Abstract
Biomarkers can be important predictors of disease severity and progression. The intense exposure to particulates and other toxins from the destruction of the World Trade Center (WTC) overwhelmed the lung’s normal protective barriers. The Fire Department of New York (FDNY) cohort not only had baseline pre-exposure lung function measures but also had serum samples banked soon after their WTC exposure. This well phenotyped group of highly exposed first responders is an ideal cohort for biomarker discovery and eventual validation. Disease progression was heterogeneous in this group in that some individuals subsequently developed abnormal lung function while others recovered. Airflow obstruction predominated in WTC exposed patients who were symptomatic. Multiple independent disease pathways may cause this abnormal FEV1 after irritant exposure. WTC exposure activates one or more of these pathways causing abnormal FEV1 in an individual. Our hypothesis was that serum biomarkers expressed within 6 months after World Trade Center (WTC) exposure reflect active disease pathways and predict subsequent development or protection from abnormal FEV1<lower limit of normal (LLN) known as WTC-LI. We utilized a nested case-cohort control design of previously healthy never smokers who sought subspecialty pulmonary evaluation to explore predictive biomarkers of WTC-LI. We have identified biomarkers of Inflammation, metabolic derangement, protease/antiprotease balance and vascular injury expressed in serum within 6 months of WTC exposure that were predictive of their FEV1 up to 7 years after their WTC exposure. Predicting future risk of airway injury after particulate exposures can focus monitoring and early treatment on a subset of patients in greatest need of these services.
Keywords: World Trade Center, Lung Injury, Predictive Biomarkers, Obstructive Airway Disease
Occupational Exposure and the Development of Obstructive Airways Disease (OAD)
There is mounting evidence that occupational exposures are a cause of OAD.1 Occupational exposures are varied but often consist of particulate matter (PM) and other toxins. Epidemiologic evidence links PM exposure to the development of vascular and pulmonary diseases. 2-4 The development of OAD from PM induced inflammation and smoke is poorly understood,5 but studies have shown that exposure to high ambient PM significantly decreases forced expiratory volume over 1 second (FEV1) after 5-7 days.6,7 Long-term exposure to air pollutants from motor vehicles and other sources impairs lung function.8-12 Upper respiratory symptoms increase by 3% per 10 mcg/m3 of PM exposure.6,13
WTC Particulate Matter Exposure
The destruction of the WTC led to the release of an estimated 10 million tons of material, exposing over 300,000 rescue workers and New York City (NYC) residents and local workers to WTC particulate matter (WTC-PM).14-16 The concentrations of airborne and respirable WTC-PM ranged from 1-100 mg/m3 immediately after the WTC collapse.17-19 One month after the event, PM2.5 (≤ 2.5 microns) concentration was 196 mcg/m3, three times the National Ambient Air Quality Standards 24 hour standard.20 The toxicology and physical properties of WTC-PM have been well described. 18,21 WTC-PM is primarily composed of pulverized concrete, plastics and other hydrocarbons. WTC-PM was found to be highly alkaline; pH 9-11. 18,22 Exposure to both fine (PM2.5) and coarse (PM53,, >53 microns) PM has been implicated in the development of lung injury. 23,24
Many rescue and recovery workers as well as residents and local workers continued to be exposed to dust for at least three months.25 Induced sputum of NYC firefighters who were exposed to WTC-PM post 9/11 showed increased amounts of PM (1-50μm), neutrophils, and eosinophils even 10 months after the original exposure.26 In a case series of symptomatic local residents and community workers that underwent lung biopsy several years after 2001, emphysematous change, and small airway abnormalities, and rare cases of interstitial fibrosis were seen. Furthermore, opaque and birefringent particles were found within the macrophages of all cases, and particulate analysis showed these particles to be similar in composition to WTC particulates.27
WTC Associated Pulmonary Function and Radiographic Phenotype
Respiratory compromise has been documented in FDNY rescue workers19,28-32, other exposed workers 33 and lower Manhattan residents.34-36 These affected WTC exposed individuals continue to have increased symptoms, medication usage, pulmonary disability and lower quality of life 13 years after exposure.19,20,22,24,28-32,37-76 Thus far, the physiologic and radiographic phenotype associated with WTC exposure has been heterogeneous in nature, Table 1. Many exposed individuals have bronchial wall thickening and air trapping on CT. Emphysema, COPD, and OAD occur, but some studies have shown that there is a significant restrictive phenotype seen.
Table 1.
Overview of the Spirometric and Radiographic Findings in the WTC Exposed Cohorts
| Source | Phenotype | Study Cohort | Findings | References |
|---|---|---|---|---|
| PFT | OAD | FDNY, Iron workers, NYPD, rescue-recovery workers and volunteers |
Decreased FEV1, Decreased FEV1/FVC |
39,41- 44,46,47,50,51,61,67, 164-168 |
| BD response | FDNY | Reversible abnormality on post-BD spirometry* |
67 | |
| MCT | FDNY | Bronchial hyperreactivity† | 31,67 | |
| Pseudo-restrictive | FDNY, iron workers | FEV1/FVC<LLN, FVC<LLN, TLC≥LLN | 33,169 | |
| Restrictive | FDNY, NYPD, Community workers | 24,27,81,170 | ||
| Small Airways | FDNY, Community workers | FVC<LLN, FEV1/FVC and TLC≥LLN | 67,81,171 | |
| Radiographic | Emphysema/COPD | FDNY, Community Workers | 38,67 | |
| BWT | FDNY | 67,81 | ||
| Air trapping | FDNY, NYPD, Technical and Construction Workers |
67,81,172 |
Abbreviations: PFT: pulmonary function test; OAD: obstructive airways disease; BD: bronchodilator; BWT: Bronchial wall thickening; FDNY: Fire Department of New York; FEV1: forced expiratory volume over 1 second; FVC: forced vital capacity; LLN: Lower limit of normal; TLC: Total lung capacity
post-BD FEV1≥12% change from pre-BD and improvement of FEV1≥200mL
Methacholine PC20≤8mg/mL, <16mg/mL
The Utility of Biomarkers of Occupational Lung Function Loss
Identification of biomarkers of disease progression is crucial to guide early intervention as well as treatment. In addition, identification of biomarkers of disease has potential to direct future research into mechanisms producing airflow obstruction. These biomarkers can also identify those at risk who would most benefit from avoidance of further exposure or aggressive management. Measuring biomarkers in serum is a powerful and cost effective approach for risk factor discovery. Immediately after 9/11, the FDNY-Bureau of Health Services (BHS) began implementing protocols for obtaining, processing, storing and retrieving serum from the WTC-exposed firefighters. This cohort of nearly 16,000 Fire Department of New York (FDNY) rescue workers (firefighters, paramedics and emergency medical technicians) form a well-characterized cohort that has been a powerful resource for documenting the impact of WTC exposure on the lung, Figure 1. Serum samples were obtained on a majority of the cohort from September, 2001 to February, 2002, at initial development of airflow obstruction. Taking advantage of this unique cohort, our group has investigated predictive biomarkers of WTC-associated lung injury.77-79 We have identified biomarker subtypes that may be indicators of associated pathways active in the development of WTC associated end-organ dysfunction. This paper is dedicated to reviewing our findings and their physiologic importance.
Figure 1. WTC-FDNY Biomarker Timeline and development of Nested Case- Cohort Control Study Design.
Summary of phenotyping that was done on the cohort and case susceptible and resistant to WTC-LI are shown in the inclusion criteria to develop the baseline cohort. Overlapping of cohort control with cases susceptible and resistant to WTC-LI is highlighted in the dashed red circle.
For biomarker discovery, we chose to focus on FEV1 as outcome and indicator of disease state. Spirometry had been measured annually in the FDNY cohort three years prior to 9/11/2001, and this cohort continued to receive annual pulmonary function tests (PFTs) as part of the FDNY-WTC-Medical Monitoring and Treatment Program (MMTP). This longitudinal surveillance allowed us to document that in the first year after 9/11, there was a decline in FEV1 in exposed FDNY rescue workers at a rate twelve times greater than that found pre-9/11.29,80 Further evaluation showed that firefighters who had never smoked lost an average of 439 mL of FEV1 in the first year post-9/11, followed by a mean annualized reduction in FEV1 of 26 mL per year in the subsequent 6.5 years.19,66 In addition, acute airway inflammation, reactive airway dysfunction, and overall decline in FEV1 have been reported in rescue workers exposed to WTC-PM.31,67
Our study cohort demonstrated a reduced FEV1 below the lower limits of normal (LLN) was consistent with an obstructive pattern67,81 with a low FEV1/FVC ratio (median 72, IQR 65-77). This suggested that FEV1<LLN could be used as a surrogate for obstruction in this population. We therefore defined WTC-Lung Injury (WTC-LI) in this population of firefighters by their FEV1<LLN measured during subspecialty visit after exposure and upon becoming symptomatic. Measurement of FEV1 is robust and easily measurable. Furthermore, FEV1 has been the primary endpoint in trials of therapeutic agents and has been used to track health outcomes in numerous lung diseases, including chronic obstructive pulmonary disease (COPD). 82
Design of the FDNY-WTC Biomarker Study
At their first FDNY medical monitoring exam (MME) post-9/11, all participants received PFT and serum aliquots were collected and deposited in a biorepository, Figure 1. Symptomatic firefighters were referred to subspecialty pulmonary evaluation between 9/12/2001 and 3/10/2008.83
Nested Case-Cohort Control Study Design
We used a nested case-cohort control strategy to define biomarker expression, Figure 1.84-87 The baseline cohort was derived from 1,720 exposed workers who needed subspecialty pulmonary evaluation and treatment within 6.5 years of 9/11. There was significant inter-individual variation in lung function with some patients recovering and others suffering loss of lung function thereby allowing us to study both outcomes in a similarly exposed cohort.46,88 A homogeneous sub-cohort of subjects without prior lung disease or tobacco use was identified after applying inclusion criteria (n=801). The two case subgroups (n=100 for each) were intended to over-sample those susceptible and resistant to WTC-LI. Those susceptible to WTC-LI had FEV1<LLN and included patients in the bottom octile of FEV1% predicted at the time of sub-specialty pulmonary exam. In contrast, the resistant population included those identified in the top octile of FEV1% predicted at the same time point, and had FEV1≥LLN. The cohort (n=801) was stratified on tertiles of FEV1 and BMI, and the cohort control (n=171) was randomly selected. This cohort control has overlapping populations with the susceptible and resistant populations, and ensures sufficient representation of the general FDNY population. All subjects signed informed Institutional Review Board-approved consent at the time of enrollment allowing analysis of their information and samples for research (Montefiore Medical Center; #07-09-320 and New York University; #11-00439). Serum samples were analyzed utilizing Luminex as previously described. 40,50 Both case definitions and cohort-controls were randomized to batches in a 1:1:2 ratio to avoid batch effect bias and analyzed contemporaneously to avoid variability in time dependent sample decay. 85,89
All demographics were obtained from the FDNY-WTC-monitoring database. Degree of exposure was self-reported at the first FDNY-WTC-monitoring and was categorized using the FDNY-WTC Exposure Intensity Index based on Arrival Time: 1. Present on the morning of 9/11/2001 2. Arrived between the afternoon of 9/11/2001 and 9/12/2001.67 Those arriving after day three were excluded from analysis as a result of their low numbers in this sample.49,90
Serum Biomarkers of WTC-LI
Biomarkers of Inflammation
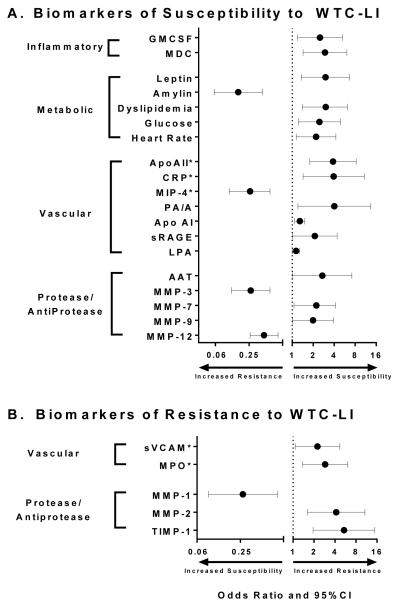
We hypothesized that individuals susceptible to airflow obstruction, induced by environmental irritants, would express different levels of pro-inflammatory cytokines compared to similarly exposed individuals in the cohort-control., We identified inflammatory cytokines and chemokines in the serum of FDNY rescue workers obtained within six months of 9/11 using commercially available multiplex kits, Figure 1. We correlated inflammatory biomarkers at monitoring entry with FEV1 at sub-specialty pulmonary exam. Cases of those susceptible to WTC-LI with FEV1<LLN were compared to controls with FEV1≥LLN. From monitoring entry to sub-specialty pulmonary exam years later, FEV1 declined 12% in cases and increased 3% in controls. Elevated Granulocyte Macrophage-Colony Stimulating Factor (GM-CSF) and Macrophage Derived Chemokine (MDC) increased the risk for subsequent FEV1<LLN by 2.5 fold and 3.0 fold in a logistic regression model adjusted for exposure, BMI, age on 9/11, and neutrophil, Figure 2A. Roles for GM-CSF and MDC in airway injury are biologically plausible in that human bronchial epithelial cells produce GM-CSF in response to PM and MDC is elevated in bronchoalveolar lavage of asthmatics. The model had sensitivity of 38% and a specificity of 88%. The low sensitivity suggested other biomarkers not yet identified were significant risk factors for accelerated decline of lung function after irritant exposure at the WTC site.
Figure 2. Overview of Biomarkers predicting WTC-LI as defined by FEV1 < LLN.
A. Utilizing a Susceptible case-cohort control design B. Utilizing a resistant case-cohort control design. *Represents relative risk †Not previously published
Metabolic Biomarkers as Predictors of WTC Lung Function Loss
Metabolic syndrome (MetSyn) is a principal contributor to systemic inflammation associated end organ damage. 91 This interaction is best understood in vascular diseases, but previous cross sectional studies have suggested associations of impaired lung function with MetSyn. 92-96
Utilizing our case-cohort control study design, we diagnosed MetSyn using World Health Organization (WHO)-modified National Heart Lung and Blood Institute/American Heart Association criteria. Subjects had to meet three of the following five criteria: i. Elevated triglycerides ≥150 mg/dL (1.7mmol/L). ii. Reduced High Density Lipoprotein (HDL)<40 mg/dL in men iii. Elevated systolic blood pressure (BP) ≥130 mm Hg or diastolic BP ≥85 mm Hg iv. Elevated fasting glucose ≥100 mg/dL.97,98 v. Body Mass Index (BMI) >30 was substituted for increased waist circumference as per WHO Criteria. 99 Concurrent fasting blood levels of HDL, Glucose, and triglycerides were assayed when the subjects’ first post-9/11 serum samples were collected and banked at monitoring entry.
We found that cases of WTC-LI had significantly higher glucose and heart rate at monitoring entry than controls. However, cases did not have significantly different triglycerides, systolic blood pressure, or HDL. There was a trend of a higher percentage of individuals with MetSyn in cases compared to controls (27% vs 16%, p=0.07). Cases had a larger proportion of individuals with higher glucose (28% vs 16%, p=0.03) and lower HDL (32% vs 20%, p=0.05). The differences in lipid profiles between cases and controls were accentuated when individuals had combined abnormalities in triglycerides and HDL (dyslipidemia, defined as triglycerides≥150mg/dL and HDL<40mg/dL) (28% vs 14%, p=0.006). In addition, we defined a heart rate above the median of 66 beats per minute (bpm) as elevated for the entire nonsmoking cohort. Cases compared to controls had a larger proportion with elevated heart rate (65% vs 48% p=0.02). We then defined cut points for metabolic analytes using the top quartile for leptin and amylin.
We assessed if any of the biomarkers with significantly different prevalence altered the odds ratio (OR) of being a case using logistic models adjusted for BMI, age on 9/11, race and WTC arrival time. When biomarkers and clinical parameters were included in the final model, dyslipidemia, elevated heart rate and elevated leptin significantly increased the odds of being a case: Dyslipidemia, OR=3.03 , heart rate≥66 bpm, OR=2.20 and leptin ≥ 10,300 pg/mL OR=3.00, Figure 2A. Elevated amylin was strongly protective, decreasing the odds of being a case by 84%, Figure 2A. We assessed the ability of the final logistic regression model to predict case status using receiver operating characteristic (ROC) analysis. The final model had an area under the curve (AUC) of 0.774 after adjusting for previously mentioned covariates.
Biologically active lipid metabolites are biologically plausible pathways of disease and can be attenuated pharmacologically. As a logical extension of our earlier work we therefore turned our attention to Lysophosphatidic acid (LPA), an LDL derivative and Apolipoprotein (Apo)A1, a component of HDL. LPA activates pathways involved in vascular injury.100-103 Vascular injury occurs early in smoking related COPD with pulmonary perfusion abnormalities and reduced blood return to the heart observed prior to development of abnormal FEV1.104,105 Similar pathophysiology likely occurs in irritant induced COPD. Pulmonary arteriopathy was present in 58% of lung biopsies from non-FDNY WTC-PM exposed individuals and in 74% with constrictive bronchiolitis after inhalational exposures suffered during military service in Iraq and Afghanistan.27,106.
To assess the relationship between LPA and ApoA1 with the outcome of being a susceptible case, we used a multivariable logistic model (adjusted for BMI, exposure intensity, pre-9/11 FEV1% predicted, age on 9/11, race, WTC exposure, dyslipidemia, platelet and neutrophil count). In the adjusted model, a 10 μM increase of LPA was associated with a 14% increase in the odds of having WTC-LI while an increase of 1 mg/mL of ApoA1 increased the odds of developing WTC-LI by 29%. These findings further demonstrate the biological relevance of lipids and their metabolites in the progression of OAD after WTC exposures.
Vascular Biomarkers of WTC-LI
Recent studies associate systemic vascular involvement with lung disease.107,108 Prospective studies have demonstrated an association between systemic inflammation, impaired lung function, and central arterial stiffness that occurs prior to the development of cardiovascular disease (CVD).109-111 Similarly, perfusion abnormalities and reduced pulmonary blood flow occur prior to development of abnormal FEV1 in smokers at risk for COPD. Lung biopsies from WTC-PM exposed individuals showed pulmonary arteriopathy and constrictive bronchiolitis secondary to inhalational exposures, and hinted at the potential use of vascular biomarkers associated with WTC-LI.27,106
Vascular biomarkers were investigated in subjects that went on to develop WTC-LI and in those that did not. Individuals with elevated Apolipoprotein (Apo)AII and C-reactive protein (CRP) levels within six months of 9/11 had significantly increased risk of developing decreased lung function over the subsequent six years while elevated Macrophage Inflammatory Protein (MIP-4) reduced the risk of susceptibility to decreased lung function, Figure 2A. Alternately, firefighters with elevated soluble Vascular Cell Adhesion Molecule (sVCAM) and low myeloperoxidase (MPO) levels within six months of 9/11/2001 predicted recovery of lung function, and showed return of FEV1 to pre-9/11 values after an acute decline post-9/11, Figure 2B.
The biologic plausibility of our findings is significantly supported by the literature. CRP is a known marker of acute systemic inflammation and CVD that has been shown to have an inverse relationship with FEV1.112-114 CRP levels were elevated in individuals with COPD independent of any CVD risks.115 Pulmonary hypertension, a disease of the pulmonary vasculature, is associated with CVD biomarkers such as apolipoprotein.116 This parallels our prior observation that dyslipidemia predicts poor outcome after WTC dust exposure.
Macrophage inflammatory protein-4 (MIP-4) was inversely associated with odds of developing WTC-LI. This finding contrasts with that of the Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) cohort, in which elevated MIP-4 was associated with concurrent COPD, increased risk of cardiovascular hospitalization and mortality.117 The difference in the association of of MIP-4 may be due to the timing of the biomarker studies; our cohort had blood biomarkers prior to disease presentation, whereas the ECLIPSE study focused on end-stage disease. MIP-4 (CCL18, PARC) is an early promoter of regulatory T-cell differentiation and may generate an anti-inflammatory counter-regulatory response that leads to protection of lung injury in our group.118,119 Finally, low levels of MPO demonstrate less neutrophil activation, an important mediator of PM-induced pulmonary and cardiovascular injury. In our group, it was associated with resistance to the damaging effects of WTC-PM exposure.120,121 Our data and other recent reports emphasize the need to better understand the mechanisms by which inhaled irritants damage pulmonary vessels.
In addition to serum biomarkers of vascular involvement, our group and others have investigated quantifiable vasculometric changes associated with OAD. An increased ratio of the pulmonary artery to aorta (PA/A) diameter measured by computed tomography (CT) has been associated with pulmonary hypertension and poor outcomes in various disease states. The elevated PA/A ratio implies relative pulmonary vascular enlargement and has been associated with past and future exacerbations in patients with moderate-to-severe COPD.122 PA/A has been associated with a decreased FEV1 in the same population. Furthermore, in subjects with CVD a PA/A ratio >1 has been associated with increased mortality.123 PA/A from chest CT scans obtained for clinical indications in our case cohort control population were calculated. Using the Youden index, a PA/A value of 0.92 was selected as the cutoff for best predicting the development of WTC-LI in a logistic regression analysis. After adjusting for age at CT, pre-9/11 FEV1, BMI at sub-specialty pulmonary evaluation and exposure, the odds of having World Trade Center Lung Injury in patients with a value of PA/A ≥0.92 was 4.02, Figure 2.
In the ECLIPSE/COPD Gene cohort, PA/A >1 was associated with disease severity and subjects had more advanced disease than in the FDNY-WTC cohort.122 Although our cohort’s mean PA and A were similar to those measured in ECLIPSE/COPD Gene cohort, 81% of our cohort did not meet GOLD COPD criteria. Therefore, it was expected that the PA/A would be less than previously reported ratios of 1.122 When comparing our cohort to the Framingham Heart Study, our case mean PA/A and PA values are similar to their 90th% upper limit of normal. 124 Our study suggests that the PA/A ratio≥0.92 may represent a marker of early vascular injury in particulate matter related lung disease and is in line with these recent publications.
Protease/ Antiprotease Balance as Biomarkers of WTC-LI
The balance of protease/anti-protease activity is the crux of many diseases including cigarette-induced chronic lung disease and other causes of accelerated lung function decline.125-128 The serine antiprotease α-1 Antitrypsin (AAT) predicts accelerated FEV1 decline in WTC exposed firefighters and is a well-studied biomarker of smoking associated lung disease.129 To determine the impact of AAT levels on FEV1 decline after WTC exposure, AAT levels and genotype were assayed were assayed in 90 randomly selected subjects. The rate of FEV1 loss increased with increasing AAT deficiency defined either by genotype or serum concentration. Moderately (MS or SZ genotype) deficient rescue workers’ FEV1 declined 110cm3/year more than normal, while mildly (MS or SS genotype) deficient rescue workers lost 32cm3/year more than normal. Rescue workers with AAT serum levels below 20 μmol/l lost 49 cm3/year of FEV1 compared to workers with AAT levels greater than or equal to 20 μmol/l. There was no impact of AAT levels or genotype on spirometric decline rates prior to 09/11/2001 suggesting an interaction between low AAT and WTC exposure.130 In data previously unreported, utilizing our case-cohort control study we found that AAT <85 mg/dL increased the risk of developing WTC-LI, Figure 2A.131-133
Matrix metalloproteinases (MMPs) can catabolize and degrade the extracellular matrix. 134 Many are known intermediates of each other, and their levels are affected by environmental factors such as hypoxia, inflammation and oxidative stress. Several MMPs can be either directly inhibited by or form complexes with TIMPs. MMP/TIMP balance is another well-defined mediator of COPD. 134
Genetic association studies with MMPs demonstrate a strong association with the development of lung disease.125 MMP-1 is induced in smokers with COPD and its overexpression in mice causes emphysema.135,136 The destructive effects of MMPs are inhibited by tissue inhibitors of matrix metalloproteinases (TIMPs). There is little data on the role of MMPs and TIMPs in the resistance to the damaging effect of dust exposure.137-140 One carefully done pathologic study demonstrated increased MMP-2 and TIMP-1 mRNA expression in surgically removed lung and predicted improved FEV1 in COPD patients.141 As serum MMP and TIMP expression is related to the development of COPD, the link between serum MMP/TIMP balance and lung function in the WTC exposed cohort became the focus of our next set of investigations.142
In investigating susceptible cases, elevated serum levels of MMP-3 and MMP-12 reduce the risk of developing WTC-LI. Increased time between 9/11 and blood draw is associated with a diminished protective effect. Specifically, early elevated expression of MMP-3 and MMP-12 in serum within 200 days after WTC exposure predicts protected lung function over the subsequent seven years (2001-2008). We found that MMP-3 and MMP-12 ranges of the cohort were comparable to other published patient populations, including healthy controls and patient cohorts with emphysema or rheumatoid arthritis.143,144 MMP-3 was found to be more protective than MMP-12, Figure 2A. Both biomarker models displayed robust predictive ability by logistic regression with AUC>0.8.
Compared to susceptible cases, the resistant cases had greater than average reduction in FEV1 immediately after exposure, but returned to pre-exposure FEV1 over the next 6.5 years. Because serum was drawn well before the pulmonary function test that demonstrated recovery, the biomarker information reflected evolving injury. All subjects in this nested case control investigation had heavy WTC dust exposure and arrived at the collapse site within 2 days of 9/11/2001. MMP-2 and TIMP-1 expression above the 75th percentile are protective biomarkers, significantly increasing the odds of resistance between 4.2 and 5.4 fold. Alternately, elevated MMP-1 is a risk factor, reducing the odds of resistance by 73%, Figure 2B. The biomarker model using serum MMP-1, MMP-2 and TIMP-1 concentration predicted resistance with a sensitivity of 74%, a specificity of 86% and a receiver operator characteristic of 0.90. 40
Innate and Humoral Mediators
The ratio of FEV1/Forced Vital Capacity (FVC) is another well-validated spirometric measure of airflow obstruction. Genome wide association studies (GWAS) observed that a set of genetic variants of chitinases are associated with only FEV1. Polymorphisms at other loci can predict FEV1/FVC and both FEV1 and FEV1/FVC.145,146 Biomarkers predicting abnormal FEV1/FVC may therefore be distinct from those predicting abnormal FEV1. Hence, we investigated if biomarkers expressed within 6 months of 9/11/2001 predicted future abnormal FEV1/FVC in this WTC exposed cohort. In this investigation, we evaluated innate and humoral mediators. We found that increased serum chitotriosidase (CHIT) reduces the odds of developing obstruction after WTC-particulate matter exposure and is associated with recovery of lung function. Alternately, elevated Immunoglobulin E (IgE) was a risk factor for airflow obstruction and progressive lung function decline.
CHIT belongs to the glycosyl hydrolase 18 gene family that binds and cleaves chitin. CHIT is part of the innate host defense against bacterial and fungal infections since chitin is a major structural component in bacteria, fungi, insects and crustaceans.147-151 CHIT is produced in mature monocyte-derived macrophages, lung macrophages and other specific subsets of tissue macrophages. 152-155 Elevated chitotriosidase expression is associated with smoking induced and fibrotic lung disease. 156,157 However, its biological function has not been clearly defined.
IgE-mediated humoral immunity is another important immune response mechanism in the respiratory tract. 158 Elevated IgE is a key immune mediator in asthma. Children and adults with asthma have higher IgE than normal controls and anti-IgE antibody is an effective asthma modulator. 159-162 Elevated serum IgE is strongly associated with low FEV1/FVC in patients with chronic obstructive lung disease. 163
SUMMARY
Identification of serum biomarkers that predict lung disease can direct future research into mechanisms producing airflow obstruction, fuel future work about their downstream effects, aid in the development of diagnostic markers and potential therapeutic targets in clinical trials. Our study cohort, with clinical information before and after a significant environmental exposure, serves as a unique opportunity to identify biomarkers associated and predictive of lung and vascular disease. Although many studies have focused on occupational biomarker identification, our study cohort has lung function assessment prior to exposure and development of disease. This is ideal for the study of predictive biomarkers, and has allowed us to establish leadership in pathophysiologic investigation of WTC-LI.77-79
We have developed a high-dimensional dataset that includes at least 130 serum and radiographic biomarkers in a cohort of firefighters with intense exposure to World Trade Center dust. We have identified multiple biomarkers of biologically plausible pathways that are active in the development of WTC-associated end-organ dysfunction. It was necessary to develop a parsimonious biomarker model that would generate reproducible and generalizable findings. To optimize identification of candidate biomarkers from a relatively small sample size we utilized a case cohort control for much of our work. Furthermore, the small sample size in a high-dimensional biomarker dataset could obscure potential pathways of interest, we studied biomarkers in separate but often related pathways to further optimize signal to noise. Future work will include repeated serum sampling to add a longitudinal component to the FDNY-NYU biorepository. This will expand our understanding of biomarker evolution and will be a resource for investigating other long latency WTC related diseases.
Development of OAD following particulate matter exposure is a major health concern worldwide. First responders and the military may be specifically more affected by the high amounts of particulate exposure during disasters and conflict. Identifying predictive biomarkers of FEV1 loss allows for early identification of at risk individuals allowing for early screening, necessary treatment and risk avoidance. While unique in many ways, the exposures on 9/11/2001 have allowed us to make observations related to lung disease progression that may translate to other occupationally and environmentally exposed populations. The processes initiated by WTC exposure impacted multiple distinct injury and repair pathways. Our prior studies and this review emphasize the utility of serum stored in the aftermath of a disaster. The insight into protein expression in OAD gained from the analysis of this serum has the potential to guide future mechanistic and therapeutic studies designed to blunt the impact of the worldwide COPD epidemic.
Acknowledgments
This work was supported by NIH-NHLBI K23HL084191(AN), NIAID (MDW) K24A1080298, NIH-R01HL057879 (MDW), and NIOSH (U10-OH008243, U10-OH008242) and UL1RR029893 (DJP). This work was also partially funded by the NYU-HHC
Clinical and Translational Science Institute is supported in part by grant UL1TR000038 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
The funding agencies did not participate in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
ABBREVIATIONS
- AT
Air Trapping
- AAT
Alpha-1 Antitrypsin
- Apo
Apolipoprotein
- AUC
Area under the curve
- BHS
Bureau of Health Services
- BMI
Body Mass Index
- BP
Blood Pressure
- BPM
Beats per Minute
- BWT
Bronchial Wall Thickening
- CHIT
Chitotriosidase
- COPD
chronic obstructive pulmonary disease
- CVD
Cardiovascular Disease
- DLCO
Diffusion Capacity of the Lung for Carbon Monoxide
- Dyslipidemia
HDL<40 mg/dL and Triglycerides≥150 mg/dL
- ECLIPSE
Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints
- FDNY
Fire Department of New York
- FEV1
Forced Expiratory Volume in one second
- FVC
Forced Vital Capacity
- GM-CSF
Granulocyte Macrophage-Colony Stimulating Factor
- GOLD
Global Initiative for Chronic Obstructive Lung disease
- GWAS
Genome wide association studies
- HDL
High Density Lipoprotein
- IgE
Immunoglobulin E
- IL-6
Interleukin-6
- LLN
Lower Limit of Normal as defined by NHANES III
- LPA
Lysophosphatidic acid
- MDC
Macrophage Derived Chemokine
- MetSyn
Metabolic Syndrome
- MME
Medical Monitoring Exam
- MIP-4
Macrophage Inflammatory Protein
- MMP
Matrix metalloproteinases
- MMTP
Medical Monitoring Treatment Program
- MPO
Myeoloperoxidase
- NAAQS
National Ambient Air Quality Standards
- NHANES
National
- NYC
New York City
- NYPD
New York Police Department
- NYU
New York University
- OAD
Obstructive Airways Disease
- OR
Odds Ratio
- PA/A
Pulmonary Artery/Aorta
- PFT
Pulmonary Function Test
- PM
Particulate Matter
- sRAGE
soluble Receptor for Advanced Glycation End Products
- ROC
Receiver Operator Characteristics
- sVCAM
soluble Vascular Cell Adhesion Molecule
- TIMP
tissue inhibitors of matrix metalloproteinases
- TLC
Total Lung Capacity
- TNF-α
Tumor Necrosis Factor-alpha
- WHO
World Health Organization
- WTC
World Trade Center
- WTC-LI
World Trade Center-Lung Injury
- WTC-PM
World Trade Center-Particulate Matter
Footnotes
Conflict of Interest
There are no conflicts of interest.
References
- 1.Blanc PD, Eisner MD, Earnest G, et al. Further exploration of the links between occupational exposure and chronic obstructive pulmonary disease. J Occup Environ Med. 2009;51:804–10. doi: 10.1097/JOM.0b013e3181a7dd4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987-1994. N Engl J Med. 2000;343:1742–9. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- 3.Thurston GD, Ito K, Hayes CG, Bates DV, Lippmann M. Respiratory hospital admissions and summertime haze air pollution in Toronto, Ontario: consideration of the role of acid aerosols. Environ Res. 1994;65:271–90. doi: 10.1006/enrs.1994.1037. [DOI] [PubMed] [Google Scholar]
- 4.Dockery DW, Pope CA, 3rd, Xu X, et al. An association between air pollution and mortality in six U.S. cities. N Engl J Med. 1993;329:1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 5.Barnes PJ. Chronic obstructive pulmonary disease: effects beyond the lungs. PLoS Med. 2010;7:e1000220. doi: 10.1371/journal.pmed.1000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hoek G, Brunekreef B. Acute effects of a winter air pollution episode on pulmonary function and respiratory symptoms of children. Arch Environ Health. 1993;48:328–35. doi: 10.1080/00039896.1993.9936721. [DOI] [PubMed] [Google Scholar]
- 7.Brunekreef B, Kinney PL, Ware JH, et al. Sensitive subgroups and normal variation in pulmonary function response to air pollution episodes. Environ Health Perspect. 1991;90:189–93. doi: 10.1289/ehp.90-1519500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann-Liebrich U, Leuenberger P, Schwartz J, et al. Lung function and long term exposure to air pollutants in Switzerland. Study on Air Pollution and Lung Diseases in Adults (SAPALDIA) Team. Am J Respir Crit Care Med. 1997;155:122–9. doi: 10.1164/ajrccm.155.1.9001300. [DOI] [PubMed] [Google Scholar]
- 9.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–42. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 10.Franco Suglia S, Gryparis A, Schwartz J, Wright RJ. Association between traffic-related black carbon exposure and lung function among urban women. Environ Health Perspect. 2008;116:1333–7. doi: 10.1289/ehp.11223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotschi T, Heinrich J, Sunyer J, Kunzli N. Long-term effects of ambient air pollution on lung function: a review. Epidemiology. 2008;19:690–701. doi: 10.1097/EDE.0b013e318181650f. [DOI] [PubMed] [Google Scholar]
- 12.Kan H, Heiss G, Rose KM, Whitsel E, Lurmann F, London SJ. Traffic exposure and lung function in adults: the Atherosclerosis Risk in Communities study. Thorax. 2007;62:873–9. doi: 10.1136/thx.2006.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostro BD, Lipsett MJ, Mann JK, Krupnick A, Harrington W. Air pollution and respiratory morbidity among adults in southern California. Am J Epidemiol. 1993;137:691–700. doi: 10.1093/oxfordjournals.aje.a116729. [DOI] [PubMed] [Google Scholar]
- 14.Landrigan PJ. Health consequences of the 11 September 2001 attacks. Environ Health Perspect. 2001;109:A514–5. doi: 10.1289/ehp.109-a514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldrich TK, Gustave J, Hall CB, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 362:1263–72. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Claudio L. Environmental aftermath. Environ Health Perspect. 2001;109:A528–36. doi: 10.1289/ehp.109-a528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen LC, Thurston G. World Trade Center cough. Lancet. 2002;360(Suppl):s37–8. doi: 10.1016/s0140-6736(02)11814-9. [DOI] [PubMed] [Google Scholar]
- 18.McGee JK, Chen LC, Cohen MD, et al. Chemical analysis of World Trade Center fine particulate matter for use in toxicologic assessment. Environ Health Perspect. 2003;111:972–80. doi: 10.1289/ehp.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prezant DJ, Weiden M, Banauch GI, et al. Cough and bronchial responsiveness in firefighters at the World Trade Center site. N Engl J Med. 2002;347:806–15. doi: 10.1056/NEJMoa021300. [DOI] [PubMed] [Google Scholar]
- 20.Rom WN, Reibman J, Rogers L, et al. Emerging exposures and respiratory health: World Trade Center dust. Proc Am Thorac Soc. 2010;7:142–5. doi: 10.1513/pats.200908-092RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lioy PJ, Weisel CP, Millette JR, et al. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110:703–14. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lioy PJ, Pellizzari E, Prezant D. The World Trade Center aftermath and its effects on health: understanding and learning through human-exposure science. Environ Sci Technol. 2006;40:6876–85. doi: 10.1021/es062980e. [DOI] [PubMed] [Google Scholar]
- 23.Gavett SH, United States. Environmental Protection Agency. Office of Research and Development. National Health and Environmental Effects Research Laboratory (U.S.) Toxicological effects of fine particulate matter derived from the destruction of the World Trade Center. National Health and Environmental Effects Research Laboratory, Office of Research and Development, U.S. Environmental Protection Agency; Research Triangle Park, N.C.: 2002. [Google Scholar]
- 24.Rom WN, Weiden M, Garcia R, et al. Acute eosinophilic pneumonia in a New York City firefighter exposed to World Trade Center dust. Am J Respir Crit Care Med. 2002;166:797–800. doi: 10.1164/rccm.200206-576OC. [DOI] [PubMed] [Google Scholar]
- 25.Landrigan PJ, Lioy PJ, Thurston G, et al. Health and environmental consequences of the world trade center disaster. Environ Health Perspect. 2004;112:731–9. doi: 10.1289/ehp.6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fireman EM, Lerman Y, Ganor E, et al. Induced sputum assessment in New York City firefighters exposed to World Trade Center dust. Environ Health Perspect. 2004;112:1564–9. doi: 10.1289/ehp.7233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caplan-Shaw CE, Yee H, Rogers L, et al. Lung pathologic findings in a local residential and working community exposed to World Trade Center dust, gas, and fumes. J Occup Environ Med. 2011;53:981–91. doi: 10.1097/JOM.0b013e31822fff60. [DOI] [PubMed] [Google Scholar]
- 28.Banauch GI, Dhala A, Alleyne D, et al. Bronchial hyperreactivity and other inhalation lung injuries in rescue/recovery workers after the World Trade Center collapse. Crit Care Med. 2005;33:S102–6. doi: 10.1097/01.ccm.0000151138.10586.3a. [DOI] [PubMed] [Google Scholar]
- 29.Banauch GI, Hall C, Weiden M, et al. Pulmonary function after exposure to the World Trade Center collapse in the New York City Fire Department. Am J Respir Crit Care Med. 2006;174:312–9. doi: 10.1164/rccm.200511-1736OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feldman DM, Baron SL, Bernard BP, et al. Symptoms, respirator use, and pulmonary function changes among New York City firefighters responding to the World Trade Center disaster. Chest. 2004;125:1256–64. doi: 10.1378/chest.125.4.1256. [DOI] [PubMed] [Google Scholar]
- 31.Banauch GI, Alleyne D, Sanchez R, et al. Persistent hyperreactivity and reactive airway dysfunction in firefighters at the World Trade Center. Am J Respir Crit Care Med. 2003;168:54–62. doi: 10.1164/rccm.200211-1329OC. [DOI] [PubMed] [Google Scholar]
- 32.Banauch GI, Dhala A, Prezant DJ. Pulmonary disease in rescue workers at the World Trade Center site. Curr Opin Pulm Med. 2005;11:160–8. doi: 10.1097/01.mcp.0000151716.96241.0a. [DOI] [PubMed] [Google Scholar]
- 33.Herbert R, Moline J, Skloot G, et al. The World Trade Center disaster and the health of workers: five-year assessment of a unique medical screening program. Environ Health Perspect. 2006;114:1853–8. doi: 10.1289/ehp.9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reibman J, Lin S, Hwang SA, et al. The World Trade Center residents' respiratory health study: new-onset respiratory symptoms and pulmonary function. Environ Health Perspect. 2005;113:406–11. doi: 10.1289/ehp.7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.From the Centers for Disease Control and Prevention Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center--Manhattan, New York, 2001. Jama. 2002;288:1466–7. [PubMed] [Google Scholar]
- 36.Self-reported increase in asthma severity after the September 11 attacks on the World Trade Center--Manhattaan, New York, 2001. MMWR Morb Mortal Wkly Rep. 2002;51:781–4. [PubMed] [Google Scholar]
- 37.Glaser MS, Webber MP, Zeig-Owens R, et al. Estimating the time interval between exposure to the world trade center disaster and incident diagnoses of obstructive airway disease. Am J Epidemiol. 2014;180:272–9. doi: 10.1093/aje/kwu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niles JK, Webber MP, Liu X, et al. The upper respiratory pyramid: Early factors and later treatment utilization in World Trade Center exposed firefighters. Am J Ind Med. 2014;57:857–65. doi: 10.1002/ajim.22326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsukiji J, Cho SJ, Echevarria GC, et al. Lysophosphatidic acid and apolipoprotein A1 predict increased risk of developing World Trade Center-lung injury: a nested case-control study. Biomarkers. 2014;19:159–65. doi: 10.3109/1354750X.2014.891047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nolan A, Kwon S, Cho SJ, et al. MMP-2 and TIMP-1 predict healing of WTC-lung injury in New York City firefighters. Respir Res. 2014;15:5. doi: 10.1186/1465-9921-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho SJ, Echevarria GC, Kwon S, et al. One airway: Biomarkers of protection from upper and lower airway injury after World Trade Center exposure. Respir Med. 2014;108:162–70. doi: 10.1016/j.rmed.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kwon S, Weiden MD, Echevarria GC, et al. Early Elevation of Serum MMP-3 and MMP-12 Predicts Protection from World Trade Center-Lung Injury in New York City Firefighters: A Nested Case-Control Study. PLoS One. 2013;8:e76099. doi: 10.1371/journal.pone.0076099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Niles JK, Webber MP, Cohen HW, et al. The respiratory pyramid: From symptoms to disease in World Trade Center exposed firefighters. Am J Ind Med. 2013;56:870–80. doi: 10.1002/ajim.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho SJ, Nolan A, Echevarria GC, et al. Chitotriosidase is a biomarker for the resistance to World Trade Center lung injury in New York City firefighters. J Clin Immunol. 2013;33:1134–42. doi: 10.1007/s10875-013-9913-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weakley J, Webber MP, Ye F, et al. Agreement between obstructive airways disease diagnoses from self-report questionnaires and medical records. Prev Med. 2013;57:38–42. doi: 10.1016/j.ypmed.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Weiden MD, Naveed B, Kwon S, et al. Cardiovascular biomarkers predict susceptibility to lung injury in World Trade Center dust-exposed firefighters. Eur Respir J. 2013;41:1023–30. doi: 10.1183/09031936.00077012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiden MD, Naveed B, Kwon S, et al. Comparison of WTC dust size on macrophage inflammatory cytokine release in vivo and in vitro. PLoS One. 2012;7:e40016. doi: 10.1371/journal.pone.0040016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soo J, Webber MP, Hall CB, et al. Pulmonary function predicting confirmed recovery from lower-respiratory symptoms in World Trade Center-exposed firefighters, 2001 to 2010. Chest. 2012;142:1244–50. doi: 10.1378/chest.11-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naveed B, Weiden MD, Kwon S, et al. Metabolic syndrome biomarkers predict lung function impairment: a nested case-control study. Am J Respir Crit Care Med. 2012;185:392–9. doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nolan A, Naveed B, Comfort AL, et al. Inflammatory biomarkers predict airflow obstruction after exposure to World Trade Center dust. Chest. 2012;142:412–8. doi: 10.1378/chest.11-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webber MP, Glaser MS, Weakley J, et al. Physician-diagnosed respiratory conditions and mental health symptoms 7-9 years following the World Trade Center disaster. Am J Ind Med. 2011;54:661–71. doi: 10.1002/ajim.20993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weakley J, Webber MP, Gustave J, et al. Trends in respiratory diagnoses and symptoms of firefighters exposed to the World Trade Center disaster: 2005-2010. Prev Med. 2011;53:364–9. doi: 10.1016/j.ypmed.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Soo J, Webber MP, Gustave J, et al. Trends in probable PTSD in firefighters exposed to the World Trade Center disaster, 2001-2010. Disaster Med Public Health Prep. 2011;5(Suppl 2):S197–203. doi: 10.1001/dmp.2011.48. [DOI] [PubMed] [Google Scholar]
- 54.Zeig-Owens R, Webber MP, Hall CB, et al. Early assessment of cancer outcomes in New York City firefighters after the 9/11 attacks: an observational cohort study. Lancet. 2011;378:898–905. doi: 10.1016/S0140-6736(11)60989-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guidotti TL, Prezant D, de la Hoz RE, Miller A. The evolving spectrum of pulmonary disease in responders to the World Trade Center tragedy. Am J Ind Med. 2011;54:649–60. doi: 10.1002/ajim.20987. [DOI] [PubMed] [Google Scholar]
- 56.Jordan HT, Stellman SD, Prezant D, Teirstein A, Osahan SS, Cone JE. Sarcoidosis diagnosed after September 11, 2001, among adults exposed to the World Trade Center disaster. J Occup Environ Med. 2011;53:966–74. doi: 10.1097/JOM.0b013e31822a3596. [DOI] [PubMed] [Google Scholar]
- 57.Niles JK, Webber MP, Gustave J, et al. The impact of the World Trade Center attack on FDNY firefighter retirement, disabilities, and pension benefits. Am J Ind Med. 2011;54:672–80. doi: 10.1002/ajim.20965. [DOI] [PubMed] [Google Scholar]
- 58.Niles JK, Webber MP, Gustave J, et al. Comorbid trends in World Trade Center cough syndrome and probable posttraumatic stress disorder in firefighters. Chest. 2011;140:1146–54. doi: 10.1378/chest.10-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Munjal KG, Silverman RA, Freese J, et al. Utilization of emergency medical services in a large urban area: description of call types and temporal trends. Prehosp Emerg Care. 2011;15:371–80. doi: 10.3109/10903127.2011.561403. [DOI] [PubMed] [Google Scholar]
- 60.Chiu S, Niles JK, Webber MP, et al. Evaluating risk factors and possible mediation effects in posttraumatic depression and posttraumatic stress disorder comorbidity. Public Health Rep. 2011;126:201–9. doi: 10.1177/003335491112600211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berninger A, Webber MP, Niles JK, et al. Longitudinal study of probable post-traumatic stress disorder in firefighters exposed to the World Trade Center disaster. Am J Ind Med. 2010;53:1177–85. doi: 10.1002/ajim.20894. [DOI] [PubMed] [Google Scholar]
- 62.Berninger A, Webber MP, Weakley J, et al. Quality of life in relation to upper and lower respiratory conditions among retired 9/11-exposed firefighters with pulmonary disability. Qual Life Res. 2010;19:1467–76. doi: 10.1007/s11136-010-9710-9. [DOI] [PubMed] [Google Scholar]
- 63.Banauch GI, Brantly M, Izbicki G, et al. Accelerated spirometric decline in New York City firefighters with alpha(1)-antitrypsin deficiency. Chest. 2010;138:1116–24. doi: 10.1378/chest.10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berninger A, Webber MP, Cohen HW, et al. Trends of elevated PTSD risk in firefighters exposed to the World Trade Center disaster: 2001-2005. Public Health Rep. 2010;125:556–66. doi: 10.1177/003335491012500411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webber MP, Lee R, Soo J, et al. Prevalence and incidence of high risk for obstructive sleep apnea in World Trade Center-exposed rescue/recovery workers. Sleep Breath. 2011;15:283–94. doi: 10.1007/s11325-010-0379-7. [DOI] [PubMed] [Google Scholar]
- 66.Aldrich TK, Gustave J, Hall CB, et al. Lung function in rescue workers at the World Trade Center after 7 years. N Engl J Med. 2010;362:1263–72. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiden MD, Ferrier N, Nolan A, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 2010;137:566–74. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Webber MP, Gustave J, Lee R, et al. Trends in respiratory symptoms of firefighters exposed to the world trade center disaster: 2001-2005. Environ Health Perspect. 2009;117:975–80. doi: 10.1289/ehp.0800291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiu S, Webber MP, Zeig-Owens R, et al. Validation of the Center for Epidemiologic Studies Depression Scale in screening for major depressive disorder among retired firefighters exposed to the World Trade Center disaster. J Affect Disord. 2010;121:212–9. doi: 10.1016/j.jad.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 70.Prezant DJ, Levin S, Kelly KJ, Aldrich TK. Upper and lower respiratory diseases after occupational and environmental disasters. Mt Sinai J Med. 2008;75:89–100. doi: 10.1002/msj.20028. [DOI] [PubMed] [Google Scholar]
- 71.Banauch GI, Izbicki G, Christodoulou V, et al. Trial of prophylactic inhaled steroids to prevent or reduce pulmonary function decline, pulmonary symptoms, and airway hyperreactivity in firefighters at the world trade center site. Disaster Med Public Health Prep. 2008;2:33–9. doi: 10.1097/DMP.0b013e318164ee0c. [DOI] [PubMed] [Google Scholar]
- 72.Prezant DJ. World Trade Center Cough Syndrome and its treatment. Lung. 2008;186(Suppl 1):S94–102. doi: 10.1007/s00408-007-9051-9. [DOI] [PubMed] [Google Scholar]
- 73.Izbicki G, Chavko R, Banauch GI, et al. World Trade Center “sarcoid-like” granulomatous pulmonary disease in New York City Fire Department rescue workers. Chest. 2007;131:1414–23. doi: 10.1378/chest.06-2114. [DOI] [PubMed] [Google Scholar]
- 74.Truncale T, Brooks S, Prezant DJ, Banauch GI, Nemery B. World Trade Center dust and airway reactivity. Am J Respir Crit Care Med. 2004;169:883–4. doi: 10.1164/ajrccm.169.7.954. author reply 4-5. [DOI] [PubMed] [Google Scholar]
- 75.Edelman P, Osterloh J, Pirkle J, et al. Biomonitoring of chemical exposure among New York City firefighters responding to the World Trade Center fire and collapse. Environ Health Perspect. 2003;111:1906–11. doi: 10.1289/ehp.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kelly KJ, Connelly E, Reinhold GA, Byrne M, Prezant DJ. Assessment of health effects in New York City firefighters after exposure to polychlorinated biphenyls (PCBs) and polychlorinated dibenzofurans (PCDFs): the Staten Island Transformer Fire Health Surveillance Project. Arch Environ Health. 2002;57:282–93. doi: 10.1080/00039890209601411. [DOI] [PubMed] [Google Scholar]
- 77.Holguin F. The metabolic syndrome as a risk factor for lung function decline. Am J Respir Crit Care Med. 2012;185:352–3. doi: 10.1164/rccm.201112-2172ED. [DOI] [PubMed] [Google Scholar]
- 78.Balmes JR. Can we predict who will develop chronic sequelae of acute inhalational injury? Chest. 2012;142:278–9. doi: 10.1378/chest.12-0126. [DOI] [PubMed] [Google Scholar]
- 79.Antao VC. The World Trade Center disaster: a tragic source of medical advancement. Eur Respir J. 2013;41:999–1001. doi: 10.1183/09031936.00181112. [DOI] [PubMed] [Google Scholar]
- 80.Naveed B, Weiden MD, Kwon S, et al. Metabolic Syndrome Biomarkers Predict Lung Function Impairment: A Nested Case-Control Study. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berger KI, Reibman J, Oppenheimer BW, Vlahos I, Harrison D, Goldring RM. Lessons from the World Trade Center disaster: airway disease presenting as restrictive dysfunction. Chest. 2013;144:249–57. doi: 10.1378/chest.12-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sin DD, Vestbo J. Biomarkers in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:543–5. doi: 10.1513/pats.200904-019DS. [DOI] [PubMed] [Google Scholar]
- 83.Weiden MD, Ferrier N, Nolan A, et al. Obstructive airways disease with air trapping among firefighters exposed to World Trade Center dust. Chest. 137:566–74. doi: 10.1378/chest.09-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rundle AG, Vineis P, Ahsan H. Design options for molecular epidemiology research within cohort studies. Cancer Epidemiol Biomarkers Prev. 2005;14:1899–907. doi: 10.1158/1055-9965.EPI-04-0860. [DOI] [PubMed] [Google Scholar]
- 85.Rosenberg L, Slone D, Shapiro S, et al. Breast cancer and alcoholic-beverage consumption. Lancet. 1982;1:267–70. doi: 10.1016/s0140-6736(82)90987-4. [DOI] [PubMed] [Google Scholar]
- 86.Van Berge-Landry HM, Bovbjerg DH, James GD. The reproducibility of ethnic differences in the proportional awake-sleep blood pressure decline among women. Am J Hum Biol. 2010;22:325–9. doi: 10.1002/ajhb.20993. [DOI] [PubMed] [Google Scholar]
- 87.Palmer IM, Schutte AE, Huisman HW. Ethnic and gender differences regarding the insulin-blood pressure relationship. Diabetes Res Clin Pract. 2009;85:102–10. doi: 10.1016/j.diabres.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 88.Naveed B, Weiden MD, Kwon S, et al. Metabolic Syndrome Biomarkers Predict Lung Function Impairment: A Nested Case-Control Study. Am J Respir Crit Care Med. 2011 doi: 10.1164/rccm.201109-1672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gude D, Naveed S. Comprehending trichotillomania. Int J Trichology. 2012;4:100–1. doi: 10.4103/0974-7753.96902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Longo KA, Govek EK, Nolan A, et al. Pharmacologic inhibition of ghrelin receptor signaling is insulin sparing and promotes insulin sensitivity. J Pharmacol Exp Ther. 2011;339:115–24. doi: 10.1124/jpet.111.183764. [DOI] [PubMed] [Google Scholar]
- 91.Chen JC, Schwartz J. Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ Health Perspect. 2008;116:612–7. doi: 10.1289/ehp.10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lazarus R, Sparrow D, Weiss ST. Baseline ventilatory function predicts the development of higher levels of fasting insulin and fasting insulin resistance index: the Normative Aging Study. Eur Respir J. 1998;12:641–5. doi: 10.1183/09031936.98.12030641. [DOI] [PubMed] [Google Scholar]
- 93.Lawlor DA, Ebrahim S, Smith GD. Associations of measures of lung function with insulin resistance and Type 2 diabetes: findings from the British Women's Heart and Health Study. Diabetologia. 2004;47:195–203. doi: 10.1007/s00125-003-1310-6. [DOI] [PubMed] [Google Scholar]
- 94.Lin WY, Yao CA, Wang HC, Huang KC. Impaired lung function is associated with obesity and metabolic syndrome in adults. Obesity (Silver Spring) 2006;14:1654–61. doi: 10.1038/oby.2006.190. [DOI] [PubMed] [Google Scholar]
- 95.Fimognari FL, Pasqualetti P, Moro L, et al. The association between metabolic syndrome and restrictive ventilatory dysfunction in older persons. J Gerontol A Biol Sci Med Sci. 2007;62:760–5. doi: 10.1093/gerona/62.7.760. [DOI] [PubMed] [Google Scholar]
- 96.Leone N, Courbon D, Thomas F, et al. Lung Function Impairment and Metabolic Syndrome: The Critical Role of Abdominal Obesity. American Journal of Respiratory and Critical Care Medicine. 2009;179:509–16. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 97.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 98.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 99.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Executive summary. Cardiol Rev. 2005;13:322–7. [PubMed] [Google Scholar]
- 100.Naveed S, Okoli K, Hollingsworth J, Kasmani R. Guillain-Barre syndrome as a paraneoplastic manifestation of small-cell carcinoma of lung. South Med J. 2010;103:156–8. doi: 10.1097/smj.0b013e3181bfd2c0. [DOI] [PubMed] [Google Scholar]
- 101.Siddiqi A, Khan DA, Khan FA, Naveed AK. Impact of CYP2C9 genetic polymorphism on warfarin dose requirements in Pakistani population. Pak J Pharm Sci. 2010;23:417–22. [PubMed] [Google Scholar]
- 102.Murph M, Mills GB. Targeting the lipids LPA and S1P and their signalling pathways to inhibit tumour progression. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000476. [DOI] [PubMed] [Google Scholar]
- 103.Smyth SS, Cheng HY, Miriyala S, Panchatcharam M, Morris AJ. Roles of lysophosphatidic acid in cardiovascular physiology and disease. Biochim Biophys Acta. 2008;1781:563–70. doi: 10.1016/j.bbalip.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodriguez-Roisin R, Drakulovic M, Rodriguez DA, Roca J, Barbera JA, Wagner PD. Ventilation-perfusion imbalance and chronic obstructive pulmonary disease staging severity. J Appl Physiol. 2009;106:1902–8. doi: 10.1152/japplphysiol.00085.2009. [DOI] [PubMed] [Google Scholar]
- 105.Liebow AA. Pulmonary emphysema with special reference to vascular changes. Am Rev Respir Dis. 1959;80:67–93. doi: 10.1164/arrd.1959.80.1P2.67. [DOI] [PubMed] [Google Scholar]
- 106.King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365:222–30. doi: 10.1056/NEJMoa1101388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taraseviciene-Stewart L, Scerbavicius R, Choe KH, et al. An animal model of autoimmune emphysema. Am J Respir Crit Care Med. 2005;171:734–42. doi: 10.1164/rccm.200409-1275OC. [DOI] [PubMed] [Google Scholar]
- 108.Voelkel N, Taraseviciene-Stewart L. Emphysema: an autoimmune vascular disease? Proc Am Thorac Soc. 2005;2:23–5. doi: 10.1513/pats.200405-033MS. [DOI] [PubMed] [Google Scholar]
- 109.Zureik M, Benetos A, Neukirch C, et al. Reduced pulmonary function is associated with central arterial stiffness in men. Am J Respir Crit Care Med. 2001;164:2181–5. doi: 10.1164/ajrccm.164.12.2107137. [DOI] [PubMed] [Google Scholar]
- 110.Tockman MS, Pearson JD, Fleg JL, et al. Rapid decline in FEV1. A new risk factor for coronary heart disease mortality. Am J Respir Crit Care Med. 1995;151:390–8. doi: 10.1164/ajrccm.151.2.7842197. [DOI] [PubMed] [Google Scholar]
- 111.Mannino DM, Thorn D, Swensen A, Holguin F. Prevalence and outcomes of diabetes, hypertension and cardiovascular disease in COPD. Eur Respir J. 2008;32:962–9. doi: 10.1183/09031936.00012408. [DOI] [PubMed] [Google Scholar]
- 112.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 113.Elbers PW, Ince C. Mechanisms of critical illness--classifying microcirculatory flow abnormalities in distributive shock. Crit Care. 2006;10:221. doi: 10.1186/cc4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cirillo DJ, Agrawal Y, Cassano PA. Lipids and pulmonary function in the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2002;155:842–8. doi: 10.1093/aje/155.9.842. [DOI] [PubMed] [Google Scholar]
- 115.Pinto-Plata VM, Mullerova H, Toso JF, et al. C-reactive protein in patients with COPD, control smokers and non-smokers. Thorax. 2006;61:23–8. doi: 10.1136/thx.2005.042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yuditskaya S, Tumblin A, Hoehn GT, et al. Proteomic identification of altered apolipoprotein patterns in pulmonary hypertension and vasculopathy of sickle cell disease. Blood. 2009;113:1122–8. doi: 10.1182/blood-2008-03-142604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sin DD, Miller BE, Duvoix A, et al. Serum PARC/CCL-18 concentrations and health outcomes in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183:1187–92. doi: 10.1164/rccm.201008-1220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vulcano M, Struyf S, Scapini P, et al. Unique regulation of CCL18 production by maturing dendritic cells. J Immunol. 2003;170:3843–9. doi: 10.4049/jimmunol.170.7.3843. [DOI] [PubMed] [Google Scholar]
- 119.Azzaoui I, Yahia SA, Chang Y, et al. CCL18 differentiates dendritic cells in tolerogenic cells able to prime regulatory T cells in healthy subjects. Blood. 2011;118:3549–58. doi: 10.1182/blood-2011-02-338780. [DOI] [PubMed] [Google Scholar]
- 120.Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. Am J Respir Crit Care Med. 1999;159:702–9. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 121.Mills NL, Tornqvist H, Gonzalez MC, et al. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N Engl J Med. 2007;357:1075–82. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 122.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367:913–21. doi: 10.1056/NEJMoa1203830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nakanishi R, Rana JS, Shalev A, et al. Mortality Risk as a Function of the Ratio of Pulmonary Trunk to Ascending Aorta Diameter In Patients With Suspected Coronary Artery Disease. Am J Cardiol. 2013 doi: 10.1016/j.amjcard.2013.01.266. [DOI] [PubMed] [Google Scholar]
- 124.Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging. 2012;5:147–54. doi: 10.1161/CIRCIMAGING.111.968610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rastogi A, Tan SH, Banerjee S, et al. ERG monoclonal antibody in the diagnosis and biological stratification of prostate cancer: delineation of minimal epitope, critical residues for binding, and molecular basis of specificity. Monoclon Antib Immunodiagn Immunother. 2014;33:201–8. doi: 10.1089/mab.2014.0026. [DOI] [PubMed] [Google Scholar]
- 126.Rosas IO, Richards TJ, Konishi K, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jones CB, Sane DC, Herrington DM. Matrix metalloproteinases: a review of their structure and role in acute coronary syndrome. Cardiovasc Res. 2003;59:812–23. doi: 10.1016/s0008-6363(03)00516-9. [DOI] [PubMed] [Google Scholar]
- 128.Death AK, Nakhla S, McGrath KC, et al. Nitroglycerin upregulates matrix metalloproteinase expression by human macrophages. J Am Coll Cardiol. 2002;39:1943–50. doi: 10.1016/s0735-1097(02)01907-1. [DOI] [PubMed] [Google Scholar]
- 129.Banauch GI, Brantly M, Izbicki G, et al. Accelerated spirometric decline in New York City firefighters with alpha-antitrypsin deficiency. Chest. 2010;138:1116–24. doi: 10.1378/chest.10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Banauch GI, Brantly M, Izbicki G, et al. Accelerated spirometric decline in New York City firefighters with alpha-antitrypsin deficiency. Chest. 138:1116–24. doi: 10.1378/chest.10-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Daroux M, Prevost G, Maillard-Lefebvre H, et al. Advanced glycation end-products: implications for diabetic and non-diabetic nephropathies. Diabetes Metab. 2010;36:1–10. doi: 10.1016/j.diabet.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 132.Tekabe Y, Luma J, Einstein AJ, et al. A novel monoclonal antibody for RAGE-directed imaging identifies accelerated atherosclerosis in diabetes. J Nucl Med. 2010;51:92–7. doi: 10.2967/jnumed.109.064659. [DOI] [PubMed] [Google Scholar]
- 133.Spiekerkoetter E, Guignabert C, de Jesus Perez V, et al. S100A4 and bone morphogenetic protein-2 codependently induce vascular smooth muscle cell migration via phospho-extracellular signal-regulated kinase and chloride intracellular channel 4. Circ Res. 2009;105:639–47. doi: 10.1161/CIRCRESAHA.109.205120. 13 p following 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Suzuki R, Miyazaki Y, Takagi K, Torii K, Taniguchi H. Matrix metalloproteinases in the pathogenesis of asthma and COPD: implications for therapy. Treat Respir Med. 2004;3:17–27. doi: 10.2165/00151829-200403010-00003. [DOI] [PubMed] [Google Scholar]
- 135.Imai K, Dalal SS, Chen ES, et al. Human collagenase (matrix metalloproteinase-1) expression in the lungs of patients with emphysema. Am J Respir Crit Care Med. 2001;163:786–91. doi: 10.1164/ajrccm.163.3.2001073. [DOI] [PubMed] [Google Scholar]
- 136.Geraghty P, Dabo AJ, D'Armiento J. TLR4 protein contributes to cigarette smoke-induced matrix metalloproteinase-1 (MMP-1) expression in chronic obstructive pulmonary disease. J Biol Chem. 2011;286:30211–8. doi: 10.1074/jbc.M111.238824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Vandenbroucke RE, Dejonckheere E, Libert C. A therapeutic role for matrix metalloproteinase inhibitors in lung diseases? Eur Respir J. 38:1200–14. doi: 10.1183/09031936.00027411. [DOI] [PubMed] [Google Scholar]
- 138.Martin MD, Matrisian LM. The other side of MMPs: protective roles in tumor progression. Cancer Metastasis Rev. 2007;26:717–24. doi: 10.1007/s10555-007-9089-4. [DOI] [PubMed] [Google Scholar]
- 139.Elkington PT, Friedland JS. Matrix metalloproteinases in destructive pulmonary pathology. Thorax. 2006;61:259–66. doi: 10.1136/thx.2005.051979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Joos L, He JQ, Shepherdson MB, et al. The role of matrix metalloproteinase polymorphisms in the rate of decline in lung function. Hum Mol Genet. 2002;11:569–76. doi: 10.1093/hmg/11.5.569. [DOI] [PubMed] [Google Scholar]
- 141.Seabrook JM, Nolan PL. The vascular interaction of noradrenaline and 5-hydroxytryptamine. Eur J Pharmacol. 1983;89:131–5. doi: 10.1016/0014-2999(83)90617-9. [DOI] [PubMed] [Google Scholar]
- 142.Bu DX, Rai V, Shen X, et al. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res. 2010;106:1040–51. doi: 10.1161/CIRCRESAHA.109.201103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D'Armiento JM, Goldklang MP, Hardigan AA, et al. Increased matrix metalloproteinase (MMPs) levels do not predict disease severity or progression in emphysema. PLoS One. 2013;8:e56352. doi: 10.1371/journal.pone.0056352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Posthumus MD, Limburg PC, Westra J, et al. Serum levels of matrix metalloproteinase-3 in relation to the development of radiological damage in patients with early rheumatoid arthritis. Rheumatology (Oxford) 1999;38:1081–7. doi: 10.1093/rheumatology/38.11.1081. [DOI] [PubMed] [Google Scholar]
- 145.Seibold MA, Donnelly S, Solon M, et al. Chitotriosidase is the primary active chitinase in the human lung and is modulated by genotype and smoking habit. J Allergy Clin Immunol. 2008;122:944–50. e3. doi: 10.1016/j.jaci.2008.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Aminuddin F, Akhabir L, Stefanowicz D, et al. Genetic association between human chitinases and lung function in COPD. Hum Genet. 2012;131:1105–14. doi: 10.1007/s00439-011-1127-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Araujo AC, Souto-Padron T, de Souza W. Cytochemical localization of carbohydrate residues in microfilariae of Wuchereria bancrofti and Brugia malayi. J Histochem Cytochem. 1993;41:571–8. doi: 10.1177/41.4.8450196. [DOI] [PubMed] [Google Scholar]
- 148.Debono M, Gordee RS. Antibiotics that inhibit fungal cell wall development. Annu Rev Microbiol. 1994;48:471–97. doi: 10.1146/annurev.mi.48.100194.002351. [DOI] [PubMed] [Google Scholar]
- 149.Fuhrman JA, Piessens WF. Chitin synthesis and sheath morphogenesis in Brugia malayi microfilariae. Mol Biochem Parasitol. 1985;17:93–104. doi: 10.1016/0166-6851(85)90130-6. [DOI] [PubMed] [Google Scholar]
- 150.Neville AC, Parry DA, Woodhead-Galloway J. The chitin crystallite in arthropod cuticle. J Cell Sci. 1976;21:73–82. doi: 10.1242/jcs.21.1.73. [DOI] [PubMed] [Google Scholar]
- 151.Shahabuddin M, Kaslow DC. Plasmodium: parasite chitinase and its role in malaria transmission. Exp Parasitol. 1994;79:85–8. doi: 10.1006/expr.1994.1066. [DOI] [PubMed] [Google Scholar]
- 152.Di Rosa M, Musumeci M, Scuto A, Musumeci S, Malaguarnera L. Effect of interferon-gamma, interleukin-10, lipopolysaccharide and tumor necrosis factor-alpha on chitotriosidase synthesis in human macrophages. Clin Chem Lab Med. 2005;43:499–502. doi: 10.1515/CCLM.2005.088. [DOI] [PubMed] [Google Scholar]
- 153.Malaguarnera L, Musumeci M, Di Rosa M, Scuto A, Musumeci S. Interferon-gamma, tumor necrosis factor-alpha, and lipopolysaccharide promote chitotriosidase gene expression in human macrophages. J Clin Lab Anal. 2005;19:128–32. doi: 10.1002/jcla.20063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Renkema GH, Boot RG, Muijsers AO, Donker-Koopman WE, Aerts JM. Purification and characterization of human chitotriosidase, a novel member of the chitinase family of proteins. J Biol Chem. 1995;270:2198–202. doi: 10.1074/jbc.270.5.2198. [DOI] [PubMed] [Google Scholar]
- 155.van Eijk M, van Roomen CP, Renkema GH, et al. Characterization of human phagocyte-derived chitotriosidase, a component of innate immunity. Int Immunol. 2005;17:1505–12. doi: 10.1093/intimm/dxh328. [DOI] [PubMed] [Google Scholar]
- 156.Agapov E, Battaile JT, Tidwell R, et al. Macrophage chitinase 1 stratifies chronic obstructive lung disease. Am J Respir Cell Mol Biol. 2009;41:379–84. doi: 10.1165/rcmb.2009-0122RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Letuve S, Kozhich A, Humbles A, et al. Lung chitinolytic activity and chitotriosidase are elevated in chronic obstructive pulmonary disease and contribute to lung inflammation. Am J Pathol. 2010;176:638–49. doi: 10.2353/ajpath.2010.090455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Postma DS, Bleecker ER, Amelung PJ, et al. Genetic susceptibility to asthma--bronchial hyperresponsiveness coinherited with a major gene for atopy. N Engl J Med. 1995;333:894–900. doi: 10.1056/NEJM199510053331402. [DOI] [PubMed] [Google Scholar]
- 159.Beeh KM, Ksoll M, Buhl R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. Eur Respir J. 2000;16:609–14. doi: 10.1034/j.1399-3003.2000.16d07.x. [DOI] [PubMed] [Google Scholar]
- 160.Sears MR, Burrows B, Flannery EM, Herbison GP, Hewitt CJ, Holdaway MD. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–71. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 161.Busse WW, Morgan WJ, Gergen PJ, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hanania NA, Alpan O, Hamilos DL, et al. Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med. 2011;154:573–82. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 163.Renkema TE, Kerstjens HA, Schouten JP, Vonk JM, Koeter GH, Postma DS. The importance of serum IgE for level and longitudinal change in airways hyperresponsiveness in COPD. Clin Exp Allergy. 1998;28:1210–8. doi: 10.1046/j.1365-2222.1998.00382.x. [DOI] [PubMed] [Google Scholar]
- 164.Farfel M, DiGrande L, Brackbill R, et al. An overview of 9/11 experiences and respiratory and mental health conditions among World Trade Center Health Registry enrollees. J Urban Health. 2008;85:880–909. doi: 10.1007/s11524-008-9317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kleinman EJ, Cucco RA, Martinez C, et al. Pulmonary function in a cohort of New York City Police Department emergency responders since the 2001 World Trade Center disaster. J Occup Environ Med. 2011;53:618–26. doi: 10.1097/JOM.0b013e31821f2c83. [DOI] [PubMed] [Google Scholar]
- 166.Salzman SH, Moosavy FM, Miskoff JA, Friedmann P, Fried G, Rosen MJ. Early respiratory abnormalities in emergency services police officers at the World Trade Center site. J Occup Environ Med. 2004;46:113–22. doi: 10.1097/01.jom.0000111612.68916.d0. [DOI] [PubMed] [Google Scholar]
- 167.Friedman SM, Maslow CB, Reibman J, et al. Case-control study of lung function in World Trade Center Health Registry area residents and workers. Am J Respir Crit Care Med. 2011;184:582–9. doi: 10.1164/rccm.201011-1909OC. [DOI] [PubMed] [Google Scholar]
- 168.Kazeros A, Maa MT, Patrawalla P, et al. Elevated peripheral eosinophils are associated with new-onset and persistent wheeze and airflow obstruction in world trade center-exposed individuals. J Asthma. 2013;50:25–32. doi: 10.3109/02770903.2012.743149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Skloot G, Goldman M, Fischler D, et al. Respiratory symptoms and physiologic assessment of ironworkers at the World Trade Center disaster site. Chest. 2004;125:1248–55. doi: 10.1378/chest.125.4.1248. [DOI] [PubMed] [Google Scholar]
- 170.Mann JM, Sha KK, Kline G, Breuer FU, Miller A. World Trade Center dyspnea: bronchiolitis obliterans with functional improvement: a case report. Am J Ind Med. 2005;48:225–9. doi: 10.1002/ajim.20196. [DOI] [PubMed] [Google Scholar]
- 171.Oppenheimer BW, Goldring RM, Herberg ME, et al. Distal airway function in symptomatic subjects with normal spirometry following World Trade Center dust exposure. Chest. 2007;132:1275–82. doi: 10.1378/chest.07-0913. [DOI] [PubMed] [Google Scholar]
- 172.Mendelson DS, Roggeveen M, Levin SM, Herbert R, de la Hoz RE. Air trapping detected on end-expiratory high-resolution computed tomography in symptomatic World Trade Center rescue and recovery workers. J Occup Environ Med. 2007;49:840–5. doi: 10.1097/JOM.0b013e3180d09e87. [DOI] [PubMed] [Google Scholar]