonogenic cells in human endometrium has opened an active area of research on endometrial stem/progenitor cells in the subsequent 10 years.
METHODS
The published literature was searched using the PubMed database with the search terms ‘endometrial stem cells and menstrual blood stem cells' until December 2014.
RESULTS
Endometrial epithelial stem/progenitor cells have been identified as clonogenic cells in human and as label-retaining or CD44+ cells in mouse endometrium, but their characterization has been modest. In contrast, endometrial mesenchymal stem/stromal cells (MSCs) have been well characterized and show similar properties to bone marrow MSCs. Specific markers for their enrichment have been identified, CD146+PDGFRβ+ (platelet-derived growth factor receptor beta) and SUSD2+ (sushi domain containing-2), which detected their perivascular location and likely pericyte identity in endometrial basalis and functionalis vessels. Transcriptomics and secretomics of SUSD2+ cells confirm their perivascular phenotype. Stromal fibroblasts cultured from endometrial tissue or menstrual blood also have some MSC characteristics and demonstrate broad multilineage differentiation potential for mesodermal, endodermal and ectodermal lineages, indicating their plasticity. Side population (SP) cells are a mixed population, although predominantly vascular cells, which exhibit adult stem cell properties, including tissue reconstitution. There is some evidence that bone marrow cells contribute a small population of endometrial epithelial and stromal cells. The discovery of specific markers for endometrial stem/progenitor cells has enabled the examination of their role in endometrial proliferative disorders, including endometriosis, adenomyosis and Asherman's syndrome. Endometrial MSCs (eMSCs) and menstrual blood stromal fibroblasts are an attractive source of MSCs for regenerative medicine because of their relative ease of acquisition with minimal morbidity. Their homologous and non-homologous use as autologous and allogeneic cells for therapeutic purposes is currently being assessed in preclinical animal models of pelvic organ prolapse and phase I/II clinical trials for cardiac failure. eMSCs and stromal fibroblasts also exhibit non-stem cell-associated immunomodulatory and anti-inflammatory properties, further emphasizing their desirable properties for cell-based therapies.
CONCLUSIONS
Much has been learnt about endometrial stem/progenitor cells in the 10 years since their discovery, although several unresolved issues remain. These include rationalizing the terminology and diagnostic characteristics used for distinguishing perivascular stem/progenitor cells from stromal fibroblasts, which also have considerable differentiation potential. The hierarchical relationship between clonogenic epithelial progenitor cells, endometrial and decidual SP cells, CD146+PDGFR-β+ and SUSD2+ cells and menstrual blood stromal fibroblasts still needs to be resolved. Developing more genetic animal models for investigating the role of endometrial stem/progenitor cells in endometrial disorders is required, as well as elucidating which bone marrow cells contribute to endometrial tissue. Deep sequencing and epigenetic profiling of enriched populations of endometrial stem/progenitor cells and their differentiated progeny at the population and single-cell level will shed new light on the regulation and function of endometrial stem/progenitor cells.
Keywords: endometrium, endometrial stem cells, mesenchymal stem cells, regenerative medicine, epithelial progenitor cells, endometriosis, adenomyosis, menstrual blood, sushi domain containing-2, immunomodulation
Introduction
It is 10 years since the first evidence for the existence of adult stem cell populations in the endometrium was published. In this study, rare clonogenic cells or colony-forming units (CFUs) were identified in purified populations of human endometrial epithelial and stromal cells isolated from hysterectomy tissue (Chan et al., 2004). Concurrently, it was reported that some epithelial and stromal cells in human endometrium of HLA-antigen-mismatched bone marrow transplant recipients were of donor origin (Taylor, 2004). Subsequently, label-retaining cells (LRCs) were identified in mouse endometrium (Chan and Gargett, 2006). This early direct evidence of stem/progenitor cells in the endometrium was then summarized in the first comprehensive review on endometrial stem/progenitor cells published in Human Reproduction Update (Gargett, 2007). Later in 2007, a second publication on murine endometrial LRCs confirmed and extended the original findings (Cervelló et al., 2007). The 2007 Human Reproduction Update review also provided a blueprint on how to identify stem/progenitor populations in tissues and organs not previously characterized for adult stem cell activity, focusing on functional assays used in other organs. These included CFU activity, self-renewal, differentiation, proliferative potential, label retention and tissue reconstitution assays. It pointed out the importance of linking stem cell markers to functional stem cell activity. It also summarized the indirect evidence for stem/progenitor cells in the highly regenerative human endometrium gleaned from the literature.
In this comprehensive review, we summarize the progress that has been made on the identification and characterization of endometrial stem/progenitor cells in both human and mouse models since this last review. We will focus on the identity and in vivo location of the stem/progenitor cells as specific markers and approaches that have now been identified for their purification, particularly for the mesenchymal stem/stromal cell (MSC) population. Specific markers also allow ‘omics' characterization of endometrial stem/progenitor cell populations. The role of bone marrow-derived and endogenous stem/progenitor cells in endometrial proliferative disorders, including endometriosis, adenomyosis, thin dysfunctional endometrium and Asherman's syndrome, will also be covered. The review will also describe the use of the endometrial MSCs (eMSCs) as potential cell-based therapies for several women's health and other diseases. Finally, we will raise unresolved issues facing the field, particularly the similarities and differences between eMSCs and endometrial stromal fibroblasts and the identity of bone marrow-derived cells involved in endometrial function.
Methods
The published literature was searched using the PubMed database with the search terms ‘endometrial stem cells and menstrual blood stem cells' until December 2014. Only original articles in English were included. The review includes human, mouse and domestic animal studies.
Identity of stem/progenitor cells in human endometrium
The immense regenerative capacity of human endometrium and its bilayer structure, in which the upper functionalis is shed at menses and regenerates from the remaining basalis in the subsequent cycle (Padykula et al., 1984; Padykula, 1991; Spencer et al., 2005; Jabbour et al., 2006), has been the motivation for investigators to identify and characterize endometrial stem/progenitor cell populations. The endometrium comprises luminal and glandular epithelial cells and a substantial vascularized stroma; hence, a number of laboratories have focused on identifying epithelial and mesenchymal stem/progenitor cells (Gargett et al., 2012).
Epithelial progenitor cells
Endometrial glands lined with a pseudostratified columnar epithelium extend from the luminal epithelium to the endometrial/myometrial junction. During menses, the basal component of the glands remains in the basalis layer and epithelial cells re-epithelialize the exposed surface and then proliferate to regenerate the new functionalis under the influence of rising estrogen levels (Gargett et al., 2012). It was hypothesized that remaining glands of the basalis contained the epithelial progenitor cell population (reviewed in Gargett, 2007). To date, epithelial progenitor cells have been identified as CFUs in cell suspensions derived from hysterectomy tissue, which includes the basalis (Chan et al., 2004; Schwab et al., 2005; Gargett et al., 2009) and they are present in the side population (SP) (see SP cells). The large, single-cell-derived epithelial CFU, comprising 0.08% of epithelial cells serially cloned at least three times, a measure of self-renewal in vitro, underwent 34 population doublings, indicating high proliferative potential, and differentiated into large gland-like structures in three-dimensional (3D) culture (Gargett et al., 2009). The 3D cultures included a stromal feeder layer, which likely provided epithelial progenitor cell niche factors that promote differentiation and morphogenesis. To date, there are no publications identifying specific markers that isolate epithelial progenitor cells in human endometrium with these stem cell properties.
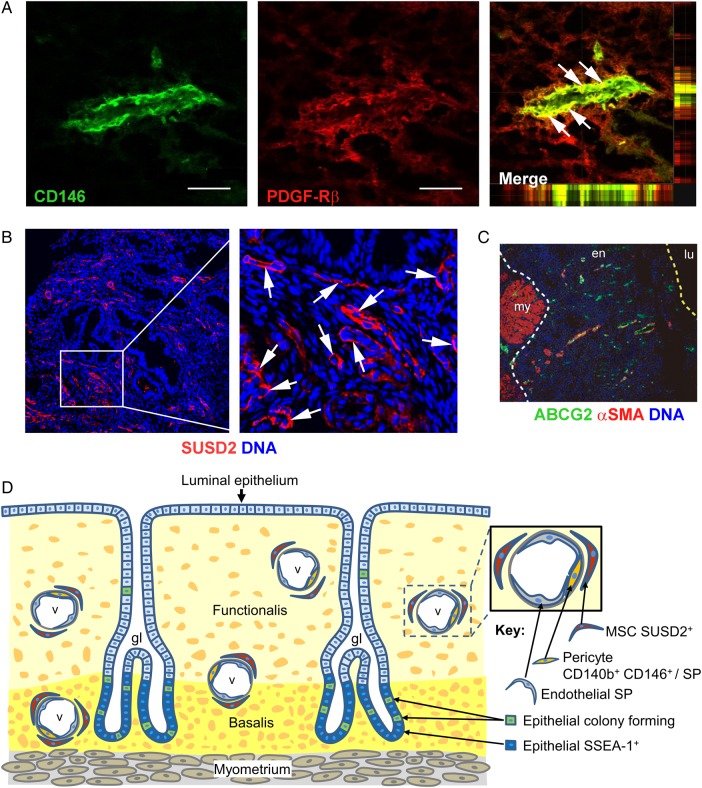
There has been progress in identifying a marker of endometrial basalis epithelium, the postulated location of endometrial epithelial stem/progenitor cells (Fig. 1D). Stage-specific embryonic antigen 1 (SSEA-1, or CD15), a Lewis X epitope of a glycoprotein expressed on differentiating human embryonic stem (hES) cells and human neutrophils (Wright and Andrews, 2009), was expressed most strongly in the basalis glands of endometrial tissue from hysterectomy samples of cycling women (Valentijn et al., 2013). SSEA-1 was also strongly expressed in the glandular epithelium of postmenopausal endometrium, which has gene profiles similar to basalis epithelium of cycling women (Gaide Chevronnay et al., 2009; Nguyen et al., 2012). The stem cell activity of human endometrial SSEA-1+ cells has not yet been examined. However, cultured SSEA-1+ endometrial epithelial cells had greater telomerase activity and longer telomeres and were more quiescent with lower proliferation rates than SSEA-1− epithelial cells, features of progenitor cell populations. They also formed spheroids in 3D culture and differentiated into spheres with polarized epithelium. SSEA-1+ cells expressed lower levels of estrogen receptor-α (ESR1) and progesterone receptor (PR) when compared with the SSEA-1− cells (Valentijn et al., 2013), suggesting a less differentiated cell phenotype and reliance on growth factors released from ESR1-expressing niche cells to mediate estrogen-induced proliferative signals. In contrast, ESR1 is detected in basalis glands of normal endometrium throughout the menstrual cycle, whereas functionalis expression is restricted to the proliferative stage (Leyendecker et al., 2002). This suggests that human endometrial epithelial progenitor cells will be a subset of the SSEA-1+ population that may reside in the functionalis abutting the basalis. The surface marker LGR5 (leucine-rich repeat-containing G-protein-coupled receptor 5), which identifies rapidly cycling murine intestinal epithelial stem cells (Barker et al., 2007), was located on rare epithelial cells in human endometrium in the lower functionalis near the basalis (Gil-Sanchis et al., 2013). LGR5 was dynamically expressed in the endometrium and was negatively regulated by estrogen and progesterone in mice (Krusche et al., 2007; Sun et al., 2009). Whether LGR5 and SSEA-1 will be markers for endometrial epithelial progenitor cells awaits assessment using stem cell assays.
Figure 1.
Localization of human endometrial mesenchymal stem cells. (A–C) Immunofluorescence images of human endometrium showing perivascular identity of human eMSCs. (A) Co-localization (white arrows) of CD146 and platelet-derived growth factor receptor beta (PDGF-Rβ) in pericytes of venules and possibly capillaries in the functionalis stroma. The x/z and y/z planes are shown on the far right and underneath the merged images demonstrating co-localization of the two surface markers. (B) Perivascular SUSD2 expression (white arrows). (C) ATP-binding cassette, subfamily G member 2 (ABCG2) and αSMA co-staining showing perivascular and endothelial identity of SP cells. The white dotted lines indicate the junction between the endometrium (en) and myometrium (my) and yellow dotted line indicates the luminal surface (lu) of the uterine epithelium. (D) Schematic showing location of stem/progenitor cells identified in the human endometrium. Epithelial progenitor cells are postulated to be a subpopulation of cells located in the base of the glands in the basalis, identified by SSEA-1. Sushi domain containing-2+ (SUSD2+) eMSCs are perivascular cells. eMSC co-expressing CD146 and PDGFRβ/CD140b are most likely pericytes, as they are located adjacent to endothelial cells in vessels (v) in both the basalis and the functionalis. SP cells are a heterogeneous population comprising CD31+ endothelial cells and CD140b+CD146+ pericytes. Scale bar in (A) = 50 µm. (A) Reprinted with permissions from Schwab and Gargett (2007). (C) Reprinted with permissions from Masuda et al. (2010). (D) Adapted from Gurung et al. (2015).
Multipotent MSCs
MSCs were originally identified in bone marrow cultures as clonogenic fibroblasts (CFU-F). It became apparent that plastic adherent bone marrow cultures comprised MSCs as well as fibroblasts, and to reflect this heterogeneity, they were recently renamed multipotent mesenchymal stromal cells and the MSC acronym was retained (Table I). MSCs have been identified in adipose tissue (Zuk et al., 2002), endometrium (Schwab and Gargett, 2007; Gargett et al., 2009) and many other organs over the last decade (Crisan et al., 2008). The defining features of bone marrow MSCs (bmMSCs) are plastic adherence, clonogenicity, multilineage differentiation into bone and marrow lineages (osteocytes, chondrocytes, adipocytes) in vitro and a surface phenotype (CD29+, CD44+, CD73+, CD90+, CD105+, CD146+, CD31−, CD34− and CD45−) distinguishing them from haematopoietic stem cells (HSCs) also resident in marrow (Dominici et al., 2006). Clonogenic endometrial cells from human (Gargett et al., 2009; Cervelló et al., 2010) and porcine (Miernik and Karasinski, 2012) species and the human CD146+PDGFR-β+ and SUSD2+ [sushi domain containing-2 (previously W5C5+)] or endometrial stromal subpopulations (Schwab and Gargett, 2007; Masuda et al., 2012; Ulrich et al., 2014c) exhibit the same in vitro properties as bmMSCs. Cultured fibroblasts from the endometrium (stromal cells), bone marrow and many organs also exhibit these classic bmMSC properties in vitro, prompting MSC biologists to question the utility of these defining features (Hematti, 2012; Bianco et al., 2013; Phinney and Sensebé, 2013). Rather, the ability for a single cell to generate heterotropic bone or bone marrow organs (ossicles) in vivo is now considered the definition of bmMSC (Sacchetti et al., 2007; Bianco et al., 2013). The analogous definition for eMSCs would be the generation of a vascularized stroma with the capacity to differentiate into decidualized stroma when transplanted into an animal at the single-cell level. This has not been achieved; however, clonally derived, purified SUSD2+ eMSCs produced endometrial stroma and incorporated into renal parenchymal blood vessels when xenografted under the kidney capsule of immunocompromised NSG mice (Masuda et al., 2012). Likewise, endometrial stromal SP cells with some bmMSC in vitro properties produced stroma in subrenal xenografts (Masuda et al., 2010; Cervelló et al., 2011). In contrast, human endometrial stromal cell (fibroblast) cultures have more limited differentiation capacity in vitro, usually into single bone, marrow or non-mesodermal lineages (Wolff et al., 2007; Dimitrov et al., 2008).
Table I.
Glossary of cell types.
| Cell type | Definition |
|---|---|
| Bone marrow mesenchymal stem/stromal cells (bmMSCs) | Multipotent, highly proliferative, self-renewing adult stromal stem cells found in the bone marrow that display immunomodulatory properties. Plastic adherent cultures are heterogeneous and contain perivascular cells and stromal fibroblasts |
| Endometrial MSCs (eMSCs) | Multipotent, highly proliferative, self-renewing adult stromal stem cells found in a perivascular location in the endometrium and distinct from endometrial stromal fibroblasts |
| Endometrial regenerative cell (ERC) | A collective term for MSC and stromal cells isolated from menstrual blood that are highly proliferative and multipotent (see Table IV for acronyms) |
| Human embryonic stem (hES) cells | Pluripotent stem cells derived from the inner cell mass of a blastocyst, able to differentiate into cells of all three germ layers |
| Haematopoietic stem cells (HSCs) | Multipotent, self-renewing non-plastic adherent stem cells that reside in the bone marrow and are responsible for producing all blood cell types |
| Induced pluripotent stem (iPS) cells | A pluripotent stem cell produced from an adult cell through reprogramming by introduction of pluripotency genes or transcription factors |
| Label retaining cells (LRCs) | A quiescent stem-like cell that retains a DNA label over a longer period of time than more mature cells |
| Multipotent mesenchymal stem/stromal cells (MSCs) | A stromal cell that exhibits characteristics of clonogenicity, multipotency and self-renewal and is responsible for tissue maintenance |
| Main population (MP) cells | A cell that does not efflux the Hoechst dye or a non-SP cell. Differentiated cells likely derived from an SP cell |
| Progenitor or transit-amplifying cells | A cell that has less potential than a stem cell, i.e. undergoes less differentiation and reduced proliferative capacity |
| Side population (SP) cells | A cell that is able to efflux the Hoechst dye, through expression of the ABCG2 transporter that pumps organic molecules out of the cell. A property of stem cells |
| Stem cell niche | The microenvironment in which stem cells are found, comprising niche cells and extracellular matrix, which directly or indirectly interact with the stem cells to control cell fate decisions regarding proliferation, self-renewal and differentiation |
| Stromal fibroblasts (fibroblasts) | Main component of stromal or connective tissue. A non-stem cell which lacks clonogenicity but can differentiate into mesodermal lineages and expresses common phenotypic cell surface markers. Gene profiling and RNA sequencing show that endometrial stromal fibroblasts are closely related but distinct from endometrial MSCs |
| SUSD2 (W5C5) | A cell surface marker that enriches for endometrial and bone marrow MSCs, also known as W5C5 and detected by the W5C5 antibody |
Markers of eMSCs
Specific markers or combinations of markers have been identified for eMSCs (Gargett and Masuda, 2010; Lv et al., 2014a) (Table II). The CD146+PDGFR-β+ subpopulation comprises 1.5% of endometrial stromal cells (Schwab and Gargett, 2007). Almost all stromal CFUs with in vitro bmMSC properties are found in the CD146+PDGFR-β+CD45− fraction (CD45 excludes leukocytes). This marker set identified an in vivo perivascular location for eMSCs in both the functionalis and basalis of human endometrium (Fig. 1A), indicating that the CD146+PDGFR-β+ subpopulation can be harvested from endometrial biopsy samples (Schüring et al., 2011; Spitzer et al., 2012) and will be shed in menstrual blood (Gargett and Masuda, 2010). Similarly, bone marrow and many other MSCs have a perivascular location in vivo (Shi and Gronthos, 2003; Sacchetti et al., 2007; Crisan et al., 2008). STRO-1, the most widely used single bmMSC marker, is also expressed by endothelial and perivascular cells in human endometrium, but failed to enrich for stromal CFUs (Schwab et al., 2008). Screening endometrial cell suspensions with a panel of perivascular markers identified a single marker, SUSD2, for isolating clonogenic eMSC from human endometrium (Masuda et al., 2012) (Fig. 1B). This marker, sometimes referred to as W5C5, recognizes the sushi domain containing-2 (SUSD2) antigen (Sivasubramaniyan et al., 2013). Purification with SUSD2 antibody-labelled magnetic beads is less damaging to the cells increasing the yield of eMSCs compared with flow cytometry sorting using the CD146+PDGFR-β+ markers, indicating the utility of magnetic bead sorting over flow cytometry sorting (Schwab and Gargett, 2007; Masuda et al., 2012). The relationship between SUSD2+ eMSC and existing markers of eMSCs was explored by flow cytometry (Masuda et al., 2012). Most SUSD2+ cells expressed PDGFR-β, whereas all SUSD2+CD146+ cells were positive for PDGFR-β. These SUSD2+CD146+ cells generated more CFUs than the CD146+PDGFR-β+ subpopulation, highly expressed SUSD2 (SUSD2hi) and were increased in proliferative endometrium, suggesting their role in growth of the endometrial functionalis stroma. The surface phenotype of SUSD2+ endometrial stromal cells indicates that they are predominantly CD90+ (93%) perivascular cells, which are CD90hi in the human endometrium (Schwab et al., 2008). SUSD2+ cells also express Stro-1 (60%) (Masuda et al., 2012).
Table II.
Surface marker phenotype of human endometrial marker-enriched mesenchymal stem cells and stromal cell populations.
| Cell type investigated | Marker expression |
Other markers investigated | References | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD29 | CD44 | CD73 | CD90 | CD105 | PDGFRβ | CD146 | CD31 | CD34 | CD45 | |||
| CD146+ PDGFRβ+ endometrial stromal cells | + | + | + | + | + | − | − | STRO-1− | Schwab and Gargett (2007) | |||
| SUSD2+ endometrial stromal cells | + | + | + | + | + | + | + | − | − | CD117+ STRO-1+ | Masuda et al. (2012) | |
| PMP SUSD2+ endometrial stromal cells | + | + | + | + | + | + | Ulrich et al. (2014c) | |||||
| Briefly cultured endometrial SP cells | − | − | CD9− CD13− | Kato et al. (2007) | ||||||||
| Endometrial SP cells | + | + | + | + | CD13+ CD49f+ EMA+ | Tsuji et al. (2008) | ||||||
| Endometrial stromal SP cells | + | + | − | − | − | CD9+ VM+ CD133− STRO-1− ESR1− PR− | Cervelló et al. (2010, 2011) | |||||
| Endometrial SP cells | + | + | + | + | + | CD10+ CD144+ CD326+ CD133− SUSD2+ | Masuda et al. (2010), Miyazaki et al. (2012) | |||||
| Endometrial stromal colonies | + | + | + | − | − | CD14− CD19− CD56/16− HLA-DR− | Dimitrov et al. (2008) | |||||
| Endometrial stromal colonies | + | + | + | + | + | + | + | − | − | − | Gargett et al. (2009) | |
| Endometrial stromal colonies | + | + | + | CD81+ | Li et al. (2010) | |||||||
| Endometrial stromal colonies | + | + | + | + | − | CD14− | Schüring et al. (2011) | |||||
| Endometrial stromal colonies | + | + | + | − | − | − | Ai et al. (2012) | |||||
| Endometrial large stromal colonies | + | + | + | − | − | VM+ CK− | Yang et al. (2014) | |||||
| Passaged endometrial stromal cells | + | + | + | + | − | − | − | CD133− | Ebrahimi-Barough et al. (2013) | |||
| Passaged endometrial stromal cells | + | + | + | − | − | Santamaria et al. (2011) | ||||||
| Passaged endometrial stromal cells | + | + | + | − | − | αSMA+(5%) | Wolff et al. (2011) | |||||
| Passaged endometrial stromal cells | + | + | − | − | CK− | Wang et al. (2012b) | ||||||
PDGFRβ+, platelet-derived growth factor receptor beta; αSMA, alpha smooth muscle actin; CK, cytokeratin; EMA, epithelial membrane antigen; ESR1, estrogen receptor alpha; PMP, postmenopausal; PR, progesterone receptor; VM, vimentin.
Other less well-known markers of human eMSCs include MSC antigen-1 (MSCA-1), which is suitable for prospective isolation of bmMSCs (Sobiesiak et al., 2010; Lv et al., 2014a). The MSCA-1 is the ectoenzyme, tissue non-specific alkaline phosphatase also expressed on hES and on endometrial CD146+ cells. It has less value for prospective isolation of eMSC as MSCA-1 is also expressed on the apical surface of endometrial glandular epithelial cells (Sobiesiak et al., 2010).
SUSD2+ cells were also identified in the atrophic and estrogen-treated postmenopausal endometrium (Ulrich et al., 2014c). Although stromal CFUs were found in the SUSD2+ subpopulation, the percentage was less than that in the premenopausal endometrium and the capacity for mesodermal in vitro differentiation was less in ageing eMSCs. Perivascular eMSCs do not express ESR1, unlike the stromal fibroblast population, showing further differences between the two populations (Ulrich et al., 2014c). Gene profiling of freshly isolated CD146+PDGFR-β+ eMSCs and CD146−PDGFR-β+ endometrial stromal cells showed 762 differentially expressed genes with 374 upregulated in eMSCs and 384 downregulated, and by implication the latter were upregulated in stromal fibroblasts (Spitzer et al., 2012). These results show that endometrial perivascular cells are a population distinct from stromal fibroblasts. The identification of specific markers of eMSCs enables their prospective isolation using magnetic bead or flow cytometry sorting for further characterization and for potential use in cell-based therapies.
SP cells
Adult stem cells have been identified in many organs by their ability to rapidly efflux Hoechst 33342 DNA binding dye (Table I). These cells are identified by a discrete SP using dual colour flow cytometry (Goodell et al., 1997; Challen and Little, 2006) and can be sorted for further characterization. Human endometrium contains up to 5% SP cells in freshly isolated (Tsuji et al., 2008; Cervelló et al., 2010; Masuda et al., 2010) and short-term cultured (Kato et al., 2007) human endometrial cells (Table II). The SP number varies considerably among subjects, although higher percentages were found in proliferative (Tsuji et al., 2008; Masuda et al., 2010) and menstrual (Kato et al., 2007) stages. Immunostaining with the SP marker, ABCG2, labelled endothelial cells lining blood vessels in both the functionalis and the basalis (Fig. 1C) (Tsuji et al., 2008; Masuda et al., 2010). Flow cytometric analysis indicated that the SP comprises a mixed cell population: CD31+ endothelial cells (51%), CD326+ (EpCAM) epithelial cells (27%) and CD10+ or PDGFR-β+ stromal cells (10–14%) (Table II) (Miyazaki et al., 2012). The main population (MP), which does not efflux Hoechst, has an epithelial and stromal composition similar to the SP, but significantly fewer CD31+ endothelial cells. Although the SP is enriched for the CD146+PDGFR-β+ perivascular eMSC population compared with the MP, SUSD2+ cells are equally distributed between the SP (11%) and the MP (14%) (Miyazaki et al., 2012). Epithelial and stromal SPs have typical in vitro characteristics of MSCs (Table III) and telomerase activity at a level intermediate between embryonic stem cells and mature cells (Cervelló et al., 2010). Similar to SUSD2+ eMSCs, the endometrial SP does not express ESR1 or PR (Cervelló et al., 2010; Masuda et al., 2010), but expresses ESR2 in keeping with their endothelial predominance. Freshly sorted human endometrial SP cells were quiescent (85% in the G0 phase of the cell cycle), a typical feature of adult stem cells, and showed little clonogenic growth in culture. Cultured SP cells were primarily in G1 and G1/M/S phases and showed enhanced clonogenicity (Tsuji et al., 2008).
Table III.
In vitro and in vivo differentiation of human endometrial marker-enriched mesenchymal stem cells and stromal cell populations.
| Cell type investigated | Adi | Ost | Chon | Myo | Neu | Functional differentiation studies | References |
|---|---|---|---|---|---|---|---|
| CD146+ PDGF-Rβ+ endometrial stromal cells | H, R | H, R | H, R | I, R | Schwab and Gargett (2007) | ||
| CD146+ PDGFRβ+ endometrial stromal cells | H | Spitzer et al. (2012) | |||||
| SUSD2+ endometrial stromal cells | H, R | H, R | H, R | I, R | In vitro: angiogenic—CD31+; in vivo: SUSD2+ cells produced endometrial stromal-like tissue under the kidney capsule of NSG mice | Masuda et al. (2012) | |
| SUSD2+ endometrial stromal cells | H, I | In vitro: PA+G scaffold—myogenic–Masson's trichrome, SM22α+ SM-MHC+; fibroblastic—Masson's trichrome, COL-1+ Tn-C+ | Su et al. (2014) | ||||
| PMP SUSD2+ endometrial stromal cells | H | H | H | I | Ulrich et al. (2014c) | ||
| Briefly cultured endometrial SP cells | In vitro: epithelial SP—CD9+ E-cadherin+ gland structures on Matrigel;Stromal SP—CD9+ VM+ clusters on Matrigel | Kato et al. (2007) | |||||
| SP endometrial cells | In vitro: decidualization—Prolactin+ IGFRB-1+, secrete prolactin | Tsuji et al. (2008) | |||||
| SP endometrial stromal and epithelial cells | H, R | I, R | In vivo: regenerated human endometrium when stromal SP cells or cell lines were transplanted into NOD-SCID mice | Cervelló et al. (2010, 2011) | |||
| SP endometrial cells | In vivo: ESP with/without EMP transplanted under kidney capsule of OVX NOG mice reconstituted VM+ CD13+ stroma and CK+ glands with human CD31+ αSMA+ vessels | Masuda et al. (2010), Miyazaki et al. (2012) | |||||
| Passaged endometrial stromal colonies | H | Dimitrov et al. (2008) | |||||
| Endometrial stromal and epithelial colonies | H, R | H, R | H, R | I, R | In vitro: large epithelial CFU—CK+ gland-like structures in 3D Matrigel | Gargett et al. (2009) | |
| Endometrial stromal colonies | H, R | H, R | H, R | I, R | In vitro: pancreatic-lineage cells—insulin-secreting, resistant to oxidative stress and IL-1β-induced apoptosis; in vivo: restored INS production in STZ-treated SCID mice | Li et al. (2010) | |
| Endometrial stromal colonies | H, R | H | Schüring et al. (2011) | ||||
| Endometrial stromal colonies | H, R | Ai et al. (2012) | |||||
| Passaged endometrial large stromal colonies | H | H | In vitro: hepatogenic—CK8+ Albumin+, demonstrated urea synthesis, ammonia removal and glycogen storage (PAS+) | Yang et al. (2014) | |||
| First passage endometrial stromal cells | H, I | Wolff et al. (2007) | |||||
| Passaged endometrial stromal cells | In vitro: pancreatic β-like cells—PDX1, PAX4, GLUT2, INS, produced INS in response to glucose; in vivo: restored INS production in STZ-treated SCID mice | Santamaria et al. (2011) | |||||
| Passaged endometrial stromal cells | I | In vitro: neurogenic—barium-sensitive K channels; in vivo: in a PD mouse model, stromal cells migrated to lesion site, differentiated and produced dopamine | Wolff et al. (2011) | ||||
| Passaged endometrial stromal cells | In vitro: CD41a+ CD42b+ megakaryocytes releasing CD62p+ functional platelets that bound fibrinogen after thrombin stimulation | Wang et al. (2012b) | |||||
| Passaged endometrial stromal cells | I | In vitro: oligodendrocyte progenitors—A2B5, Nestin, O4, Olig2, PDGFRα, SOX10 | Ebrahimi-Barough et al. (2013) |
Adi, adipocyte; Ost, osteocyte; Chon, chondrocyte; Myo, smooth muscle myocyte; Neu, neural; AFP, alpha-fetoprotein; CFU, colony-forming unit; CK, cytokeratin; COL-1, collagen 1; EMP, endometrial main population cells; ESP, endometrial side population cells; GLUT1, glucose transporter 1; H, histology stain; I, immunohistochemistry; INS, insulin; NSG/NOG, NOD/SCID/IL-2Rγchainnull; OVX, ovariectomized; PA+G, polyamide and gelatin-composite meshes; PAS, periodic acid-Schiff; PD, 1-methyl 4-phenyl 1,2,3,6-tetrahydro pyridine-induced animal model of Parkinson's disease; PAX4, paired box 4; PDX1, pancreatic and duodenal homeobox 1; PMP, postmenopausal; R, mRNA expression; RedFluc, red-emitting firefly luciferase; SP, side population; STZ, streptozotocin; TdTom, Tandem Tomato; TH, tyrosine hydroxylase; Tn-C, tenascin-C; VM, vimentin.
The SP reconstitutes endometrial tissue in vivo when transplanted underneath the kidney capsule of immunocompromised mice (Table III) (Masuda et al., 2010; Cervelló et al., 2011). Lentiviral labelling with the red fluorescent protein Tandem Tomato (TdTom) has enabled tracing of xenografted SP and MP cells in vivo (Miyazaki et al., 2012). Non-labelled, unfractionated endometrial cell suspensions were mixed with the SP or MP to provide niche cells for the transplanted cells. The image analysis revealed that the SP contributed significantly more TdTom+ vimentin+ stroma, TdTom+ cytokeratin+ epithelium and TdTom+ CD31+ endothelial cells in the transplants when compared with the xenografted MP (Miyazaki et al., 2012), suggesting that the SP was enriched for the progenitor populations of these three lineages. However, it does not indicate whether a single stem/progenitor cell in the SP can differentiate into these lineages. These features and the marker profiles of the SP suggest that vascular and perivascular cells are enriched in the SP and are the main contributors to their tissue reconstitution capacity.
Differentiation of eMSCs
Several studies have demonstrated the differentiation potential of various eMSC populations and of cultured endometrial stromal fibroblasts (Table III). Most have focused on mesodermal differentiation, particularly bone marrow lineages to satisfy the minimal criteria for MSC status (Dominici et al., 2006). Both clonogenic (Gargett et al., 2009), CD146+PDGFR-β+ (Schwab and Gargett, 2007) and SUSD2+ (Masuda et al., 2012) stromal cells exhibited trilineage differentiation into adipocyte, osteoblast and chondrocyte lineages in vitro. They also differentiated into smooth muscle cells and fibroblasts (Masuda et al., 2012; Su et al., 2014). Endometrial SP cells differentiated into adipocyte and osteoblast lineages (Cervelló et al., 2010, 2011), whereas some studies have shown single lineage differentiation for clonogenic or CD146+PDGFR-β+ cells (Dimitrov et al., 2008; Spitzer et al., 2012).
Endometrial stromal fibroblast cell differentiation
Cultured endometrial stromal fibroblasts undergo mesodermal lineage differentiation, usually to single lineages and to a lesser extent than purified eMSC or clonogenic populations (Table III). Cell types produced include chondrocytes (Wolff et al., 2007) and adipocytes (Ai et al., 2012; Ebrahimi-Barough et al., 2013). Bovine endometrial stromal fibroblasts showed osteogenic lineage differentiation (Donofrio et al., 2008). Cultured human endometrial stromal fibroblasts also differentiated into a haematopoietic lineage generating CD41a+ and CD42b+ polyploid megakaryocytes that released platelets in vitro, indicating the plasticity of cultured endometrial stromal fibroblasts (Wang et al., 2012b). It is clear that endometrial stromal fibroblasts have broad mesodermal multipotency.
Endometrial stromal fibroblasts also show differentiation potential across embryonic lineage boundaries (Table III). They differentiated into endodermal pancreatic lineages in vitro and in vivo (Li et al., 2010; Santamaria et al., 2011). Passaged endometrial stromal fibroblasts (Santamaria et al., 2011) or 3D spheroid cultures (Li et al., 2010) produced differentiated cells that secreted insulin and expressed β-cell pancreatic genes. Glucagon-producing cells were also generated (Li et al., 2010). Xenografting these differentiated cells into an immunocompromised mouse model of diabetes reduced hyperglycaemia, and human insulin was detected in the mouse serum (Li et al., 2010). These studies indicate that in vitro differentiated endometrial stromal fibroblasts have relevant functional properties in vivo. Clonogenic eMSCs also differentiated into a hepatocyte-like lineage in a four-step in vitro hepatogenic differentiation protocol (Yang et al., 2014). The differentiated cells generated urea and metabolized ammonia.
Cultured endometrial stromal fibroblasts have been differentiated into an ectodermal lineage; dopaminergic neuron-like cells expressing neural stem cell markers and tyrosine hydroxylase, the rate-limiting enzyme for dopamine synthesis (Wolff et al., 2011). Only the differentiated cells produced potassium currents. In a mouse model of Parkinson's disease, cultured human endometrial stromal fibroblasts transplanted directly into the striatum migrated to the lesioned substantia nigra and either differentiated into dopamine-secreting neurons or promoted endogenous neuronal function, partially restoring dopamine levels. Stromal fibroblasts also differentiated into oligodendrocyte progenitor cells, the myelinating cells of the central nervous system, and expressed oligodendrocyte progenitor cell proteins (Ebrahimi-Barough et al., 2013). Whether the perivascular eMSC population demonstrates ectodermal lineage differentiation is unknown.
Differentiation of eMSCs into decidual cells
Decidualization is the physiological differentiation pathway of eMSCs around the spiral arterioles and the subepithelial stroma. Progesterone mediates decidualization, a process critically important in establishing the feto-maternal interface of pregnancy. The relative roles of cultured perivascular SUSD2+ eMSCs and SUSD2− endometrial stromal fibroblasts were examined by RNA sequencing, following decidualization induction in vitro (Murakami et al., 2014). Despite the SUSD2− cells upregulating SUSD2 expression in culture, the two cell types retained distinct gene expression profiles, with the SUSD2+ cells enriched in novel and known endometrial perivascular signature genes. In the undifferentiated state, SUSD2+-derived decidual cells produced lower levels of inflammatory mediators and certain chemokines when compared with the SUSD2− stromal fibroblasts. An even greater divergence in the secretomes of the two cell types was observed upon decidual differentiation. Decidualized SUSD2+ cells were the major source of several cytokines, including an 18-fold greater production of leukaemia inhibitory factor and a 43-fold increase in chemokine (C-C motif) ligand 7 when compared with 4–5-fold increases in the differentiated SUSD2− cells (Murakami et al., 2014). This differential molecular response in decidualizing SUSD2+ and SUSD2− cells further emphasizes the differences between perivascular eMSCs and stromal fibroblasts.
Profiling eMSCs and stromal fibroblast populations
Specific markers that purify endometrial stem/progenitor cell populations enable the identification of gene expression signatures. Gene profiling of three freshly isolated cell populations sorted from CD146 and PDGFR-β co-labelled cells confirmed that the CD146+ PDGFR-β+ population was clonogenic, perivascularly located, differentiated into adipocytes and expressed pericyte markers and genes associated with angiogenesis and vasculogenesis (Spitzer et al., 2012). The CD146+PDGFR-β+ eMSC selectively expressed high levels of the SUSD2 gene (Wells et al., 2013), confirming flow cytometry data (Masuda et al., 2012). They also expressed genes involved in steroid hormone and hypoxia responses, inflammation, immunomodulation and cell communication, emphasizing their role in tissue homeostasis and immune tolerance required for embryo implantation and placental development. Increased expression of Notch, Hedgehog, insulin-like growth factor (IGF), transforming growth factor β (TGFβ) and G-protein-coupled receptor signalling pathway genes suggest stem cell function in self-renewal and differentiation. The gene profile of CD146+PDGFR-β+ eMSCs clustered with CD146−PDGFR-β+ endometrial fibroblasts, but was distinct from CD146+PDGFR-β− endothelial cells, indicating that their main differentiated lineage is the endometrial stromal fibroblast (Spitzer et al., 2012). RNAseq analysis comparing cultured SUSD2+ and SUSD2− cells confirmed the pericyte phenotype of eMSCs and a role for Notch in regulating SUSD2 expression (Murakami et al., 2014). The secretome of in vitro decidualized SUSD2+ cells revealed greater production of chemokines and inflammatory modulators, compared with decidualized SUSD2− stromal fibroblasts. This suggests that the SUSD2+ perivascular cells establish a specific chemokine microenvironment around the endometrial vasculature, likely crucial in establishing early pregnancy through recruitment of leukocyte populations to the materno-foetal interface for mediating maternal immune tolerance and promoting trophoblast invasion of the spiral arterioles (Murakami et al., 2014).
Gene profiling of cultured epithelial and stromal SP cells generated a gene signature showing considerable overlap of differentially regulated genes, suggesting a common gene signature and possibly a single stem/progenitor cell phenotype (Cervelló et al., 2010). However, the lack of purity of the epithelial and stromal SP cultures may have contributed to this similar gene signature. Several common endometrial SP genes were differentially expressed compared with bmMSCs, including interleukin-1B (IL-1B), growth differentiation factor 15, von Willebrand factor (VWF), matrix metalloproteinase 3, colony stimulating factor 2, intercellular adhesion molecule 1 (Wang et al., 2012a; Gaafar et al., 2014) exemplifying the uniqueness of MSCs derived from different sources.
Gene profiling of bmMSCs differentiated towards endometrial decidual cells by a cyclic AMP analogue showed that the culture process generates a distinct gene expression pattern (Aghajanova et al., 2010). Among the upregulated genes of cAMP-regulated, decidualized bmMSCs were the typical decidual markers IGFBP1 and prolactin, correlating with observed phenotypic changes. Several c-AMP-regulated genes with roles in endometrial function were also upregulated, including IGF1, inhibin βA, vascular endothelial growth factor (VEGF) A, pappalysin and parathyroid hormone-like hormone. Comparison of c-AMP-treated bmMSCs with cultured human endometrial stromal fibroblasts revealed 20 common genes, all of which are involved in the endometrial function. Identification of a gene signature for bmMSCs and eMSCs distinct from endometrial stromal fibroblasts is important in determining the role of endogenous or bone marrow-derived MSCs in endometrial regeneration and differentiation (Aghajanova et al., 2010; Spitzer et al., 2012).
Profiling studies confirmed the similarity between cultured endometrial stromal fibroblasts from hysterectomy tissue and menstrual blood and skin fibroblasts (Wang et al., 2012a). Similarly, there was a core gene signature common to bmMSCs and cultured endometrial stromal fibroblasts, although there were also distinct differences, particularly in inflammatory, immunomodulatory and angiogenesis genes (Wang et al., 2012a; Gaafar et al., 2014). Although these two studies compared bmMSCs with menstrual blood or endometrial stromal fibroblasts, only two overlapping gene expression differences were observed: ITGA10 (integrin-α10) and VCAM1 (vascular cell adhesion molecule 1—CD106), both downregulated in menstrual blood and endometrial stromal fibroblasts compared with bmMSCs. Lack of correlation between these similar studies may be due to the different platforms used for gene profiling, as one used focused arrays (Gaafar et al., 2014) and the other whole-genome microarrays (Wang et al., 2012a).
Stem/progenitor cells in endometrial decidua
Human endometrial stroma terminally differentiates into the decidua during the mid-late secretory stage of the menstrual cycle. Decidualization commences in the perivascular cells of the spiral arterioles and spreads to the subepithelial stroma. The decidua of pregnancy may therefore harbour a subpopulation of undifferentiated MSCs related to eMSCs (Kyurkchiev et al., 2010). Indeed, clonogenic SP cells were identified in the first trimester decidua (Tsuji et al., 2008; Guo et al., 2010; Wang et al., 2013) comprising 0.03–1.4% of cells, a lower abundance than their endometrial counterparts. SP cells sorted from short-term cultured human decidual cells expressed neither CD31 (endothelial marker) nor CD146 (MSC marker) (Wang et al., 2013), but differentiated into endothelial cells in vitro and induced neovascularization following intramascular injection in a mouse ischaemic hind limb injury model, rescuing the limb (Wang et al., 2013). This CD31−CD146− SP proliferated more rapidly than MP cells when cultured in 0.2% serum-containing media supplemented with either epidermal growth factor (EGF) or fibroblast growth factor 2 (FGF2), similar to clonogenic eMSCs (Chan et al., 2004). They also proliferated in IGF-1- and VEGF-containing media (Wang et al., 2013). In contrast, short-term cultured decidual SP cells required IL-6, stem cell factor and thrombopoietin for growth in serum-free (SF) medium (Guo et al., 2010). The clonogenic cells appeared more heterogeneous than endometrial clones and differentiated into prolactin-staining cells following treatment with cAMP. Confocal analysis of the decidua parietalis using specific markers for purifying bmMSC or eMSC demonstrated a vascular niche for decidua MSCs (Castrechini et al., 2012). STRO-1 co-localized with vWF (endothelial marker), in agreement with recent reports that STRO-1 was an endothelial marker in adipose tissue arterioles and capillaries (Lin et al., 2011). In contrast, CD146 was perivascular with partial overlap with vWF.
Cultured stromal fibroblasts from first trimester and term decidua demonstrated characteristic MSC properties: clonogenicity (2–18%), mesodermal lineage differentiation and surface marker phenotype (Dimitrov et al., 2010; Castrechini et al., 2012). Cultured placental decidua basalis stromal fibroblasts differentiated into pancreatic cells in vitro when transfected with a microRNA involved in pancreas development (Shaer et al., 2014), indicating that decidua basalis stromal fibroblasts are equally as capable of differentiating across germ lineage boundaries as endometrial stromal fibroblasts.
Decidual SP cells and stromal fibroblasts respond to sex steroid hormones. Both estrogen and progesterone dose dependently stimulated greater proliferation and migration of the decidual CD31−CD146− cells in vitro than MP cells (Wang et al., 2013). High concentrations of progesterone (7–30 μM) upregulated HLA-G on a small proportion (5.3%) of decidual stromal fibroblasts, suggesting that they may function in immunomodulation of the implanting embryo (Ivanova-Todorova et al., 2009). It is unknown whether this HLA-G-expressing subpopulation are decidual perivascular MSCs. Nor is it known if the perivascular decidual stromal cells or HLA-G-expressing cells are SUSD2+. More studies are required using specific markers and profiling technologies to determine the relationship among eMSCs, decidual MSCs, endometrial stromal fibroblasts and decidual stromal fibroblasts.
Endometrial stem/progenitor cells in menstrual blood
The markers used to enrich for eMSCs (co-expression of CD146 and PDGFRβ or SUSD2) revealed their perivascular location in both the basalis and the functionalis of human endometrium, indicating that eMSCs would be shed in menstrual blood (Gargett and Masuda, 2010). Several laboratories have identified and characterized an MSC-like population in menstrual blood. Generally, menstrual blood was collected in menstrual cups (Koks et al., 1999) and cultured directly onto plastic culture dishes in a manner similar to bmMSCs; similarly, they comprise a mix of eMSCs and stromal fibroblasts. One group used c-KIT (CD117) to further purify the cultured cells (Patel et al., 2008). CD117 is induced during culture as freshly isolated endometrial stromal fibroblasts are CD117−, but cultured SUSD2+ cells are CD117+ (Masuda et al., 2012).
Cells cultured from menstrual blood have been given various names (Table IV): endometrial regenerative cells (ERCs) (Meng et al., 2007), endometrial menstrual MSCs (Patel et al., 2008), menstrual blood MSCs (mbMSCs) (Gargett and Masuda, 2010), endometrial decidual tissue MSCs (EDT-MSCs) (Rossignoli et al., 2013), menstrual blood-derived MSCs (MMSCs) (Hida et al., 2008) and menstrual blood progenitor cells (MBPCs) (Wu et al., 2014b). In this review, cultured menstrual blood cells will be referred to as ERCs, a term that encompasses both the stromal fibroblast and MSC composition of these isolates. Epithelial cells were not generally observed in cultured menstrual blood, because they were not present, were overlooked or had been overgrown by the stromal fibroblast populations (Musina et al., 2008), suggesting that epithelial progenitors are more likely located in the basalis and not normally shed during menstruation (Gargett and Masuda, 2010).
Table IV.
Surface marker phenotype of human menstrual blood stem/progenitor cell and stromal cell populations.
| Menstrual blood stem cell name | Acronym | Cell type investigated | Marker expression |
Other markers investigated | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD29 | CD44 | CD73 | CD90 | CD105 | OCT4 | CD34 | CD45 | |||||
| Endometrial decidual tissue MSC | EDT-MSC | Adherent MB cells | + | + | CD146+ SSEA-4+ (1–19.4%) | Rossignoli et al. (2013) | ||||||
| Endometrial regenerative cells | ERC | Created ERC cell lines from MB | + | + | + | + | + | + | − | − | CD59+ hTERT+ MMPs+ CD133− NANOG− SSEA-4− STRO-1− | Meng et al. (2007) |
| Menstrual blood MSC | mbMSC | Adherent MB cells | + | + | + | − | − | Musina et al. (2008) | ||||
| Menstrual blood-derived mesenchymal cells | MMC | Adherent MB cells | + | + | + | CD55+ CD59+ CD166+ | Hida et al. (2008) | |||||
| Passaged adherent MB cells | − | + | + | + | − | − | CD13+ CD54+ CD146+ CD166+ CD14− CD16− CD19− HLA-DR− | Sugawara et al. (2014) | ||||
| Menstrual blood progenitor cells | MBPC | Adherent MB cells | + | CD117− SSEA-4− | Wu et al. (2014b) | |||||||
| Menstrual blood-derived (stem) cells | Passaged (P6–P9) adherent MB cells | + | CXCR4+ NANOG+ SSEA+ | Borlongan et al. (2010) | ||||||||
| Menstrual stromal stem cells | MenSC | P5 adherent MB cells selected for C-KIT | + | + | + | + | + | − | − | CD9+ CD49f+ CD166+ C-KIT+ CXCR4+ MHC -I+ SSEA-4+ CD38− CD133− MHC-II− LIN− | Patel et al. (2008) | |
| From S-Evans Biosciences (China) | + | + | + | − | CD14− CD19− CD35− HLA-DR− | Hu et al. (2014) | ||||||
| Passaged adherent MB cells | + | + | + | + | + | − | − | CD9+ CD10+ CD146+ CD38− CD133− C-KIT− STRO-1− | Darzi et al. (2012), Kazemnejad et al. (2012), Khanjani et al. (2014), Khanmohammadi et al. (2014) | |||
MB, menstrual blood; MHC, major histocompatibility complex; MMP, matrix metalloproteinase.
Several recent reviews summarize the properties of ERCs and their potential for cellular therapies (Ulrich et al., 2013; Khoury et al., 2014). ERCs cultured from menstrual blood are clonogenic (Musina et al., 2008), highly proliferative, with a short population doubling time of 20 h (Meng et al., 2007; Patel et al., 2008; Rossignoli et al., 2013; Wu et al., 2014b) and underwent 25–30 population doublings (Hida et al., 2008; Rossignoli et al., 2013). Clonogenic ERCs retained a stable karyotype for 68 passages (Meng et al., 2007; Wu et al., 2014b).
Markers of menstrual blood ERCs
Cultured ERCs express telomerase reverse transcriptase (hTERT) and demonstrate telomerase activity, as well as typical MSC phenotypic markers (Table IV), but like eMSCs, they do not express the specific bmMSC marker STRO-1 (Cui et al., 2007; Meng et al., 2007; Hida et al., 2008; Patel et al., 2008; Khanmohammadi et al., 2014). Pluripotency marker expression has been demonstrated in ERC, including OCT-4 (Patel et al., 2008; Borlongan et al., 2010; Darzi et al., 2012; Wu et al., 2014b), SSEA-4 (Patel et al., 2008; Rossignoli et al., 2013) and NANOG (Borlongan et al., 2010). However, these markers were not found consistently on ERC and OCT4 was cytoplasmic by immunofluorescence (Borlongan et al., 2010), indicating that the ERCs are not truly pluripotent.
Differentiation of menstrual blood ERCs
ERCs have broad in vitro differentiation capacity (Table V) and, under appropriate conditions, differentiated into typical mesodermal lineages: adipogenic, chondrogenic, osteogenic (Meng et al., 2007; Patel et al., 2008; Darzi et al., 2012; Rossignoli et al., 2013) and skeletal and cardiac muscle (Cui et al., 2007; Hida et al., 2008). Compared with bmMSCs, mesodermal differentiation was less robust for ERCs; however, greater differentiation was achieved for the adipogenic lineage using retinoic acid (Khanmohammadi et al., 2014), the osteogenic lineage with human platelet releasate (Darzi et al., 2012) and cardiomyogenic lineage in SF medium containing thyroxine and insulin (Ikegami et al., 2010). Co-culture of ERCs with mouse foetal cardiomyocytes generated spontaneously beating cells, showing striations and expressing cardiac-specific Troponin 1 (Hida et al., 2008; Khanmohammadi et al., 2014). ERCs co-cultured with human nucleus pulposus cells in 2% oxygen differentiated into nucleus pulposus-like cells (Hu et al., 2014). Cultured ERCs, bmMSCs and amnion-derived MSCs differentiated into decidua-like cells expressing prolactin and IGFBP1 when induced by a cAMP analogue, but not estrogen and/or progesterone, despite expressing ESR1 and PR (Sugawara et al., 2014). This suggests that decidualized cells originally cultured from menstrual blood de-differentiated during culture expansion and required a strong decidual stimulus to redifferentiate.
Table V.
In vitro and in vivo differentiation of human menstrual blood stem/progenitor cells and stromal cell populations.
| Cell type investigated | Adi | Ost | Cho | Myo | Neu | Functional differentiation studies | References |
|---|---|---|---|---|---|---|---|
| Adherent MB cells | In vivo: DMD mouse model—improvement via fusion with host myocytes | Cui et al. (2007) | |||||
| Created ERC cell lines from MB | I | H | I | I | In vitro: angiogenic—CD34+ CD62+; hepatocytic—Albumin+; pancreatic—Insulin+; respiratory epithelial—proSP-C+; cardiogenic—Troponin I+ | Meng et al. (2007) | |
| Adherent MB cells | In vitro: cardiogenic via co-culture—troponin-I+ AP+, beating MMCsIn vivo: improvement of cardiac function following MI after MMC Tx | Hida et al. (2008) | |||||
| Adherent MB cells | H | H | Musina et al. (2008) | ||||
| P5 adherent MB cells selected for C-KIT | H | H | H | I, R | In vitro: cardiogenic—Actin+ Troponin+ Connexin 43+ ANP+ Mef2C+ | Patel et al. (2008) | |
| Passaged adherent MB cells | In vivo: ERC inhibited glioma volume by inhibition of angiogenesis in Sprague-Dawley rat model | Han et al. (2009) | |||||
| Passaged adherent MB cells | In vivo: ERC Tx into four MS patients—no immune/adverse effects at 1 year | Zhong et al. (2009) | |||||
| Passaged (P6–P9) adherent MB cells | In vitro: OGD-exposed rat neuron co-culture—reduced cell deathIn vivo: Tx into rat stroke model—motor/neurological improvements | Borlongan et al. (2010) | |||||
| Adherent MB cells | In vitro: cardiogenic via co-culture—Troponin-I+, beating MMCs | Ikegami et al. (2010) | |||||
| Passaged adherent MB cells | H, R | H, R | I | I, R | In vitro: hepatocytes—PAS, Glycogen+ ALB+ CK-18+ TAT+; chondrocytes on 3D nanofibrous scaffold—collagen type II+ AB+ sGAG+ | Darzi et al. (2012), Kazemnejad et al. (2012), Khanjani et al. (2014), Azedi et al. (2014), Khanmohammadi et al. (2014) | |
| Adherent MB cells | H | H, R | H | In vitro: adipogenic, osteogenic and chondrogenic lineages | Rossignoli et al. (2013) | ||
| MenSCs from S-Evans Biosciences (Hangzhou, China) | In vitro: MenSC co-cultured with NP cells from intravertebral disc tissue expressed NP markers and ECM accumulation | Hu et al. (2014) | |||||
| Passaged adherent MB cells | In vitro: decidualization—Prolactin+ IGFBP1+ | Sugawara et al. (2014) | |||||
| Adherent MB cells | H | H | H | In vivo: IV MBPC ameliorated diabetic symptoms in a T1DM murine model through differentiation of endogenous progenitor cells | Wu et al. (2014b) |
ALB, albumin; ANP, atrial natriuretic peptide; AP, action potential; DMD, Duchenne muscular dystrophy; ECM, extracellular matrix; ERC, endometrial regenerative cells; H, histology stain; I, immunohistochemistry; IV, intravenous; Mef2C, myocyte enhancer factor 2C; MI, myocardial infarction; MMC/MenSC, menstrual blood stem cells; MS, multiple sclerosis; NP, nucleus pulposus; OGD, oxygen glucose deprivation; PAS, periodic acid-Schiff; proSP-C, ProSurfactant protein C; R, mRNA expression; sGAG, sulphated glycosaminoglycan; T1DM, type 1 diabetes mellitus; TAT1, TAT amino acid transporter 1; Tx, transplantation.
ERCs also differentiated into several neural lineages in vitro, similar to endometrial stromal fibroblasts (Patel et al., 2008; Azedi et al., 2014). In SF medium, a small proportion of cultured ERCs and bmMSCs generated neurosphere-like structures, which upon dissociation produced further neurospheres derived from single cells (Azedi et al., 2014). Retinoic acid and PDGF induced these secondary neurospheres to differentiate into glial cells, although to a lesser extent than bmMSCs. In another co-culture model, ERCs protected primary rat neurons from oxygen and glucose deprivation-induced loss of viability (Borlongan et al., 2010). These ERCs, delivered either intravenously (4 × 106 cells) or intracerebrally (4 × 105 cells), into a rat stroke model survived and increased the survival of host cells in the ischaemic penumbra after 14 days, improving behavioural and histological scores compared with vehicle controls.
Similar to endometrial stromal fibroblasts, ERCs differentiated into endodermal lineages in vitro and in vivo. ERCs differentiated into hepatocyte-like cells (Khanjani et al., 2014), expressing hepatocyte genes and proteins, secreted albumin and accumulated glycogen in greater quantities than similarly differentiated bmMSC. In a type 1 diabetes mellitus mouse model, intravenously administered ERCs (3 × 105 cells) migrated to the damaged pancreas and promoted the differentiation of endogenous endocrine progenitors into functional β-cells, reversing hyperglycaemia (Wu et al., 2014b). Dye-labelled ERCs were found in the pancreas, lungs and liver 3 days after transplantation and were still detectable in the pancreas after 14 days. Immunofluorescence co-localization studies showed that the ERC did not differentiate into pancreatic progenitors or insulin-producing β-cells, nor was human insulin detected in the mouse serum. Several pancreatic developmental genes and mature β-cell genes were sequentially upregulated in ERC-transplanted mice compared with vehicle controls. ERCs were more potent in reversing hyperglycaemia in this type 1 diabetes mouse model than bmMSCs or umbilical cord MSCs, indicating their potential for cell-based therapies.
Identity and regulation of stem/progenitor cells in mouse endometrium
In the absence of specific markers for identifying mouse endometrial stem/progenitor cells, label retention was initially used to characterize their phenotype and in vivo location.
LRCs
The quiescent or slow-cycling phenotype of many quiescent adult stem cells allows their identification by label retention assays in mice (Table I). The thymidine analogue bromodeoxyuridine (BrdU) is typically delivered as a pulse during development or remodelling and incorporates into the DNA of actively dividing cells. A chase period follows, when actively dividing cells dilute the label below detectable levels while quiescent and slow-cycling cells retain detectable label. The timing of the initial pulse and the length of the chase are critical variables in determining which cells incorporate and retain detectable label (Gargett et al., 2007). Several labelling and chase regimes have been used to identify LRC in the stroma and epithelium of mouse endometrium (Chan and Gargett, 2006; Cervelló et al., 2007; Chan et al., 2012; Patterson and Pru, 2013; Cao et al., 2014) (Table VI). A transgene-based label retention system was also used in the murine female reproductive tract, with labelling initiated by antibiotic-inducible expression of green fluorescent protein (GFP)-labelled histones (H2B-GFP) (Wang et al., 2012c; Patterson and Pru, 2013).
Table VI.
Summary of label retention papers published 2006–2014.
| Label | Pulse | Chase | LRC present after chase | Comments | References |
|---|---|---|---|---|---|
| BrdU | PND 3–5P PND 19–21 |
Up to 12 weeks Up to 10 weeks |
L, G, S L, S |
Epithelial LRCs are Esr1− Stromal LRCs are Esr1+/−, αSMA+, Sca-1−a |
Chan and Gargett (2006) |
| BrdU | PND 3–5 | 8–10 weeks | S | Some LRCs express Oct-4 and c-Kit | Cervelló et al. (2007) |
| BrdU | Adult, model of menstrual breakdown and repair | 4.5–8.5 days | L, G | Glandular epithelial LRCs proliferate following epithelial repair | Kaitu'u-Lino et al. (2010) |
| BrdU | PND 3–5 | 4 and 8 weeks | L, S | Epithelial LRCs proliferate after estrogen LRCs initiate epithelial proliferation in prepubertal endometrium 12% of stromal LRCs proliferate after estrogen |
Chan et al. (2012) |
| BrdU | PND 19–22 | Up to 11 weeks | L, S | Luminal epithelial LRCs at 5 weeks chase Stromal LRCs persist through pregnancy and proliferate postpartum Stromal LRCs express CD140b (46%), CD146 (2%), CD44 (24%), CD90 (45%), Sall4 (34%), Sca-1 (72%); ABCG2− |
Cao et al. (2014) |
| H2B-GFP | Adult cycling | Up to 47 weeks | G, S | Endometrial epithelial LRCs lost within 4 weeks Long-term epithelial LRCs in distal oviduct Long-term epithelial LRCs are Esr1−, CD44−, Sca-1−, Lgr5−, c-Kit− |
Wang et al. (2012c) |
| H2B-GFP | ED 13.5–PND 21 PND 21–40 |
Up to 47 weeks 8 months |
L, G, S G |
Endometrial LRCs are short-lived (<5 weeks) Long-term epithelial LRCs in distal oviduct and endocervical transition zone Epithelial LRCs persist |
Patterson and Pru (2013) |
ED, embryonic day; PND, postnatal day; L, luminal epithelial; G, glandular epithelial; S, stromal.
aAbout16% stromal LRCs are Esr1+ and 84% Esr1−.
Stromal LRCs
Postnatal (days 3–5) or prepubertal (days 19–21) administration of BrdU provides a window to label developmentally active stem/progenitor cells expected to reside in the endometrium. Stromal LRCs produced by this protocol were detectable after a chase in excess of 9 weeks (Chan and Gargett, 2006; Cervelló et al., 2007) (Table VI). Label retention studies using the H2B-GFP system during embryonic, early postnatal development and adulthood also produced stromal LRCs after a 3–8-week chase (Wang et al., 2012c; Patterson and Pru, 2013). Stromal LRCs from postnatal or prepubertal BrdU labelling were detected at the endometrial–myometrial junction, beneath the luminal epithelium, or in a perivascular location near CD31+ endothelial cells (Chan and Gargett, 2006). LRCs did not express CD45, demonstrating that they were not infiltrating leukocytes (Chan and Gargett, 2006). Stromal LRCs expressed the stem cell markers Oct-4, c-Kit (Cervelló et al., 2007), CD140b, CD146, CD44, CD90 and Sall4 (Chan and Gargett, 2006; Cao et al., 2014). Sca1 was absent from postnatal-derived LRCs but expressed in prepubertal-derived LRCs (Chan and Gargett, 2006; Cao et al., 2014). Postnatal-derived LRCs in the perivascular zone expressed α-smooth muscle actin (αSMA), suggesting that they are perivascular cells or pericytes (Chan and Gargett, 2006). A small proportion (16%) of stromal postnatal or prepubertal-derived LRCs expressed Esr1, the predominant ESR involved in estrogen-mediated endometrial regeneration. In summary, stromal LRCs in postnatal and prepubertal models are heterogeneous populations, and further investigation is required to determine whether subpopulations of LRCs are the MSCs of the mouse endometrium. Examining LRCs in mouse models of endometrial regeneration may identify which subpopulation of stromal LRCs functions in generating new stromal vascular tissue (see later section).
Epithelial LRCs
Epithelial LRCs in postnatal and prepubertal models are absent or very rare after a 3–4-week chase (Table VI) (Chan and Gargett, 2006; Cervelló et al., 2007; Patterson and Pru, 2013). The shorter persistence of epithelial LRCs is due to higher rates of epithelial cell proliferation, particularly under the influence of estrogen once estrous cycling begins at ∼4 weeks of age (Chan and Gargett, 2006). Epithelial LRCs were predominantly in the luminal rather than glandular epithelium, reflecting the higher turnover of luminal epithelium during development that facilitates labelling and subsequent dilution (Chan and Gargett, 2006). These luminal epithelial LRCs did not express Esr1, unlike most non-labelled epithelial cells. Epithelial LRCs do, however, proliferate in response to estrogen, pointing to an indirect effect mediated via neighbouring Esr1+ cells (Chan and Gargett, 2006; Chan et al., 2012). Glandular epithelial LRCs were rare in postnatal or prepubertal models and have not been characterized in detail.
H2B-GFP labelling spanning embryonic development to postnatal day 21 yielded highly persistent epithelial LRCs (9–13-week chase) in the distal oviduct and endocervical transition zone, but not endometrium (Wang et al., 2012c). Peripubertal H2B-GFP labelling (postnatal days 21–40) gave rise to glandular LRCs that persisted for 8 months and through several pregnancies, further emphasizing differences between glandular and luminal LRCs (Patterson and Pru, 2013). In contrast, H2B-GFP labelling in adult cycling mice did not produce long-term glandular LRC in the endometrium, suggesting that the peripubertal phase is a unique developmental window when some glandular epithelial development is permanently completed. Long-term epithelial H2B-GFP LRCs were reported in the distal oviduct after labelling of adult cycling mice (Wang et al., 2012c).
The LRC approach does not definitively identify stem/progenitor populations. It does, however, provide insight into patterns of development, rates of cell turnover and reactivation during endometrial regeneration and repair. LRC experiments highlight the higher turnover of luminal epithelium, relative to glandular epithelium and the stromal compartment. These observations suggest that luminal epithelium may be replenished from glandular epithelial or a stromal stem/progenitor population, but this is currently unclear (Kaitu'u-Lino et al., 2010; Huang et al., 2012; Patterson and Pru, 2013). The location of many stromal LRCs directly under the luminal epithelium (Chan and Gargett, 2006) may represent a snapshot of the ‘mesenchymal-to-epithelial transition’ (MET) believed to occur in the endometrium (Huang et al., 2012; Patterson and Pru, 2013). The perivascular location of other stromal LRCs (Kaitu'u-Lino et al., 2012) suggests a link to the perivascularly located human eMSCs. Unfortunately, the functional properties of BrdU-LRC have been impossible to assess directly because BrdU detection assays require fixation and treatment that kills the tissue being examined. This limitation is circumvented by the use of the transgenic H2B-GFP system, which allows the isolation of living LRCs. This transgenic system has only recently been used in the study of LRCs in the female reproductive tract (Wang et al., 2012c; Patterson and Pru, 2013) and is compatible with in vitro and in vivo assays that could clarify the identity and potential of quiescent putative mouse endometrial stem/progenitor populations.
SP cells
SP cells have been identified in murine postpartum but not in the normal cycling endometrium (Hu et al., 2010). The postpartum endometrial SP was enriched in clonogenic cells, which expressed Esr1 and tended to differentiate on exposure to estrogen in culture (Hu et al., 2010). However, unlike human endometrial SP, the mouse endometrial SP was not enriched for endothelial cells and its exact identity remains unclear.
CD44 as an epithelial progenitor marker in mouse endometrium
Compared with the human, cell surface markers for stem/progenitor cells are less well characterized in the mouse endometrium. CD44 is a transmembrane protein expressed on many cell types, including HSCs, MSCs and cancer stem cells (Zöller, 2011). In the mouse endometrium, CD44-expressing epithelial cells constituted an epithelial progenitor population, which lacked Esr1 or PR (Janzen et al., 2013). This epithelial population survived hormonal deprivation, possibly due to Wnt pathway activation. CD44+ epithelial cells generated more gland-like structures than CD44− cells in a tissue reconstitution assay in immunocompromised mice. CD44+ cells were also proliferative, suggesting that they are distinct from slow-cycling epithelial LRCs.
Role of endometrial stem/progenitor cells in endometrial regeneration
The human endometrium not only regenerates each month as part of the menstrual cycle, but also following parturition, almost complete resection and in postmenopausal women taking estrogen-based hormone replacement therapy (Gargett et al., 2012).
Tissue reconstituting cells regenerate human endometrium
Unfractionated single-cell suspensions of endometrium cells from hysterectomy tissue have regenerated endometrial tissue following xenografting beneath the kidney capsule of severely immunocompromised NOG mice lacking T, B and natural killer (NK) cells (Masuda et al., 2007b). The uterine cells organized into endometrial and myometrial tissue layers, comprising cytokeratin+CD9+ glandular structures, CD10+CD13+ stroma and αSMA myometrial-like tissue. The endometrial xenografts responded to cyclical estrogen and progesterone administration, mimicking the human menstrual cycle in ovariectomized-recipient mice. Estrogen stimulated epithelial and stromal proliferation, whereas progesterone induced tortuous glands and decidualized the stroma. When progesterone was withdrawn, large blood-filled cysts formed, suggestive of menstruation. Cells labelled with a lentiviral luciferase vector prior to xenografting enabled non-invasive bioluminescence imaging during hormonally induced ‘menstrual’ cycles, showing growth and regression of the human endometrial tissue generated in vivo (Masuda et al., 2007b; Maruyama et al., 2010).
This xenograft model has been used to examine the tissue reconstitution activity of candidate endometrial stem/progenitor cell populations, including SP cells. As noted in the SP cells section, xenografting the SP, but not the MP alone, generated endometrium when transplanted under the kidney capsule of immunocompromised mice (Cervelló et al., 2010; Masuda et al., 2010). Freshly isolated SP cells generated vasculature and migrating ERβ+ (ESR2) endothelial cells, comprising 80% of the grafts. Stromal and glandular components comprised 13 and 8%, respectively. Clonally derived human endometrial epithelial and stromal SP cells also generated endometrial tissue when xenografted into immunocompromised mice (Cervelló et al., 2010). These xenografts immunostained for stroma (vimentin) and epithelium (CD9), but not for endothelium (CD31). Organized endometrial glands were not easily distinguished, possibly due to prolonged clonal culture of the SP prior to xenografting. The regenerated endometrial tissue did not express ESR1, but some cells expressed PR. These studies indicate that endometrial cell subpopulations with stem/progenitor cell activity regenerate endometrial tissue in vivo. As indicated earlier, further research is required to more precisely identify the cell type(s) in the transplanted SPs that generate human endometrial tissue (Gargett and Ye, 2012).
Regeneration of postmenopausal endometrium
The atrophic endometrium of postmenopausal women regenerates to a thickness similar to premenopausal endometrium by the administration of estradiol valerate for 8 weeks (Ettinger et al., 1997; Ulrich et al., 2014c). Clonogenic SUSD2+ eMSCs have been identified in the regenerated endometrium, with similar self-renewal and multipotency to premenopausal eMSCs (Ulrich et al., 2014c). Comparable vascular densities of endometrium from postmenopausal women treated with or without estrogen suggested that perivascular SUSD2+ cells have a role in the stromal vascular regeneration of postmenopausal endometrium. Although SSEA-1, the marker for basalis epithelium, is present on all postmenopausal epithelial cells, its role in regenerating the glandular and luminal epithelium is unknown (Valentijn et al., 2013). Similarly, clonogenic epithelial cells or SP cells have not yet been identified in postmenopausal endometrium, nor has their role in regenerating epithelial tissue been investigated.
Mouse models of endometrial repair and regeneration
Although the mouse estrus cycle does not involve menstruation and regeneration of a functionalis, mice undergo up to 80 estrus cycles and/or produce 8–10 litters during reproductive life, indicating that repair and regeneration are important features of the endometrial mucosa (Gargett et al., 2012). The BrdU label retention system was utilized to determine the role of LRCs in models of endometrial epithelial repair following a menstruation-like event (Kaitu'u-Lino et al., 2010, 2012), estrogen-induced endometrial regeneration in ovariectomized mice (Chan et al., 2012) and in postpartum repair and regeneration (Cao et al., 2014) (Table VI).
Epithelial LRCs were only observed in the glands in a modified mouse model of menstrual breakdown and epithelial repair, incorporating a BrdU pulse in adult mice during estrogen priming and a short 7–9-day chase during progesterone-mediated differentiation and rapid epithelial re-epithelialization (Kaitu'u-Lino et al., 2010). During endometrial shedding, the luminal epithelium and the subsequent estrogen-independent epithelial repair rapidly lost the BrdU label, whereas the glandular epithelium remained quiescent. Following complete re-epithelialization, >30% of the glandular LRCs proliferated, indicating a role for glandular LRCs following endometrial epithelial repair. In longitudinal endometrial profiles, these glands were located close to the endometrial–myometrial junction, similar to the basalis in humans. These glands showed features of basalis epithelium, relative quiescence as shown by lower BrdU pulse labelling, slower BrdU dilution and higher Esr1 expression, compared with luminal epithelium. A subpopulation of these glandular epithelial LRCs may be the candidate epithelial progenitors of the mouse endometrium rather than the luminal LRCs identified in pulse-labelled neonatal mice. However, the chase period was insufficient to fully dilute the label in the glands, which can be achieved by hormonal manipulation to simulate estrus cycling following re-epithelialization. Perivascular LRCs were also identified in this model, which may have a role in considerable remodelling of the stromal vascular fraction during decidualization, breakdown and epithelial repair (Kaitu'u-Lino et al., 2012).
A functional role for mouse endometrial LRCs in driving endometrial regeneration was demonstrated in an estrogen replacement model using postnatal pulse labelling in subsequently ovariectomized mice (Chan et al., 2012). In this kinetic study, endometrial LRCs of 4-week chased peripubertal mice localized to the luminal epithelium. Eight hours following estrogen replacement, these LRCs initiated epithelial cell proliferation, indicating their role in driving tissue regeneration. In contrast, in postnatal-labelled, 8-week chased adult mice, both luminal epithelial LRCs and non-LRCs initiated proliferation 2 h after estrogen treatment. This suggests that neonatally labelled luminal epithelial LRCs play a larger role in estrogen-induced proliferation in the peripubertal endometrium relative to the adult cycling endometrium (Chan et al., 2012). A subpopulation of perivascular stromal LRCs (12%) also proliferated in response to estrogen stimulation, but the stromal proliferative response was modest (Chan and Gargett, 2006; Chan et al., 2012). These findings suggests that a minority of stromal LRCs in the 8-week chase of postnatal-labelled mice are likely the stem/progenitor cells and that longer chase periods are required to identify them (Gargett et al., 2012).
Prepubertal (postnatal days 19–22) BrdU labelling allows the chase period to extend into pregnancy and the postpartum period, phases of major endometrial remodelling, repair and regeneration. Endometrial stromal LRC numbers were maintained during pregnancy but decreased in the postpartum period coinciding with repair, regeneration and increased levels of proliferation (Cao et al., 2014). On postpartum day 1, there was a peak of proliferating stromal LRCs in the endometrium, suggesting a role in postpartum repair. In contrast, there was no evidence that distal oviduct and endocervical junctional zone (JZ) and long-term glandular LRCs identified by H2B-GFP labelling participated in endometrial repair and regeneration following endometrial shedding or in the postpartum period (Patterson and Pru, 2013). However, distal oviduct H2B-GFP LRCs form spheroids in vitro and have differentiation capacity (Wang et al., 2012c), suggesting that they may be reserve cells with potential to contribute to endometrial repair and/or regeneration, possibly under circumstances yet to be experimentally tested.
Genetic lineage tracing has provided evidence that a stromal sub-population derived from anti-Mullerian hormone (AMH) receptor type II expressing cells contributed to epithelial repair and regeneration via MET (Huang et al., 2012; Patterson et al., 2013). A study of cellular dynamics and gene expression in an experimental model of endometrial re-epithelialization supports the importance of MET (Cousins et al., 2014); however, the exact stromal cell type involved remains to be determined.
In summary, the LRC approach has identified small populations of epithelial and stromal cells with label retention properties, some of which appear to participate in endometrial remodelling and regenerative events. However, functional studies of living LRCs using transgenic labels, such as H2B-GFP, lineage tracing and additional models, and stem cell assays are required to elucidate the role of quiescent putative epithelial and stromal stem/progenitor populations in the murine endometrium.
Role of bone marrow-derived cells in endometrial regeneration
Bone marrow has been proposed as a source of cells that cross lineage barriers to differentiate into specialized cell types of several organs including the endometrium (Krause et al., 2001; Du and Taylor, 2009). Bone marrow comprises small populations of haematopoietic, mesenchymal and endothelial stem/progenitor cells and large numbers of myeloid cells at various stages of differentiation. Several reports suggest that bone marrow gives rise to endometrial stromal, epithelial and endothelial cells. These studies used donor-specific markers to identify cells from transplanted bone marrow in the endometrium of recipient patients, mice and baboons. As the endometrium is an immunologically active tissue that recruits bone marrow-derived immune (myeloid and lymphoid) cells, the identity of bone marrow-derived endometrial cells needs to be carefully verified by the absence of immune cell markers and/or the presence of endometrial cell-specific markers to distinguish between a stem cell and immune cell origin.
Human studies
The first study to report bone marrow-derived endometrial cells examined endometrium from patients who had received HLA-mismatched bone marrow transplants (Taylor, 2004). Donor-derived cells were detected by reverse transcriptase–polymerase chain reaction and immunostaining for their HLA type and their phenotype determined by location and morphology. Bone marrow-derived cells accounted for up to 48% of the epithelial cells and 52% stromal cells. Subsequent studies using the Y-chromosome as a marker of bone marrow-derived cells in the endometrium of patients receiving sex-mismatched bone marrow transplants also reported contributions to epithelia and stroma, albeit at more modest levels of <10% (Ikoma et al., 2009; Cervelló et al., 2012). In keeping with reports of bone marrow-derived endothelial progenitors, bone marrow-derived endometrial endothelial cells have also been reported (Mints et al., 2008). However, bone marrow-derived cells did not contribute to the endometrial SP of putative stem/progenitor cells in the human endometrium (Cervelló et al., 2012). Thus, the question still remains whether the bone marrow-derived cells incorporating into the human endometrium are stem cells or immune cells.
Mouse models
Mouse models have also been used to examine the role of bone marrow-derived cells in endometrial regeneration. Bone marrow transplantation studies, using conditioning to ablate host marrow stem cells and allow haematopoietic engraftment, reported donor bone marrow-derived stromal, epithelial and endothelial cells in the mouse endometrium (Bratincsák et al., 2007; Du and Taylor, 2007; Mints et al., 2008; Du et al., 2012; Morelli et al., 2013). Factors driving the formation of bone marrow-derived endometrial cells include estrogen, which increased the incorporation of bone marrow-derived endothelial progenitors into uterine vasculature (Masuda et al., 2007a). Hormone-driven endometrial cycling showed no detectable effect on rates of bone marrow cell integration into endometrial stroma and epithelium (Du et al., 2012), arguing against a proposed role for bone marrow-derived cells in cyclic regeneration. However, uterine ischaemia or trauma approximately doubled the rates of stromal engraftment, without increasing epithelial engraftment (Du et al., 2012; Alawadhi et al., 2014). These observations suggest a role for bone marrow-derived stromal cells in repairing endometrial injury, rather than normal cyclic regeneration. Exposure to cigarette smoke decreases the recruitment of both stromal and epithelial bone marrow-derived cells, a finding linked to both infertility and reduced rates of endometriosis in smokers (Zhou et al., 2011). An important aspect of this type of study is the ability to clearly distinguish endometrial cells from bone marrow derived-leukocytes through co-immunolocalization and confocal microscopy. Several micrographs of uterine CD45 immunostaining in some studies of bone marrow-derived endometrial cells (Du et al., 2012; Alawadhi et al., 2014) are unusually devoid of CD45+ cells, suggesting a low detection rate for leukocytes, possibly leading to misclassification of bone marrow-derived leukocytes as stromal and epithelial cells.
Most studies of bone marrow-derived endometrial cells have not addressed the type of bone marrow cells contributing to the endometrium. Protocols used have been typically designed to facilitate engraftment of HSCs. HSCs have been widely reported to give rise to non-haematopoietic lineages in other tissues (Krause et al., 2001), but this has been disputed (Wagers and Weissman, 2004). In support of HSCs as the source of bone marrow-derived endometrial cells, transgenic mouse models tracing the CD45+ bone marrow haematopoietic lineage demonstrated the existence of bone marrow-derived endometrial epithelium in a small number of animals (Bratincsák et al., 2007). In contrast, human patients and baboons transplanted with mobilized and purified HSCs failed to exhibit evidence of HSC-derived endometrial stroma (Wolff et al., 2013). Also arguing against HSC-derived contributions to the endometrium is the finding that granulocyte-colony stimulating factor, which mobilizes bone marrow HSCs, reduced the engraftment of bone marrow-derived stromal cells in the endometrium (Du et al., 2012). It was proposed that bmMSC, a population not mobilized by granulocyte-colony stimulating factor, rather than HSC, generated endometrial stroma. Interpretation of these studies is further complicated by the finding of a resident population of uterine haemangioblasts that generate HSCs and vascular cells in the murine endometrium (Sun et al., 2010).
Challenges in defining the role of bone marrow-derived cells in endometrial regeneration
The concept of bone marrow as a source of transdifferentiating cells responsible for cellular replacement has been investigated in many organs including lung, liver, kidney, intestine and skin (Krause et al., 2001). Studies of these organs tell a cautionary tale relevant to ongoing work on bone marrow-derived cells in endometrial repair and regeneration. Initial enthusiasm for the concept of bone marrow transdifferentiation (Petersen et al., 1999; Krause et al., 2001; Gupta et al., 2002) was tempered by subsequent reports that genuine transdifferentiation of bone marrow-derived cells is either very rare or cannot be detected (Wagers et al., 2002; Duffield et al., 2005; Kotton et al., 2005). Cell fusion, artefactual marker staining and overlaying leukocytes are commonly cited as sources of cells that may be misinterpreted as transdifferentiated cells (Wang et al., 2003; Duffield and Bonventre, 2005). During the ongoing debate on bone marrow cell transdifferentiation, a common emerging theme is that rigorous technical analysis and multiple markers must be used to definitively identify and determine the phenotype of candidate bone marrow-derived cells in any organ (Duffield and Bonventre, 2005; Kassmer and Krause, 2010). The existence of bone marrow-derived endometrial cells has yet to receive the level of scrutiny applied to many other organs.
Generating endometrial epithelial-like tissue from hES cells
In contrast to adult stem/progenitor cells, pluripotent hES cells are available in large numbers, and many protocols have been developed for their differentiation into clinically relevant cell types (Trounson, 2006), including endometrial cells (Song et al., 2015). Induced pluripotent stem (iPS) cells (Table I) offer a more attractive source of pluripotent cells to generate cells for tissue repair as they can be derived from the patient's own cells, overcoming immunological barriers associated with hES cell derivatives (Takahashi et al., 2007). Endometrial cells are an attractive source of cells for reprogramming into iPS cells as they express elevated levels of pluripotent factors and more efficiently generate iPS cells when compared with conventional cells (Park et al., 2011). Human endometrial epithelial-like tissue has been derived from hES cells by recapitulating the stages of reproductive tract development using a two-stage strategy (Ye et al., 2011). GFP-tagged hES cells were initially partially differentiated to intermediate and lateral plate mesoderm in vitro using bone morphogenetic protein 4 and activin A during embryoid body formation. This was followed by further differentiation in vivo by induction with neonatal mouse uterine mesenchyme as a tissue recombinant and transplanted into immune-compromised NSG mice. Differentiation during this 9-week process was monitored by assessing transcripts and protein for specific mesodermal differentiation markers in the differentiating embryoid bodies, Müllerian duct markers (LIM homeobox protein 1, LIM1; paired box 2, PAX2; homeobox A10, HOXA10) in early xenografts and endometrial epithelial markers (ovarian cancer-related tumour marker, CA125; ESR1, β-tubulin on ciliated cells) in late harvest xenografts (Ye et al., 2011, 2012). These endometrial gland-like profiles were functional and proliferated in response to exogenous estrogen and upregulated glycodelin A. The ability of uterine stroma to direct the differentiation of hES cell-derived mesodermal derivatives could be used to differentiate endometrial stromal cell-derived iPS cells into endometrial epithelial cells for potential use in tissue engineering applications to regenerate endometrial tissue in Asherman's syndrome (Gargett and Ye, 2012).
Endometrial stem/progenitor cells in disorders of endometrial proliferation
As adult stem cells have a key role in maintaining tissue homeostasis, it is likely that their function is aberrant in benign gynaecological disease associated with altered endometrial proliferation. Stem/progenitor cells are regulated by the stem cell niche, which may also have roles in the development and progression of endometriosis, adenomyosis, thin dysfunctional endometrium and Asherman's syndrome (Table VII).
Table VII.
Endometrial diseases in which endometrial stem/progenitor cells may play a role.
| Endometrial disease | Description |
|---|---|
| Adenomyosis | A benign disease involving extensive growth and invasion of basalis endometrial tissue into the uterine myometrium with associated smooth muscle hyperplasia, resulting in an enlarged uterus and painful, heavy or prolonged periods |
| Asherman's syndrome and intrauterine adhesions (IUAs) | An acquired uterine condition characterized by complete obliteration of the endometrium with fibrotic intrauterine adhesions (IUAs) causing amenorrhea and infertility. IUA is a less severe condition involving partial replacement of the endometrium with fibrous tissue, causing hypomenorrhea, infertility and pregnancy loss. It results from trauma to the basalis endometrium following dilation and curettage (D&C) due to miscarriage, abortion or retained placenta in a setting of low estrogen and/or infection |
| Endometriosis | A benign disease affecting reproductive aged women in whom endometrial tissue grows outside the uterine cavity, most often in the pelvic cavity, around/on the ovaries and in the rectovaginal septum, resulting in inflammation, infertility and severe pelvic pain |
| Thin dysfunctional endometrium | Endometrial tissue that does not respond to estrogen stimulation and fails to reach at least 7 mm in thickness necessary for embryo implantation and maintenance of an ongoing pregnancy |
Endometriosis
Endometriosis is characterized by the growth of endometrial tissue outside the uterine cavity (Giudice and Kao, 2004; Viganó et al., 2004). The most widely accepted theory for the pathogenesis of endometriosis is that retrograde menstruation deposits viable endometrial fragments into the pelvic cavity which attach to and invade the peritoneal mesothelium to establish ectopic growth of endometrial tissue (Sampson, 1927). Despite most women experiencing retrograde menstruation, it is not known why only 6–10% of the reproductive age women develop endometriosis (Halme et al., 1984). Following the discovery of endometrial stem/progenitor cells, Sampson's hypothesis was extended to address this disparity. It is proposed that endometrial stem/progenitor cells with associated niche cells are abnormally shed during menses, where they gain access to the peritoneal cavity by retrograde menstruation and establish ectopic implants, causing endometriosis (Starzinski-Powitz et al., 2001; Leyendecker et al., 2002; Gargett, 2007; Sasson and Taylor, 2008; Gargett and Masuda, 2010). It was also proposed that endometriosis lesions initiated by endometrial stem/progenitor cells would be more severe and invasive than lesions initiated by more differentiated transit-amplifying cells, explaining the different grades of endometriosis (Gargett, 2007). Alternatively, endometrial stem/progenitor cells, with yet to be identified intrinsic abnormalities, such as carrying one of the endometriosis susceptibility alleles (Nyholt et al., 2012), may have increased propensity to implant and establish an ectopic colony. Normal endometrial stem/progenitor cells may also implant more readily on an abnormal peritoneal mesothelium (Gargett and Chan, 2006; Gargett, 2007). It was also hypothesized that endometrial stem/progenitor cells may be involved in the pathogenesis of premenarcheal and adolescent endometriosis through retrograde neonatal uterine bleeding due to maternal progesterone withdrawal at birth (Brosens and Benagiano, 2013; Brosens et al., 2013; Gargett et al., 2014). Endometrial stem/progenitor cells, together with niche cells, would remain dormant beneath the peritoneum until rising estrogen levels triggered at menarche activate them to initiate clonal growths of ectopic endometrium and establish early onset endometriosis prior to menstruation (Fig. 2).
Figure 2.
Schematic describing the hypothesis that endometrial stem/progenitor cells shed in neonatal uterine bleeding may play a role in early onset endometriosis. Neonatal uterine bleeding occurs in 5% of neonates. It is hypothesized that retrograde neonatal bleeding occurs because thick mucus obstructs the long neonatal cervix. Fragments of shed endometrial tissue are postulated to contain an endometrial epithelial progenitor cell (pink) and a perivascular MSC (pink) together with niche cells. These rapidly adhere to the neonatal mesothelium, invade and/or become contiguous with the mesothelial lining where they remain quiescent for ∼10 years. Rising estrogen (E2) levels associated with thelarche and menarche reactivate the stem/progenitor cells to initiate growth of endometriosis lesions on the surface of or below the peritoneal mesothelium, resulting in early onset endometriosis. Reprinted with permissions from Gargett et al. (2014).
No direct evidence for the role of endometrial stem/progenitor cells in the pathogenesis of endometriosis has yet been reported. Several studies support a role for shedding of endometrial epithelial progenitor cells from the basalis in women with endometriosis. Fragments of basalis endometrium were identified more often in menstrual blood of women with than without endometriosis (Leyendecker et al., 2002). SSEA-1, a marker of endometrial basalis epithelial cells, was found in endometriotic lesions (Valentijn et al., 2013). The monoclonality of ectopic endometrial epithelial cells (Jimbo et al., 1997; Tamura et al., 1998; Wu et al., 2003) and identification of DNA losses/genomic imbalances demonstrating clonal proliferation (Silveira et al., 2012) in endometriotic lesions suggest the involvement of progenitor cells in the pathogenesis of endometriosis. Stem cell genes and proteins OCT4, SOX2, NANOG, Musashi and C-KIT have been observed in endometriotic lesions (Götte et al., 2008; Forte et al., 2009; Song et al., 2014). Cultured ectopic, clonally derived eMSCs expressed OCT-4 and demonstrated MSC phenotypic surface markers (Kao et al., 2011). Together, these studies suggest that women with endometriosis shed basalis endometrium at menstruation, increasing the likelihood of endometrial epithelial progenitor cells gaining access to the peritoneal cavity to initiate and develop into endometriotic lesions. However, these findings do not explain a role for eMSCs, which are present in both functionalis and basalis.
Recent functional human studies identified stem/progenitor cells in ectopic endometriotic lesions, suggesting their potential role. Similar to eutopic endometrial stem/progenitor cells, endometriotic epithelial and stromal CFUs were observed, and these serially cloned two to three times (Chan et al., 2011). Ectopic stromal CFUs were multipotent (Chan et al., 2011; Kao et al., 2011) and underwent more than 25 population doublings before senescence (Kao et al., 2011; Moggio et al., 2012), similar to eutopic stromal CFUs (Gargett et al., 2009). Ectopic endometriotic stromal cell lines differentiated into cytokeratin- and E-cadherin-expressing epithelial cells in culture (Moggio et al., 2012). Ectopic MSCs demonstrated greater migration and invasion than eutopic MSCs with increased angiogenesis and invasion into surrounding tissue in a scaffold transplantation mouse model (Kao et al., 2011). Together, these studies support the presence of endometrial stem/progenitor cells in ectopic endometriotic lesions. The peritoneal fluid of menstruating women with and without endometriosis contained similar numbers of endometrial cells (Bokor et al., 2009), although markers used to identify them were not specific and also immunostained resident peritoneal mesothelial cells. As endometrial stromal cells rapidly attach to peritoneal mesothelial cells in vitro (Lucidi et al., 2005), their concentration in peritoneal fluid may not accurately represent those retrogradely shed during menstruation. The presence of endometrial stem/progenitor cells in peritoneal fluid has not yet been reported, which is crucial for identifying their role in the pathogenesis of endometriosis. The identification of the specific eMSC marker SUSD2 will enable their identification in shedding endometrium and peritoneal fluid. However, markers are currently lacking for endometrial epithelial progenitor cells.
Endometriosis models in mice, rats, baboons and marmosets (Grümmer, 2006) exist, but these have focused on pathophysiological mechanisms involved in the development of disease, rather than the involvement of stem/progenitor cells, although bone marrow-derived cells may have contributed to the progression of established endometriosis lesions (Taylor, 2004). However, it can be inferred that endometrial stem/progenitor cells are likely responsible for lesion formation in animal models, in which menstrual debris was transplanted into the peritoneal cavity (Greaves et al., 2014) or endometrial cells/tissues were injected subcutaneously (Wang et al., 2014). Genetic labelling of endometrial stem/progenitor cells with fluorescent tags is needed to exploit these animal models for cell tracking to determine their role in ectopic lesion formation. This approach was used to label all endometrial cells with adenovirus encoding red fluorescent protein (Wang et al., 2014) or recombinant lentivirus conferring yellow fluorescent protein or click beetle red-emitting luciferase (Masuda et al., 2007b) for non-invasive monitoring of cell xenografts over several menstrual-like cycles. These animal models need further adapting to monitor labelled endometrial stem/progenitor cells, transplanted into the peritoneal cavity and subjected to a similar hormonal regime as the mouse model of menstruation (Brasted et al., 2003) for several cycles to examine the role of endometrial stem/progenitor cells in the initiation and progression of endometriosis.
Adenomyosis
Adenomyosis is considered a dichotomous disease characterized by thickening and disruption of the endo-myometrial JZ structure (Benagiano et al., 2014). Little is known about the pathophysiology of adenomyosis, which mainly affects parous women. Theories suggest that chronic microtrauma to the JZ from chronic peristaltic myometrial contractions causes repeated cycles of tissue injury and repair (Leyendecker et al., 2009). A vicious cycle is established in which local estrogen production promotes uterine hyperperistalsis and further auto-traumatization, allowing basal endometrial glands and stroma to penetrate the myometrium and proliferate to form pockets of adenomyosis within the uterine muscle. Tissue injury typically activates adult stem cells, which may establish ectopic endometrial lesions through disruption of endometrial stem/progenitor cell niches (Gargett, 2007). Abnormal differentiation of eMSCs may be responsible for the smooth muscle hyperplasia (Gargett, 2007). In support of this concept, stromal cells cultured from adenomyotic tissue differentiated into typical mesodermal lineages and expressed MSC surface markers (Chen et al., 2010). Whether adenomyotic stromal cells exhibit MSC properties of clonogenicity and self-renewal or contain a subpopulation of perivascular CD146+PDGFRβ+ or SUSD2+ or SP cells is unknown. Gene expression profiling identified differences between adenomyotic and normal endometrial stromal cells (Chen et al., 2010), particularly COX-2 overexpression. COX-2 has a role in the tissue injury repair cycle and local estrogen production (Leyendecker et al., 2009). In an attempt to identify endometrial stem cells in adenomyotic tissue, immunostaining with an adult stem cell marker, Musashi-1, was undertaken (Chen et al., 2014). However, most epithelial cells and a proportion of stromal cells were Musashi-1+, in contrast to observations of rare immunostained cells in the normal endometrium (Götte et al., 2008). Given this discrepancy and that Musashi-1+ endometrial stromal cells in eutopic and ectopic adenomyosis do not appear in a perivascular location, it seems unlikely that Musashi-1-expressing cells are endometrial stem/progenitor cells in adenomyosis. It also indicates that functional stem cell assays should accompany studies examining stem cell marker expression. More research is required to establish a role for endometrial stem/progenitor cells in the initiation and progression of adenomyosis.
Thin endometrium and Asherman's syndrome
Dysfunctional endometrial stem/progenitor cells may be responsible for the inability of some women to generate a sufficiently thick endometrium (>7–8 mm) to support embryo implantation (Yu et al., 2008). Thin dysfunctional endometrium unresponsive to estrogen stimulation is particularly challenging in IVF clinics. In Asherman's syndrome, defined as the complete obliteration of the uterine cavity with adhesions, we hypothesize a complete loss of endometrial stem/progenitor cells. Intrauterine adhesions (IUAs) represent a continuum between Asherman's syndrome and thin dysfunctional endometrium, where we postulate that there are insufficient endometrial stem/progenitor cells, which may or may not be dysfunctional, residing in the pockets of remaining endometrial tissue (Gargett and Ye, 2012). Between 2 and 22% of infertile women have Asherman's syndrome or IUA (Yu et al., 2008; Panayotidis et al., 2009). Trauma to the endometrial basalis and JZ from termination of pregnancy, spontaneous miscarriage and postpartum curettage in a setting of low circulating estrogen levels hinder endometrial regeneration. Any remaining endometrial stem/progenitor cells survive a low estrogen environment, but their niche cells require an estrogen-rich milieu to activate them. When infection and inflammation also occur, the inflammatory products may damage endometrial stem/progenitor cells, critically reducing their numbers and limiting their capacity to regenerate sufficient endometrium (Gargett and Ye, 2012).
A mouse model of Asherman's syndrome has been developed using a needle to traumatize the lumen of both uterine horns (Alawadhi et al., 2014). Immediately following the damage, unfractionated male mouse bone marrow cells or saline was administered intravenously and the mice were examined 3 months later. Histological analysis showed reduced fibrosis and pregnancies with normal litter sizes in 90% of the bone marrow-transplanted animals, compared with 30% of saline-treated mice. A small number (0.1%) of non-leukocyte (CD45−) Y chromosome+ cells were detected in the endometrium of the transplanted mice, but the original identity of the incorporated cells is unknown. The effective repair of damaged endometrium by this small number of cells suggests either an immediate systemic cytokine effect or an indirect activation of endogenous endometrial stem/progenitor cells or their niches.
In a rat model of Asherman's syndrome in which trichloroacetic acid was instilled into the uterine lumen, cultured male rat adipose-derived MSCs were injected into one uterine horn and then intraperitoneally at 5 day intervals. Histological assessment of the endometrium revealed that 4–6% of the stroma was BrdU-labelled cells (Kilic et al., 2014). MSCs alone increased vascular and cellular proliferation and VEGF immunostaining, but had no effect on inflammation or fibrosis. When co-administered with oral estrogen, the MSCs also reduced fibrosis. In contrast to systemic delivery, local injection of allogeneic MSCs to the injured endometrium enabled a proportion to incorporate into the tissue with apparent promotion of tissue regeneration, although neither tissue architecture nor endometrial function in supporting implantation or pregnancy was assessed.
Several case studies have reported the instillation of fresh CD34+ or cultured autologous bone marrow cells into the uterine cavity or subendometrial region of women with Asherman's syndrome or IUA (Nagori et al., 2011; Singh et al., 2014). Very moderate increases in endometrial thickness and evidence of menstruation or pregnancy were reported. However, these must be viewed with caution as there were no control subjects; a lack of detail on bone marrow cell preparation and the increase in endometrial thickness was generally below that required for successful embryo implantation (Gargett and Healy, 2011). Other sources of cells should also be considered in further development of a stem cell approach to treating thin dysfunctional endometrium, for example, allogeneic endometrial stem/progenitor cells or autologous endometrial cells derived from iPS cells. Endometrial-like tissue has been generated from hESCs (Ye et al., 2011), and iPS cells are easily created from human endometrial stromal cells (Park et al., 2011) or shed menstrual blood cells due to their plasticity and regenerative capacity (de Carvalho Rodrigues et al., 2012; Li et al., 2013). Further development of the animal model is required to determine the mechanism of action before cell-based therapies for Asherman's syndrome or thin dysfunctional endometrium are trialled in humans.
Clinical use of endometrial and mbMSCs for regenerative medicine
The ease with which endometrial tissue can be obtained by Pipelle biopsy without anaesthetic in comparison to the collection of bone marrow aspirates or adipose tissue liposuctions makes it an attractive source of MSCs for regenerative medicine (Ulrich et al., 2013). Menstrual blood is an even easier source for collection of MSC-like cells, although greater attention is required to ensure sterility of the cell product and methods for the purification of eMSCs from this source have not been refined.
Non-homologous use of menstrual blood stromal cells (ERCs)
The potential of cultured menstrual blood ERCs as a regenerative medicine therapy for a range of allogeneic non-homologous applications has been examined in several preclinical animal models. In a rat model, cell sheet technology was used to patch an infarcted heart, resulting in ERC incorporation and differentiation into cardiomyocytes, and improved cardiac function (Hida et al., 2008). Similarly, ERC transdifferentiated into skeletal muscle myocytes by fusion in a Duchenne muscular dystrophy mouse model (Cui et al., 2007). In a rat stroke model, cultured ERCs sorted for CD117 expression showed neuroprotection by differentiating into neuronal cells, improving motor and neurologic impairments (Borlongan et al., 2010). Cultured ERCs have reversed ovarian damage in a cyclophosphamide-induced mouse model of premature ovarian failure (Liu et al., 2014). In this model, locally injected ERC labelled with a fluorescent DiO dye showed greater retention than DiO-labelled human fibroblasts (source unknown) in the ovaries after 14 days. The DiO-labelled cells also expressed ovarian markers, AMH, FSH receptor, inhibin-α and -β. cDNA profiling revealed greater similarity in gene expression with human ovaries for mice treated with ERCs compared with human fibroblasts. Ovarian weight increased and function improved as judged by normalized circulating estradiol and FSH levels in ERC-treated mice. There were also fewer atretic follicles and more normal follicles. These studies indicate that ERCs have superior retention and reparative action than fibroblasts (presumably dermal) when delivered locally to injured ovaries.
Tissue engineering principles are being developed using ERC for eventual clinical translation. ERCs cultured on polycaprolactone nanofibres showed greater proliferative capacity and ability to differentiate into chondrocytes than bmMSCs (Kazemnejad et al., 2012). In evaluating ERCs for a potential cardiac tissue engineering application, a greater ability was observed to penetrate silk fibroin scaffolds and proliferate than bmMSCs (Rahimi et al., 2014). Further development of these tissue engineering constructs and their evaluation in vivo are warranted for ERCs, given their greater proliferation rates and easier acquisition than other sources of MSCs. Summarized in a recent review (Ulrich et al., 2013) are several case reports on the treatment of patients with various disorders using systemically delivered, cultured ERCs. In addition, a phase 2 clinical trial, the RECOVER-ERC launched by Medistem for treating congestive cardiac failure (Bockeria et al., 2013), is also detailed. Although there have been no side effects reported, the clinical data have not yet been published. The potential of menstrual blood stromal fibroblasts for regenerative medicine purposes looks promising for non-homologous use. However, more research is required to compare local versus systemic delivery and to determine the mechanism of action by using genetic labelling to track the cells more accurately in appropriate preclinical animal models to generate sufficient confidence for translating this potential into the clinic.
Autologous use of eMSCs as a therapy for pelvic organ prolapse
Pelvic organ prolapse (POP) is a major hidden disease burden for women, a legacy of vaginal birth for 50% of postmenopausal parous women (Nygaard et al., 2008). POP is the herniation of the pelvic organs into the vagina causing urinary and faecal incontinence, voiding and sexual dysfunction. Many women (19%) have a lifetime risk of undergoing reconstructive surgery for POP (Smith et al., 2010; Wu et al., 2014a) and ∼30% require additional operations due to failure of native tissue surgery or from significant adverse events associated with the use of vaginal mesh (Olsen et al., 1997; FDA, 2011). It has been proposed that autologous eMSCs used together with new mesh designs matching the biomechanical properties of vaginal tissue (Ulrich et al., 2012; Edwards et al., 2013) in a tissue engineering construct may improve surgical outcomes of vaginal mesh surgery for POP (Gargett, 2006; Ulrich et al., 2013). In a proof-of-principle experiment, culture-expanded SUSD2+ DiO-labelled eMSC seeded on new mesh (polyamide/gelatin composite, 2.5 × 105 cells) improved tissue integration of the mesh and the biomechanical outcomes of the tissue/mesh complex after 90 days of implantation in a fascial defect wound of immunocompromised nude rats, despite surviving at the site for only 14 days (Ulrich et al., 2014a). The eMSC promoted early neovascularization around the implanted mesh (7 days). An early inflammatory response was also observed around the mesh with eMSCs, characterized by increased numbers of M1 macrophages, but by 30 days these had differentiated into the M2 wound-healing phenotype and at 90 days the chronic macrophage response was significantly reduced. Similar quantities of new tissue collagen were deposited whether or not eMSCs were present, but more physiological, crimped collagen was observed in the mesh with eMSCs (Edwards et al., 2015). This led to a more compliant, less stiff mesh/tissue complex than mesh alone, addressing one problem associated with the use of clinical polypropylene mesh (FDA, 2011). This promising result in a heterologous small animal model of POP repair surgery has led to the development of an autologous large animal, ovine preclinical model of POP using ovine eMSCs (Ulrich et al., 2014b). This animal model is required to determine whether the mechanism of action of locally delivered eMSCs is due to paracrine release of factors promoting wound repair or whether they differentiate and incorporate into the dermis and smooth muscles of the vaginal wall (Atala, 2009; von Bahr et al., 2012). In vitro, TGFβ1 and platelet-derived growth factor BB (PDGF-BB) induced differentiation of SUSD2+ eMSCs seeded on the polyamide/gelatin meshes into SUSD2− smooth muscle cells expressing SM22α and smooth muscle myosin heavy chain, intermediate and late smooth muscle cell differentiation markers (Su et al., 2014). Connective tissue growth factor also differentiated eMSCs into collagen-producing fibroblasts. It remains to be determined whether autologous eMSCs delivered on the new mesh will undergo differentiation to these tissue-forming cells in vivo.
Clinical good manufacturing practice production of eMSCs
To prepare eMSCs or ERCs for cell-based therapies, it is necessary to incorporate the principles of Good Manufacturing Practice guidelines into the isolation and culture expansion process to ensure that the cells produced are of desired quality, safety and efficacy for each batch (Eaker et al., 2013; Hunsberger et al., 2015). This requires the removal of all animal products (xeno-free, XF) used in the dissociation of tissue, cell purification, cryopreservation and in culture expansion protocols. In particular, bovine foetal calf serum needs to be replaced in the development of optimized SF culture medium, an area of active development for MSCs from other sources. Initial steps towards the optimization and scale-up or scale-out culture of CD146+PDGFRβ+ eMSCs examining commercial and in-house XF and SF media formulations found that eMSC attachment and proliferation were optimal on a fibronectin matrix in an in-house Dulbecco's modified Eagle's medium/F-12 SF medium containing FGF2 and EGF in physiological hypoxia (5% oxygen) (Rajaraman et al., 2013). eMSCs cultured under these optimized conditions retained their MSC properties. The specific markers used to enrich eMSCs (PDGFRβ, CD146 and SUSD2) were more informative in discriminating between the various media and matrix combinations tested than the classic MSC markers (CD29, CD44, CD73 and CD105), pointing to the value of more specific perivascular markers. Further research is needed to determine the optimal XF conditions for eMSC and ERC culture expansion.
Immunomodulatory properties of eMSCs and ERCs and potential therapeutic use
In addition to their progenitor properties, MSCs have anti-inflammatory and immunomodulatory properties, regulating both the innate and adaptive immune systems, that have been exploited clinically (Le Blanc and Mougiakakos, 2012; Bianco et al., 2013). Tissue fibroblasts also have these anti-inflammatory and immunomodulatory properties (Haniffa et al., 2009; Bianco et al., 2013), and this is why cultures of unfractionated adherent bone marrow cells containing predominantly stromal fibroblasts have been effective in many studies and clinical trials.
In a proinflammatory environment, resident MSCs exposed to the inflammatory cytokine tumour necrosis factor-α and/or interferon-γ are activated to secrete anti-inflammatory mediators (Krampera, 2011). These mediators include IL-10, prostaglandin E2, TGFβ, HLA-G, indoleamine oxidase and nitric oxide, which together with cell–cell interaction suppress dendritic, T, B and NK cell activation (Le Blanc and Mougiakakos, 2012). The low level of expression of major histocompatibility complex II and co-stimulatory molecules confers MSCs with low alloreactivity, enabling them to be used for allogeneic transplantation. MSCs can also switch macrophages from an inflammatory M1 to a reparative M2 phenotype (Krampera, 2011).
Although no in vitro studies have examined the immunoregulatory function of human eMSCs or ERCs, transcriptional profiling of fresh CD146+PDGFRβ+ and cultured SUSD2+ and SUSD2− cells revealed that eMSCs expressed inflammatory and immunomodulatory genes (Spitzer et al., 2012; Murakami et al., 2014). The SUSD2− stromal fibroblasts secreted more chemokines and inflammatory mediators than SUSD2+ eMSCs, but both cell types secreted similar levels of many cytokines and chemokines including the Th-2-associated IL-4 and anti-inflammatory IL-10 (Murakami et al., 2014). In contrast, in vivo studies showed that eMSCs and ERCs have anti-inflammatory and immunomodulatory properties. Locally delivered eMSCs reduced the chronic inflammatory response to implanted synthetic mesh in a nude rat fascial wound repair model and promoted the switch of M1 to M2 macrophages, resulting in improved biocompatibility compared with controls (Ulrich et al., 2014a). Cultured endometrial stromal cells and ERCs suppressed neuroinflammation in an autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis (Peron et al., 2012) and bowel inflammation in a colitis model (Lv et al., 2014b), respectively. In the multiple sclerosis model, endometrial stromal fibroblasts were delivered intraperitoneally 24 h prior to EAE induction. In the colitis model, ERCs were systemically delivered several times after disease induction. Both models showed improved clinical symptoms and histological scores, reduced leukocyte infiltrates, lower inflammatory cytokine transcription in the damaged organs and upregulated anti-inflammatory cytokine transcripts in the spleens. There were also fewer active T cells, cytotoxic T cells and dendritic cells and more regulatory T cells in the spleens of animals receiving cells. In these models, the eMSCs, endometrial stromal fibroblasts and ERCs acted in a paracrine manner to suppress inflammation, with the latter two cell types also downregulating immune responses. These findings concur with many observations reported for MSCs derived from other sources, suggesting that eMSC-like cells have similar anti-inflammatory and immunoregulatory properties.
Unresolved issues in endometrial stem cell research
A key issue facing the endometrial stem/progenitor cell field is the use of numerous terms to describe the various stem/progenitor cell types (Tables I, II and IV). A common nomenclature recognizing the species, type of cell and its source is needed. It will be important that any nomenclature fits with that used in the broader stem cell discipline. This particularly applies to the universal definition of MSC (Dominici et al., 2006), which is increasingly recognized as inadequate for defining MSC from tissues other than bone marrow (Bianco et al., 2013). The nomenclature will need to reflect the different cell types studied, perivascular cells versus the endometrial stromal fibroblast, clonogenic versus non-clonogenic stromal fibroblasts, SP versus MP and those identified with specific markers enriching for clonogenic, self-renewing and multipotent progenitors versus unfractionated multipotent stromal fibroblasts.
Although it is apparent that bone marrow-derived cells incorporate in low numbers into the endometrium, the nature of these cells is currently unknown. Careful studies using transgenic tools to track cells are needed. Together with known markers of endometrial stem/progenitor cells and those to be identified in future will enable mechanistic studies to more precisely determine the role of bone marrow-derived cells in normal endometrial physiology and in endometrial diseases.
The hierarchical relationship among the clonogenic epithelial progenitor cells, endometrial and decidual SP cells, CD146+PDGFR-β+ and SUSD2+ cells and menstrual blood ERCs is not fully known. This requires more defining markers or gene signatures and determination of the in vivo activity for the various cell types. As these data are generated, it may be possible to determine whether MET links the various stem/progenitor cell types and whether this process occurs during endometrial regeneration.
Future perspectives
Identification of specific markers for eMSCs enables profiling of these stem/progenitor cells and their differentiated progeny to identify their signatures. Comparison with bmMSCs, adipose MSCs and placental MSCs also becomes possible. This work has commenced and already shows differences between the perivascular-derived MSCs and the stromal fibroblast and their decidualized derivatives (Spitzer et al., 2012; Murakami et al., 2014). RNA sequencing will uncover regulatory pathways involved in the endometrial stem/progenitor cell function and their differentiation pathways allowing comparisons between cells from normal and dysfunctional endometrium and ectopic endometrial lesions of adenomyosis and endometriosis. Epigenetics governs cellular phenotype, particularly during cellular differentiation. Interrogation of the epigenetic profiles of endometrial stem/progenitor cells and stromal fibroblasts will uncover regulatory pathways, governing the function of these cell types. Similarly, once markers for endometrial epithelial progenitors are determined, their chromatin state can be determined and compared between well-characterized cell phenotypes, shedding light on the regulation of the adult stem cell state and its differentiation. These advances in sequencing technologies are matched by the availability of single-cell analysis using Fluidigm® technologies, enabling investigations into cell heterogeneity. For example, SUSD2 has a wide range of expressions on individual cells, but the function and phenotype of low versus high expression are currently unknown (unpublished), but could be determined using single-cell analysis and deep sequencing or epigenetic profiling.
eMSCs and menstrual blood ERCs are showing promise for cell-based therapies for gynaecological disease and non-homologous use. The properties of eMSCs are favourable for further development in regenerative medicine applications as they are relatively easy to obtain compared with the current commonly used sources. Regenerative medicine frequently requires biomaterials and scaffolds to deliver cells to the injured site. These can be fabricated (Edwards et al., 2013) or can be derived from decellularized uterine matrix for endometrial applications (Miyazaki and Maruyama, 2014), the latter with potential application for Asherman's syndrome or IUA. Once epithelial progenitor markers are identified, these and the MSCs could be used to seed these decellularized matrices.
Concluding remarks
In the 10-year history of endometrial stem/progenitor cells, substantial progress has been made in developing assays for their evaluation and for identifying specific markers and characterizing these populations. The in vivo identity of eMSC and SP cells has been determined, and examination of their role in endometriosis and adenomyosis has commenced. The ease with which eMSCs and ERCs can be obtained makes them attractive candidates for cell-based therapies for gynaecological disease and other regenerative medicine applications.
Authors' roles
C.E.G. was involved in conception and design, acquisition of data, analysis and interpretation of data, writing the article and revising it critically for important intellectual content plus gave final approval of the version to be published. K.E.S. provided data acquisition, analysis and interpretation of data, drafting the article and gave final approval of the version to be published. J.A.D. was involved in acquisition of data, analysis and interpretation of data, writing the article and revising it critically for important intellectual content plus gave final approval of the version to be published.
Funding
This study was funded by National Health and Medical Research Council of Australia grants 1085435 (C.E.G. and J.A.D.) and 1081944 (C.E.G.) and Senior Research Fellowship (1042298, C.E.G.), Royal Australian and New Zealand College of Obstetricians and Gynaecologists (K.E.S.) and Victorian Infrastructure Support Program. Funding to pay the open access publication charges for this article was provided by the National Health and Medical Research Council of Australia grants ID1085435 and ID1042298.
Conflict of interest
The authors have nothing to declare.
Acknowledgements
The authors wish to thank Dr Fiona Cousins for critical reading of the manuscript.
References
- Aghajanova L, Horcajadas JA, Esteban FJ, Giudice LC. The bone marrow-derived human mesenchymal stem cell: potential progenitor of the endometrial stromal fibroblast. Biol Reprod 2010;82:1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J, Shahverdi AR, Barough SE, Kouchesfehani HM, Heidari S, Roozafzoon R, Verdi J, Khoshzaban A. Derivation of adipocytes from human endometrial stem cells (EnSCs). J Reprod Infertil 2012;13:151–157. [PMC free article] [PubMed] [Google Scholar]
- Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of Asherman's syndrome. PLoS ONE 2014;9:e96662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atala A. Engineering organs. Curr Opin Biotechnol 2009;20:575–592. [DOI] [PubMed] [Google Scholar]
- Azedi F, Kazemnejad S, Zarnani AH, Behzadi G, Vasei M, Khanmohammadi M, Khanjani S, Edalatkhah H, Lakpour N. Differentiation potential of menstrual blood versus bone marrow-stem cells into glial-like cells. Cell Biol Int 2014;38:615–624. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007;449:1003–1007. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update 2014;20:386–402. [DOI] [PubMed] [Google Scholar]
- Bianco P, Cao X, Frenette PS, Mao JJ, Robey PG, Simmons PJ, Wang CY. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med 2013;19:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockeria L, Bogin V, Bockeria O, Le T, Alekyan B, Woods EJ, Brown AA, Ichim TE, Patel AN. Endometrial regenerative cells for treatment of heart failure: a new stem cell enters the clinic. J Transl Med 2013;11:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokor A, Debrock S, Drijkoningen M, Goossens W, Fülöp V, D'Hooghe T. Quantity and quality of retrograde menstruation: a case control study. Reprod Biol Endocrinol 2009;7:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlongan CV, Kaneko Y, Maki M, Yu SJ, Ali M, Allickson JG, Sanberg CD, Kuzmin-Nichols N, Sanberg PR. Menstrual blood cells display stem cell-like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev 2010;19:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasted M, White CA, Kennedy TG, Salamonsen LA. Mimicking the events of menstruation in the murine uterus. Biol Reprod 2003;69:1273–1280. [DOI] [PubMed] [Google Scholar]
- Bratincsák A, Brownstein MJ, Cassiani-Ingoni R, Pastorino S, Szalayova I, Tóth ZE, Key S, Németh K, Pickel J, Mezey E. CD45-positive blood cells give rise to uterine epithelial cells in mice. Stem Cells 2007;25:2820–2826. [DOI] [PubMed] [Google Scholar]
- Brosens I, Benagiano G. Is neonatal uterine bleeding involved in the pathogenesis of endometriosis as a source of stem cells? Fertil Steril 2013;100:622–623. [DOI] [PubMed] [Google Scholar]
- Brosens I, Brosens J, Benagiano G. Neonatal uterine bleeding as antecedent of pelvic endometriosis. Hum Reprod 2013;28:2893–2897. [DOI] [PubMed] [Google Scholar]
- Cao M, Chan RW, Yeung WS. Label-retaining stromal cells in mouse endometrium awaken for expansion and repair after parturition. Stem Cells Dev 2014;24:768–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrechini NM, Murthi P, Qin S, Kusuma GD, Wilton L, Abumaree M, Gronthos S, Zannettino A, Gude NM, Brennecke SP et al. Decidua parietalis-derived mesenchymal stromal cells reside in a vascular niche within the choriodecidua. Reprod Sci 2012;19:1302–1314. [DOI] [PubMed] [Google Scholar]
- Cervelló I, Martínez-Conejero JA, Horcajadas JA, Pellicer A, Simón C. Identification, characterization and co-localization of label-retaining cell population in mouse endometrium with typical undifferentiated markers. Hum Reprod 2007;22:45–51. [DOI] [PubMed] [Google Scholar]
- Cervelló I, Gil-Sanchis C, Mas A, Delgado-Rosas F, Martínez-Conejero JA, Galán A, Martínez-Romero A, Martínez S, Navarro I, Ferro J et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS ONE 2010;5:e10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervelló I, Mas A, Gil-Sanchis C, Peris L, Faus A, Saunders PT, Critchley HO, Simón C. Reconstruction of endometrium from human endometrial side population cell lines. PLoS ONE 2011;6:e21221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervelló I, Gil-Sanchis C, Mas A, Faus A, Sanz J, Moscardó F, Higueras G, Sanz MA, Pellicer A, Simón C. Bone marrow-derived cells from male donors do not contribute to the endometrial side population of the recipient. PLoS ONE 2012;7:e30260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challen GA, Little MH. A side order of stem cells: the SP phenotype. Stem Cells 2006;24:3–12. [DOI] [PubMed] [Google Scholar]
- Chan RW, Gargett CE. Identification of label-retaining cells in mouse endometrium. Stem Cells 2006;24:1529–1538. [DOI] [PubMed] [Google Scholar]
- Chan RW, Schwab KE, Gargett CE. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod 2004;70:1738–1750. [DOI] [PubMed] [Google Scholar]
- Chan RW, Ng EH, Yeung WS. Identification of cells with colony-forming activity, self-renewal capacity, and multipotency in ovarian endometriosis. Am J Pathol 2011;178:2832–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan RW, Kaitu'u-Lino T, Gargett CE. Role of label-retaining cells in estrogen-induced endometrial regeneration. Reprod Sci 2012;19:102–114. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Li HY, Chang YL, Yuan CC, Tai LK, Lu KH, Chang CM, Chiou SH. Suppression of migratory/invasive ability and induction of apoptosis in adenomyosis-derived mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil Steril 2010;94:1972–1979. [DOI] [PubMed] [Google Scholar]
- Chen YZ, Wang JH, Yan J, Liang Y, Zhang XF, Zhou F. Increased expression of the adult stem cell marker Musashi-1 in the ectopic endometrium of adenomyosis does not correlate with serum estradiol and progesterone levels. Eur J Obstet Gynecol Reprod Biol 2014;173:88–93. [DOI] [PubMed] [Google Scholar]
- Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HO, Saunders PT. Evidence from a mouse model that epithelial cell migration and mesenchymal–epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS ONE 2014;9:e86378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313. [DOI] [PubMed] [Google Scholar]
- Cui CH, Uyama T, Miyado K, Terai M, Kyo S, Kiyono T, Umezawa A. Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol Biol Cell 2007;18:1586–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzi S, Zarnani AH, Jeddi-Tehrani M, Entezami K, Mirzadegan E, Akhondi MM, Talebi S, Khanmohammadi M, Kazemnejad S. Osteogenic differentiation of stem cells derived from menstrual blood versus bone marrow in the presence of human platelet releasate. Tissue Eng Part A 2012;18:1720–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Rodrigues D, Asensi KD, Vairo L, Azevedo-Pereira RL, Silva R, Rondinelli E, Goldenberg RC, Campos de Carvalho AC, Urményi TP. Human menstrual blood-derived mesenchymal cells as a cell source of rapid and efficient nuclear reprogramming. Cell Transplant 2012;21:2215–2224. [DOI] [PubMed] [Google Scholar]
- Dimitrov R, Timeva T, Kyurkchiev D, Stamenova M, Shterev A, Kostova P, Zlatkov V, Kehayov I, Kyurkchiev S. Characterization of clonogenic stromal cells isolated from human endometrium. Reproduction 2008;135:551–558. [DOI] [PubMed] [Google Scholar]
- Dimitrov R, Kyurkchiev D, Timeva T, Yunakova M, Stamenova M, Shterev A, Kyurkchiev S. First-trimester human decidua contains a population of mesenchymal stem cells. Fertil Steril 2010;93:210–219. [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop D, Horwitz E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- Donofrio G, Franceschi V, Capocefalo A, Cavirani S, Sheldon IM. Bovine endometrial stromal cells display osteogenic properties. Reprod Biol Endocrinol 2008;6:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007;25:2082–2086. [DOI] [PubMed] [Google Scholar]
- Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci 2009;16:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev 2012;21:3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int 2005;68:1956–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffield JS, Park KM, Hsiao LL, Kelley VR, Scadden DT, Ichimura T, Bonventre JV. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J Clin Invest 2005;115:1743–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker S, Armant M, Brandwein H, Burger S, Campbell A, Carpenito C, Clarke D, Fong T, Karnieli O, Niss K et al. Concise review: guidance in developing commercializable autologous/patient-specific cell therapy manufacturing. Stem Cells Transl Med 2013;2:871–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimi-Barough S, Kouchesfahani HM, Ai J, Massumi M. Differentiation of human endometrial stromal cells into oligodendrocyte progenitor cells (OPCs). J Mol Neurosci 2013;51:265–273. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Werkmeister JA, Rosamilia A, Ramshaw JA, White JF, Gargett CE. Characterisation of clinical and newly fabricated meshes for pelvic organ prolapse repair. J Mech Behav Biomed Mater 2013;23:53–61. [DOI] [PubMed] [Google Scholar]
- Edwards SL, Ulrich D, White JF, Su K, Rosamilia A, Ramshaw JA, Gargett CE, Werkmeister JA. Temporal changes in the biomechanical properties of endometrial mesenchymal stem cell seeded scaffolds in a rat model. Acta Biomater 2015;13:286–294. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Bainton L, Upmalis DH, Citron JT, VanGessel A. Comparison of endometrial growth produced by unopposed conjugated estrogens or by micronized estradiol in postmenopausal women. Am J Obstet Gynecol 1997;176:112–117. [DOI] [PubMed] [Google Scholar]
- FDA. Urogynecologic surgical mesh: update on the safety and effectiveness of transvaginal placement for pelvic organ prolapse 2011. http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf.
- Forte A, Schettino MT, Finicelli M, Cipollaro M, Colacurci N, Cobellis L, Galderisi U. Expression pattern of stemness-related genes in human endometrial and endometriotic tissues. Mol Med 2009;15:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaafar T, Osman O, Osman A, Attia W, Hamza H, El Hawary R. Gene expression profiling of endometrium versus bone marrow-derived mesenchymal stem cells: upregulation of cytokine genes. Mol Cell Biochem 2014;395:29–43. [DOI] [PubMed] [Google Scholar]
- Gaide Chevronnay HP, Galant C, Lemoine P, Courtoy PJ, Marbaix E, Henriet P. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 2009;150:5094–5105. [DOI] [PubMed] [Google Scholar]
- Gargett CE. Identification and characterisation of human endometrial stem/progenitor cells. Aust N Z J Obstet Gynaecol 2006;46:250–253. [DOI] [PubMed] [Google Scholar]
- Gargett CE. Uterine stem cells: what is the evidence? Hum Reprod Update 2007;13:87–101. [DOI] [PubMed] [Google Scholar]
- Gargett BE, Chan RW. Endometrial stem/progenitor cells and proliferative disorders of the endometrium. Minerva Ginecol 2006;58:511–526. [PubMed] [Google Scholar]
- Gargett CE, Healy DL. Generating receptive endometrium in Asherman's syndrome. J Hum Reprod Sci 2011;4:49–52. [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Masuda H. Adult stem cells in the endometrium. Mol Hum Reprod 2010;16:818–834. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Ye L. Endometrial reconstruction from stem cells. Fertil Steril 2012;98:11–20. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Chan RW, Schwab KE. Endometrial stem cells. Curr Opin Obstet Gynecol 2007;19:377–383. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod 2009;80:1136–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gargett CE, Nguyen HP, Ye L. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord 2012;13:235–251. [DOI] [PubMed] [Google Scholar]
- Gargett CE, Schwab KE, Brosens JJ, Puttemans P, Benagiano G, Brosens I. Potential role of endometrial stem/progenitor cells in the pathogenesis of early-onset endometriosis. Mol Hum Reprod 2014;20:591–598. [DOI] [PubMed] [Google Scholar]
- Gil-Sanchis C, Cervelló I, Mas A, Faus A, Pellicer A, Simón C. Leucine-rich repeat-containing G-protein-coupled receptor 5 (Lgr5) as a putative human endometrial stem cell marker. Mol Hum Reprod 2013;19:407–414. [DOI] [PubMed] [Google Scholar]
- Giudice LC, Kao LC. Endometriosis. Lancet 2004;364:1789–1799. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, Marks DF, DeMaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP. Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat Med 1997;3:1337–1345. [DOI] [PubMed] [Google Scholar]
- Götte M, Wolf M, Staebler A, Buchweitz O, Kelsch R, Schüring AN, Kiesel L. Increased expression of the adult stem cell marker Musashi-1 in endometriosis and endometrial carcinoma. J Pathol 2008;215:317–329. [DOI] [PubMed] [Google Scholar]
- Greaves E, Cousins FL, Murray A, Esnal-Zufiaurre A, Fassbender A, Horne AW, Saunders PT. A novel mouse model of endometriosis mimics human phenotype and reveals insights into the inflammatory contribution of shed endometrium. Am J Pathol 2014;184:1930–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grümmer R. Animal models in endometriosis research. Hum Reprod Update 2006;12:641–649. [DOI] [PubMed] [Google Scholar]
- Guo C, Zhu H, Huang W, Li S, Qu W, Liu Y, Tan A. Side population cells in the human decidua of early pregnancy exhibit stem/progenitor cell-like characteristics. Reprod Biomed Online 2010;21:783–793. [DOI] [PubMed] [Google Scholar]
- Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME. A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int 2002;62:1285–1290. [DOI] [PubMed] [Google Scholar]
- Gurung S, Deane JA, Masuda H, Maruyama T, Gargett CE. Stem cells in endometrial physiology. Semin Reprod Med 2015;33:326–332. [DOI] [PubMed] [Google Scholar]
- Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol 1984;64:151–154. [PubMed] [Google Scholar]
- Han X, Meng X, Yin Z, Rogers A, Zhong J, Rillema P, Jackson JA, Ichim TE, Minev B, Carrier E et al. Inhibition of intracranial glioma growth by endometrial regenerative cells. Cell Cycle 2009;8:606–610. [DOI] [PubMed] [Google Scholar]
- Haniffa MA, Collin MP, Buckley CD, Dazzi F. Mesenchymal stem cells: the fibroblasts’ new clothes? Haematologica 2009;94:258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy 2012;14:516–521. [DOI] [PubMed] [Google Scholar]
- Hida N, Nishiyama N, Miyoshi S, Kira S, Segawa K, Uyama T, Mori T, Miyado K, Ikegami Y, Cui C et al. Novel cardiac precursor-like cells from human menstrual blood-derived mesenchymal cells. Stem Cells 2008;26:1695–1704. [DOI] [PubMed] [Google Scholar]
- Hu FF, Jing X, Cui YG, Qian XQ, Mao YD, Liao LM, Liu JY. Isolation and characterization of side population cells in the postpartum murine endometrium. Reprod Sci 2010;17:629–642. [DOI] [PubMed] [Google Scholar]
- Hu X, Zhou Y, Zheng X, Tian N, Xu C, Wu W, Li F, Zhu S, Zheng Y, Xue E et al. Differentiation of menstrual blood-derived stem cells toward nucleus pulposus-like cells in a coculture system with nucleus pulposus cells. Spine 2014;39:754–760. [DOI] [PubMed] [Google Scholar]
- Huang CC, Orvis GD, Wang Y, Behringer RR. Stromal-to-epithelial transition during postpartum endometrial regeneration. PLoS ONE 2012;7:e44285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunsberger J, Harrysson O, Shirwaiker R, Starly B, Wysk R, Cohen P, Allickson J, Yoo J, Atala A. Manufacturing road map for tissue engineering and regenerative medicine technologies. Stem Cells Transl Med 2015;4:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami Y, Miyoshi S, Nishiyama N, Hida N, Okamoto K, Miyado K, Segawa K, Ogawa S, Umezawa A. Serum-independent cardiomyogenic transdifferentiation in human endometrium-derived mesenchymal cells. Artif Organs 2010;34:280–288. [DOI] [PubMed] [Google Scholar]
- Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, Inoue M. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol 2009;201:608.e1–608.e8. [DOI] [PubMed] [Google Scholar]
- Ivanova-Todorova E, Mourdjeva M, Kyurkchiev D, Bochev I, Stoyanova E, Dimitrov R, Timeva T, Yunakova M, Bukarev D, Shterev A et al. HLA-G expression is up-regulated by progesterone in mesenchymal stem cells. Am J Reprod Immunol 2009;62:25–33. [DOI] [PubMed] [Google Scholar]
- Jabbour HN, Kelly RW, Fraser HM, Critchley HO. Endocrine regulation of menstruation. Endocr Rev 2006;27:17–46. [DOI] [PubMed] [Google Scholar]
- Janzen DM, Cheng D, Schafenacker AM, Paik DY, Goldstein AS, Witte ON, Jaroszewicz A, Pellegrini M, Memarzadeh S. Estrogen and progesterone together expand murine endometrial epithelial progenitor cells. Stem Cells 2013;31:808–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimbo H, Hitomi Y, Yoshikawa H, Yano T, Momoeda M, Sakamoto A, Tsutsumi O, Taketani Y, Esumi H. Evidence for monoclonal expansion of epithelial cells in ovarian endometrial cysts. Am J Pathol 1997;150:1173–1178. [PMC free article] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Ye L, Gargett CE. Reepithelialization of the uterine surface arises from endometrial glands: evidence from a functional mouse model of breakdown and repair. Endocrinology 2010;151:3386–3395. [DOI] [PubMed] [Google Scholar]
- Kaitu'u-Lino TJ, Ye L, Salamonsen LA, Girling JE, Gargett CE. Identification of label-retaining perivascular cells in a mouse model of endometrial decidualization, breakdown, and repair. Biol Reprod 2012;86:184. [DOI] [PubMed] [Google Scholar]
- Kao AP, Wang KH, Chang CC, Lee JN, Long CY, Chen HS, Tsai CF, Hsieh TH, Tsai EM. Comparative study of human eutopic and ectopic endometrial mesenchymal stem cells and the development of an in vivo endometriotic invasion model. Fertil Steril 2011;95:1308–1315.e1. [DOI] [PubMed] [Google Scholar]
- Kassmer SH, Krause DS. Detection of bone marrow-derived lung epithelial cells. Exp Hematol 2010;38:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Yoshimoto M, Kato K, Adachi S, Yamayoshi A, Arima T, Asanoma K, Kyo S, Nakahata T, Wake N. Characterization of side-population cells in human normal endometrium. Hum Reprod 2007;22:1214–1223. [DOI] [PubMed] [Google Scholar]
- Kazemnejad S, Akhondi MM, Soleimani M, Zarnani AH, Khanmohammadi M, Darzi S, Alimoghadam K. Characterization and chondrogenic differentiation of menstrual blood-derived stem cells on a nanofibrous scaffold. Int J Artif Organs 2012;35:55–66. [DOI] [PubMed] [Google Scholar]
- Khanjani S, Khanmohammadi M, Zarnani AH, Akhondi MM, Ahani A, Ghaempanah Z, Naderi MM, Eghtesad S, Kazemnejad S. Comparative evaluation of differentiation potential of menstrual blood versus bone marrow-derived stem cells into hepatocyte-like cells. PLoS ONE 2014;9:e86075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanmohammadi M, Khanjani S, Edalatkhah H, Zarnani AH, Heidari-Vala H, Soleimani M, Alimoghaddam K, Kazemnejad S. Modified protocol for improvement of differentiation potential of menstrual blood-derived stem cells into adipogenic lineage. Cell Prolif 2014;47:615–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury M, Alcayaga-Miranda F, Illanes SE, Figueroa FE. The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front Immunol 2014;5:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilic S, Yuksel B, Pinarli F, Albayrak A, Boztok B, Delibasi T. Effect of stem cell application on Asherman syndrome, an experimental rat model. J Assist Reprod Genet 2014;31:975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koks CA, Groothuis PG, Dunselman GA, de Goeij AF, Evers JL. Adhesion of shed menstrual tissue in an in-vitro model using amnion and peritoneum: a light and electron microscopic study. Hum Reprod 1999;14:816–822. [DOI] [PubMed] [Google Scholar]
- Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol 2005;33:328–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera M. Mesenchymal stromal cell ‘licensing’: a multistep process. Leukemia 2011;25:1408–1414. [DOI] [PubMed] [Google Scholar]
- Krause DS, Theise ND, Collector MI, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis SJ. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell 2001;105:369–377. [DOI] [PubMed] [Google Scholar]
- Krusche CA, Kroll T, Beier HM, Classen-Linke I. Expression of leucine-rich repeat-containing G-protein-coupled receptors in the human cyclic endometrium. Fertil Steril 2007;87:1428–1437. [DOI] [PubMed] [Google Scholar]
- Kyurkchiev S, Shterev A, Dimitrov R. Assessment of presence and characteristics of multipotent stromal cells in human endometrium and decidua. Reprod Biomed Online 2010;20:305–313. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 2012;12:383–396. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod 2002;17:2725–2736. [DOI] [PubMed] [Google Scholar]
- Leyendecker G, Wildt L, Mall G. The pathophysiology of endometriosis and adenomyosis: tissue injury and repair. Arch Gynecol Obstet 2009;280:529–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HY, Chen YJ, Chen SJ, Kao CL, Tseng LM, Lo WL, Chang CM, Yang DM, Ku HH, Twu NF et al. Induction of insulin-producing cells derived from endometrial mesenchymal stem-like cells. J Pharmacol Exp Ther 2010;335:817–829. [DOI] [PubMed] [Google Scholar]
- Li Y, Li X, Zhao H, Feng R, Zhang X, Tai D, An G, Wen J, Tan J. Efficient induction of pluripotent stem cells from menstrual blood. Stem Cells Dev 2013;22:1147–1158. [DOI] [PubMed] [Google Scholar]
- Lin J, Xiang D, Zhang JL, Allickson J, Xiang C. Plasticity of human menstrual blood stem cells derived from the endometrium. J Zhejiang Univ Sci B 2011;12:372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Huang Y, Zhang J, Qin W, Chi H, Chen J, Yu Z, Chen C. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem Cells Dev 2014;23:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril 2005;84:16–21. [DOI] [PubMed] [Google Scholar]
- Lv FJ, Tuan RS, Cheung KM, Leung VY. Concise review: the surface markers and identity of human mesenchymal stem cells. Stem Cells 2014a;32:1408–1419. [DOI] [PubMed] [Google Scholar]
- Lv Y, Xu X, Zhang B, Zhou G, Li H, Du C, Han H, Wang H. Endometrial regenerative cells as a novel cell therapy attenuate experimental colitis in mice. J Transl Med 2014b;12:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Masuda H, Ono M, Kajitani T, Yoshimura Y. Human uterine stem/progenitor cells: their possible role in uterine physiology and pathology. Reproduction 2010;140:11–22. [DOI] [PubMed] [Google Scholar]
- Masuda H, Kalka C, Takahashi T, Yoshida M, Wada M, Kobori M, Itoh R, Iwaguro H, Eguchi M, Iwami Y et al. Estrogen-mediated endothelial progenitor cell biology and kinetics for physiological postnatal vasculogenesis. Circ Res 2007a;101:598–606. [DOI] [PubMed] [Google Scholar]
- Masuda H, Maruyama T, Hiratsu E, Yamane J, Iwanami A, Nagashima T, Ono M, Miyoshi H, Okano HJ, Ito M et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Natl Acad Sci USA 2007b;104:1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Matsuzaki Y, Hiratsu E, Ono M, Nagashima T, Kajitani T, Arase T, Oda H, Uchida H, Asada H et al. Stem cell-like properties of the endometrial side population: implication in endometrial regeneration. PLoS ONE 2010;5:e10387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Anwar SS, Bühring HJ, Rao JR, Gargett CE. A novel marker of human endometrial mesenchymal stem-like cells. Cell Transplant 2012;21:2201–2214. [DOI] [PubMed] [Google Scholar]
- Meng X, Ichim TE, Zhong J, Rogers A, Yin Z, Jackson J, Wang H, Ge W, Bogin V, Chan KW et al. Endometrial regenerative cells: a novel stem cell population. J Transl Med 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miernik K, Karasinski J. Porcine uterus contains a population of mesenchymal stem cells. Reproduction 2012;143:203–209. [DOI] [PubMed] [Google Scholar]
- Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, Palmblad J. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod 2008;23:139–143. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Maruyama T. Partial regeneration and reconstruction of the rat uterus through recellularization of a decellularized uterine matrix. Biomaterials 2014;35:8791–8800. [DOI] [PubMed] [Google Scholar]
- Miyazaki K, Maruyama T, Masuda H, Yamasaki A, Uchida S, Oda H, Uchida H, Yoshimura Y. Stem cell-like differentiation potentials of endometrial side population cells as revealed by a newly developed in vivo endometrial stem cell assay. PLoS ONE 2012;7:e50749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moggio A, Pittatore G, Cassoni P, Marchino GL, Revelli A, Bussolati B. Sorafenib inhibits growth, migration, and angiogenic potential of ectopic endometrial mesenchymal stem cells derived from patients with endometriosis. Fertil Steril 2012;98:1521–1530 e1522. [DOI] [PubMed] [Google Scholar]
- Morelli SS, Rameshwar P, Goldsmith LT. Experimental evidence for bone marrow as a source of nonhematopoietic endometrial stromal and epithelial compartment cells in a murine model. Biol Reprod 2013;89:7. [DOI] [PubMed] [Google Scholar]
- Murakami K, Lee YH, Lucas ES, Chan YW, Durairaj RP, Takeda S, Moore JD, Tan BK, Quenby S, Chan JK et al. Decidualization induces a secretome switch in perivascular niche cells of the human endometrium. Endocrinology 2014;155:4542–4553. [DOI] [PubMed] [Google Scholar]
- Musina RA, Belyavski AV, Tarusova OV, Solovyova EV, Sukhikh GT. Endometrial mesenchymal stem cells isolated from the menstrual blood. Bull Exp Biol Med 2008;145:539–543. [DOI] [PubMed] [Google Scholar]
- Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe Asherman's syndrome. J Hum Reprod Sci 2011;4:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HP, Sprung CN, Gargett CE. Differential expression of Wnt signaling molecules between pre- and postmenopausal endometrial epithelial cells suggests a population of putative epithelial stem/progenitor cells reside in the basalis layer. Endocrinology 2012;153:2870–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nygaard I, Barber MD, Burgio KL, Kenton K, Meikle S, Schaffer J, Spino C, Whitehead WE, Wu J, Brody DJ et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA 2008;300:1311–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NG et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet 2012;44:1355–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 1997;89:501–506. [DOI] [PubMed] [Google Scholar]
- Padykula HA. Regeneration in the primate uterus: the role of stem cells. Ann N Y Acad Sci 1991;622:47–56. [DOI] [PubMed] [Google Scholar]
- Padykula HA, Coles LG, McCracken JA, King NW Jr, Longcope C, Kaiserman-Abramof IR. A zonal pattern of cell proliferation and differentiation in the rhesus endometrium during the estrogen surge. Biol Reprod 1984;31:1103–1118. [DOI] [PubMed] [Google Scholar]
- Panayotidis C, Weyers S, Bosteels J, van Herendael B. Intrauterine adhesions (IUA): has there been progress in understanding and treatment over the last 20 years? Gynecol Surg 2009;6:197–211. [Google Scholar]
- Park JH, Daheron L, Kantarci S, Lee BS, Teixeira JM. Human endometrial cells express elevated levels of pluripotent factors and are more amenable to reprogramming into induced pluripotent stem cells. Endocrinology 2011;152:1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AN, Park E, Kuzman M, Benetti F, Silva FJ, Allickson JG. Multipotent menstrual blood stromal stem cells: isolation, characterization, and differentiation. Cell Transplant 2008;17:303–311. [DOI] [PubMed] [Google Scholar]
- Patterson AL, Pru JK. Long-term label retaining cells localize to distinct regions within the female reproductive epithelium. Cell Cycle 2013;12:2888–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AL, Zhang L, Arango NA, Teixeira J, Pru JK. Mesenchymal-to-epithelial transition contributes to endometrial regeneration following natural and artificial decidualization. Stem Cells Dev 2013;22:964–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peron JP, Jazedje T, Brandão WN, Perin PM, Maluf M, Evangelista LP, Halpern S, Nisenbaum MG, Czeresnia CE, Zatz M et al. Human endometrial-derived mesenchymal stem cells suppress inflammation in the central nervous system of EAE mice. Stem Cell Rev 2012;8:940–952. [DOI] [PubMed] [Google Scholar]
- Petersen BE, Bowen WC, Patrene KD, Mars WM, Sullivan AK, Murase N, Boggs SS, Greenberger JS, Goff JP. Bone marrow as a potential source of hepatic oval cells. Science 1999;284:1168–1170. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Sensebé L. Mesenchymal stromal cells: misconceptions and evolving concepts. Cytotherapy 2013;15:140–145. [DOI] [PubMed] [Google Scholar]
- Rahimi M, Mohseni-Kouchesfehani H, Zarnani AH, Mobini S, Nikoo S, Kazemnejad S. Evaluation of menstrual blood stem cells seeded in biocompatible Bombyx mori silk fibroin scaffold for cardiac tissue engineering. J Biomater Appl 2014;29:199–208. [DOI] [PubMed] [Google Scholar]
- Rajaraman G, White J, Tan KS, Ulrich D, Rosamilia A, Werkmeister J, Gargett CE. Optimization and scale-up culture of human endometrial multipotent mesenchymal stromal cells: potential for clinical application. Tissue Eng Part C Methods 2013;19:80–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignoli F, Caselli A, Grisendi G, Piccinno S, Burns JS, Murgia A, Veronesi E, Loschi P, Masini C, Conte P et al. Isolation, characterization, and transduction of endometrial decidual tissue multipotent mesenchymal stromal/stem cells from menstrual blood. Biomed Res Int 2013;2013:901821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacchetti B, Funari A, Michienzi S, Di Cesare S, Piersanti S, Saggio I, Tagliafico E, Ferrari S, Robey PG, Riminucci M et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 2007;131:324–336. [DOI] [PubMed] [Google Scholar]
- Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:442–469. [Google Scholar]
- Santamaria X, Massasa EE, Feng Y, Wolff E, Taylor HS. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther 2011;19:2065–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci 2008;1127:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüring AN, Schulte N, Kelsch R, Röpke A, Kiesel L, Götte M. Characterization of endometrial mesenchymal stem-like cells obtained by endometrial biopsy during routine diagnostics. Fertil Steril 2011;95:423–426. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Gargett CE. Co-expression of two perivascular cell markers isolates mesenchymal stem-like cells from human endometrium. Hum Reprod 2007;22:2903–2911. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Chan RW, Gargett CE. Putative stem cell activity of human endometrial epithelial and stromal cells during the menstrual cycle. Fertil Steril 2005;84(Suppl. 2):1124–1130. [DOI] [PubMed] [Google Scholar]
- Schwab KE, Hutchinson P, Gargett CE. Identification of surface markers for prospective isolation of human endometrial stromal colony-forming cells. Hum Reprod 2008;23:934–943. [DOI] [PubMed] [Google Scholar]
- Shaer A, Azarpira N, Vahdati A, Karimi MH, Shariati M. miR-375 induces human decidua basalis-derived stromal cells to become insulin-producing cells. Cell Mol Biol Lett 2014;19:483–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 2003;18:696–704. [DOI] [PubMed] [Google Scholar]
- Silveira CG, Abrão MS, Dias JA Jr, Coudry RA, Soares FA, Drigo SA, Domingues MA, Rogatto SR. Common chromosomal imbalances and stemness-related protein expression markers in endometriotic lesions from different anatomical sites: the potential role of stem cells. Hum Reprod 2012;27:3187–3197. [DOI] [PubMed] [Google Scholar]
- Singh N, Mohanty S, Seth T, Shankar M, Bhaskaran S, Dharmendra S. Autologous stem cell transplantation in refractory Asherman's syndrome: a novel cell based therapy. J Hum Reprod Sci 2014;7:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivasubramaniyan K, Harichandan A, Schumann S, Sobiesiak M, Lengerke C, Maurer A, Kalbacher H, Bühring HJ. Prospective isolation of mesenchymal stem cells from human bone marrow using novel antibodies directed against Sushi domain containing 2. Stem Cells Dev 2013;22:1944–1954. [DOI] [PubMed] [Google Scholar]
- Smith FJ, Holman CD, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstet Gynecol 2010;116:1096–1100. [DOI] [PubMed] [Google Scholar]
- Sobiesiak M, Sivasubramaniyan K, Hermann C, Tan C, Örgel M, Treml S, Cerabona F, de Zwart P, Ochs U, Müller CA et al. The mesenchymal stem cell antigen MSCA-1 is identical to tissue non-specific alkaline phosphatase. Stem Cells Dev 2010;19:669–677. [DOI] [PubMed] [Google Scholar]
- Song Y, Xiao L, Fu J, Huang W, Wang Q, Zhang X, Yang S. Increased expression of the pluripotency markers sex-determining region Y-box 2 and Nanog homeobox in ovarian endometriosis. Reprod Biol Endocrinol 2014;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song T, Zhao X, Sun H, Li X, Lin N, Ding L, Dai J, Hu Y. Regeneration of uterine horns in rats using collagen scaffolds loaded with human embryonic stem cell-derived endometrium-like cells. Tissue Eng Part A 2015;21:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer TE, Hayashi K, Hu J, Carpenter KD. Comparative developmental biology of the mammalian uterus. Curr Top Dev Biol 2005;68:85–122. [DOI] [PubMed] [Google Scholar]
- Spitzer TL, Rojas A, Zelenko Z, Aghajanova L, Erikson DW, Barragan F, Meyer M, Tamaresis JS, Hamilton AE, Irwin JC et al. Perivascular human endometrial mesenchymal stem cells express pathways relevant to self-renewal, lineage specification, and functional phenotype. Biol Reprod 2012;86:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starzinski-Powitz A, Zeitvogel A, Schreiner A, Baumann R. In search of pathogenic mechanisms in endometriosis: the challenge for molecular cell biology. Curr Mol Med 2001;1:655–664. [DOI] [PubMed] [Google Scholar]
- Su K, Edwards SL, Tan KS, White JF, Kandel S, Ramshaw JA, Gargett CE, Werkmeister JA. Induction of endometrial mesenchymal stem cells into tissue-forming cells suitable for fascial repair. Acta Biomater 2014;10:5012–5020. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Hamatani T, Yamada M, Ogawa S, Kamijo S, Kuji N, Akutsu H, Miyado K, Yoshimura Y, Umezawa A. Derivation of human decidua-like cells from amnion and menstrual blood. Sci Rep 2014;4:4599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Jackson L, Dey SK, Daikoku T. In pursuit of leucine-rich repeat-containing G protein-coupled receptor-5 regulation and function in the uterus. Endocrinology 2009;150:5065–5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Zhang Y, Brunt KR, Wu J, Li SH, Fazel S, Weisel RD, Keating A, Li RK. An adult uterine hemangioblast: evidence for extramedullary self-renewal and clonal bilineage potential. Blood 2010;116:2932–2941. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- Tamura M, Fukaya T, Murakami T, Uehara S, Yajima A. Analysis of clonality in human endometriotic cysts based on evaluation of X chromosome inactivation in archival formalin-fixed, paraffin-embedded tissue. Lab Invest 1998;78:213–218. [PubMed] [Google Scholar]
- Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004;292:81–85. [DOI] [PubMed] [Google Scholar]
- Trounson A. The production and directed differentiation of human embryonic stem cells. Endocr Rev 2006;27:208–219. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Yoshimoto M, Takahashi K, Noda Y, Nakahata T, Heike T. Side population cells contribute to the genesis of human endometrium. Fertil Steril 2008;90:1528–1537. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Edwards SL, White JF, Supit T, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE. A preclinical evaluation of alternative synthetic biomaterials for fascial defect repair using a rat abdominal hernia model. PLoS ONE 2012;7:e50044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Muralitharan R, Gargett CE. Toward the use of endometrial and menstrual blood mesenchymal stem cells for cell-based therapies. Expert Opin Biol Ther 2013;13:1387–1400. [DOI] [PubMed] [Google Scholar]
- Ulrich D, Edwards SL, Su K, Tan KS, White JF, Ramshaw JA, Lo C, Rosamilia A, Werkmeister JA, Gargett CE. Human endometrial mesenchymal stem cells modulate the tissue response and mechanical behavior of polyamide mesh implants for pelvic organ prolapse repair. Tissue Eng Part A 2014a;20:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Edwards SL, Su K, White JF, Ramshaw JA, Jenkin G, Deprest J, Rosamilia A, Werkmeister JA, Gargett CE. Influence of reproductive status on tissue composition and biomechanical properties of ovine vagina. PLoS ONE 2014b;9:e93172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich D, Tan KS, Deane J, Schwab K, Cheong A, Rosamilia A, Gargett CE. Mesenchymal stem/stromal cells in post-menopausal endometrium. Hum Reprod 2014c;29:1895–1905. [DOI] [PubMed] [Google Scholar]
- Valentijn AJ, Palial K, Al-Lamee H, Tempest N, Drury J, Von Zglinicki T, Saretzki G, Murray P, Gargett CE, Hapangama DK. SSEA-1 isolates human endometrial basal glandular epithelial cells: phenotypic and functional characterization and implications in the pathogenesis of endometriosis. Hum Reprod 2013;28:2695–2708. [DOI] [PubMed] [Google Scholar]
- Viganó P, Parazzini F, Somigliana E, Vercellini P. Endometriosis: epidemiology and aetiological factors. Best Pract Res Clin Obstet Gynaecol 2004;18:177–200. [DOI] [PubMed] [Google Scholar]
- von Bahr L, Batsis I, Moll G, Hägg M, Szakos A, Sundberg B, Uzunel M, Ringden O, Le Blanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells 2012;30:1575–1578. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell 2004;116:639–648. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science 2002;297:2256–2259. [DOI] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature 2003;422:897–901. [DOI] [PubMed] [Google Scholar]
- Wang H, Jin P, Sabatino M, Ren J, Civini S, Bogin V, Ichim TE, Stroncek DF. Comparison of endometrial regenerative cells and bone marrow stromal cells. J Transl Med 2012a;10:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chen S, Zhang C, Stegeman S, Pfaff-Amesse T, Zhang Y, Zhang W, Amesse L, Chen Y. Human endometrial stromal stem cells differentiate into megakaryocytes with the ability to produce functional platelets. PLoS ONE 2012b;7:e44300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sacchetti A, van Dijk MR, van der Zee M, van der Horst PH, Joosten R, Burger CW, Grootegoed JA, Blok LJ, Fodde R. Identification of quiescent, stem-like cells in the distal female reproductive tract. PLoS ONE 2012c;7:e40691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Shen L, Huang W, Song Y, Xiao L, Xu W, Liu Y. Vasculogenesis of decidua side population cells of first-trimester pregnancy. Stem Cell Res Ther 2013;4:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N, Hong S, Tan J, Ke P, Liang L, Fei H, Liu B, Liu L, Liu Y, Yu B. A red fluorescent nude mouse model of human endometriosis: advantages of a non-invasive imaging method. Eur J Obstet Gynecol Reprod Biol 2014;176:25–30. [DOI] [PubMed] [Google Scholar]
- Wells CA, Mosbergen R, Korn O, Choi J, Seidenman N, Matigian NA, Vitale AM, Shepherd J. Stemformatics: visualisation and sharing of stem cell gene expression. Stem Cell Res 2013;10:387–395. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Wolff AB, Hongling D, Taylor HS. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci 2007;14:524–533. [DOI] [PubMed] [Google Scholar]
- Wolff EF, Gao XB, Yao KV, Andrews ZB, Du H, Elsworth JD, Taylor HS. Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med 2011;15:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff EF, Uchida N, Donahue RE, Metzger ME, Hsieh MM, Libfraind LL, Hill MJ, Tisdale JF. Peripheral blood stem cell transplants do not result in endometrial stromal engraftment. Fertil Steril 2013;99:526–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright AJ, Andrews PW. Surface marker antigens in the characterization of human embryonic stem cells. Stem Cell Res 2009;3:3–11. [DOI] [PubMed] [Google Scholar]
- Wu Y, Basir Z, Kajdacsy-Balla A, Strawn E, Macias V, Montgomery K, Guo SW. Resolution of clonal origins for endometriotic lesions using laser capture microdissection and the human androgen receptor (HUMARA) assay. Fertil Steril 2003;79(Suppl. 1):710–717. [DOI] [PubMed] [Google Scholar]
- Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014a;123:1201–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Luo Y, Chen J, Pan R, Xiang B, Du X, Xiang L, Shao J, Xiang C. Transplantation of human menstrual blood progenitor cells improves hyperglycemia by promoting endogenous progenitor differentiation in type 1 diabetic mice. Stem Cells Dev 2014b;23:1245–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XY, Wang W, Li X. In vitro hepatic differentiation of human endometrial stromal stem cells. In Vitro Cell Dev Biol Anim 2014;50:162–170. [DOI] [PubMed] [Google Scholar]
- Ye L, Mayberry R, Lo CY, Britt KL, Stanley EG, Elefanty AG, Gargett CE. Generation of human female reproductive tract epithelium from human embryonic stem cells. PLoS ONE 2011;6:e21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Evans J, Gargett CE. Lim1/LIM1 is expressed in developing and adult mouse and human endometrium. Histochem Cell Biol 2012;137:527–536. [DOI] [PubMed] [Google Scholar]
- Yu D, Wong YM, Cheong Y, Xia E, Li TC. Asherman syndrome—one century later. Fertil Steril 2008;89:759–779. [DOI] [PubMed] [Google Scholar]
- Zhong Z, Patel AN, Ichim TE, Riordan NH, Wang H, Min WP, Woods EJ, Reid M, Mansilla E, Marin GH et al. Feasibility investigation of allogeneic endometrial regenerative cells. J Transl Med 2009;7:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gan Y, Taylor HS. Cigarette smoke inhibits recruitment of bone-marrow-derived stem cells to the uterus. Reprod Toxicol 2011;31:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöller M. CD44: can a cancer-initiating cell profit from an abundantly expressed molecule? Nat Rev Cancer 2011;11:254–267. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell 2002;13:4279–4295. [DOI] [PMC free article] [PubMed] [Google Scholar]