Abstract
Wheat stem rust caused by Puccinia graminis f. sp. tritici, can cause significant yield losses. To combat the disease breeders have deployed resistance genes both individually and in combinations to increase resistance durability. A new race, TTKSK (Ug99), identified in Uganda in 1999, is virulent on most of the resistance genes currently deployed, and is rapidly spreading to other regions of the world. It is therefore important to identify, map, and deploy resistance genes that are still effective against TTKSK. One of these resistance genes, Sr13, was previously assigned to the long arm of chromosome 6A, but its precise map location was not known. In this study, the genome location of Sr13 was determined in four tetraploid wheat (T. turgidum ssp. durum) mapping populations involving the TTKSK resistant varieties Kronos, Kofa, Medora and Sceptre. Our results showed that resistance was linked to common molecular markers in all four populations, suggesting that these durum lines carry the same resistance gene. Based on its chromosome location and infection types against different races of stem rust, this gene is postulated to be Sr13. Sr13 was mapped within a 1.2 to 2.8 cM interval (depending on the mapping population) between EST markers CD926040 and BE471213, which corresponds to a 285-kb region in rice chromosome 2, and a 3.1-Mb region in Brachypodium chromosome 3. These maps will be the foundation for developing high-density maps, identifying diagnostic markers, and positional cloning of Sr13.
Keywords: durum wheat, stem rust, disease resistance, genetic mapping, plant pathogen
Introduction
Puccinia graminis Pers.:Pers f. sp. tritici Eriks. & E. Henn., the causal agent of wheat stem rust, is found in all major wheat growing areas and has been responsible for widespread yield losses during the first half of the previous century. A large-scale campaign to eradicate the alternate host, barberry (Berbis vulgaris L), in the early 1920’s was later found insufficient to end the stem rust epidemics (Leonard 2001). The barberry eradication efforts were then complemented by the deployment of stem rust resistance genes in the released wheat varieties bringing the stem rust under control during the last half of the twentieth century.
In 1999 a new race of stem rust virulent on most previously resistant lines was identified in Uganda (Pretorius et al. 2000). This new race, designated Ug99 or TTKS (Wanyera et al. 2006), spread to Kenya in 2001 and to Ethiopia in 2003 (Singh et al. 2006). By 2006, TTKS was identified in Sudan and Yemen (www.fao.org), and in 2008, its presence was confirmed in Iran (Nazari et al. 2009). Three different races within the TTKS lineage were since identified from isolates in Kenya that led to the re-designation of the original race as TTKSK, and the other two races as TTKST (with additional virulence on Sr24) (Jin et al. 2008) and TTTSK (with additional virulence on Sr36) (Jin et al. 2009).
Several resistance genes are still effective against the three TTKS lineages in both tetraploid (T. turgidum ssp. durum L.) and hexaploid wheat (T. aestivum L.) cultivars (Jin et al. 2007). Among them, Sr13 is the only known gene with effective resistance to the TTKS races present within the US durum wheat adapted cultivars. The Ethiopian land race ST464, and the domesticated emmer wheat (T. turgidum ssp. dicoccon L.) Khapli are the two major sources of Sr13 in durum (Knott 1962; Klindworth et al. 2007). The Sr13 resistance gene from Khapli was transferred to the common wheat variety Khapstein from the cross Steinwedel x Khapli, and was subsequently mapped on the distal region of the long arm of chromosome 6A by McIntosh (1972). Tests of Khapstein with several North American races of stem rust showed that the transferred gene was a useful source of resistance to the prevalent races of stem rust at that time, but that Khapstein did not carry all of the resistance of Khapli (Watson and Stewart 1956), in particular the Sr7a gene as stated by McIntosh et al. (1995).
The moderate resistance of Sr13 to TTKS makes it a good candidate for gene pyramiding with other stem rust resistance genes. Gene pyramiding is a strategy commonly used by wheat breeders to extend the durability of the deployed resistance genes. However, the generation of these resistance gene pyramids based on marker selection will require the development of markers closely linked to the target genes. To date, molecular markers have been identified for several stem rust resistance genes including Sr2 (Spielmeyer et al. 2003), Sr6 (Tsilo et al. 2009), Sr9a (Tsilo et al. 2007), Sr24 and Sr26 (Mago et al. 2005), Sr25 and Sr26 (Liu et al. 2009), Sr31 (Mago et al. 2002), Sr36 (Tsilo et al. 2008), Sr38 (Seah et al. 2001), Sr39 (Mago et al. 2009), and Sr40 (Wu et al. 2009), but many more stem rust resistance genes including Sr13 remain to be mapped. The objectives of this research were to map Sr13 in a durum wheat genetic background and to identify closely linked markers that can be used to screen germplasm carrying the resistance gene.
Materials and methods
Plant materials
Four segregating populations were used to map Sr13 resistance gene in durum wheat. The first population included 93 recombinant inbred lines (RILs) derived from the cross between UC1113 and Kofa (denoted as UK). Kofa is a Desert Durum® variety developed by WestBred (now owned by Monsanto) that is resistant to TTKSK and has excellent pasta quality. Kofa originated from a Male Sterile Facilitated Recurrent Selection (MSFRS) population in which T. dicoccon lines with high protein were crossed onto Desert Durum® adapted MSFRS population. The exact pedigree of Kofa is not known, thus, either T. dicoccon or the adapted Desert Durum lines included in the MSFRS could be the source of the observed resistance gene. UC1113 is a breeding line from the UC Davis wheat breeding program selected from a CIMMYT cross CD52600 (KIFS//RSS/BD1419/3/MEXIS-CP/4/WAHAS/5/YAV79) that is susceptible to TTKSK, and has excellent agronomic performance but intermediate pasta qualities (Zhang et al 2008). Evaluations for adult plant stem rust resistance performed in Kenya in 2007 and 2008 showed that Kofa was resistant (10 to 50 R/MR) whereas UC1113 was highly susceptible (50 MS/S to 70 MS/S) to TTKSK. Seedling tests performed at the USDA-ARS Cereal Disease Laboratory and University of Minnesota confirmed that in Kofa resistance genes against TTKSK also confer resistance to variants TTKST (Sr24 virulence) and TTTSK (Sr36 virulence) and five other races of stem rust (QTHJ, RCRS, RKQQ, TPMK, and TTTT). UC1113 was susceptible to all three TTKS variants and to races TPMK and TTTT, but was resistant to the North American races QTHJ, RCRS, and RKQQ.
The other two populations were F2 progeny from crosses between Mindum x Medora (denoted as MM, 97 lines) and Mindum x Sceptre (denoted as MS, 80 lines). Mindum, susceptible to race TTKSK, was selected from the field in Turkey and released in 1917 from University of Minnesota, whereas both Medora (Ward/Macoun) and Sceptre (D72110/Coulter, D72110 = D65150/Leeds//Ward) were Canadian cultivars released in 1981 and 1985, respectively, that showed resistance to TTKSK with infection type of 2 when they were evaluated at the seedling stage. While the likely resistance source for Medora was Khapli, either ST464 or Khapli may have contributed to the resistance in Sceptre (Fig. S1).
A fourth population was developed from the cross Kronos x Rusty (denoted as KR) to link the previous three populations using common polymorphic markers. Rusty is a tetraploid genetic stock susceptible to almost all stem rust races (Klindworth et al. 2006); whereas Kronos is a Desert Durum® variety developed by Arizona Plant Breeders that is resistant to TTKSK and TRTT (a virulent race from Yemen), and has excellent pasta quality and yield potential.
To determine the usefulness of the markers linked to Sr13, a set of 34 durum cultivars and breeding lines, mostly derived from North Dakota durum wheat breeding program and previously assessed for their resistance against TTKSK, was included in this study (Table 1). The set of 34 ND durum germplasm was selected for marker validation based on their similar breeding history and common resistance sources with the resistance parents, Medora and Sceptre (Fig. S1). To further validate the identification of the resistance gene mapped in this study as Sr13, one of the reference genetic stocks for Sr13, Khapstein/9*LMPG-6 (Knott 1990), along with Khapstein (PI 210125) and the original Sr13 donor Khapli (CItr4013), were genotyped with markers linked to Sr13.
Table 1.
A list of durum wheat germplasm used for haplotype analysis in this study.
| Accession | Origin | wmc580 | CK207347 | CD926040 | BE403950 | dupw167 | TTKSK |
|---|---|---|---|---|---|---|---|
| D98529 | ND | 317 | 1000 | 855 | 723 | 230 | ; |
| D98530 | ND | 317 | 1000 | - | 713 | 230 | ; |
| D99983 | ND | 317 | 1000 | 855 | 723 | 230 | ; |
| DH01039 | ND | 317 | 1000 | 855 | 723 | 230 | ; |
| Lloyd | ND | 317 | 1000/1135 | 851 | 691/723 | 230 | ; |
| D00624 | ND | 317 | 1000 | 855 | 723 | 230 | 1 |
| DH01060 | ND | 317 | 1000 | 855 | 723 | 249 | 1 |
| Munich | ND | 317 | 1000 | 855 | 723 | 230 | 1 |
| Pierce | ND | 317 | 1000 | 855 | 723 | 230 | 1 |
| Plaza | ND | 317 | 1000 | 855 | 723 | 230 | 1 |
| D99073 | ND | 317 | 1000 | 855 | 723 | 245 | 1+ |
| D99541 | ND | 317 | 1000 | 855 | 723 | 230 | 1+ |
| D00534 | ND | 317 | 1000 | 855 | 723 | 230 | 12 |
| D00095 | ND | 317 | 1000 | 855 | 723 | 230 | ;12 |
| D00752 | ND | 317 | 1135 | 855 | - | 219 | 2 |
| D00969 | ND | 317 | 1000 | 855 | 723 | 230 | 2 |
| D99637 | ND | 317 | 1000 | 855 | 723 | 230 | 2 |
| D99656 | ND | 319 | 1000 | - | 713 | 230 | 2 |
| DH01066 | ND | 317 | 1000 | 855 | 723 | 230 | 2 |
| * Khapli (CItr4013) | NSGCa | 293 | 1000 | 851 | 691 | 230 | ;2− |
| * W2691Sr13 | NSGCa | 326 | 1000 | 851 | 691 | 245 | 2 |
| * Langdon | ND | 293 | 1000 | - | 691 | 249 | 2 |
| * Kofa | WestBred | 293 | 1000 | 855 | 723 | 230 | 2 |
| * Kronos | CA-APBb | 293 | 1135 | 855 | - | 245 | 2+ |
| * Medora | Canada | 317 | 1000 | 855 | 723 | 230 | 2 |
| * Sceptre | Canada | 317 | 1000 | 855 | 723 | 230 | 2 |
| * ST464-C1 | ND | 293 | 1000 | - | 691 | 249 | 2+ |
| Ben | ND | 317 | 1000 | 855 | 723 | 230 | 2− |
| Maier | ND | 317 | 1135 | 855 | - | 245 | 2− |
| Renville | ND | 293 | 1135 | 855 | - | 252 | 2− |
| Leeds | ND | 317 | 1000 | 855 | 723 | 230 | 2+ |
| D00622 | ND | 317 | 1000 | 855 | 723 | 230 | 3 |
| Grenora | ND | 317 | 1000 | 855 | 723 | 245 | 3 |
| Alkabo | ND | 317 | 1000 | - | 723 | 249 | 3− |
| Belzer | ND | 317 | 1000 | 855 | 723 | 249 | 3+ |
| D001097 | ND | 317 | 1000 | 855 | 723 | 230 | 3+ |
| D00767 | ND | 317 | 1000 | 855 | 723 | 249 | 4 |
| D97643 | ND | 317 | 1000 | 855 | 723 | 249 | 4 |
| Dilse | ND | 317 | 1000 | 855 | 723 | 249 | 4 |
| Divide | ND | 317 | 1000 | 855 | 723 | 249 | 4 |
| Rusty | ND | 293 | 993 | 845 | - | 230 | 4 |
| Mindum | MN | 335 | 1135 | 851 | 691 | 230 | 4 |
| UC1113 | CA | 317 | 1000 | 855 | 723 | 249 | 4 |
NSGC – USDA-ARS, National Small Grains Collection, Aberdeen, ID.
CA-APB – California – Arizona Plant Breeders
Lines are known to carry Sr13 gene.
Evaluation for resistance
All parents, RILs and F3 lines of the UK, MM, and MS populations were evaluated for their reaction to race TTKSK isolate 04KEN156/04 collected in Kenya in 2004. The KR population was evaluated separately with stem rust races TTTTF and TPMKC. Urediniospores from long-term storage in a −80°C freezer were heat shocked at 40°C for 15 min and placed in a rehydration chamber for 2 to 4h, where approximately 80% relative humidity was maintained by a KOH solution (Rowell, 1984). The urediniospores were then suspended in a light mineral oil (Soltrol 170) and inoculated onto the fully expanded primary leaves of 7- to 9-day-old seedlings of wheat lines. Seedlings were incubated in a dew chamber for 12–16 h at 18°C in the dark, and then for an additional period of 3 to 4 h under fluorescent light. The inoculated plants were placed on a greenhouse bench at 18 ± 2°C with a photoperiod of 16 h. Infection types, described by Stakman et al. (1962), were assessed 14 days post inoculation. From each genotype, 12 seedlings were screened. Infection types 0, ;, 1, 2, or combinations thereof were considered low infections, indicating resistance; whereas infection types 3 to 4 were considered high infections and plants considered susceptible. Families were classified as homozygous resistant, segregating, or homozygous susceptible to race TTKSK.
Genetic map construction
A complete genetic map of the UK segregating population including over 269 simple sequence repeat (SSR), single nucleotide polymorphism (SNP), restriction fragment length polymorphism (RFLP), and sequence tagged site (STS) markers has already been published by Zhang et al. (2008).
Genomic DNA of Mindum, Medora and Sceptre were first screened with 1440 wheat SSRs to detect polymorphic markers. Twelve wheat EST-derived SNP markers previously mapped to chromosome 6A (Chao et al. 2009) were screened and polymorphic ones were also included in the map. Additionally, primers for STS markers designed from the Sr13 gene region by aligning unmapped wheat ESTs with rice genome sequences were also used. Genotyping of SSR and STS markers followed the same protocols previously developed for SSR markers using a capillary gel system from Applied Biosystems (Foster City, CA) (Chao et al. 2007). The method used for SNP genotyping was based on template-directed dye-terminator incorporation assay with fluorescence polarization detection (FP-TDI) (Chen et al. 1999). The KR population was used to correlate the UK population with the MM and MS populations and, therefore, was screened only with molecular markers previously mapped in these three populations.
Genetic maps of the Sr13 region were constructed for the three populations using MAPMAKER software version 3.0b (Lander et al. 1987). Genetic distances were calculated using the Kosambi function. Initial maps were assembled at LOD 3. Additional markers were then placed in the most likely location using the TRY command. Regions where groups of markers ordered at LOD scores lower than 2 were denoted as asterisks (*) shown on the maps. The order was refined using the RIPPLE command.
Results
Gene postulation
Domesticated emmer wheat Khapli, has been previously shown to carry Sr7a, Sr13, and Sr14 and at least one additional un-described stem rust resistance gene (Knott 1962; Williams and Gough 1965). Khapli is shown here to be resistant to race TTKSK (Table 2). Since Sr7a and Sr14 are known to be susceptible to TTKSK (Table 2; Jin et al. 2007), the resistance in Khapli to this race is likely conferred by Sr13.
Table 2.
Eleven wheat lines with previously determined stem rust resistance genes and infection types to race TTKSK of Puccinia graminis f. sp. tritici
| Line | Known Sr genes | TTKSK |
|---|---|---|
| UC1113 | 4 | |
| Kofa | 2 | |
| Mindum | 9d, X, +a | 4 |
| Medora | 2 | |
| Sceptre | 2 | |
| Khapli | 7a, 13, 14, +a | 2 |
| Kenya Governor/10*MQ//8*LMPG | 7a | 3+ |
| W2691Sr13 | 13 | 2+ |
| St464Sr13 (PI 192334) | 13 | 2+ |
| Khapstein/9*LMPG | 13 | 2+ |
| Line A selection | 14 | 4 |
+ indicates additional un-described stem rust resistance genes are present in these lines.
Sr13 is the only known stem rust resistance gene effective against race TTKSK in the US adapted durum wheat cultivars. Typical TTKSK infection types of lines carrying Sr13 range from 2 to 2+ (Table 2). We observed similar infection types to race TTKSK in the durum lines Kofa, Medora, and Sceptre (Table 2), and postulated that these low infections to TTKSK were conferred by Sr13. This gene postulation is consistent with the map location on chromosome 6AL described below.
Genetic maps
The ratio of phenotypic scores against race TTKSK agreed with single gene segregation in all three populations (χ2 = 0.10, P = 0.76 for UK, χ2 = 0.22, P = 0.90 for MS, and χ2 = 0.84, P = 0.66 for MM). Mapping of resistance in the crosses UK, MM, and MS resulted in the localization of a single resistance gene against TTKSK on the distal region of the long arm of chromosome 6A (Fig. 1). These results agree with the previously reported location of Sr13 (McIntosh 1972). Therefore, this resistance gene will be referred to as Sr13 hereafter.
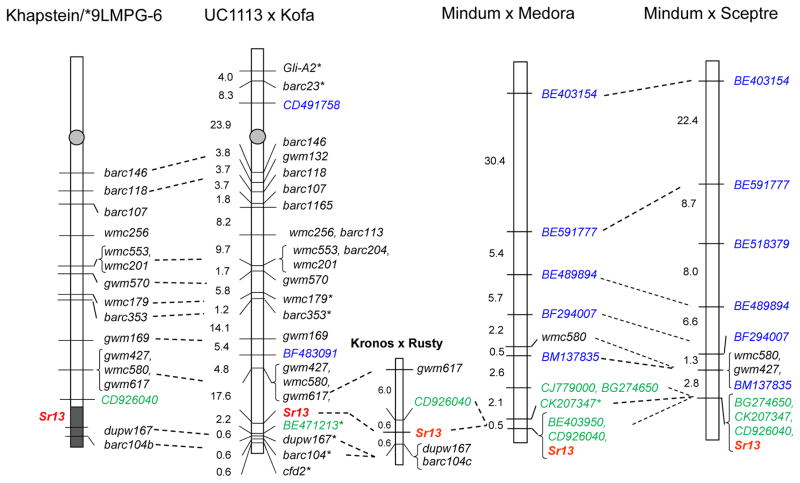
Fig. 1.
Genetic maps of Sr13 resistance gene on chromosome 6A developed from three mapping populations, UC1113 x Kofa, Mindum x Sceptre, and Mindum x Medora. DNA markers shown in blue were SNP-based, and those shown in green were STS markers. Asterisks (*) indicate markers mapped at LOD scores lower than 2.0. The shaded area at the distal end of chromosome 6AL in Khapstein/*9LMPG-6 denotes the region transferred from Khapstein into LMPG-6 including the Sr13 gene. Markers outside the shaded area are not polymorphic between LMPG-6 and Khapstein/*9LMPG-6, but are polymorphic in Khapstein (PI 210125). Markers barc104, barc104b and barc104c represent different primers (Table 3) that amplify the same locus, but that are more efficient to detect polymorphisms in different genetic backgrounds.
In the UK segregating population resistance to TTKSK was initially mapped on the distal region of chromosome arm 6AL between SSR markers gwm617 and dupw167, more than 85 cM from the centromere (Fig. 1). Later, an STS marker derived from EST BE471213 (see Table 3 for primers and PCR conditions) was mapped between Sr13 and dupw167.
Table 3.
SNP primers, primer pairs developed for STS markers, and annealing temperature used for PCR amplifications performed at either constant or touchdown (td) conditions
| Wheat EST | Forward primer (SNP detection primer) | SNP | Reverse primer | Temp |
|---|---|---|---|---|
| CD491758 | AGCTTGCCATGTTTATTATGTTAC | TGGACCATTACTATGTAGAGC | 62 | |
| BF483091 | GCAAAAAATATCTGTTAGAACAAGACTG | CACCATTGCCATCACAAGAG | 58 | |
| (ATTGTGGCTTATGTTGCTATACAAGGCA) | G/A | 60 | ||
| BE471213 | GTCTTTCTCCTTGGCTGTCG | TCATATCCTCTGCTTTCCTGAAA | 58 | |
| barc104b* | GCGCTTCCAAGGCTTAGAGGCT | GGACCAGGCATGTCTACCCT | 50 | |
| barc104c* | GCATGTTTCCCATCCCTTTA | GCCTTCCTCCCTTTTGAAAC | 50 | |
| BE403154 | CAGGTAAATCTGTAATTTTTTAAGGGAAGAC | TGTTGTCGAAGGCTCCACCA | 60 | |
| (GACCATCTTACCTTGTGTAGCATAGATG) | C/T | 60 | ||
| BE591777 | CGTGCGTTCAGTATTCAAAA | CAGCGACAACAGACTCAAGCAAA | 60 | |
| (GGGAAAAAGATTACCACACTTGCACAA) | C/T | 60 | ||
| BE518379 | AGGTGAGCACCCTGCAGTT | TGTGTTCATACTATTGAGCCAGTTCTAT | 60 | |
| (TCCGAACCATACAATCACCGGACAGGAG) | G/C | 60 | ||
| BE489894 | CAGTGGAAGGCAAGGTGTAC | TGGTTGTCAAGAGTTCCCTAAGCCT | 60 | |
| (CATTGCCCCAGGGACCCGTCTCGACTT) | G/A | 60 | ||
| BE294007 | CACACCGTAGGAGAACCAGG | ATCCACAGGCTCCATAGCAC | 60 | |
| (GATAACTTCCTTTGTACTAGAAAGTAGAT) | C/T | 60 | ||
| BM137835 | CAGGGCACAGTTCCATGATTTA | GGAATATTTTTTCCTAGGTACCAT | 60 | |
| (CTTTGGTAAGAAAATTGTGCATAGCAACT) | G/A | 60 | ||
| CK207347 | TTACGGGCCACAAACAATCT | AGCTCTCATCCATCCAGGAA | 60–55 td | |
| BG274650 | TCCTTTCTTTCACCGTGGAG | ACTCTGAGCAGCGACCAAAT | 60–55 td | |
| CJ779000 | GATGTTGCCGCCAGAATAAT | TATGCAAAGGCCTCCACTTC | 60–55 td | |
| CD926040 | GTTGGCTTGGCTACTGCTTT | AGCATTCAGCTCTGTGAGCA | 60–55 td | |
| BE403950 | GGAACATGTTGACGCTGTTG | AACACTGTTCCCGAAGTTGG | 60–55 td |
barc104b and barc104c amplify the same locus as the original barc104 SSR but use primers that are optimized for different genetic backgrounds.
The 25 RILs showing recombination between the flanking markers and Sr13 were sent to the USDA-ARS Cereal Disease Laboratory for a second blind evaluation. All 25 RILs showed identical TTKSK infection types as observed in the first evaluation, confirming the location of Sr13. In summary, in the UK segregating population Sr13 was mapped to a 20 cM interval defined by gwm617 and BE471213.
In the two other crosses involving Mindum as the susceptible parent, the screening with SSR markers revealed low levels of polymorphism for markers previously mapped in chromosome 6A. The linkage mapping analysis showed that Sr13 was located 2.8 cM distal to wmc580 in the MS population (Fig. 1). A similar result was found in the MM population where wmc580 was mapped 5.7cM proximal to Sr13 (Fig. 1). SNP markers developed from 12 ESTs previously bin-mapped to chromosome 6AL were further evaluated, and the polymorphic ones were all mapped proximal to, or tightly linked to the Sr13 gene in both MM and MS populations (Fig. 1). All the ESTs distal to BE403154 (Fig. 1) have been previously assigned to the distal 6AL-8 bin (BE591777, BE513379, BE489894, BF294007, BM137835, BE403950, and BE471213), thus, the Sr13 region is included in the 6AL-8 bin.
None of the markers distal to Sr13 in the UK population were polymorphic in the MM and MS populations. Similarly, attempts to transfer the EST markers mapped closely linked to Sr13 in the MM and MS to the UK population were not successful due to lack of polymorphism. We were able to develop genome specific primers for seven 6AL ESTs (BE403950, BE636872, BG274650, BQ802161A, BQ841735, CK207347) but no polymorphisms were detected in the 6,790 bp sequenced from both UC1113 and Kofa. In summary, gwm427 and wmc580 are the closer common markers to Sr13 between the UK and MM-MS populations.
To generate additional common markers closer to Sr13 we developed a fourth population from the cross Kronos x Rusty (denoted as KR), which were polymorphic for both distal SSR markers, barc104b and dupw167, and the tightly linked proximal EST-derived marker, CD926040. The results confirmed segregation for a single gene conferring resistance to stem rust races TTTTF and TPMKC (16 homozygous resistant, 37 heterozygous, 29 homozygous susceptible, 1:2:1 proportion χ2 test P = 0.08) in the KR population. Resistance to both races was mapped within a 1.2 cM interval flanked by markers CD926040 (0.6 cM) and dupw167-barc104b (0.6cM), which were also closely linked to the resistance genes in the other three populations. Close linkage with common molecular makers suggests that these four resistant parents carry the same resistance gene, which is most likely Sr13. The Sr13 gene postulation in the KR population was also supported by an infection type 2 in Kronos tested with stem rust races TTKSK and TRTT (virulent race from Yemen).
The identification of the homologous region in the sequenced grass species provided a starting point to discover additional markers in the Sr13 region. The rice homologues to the wheat Sr13 gene region flanked by ESTs BF483091 and BE471213 in the UK population defined a region on rice chromosome 2 (R2) of approximately 1Mb (R2 34,885 to 35,805 kb). Wheat ESTs located within this region were identified and used to generate STS markers near the Sr13 gene in the different populations. While EST BE403950 (R2 35,512 kb) was completely linked to Sr13 in the MM population, EST CD926040 (R2 35,521 kb) was found co-segregating with Sr13 in both MM and MS populations but was proximal to the resistance gene in the KR population determining a close proximal marker for Sr13 (Fig. 1). Similarly, the CK207347 EST (R2 35,501 kb) detected no recombination event with Sr13 in the MS population but was mapped 0.5 cM proximal to Sr13 in the MM population. EST BG274650 (R2 35,300 kb) was closely linked to Sr13 in the MS population but was mapped 2.1 cM proximal to Sr13 in the MM population and linked to EST CJ779000 (R2 35,410 kb). Taken together, the results from the four mapping populations suggest that the wheat Sr13 gene region on chromosome 6AL is delimited by flanking EST-derived markers CD926040 and BE471213. The orthologs of these wheat genes correspond to the collinear regions of 285 kb on rice chromosome 2 (35,521 kb to 35,805 kb), and 3.1 Mb on Brachypodium chromosome 3 (56,534 to and 59,627) (Table 4).
Table 4.
Wheat EST markers mapped in this study and their corresponding collinear regions of rice and Brachypodium chromosomes
| Map order (population) | Rice Chr2 * | Brachypodium Chr3 ** |
|---|---|---|
| BE518379 (MS) | 34,632 kb | 55,278 kb |
| BE489894 (MS & MM) | 34,617 kb | 55,306 kb |
| BE483091 (UK) | 34,885 kb | 55,422 kb |
| BF294007 (MS & MM) | 35,006 kb | 55,579 kb |
| BM137835 (MS & MM) | 35,051 kb | 55,649 kb |
| BG274650 (MS & MM) | 35,300 kb | 56,091 kb |
| CJ779000 (MM) | 35,410 kb | 56,011 kb |
| CK207347 (MS & MM) | 35,501 kb | 56,148 kb |
| BE403950 (MM) | 35,512 kb | 56,554 kb |
| CD926040 (MM & MS & KR) | 35,521 kb | 56,534 kb |
| BE471213 (UK) | 35,805 kb | 59,627 kb |
Coordinates based on Gramene Gene build January 2009
Coordinates based on Brachypodium 8X release
Genetic stocks for Sr13
The Sr13 gene name is defined by several genetic stocks, including Khapstein/9*LMPG-6 (Knott 1990; McIntosh et al. 2008). To test if the chromosome region where the resistance to TTKSK mapped in the four durum populations was also present in Khapstein/9*LMPG-6, markers from these four maps were evaluated in Khapstein/9*LMPG-6 and its recurrent susceptible parent LMPG-6 (Knott 1990), as well as in the tetraploid (Khapli) and hexaploid (Khapstein) Sr13 donors (Fig. 2). Of the 19 SSR markers evaluated on 6AL, only dupw167 and barc104b were polymorphic between LMPG-6 and Khapstein/9*LMPG-6, and the same alleles present in the latter were detected in Khapstein (PI 210125) (Fig. 2, barc104b; data not shown for dupw167), indicating that the distal 6AL chromosome region in Khapstein/9*LMPG-6 was transferred from Khapstein (Fig. 1, shaded area). Among the other 17 non-polymorphic markers between LMPG-6 and Khapstein/9*LMPG-6, 13 (including CD926040) were polymorphic with Khapstein (PI 210125) (Fig. 1, non-shaded area), suggesting that this chromosome region was not transferred from Khapstein to Khapstein/9*LMPG-6. The other four markers, gwm132, barc1165, barc113, and barc204, were not polymorphic between Khapstein and LMPG-6 and were not included in the map since they were not informative.
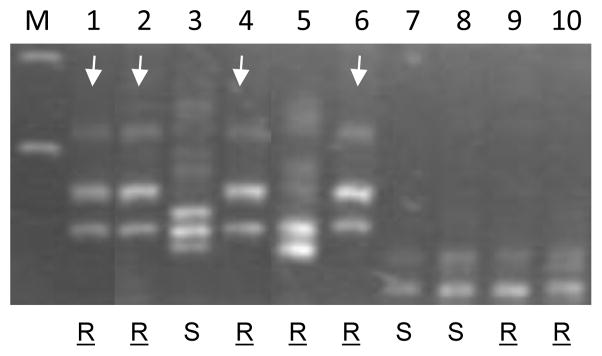
Fig. 2.
Alleles of barc104b detected among ten wheat lines, 1. Khapstein (PI 210125); 2. Khapstein/9*LMPG-6; 3. LMPG-6; 4. Khapli (C.I.4013); 5. Kofa; 6. Kronos; 7. Rusty; 8. Mindum; 9. Medora; and 10. Sceptre. “R” and “S” denote resistance and susceptibility to TTKSK, respectively. Underlined “R” indicates that the line is a known carrier of Sr13. Arrows indicate Sr13 resistant genotypes carrying the same barc104b allele as Khapli (C.I.4013).
It should be noted that heterogeneity was observed among the three different accessions of Khapstein available from the National Small Grains Collection (USDA-ARS, Aberdeen, Idaho). As a result, the actual donor source used to produce Khapstein/9*LMPG-6 is not certain. However, the fact that the accession PI 210125 shared the same dupw167 and barc104b alleles with Khapstein/9*LMPG-6 suggests that this Khapstein accession is the most similar to the original source of Sr13 in Khapstein/9*LMPG-6. Khapli accession C.I. 4013 showed the same barc104b allele as Khapstein and Khapstein/9*LMPG-6, but had a different dupw167 allele (data not shown), implying that a different Khapli accession was used in the generation of Khapstein.
The Sr13 resistant lines, Kofa, Medora, and Sceptre carry different barc104b alleles from those present in Khapli, Khapstein, Khapstein/9*LMPG-6 and Kronos (Fig. 2), but they share the same dupw167 alleles with Khapli C.I.4013, suggesting either different tetraploid sources of the Sr13 gene or independent recombination events between the resistance gene and the markers used in this study. Characterization of EST markers mapped near the Sr13 gene further revealed that CD926040 is not polymorphic between Khapstein/9*LMPG-6 and LMPG-6, but detected a different allele in Khapstein, implying that this marker is located outside the region containing Sr13 (Fig. 1). The proximal location of CD926040 relative to Sr13 is consistent with its map location in the KR population (Fig. 1).
Marker haplotypes of durum wheat germplasm
To determine if closely linked markers identified in MM and MS populations are robust enough to predict the disease phenotype in the durum germplasm, a set of 34 durum wheat cultivars from North Dakota that shared similar genetic background and Sr13 resistance sources as Medora and Sceptre (Fig. S1) was genotyped with five flanking markers, wmc580, CK207347, BE403950, CD926040, and dupw167. This germplasm set included 10 and 24 lines susceptible and resistant to TTKSK, respectively. The results showed that a predominant allele was generally found present in both susceptible and resistant accessions for all five markers investigated, and that no particular haplotype was associated with the TTKSK susceptible or resistant phenotypes (Table 1). We confirmed the lack of association between stem rust resistance and haplotypes based on these five markers by examining additional genetic stocks known to carry Sr13, such as W2691Sr13 (Table 1). Therefore, the markers identified in this study, albeit tightly linked, are not diagnostic for the Sr13 gene. It’s worth noting that for the susceptible lines, the absence of Sr13 can be predicted with a high level of confidence, but for the resistant lines that have not been analyzed genetically, we can’t rule out the presence of stem rust resistance genes different from the prevalent Sr13.
Discussion
Sr13 mapping and gene designation
In this study, we present three lines of evidence to suggest that the resistance gene against TTKSK mapped in our study is Sr13. First, the infection type of 2 against TTKSK obtained for the resistant parental lines Kronos, Kofa, Medora, and Sceptre used in this study was within the range of 2 to 2+ observed for typical infections in lines carrying Sr13. Second, consistent with a previous telocentric analysis that assigned Sr13 to the long arm of chromosome 6A with no linkage with the centromere (McIntosh, 1972) the resistance gene segregating in all four populations evaluated in this study was mapped in the distal region of chromosome arm 6AL. Finally, the presence of different dupw167 and barc104b alleles in LMPG-6 from those present in both Khapstein and Khapstein/9*LMPG-6 further supports the hypothesis that the gene mapped in this study is Sr13. The genetic stock Khapstein/9*LMPG-6, used to define the Sr13 gene name, is a resistant isogenic line selected from backcrossing Khapstein nine generations into the susceptible line LMPG-6. During the backcross process, a recombination event between CD926040 and Sr13 likely restored most of the LMPG-6 in the 6AL proximal region, and reduced the Khapstein segment to the distal 6AL region in Khapstein/9*LMPG-6. Taken together, these three sources of evidence strongly indicate that the TTKSK resistance gene mapped in this study is Sr13.
Characterization of germplasm with Sr13 linked markers
Khapli and ST464 were involved in the production of Langdon, Wells and Leeds (Fig S1). Since these lines or their derivatives were present in most of the lineages of durum wheat cultivars released from North Dakota after 1978 (Klindworth et al. 2007), a large proportion of the ND germplasm is expected to carry the Sr13 gene, which is in general agreement with the high proportion of TTKSK resistant lines found among the North Dakota germplasm included in this study (70%).
While the Sr13 resistance source can be traced from the pedigree in Medora back to Khapli, the Sr13 source for Sceptre can be either Khapli or ST464 (Fig S1). Our mapping results also confirmed the presence of Sr13 in Kofa and Kronos, but the use of a Male Sterile Facilitated Recurrent Selection population with multiple parental lines complicates the identification of the Sr13 donor in these two varieties. The presence of the same bar104b allele in Khapli and Kronos (Fig. 2) suggests that Khapli might have been the donor of Sr13 in Kronos, but more closely linked diagnostic markers will be required for a more precise identification of the sources of Sr13 in Kronos, and in the other three resistant parents as well.
Contrary to its wide distribution among ND durum wheat cultivars and germplasm, Sr13 has not been exploited extensively in common wheat breeding programs (Knott 1989), with the exception of Australian wheats (McIntosh et al. 1995). Due to its moderate resistance and effectiveness against TTKSK, Sr13 would be a valuable source of resistance for pyramiding with other resistance genes to provide durable resistance against stem rust. The use of molecular markers will assist breeders in the efforts of combining multiple resistance genes during the breeding process. The tightly linked markers identified in this study (e.g. BE403950, CK207347, or CD926040), would be useful for marker assisted selection efforts for Sr13 only in targeted populations generated from parental lines with known Sr13 alleles.
The haplotype analysis of the set of 34 North Dakota durum lines revealed that the markers developed in this study are not diagnostic to predict the presence or absence of Sr13 among germplasm, even though some of these markers were closely linked to Sr13. Low levels of polymorphism in the Sr13 region in the UK, MM and MS populations delayed our initial efforts to develop closely linked markers. The recently developed KR mapping population showed higher levels of polymorphism than the previous populations, and will be the focus of future high-density mapping and positional cloning efforts. The collinear region from rice and Brachypodium will provide useful resources of new markers to saturate the wheat Sr13 region. A dedicated positional cloning effort in wheat will be necessary not only to identify the Sr13 gene, but also to understand the mechanisms by which it confers moderate resistance to TTKSK. A better understanding of the different stem rust resistance mechanisms and the different genes involved in each of them will be useful to design more durable gene combinations to control this devastating disease.
Supplementary Material
Resistance sources used, and the pedigree and year of release information for cultivars and germplasm developed in North Dakota durum wheat breeding program. Boxes indicate founders for the germplasm used in the study, and circles indicate the resistant parents used in the mapping populations.
Acknowledgments
This project was supported in part by funds provided through a grant from the Bill & Melinda Gates Foundation to Cornell University for the Borlaug Global Rust Initiative (BGRI) Durable Rust Resistance in Wheat (DRRW) Project, in part by the National Research Initiative Competitive Grant no. 2009-65300-05640 from the USDA National Institute of Food and Agriculture, and in part by USDA-ARS CRIS project 5442-22000-030-00D.
References
- Chao S, Zhang W, Dubcovsky J, Sorrells M. Evaluation of genetic diversity and genome-wide linkage disequilibrium among US wheat (Triticum aestivum L.) germplasm representing different market classes. Crop Sci. 2007;47:1018–1030. [Google Scholar]
- Chao S, Zhang W, Akhunov E, Sherman J, Ma Y, Luo M-C, Dubcovsky J. Analysis of gene-derived SNP marker polymorphism in US wheat (Triticum aestivum L.) cultivars. Mol Breeding. 2009;23:23–33. [Google Scholar]
- Campbell CL, Long DL. The campaign to eradicate the common barberry in the United States. In: Peterson PD, editor. Stem rust of wheat: From ancient enemy to modern foe. The American Phytopathological Society; St. Paul, MN: 2001. pp. 16–50. [Google Scholar]
- Chen X, Levine L, Kwok P-Y. Fluorescence polarization in homogeneous nucleic acid analysis. Genome Res. 1999;9:492–498. [PMC free article] [PubMed] [Google Scholar]
- Jin Y, Singh RP, Ward RW, Wanyera R, Kinyua MG, Njau P, Fetch T, Jr, Pretorius ZA, Yahyaoui A. Characterization of seedling infection types and adult plant infection responses of monogenic Sr gene lines to race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2007;91:1096–1099. doi: 10.1094/PDIS-91-9-1096. [DOI] [PubMed] [Google Scholar]
- Jin Y, Szabo LJ, Pretorius ZA, Singh RP, Ward R, Fetch T., Jr Detection of virulence to resistance gene Sr24 within race TTKS of Puccinia graminis f. sp. tritici. Plant Dis. 2008;92:923–926. doi: 10.1094/PDIS-92-6-0923. [DOI] [PubMed] [Google Scholar]
- Jin Y, Szabo L, Rouse M, Fetch T, Jr, Pretorius ZA, Wanyera R, Njau P. Detection of virulence to resistance gene Sr36 within race TTKS lineage of Puccinia graminis f. sp. tritici. Plant Dis. 2009;93:367–370. doi: 10.1094/PDIS-93-4-0367. [DOI] [PubMed] [Google Scholar]
- Klindworth DL, Miller JD, Xu SS. Registration of rusty durum wheat. Crop Sci. 2006;46:1012–1013. [Google Scholar]
- Klindworth DL, Miller JD, Jin Y, Xu SS. Chromosomal locations of genes for stem rust resistance in monogenic lines derived from tetraploid wheat accession ST464. Crop Sci. 2007;47:1441–1450. [Google Scholar]
- Knott DR. The inheritance of rust resistance: IX. The inheritance of resistance to races 15B and 56 of stem rust in the wheat variety Khapstein. Can J Plant Sci. 1962;42:415–419. [Google Scholar]
- Knott DR. Near-isogenic lines of wheat carrying genes for stem rust resistance. Crop Sci. 1990;30:901–905. [Google Scholar]
- Lander ES, Green P, Abrahamson J, Barlow A, Daly MJ, Lincoln SE, Nerburg L. MAPMAKER: an interactive computer package for constructing primary genetic linkage maps of experimental and natural populations. Genomics. 1987;1:174–181. doi: 10.1016/0888-7543(87)90010-3. [DOI] [PubMed] [Google Scholar]
- Leonard KJ. Stem rust – future enemy? In: Peterson PD, editor. Stem rust of wheat: From ancient enemy to modern foe. The American Phytopathological Society; St. Paul, MN: 2001. pp. 119–146. [Google Scholar]
- Liu S, Yu L-X, Singh RP, Jin Y, Sorrells ME, Anderson JA. Diagnostic and co-dominant PCR markers for wheat stem rust resistance genes Sr25 and Sr26. Theor Appl Genet. 2009;120:691–697. doi: 10.1007/s00122-009-1186-z. [DOI] [PubMed] [Google Scholar]
- Mago R, Spielmeyer W, Lawrence GJ, Lagudah ES, Ellis JG, Pryor A. Identification and mapping of molecular markers linked to rust resistance genes located on chromosome 1RS of rye using wheat-rye translocation lines. Thero Appl Genet. 2002;104:1317–1324. doi: 10.1007/s00122-002-0879-3. [DOI] [PubMed] [Google Scholar]
- Mago R, Bariana HS, Dundas IS, Spielmeyer W, Lawrence GJ, Pryor AJ, Ellis JG. Development of PCR markers for the selection of wheat stem rust resistance genes Sr24 and Sr26 in diverse wheat germplasm. Theor Appl Genet. 2005;111:496–504. doi: 10.1007/s00122-005-2039-z. [DOI] [PubMed] [Google Scholar]
- Mago R, Zhang P, Bariana HS, Verlin DC, Bansal UK, Ellis JG, Dundas IS. Development of wheat lines carrying stem rust resistance gene Sr39 with reduced Aegilops speltoides chromatin and simple PCR markers for marker-assisted selection. Theor Appl Genet. 2009;119:1441–1450. doi: 10.1007/s00122-009-1146-7. [DOI] [PubMed] [Google Scholar]
- McIntosh RA. Cytogentical studies in wheat: VI. Chromosome location and linkage studies involving Sr13 and Sr8 for reaction to Puccinia graminis f. sp. tritici. Aust J Biol Sci. 1972;25:765–763. [Google Scholar]
- McIntosh RA, Wellings CR, Park RF. Wheat rusts: An atlas of resistance genes. CSIRO Australia. 1995:108–109. [Google Scholar]
- McIntosh RA, Yamazaki Y, Dubcovsky J, Rogers WJ, Morris CF, Somers D, Appels R, Devos KM. McIntosh RA, editor. Catalogue of gene symbols for wheat. 2008 http://wheat.pw.usda.gov/GG2/Triticum/wgc/2008/GeneSymbol.pdf.
- Nazari K, Mafi M, Yahyaoui A, Singh RP, Park RF. Detection of wheat stem rust (Puccinia graminis f. sp. tritici) race TTKSK (Ug99) in Iran. Plant Dis. 2009;93:317. doi: 10.1094/PDIS-93-3-0317B. [DOI] [PubMed] [Google Scholar]
- Pretorius ZA, Singh RP, Wagoire WW, Payne TS. Detection of virulence to wheat stem rust resistance gene Sr31 in Puccinia graminis f. sp. tritici in Uganda. Plant Dis. 2000;84:203. doi: 10.1094/PDIS.2000.84.2.203B. [DOI] [PubMed] [Google Scholar]
- Rowell JB. Controlled infection by Puccinia graminis f. sp. tritici under artificial conditions. In: Bushnell WR, Roelf AP, editors. The cereal rusts, vol 1, Origins, specificity, structure, and physiology. Academic Press; Orlando: 1984. pp. 292–332. [Google Scholar]
- Seah S, Bariana H, Jahier J, Sivasithamparam K, Lagudah ES. The introgressed segment carrying rust resistance genes Yr17, Lr37 and Sr38 in wheat can be assayed by a cloned disease resistance gene-like sequence. Theor Appl Genet. 2001;102:600–605. [Google Scholar]
- Singh RP, Hodson DP, Jin Y, Huerta-Espino J, Kinyua MG, Wanyera R, Njau P, Ward RW. Current status, likely migration and strategies to mitigate the threat to wheat production from race Ug99 (TTKS) of stem rust pathogen. CAB Reviews: Perspectives in agriculture, veterinary science, nutrition and natural resources. 2006;1(054) [Google Scholar]
- Spielmeyer W, Sharp PJ, Lagudah ES. Identification and validation of markers linked to broad-spectrum stem rust resistance gene Sr2 in wheat (Triticum aestivum L.) Crop Sci. 2003;43:333–336. [Google Scholar]
- Stakman EC, Steward DM, Loegering WQ. USDA Agric Res Serv E-617. 1962. Identification of physiologic races of Puccinia graminis var. tritici. [Google Scholar]
- Tsilo TJ, Jin Y, Anderson JA. Microsatellite markers linked to stem rust resistance allele Sr9a in wheat. Crop Sci. 2007;47:2013–2020. [Google Scholar]
- Tsilo TJ, Jin Y, Anderson JA. Diagnostic microsatellite markers for the detection of stem rust resistance gene Sr36 in diverse genetic backgrounds of wheat. Crop Sci. 2008;48:253–261. [Google Scholar]
- Tsilo TJ, Chao S, Jin Y, Anderson JA. Identification and validation of SSR markers linked to the stem rust resistance gene Sr6 on the short arm of chromosome 2D in wheat. Theor Appl Genet. 2009;118:515–524. doi: 10.1007/s00122-008-0917-x. [DOI] [PubMed] [Google Scholar]
- Wanyera R, Kinyua MG, Jin Y, Singh R. The spread of stem rust caused by Puccinia graminis f. sp. tritici, with virulence on Sr31 in wheat in Eastern Africa. Plant Dis. 2006;90:113. doi: 10.1094/PD-90-0113A. [DOI] [PubMed] [Google Scholar]
- Watson IA, Steward DM. Sources of wheat stem rust resistance. Agron J. 1956;48:526–527. [Google Scholar]
- Williams ND, Gough FJ. Inheritance of stem rust reaction in a Khapli emmer cross. Crop Sci. 1965;5:145–147. [Google Scholar]
- Wu S, Pumphrey M, Bai G. Molecular mapping of stem-rust-resistance gene Sr40 in wheat. Crop Sci. 2009;49:1681–1686. [Google Scholar]
- Zhang W, Chao S, Manthey F, Chicaiza O, Brevis JC, Echenique V, Dubcovsky J. QTL analysis of pasta quality using a composite microsatellite - SNP map of durum wheat. Theor Appl Genet. 2008;117:1361–1377. doi: 10.1007/s00122-008-0869-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Resistance sources used, and the pedigree and year of release information for cultivars and germplasm developed in North Dakota durum wheat breeding program. Boxes indicate founders for the germplasm used in the study, and circles indicate the resistant parents used in the mapping populations.