Abstract
Erythrocyte drug encapsulation is one of the most promising therapeutic alternative approaches for the administration of toxic or rapidly cleared drugs. Drug-loaded erythrocytes can operate through one of the three main mechanisms of action: extension of circulation half-life (bioreactor), slow drug release, or specific organ targeting. Although the clinical development of erythrocyte carriers is confronted with regulatory and development process challenges, industrial development is expanding. The manufacture of this type of product can be either centralized or bedside based, and different procedures are employed for the encapsulation of therapeutic agents. The major challenges for successful industrialization include production scalability, process validation, and quality control of the released therapeutic agents. Advantages and drawbacks of the different manufacturing processes as well as success key points of clinical development are discussed. Several entrapment technologies based on osmotic methods have been industrialized. Companies have already achieved many of the critical clinical stages, thus providing the opportunity in the future to cover a wide range of diseases for which effective therapies are not currently available.
Keywords: red blood cell, encapsulation method, drug carrier, industrial development, clinical use
Introduction
The development of drug encapsulation systems has become an important approach to overcome problems of poorly stable, fragile, or potentially immunogenic drugs. Over the past two decades, the application of the erythrocyte as drug carrier has gained extensive interest. Indeed, this biocompatible vehicle exhibits many advantageous features compared to colloidal or synthetic carriers such as liposomes, nanoparticles, emulsions, viruses, and pegylated formulations.1 In addition to its long life span in circulation (80–130 days), the erythrocyte displays a relatively inert intracellular environment and offers specific targeting to the reticuloendothelial system (RES).2–7 This new therapeutic approach may palliate some of the unmet needs in the fields of oncology, rare diseases, and inflammatory conditions.
Technically, there are two main approaches to combine drugs with erythrocytes. The first one is by means of forming transient pores in the erythrocyte membrane, thus allowing for the drug to enter the erythrocyte. This intraerythrocytic loading method is used with the aim to greatly enhance the half-life of the drug and to avoid hypersensitive reactions in patients by shielding the drug from the immune system.8 The second strategy consists of coupling the active drug to the erythrocyte membrane, thus exposing it at the surface of the cell. Although this latter method has shown efficiency in the slow release of drugs,6 it cannot be used to target the RES or to reduce allergenic reactions by protecting the drug from the extracellular environment. The present discussion essentially focuses on the encapsulation of drugs within erythrocytes, as it meets a greater number of therapeutic needs. Different methods have been developed for the incorporation of active ingredients within erythrocytes.9 Among these methods, the most popular are osmotic based, electroporation and endocytosis, while other methods, such as chemical perturbation of the membrane,10 electric cell fusion,11 and lipid fusion,12 remain less explored. The ultimate goal in drug development is to enter the clinic, thus requiring large-scale production. The aims of this paper are to provide an overview of the most suitable encapsulation methods for industrial development and to discuss the main key milestones toward marketing approval.
Overview of drug encapsulation methods
The development of clinical therapies based on erythrocyte carriers necessitates an automation of the encapsulation method. In addition, while some conditions need treatments that could employ only a few milliliters of processed cells, other treatments such as amino acid depletion for tumor starvation require the processing of large volumes of blood (250–350 mL). These technical constraints deeply reduce the spectrum of clinical applications that may emerge from laboratory experiments. The following section provides an overview of the advantages and disadvantages of each encapsulation approach and provides a discussion on the most suitable approaches for industrialization.
Endocytosis
Ginn et al discovered that endocytosis may be induced in mature erythrocytes by drugs of a specific chemical structure.13 Molecules intercalate passively into the inner membrane of the phospholipid bilayer of the erythrocytes. This process ends with the formation of vacuoles (containing the drug) within the intraerythrocytic compartment. The performance of the process is dependent on drug concentration, pH (7.9–8.1), and temperature (37°C). However, the method is only suitable for the entrapment of cations or anions, which have both hydrophobic and hydrophilic groups.14–17 This limitation combined with an evident lack of process reproducibility makes it incompatible with an industrial development.
Conjugation to cell-penetrating peptides
This method consists of the covalent linking of cell-penetrating peptide (CPP) to the molecule of interest via a disulfide linkage. The transfer of the peptide across the cell membrane is made through a process of protein transduction, delivering the therapeutic molecule within the cell. Different types of cells can internalize the CPP-conjugated molecule. This process was specifically applied to erythrocytes with the encapsulation of CPP-conjugated L-asparaginase, an enzyme that displays therapeutic benefits in the treatment of acute lymphoblastic leukemia.18,19 Within the erythrocyte, CPP is dissociated from L-asparaginase via reduction in disulfide bonds by intraerythrocytic glutathione and reductases. Although this entrapment method presents the advantage of being noninvasive toward the erythrocyte, its use for clinical applications may be compromised by several aspects. First, it has not been demonstrated that CPP-mediated entry is irreversible and CPPs have been found to be enriched in the extracellular medium supporting a reversibility of the phenomenon. Second, the reproducibility of encapsulation is highly dependent on the cellular reductase activities that may be difficult to handle at the industrial level. Third, although the last generation of CPP such as low molecular weight protamine appears to be nonimmunogenic and less toxic, this safety aspect needs careful consideration in view of chronic exposure in patients.
Dimethyl sulfoxide osmotic pulse
This method was essentially used for the incorporation of inositol hexaphosphate (IHP), an allosteric effector of hemoglobin.20,21 The method is performed using four steps: first, dimethyl sulfoxide (DMSO) is added to red blood cells (RBCs) to raise the osmolality of suspension (between 1,000 mOsmol/kg and 1,500 mOsmol/kg, depending upon DMSO concentration). Then, erythrocytes are diluted with an isotonic solution of the drug. This induces a rapid decrease in DMSO concentration in the extracellular compartment and a transient gradient across the erythrocyte membrane. This gradient is accompanied by an influx of water and drug loading until equilibrium between intra- and extraerythrocytic DMSO concentrations is reached. This mechanism is possible because the DMSO escape from the cell is slower than the entry of water into the cell. The DMSO method results not only in highly loaded RBCs but only in a small fraction of cells (a total of 9%–25%).22 To isolate this “IHP-enriched” cell fraction, some researchers conducted a Percoll separation but this technique is time-consuming and costly, and therefore, this technique may be only applicable at the laboratory scale.
Electroporation
This method has been applied to numerous living cells by Tsong23 and by Tsong and Kinosita.24 It consists of generating an electric shock to induce erythrocyte membrane permeability in isotonic solutions. The potential difference created across the membrane leads to the formation of pores in the erythrocyte membrane. Ions are rapidly distributed between the intra- and extracellular compartments causing erythrocyte swelling and drug entry. This method has been successfully used for the encapsulation of several molecules and macromolecules in human erythrocytes.25–27 In 1995, Nicolau et al developed a large-scale, continuous-flow electroporation system to load IHP.28–30 Unfortunately, the device was not suitable for processing large volumes of blood (only 20–50 mL) which may limit clinical application.
Osmotic-based methods
On the contrary to the methods mentioned earlier, osmotic-based methods present flexible process parameters allowing the development of specialized equipment capable of handling large volumes of blood. These methods are all based on the osmotic pressure difference between the inside and outside of the erythrocyte. Among the three existing methods (hypotonic preswelling, hypotonic dilution, and hypotonic dialysis), the hypotonic preswelling and the hypotonic dialysis processes remain the more scalable ones. The common sequential steps to obtain lysed–resealed erythrocytes by these techniques include: 1) isotonic washings of cells, 2) contact with hypotonic medium permitting pore opening in the erythrocyte membrane diffusion of drug in the intracellular compartment, and 3) after hypertonic/iso-osmotic buffering, resealing of pore restoration of isotonicity. The concept and the robustness of osmotic methods have led to several companies embarking on the industrial development of drug-loaded human erythrocytes and the development of apparatus suitable for clinical applications.
Hypotonic preswelling
This two-step technique is based on the controlled swelling of erythrocytes. First, cells are washed with slightly hypotonic buffered solution. Under these conditions, the most fragile cells hemolyze and the remaining intact ones swell until reaching roughly 150% of their initial volume. After centrifugation, the supernatant is discarded and the pellet of preswelled RBCs is isolated. In the second step, a hypotonic aqueous solution of the drug is prepared and carefully added to the washed erythrocytes in small volumes. Between each drug addition step, the suspension is centrifuged. The process is continued until a lysis point is reached (disappearance of distinct frontier between packed cells and the supernatant after centrifugation). The slow process of swelling results in a good retention of cytoplasmic components. RBCs are then resealed by adding a calculated volume of isotonic buffer and then incubation at 37°C.31 Small molecules have been successfully encapsulated at the laboratory scale using this method.32,33 In 1998, Magnani et al developed a medical device for erythrocyte loading termed the “Red Cell Loader” (proprietary technology of EryDel S.p.A., Urbino, Italy). The method of entrapment is based on the “preswell” of erythrocytes with two sequential hypotonic dilutions of washed erythrocytes followed by a concentration step using a hemofilter. The operation takes roughly 2 hours.34 The process is usually performed in the blood transfusion service of the hospital using homologous or autologous erythrocytes and specific disposable kits depending on the procedure to apply. By allowing rapid administration of loaded erythrocytes to the patient, the use of Red Cell Loader avoids problems of in vitro drug leakage. This strategy may be useful for the administration of drugs for which slow and prolonged release into circulation is needed.35 The limitation of this industrial procedure is the volume of processed erythrocytes which has been standardized to only 50 mL in order to prevent discomfort of patients (patents WO 2011/135429 A1 and WO 2011/135431 A1).36 Such low volumes may not be suitable for enzyme therapies employed for the purpose of systemic detoxification.
Hypotonic dialysis
This method consists of using semipermeable membranes for drug incorporation. In the majority of cases, the molecule to be entrapped has a molecular weight greater than the cutoff of the dialysis membrane and is added to erythrocyte suspension at the beginning of the process. Technically, there are two main ways to perform hypotonic dialysis. The first method uses dialysis bags. The dialysis bag containing a buffered suspension of erythrocytes is immersed into 10–20 volumes of a hypotonic buffer and gently mixed for at least 1 hour to allow pore opening and drug entry.37 This procedure has been successfully used clinically. Over a period of 17 years, a patient suffering from adenosine deaminase (ADA) deficiency was treated at 2–3 weekly intervals with ADA-loaded autologous erythrocytes (prepared in dialysis bags). The treatment proved to be metabolically and clinically effective.38 The same process was used to load thymidine phosphorylase (TP), and therapeutic benefits were observed in patients affected with mitochondrial neurogastrointestinal encephalopathy (MNGIE).39 Both ADA-RBCs and TP-RBCs were manufactured according to the current good manufacturing practice (cGMP) guidelines in a pharmaceutical environment. However, in order to reach industrial manufacturing, this loading process using dialysis bags requires optimization in terms of shelf life, automation, and large-scale feasibility.
The second method, which is far more rapid and effective, is little described in the literature. With this method, the dialysis bag is replaced by a dialysis cartridge composed of multiple hollow fibers (hemodialyzer) and is much more convenient for treating large volumes of blood. The buffered RBC suspension containing the drug is introduced into the blood compartment of the dialyzer by the means of peristaltic pump. Drug entry into erythrocytes is performed by contact of the cell suspension with a hypotonic buffer running at countercurrent into the hemodialyzer.40,41 In 1982, Ropars et al patented an automated process for the internalization of drugs into erythrocytes using the encapsulation of IHP as an illustration.42 Although the apparatus guarantees pyrogen-free and sterile erythrocyte suspensions, it was not tested in the clinic. In 2006, Godfrin optimized this process and developed an apparatus termed the “ERY-caps®” for industrial application (proprietary technology of ERYTECH Pharma, Lyon, France).43 On the contrary to the Red Cell Loader described previously, the ERYcaps® provides drug-loaded RBCs prepared from homologous erythrocytes in a dedicated cGMP pharmaceutical unit. The whole process takes roughly 3 hours and erythrocyte pellets of 250–350 mL can be treated (patented process).43 This strategy can be successfully used to prolong the circulatory half-life of active macromolecules compared to that of erythrocyte half-life.
In the “Overview of drug encapsulation methods” section, we made an inventory of existing loading procedures and discussed the feasibility of industrial transposition. The arguments were put forward to show that osmotic-based methods (preswelling and hypotonic dialysis) are the best candidates for industrial development, providing greater ease to build customized and automated machinery as compared to other encapsulation methods. Several companies have invested in this innovative technology with the aim of offering patients the benefits of erythrocytes as drug carriers.
Focus on promising therapeutic applications
Although the erythrocyte is anucleate, it remains a living cell. Therefore, the success of drug encapsulation is conditioned by two main biological parameters:
The diameter of pores opened in the erythrocyte membrane.
The chemical nature of the erythrocyte membrane.
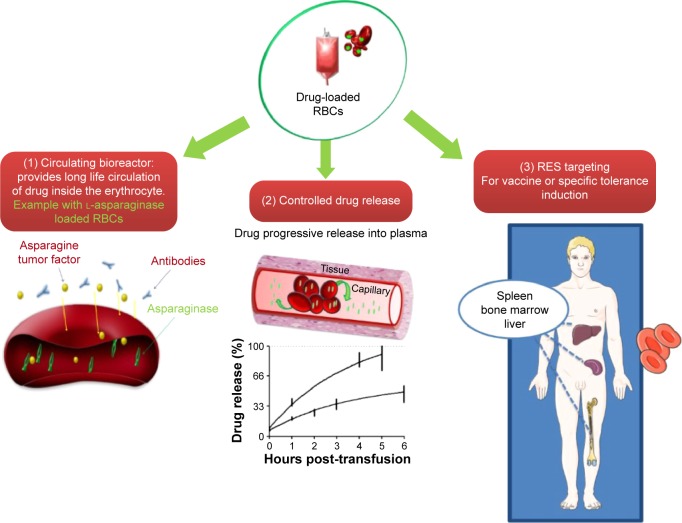
In our experience, the most “incorporable” molecules are macromolecules ranging roughly from 1,000 Da to 500,000 Da. The encapsulation of small molecules is associated with an increased probability of drug leakage unless the drug presents phosphate groups that facilitate drug retention within the cell, for example, IHP. Therefore, drug-loaded erythrocytes tend to be naturally designed to offer potential therapies for metabolic diseases and enzyme deficiencies, needing the encapsulation of macromolecules, rather than for infectious diseases or hepatic metastases for which reference drugs are often nonencapsulable small molecules (ie, nonpolar antibiotics and 5-fluorouracil, respectively). In addition, the mechanism by which the erythrocytes are exploited is very important for successful industrial development. There are three major possible uses of erythrocytes as drug carriers (Figure 1).
Figure 1.
Schematic of possible therapeutic applications of erythrocyte drug carriers: (1) as a circulating bioreactor, (2) for controlled drug release, and (3) as for targeting RES.
Abbreviations: RES, reticuloendothelial system; RBCs, red blood cells.
Drug-loaded erythrocytes as circulating “bioreactors”
The objective of using erythrocytes as bioreactors is the clearance of undesired molecules from the bloodstream. For instance, L-asparaginase-loaded RBCs were developed for the degradation of plasmatic asparagine. It is the simplest application and the more natural one since it does not require any cell treatment in addition to the loading procedure. In this system, the erythrocyte is actively involved in the efficacy of the medicinal product via:
Drug-loaded erythrocytes as a slow-release system
The use of erythrocytes as a controlled release of drug is predominantly devoted to small molecules that are minimally retained within erythrocytes and progressively leak from the cell into the plasmatic compartment. The major problem with the slow release is that once encapsulation is performed, the molecule may escape rapidly. To address this problem, Magnani et al patented a procedure that retains small drugs within the intra-erythrocytic compartment by means of high-affinity intracellular components.46 Immunophilin proteins that noncovalently bind to immunosuppressive drugs are entrapped within erythrocytes to increase drug retention.47 Although clever and suitable for industrial application, this method is restricted to molecules that are ligands of proteins. Another possibility is to encapsulate a prodrug that will be progressively cleaved by intraerythrocytic enzymes to deliver the active drug. This method has been successfully applied to the entrapment of dexamethasone-21-phosphate, the active drug (dexamethasone) being released after the removal of the phosphate group by intraerythrocytic esterases. For other drug classes, researchers have resorted to crosslinking agents such as glutaraldehyde (GA) and bis(sulfosuccinimidyl) suberate (BS3) to rigidify the erythrocyte membrane and reduce leakage.15,48–51 The resulting chemically modified erythrocytes present a marked decrease in deformability and membrane fluidity. This membrane alteration reduces erythrocyte biocompatibility and leads to their rapid elimination by the monocyte–macrophage system of the spleen.52 Thus, the employment of crosslinkers to induce “slow drug release” is not recommended for clinical use. Recently, the coating of loaded erythrocytes with biocompatible polyelectrolytes (poly-L-lysine hydrobromide and dextran sulfate) has been explored to counterbalance the inability of erythrocytes to control protein and drug release.53 It was demonstrated that adjusting the number of polyelectrolyte layers could regulate the release profile of the encapsulated protein. However, the industrial feasibility of polyelectrolyte layers may be an issue.
Drug-loaded erythrocytes for RES targeting
The third application consists of using RBCs to target the RES, also referred to as the monocyte–macrophage system. Like many other cells, erythrocytes express phosphatidyl serines when entering apoptotic phase which act as cell surface signals for recognition by phagocytic cells. After erythrocyte engulfment by the macrophage, the encapsulated protein is degraded into the lysosomal compartment in order to be presented to the immune cells. The senescent RBC is thus an excellent antigen carrier for vaccination or tolerance induction. Ex vivo, the erythrocytes may be artificially pre-aged to increase their uptake by phagocytic cells. The methods of pre-aging can be enzymatic, thermal, or chemical. In preclinical studies, the use of neuraminidase enzyme to remove sialic acids from erythrocyte membranes in order to induce cell senescence has been explored.54 The heterogeneity of the enzymatic activity, however, may lead to nonreproducible drug-loaded suspensions. Heat treatment (ie, 42°C for 90 minutes or 48°C for 30 minutes) has also been proposed for the pre-aging of processed cells by energy depletion.54,55 As previously mentioned, crosslinkers may also be used to target the spleen and the liver. GA reacts extensively with proteins in the erythrocyte membrane. The resulting erythrocytes are characterized by an extreme rigidity compromising their use for in vivo applications. Using BS3 agent, the treatment is less harsh and leads to the crosslinking of specific proteins such as Band 3.56 BS3-treated erythrocytes are less rigid than GA-treated cells and thus appear more suitable for clinical use. It is also possible to enhance erythrocyte senescence by using a BS3/ZnCl2 combination which is described to induce autologous IgG binding and complement fixation (opsonization), thus favoring the phagocytosis of ZnCl2/BS3-treated cells by macrophages.57
The two main applications of the RES-targeting strategy by erythrocytes are as follows:
The delivery of small chemical entities such as antiretroviral nucleoside analogs.58,59
The encapsulation of antigens for vaccination or tolerance induction.41,55,60–64
Because of protein degradation by lysozyme, the delivery of intact therapeutic proteins is more challenging. However, among possible applications of delivery with large macromolecules, one can cite enzyme replacement therapies for lysosomal storage diseases for which the targeted compartment is the lysosome itself.
Erythrocyte carriers present a large number of therapeutic applications. This innovative technology is expected to alleviate treatment regimens and to reduce hospitalization frequency and duration.65 The success of the pharmaceutical development essentially depends on how well the companies heighten the clinician community’s understanding of this exciting technology and stimulate its market uptake. At present, the therapeutic applications of RBCs for controlled drug release as circulating bioreactors are undoubtedly the two best approaches to accelerate market entry. The use of the erythrocyte carrier for vaccination or tolerance will require specialized development steps.
Product quality
Cell material source
Drug-loaded erythrocytes can be prepared from autologous or homologous donated blood. Autologous erythrocyte processing is usually performed using bedside medical devices. In the case of homologous transfusion, the cell material source consists of erythrocyte pellets provided by blood banks. Erythrocytes are prepared from whole blood collected from healthy donors and subjected to leukocyte depletion (criterion of acceptance <5×106/unit). The appropriate erythrocyte pellet is selected according to patient’s ABO blood type and valid irregular antibody screening. In order to avoid the processing of pellets that present storage lesions, erythrocyte units of <10 days on the day of processing are used.
Qualification of the drug to be encapsulated
Since the manufacturing process of drug-loaded erythrocytes is particularly new for regulatory bodies, existing guidelines are not well adapted and it is difficult to qualify the status of both intermediates and final products. The drug to be encapsulated may be considered as an active pharmaceutical ingredient (API) starting material. If the drug is already on the market, all pharmaceutical information will be available on a certificate of analysis provided by the manufacturer. In other cases, it is the responsibility of the company developing the erythrocyte carrier to manufacture the active drug in compliance with cGMP and to ensure its full characterization with analytical procedures meeting all the requirements laid down in ICH guidelines Q2A, Q2B, Q3A, Q3C, and Q6A.66
Scale-up
The scaling-up process from laboratory scale to clinical production may be critical in the manufacturing of drug-loaded erythrocytes. The aim of the scale-up process in erythrocytes manufacturing is to encapsulate the therapeutic substance in an efficient and fast way to produce drug-loaded erythrocytes with good yield. Although drug-loaded erythrocytes are biological products, the process can be long and difficult.
Manufacturing process
The manufacturing of drug-loaded erythrocytes can be either centralized or performed in the clinic. In each case, all safety measures must be taken to maintain the sterility of the cell suspension, through the use of sterile disposable materials and closed circuit procedures. In addition, apparatus such as the Red Cell Loader is required to be in compliance with the European Medical Devices Directive 93/42. For the centralized manufacturing of loaded erythrocytes, the facility must be maintained under A/B air grade and fully compatible with current pharmaceutical requirements.
The regulatory aspects at European level are reflected in European Medicines Agency guidelines for advanced therapy medicinal products.67 In addition, cGMPs for the manipulation of cells for clinical use are also based on the Code of Federal Regulations, 21 Part 211 through the US Food and Drug Administration Tissue Reference Group and the Japanese guidelines for the use of medical devices based on human autologous cells.68–71
The irradiation of final product to lower the risk of graft-versus-host disease appears to be unnecessary due to the significant reduction in leukocyte numbers achieved with the manufacturing process (80-fold leukocyte reduction as compared to classical stored erythrocytes using ERYcaps® manufacturing process).
Product storage
Storage properties may differ depending on the preservative solution and the container used for final product. The storage containers commonly used for erythrocyte products are plasticized polyvinyl chloride bags. Care should be taken to select bags that contain plasticizers which are essentially non-extractable in blood. In order to strengthen product safety, the evaluation of extracted substances from selected final container would provide more confidence with regard to the medical use of the erythrocyte product. Unlike long shelf-life drugs, drug-loaded erythrocytes must be rapidly administered to patients to avoid storage-induced RBC degradation. The nature of the solution used to preserve the product plays a major role in the quality and stability of modified cells. Although SAGM (saline, adenine, glucose, and mannitol) is the most widely used erythrocyte storage solutions, it has not been licensed by the US Food and Drug Administration, and hence is not used in the USA. Other solutions have been developed and commercialized: AS-1, AS-3, AS-5, MAP, erythrosol-4, PAGGGM, and PAGGSM, some of which have been demonstrated to reduce hemolysis upon storage as compared to SAGM.72 Recently, an experimental filter has been developed to improve the quality of the erythrocytes during storage.73
In vitro characterization of product
In the past decade, many cellular therapeutics have failed to obtain marketing approval due to an inadequate characterization of the end product.74 Therefore, it is extremely important to conduct an extensive characterization during clinical development in order to consolidate knowledge of the product and to provide regulatory authorities with reassurance with regard to its safety. As drug-loaded erythrocytes are considered as new products, the safety and quality questions raised are essentially related to the impact of manufacturing process on erythrocyte functionality and viability. To answer those questions, a battery of laboratory tests must be performed on the product to catalog any physical or biochemical changes in the erythrocyte as a result of processing. Changes that usually occur following the osmotic entrapment process include erythrocyte hemolysis, loss of intraerythrocytic components, reduction in erythrocyte membrane plasticity, and decrease in mean cell volume. The manufacturing process should not lead to changes >20% in order to retain as much as possible the integrity of the erythrocytic cell.
The loss of intracellular material upon processing may be evaluated with the assessment of cellular hemoglobin content. Changes in erythrocyte deformability may be explored by ektacytometry, a technique that measures the elongation of suspended RBCs subjected to a range of shear stresses.75 It has been reported that human erythrocytes loaded with IHP-RBCs have a reduced deformability and aggregability and increased viscosity as compared to normal erythrocyte suspension at the same hematocrit.76 The osmotic fragility may be determined to have an in vitro evaluation of erythrocyte viability, though it may not be predictive of product in vivo behavior.77 It is also important to measure concentrations of essential metabolites such as adenosine triphosphate and glucose and to have recourse to supplementation in case of deficiencies in the final product. In addition, erythrocyte functionality (oxygen-carrying capacity) should also be controlled with the setup of specific tests including plotting the oxygen dissociation curve (for the evaluation of P50 value and arteriovenous oxygen saturation differences) and the assay of intraerythrocytic content of 2,3-diphosphoglycerate (natural allosteric effector of hemoglobin).78 If the measured values are significantly decreased as compared to physiological values, a “rejuvenation” procedure should be included in the process.
Clinical batch release
For clinical batch release, it is crucial to conduct a drug assay and to measure extracellular hemoglobin level to guarantee the quality and the safety of the product at the time of patient infusion. In the case of centralized manufacturing, the clinical batch is characterized on-site in the quality control laboratory. If the product conforms to specifications, it will be released by the head pharmacist and sent to the hospital as a ready-to-use blood unit with a 72-hour shelf life.39 In the case of bedside apparatus, each hospital using the technology is under obligation to validate analytical methods and to have access to qualified personnel in order to be able to release the clinical batch. For products prepared from autologous erythrocytes, the major hurdle is the short time frame from blood collection to reinfusion leaving very little time for characterization.
In vivo characterization of product
Because of the difficulty of extrapolating in vitro results, in vivo studies remain the gold standard to evaluate the quality of new erythrocyte products. The primary test is based on the evaluation of percentage of red cells recovered 24 hours following transfusion. Indeed, poorly viable cells are usually removed from blood circulation within that period of time. The method usually recommended to assess the survival of stored RBCs is the sodium [51Cr] chromate radiolabeling technique.79,80 However, this method requires pretransfusional manipulations of cells including room temperature incubation and successive washings that may damage the blood product. In addition, it was reported that [51Cr] chromate elutes at a rapid rate during the 24–48 hours following transfusion leading to an underestimation of 24-hour cell survival as well as erythrocyte life span (3.5-fold reduction in life span in baboon as compared to nonradioactive method).80,81 Therefore, a nonradioactive and noninvasive method is preferred. In 2003, Zeiler et al exploited antigen mismatching for the quantification of different RBC populations by flow cytometry.82 Thus, drug-loaded erythrocytes prepared with RhD(−) erythrocytes and transfused to RhD(+) patients could easily be detected by flow cytometry using anti-antigen D antibodies. No pretransfusional treatment is required. In addition, withdrawn blood samples can be directly labeled with fluorochrome-coupled primary antibodies avoiding cell loss due to washings/centrifugation steps and thus increasing accuracy of method.
According to published data, both autologous erythrocytes loaded with dexamethasone-21-P (prepared with Red Cell Loader) and homologous RBC loaded with L-asparaginase (prepared with ERYcaps®) have 24-hour survival above the recommended threshold of 75%. In addition to the 24-hour survival, the evaluation of the pharmacokinetic (PK) and the pharmacodynamic parameters is requested as for any other drug. This includes the determination of in vivo half-life as well as the monitoring of drug efficiency. As a result of product novelty, very few data are published on the PK parameters of drug-loaded erythrocytes. The half-life of L-asparaginase encapsulated into erythrocytes was reported to be ~28 days.65 Erythrocytes loaded with dexamethason-21-P was slightly lower at 23 days.34 These values are close to values found for conventionally stored erythrocytes (28±2 days).
The industrial challenge
Companies developing erythrocyte carriers face three key challenges.
A high-performance and reproducible process
One of the main challenges for industrialization success is to develop a reproducible loading procedure. For that purpose, it is indispensable to take into account the source cell variability. Indeed, significant differences in erythrocyte resistance to lysis (osmotic fragility) may be observed between batches of erythrocyte starting material (from 2 g/L to 5 g/L) depending on multiple parameters such as donor age or blood storage conditions. In order to avoid substantial differences in quality of end products and to guarantee a consistent encapsulation rate, process operating conditions should be adjusted (ie, erythrocyte flow rate and osmolality of buffers) according to the osmotic fragility of the initial donor erythrocyte pellet.83 In addition, in the patented manufacturing process based on hypotonic dialysis (WO 2006/016247), it was postulated that the osmotic fragility should not be measured on the initial RBC pellet but on washed RBCs in the presence of the drug of interest. Indeed, this latter suspension is fully representative of the suspension intended for lysis–resealing procedure and allows consideration of the possible toxic or protective effects of drug toward the erythrocytic cell.43 Considering the application of carrier erythrocytes for human therapies, quality-by-design (QbD) criteria should also be considered to identify critical points and reduce risks during the manufacturing processes.
Low cost of goods
The price of the API starting material may vary widely depending on whether the drug is under patent protection, commercially available as a reference drug or generic form, or whether it requires cGMP manufacturing. Indeed, for the encapsulation of commercially unavailable macromolecules, the outsourcing of cGMP manufacturing to a contract manufacturing organization may bring an additional cost of ~€500k–1,500k to product development. This may significantly impact on the price of the final drug-loaded product and compromise project viability. Moreover, additional costs such as infrastructure costs should be considered. This forces industry to reach high encapsulation rates to minimize the waste of API starting material and to render products more profitable. According to published data, the ERYcaps® and the Red Cell Loader encapsulating procedures present similar encapsulation rates of ~30%.34,65 Improving this yield should reduce the cost of goods.
An efficient logistic
Companies should develop efficient logistics to enable personalized medicine requirements to be met. The stability of the product is a key parameter to manage both clinician demand and manufacturing constraints. The security and the traceability of the cold-chain distribution also constitute major challenges, especially for centralized manufacturing with a supply chain covering international distances. Specialized logistics companies are now providing temperature sensors that detect and transmit data on temperature upon product shipment.
Current clinical status of erythrocyte carriers
Due to the leaders in the industrial development of erythrocytes carriers, the technology is now reaching the late stage of pharmaceutical development. Companies have end-stage products in their pipeline and sufficient clinical evidence to overcome the last but not least step toward marketing approval.
ERYTECH Pharma based in (Lyon, France) developed a therapeutic solution (GR-ASPA) for the treatment of acute lymphoblastic leukemia: positive results were obtained in children and adults (Phase I/II8 and randomized international Phase III [NCT01518517] studies) as well as in elderly patients (Phase II trial).84 Patients administered with homologous erythrocytes loaded with L-asparaginase (GR-ASPA) showed a reduction in the number and the severity of allergic reactions as compared to the free enzyme.8,65 A Phase I clinical study was launched in the USA (NCT0181010705). Other clinical trials were initiated in Europe to extend GR-ASPA therapeutic applications. A trial IIb is ongoing in acute myeloid leukemia (NCT01810705). Preclinical animal studies showed the potential therapeutic benefit of this product in solid tumors. An orphan drug designation (ODD) status was granted for pancreatic cancer and a Phase I escalade dose trial was recently completed (NCT01523808). An early-stage product named ENHOXY® has also been developed as a powerful oxygen enhancer in the fields of sickle cell anemia (ODD granted in 2012) and more generally hypoxia-related diseases.85
EryDel, in Italy, has developed EryDex, a system to deliver dexamethasone at stable low systemic levels over an extended period of time. The benefits of treatment were demonstrated in the frame of clinical pilot studies in patients with chronic obstructive pulmonary disease,86 cystic fibrosis,87 ulcerative colitis, and Crohn’s disease.88 More recently, the product presented a great therapeutic interest for ataxia telangiectasia, a rare autosomal recessive neurological disorder with high mortality.89 A pilot Phase II trial demonstrated statistically significant efficacy of product (NCT01255358).90,91 The product obtained ODD status in 2013. A Phase I PK study of EryDex is ongoing in the USA, and a pivotal Phase III trial should be initiated soon.
Orphan Technologies Ltd (Rapperswil, Switzerland), is a newly created company dedicated to the treatment of rare and ultrarare diseases. The company recently acquired the license for developing OT-15-MNGIE (TP-loaded erythrocytes for the treatment of MNGIE) and OT-81-ADA-SCID (ADA-loaded erythrocytes) for the treatment of severe combined immunodeficiency. Both products are currently administered to humans under compassionate use and have not yet entered Phase I clinical trials.
Conclusion
This review addresses the use of resealed erythrocytes for clinical application. Although the field of potential therapeutic applications described by laboratory experiments is broad, the pathway to the clinics is long and it is therefore important to address both industrial feasibility and safety considerations early on. In the present paper, we described the most commonly used entrapment methods. All procedures have benefits and drawbacks. Osmotic-based methods are associated with high process performance and reproducibility and are therefore the most suitable ones for industrial development. The main daunting challenges for manufacturing success include production scalability, process validations, and quality control of released therapeutic. Several companies embarked on the development of drug-loaded erythrocytes with specific apparatus. The Red Cell Loader and the ERYcaps® are two promising technologies with complementary therapeutic applications. The Red Cell Loader was successfully used for controlled drug release. Enzyme-loaded erythrocytes through the ERYcaps® have already proven clinical therapeutic benefits as compared to the free enzyme, reducing the dosage amount and frequency and improving patients’ quality of life. The use of the erythrocyte as drug carrier is a fundamental advancement in the current exploitation of blood and may be applicable in medical fields for which no effective therapy is currently available.
Footnotes
Disclosure
Vanessa Bourgeaux is an employee of ERYTECH Pharma. Yann Godfrin is the Vice President of ERYTECH Pharma. Bridget E Bax is a member of the Scientific Advisory Board of ERYTECH Pharma. The authors report no other conflicts of interest in this work.
References
- 1.Pierige F, Serafini S, Rossi L, et al. Cell-based drug delivery. Adv Drug Deliv Rev. 2008;60(2):286–295. doi: 10.1016/j.addr.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 2.Patel RP, Patel MJ, Patel NA. An overview of resealed erythrocyte drug delivery. J Pharm Res. 2009;2(6):1008–1012. [Google Scholar]
- 3.Millan CG, Marinero ML, Castaneda AZ, et al. Drug, enzyme and peptide delivery using erythrocytes as carriers. J Control Release. 2004;95(1):27–49. doi: 10.1016/j.jconrel.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Gothoskar AV. Resealed erythrocytes: a review. Pharmaceutical Technology [serial on the internet]; 2004. [Accessed December 6, 2015]. pp. 140–158. Available from: http://www.pharmtech.com. [Google Scholar]
- 5.Harisa GE, Ibrahim MF, Alanazi FK, et al. Application and safety of erythrocytes as a novel drug delivery system. Asian J Biochem. 2011;6:309–321. [Google Scholar]
- 6.Muzykantov VR. Drug delivery by red blood cells: vascular carriers designed by mother nature. Expert Opin Drug Deliv. 2010;7(4):403–427. doi: 10.1517/17425241003610633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gutierrez MC, Colino Gandarillas CI, Sayalero Marinero ML, et al. Cell-based drug-delivery platforms. Ther Deliv. 2012;3(1):25–41. doi: 10.4155/tde.11.141. [DOI] [PubMed] [Google Scholar]
- 8.Domenech C, Thomas X, Chabaud S, et al. l-asparaginase loaded red blood cells in refractory or relapsing acute lymphoblastic leukaemia in children and adults: results of the GRASPALL 2005-01 randomized trial. Br J Haematol. 2011;153:58–65. doi: 10.1111/j.1365-2141.2011.08588.x. [DOI] [PubMed] [Google Scholar]
- 9.Tirupathi Rao K, Suria Prabha K, Muthu Prasanna P. Resealed erythrocytes: as a specified tool in novel drug delivery carrier system. Res J Pharm Biol Chem Sci. 2011;2(4):496–512. [Google Scholar]
- 10.Kitao T, Hattori K. Erythrocyte entrapment of daunomycin by amphotericin B without hemolysis. Cancer Res. 1980;40(4):1351–1353. [PubMed] [Google Scholar]
- 11.Li LH, Hensen ML, Zhao YL, et al. Electrofusion between heterogeneous-sized mammalian cells in a pellet: potential applications in drug delivery and hybridoma formation. Biophys J. 1996;71(1):479–486. doi: 10.1016/S0006-3495(96)79249-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicolau C, Gersonde K. Incorporation of inositol hexaphosphate into intact red blood cells. I. Fusion of effector-containing lipid vesicles with erythrocytes. Naturwissenschaften. 1979;66(11):563–566. doi: 10.1007/BF00368810. [DOI] [PubMed] [Google Scholar]
- 13.Ginn FL, Hochstein P, Trump BF. Membrane alterations in hemolysis: internalization of plasmalemma induced by primaquine. Science. 1969;164(3881):843–845. doi: 10.1126/science.164.3881.843. [DOI] [PubMed] [Google Scholar]
- 14.Harisa GE, Ibrahim MF, Alanazi FK. Characterization of human erythrocytes as potential carrier for pravastatin: an in vitro study. Int J Med Sci. 2011;8(3):222–230. doi: 10.7150/ijms.8.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talwar N, Jain NK. Erythrocyte based delivery system of primaquine: in vitro characterization. J Microencapsul. 1992;9(3):357–364. doi: 10.3109/02652049209021250. [DOI] [PubMed] [Google Scholar]
- 16.Schrier SL, Junga I. Entry and distribution of chlorpromazine and vinblastine into human erythrocytes during endocytosis. Proc Soc Exp Biol Med. 1981;168(2):159–167. doi: 10.3181/00379727-168-41252. [DOI] [PubMed] [Google Scholar]
- 17.Greenwalt TJ, Lau FO, Swierk EM, et al. Studies of erythrocyte membrane loss produced by amphipathic drugs and in vitro storage. Br J Haematol. 1978;39(4):551–557. doi: 10.1111/j.1365-2141.1978.tb03625.x. [DOI] [PubMed] [Google Scholar]
- 18.Kwon YM, Chung HS, Moon C, et al. L-Asparaginase encapsulated intact erythrocytes for treatment of acute lymphoblastic leukemia (ALL) J Control Release. 2009;139(3):182–189. doi: 10.1016/j.jconrel.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He H, Ye J, Wang Y, et al. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J Control Release. 2014;176:123–132. doi: 10.1016/j.jconrel.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Franco RS, Weiner M, Wagner K, et al. Incorporation of inositol hexaphosphate into red blood cells mediated by dimethyl sulfoxide. Life Sci. 1983;32(24):2763–2768. doi: 10.1016/0024-3205(83)90397-1. [DOI] [PubMed] [Google Scholar]
- 21.Franco R, Barker R, Mayfield G, et al. The in vivo survival of human red cells with low oxygen affinity prepared by the osmotic pulse method of inositol hexaphosphate incorporation. Transfusion. 1990;30(3):196–200. doi: 10.1046/j.1537-2995.1990.30390194336.x. [DOI] [PubMed] [Google Scholar]
- 22.Mosca A, Paleari R, Russo V, et al. IHP entrapment into human erythrocytes: comparison between hypotonic dialysis and DMSO osmotic pulse. Adv Exp Med Biol. 1992;326:19–26. doi: 10.1007/978-1-4615-3030-5_2. [DOI] [PubMed] [Google Scholar]
- 23.Tsong TY. Electroporation of cell membranes. Biophys J. 1991;60(2):297–306. doi: 10.1016/S0006-3495(91)82054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsong TY, Kinosita K., Jr Use of voltage pulses for the pore opening and drug loading, and the subsequent resealing of red blood cells. Bibl Haematol. 1985;51:108–114. doi: 10.1159/000410233. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell DH, James GT, Kruse CA. Bioactivity of electric field-pulsed human recombinant interleukin-2 and its encapsulation into erythrocyte carriers. Biotechnol Appl Biochem. 1990;12(3):264–275. [PubMed] [Google Scholar]
- 26.Lizano C, Sanz S, Luque J, et al. In vitro study of alcohol dehydrogenase and acetaldehyde dehydrogenase encapsulated into human erythrocytes by an electroporation procedure. Biochim Biophys Acta. 1998;1425(2):328–336. doi: 10.1016/s0304-4165(98)00085-3. [DOI] [PubMed] [Google Scholar]
- 27.Dong Q, Jin W. Monitoring diclofenac sodium in single human erythrocytes introduced by electroporation using capillary zone electrophoresis with electrochemical detection. Electrophoresis. 2001;22(13):2786–2792. doi: 10.1002/1522-2683(200108)22:13<2786::AID-ELPS2786>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 28.Dzekunov SM, Wang SH, Hanson DH, inventors. Maxcyte Inc., assignee Apparatus and method for electroporation of biological samples. 7186559. United States patent US. 2007 Mar 6;
- 29.Nicolau C, Bruggemann U, Mouneimne Y, Conrad Roux E, inventors. Cbr Laboratories Inc., assignee Method and apparatus for encapsulation of biologically-active substances. 1466968. Patent EP. 2006 Oct 4;
- 30.Bruggemann U, Roux EC, Hannig J, et al. Low-oxygen-affinity red cells produced in a large-volume, continuous-flow electroporation system. Transfusion. 1995;35(6):478–486. doi: 10.1046/j.1537-2995.1995.35695288766.x. [DOI] [PubMed] [Google Scholar]
- 31.Rechsteiner MC. Uptake of proteins by red blood cells. Exp Cell Res. 1975;93(2):487–492. doi: 10.1016/0014-4827(75)90478-4. [DOI] [PubMed] [Google Scholar]
- 32.Hamidi M, Zarrin AH, Foroozesh M, et al. Preparation and in vitro evaluation of carrier erythrocytes for RES-targeted delivery of interferon-alpha 2b. Int J Pharm. 2007;341(1–2):125–133. doi: 10.1016/j.ijpharm.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Hamidi M, Tajerzadeh H, Dehpour AR, et al. In vitro characterization of human intact erythrocytes loaded by enalaprilat. Drug Deliv. 2001;8(4):223–230. doi: 10.1080/107175401317245903. [DOI] [PubMed] [Google Scholar]
- 34.Magnani M, Rossi L, D’ascenzo M, et al. Erythrocyte engineering for drug delivery and targeting. Biotechnol Appl Biochem. 1998;28(pt 1):1–6. [PubMed] [Google Scholar]
- 35.Rossi L, Serafini S, Pierigé F, et al. Erythrocytes as a controlled drug delivery system: clinical evidences. J Control Release. 2006;116(2):e43–e45. doi: 10.1016/j.jconrel.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 36.Biagiotti S, Paoletti MF, Fraternale A, et al. Drug delivery by red blood cells. IUBMB Life. 2011;63(8):621–631. doi: 10.1002/iub.478. [DOI] [PubMed] [Google Scholar]
- 37.Bax BE, Bain MD, Fairbanks LD, et al. In vitro and in vivo studies with human carrier erythrocytes loaded with polyethylene glycol-conjugated and native adenosine deaminase. Br J Haematol. 2000;109(3):549–554. doi: 10.1046/j.1365-2141.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- 38.Bax BE, Bain MD, Fairbanks LD, et al. A 9-yr evaluation of carrier erythrocyte encapsulated adenosine deaminase (ADA) therapy in a patient with adult-type ADA deficiency. Eur J Haematol. 2007;79(4):338–348. doi: 10.1111/j.1600-0609.2007.00927.x. [DOI] [PubMed] [Google Scholar]
- 39.Godfrin Y, Bax BE. Enzyme bioreactors as drugs. Drugs Future. 2012;37(4):263–272. [Google Scholar]
- 40.Bourgeaux V, Hequet O, Campion Y, et al. Inositol hexaphosphate-loaded red blood cells prevent in vitro sickling. Transfusion. 2010;50(10):2176–2184. doi: 10.1111/j.1537-2995.2010.02663.x. [DOI] [PubMed] [Google Scholar]
- 41.Banz A, Cremel M, Rembert A, et al. In situ targeting of dendritic cells by antigen-loaded red blood cells: a novel approach to cancer immunotherapy. Vaccine. 2010;28(17):2965–2972. doi: 10.1016/j.vaccine.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Ropars C, Nicolau C, Chassaigne M, inventors. CNRS, Centre Hospitalier Regional De Tours, Studiengesellschaft Kohle mbH, assignees Procédé et dispositif pour l’encapsulation dans les érythrocytes d’au moins une substance à activité biologique, notamment des effecteurs allostériques de l’hémoglobine et érythrocytes ainsi obtenus [Process and device for the encapsulation in erythrocytes of at least one biologically active substance, in particular hemoglobin allosteric effectors, and erythrocytes so obtained] 0101341. Patent EP. 1986 Nov 5; French.
- 43.Godfrin Y, inventor. Erytech Pharma, assignee Lysis/resealing process and device for incorporating an active ingredient, in particular asparaginase or inositol hexaphosphate, in erythrocytes. 2006016247. Patent WO. 2006 Feb 16;
- 44.Darghouth D, Koehl B, Heilier JF, et al. Alterations of red blood cell metabolome in overhydrated hereditary stomatocytosis. Haematologica. 2011;96(12):1861–1865. doi: 10.3324/haematol.2011.045179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bernhardt I, Llory JC. Red Cell Membrane Transport in Health and Disease. Berlin: Springer-Verlag; 2003. [Google Scholar]
- 46.Magnani M, Rossi L, Bagiotti S, Bianchi M, inventors Drug delivery systems. WO2010145849 A3. United States patent US. 2011 Dec 15;
- 47.Biagiotti S, Rossi L, Bianchi M, et al. Immunophilin-loaded erythrocytes as a new delivery strategy for immunosuppressive drugs. J Control Release. 2011;154(3):306–313. doi: 10.1016/j.jconrel.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 48.Foroozesh M, Hamidi M, Zarrin A, et al. Preparation and in-vitro characterization of tramadol-loaded carrier erythrocytes for long-term intravenous delivery. J Pharm Pharmacol. 2011;63(3):322–332. doi: 10.1111/j.2042-7158.2010.01207.x. [DOI] [PubMed] [Google Scholar]
- 49.Tonetti M, Astroff AB, Satterfield W, et al. Pharmacokinetic properties of doxorubicin encapsulated in glutaraldehyde-treated canine erythrocytes. Am J Vet Res. 1991;52(10):1630–1635. [PubMed] [Google Scholar]
- 50.Tonetti M, Astroff B, Satterfield W, et al. Construction and characterization of adriamycin-loaded canine red blood cells as a potential slow delivery system. Biotechnol Appl Biochem. 1990;12(6):621–629. [PubMed] [Google Scholar]
- 51.DeLoach JR, Barton C. Glutaraldehyde-treated carrier erythrocytes for organ targeting of methotrexate in dogs. Am J Vet Res. 1981;42(11):1971–1974. [PubMed] [Google Scholar]
- 52.Baskurt OK. The role of spleen in suppressing the rheological alterations in circulating blood. Clin Hemorheol Microcirc. 1999;20(3):181–188. [PubMed] [Google Scholar]
- 53.Luo R, Mutukumaraswamy S, Venkatraman SS, et al. Engineering of erythrocyte-based drug carriers: control of protein release and bioactivity. J Mater Sci Mater Med. 2012;23(1):63–71. doi: 10.1007/s10856-011-4485-2. [DOI] [PubMed] [Google Scholar]
- 54.Zocchi E, Guida L, Benatti U, et al. Hepatic or splenic targeting of carrier erythrocytes: a murine model. Biotechnol Appl Biochem. 1987;9(5):423–434. [PubMed] [Google Scholar]
- 55.Banz A, Cremel M, Mouvant A, et al. Tumor growth control using red blood cells as the antigen delivery system and poly(I: C) J Immunother. 2012;35(5):409–417. doi: 10.1097/CJI.0b013e3182594352. [DOI] [PubMed] [Google Scholar]
- 56.Sprandel U, Way JL, editors. Erythrocytes as Drug Carriers in Medicine. New York, NY: Plenum Press; 1996. [Google Scholar]
- 57.Chiarantini L, Rossi L, Fraternale A, et al. Modulated red blood cell survival by membrane protein clustering. Mol Cell Biochem. 1995;144(1):53–59. doi: 10.1007/BF00926740. [DOI] [PubMed] [Google Scholar]
- 58.Magnani M, Rossi L, Brandi G, et al. Targeting antiretroviral nucleoside analogues in phosphorylated form to macrophages: in vitro and in vivo studies. Proc Natl Acad Sci U S A. 1992;89(14):6477–6481. doi: 10.1073/pnas.89.14.6477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magnani M, Casabianca A, Fraternale A, et al. Synthesis and targeted delivery of an azidothymidine homodinucleotide conferring protection to macrophages against retroviral infection. Proc Natl Acad Sci U S A. 1996;93(9):4403–4408. doi: 10.1073/pnas.93.9.4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Magnani M. In: Erythrocyte Engineering for Drug Delivery and Targeting. Magnani M, editor. New York, NY: Springer, Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 61.Hamidi M, Zarei N, Zarrin AH, et al. Preparation and in vitro characterization of carrier erythrocytes for vaccine delivery. Int J Pharm. 2007;338(1–2):70–78. doi: 10.1016/j.ijpharm.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 62.Polvani C, Gasparini A, Benatti U, et al. Murine red blood cells as efficient carriers of three bacterial antigens for the production of specific and neutralizing antibodies. Biotechnol Appl Biochem. 1991;14(3):347–356. [PubMed] [Google Scholar]
- 63.Magnani M, Chiarantini L, Vittoria E, et al. Red blood cells as an antigen-delivery system. Biotechnol Appl Biochem. 1992;16(2):188–194. [PubMed] [Google Scholar]
- 64.Cremel M, Guerin N, Horand F, et al. Red blood cells as innovative antigen carrier to induce specific immune tolerance. Int J Pharm. 2013;443(1–2):39–49. doi: 10.1016/j.ijpharm.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 65.Godfrin Y, Bertrand Y. L-asparaginase introduced into erythrocytes for the treatment of leukemia (ALL) Oncol News. 2006;1(1):2–4. [Google Scholar]
- 66.Mollër H, Oldenhof C. The active pharmaceutical ingredients starting material (APISM) and other materials in API manufacture. Drug Inf J. 1999;33:755–761. [Google Scholar]
- 67.European Medicines Agency (EMA) Report of Committee for Advanced Therapies (Draft) London: 2012. Reflection paper on clinical aspects related to tissue engineered products [report] (Report No 139870). [Google Scholar]
- 68.Burger SR. Design and operation of a current good manufacturing practices cell-engineering laboratory. Cytotherapy. 2000;2(2):111–122. doi: 10.1080/146532400539116. [DOI] [PubMed] [Google Scholar]
- 69.Casaroli-Marano RP, Tabera J, Vilarrodona A, et al. Regulatory issues in cell-based therapy for clinical purposes. Dev Ophthalmol. 2014;53:189–200. doi: 10.1159/000357766. [DOI] [PubMed] [Google Scholar]
- 70.U.S. Department of Health and Human Services Food and Drug Administration . Guidance for Industry and FDA Staff: Minimal Manipulation of Structural Tissue Jurisdictional Update [guideline] Silver Spring, MD: U.S. Department of Health and Human Services Food and Drug Administration; 2006. [Google Scholar]
- 71.National Institute of Health Science (NIHS) Guideline for Quality and Safety Assurance of Pharmaceuticals and Medical devices Based on Human Autologous Cells or Tissue. Research Triangle Park, NC: National Institute of Health Science (NIHS); 2008. guideline in Japanese. [Google Scholar]
- 72.Sparrow RL, Sran A, Healey G, et al. In vitro measures of membrane changes reveal differences between red blood cells stored in saline-adenine-glucose-mannitol and AS-1 additive solutions: a paired study. Transfusion. 2014;54(3):560–568. doi: 10.1111/trf.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sowemimo-Coker SO. Evaluation of an experimental filter designed for improving the quality of red blood cells (RBCs) during storage by simultaneously removing white blood cells and immunomodulators and improving RBC viscoelasticity and Band 3 proteins. Transfusion. 2014;54(3):592–601. doi: 10.1111/trf.12330. [DOI] [PubMed] [Google Scholar]
- 74.Brandenberger R, Burger S, Campbell A, et al. Cell therapy bioprocessing. Integrating process and product development for the next generation of biotherapeutics. BioProcess Int. 2011;9(S1):30–37. [Google Scholar]
- 75.Bessis M, Mohandas N, Feo C. Automated ektacytometry: a new method of measuring red cell deformability and red cell indices. Blood Cells. 1980;6(3):315–327. [PubMed] [Google Scholar]
- 76.Lamarre Y, Bourgeaux V, Pichon A, et al. Effect of inositol hexaphosphate-loaded red blood cells (RBCs) on the rheology of sickle RBCs. Transfusion. 2013;53(3):627–636. doi: 10.1111/j.1537-2995.2012.03779.x. [DOI] [PubMed] [Google Scholar]
- 77.Dacie JV, Lond MB, Vaughan JM, et al. The fragility of the red blood cells: its measurement and significance. J Pathol Bacteriol. 1938;46(2):341–356. [Google Scholar]
- 78.Guarnone R, Centenara E, Barosi G. Performance characteristics of Hemox-Analyzer for assessment of the hemoglobin dissociation curve. Haematologica. 1995;80(5):426–430. [PubMed] [Google Scholar]
- 79.Moroff G, Sohmer PR, Button LN. Proposed standardization of methods for determining the 24-hour survival of stored red cells. Transfusion. 1984;24(2):109–114. doi: 10.1046/j.1537-2995.1984.24284173339.x. [DOI] [PubMed] [Google Scholar]
- 80.International Committee for Standardization in Haematology Recommended method for radioisotope red-cell survival studies. Br J Haematol. 1980;45(4):659–666. doi: 10.1111/j.1365-2141.1980.tb07189.x. [DOI] [PubMed] [Google Scholar]
- 81.Valeri CR, MacGregor H, Giorgio A, et al. Comparison of radioisotope methods and a nonradioisotope method to measure the RBC volume and RBC survival in the baboon. Transfusion. 2003;43(10):1366–1373. doi: 10.1046/j.1537-2995.2003.00528.x. [DOI] [PubMed] [Google Scholar]
- 82.Zeiler T, Muller J, Kretschmer V. Flow cytometric determination of survival time and 24-hour recovery of transfused Red blood cells. Transfus Med Hemother. 2003;30:14–19. [Google Scholar]
- 83.Boucher L, Chassaigne M, Ropars C. Internalization and distribution of inositol hexakisphosphate in red blood cells. Biotechnol Appl Biochem. 1996;24(pt 1):73–78. [PubMed] [Google Scholar]
- 84.Hunault-Berger M, Leguay T, Huguet F, et al. A Phase 2 study of L-asparaginase encapsulated in erythrocytes in elderly patients with Philadelphia chromosome negative acute lymphoblastic leukemia: the GRASPALL/GRAALL-SA2-2008 study. Am J Hematol. 2015;90(9):811–818. doi: 10.1002/ajh.24093. [DOI] [PubMed] [Google Scholar]
- 85.Bourgeaux V, Aufradet E, Campion Y, et al. Efficacy of homologous inositol hexaphosphate-loaded red blood cells in sickle transgenic mice. Br J Haematol. 2012;157:357–369. doi: 10.1111/j.1365-2141.2012.09077.x. [DOI] [PubMed] [Google Scholar]
- 86.Rossi L, Serafini S, Cenerini L, et al. Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease. Biotechnol Appl Biochem. 2001;33(pt 2):85–89. doi: 10.1042/ba20000087. [DOI] [PubMed] [Google Scholar]
- 87.Rossi L, Castro M, D’Orio F, et al. Low doses of dexamethasone constantly delivered by autologous erythrocytes slow the progression of lung disease in cystic fibrosis patients. Blood Cells Mol Dis. 2004;33(1):57–63. doi: 10.1016/j.bcmd.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Castro M, Rossi L, Papadatou B, et al. Long-term treatment with autologous red blood cells loaded with dexamethasone 21-phosphate in pediatric patients affected by steroid-dependent Crohn disease. J Pediatr Gastroenterol Nutr. 2007;44(4):423–426. doi: 10.1097/MPG.0b013e3180320667. [DOI] [PubMed] [Google Scholar]
- 89.Menotta M, Biagiotti S, Bianchi M, et al. Dexamethasone partially rescues ataxia telangiectasia-mutated (ATM) deficiency in ataxia telangiectasia by promoting a shortened protein variant retaining kinase activity. J Biol Chem. 2012;287(49):41352–41363. doi: 10.1074/jbc.M112.344473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chessa L, Leuzzi V, Plebani A, et al. Intra-erythrocyte infusion of dexamethasone reduces neurological symptoms in ataxia teleangiectasia patients: results of a phase 2 trial. Orphanet J Rare Dis. 2014;9(1):5. doi: 10.1186/1750-1172-9-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Leuzzi V, Micheli R, D’Agnano D, et al. Positive effect of erythrocyte-delivered dexamethasone in ataxia-telangiectasia. Neurol Neuroimmunol Neuroinflamm. 2015;2(3):e98. doi: 10.1212/NXI.0000000000000098. [DOI] [PMC free article] [PubMed] [Google Scholar]