Abstract
Myopia is the most common type of refractive errors and one of the world’s leading causes of blindness. Visual manipulations in animal models have provided convincing evidence for the role of environmental factors in myopia development. These models along with in vitro studies have provided important insights into underlying mechanisms. The key locations of the retinal pigment epithelium (RPE) and choroid make them plausible conduits for relaying growth regulatory signals originating in the retina to the sclera, which ultimately determines eye size and shape. Identifying the key signal molecules and their targets may lead to the development of new myopia control treatments. This section summarizes findings implicating the RPE and choroid in myopia development. For RPE and/or choroid, changes in morphology, activity of ion channels/transporters, as well as in gene and protein expression, have been linked to altered eye growth. Both tissues thus represent potential targets for novel therapies for myopia.
1. INTRODUCTION
Uncorrected refractive errors represent one of the world’s leading causes of blindness and a significant contributor to the global burden of eye diseases.1,2 For children and young adults, myopia, hyperopia, and astigmatism represent the categories of refractive errors encountered; these same conditions may be found in older adults, with presbyopia representing an additional potential cause of vision loss for this group.
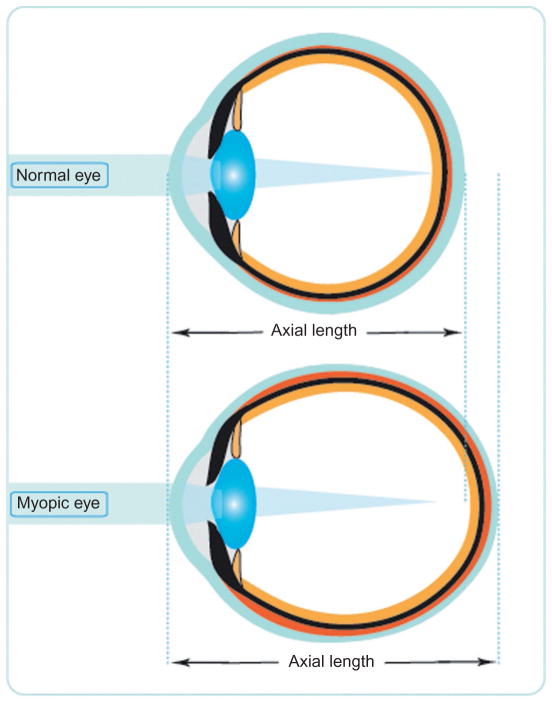
Myopia (near-sightedness) describes the condition in which the image of a distant object is focused in front of the retina, resulting in blurred distance vision when left uncorrected. Myopia reflects the mismatch between the refracting power of the eye and its optical axial length. Most myopia is caused by excessive ocular elongation, with refracting power being near normal (Fig. 1).3 Myopia carries an increased risk of a variety of sight-threatening pathologies, including myopic maculopathy, retinal detachment, choroidal neovascularization, cataract, and glaucoma, with high myopes (classically defined as spherical equivalent refractive errors equal to or greater than −6 D), being at greatest risk.4,5
Figure 1.
Schematic diagram illustrating the principal gross anatomical differences between human emmetropic and myopic eyes, the latter typically being longer and more prolate, with a longer vitreous chamber.
Myopia is now the most common type of refractive error and one of the world’s leading causes of functional blindness due to lack of access to optical corrections.6 A figure of 41.6% for persons aged 12–54 years is given in the most recently published myopia prevalence data for the United States,7 while even higher, epidemic levels of myopia have been reported for many Asia countries, e.g., 96.5% in young Korean males, along with increases in the average amount of myopia.8,9 Thus, myopia now represents a significant public health problem worldwide, both socially and economically.1,10 These climbing prevalence statistics are driving research aimed at effective therapeutic interventions to prevent the development of myopia and/or slow its progression.
It is now generally accepted that both genetic and environmental factors play roles in the development of human myopia.5,11,12 Genetic studies of myopia, using linkage and genome-wide association approaches, have now identified multiple myopic loci and candidate genes for high myopia and the most common form of juvenile myopia.12–14 Nonetheless, human epidemiological studies have also provided convincing evidence for environmental influences, with near work and outdoor activities being among the factors identified to affect myopia prevalence.15
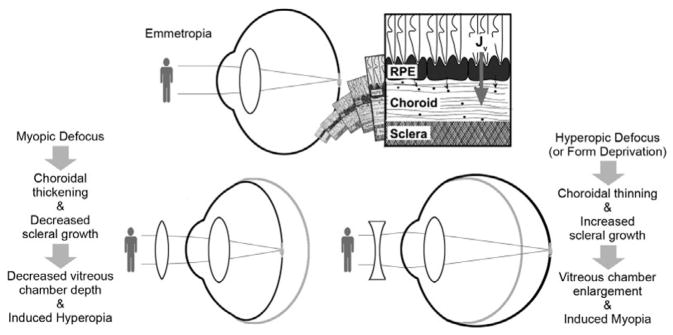
That environmental factors influence ocular growth regulation and thus refractive errors is further supported by animal studies in which the visual environment is manipulated to alter optical defocus and/or the quality of the retinal image. Specifically, both negative defocusing lenses, used to move the plane of focus behind the retina, and form-deprivation strategies, e.g., achieved using diffusers to cover the eyes, accelerate eye growth in young animals, thereby inducing myopia (Fig. 2). Chickens, guinea pigs, tree shrews, and monkeys represent the most widely studied models,11 with the mouse and zebrafish also making appearances in select studies.17,18 Such models represent important tools for investigating the molecular and cellular signaling pathways mediating ocular growth regulation, which may, in turn, lead to the development of new myopia therapies.19,20 Fortuitously, ocular growth appears to be largely regulated by local ocular mechanisms.11 Thus, related studies have focused on retino-scleral signaling cascades linking the retina, the presumed source of ocular growth signals, to the choroid and sclera, whose growth/remodeling ultimately determines the physical dimensions of the vitreous chamber and the location of the retina (Fig. 3).21,22 Through investigations into the molecular and cellular components of these signaling pathways and the changes linked to altered ocular growth, and the cellular and biochemical events mediating the changes in the choroid and sclera, it is plausible that novel pharmacological treatments for controlling myopia be forthcoming.11
Figure 2.

Chicks fitted with either a translucent, form-depriving goggle (left), or a defocusing spectacle lens (right). From Ref. 16.
Figure 3.
Schematic diagram summarizing models used to study eye growth regulation, including key ocular features of these models; plausible local ocular growth regulatory signal pathway included. From Ref. 16.
This chapter covers the roles of the retinal pigment epithelium (RPE) and choroid in ocular growth regulation and refractive error development, including myopia, and encompasses molecular, biochemical and cellular mechanisms. Please refer further to the following chapters, “Molecular and Biochemical Aspects of the Retina on Refraction” (written by Ranjay Chakraborty and Machelle Pardue), “Scleral Mechanisms Underlying Ocular Growth and Myopia” (written by Ravikath Metlapally and Christine F. Wildsoet), and “Genetics of Refraction and Myopia” (written by Qingjiong Zhang).
2. THE ROLE OF THE RPE IN EYE GROWTH REGULATION
The RPE is a monolayer of highly specialized pigmented cells, separating the neural retina from the vascular choroid. Being pigmented, these cells serve to absorb stray light within the eye. Being also interconnected by tight junctions, these cells represent a critical component of the blood–retina barrier (Fig. 4), with essential roles in the maintenance of retinal integrity.23–25 These cells also show other specializations that reflect their role in maintaining retinal homeostasis. Thus, they are polarized, with asymmetric distributions of specialized transport proteins and channels over their apical and basolateral membranes, allowing for the tight regulation of exchange between the retina and the choroid of many molecules, including ions, water, nutrients, and waste products. In addition, the RPE plays a critical role in the maintenance of photoreceptor function; related functions include the phagocytosis of photoreceptor outer segments, which follows a diurnal cycle, and uptake and recycling of retinal as a critical step in photopigment regeneration. These various, well-recognized functions of the RPE have long been the subject of study. However, more recent research has uncovered additional functions for the RPE. For example, it is now known to be a major source of cytokines and growth factors, with important roles in maintaining retinal integrity, in establishing the immune privilege of the eye and potentially in early eye growth regulation.
Figure 4.
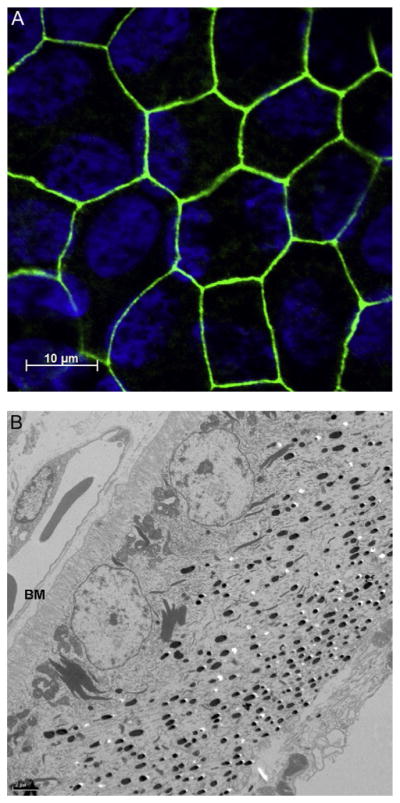
(A) Cultured human fetal RPE cells. Tight junctions, shown stained for ZO-1 (zonula occludens-1, in green), ensure that the RPE functions as an effective barrier between the retina and the choroid, with exchange tightly regulated through ion channels and transporters. (B) Transmission electron micrographs of chick RPE; cells show a distinct asymmetry, with nuclei located in the basal region adjacent Bruch’s membrane (BM) and choriocapillaris (CC), and melanin granules more concentrated in the apical (retinal) region.
In the context of myopia development, it has been demonstrated that postnatal eye growth is largely controlled locally. Evidence comes from lesioning studies involving optic nerve section and related pharmacological studies; thus, even when the retina–brain link is disrupted, myopia may be induced with appropriate experimental manipulations in both chickens and guinea pigs.11,26,27 This model of local ocular growth regulation also explains observations of localized ocular shape changes in response to localized manipulation of retinal images.11 The RPE’s key location between the retina and the choroid makes it a possible conduit for relaying growth regulatory signals originating in the retina to the choroid and sclera.16 The RPE is known to have receptors for many of the signaling molecules that have been implicated in eye growth regulation, including dopamine (DA), acetylcholine, vasoactive intestinal peptide (VIP), and glucagon.16,28–32 In addition, the transepithelial transport of ions and/or fluid across the RPE may have implications for choroidal thickness, which appears to be one of the targets of ocular growth signals.33 Finally, recent studies have demonstrated differential expression of a number of genes in the RPE of eyes undergoing experimental manipulations perturbing normal eye growth.34,35
2.1 Morphological Features of RPE in Myopic and “Recovering” Eyes and Potential Role in Myopia-Related Pathology
Morphological changes have been reported in the RPE of experimental animals with induced myopia.36–38 For example, in form-deprived chicks, the increase in total area of the RPE layer in their myopic eyes was coupled to an increase in the surface area of individual RPE cells, by way of maintaining coverage of the expanded vitreous chamber.36 Nonetheless, the majority of the RPE cells in the form-deprived eyes retained near-normal, hexagonal shapes despite their expanded surface areas.36 Interestingly, while in normal eyes, RPE cell expansion was limited mainly in the peripheral regions, the expansion in surface area in myopic chick eyes encompassed all but a temporal region.36 Enlarged RPE cells but not increases in cell numbers have also been reported in the lid-sutured eyes of a mammalian model, although the distribution of multinucleated RPE cells was reported to be significantly altered.37,38 The preceding changes do not in themselves suggest pathological changes in the RPE. However, the observations of Liang et al. may be interpreted as indirect evidence of altered RPE function. Specifically, in chick eyes allowed to recovery from form-deprivation-induced myopia, they reported significant edema and altered basal in-foldings in the RPE along with thickening of Bruch’s membrane.39 Nonetheless, studies of the RPE of eyes with experimentally-induced myopia in the context of related pathologies have been very limited to-date, and the following sections will focus on its potential role in eye growth regulation.
2.2 Ion and Fluid Transport Across the RPE and Implications for Eye Growth Regulation
As noted earlier, RPE cells are interconnected by tight junctions, which prevent the free exchange of ions and water between the retina and the choroid. However, the RPE has specializations that allow for regulated fluid exchange between the retina and the choroid, and specifically, transport of ions and water from the subretinal space to the blood, as is critical for maintaining retinal homeostasis.16,23 Among relevant channels present on the RPE are potassium (K+) and chloride (Cl−) channels, which are known to regulate transepithelial fluid movement. Many show asymmetric distribution, consistent with their functions. Thus, Cl− channels and a cystic fibrosis transmembrane conductance regulator have been localized to the basolateral side of RPE cells, while Na+, K+-ATPase, the source of energy for trans-epithelial transport, is located on the apical membrane.16,23,25,40 While the role of the RPE in maintaining retinal homeostasis is likely to be common to all species, species differences in the distribution of ion channels and function have been reported. For the chick, which is a widely used model in myopia research, a detailed summary for its ion and fluid transport in the RPE and its potential role in eye growth regulation is contained in reviews by Rymer et al. and Crewther.16,41
In the context of eye growth regulation, the roles of RPE ion and fluid channels are not well understood, although the observation of early, rapid changes in choroidal thickness during the development of myopia and hyperopia in chicks provides a plausible link between ion and fluid transport across the RPE and eye growth regulation.21,42 Furthermore, potassium (K+) and phosphate levels are reported to be decreased, and chloride (Cl−) elevated, in the vitreous of form-deprivation myopic chicks.43 The genes encoding the Cl− transporter and channel were down-regulated in the RPE with lens-induced myopia.33 These results open the possibility that the choroid thinning observed during the early phase of experimental myopia induction may be a product of decreased ion and fluid transport to the choroid. That the concentrations of K+, Na+, and Cl− ions are reported to be elevated in RPE-photoreceptor outer segment regions of freeze-dried preparation from eyes allowed to recover from induced myopia, represents further indirect evidence implicating ion transport in eye growth regulation.44,45 Energy dispersive X-ray microanalysis was used for the latter assays. Note also that these changes were detected only early in the recovery process, with levels more closely paralleling those of fellow eyes with longer recovery periods. More direct evidence implicating ion transport in eye growth regulation is provided by studies involving pharmacological manipulation in chicks.46 Thus, intravitreal injection of barium chloride, a nonspecific potassium channel inhibitor, was found to inhibit the compensatory ocular growth responses to imposed optical defocus, be they elicited with positive lenses or negative lenses, and bumetanide, a selective sodium–potassium–chloride cotransporter inhibitor, selectively inhibited the response to negative lens.
The premise that altered ion transport across the RPE is a feature of eye growth regulation, as discussed above, leaves open the identity of signal molecules responsible. Among possibilities are one or more retinal neuro-transmitters, including DA, which has been linked to eye growth regulation and for which there are receptors on the RPE.47,48
2.3 Neurotransmitters as Plausible Signal Molecules for RPE-Mediated Eye Growth Regulation
Retinal neurotransmitters including DA, acetylcholine, and glucagon have been the focus of many studies in relation to their roles in retinal functions in the context of eye growth regulation. Below, we consider the possibility that the RPE could be the site of action of such molecules, serving as signal molecules for eye growth regulation, on the basis that many of these molecules also have receptors on RPE and are known to affect RPE physiology.16
2.3.1 Dopamine
In the retina, DA, serving as a neurotransmitter and neuromodulator, plays important roles in retinal function.49 This topic is explored in more detail in chapter “Molecular and Biochemical Aspects of the Retina on Refraction” (written by Ranjay Chakraborty and Machelle Pardue) of this volume. Here, the possible role of DA in eye growth regulation, acting via the RPE is discussed, along with related physiology.
There are five subtypes of DA receptors (D1–D5), which, based on their biochemical and pharmacological properties, have been further categorized into D1-like (D1, D5) and D2-like (D2–D4) subfamilies.50,51 DA receptors are known to be widely distributed in the eye, including on neural cells within the retina and RPE.32,49,52–54 DA receptors have been identified on human RPE cells as well as teleost, chick, cat, and bovine RPE.31,32,52,53 Both D1 and D2 DA receptors have been identified in cultured human RPE.55,56 In chicks, D2/3 receptors have been identified on the basal side of the RPE. However, due to the use of in situ hybridization and immunocytochemistry in the study in chick, the presence of DA receptors on the apical surface of RPE cells was left unresolved due to heavy pigmentation in this region.32
In vivo animal studies, mostly involving the chick model, have consistently reported inhibitory effects on experimental myopia of DA receptor agonists, typically delivered intravitreally.57–59 Indirect support for the possibility that RPE is the site of action of these drugs is provided by two studies in chicks. First, [3H]-spiperone, a D2-receptor antagonist, was demonstrated to reach the RPE when administered by either intravitreal or subconjunctival injection.58 Second, in vitro electrophysiology studies using retina–RPE–choroid preparations showed that RPE function was altered differently with retinal versus choroidal perfusion of DA. Note also that DA has been named as a likely modulation of basolateral Cl− channels in the RPE, acting through different populations of DA receptors on the apical and basolateral membranes of RPE.47
2.3.2 Acetylcholine
Acetylcholine (ACh) receptors fall into two broad categories, of membrane receptors, metabotropic muscarinic acetylcholine receptors (mAChRs) and ionotropic nicotinic acetylcholine receptors (nAChR).60 Of these two types of receptors, muscarinic receptors are a family of G-protein-coupled receptors, which comprise five receptor subtypes (M1–M5) in mammals. In chicks, only M2–M5 receptor subtypes have been identified.61,62 In addition to serving as a retinal neurotransmitter, ACh appears to play an important role in the developing retina.63,64 There is now substantial evidence that ACh, acting via both muscarinic and nicotinic receptors (mAChR and nAChR), is involved in eye growth regulation.16,48,65,66 Discussion here is limited to the more widely studied mAChR-mediated effects.
Studies linking ACh and muscarinic receptors with eye growth regulation and specifically myopia are many. In humans, atropine, a nonselective mAChR antagonist, has a long history of use for myopia control, dating back to the middle of the last century. Today, it remains the mostly widely used pharmacological agent clinically for this purpose, despite evidence of rebound effects after treatment is terminated, at least for higher doses.65,67,68 Animal models studied in this context include the mouse, chicks, guinea pigs, tree shrews, and monkeys, with consistent antimyopia effects of muscarinic receptor antagonists being reported.16,66,69,70 For example, in chicks, both intravitreal and subconjunctival injection of atropine were found to inhibit the development of form-deprivation and lens-induced myopia.59,71,72 Other, more selective antimuscarinic drugs including pirenzepine and himbacine have also been reported to inhibit the development of myopia, with M4 receptors being favored as the receptor subtype mediating these effects based on the selectivity profiles of the latter drugs, i.e., M1/M4 (mammal) or M2/M4 (chick) for pirenzepine and M2/M4 for himbacine.66,72–75
As with DA receptors, the wide distribution of muscarinic receptors throughout ocular tissues offers multiple candidate tissues, and the sites of action for the antimyopia effects of muscarinic receptor antagonists include but are not limited to retina, choroid, and sclera.16,71,72 The RPE also expresses muscarinic receptors and thus is a candidate tissue.76,77 In chick RPE, the relevant receptor subtypes, M2, M3, and M4 receptors, have all been identified.61 Physiological actions mediated by mAChRs activation include increased phosphoinositide turnover and intracellular Ca2+, as seen in cultured human RPE.28,77,78 However, at this time, other supporting evidence for the RPE being the site of action of the described antimyopia actions is lacking.
In addition to the two neurotransmitter receptors discussed above, the RPE is also reported to possess receptors for a number of other neurotransmitters, including glucagon and VIP, which have been implicated in eye growth regulation.16,29,30,79 In the case of glucagon, the RPE has been considered a plausible site of action in two separate studies in chicks,80,81 and in the case of VIP, the observation of polarized secretion of macromolecules with application of VIP to cultured RPE cells provides a plausible mechanism for growth regulation of the nearby choroid and/or sclera.82
2.4 RPE-Derived Growth Factors and Cytokines as Plausible Eye Growth Signal Molecules or Regulators
The RPE represents a major source of growth factors and cytokines, including insulin-like growth factor-1, transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), and vascular endothelial growth factor (VEGF).16,23 Synthesized locally and subsequently secreted, they have been attributed roles in the maintenance of the structure and homeostasis of retina and choroid. Note that depending on the direction of their secretion, i.e., toward the retina and/or choroid side, these growth factors have potential to effect changes that are limited to the retina or choroid/sclera.
So far, there has been only limited study of the roles of RPE-derived growth factors and cytokines in postnatal eye growth regulation,34,35 and results were not always conclusive due to the approaches used. For example, the combined tissue collection of the RPE and retina in early gene expression studies involving the chick precludes conclusion about the site of observed changes in expression.83,84 Nonetheless, the report of down-regulation of Bone Morphogenetic Protein (BMP2) gene expression in retina/RPE after form deprivation of chicks for either 6 h or 3 days is consistent with findings in isolated chick RPE with prolonged negative lens treatment.85 These studies made use of high-throughput gene expression profiling with DNA microarray screening. In follow-up studies of isolated chick RPE, the gene expression of BMP-2, -4, and -7 was found to be bidirectionally regulated, with opposite signs of imposed defocus eliciting opposite responses (decreased with negative lenses, increased with positive lenses), even with exposures as short as 2 h and 2 days.34,35 The rapidity of these responses suggests roles for these BMPs in the initiation and early phases of altered eye growth. Additional RPE gene expression studies have implicated in defocus-induced “myopic growth,” TGF-β2, which belongs to the same superfamily of growth factors as BMPs, and has been previously linked to eye growth regulation in studies involving chicks and tree shrews.86–89 Further characterization of the signal pathways involving these RPE-derived growth factors/cytokines, to identify both up- and downstream components, may well uncover novel treatments for myopia control.
3. THE ROLE OF CHOROID IN EYE GROWTH REGULATION
The choroid lies between the RPE and the sclera. In most species, it can be divided into five layers histologically, starting from the inner (retinal) side: Bruch’s membrane, choroicapillaris, Haller’s layer, Sattler’s layer, and the suprachoroidea, with all but the first layer being largely vascular,21 although in birds, the suprachoroid contains large, endothelium-lined spaces (lacunae), which resemble lymphatic vessels.90,91 The choroid also contains a variety of nonvascular resident cells, including melanocytes, fibroblasts, nonvascular smooth muscle cells, and immunocompetent cells, supported by collagenous and elastic elements.21 Traditionally, the choroid has been assigned as its major functions, supply of oxygen and nutrients to the outer retina, light absorption (pigmented choroid), thermoregulation, and modulation of intraocular pressure. However, recent studies also point to a role for the choroid in ocular focus adjustment, including emmetropization, and thus eye growth regulation, opening up the possibility of novel therapeutic approaches for myopia control.21 Elucidating underlying signal pathways and mechanisms are essential first steps.
Referred to sometimes as choroidal accommodation, changes in choroidal thickness in response to imposed defocus were first described in young chicks, which also show the most dramatic changes of all animals studied. These changes serve to move the retina toward the altered plane of focus. Thus, while the choroid of young chicks is about 250 μm thick centrally and 100 μm thick peripherally, similar to mammals and primates, in response to substantial imposed myopic defocus, e.g., with +15 D lenses, the choroid of the chick eye increases its thickness significantly, effecting a correspondingly large, compensatory change in refraction.21,42,92–94 With imposed hyperopic defocus, the choroid thins instead of thickening, pulling the retina backward toward the altered image plane. In refractive terms, the net effects are induced hyperopia and myopia, respectively. Form deprivation, which also induces myopia, also causes choroidal thinning, although here, the adjustment to the position of the retina serves no compensatory role. These changes in choroidal thickness occur very rapidly, being detectible with high-frequency ultrasonography in a matter of minutes in young chicks.11,34,42,94,95 Similar choroidal responses have been documented in other animals, including guinea pigs, marmosets, macaques, and humans most recently, although the scale of the changes are much smaller than those observed in chicks in all cases.21,96
The mechanisms underlying the above choroidal thickness changes remain to be fully elucidated and it is possible that different mechanisms underlie the thickening and thinning responses. To-date, related changes in blood flow and structure have been described in chicks, along with bidirectional changes in the permeability of the choroidal vasculature, i.e., decreased during form deprivation and increased during recovery from form-deprivation myopia.97–99 The protein content of suprachoroidal fluids has also been reported to be decreased in form-deprived eyes, and increased after normal vision is restored, consistent with anatomical localization of the thickness changes to the outer choroidal lacunae in chicks.21,97 Presumably, these proteins serve as osmotic agents to regulate the water content and thus thickness of the outer choroid, with proteoglycans being among identified molecules reported to be elevated in eyes wearing positive lenses or in recovery (after diffuser removal).94 It has also been speculated that non-vascular smooth muscle cells contribute to choroidal thickness changes, by contracting or relaxing as appropriate.94 The extent to which observed changes in choroidal blood flow contribute to thickness changes via related changes in vessel diameters remains to be clarified, but may be significant, at least in mammals and primates whose choroids appear to lack lacunae. The role of the RPE as a regulator of one or more of these events also remains to be established. At the most basic level, it is possible that the RPE, by regulating ion and fluid exchange between the retina and the choroid, contributes to the regulation of choroidal thickness.16,21 Alternatively, more complex signal cascades may be involved. For example, two DA receptor agonists, apomorphine and quinpirole, administered by intravitreal injection, have been linked to transient choroidal thickening; both also inhibit lens-induced myopia and both have potential access to receptors on the RPE, although retinal sites of action are plausible alternatives.100 Likewise, retinal glucagon has been linked to altered eye growth, and intravitreal injection of exogenous glucagon is reported to modulate the choroidal thickness changes induced by visual manipulations.101
Of available animal models for myopia, the chick has been mostly widely studied in terms of regulatory mechanisms, with glucagon and retinoic acid (RA) being the subject of a number of studies. The pictures for both are complex. In the case of glucagon, the chick choroid as well as retina expresses glucagon and its receptors,30 and choroidal glucagon protein levels are reported to increase with short term (up to 1 day) positive lens wear, and be unaltered by negative lenses.102 Furthermore, insulin, which generally has opposing actions to glucagon, also appears to modulate choroidal thickness, apparently through an RPE-dependent mechanism, as demonstrated in vitro with chick eyecup preparations, in which added insulin thinned the choroid, in the presence of either RPE or RPE-conditioned medium.101,103 There is also strong evidence implicating retinal and choroidal RA in eye growth regulation.21 In relation to the choroid, RA shows bidirectional changes in response to visual manipulations that slow (positive lens and removal of diffusers) or accelerate (negative lens or diffuser) eye growth.104 Choroidal expression of the RA-synthesizing enzyme, retinaldehyde dehydrogenase 2, also exhibits differential regulation with negative and positive lens treatment, as well as recovery from form deprivation.105,106
In addition to serving as a focusing mechanism, the choroid may also play an important role in regulating scleral growth and remodeling. Modulation of scleral proteoglycan synthesis appears to be one of the targets of choroidal RA.106,107 In addition, the choroid expresses and synthesizes a variety of growth factors and enzymes, including bFGF, TGF-β, tissue plasminogen activator (t-PA), and matrix metalloproteinases, all of which have been linked to scleral remodeling and/or eye growth regulation.22,105,108–111 For example, during the development of myopia, TGF-β gene has been shown to be differentially expressed in the choroid in chicks, albeit not in tree shrews.105,111 Despite the difference between chicks and tree shrews noted in relation to choroidal TGF-β gene expression, other studies of gene expression in both tree shrews and marmosets point to involvement of the choroid in eye growth regulation. Microarray gene profiling applied to RPE/choroid preparations from marmosets undergoing lens treatment of opposite signs, revealed altered expression of a number of the 204 screened genes, including protein tyrosine phosphatase receptor type B, TGF-β-induced, and FGF-2.112 Interestingly, in the tree shrew, similar differential gene expression patterns were observed in the choroid with three different visual manipulations (negative lens, form deprivation, and continuous darkness), implying a common myopia-inducing molecular signaling cascade, at least within the choroid.113,114
Recent studies in chick also provide an interesting perspective on the potential role of members of the VEGFs family in eye growth regulation. They are best known for their roles in angiogenesis, and VEGF antagonists such as bevacizumab, an antibody against human VEGF, are now widely used clinically in the treatment of wet age-related maculopathy. However, recent years have seen an expansion of their clinical use to include other macular pathologies, including myopic maculopathy.115 Thus, the findings that members of the VEGF family and their receptors are expressed in chick choroid, and intravitreal injection of bevacizumab inhibits both the development of form-deprivation myopia and the choroidal thickening during the recovery from form-deprivation myopia in chicks implies a fundamental role for this family in regulating choroidal function.116,117
Further studies into the role of choroid in eye growth regulation and underlying signal pathways and mechanisms may lead to the development of new therapeutic approaches for myopia treatment through the modulation of choroidal functions.
Please refer further to chapter, “Scleral Mechanisms Underlying Ocular Growth and Myopia” (written by Ravi Metlapally and Christine F. Wildsoet).
References
- 1.Ono K, Hiratsuka Y, Murakami A. Global inequality in eye health: country-level analysis from the Global Burden of Disease Study. Am J Public Health. 2010;100:1784–1788. doi: 10.2105/AJPH.2009.187930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bull World Health Organ. 2008;86:63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBrien NA, Millodot M. A biometric investigation of late onset myopic eyes. Acta Ophthalmol (Copenh) 1987;65:461–468. doi: 10.1111/j.1755-3768.1987.tb07024.x. [DOI] [PubMed] [Google Scholar]
- 4.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25:381–391. doi: 10.1111/j.1475-1313.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- 5.Flitcroft DI. The complex interactions of retinal, optical and environmental factors in myopia aetiology. Prog Retin Eye Res. 2012;31:622–660. doi: 10.1016/j.preteyeres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 7.Vitale S, Sperduto RD, Ferris FL., 3rd Increased prevalence of myopia in the United States between 1971–1972 and 1999–2004. Arch Ophthalmol. 2009;127:1632–1639. doi: 10.1001/archophthalmol.2009.303. [DOI] [PubMed] [Google Scholar]
- 8.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32:3–16. doi: 10.1111/j.1475-1313.2011.00884.x. [DOI] [PubMed] [Google Scholar]
- 9.Jung SK, Lee JH, Kakizaki H, Jee D. Prevalence of myopia and its association with body stature and educational level in 19-year-old male conscripts in Seoul, South Korea. Invest Ophthalmol Vis Sci. 2012;53:5579–5583. doi: 10.1167/iovs.12-10106. [DOI] [PubMed] [Google Scholar]
- 10.Vitale S, Cotch MF, Sperduto R, Ellwein L. Costs of refractive correction of distance vision impairment in the United States, 1999–2002. Ophthalmology. 2006;113:2163–2170. doi: 10.1016/j.ophtha.2006.06.033. [DOI] [PubMed] [Google Scholar]
- 11.Wallman J, Winawer J. Homeostasis of eye growth and the question of myopia. Neuron. 2004;43:447–468. doi: 10.1016/j.neuron.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 12.Wojciechowski R. Nature and nurture: the complex genetics of myopia and refractive error. Clin Genet. 2011;79:301–320. doi: 10.1111/j.1399-0004.2010.01592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Solouki AM, Verhoeven VJ, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2012;42:897–901. doi: 10.1038/ng.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2012;42:902–905. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379:1739–1748. doi: 10.1016/S0140-6736(12)60272-4. [DOI] [PubMed] [Google Scholar]
- 16.Rymer J, Wildsoet CF. The role of the retinal pigment epithelium in eye growth regulation and myopia: a review. Vis Neurosci. 2005;22:251–261. doi: 10.1017/S0952523805223015. [DOI] [PubMed] [Google Scholar]
- 17.Pardue MT, Faulkner AE, Fernandes A, et al. High susceptibility to experimental myopia in a mouse model with a retinal on pathway defect. Invest Ophthalmol Vis Sci. 2008;49:706–712. doi: 10.1167/iovs.07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veth KN, Willer JR, Collery RF, et al. Mutations in zebrafish lrp2 result in adult-onset ocular pathogenesis that models myopia and other risk factors for glaucoma. PLoS Genet. 2011;7:e1001310. doi: 10.1371/journal.pgen.1001310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sivak J. The cause(s) of myopia and the efforts that have been made to prevent it. Clin Exp Optom. 2012;95:572–582. doi: 10.1111/j.1444-0938.2012.00781.x. [DOI] [PubMed] [Google Scholar]
- 20.Aller TA. Clinical management of progressive myopia. Eye (Lond) 2014;28:147–153. doi: 10.1038/eye.2013.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 23.Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 24.Boulton M, Dayhaw-Barker P. The role of the retinal pigment epithelium: topographical variation and ageing changes. Eye (Lond) 2001;15:384–389. doi: 10.1038/eye.2001.141. [DOI] [PubMed] [Google Scholar]
- 25.Marmor M, Wolfensberger T. The Retinal Pigment Epithelium: Function and Disease. 1. New York, NY: Oxford University Press; 1998. pp. 1–745. [Google Scholar]
- 26.Wildsoet CF. Active emmetropization—evidence for its existence and ramifications for clinical practice. Ophthalmic Physiol Opt. 1997;17:279–290. [PubMed] [Google Scholar]
- 27.McFadden SA, Wildsoet CF. Mammalian eyes need an intact optic nerve to detect the sign of defocus during emmetropisation. ARVO Abstract. 2009;50:E-Abstract 1620. [Google Scholar]
- 28.Friedman Z, Hackett SF, Campochiaro PA. Human retinal pigment epithelial cells possess muscarinic receptors coupled to calcium mobilization. Brain Res. 1988;446:11–16. doi: 10.1016/0006-8993(88)91291-7. [DOI] [PubMed] [Google Scholar]
- 29.Koh SW, Chader GJ. Elevation of intracellular cyclic AMP and stimulation of adenylate cyclase activity by vasoactive intestinal peptide and glucagon in the retinal pigment epithelium. J Neurochem. 1984;43:1522–1526. doi: 10.1111/j.1471-4159.1984.tb06072.x. [DOI] [PubMed] [Google Scholar]
- 30.Buck C, Schaeffel F, Simon P, Feldkaemper M. Effects of positive and negative lens treatment on retinal and choroidal glucagon and glucagon receptor mRNA levels in the chicken. Invest Ophthalmol Vis Sci. 2004;45:402–409. doi: 10.1167/iovs.03-0789. [DOI] [PubMed] [Google Scholar]
- 31.Versaux-Botteri C, Gibert JM, Nguyen-Legros J, Vernier P. Molecular identification of a dopamine D1b receptor in bovine retinal pigment epithelium. Neurosci Lett. 1997;237:9–12. doi: 10.1016/s0304-3940(97)00783-0. [DOI] [PubMed] [Google Scholar]
- 32.Rohrer B, Stell WK. Localization of putative dopamine D2-like receptors in the chick retina, using in situ hybridization and immunocytochemistry. Brain Res. 1995;695:110–116. doi: 10.1016/0006-8993(95)00700-z. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Wong CL, Shan SW, et al. Characterisation of Cl(−) transporter and channels in experimentally induced myopic chick eyes. Clin Exp Optom. 2011;94:528–535. doi: 10.1111/j.1444-0938.2011.00611.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Y, Liu Y, Wildsoet CF. Bidirectional, optical sign-dependent regulation of BMP2 gene expression in chick retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2012;53:6072–6080. doi: 10.1167/iovs.12-9917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang Y, Liu Y, Ho C, Wildsoet CF. Effects of imposed defocus of opposite sign on temporal gene expression patterns of BMP4 and BMP7 in chick RPE. Exp Eye Res. 2013;109:98–106. doi: 10.1016/j.exer.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin T, Grimes PA, Stone RA. Expansion of the retinal pigment epithelium in experimental myopia. Vis Res. 1993;33:1881–1885. doi: 10.1016/0042-6989(93)90015-o. [DOI] [PubMed] [Google Scholar]
- 37.Harman AM, Hoskins R, Beazley LD. Experimental eye enlargement in mature animals changes the retinal pigment epithelium. Vis Neurosci. 1999;16:619–628. doi: 10.1017/s0952523899164022. [DOI] [PubMed] [Google Scholar]
- 38.Fleming PA, Harman AM, Beazley LD. Changing topography of the RPE resulting from experimentally induced rapid eye growth. Vis Neurosci. 1997;14:449–461. doi: 10.1017/s0952523800012128. [DOI] [PubMed] [Google Scholar]
- 39.Liang H, Crewther SG, Crewther DP, Pirie B. Morphology of the recovery from form deprivation myopia in the chick. Aust NZ J Ophthalmol. 1996;24:41–44. doi: 10.1111/j.1442-9071.1996.tb00991.x. [DOI] [PubMed] [Google Scholar]
- 40.Maminishkis A, Chen S, Jalickee S, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crewther DP. The role of photoreceptors in the control of refractive state. Prog Retin Eye Res. 2000;19:421–457. doi: 10.1016/s1350-9462(00)00004-5. [DOI] [PubMed] [Google Scholar]
- 42.Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vis Res. 1995;35:1175–1194. doi: 10.1016/0042-6989(94)00233-c. [DOI] [PubMed] [Google Scholar]
- 43.Seko Y, Shimokawa H, Pang J, Tokoro T. Disturbance of electrolyte balance in vitreous of chicks with form-deprivation myopia. Jpn J Ophthalmol. 2000;44:15–19. doi: 10.1016/s0021-5155(99)00177-x. [DOI] [PubMed] [Google Scholar]
- 44.Crewther SG, Liang H, Junghans BM, Crewther DP. Ionic control of ocular growth and refractive change. Proc Natl Acad Sci USA. 2006;103:15663–15668. doi: 10.1073/pnas.0607241103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang H, Crewther SG, Crewther DP, Junghans BM. Structural and elemental evidence for edema in the retina, retinal pigment epithelium, and choroid during recovery from experimentally induced myopia. Invest Ophthalmol Vis Sci. 2004;45:2463–2474. doi: 10.1167/iovs.03-1009. [DOI] [PubMed] [Google Scholar]
- 46.Crewther SG, Murphy MJ, Crewther DP. Potassium channel and NKCC cotransporter involvement in ocular refractive control mechanisms. PLoS One. 2008;3:e2839. doi: 10.1371/journal.pone.0002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gallemore RP, Steinberg RH. Effects of dopamine on the chick retinal pigment epithelium. Membrane potentials and light-evoked responses. Invest Ophthalmol Vis Sci. 1990;31:67–80. [PubMed] [Google Scholar]
- 48.Stone RA, Sugimoto R, Gill AS, Liu J, Capehart C, Lindstrom JM. Effects of nicotinic antagonists on ocular growth and experimental myopia. Invest Ophthalmol Vis Sci. 2001;42:557–565. [PubMed] [Google Scholar]
- 49.Reis RA, Ventura AL, Kubrusly RC, de Mello MC, de Mello FG. Dopaminergic signaling in the developing retina. Brain Res Rev. 2007;54:181–188. doi: 10.1016/j.brainresrev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 50.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 51.Vallone D, Picetti R, Borrelli E. Structure and function of dopamine receptors. Neurosci Biobehav Rev. 2000;24:125–132. doi: 10.1016/s0149-7634(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 52.Dearry A, Burnside B. Stimulation of distinct D2 dopaminergic and alpha 2-adrenergic receptors induces light-adaptive pigment dispersion in teleost retinal pigment epithelium. J Neurochem. 1988;51:1516–1523. doi: 10.1111/j.1471-4159.1988.tb01120.x. [DOI] [PubMed] [Google Scholar]
- 53.Bruinink A, Dawis S, Niemeyer G, Lichtensteiger W. Catecholaminergic binding sites in cat retina, pigment epithelium and choroid. Exp Eye Res. 1986;43:147–151. doi: 10.1016/s0014-4835(86)80082-3. [DOI] [PubMed] [Google Scholar]
- 54.Nguyen-Legros J, Versaux-Botteri C, Vernier P. Dopamine receptor localization in the mammalian retina. Mol Neurobiol. 1999;19:181–204. doi: 10.1007/BF02821713. [DOI] [PubMed] [Google Scholar]
- 55.Dearry A, Falardeau P, Shores C, Caron MG. D2 dopamine receptors in the human retina: cloning of cDNA and localization of mRNA. Cell Mol Neurobiol. 1991;11:437–453. doi: 10.1007/BF00734808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Maminishkis A, Zhi C, et al. Apomorphine regulates TGF-β1 and TGF-β2 expression in human fetal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2009;50:E-Abstract 3845. [Google Scholar]
- 57.Stone RA, Lin T, Laties AM, Iuvone PM. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA. 1989;86:704–706. doi: 10.1073/pnas.86.2.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rohrer B, Spira AW, Stell WK. Apomorphine blocks form-deprivation myopia in chickens by a dopamine D2-receptor mechanism acting in retina or pigmented epithelium. Vis Neurosci. 1993;10:447–453. doi: 10.1017/s0952523800004673. [DOI] [PubMed] [Google Scholar]
- 59.Schmid KL, Wildsoet CF. Inhibitory effects of apomorphine and atropine and their combination on myopia in chicks. Optom Vis Sci. 2004;81:137–147. doi: 10.1097/00006324-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 60.Fukuda K, Kubo T, Akiba I, Maeda A, Mishina M, Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987;327:623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- 61.Fischer AJ, McKinnon LA, Nathanson NM, Stell WK. Identification and localization of muscarinic acetylcholine receptors in the ocular tissues of the chick. J Comp Neurol. 1998;392:273–284. [PubMed] [Google Scholar]
- 62.Yin GC, Gentle A, McBrien NA. Muscarinic antagonist control of myopia: a molecular search for the M1 receptor in chick. Mol Vis. 2004;10:787–793. [PubMed] [Google Scholar]
- 63.Hutchins JB. Acetylcholine as a neurotransmitter in the vertebrate retina. Exp Eye Res. 1987;45:1–38. doi: 10.1016/s0014-4835(87)80075-1. [DOI] [PubMed] [Google Scholar]
- 64.Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Vis Neurosci. 2012;29:61–71. doi: 10.1017/S0952523811000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113:2285–2291. doi: 10.1016/j.ophtha.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 66.Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song YY, Wang H, Wang BS, Qi H, Rong ZX, Chen HZ. Atropine in ameliorating the progression of myopia in children with mild to moderate myopia: a meta-analysis of controlled clinical trials. J Ocul Pharmacol Ther. 2011;27:361–368. doi: 10.1089/jop.2011.0017. [DOI] [PubMed] [Google Scholar]
- 68.Tong L, Huang XL, Koh AL, Zhang X, Tan DT, Chua WH. Atropine for the treatment of childhood myopia: effect on myopia progression after cessation of atropine. Ophthalmology. 2009;116:572–579. doi: 10.1016/j.ophtha.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 69.Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic receptors in mouse scleral fibroblasts: prior to and after induction of experimental myopia with atropine treatment. Mol Vis. 2011;17:680–692. [PMC free article] [PubMed] [Google Scholar]
- 70.Zou L, Liu R, Zhang X, et al. Upregulation of regulator of G-protein signaling 2 in the sclera of a form deprivation myopic animal model. Mol Vis. 2014;20:977–987. [PMC free article] [PubMed] [Google Scholar]
- 71.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci. 1993;34:205–215. [PubMed] [Google Scholar]
- 72.Stone RA, Lin T, Laties AM. Muscarinic antagonist effects on experimental chick myopia. Exp Eye Res. 1991;52:755–758. doi: 10.1016/0014-4835(91)90027-c. [DOI] [PubMed] [Google Scholar]
- 73.Luft WA, Ming Y, Stell WK. Variable effects of previously untested muscarinic receptor antagonists on experimental myopia. Invest Ophthalmol Vis Sci. 2003;44:1330–1338. doi: 10.1167/iovs.02-0796. [DOI] [PubMed] [Google Scholar]
- 74.Cottriall CL, Truong HT, McBrien NA. Inhibition of myopia development in chicks using himbacine: a role for M(4) receptors? Neuroreport. 2001;12:2453–2456. doi: 10.1097/00001756-200108080-00033. [DOI] [PubMed] [Google Scholar]
- 75.Leech EM, Cottriall CL, McBrien NA. Pirenzepine prevents form deprivation myopia in a dose dependent manner. Ophthalmic Physiol Opt. 1995;15:351–356. [PubMed] [Google Scholar]
- 76.Salceda R. Muscarinic receptors binding in retinal pigment epithelium during rat development. Neurochem Res. 1994;19:1207–1210. doi: 10.1007/BF00965157. [DOI] [PubMed] [Google Scholar]
- 77.Osborne NN, FitzGibbon F, Schwartz G. Muscarinic acetylcholine receptor-mediated phosphoinositide turnover in cultured human retinal pigment epithelium cells. Vis Res. 1991;31:1119–1127. doi: 10.1016/0042-6989(91)90038-7. [DOI] [PubMed] [Google Scholar]
- 78.Sekiguchi-Tonosaki M, Obata M, Haruki A, Himi T, Kosaka J. Acetylcholine induces Ca2+ signaling in chicken retinal pigmented epithelial cells during dedifferentiation. Am J Physiol. 2009;296:C1195–C1206. doi: 10.1152/ajpcell.00423.2008. [DOI] [PubMed] [Google Scholar]
- 79.Ekman R, Tornqvist K. Glucagon and VIP in the retina. Invest Ophthalmol Vis Sci. 1985;26:1405–1409. [PubMed] [Google Scholar]
- 80.Beloukhina N, Vessey K, Stell WK. Glucagon prevents myopia via distal retina or RPE. IOVS. 2005;46:ARVO E-Abstract 3337. [Google Scholar]
- 81.Zhu X, Liu Y, Wallman J. Glucagon increases choroidal thickness of chick eyes by acting on the retinal pigment epithelium. IOVS. 2005;46:ARVO E-Abstract 3338. [Google Scholar]
- 82.Koh SW. VIP stimulation of polarized macromolecule secretion in cultured chick embryonic retinal pigment epithelium. Exp Cell Res. 1991;197:1–7. doi: 10.1016/0014-4827(91)90472-7. [DOI] [PubMed] [Google Scholar]
- 83.McGlinn AM, Baldwin DA, Tobias JW, Budak MT, Khurana TS, Stone RA. Form-deprivation myopia in chick induces limited changes in retinal gene expression. Invest Ophthalmol Vis Sci. 2007;48:3430–3436. doi: 10.1167/iovs.06-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stone RA, Khurana TS. Gene profiling in experimental models of eye growth: clues to myopia pathogenesis. Vis Res. 2010;50:2322–2333. doi: 10.1016/j.visres.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Y, Liu Y, Xu J, Nimri N, Wildsoet CF. Microarray analysis of RPE gene expression in chicks during long-term imposed hyperopic defocus. Invest Ophthalmol Vis Sci. 2010;51:E-Abstract 3680. [Google Scholar]
- 86.Zhang Y, Liu Y, Ho C, Hammond D, Wildsoet CF. Differential expression of BMP7, TGF-β2, and noggin in chick RPE after imposed optical defocus. Invest Ophthalmol Vis Sci. 2012;53:E-Abstract 3458. [Google Scholar]
- 87.Wagner DO, Sieber C, Bhushan R, Borgermann JH, Graf D, Knaus P. BMPs: from bone to body morphogenetic proteins. Sci Signal. 2010:3. mr1. doi: 10.1126/scisignal.3107mr1. [DOI] [PubMed] [Google Scholar]
- 88.Rohrer B, Stell WK. Basic fibroblast growth factor (bFGF) and transforming growth factor beta (TGF-beta) act as stop and go signals to modulate postnatal ocular growth in the chick. Exp Eye Res. 1994;58:553–561. doi: 10.1006/exer.1994.1049. [DOI] [PubMed] [Google Scholar]
- 89.McBrien NA. Regulation of scleral metabolism in myopia and the role of transforming growth factor-beta. Exp Eye Res. 2013;114:128–140. doi: 10.1016/j.exer.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 90.De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Vascularization, supporting tissue and innervation. Anat Embryol. 1997;195:393–418. doi: 10.1007/s004290050060. [DOI] [PubMed] [Google Scholar]
- 91.De Stefano ME, Mugnaini E. Fine structure of the choroidal coat of the avian eye. Lymphatic vessels. Invest Ophthalmol Vis Sci. 1997;38:1241–1260. [PubMed] [Google Scholar]
- 92.Ramrattan RS, van der Schaft TL, Mooy CM, de Bruijn WC, Mulder PG, de Jong PT. Morphometric analysis of Bruch’s membrane, the choriocapillaris, and the choroid in aging. Invest Ophthalmol Vis Sci. 1994;35:2857–2864. [PubMed] [Google Scholar]
- 93.Krebs W, Krebs I. Primate Retina and Choroid: Atlas of Fine Structure in Man and Monkey. New York: Springer-Verlag; 1991. [Google Scholar]
- 94.Wallman J, Wildsoet C, Xu A, et al. Moving the retina: choroidal modulation of refractive state. Vis Res. 1995;35:37–50. doi: 10.1016/0042-6989(94)e0049-q. [DOI] [PubMed] [Google Scholar]
- 95.Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005;46:2238–2241. doi: 10.1167/iovs.04-0956. [DOI] [PubMed] [Google Scholar]
- 96.Chakraborty R, Read SA, Collins MJ. Monocular myopic defocus and daily changes in axial length and choroidal thickness of human eyes. Exp Eye Res. 2012;103:47–54. doi: 10.1016/j.exer.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 97.Pendrak K, Papastergiou GI, Lin T, Laties AM, Stone RA. Choroidal vascular permeability in visually regulated eye growth. Exp Eye Res. 2000;70:629–637. doi: 10.1006/exer.2000.0825. [DOI] [PubMed] [Google Scholar]
- 98.Junghans BM, Crewther SG, Liang H, Crewther DP. A role for choroidal lymphatics during recovery from form deprivation myopia? Optom Vis Sci. 1999;76:796–803. doi: 10.1097/00006324-199911000-00028. [DOI] [PubMed] [Google Scholar]
- 99.Hirata A, Negi A. Morphological changes of choriocapillaris in experimentally induced chick myopia. Graefes Arch Clin Exp Ophthalmol. 1998;236:132–137. doi: 10.1007/s004170050053. [DOI] [PubMed] [Google Scholar]
- 100.Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res. 2010;91:715–720. doi: 10.1016/j.exer.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhu X, Wallman J. Opposite effects of glucagon and insulin on compensation for spectacle lenses in chicks. Invest Ophthalmol Vis Sci. 2009;50:24–36. doi: 10.1167/iovs.08-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Feldkaemper MP, Schaeffel F. Evidence for a potential role of glucagon during eye growth regulation in chicks. Vis Neurosci. 2002;19:755–766. doi: 10.1017/s0952523802196064. [DOI] [PubMed] [Google Scholar]
- 103.Sheng C, Zhu X, Wallman J. In vitro effects of insulin and RPE on choroidal and scleral components of eye growth in chicks. Exp Eye Res. 2013;116:439–448. doi: 10.1016/j.exer.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 104.Mertz JR, Wallman J. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000;70:519–527. doi: 10.1006/exer.1999.0813. [DOI] [PubMed] [Google Scholar]
- 105.Simon P, Feldkaemper M, Bitzer M, Ohngemach S, Schaeffel F. Early transcriptional changes of retinal and choroidal TGFbeta-2, RALDH-2, and ZENK following imposed positive and negative defocus in chickens. Mol Vis. 2004;10:588–597. [PubMed] [Google Scholar]
- 106.Rada JA, Hollaway LR, Lam W, Li N, Napoli JL. Identification of RALDH2 as a visually regulated retinoic acid synthesizing enzyme in the chick choroid. Invest Ophthalmol Vis Sci. 2012;53:1649–1662. doi: 10.1167/iovs.11-8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Troilo D, Nickla DL, Mertz JR, Summers Rada JA. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Invest Ophthalmol Vis Sci. 2006;47:1768–1777. doi: 10.1167/iovs.05-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hu W, Criswell MH, Fong SL, et al. Differences in the temporal expression of regulatory growth factors during choroidal neovascular development. Exp Eye Res. 2009;88:79–91. doi: 10.1016/j.exer.2008.10.014. [DOI] [PubMed] [Google Scholar]
- 109.Lambert V, Munaut C, Jost M, et al. Matrix metalloproteinase-9 contributes to choroidal neovascularization. Am J Pathol. 2002;161:1247–1253. doi: 10.1016/S0002-9440(10)64401-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y, Gillies C, Cone RE, O’Rourke J. Extravascular secretion of t-PA by the intact superfused choroid. Invest Ophthalmol Vis Sci. 1995;36:1625–1632. [PubMed] [Google Scholar]
- 111.Jobling AI, Wan R, Gentle A, Bui BV, McBrien NA. Retinal and choroidal TGF-beta in the tree shrew model of myopia: isoform expression, activation and effects on function. Exp Eye Res. 2009;88:458–466. doi: 10.1016/j.exer.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 112.Shelton L, Troilo D, Lerner MR, Gusev Y, Brackett DJ, Rada JS. Microarray analysis of choroid/RPE gene expression in marmoset eyes undergoing changes in ocular growth and refraction. Mol Vis. 2008;14:1465–1479. [PMC free article] [PubMed] [Google Scholar]
- 113.He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid during lens-induced myopia and recovery. Exp Eye Res. 2014;123:56–71. doi: 10.1016/j.exer.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.He L, Frost MR, Siegwart JT, Jr, Norton TT. Gene expression signatures in tree shrew choroid in response to three myopiagenic conditions. Vis Res. 2014;102:52–63. doi: 10.1016/j.visres.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ruiz-Moreno JM, Montero JA. Intravitreal bevacizumab to treat myopic choroidal neovascularization: 2-year outcome. Graefes Arch Clin Exp Ophthalmol. 2010;248:937–941. doi: 10.1007/s00417-010-1340-y. [DOI] [PubMed] [Google Scholar]
- 116.Feldkaemper M, Schaeffel F, Fuchs L. Vascular endothelial growth factor A, C, D and vascular endothelial growth factor receptor 1, 2, 3 mRNA expression in the chicken retina, RPE and choroid. Invest Ophthalmol Vis Sci. 2013;54:E-Abstract 5171. [Google Scholar]
- 117.Mathis U, Ziemssen F, Schaeffel F. Effects of a human VEGF antibody (Bevacizumab) on deprivation myopia and choroidal thickness in the chicken. Exp Eye Res. 2014;127:161–169. doi: 10.1016/j.exer.2014.07.022. [DOI] [PubMed] [Google Scholar]