Abstract
Prescription opiate use and abuse has increased dramatically over the past two decades, including increased use in adolescent populations. Recently, it has been proposed that use during this critical period may affect future offspring even when use is discontinued prior to conception. Here, we utilize a rodent model to examine the effects of adolescent morphine exposure on the reward functioning of the offspring. Female Sprague Dawley rats were administered morphine for 10 days during early adolescence (post-natal day 30–39) using an escalating dosing regimen. Animals then remained drug free until adulthood at which point they were mated with naïve males. Adult offspring (F1 animals) were tested for their response to morphine-induced (0, 1, 2.5, 5, and 10 mg/kg, s.c.) conditioned place preference (CPP) and context-independent morphine-induced sensitization. Naïve littermates were used to examine mu opiate receptor expression in the nucleus accumbens and ventral tegmental area. Results indicate that F1 females whose mothers were exposed to morphine during adolescence (Mor-F1) demonstrate significantly enhanced CPP to the lowest doses of morphine compared with Sal-F1 females. There were no differences in context-independent sensitization between maternal treatment groups. Protein expression analysis showed significantly increased levels of accumbal mu opiate receptor in Mor-F1 offspring and decreased levels in the VTA. Taken together, these findings demonstrate a shift in the dose response curve with regard to the rewarding effects of morphine in Mor-F1 females which may in part be due to altered mu opiate receptor expression in the nucleus accumbens and VTA.
Keywords: opiates, opioids, conditioned place preference, adolescence, morphine, sensitization, transgenerational, mu opioid receptor, VTA, NAc
Introduction
Currently, the United States is in the midst of an opioid addiction crisis (Calcaterra et al., 2013; Johnston, 2015). Prescription opioid use has dramatically increased over the past two decades, an increase that is mirrored by rising rates of opioid abuse and addiction. Indeed, reports of opioid overdoses are at an all-time high (Calcaterra et al., 2013). In this climate, adolescent populations are also increasingly being exposed to opioids, with drugs within this class (e.g. Vicodin or Percocet) frequently prescribed following minor surgery, dental work, or even to treat the common cold (Holman et al., 2013; Holman et al., 2014; Woolf and Greco, 2014). Moreover, recreational use and abuse of opioids has begun to trend into younger populations (Fiellin, 2008; Heyman and Adger, 1997; SAMHSA, 2011). One negative outcome associated with use during adolescent developmental is an increased risk of addiction and dependence (DuRant et al., 1999; Schwartz, 1998). Moreover increased incidences of structural abnormalities have been discerned in brains of drug users who initiated use during adolescence when compared to those that initiated during adulthood (Hwang et al., 2013). The full extent of the damage that adolescent drug exposure causes is unknown. Recently, it has been proposed that use during this critical period may affect not only the individual user but future generations as well (for review see (Vassoler et al., 2013; Yohn et al., 2015)).
In humans, parental drug use is correlated with increased risk in offspring (Cadoret et al., 1986; Kendler et al., 2003). While genetics likely play a significant role, it cannot provide the sole explanation for increased drug use within families (Merikangas et al., 1998). Thus, environmental components also come into play. One such factor that may affect intergenerational patterns of addiction is drug use before pregnancy (preconception). Currently, there is a paucity of human literature describing the effects of preconception drug use followed by a period of abstinence on adult children. Rather, the literature focuses on in utero drug exposure or familial drug use without identifying adolescent exposure. This is likely due to the difficulty of teasing apart the large number of variables and complexities associated with drug addiction in human populations. In contrast, carefully controlled rodent studies allow direct investigation of the potential effects of preconception drug use on future generations. Indeed, a number of preclinical models have been developed to examine the effects of adolescent drug exposure on subsequent offspring. For example, adolescent cannabinoid exposure causes alterations in both opioid-induced CPP and heroin self-administration in future adult offspring; effects that occur despite a prolonged period of abstinence prior to conception (Byrnes et al., 2012; Szutorisz et al., 2014). Moreover, adolescent opioid exposure has been shown to have effects on anxiety-like behavior and hippocampal dendritic retraction, an effect that is mitigated by environmental enrichment of the offspring (Byrnes, 2005; Li et al., 2014). With growing rates of use and abuse in adolescent populations, it is critical to better understand the possible effects this use could have on future generations.
While drug intake in one generation could potentially impact multiple modalities, one primary area of interest is a propensity towards drug addiction in the offspring. We hypothesize that preconception morphine exposure will cause changes in the rewarding propensity of opioids in subsequent generations. In order to test this hypothesis, we administered the prototypical mu opioid receptor agonist, morphine, in increasing doses to female rats from postnatal day (P) 30-P39. This time period was chosen to represent a period coincident with adolescence in humans (McCutcheon and Marinelli, 2009; Spear, 2000). As with girls, female rats enter puberty at an earlier age than males, thus the period of adolescence around the time of puberty in female rats is representative of early-middle female adolescence. Following a prolonged abstinence period, females were mated with drug-naive colony males. The offspring (F1 animals) were then examined for their sensitivity to the effects of opioids. Previous work with this model has shown that there are alterations in various aspects of both behavior and physiology in the offspring. For example, it was shown that adult offspring from females exposed to morphine during adolescence demonstrate increased sensitivity to the analgesic properties of morphine, develop tolerance more rapidly, have alterations in play behavior, and dopamine D2 receptor sensitivity (Byrnes et al., 2011; Byrnes et al., 2013; Johnson et al., 2011; Vassoler et al., 2014). However, it is unclear if there are changes in the reward system in response to morphine. We utilized conditioned place preference (CPP) to test the rewarding properties of morphine and locomotor sensitization to test the locomotor response to repeated morphine in morphine F1 animals.
The primary rewarding mechanisms of opioid drugs are mediated by mu opioid receptors in the ventral tegmental area (VTA) and the nucleus accumbens (NAc) (Britt and Wise, 1983; Schulteis and Koob, 1996; Vaccarino et al., 1985). In order to determine if the mu opioid receptor was altered in either the VTA or the NAc, we examined protein expression levels in both areas in naïve animals. Our hypothesis for these experiments was that adolescent morphine exposure would enhance the propensity towards addiction-like behaviors in adult offspring via an alteration in opioid-mediated reward.
Materials and Methods
Animals and Housing
All animals were housed in standard acrylic laboratory cages (40 cm × 20 cm × 18 cm) at Cummings School for Veterinary Medicine at Tufts University. Animals were maintained on a 12-hour light/dark cycle with lights on at 7:00 am and all procedures were performed during the light phase. Food and water was available ad libitum. Animals were acclimated to the housing conditions for at least seven days prior to experimentation. All procedures were approved by the Institutional Animal Care and Use Committee of Tufts University, and were carried out in accordance with the National Research Council (NRC) Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize animal distress and reduce the number of animals used.
F0 Animals
For all experiments, post-natal day 23 (PND23) female Sprague-Dawley rats [Crl:CD(SD)BR] were purchased from Charles River Breeding Laboratories. All animals were housed 3–4 per cage. Beginning at PND30 females (n=26) were injected (s.c.) once daily with morphine sulfate (MS) for a total of 10 days using an increasing dosing regimen with doses increased every other day (5, 5, 10, 10, 15, 15, 20, 20, 25, 25 mg/kg, see Figure 1). Age-matched control animals (n=26) received saline injections (s.c) with volumes adjusted to match those of drug-treated females. On PND 60–80 (3 to 6 weeks after their final injection), females were mated with drug-naïve colony males. Each male was placed with 4 age-matched females (2 morphine treated and 2 saline treated). Once an animal was visibly pregnant (approximately E16–E20) she was housed singly. On PND1 (day of birth = PND0) all litters were culled to ten pups (5 male, 5 female). The weight of the pups and the dam was recorded. All litters were weighed and weaned on PND21 and housed with same-sex littermates. No differences in bodyweights were observed at either time point (data not shown). Offspring of morphine-exposed females are designated MOR-F1. Offspring of saline controls are designated SAL-F1. All testing was conducted once SAL-F1 and MOR-F1 animals were at least 60 days of age. In all of the reported findings only one offspring per litter was used in any individual treatment group.
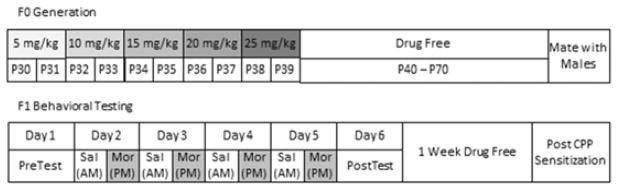
Figure 1.
Visual representation of the experimental paradigm used for the F0 and F1 generations. P=post natal day. Pretest and post test, as well as the days in between refer to the conditioned place preference paradigm. All F1 animals were at least P60 at the time of testing.
Conditioned Place Preference
Conditioning and preference testing was conducted using an automated three chamber apparatus (side A, middle chamber, side B), equipped with infrared photo beams to track animal position and movement (Hamilton-Kinder, Poway, CA). Each side of the chamber was fitted with a removable plastic insert decorated with black and white vertical stripes (side A) or all white (side B). The floor was either textured (side A) or smooth (side B). The center compartment had grey walls and a metal floor and was equipped with guillotine doors with access to both side A and side B. Each group was counterbalanced in an unbiased fashion. Thus, regardless of the performance during the preconditioning session the animal was randomly assigned to side A or side B for conditioning. The CPP paradigm consisted of a preconditioning test day (15 min), 4 conditioning days (2x a day, 30 min each), and a post conditioning test day (15 min). On Day 1 (preconditioning) each animal was placed into the middle chamber and allowed access to all three chambers. Time (s) spent in each chamber as well as distance travelled (cm) was measured for 15 minutes. Between each animal the apparatus was thoroughly wiped with 50% ethanol. The pretest and posttest for an individual animal occurred at the same time. All animals were tested between 8 and noon. The purpose of the precondition test was to determine if there was a preexisting bias for one side or the other and to examine baseline locomotor activity in a novel environment. There were no animals that were removed due to a bias (<100 seconds on either side). On Days 2–5, all animals were weighed and administered saline (1ml/kg, s.c.) and immediately confined in the saline-paired chamber in the morning (counterbalanced between side A and B) for 30 minutes. In the afternoon, approximately 5 hours later, they were administered the conditioning treatment (0, 1, 2.5, 5, or 10 mg/kg) of morphine and immediately confined in the drug-paired side (the opposite side from the morning) for 30 minutes. On Day 6 (the posttest day) all animals were placed in the middle chamber of the apparatus and allowed access to all three chambers for 15 minutes. Time (s) spent in each chamber as well as distance traveled was measured. CPP score was calculated as the difference in time spent in the drug-paired chamber from the saline-paired chamber (ie. conditioned side – unconditioned side). There were no animals removed from this study, although the animals were run through the paradigm in 7 separate cohorts. The number of animals for each group is as follows:
| mg/kg | Sal-F1 male | Mor-F1 male | Sal-F1 female | Mor-F1 female |
|---|---|---|---|---|
| 0 | 9 | 9 | 12 | 11 |
| 1 | 9 | 11 | 12 | 9 |
| 2.5 | 11 | 15 | 11 | 11 |
| 5 | 13 | 12 | 15 | 15 |
| 10 | 9 | 10 | 9 | 9 |
Context-Independent Morphine-Induced Sensitization
In order to test if the repeated doses of saline or morphine the animals’ received during CPP caused locomotor sensitization, we tested locomotor activity in a context-independent manner. One week following the last saline or morphine injection of the CPP paradigm, animals were tested for locomotor sensitization. Therefore, the animals that were tested for locomotor sensitization had all received 4 injections of either 0, 1, 2.5, 5, or 10 mg/kg morphine during conditioning. It is important to note that the group that received saline in both sides of the CPP apparatus was included in the locomotor sensitization experiment and served as the repeated saline control group. To measure context-independent locomotor sensitization animals were placed in a novel cage and activity was monitored during two 90 minutes sessions using an automated system (SmartFrame® Activity Cage Rack System; Hamilton-Kinder, Poway, CA, USA). In the morning, all animals were weighed, administered a saline injection (1 ml/kg, s.c.) and placed immediately in the activity chamber (40 cm × 20 cm × 18 cm) for 90 minutes. The animal was then removed, injected with a low dose of morphine (2 mg/kg, s.c.) and placed back in the locomotor chamber with activity measured for an additional 90 minutes. Data are expressed as distance traveled (cm) post morphine – distance traveled post saline on the sensitization test day. This design controls for any non-specific increase in locomotor activity related to novelty and/or injection stress. All animals that went through conditioned place preference were tested in context-independent sensitization except for one cohort which was omitted due to experimenter error. The N’s for these data are as follows:
| mg/kg | Sal-F1 male | Mor-F1 male | Sal-F1 female | Mor-F1 female |
|---|---|---|---|---|
| 0 | 9 | 9 | 12 | 11 |
| 1 | 9 | 10 | 10 | 9 |
| 2.5 | 10 | 13 | 10 | 10 |
| 5 | 12 | 10 | 10 | 11 |
| 10 | 9 | 10 | 9 | 9 |
Western blot analyses
Naïve F1 animals were sacrificed between PND 65–70 by brief exposure to CO2 followed by decapitation. The brains were rapidly removed and frozen with 2-methylbutane on dry ice. To collect samples for analysis of protein expression, frozen brains were mounted on a cryostat and bilateral micropunches were taken from the NAc (1.0 mm; starting at: +2.5 mm A/P, +/− 1.5 mm M/L, −7 mm D/V, relative to bregma, 2.0 mm thick; a region encompassing both core and shell) and the VTA (1 mm; starting at: −4.7 mm A/P, +/− 1.0 mm M/L, −8.2 mm D/V, relative to bregma, 1.5 mm thick). The tissue punches were then weighed and homogenized in RIPA buffer with 1 mM PMSF (60 ul buffer/1mg tissue). Following homogenization, the samples were maintained under constant agitation for 2 hours at 4 C°. They were then centrifuged for 20 minutes at 12,000 rpm at 4 C° and the supernatant was collected and the protein concentration was determined using a standard Pierce Assay (Thermo Scientific, Carlsbad, CA) with bovine serum albumin (BSA) as a protein standard. Ten μg of protein was prepared for loading using Bolt reducing agent and Bolt running buffer (Life Technologies, Carlsbad, CA). The sample was heated to 85 °C for 2 minutes, loaded onto 4–20% Tris Glycine gels (Life Technologies, Carlsbad, CA) and run for 40 minutes. The protein was transferred to nitrocellulose membrane using the iblot transfer stack system (Life Technologies, Carlsbad, CA). The membrane was then washed briefly in TBST and blocked in 5% BSA TBST for 1 hour. Following 3 5-minute washes in TBST the membrane was placed in the mu opioid receptor primary antibody (1:500, Novus Biologicals, NB100-1620, Littleton, CO) overnight at 4° C. The secondary antibody used was horseradish peroxidase conjugated mouse anti rabbit (Abcam, Campbridge, MA). Chemiluminescence was used to visualize the proteins within a Universal Hood II (Biorad, Hercules, CA). The blot was stripped and reprobed for beta actin (1:5,000, Abcam, Cambridge, MA). Protein intensity (int*mm2) was measured with Quantity One program volume analysis tool. Data is expressed as % of control normalized to beta actin. The band was analyzed at 45 kDa.
Statistical analyses
Pretest locomotor activity data were analyzed using a two-way analysis of variance (ANOVA) with sex and maternal treatment as factors. However, due to known sex differences in response to opioids coupled with sex differences in locomotor activity, both the CPP and locomotor sensitization data were analyzed separately by sex (Cataldo et al., 2010; Djurendic-Brenesel et al., 2010; Wang et al., 2006). Thus, these data were analyzed using two-way ANOVAs with dose and maternal treatment as factors. The post hoc test used was Sidak’s multiple comparison tests. The western blot data were analyzed with two-way ANOVAs with sex and maternal treatment as factors. Significance was defined as p < 0.05.
Results
F1 animals demonstrate increased sensitivity to the rewarding effects of morphine as measured by CPP
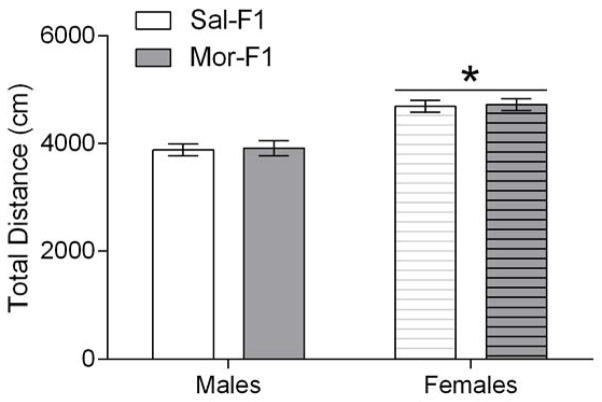
Adult male and female F1 animals were tested using 5 doses of morphine (0, 1, 2.5, 5, and 10 mg/kg; s.c.) in a 4-day unbiased 3 chamber CPP design (see Figure 1). The locomotor activity during the pretest was analyzed with a two-way ANOVA with sex and maternal treatment as between subjects’ factors. The analysis revealed a significant main effect of sex [F(1, 198)=48.01; p<0.0001] but no effect of maternal treatment [F(1, 198)=0.07184; p=0.78] nor an interaction [F(1, 198)=0.0002; p=0.98]. The females showed significantly increased locomotor activity (Figure 2.)
Figure 2.
Locomotor activity in a novel environment. Data are expressed as mean +/− SEM of the total distance travelled on the pretest day in the three chambered conditioning apparatus. * p<0.05; main effect of sex. N=9–15/group
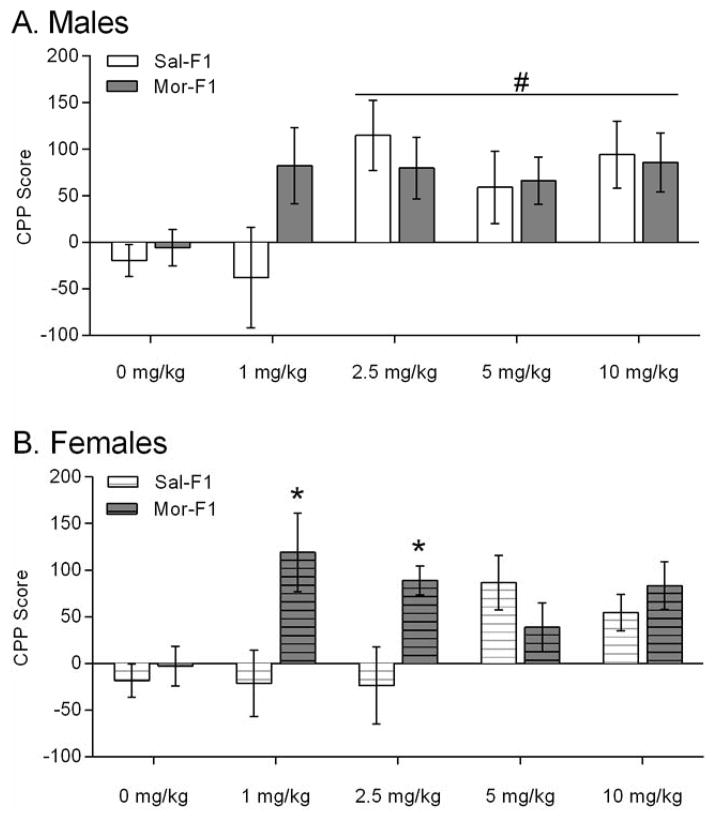
The CPP score was calculated as time spent in drug-paired side during the post test – time spent in the saline-paired side during the post test. In male offspring (Figure 3A), there was a main effect of dose [F(4, 97)=3.08; p=0.01] but no main effect of maternal treatment [F(1, 97)=0.72; p=0.39] and no interaction [F(4,97)=1.34; p=0.25]. Post-hoc analysis of the main effect of dose showed that 2.5, 5, and 10 mg/kg doses were significantly greater than the 0 mg/kg dose. The results from female offspring (Figure 3B) revealed a trend towards an effect of dose [F(4, 104)=2.39; p=0.055], a significant effect of maternal treatment [F(1, 104)=7.13; p=0.008], and a significant interaction [F(4,104)=3.69; p=0.007]. Post hoc tests revealed that the Mor-F1 females had significantly greater CPP than the Sal-F1 females at both the 1 mg/kg dose and the 2.5 mg/kg dose [t(104)=3.243; p<0.01] and [t(104)=2.687; p<0.05] respectively. Because a 3-chambered apparatus was used, the time spent in the middle was also analyzed. The data were analyzed with a three-way ANOVA with sex, maternal treatment and dose as factors. The analysis revealed a significant main effect of sex [F(1,201)=25.758; p<0.001] and a significant effect of dose [F(4, 201)=5.835; p<0.001]. The mean (SEM) for the males was 257.21 (11.18) and the females was 336.75 (11.63). The data are shown in Table 1.
Figure 3.
Conditioned place preference data expressed as the CPP score (Time spent in the conditioned side – time spent in non-conditioned side on the day of the 15-minute post test). Data are expressed as mean +/− SEM. Panel A shows the male data and Panel B shows the female data. * p<0.05 compared to Sal-F1 at the same dose. #p<0.05 main effect of dose. N=9–15/group
Table 1.
Time spent in center compartment during the post test. Data are shown as mean in one column and SEM in the adjacent column. There was a main effect of sex and dose.
| Sal-F1 male | Mor-F1 male | Sal-F1 female | Mor-F1 female | |||||
|---|---|---|---|---|---|---|---|---|
| mg/kg | MEAN | SEM | MEAN | SEM | MEAN | SEM | MEAN | SEM |
| 0 | 291.8 | 22.1 | 288.9 | 13.2 | 280.4 | 16.9 | 250.0 | 13.8 |
| 1 | 487.9 | 19.8 | 406.1 | 38.1 | 285.3 | 24.7 | 332.4 | 60.4 |
| 2.5 | 390.9 | 37.9 | 388.5 | 29.5 | 256.6 | 23.3 | 296.5 | 19.9 |
| 5 | 285.8 | 21.0 | 291.8 | 29.3 | 299.8 | 22.9 | 271.5 | 21.2 |
| 10 | 296.4 | 36.6 | 314.0 | 32.1 | 305.4 | 16.6 | 272.1 | 30.9 |
There is no difference in context-independent sensitization between Sal-F1 and Mor-F1 animals
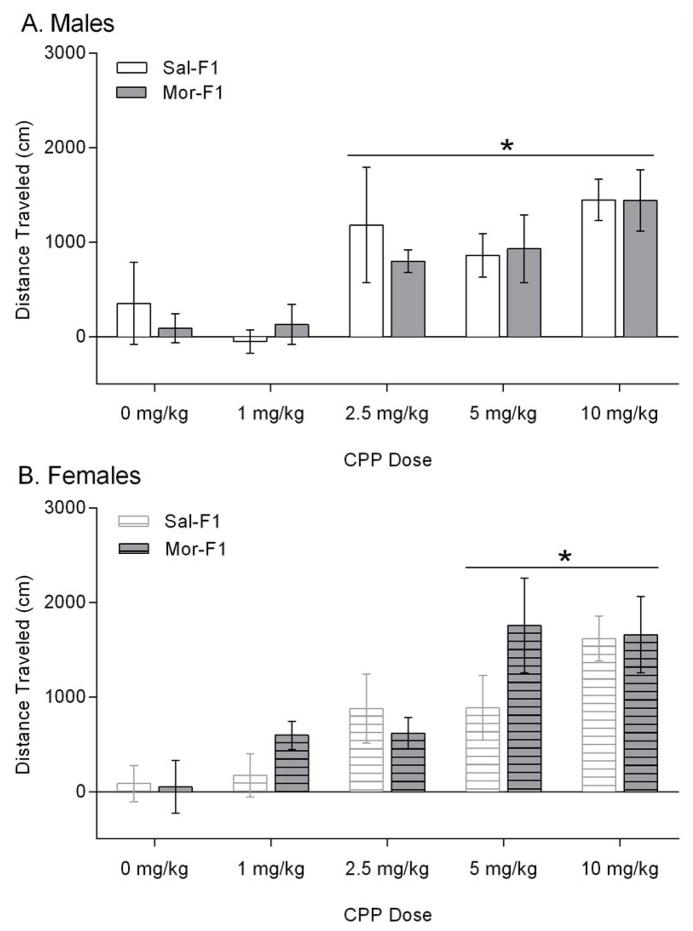
One week following the last morphine or saline injection of the CPP paradigm (6 days after the posttest), animals were tested for locomotor sensitization in a novel environment. In this context-independent sensitization paradigm, the repeated injections were administered during the CPP paradigm. Thus animals received either saline or morphine for 4 days and were tested one week later for saline- and morphine-induced locomotor activity in a different environment. The data following a saline injection were analyzed with a 3 way ANOVA, which revealed a significant main effect of sex [F(1,182)=4.894, p=0.028]. There was no effect of maternal treatment nor dose (data not shown)). The data expressed as distance traveled post morphine – post saline is shown in Figure 4. In the male offspring (Figure 4A), there was a significant main effect of dose [F(4,91)=6.450; p=0.0001] but no effect of maternal treatment [F(1,91)=0.1571; p=0.69] nor an interaction [F(4,91)=0.2580; p=0.90]. Further analysis of the dose effect revealed that the male animals demonstrated significant sensitization at the 2.5, 5, and 10 mg/kg doses (p<0.05). A similar effect was observed in the females (Figure 4B), with a significant dose effect [F(4, 91)=9.201; p<0.0001] but no effect of maternal treatment [F(1, 91)=1.150; p=0.2865] and no interaction [F(4, 91)=1.068; p=0.37]. Further analysis of the dose effect revealed that the females showed significant sensitization to the 5 and 10 mg/kg doses (p<0.05).
Figure 4.
Context-independent locomotor sensitization. Data are expressed as mean +/− SEM distance travelled following a morphine injection (2 mg/kg) – a saline injection. Panel A shows the data from male animals and panel B shows the data from female animals. *p<0.05 main effect of dose. N=9–15/group
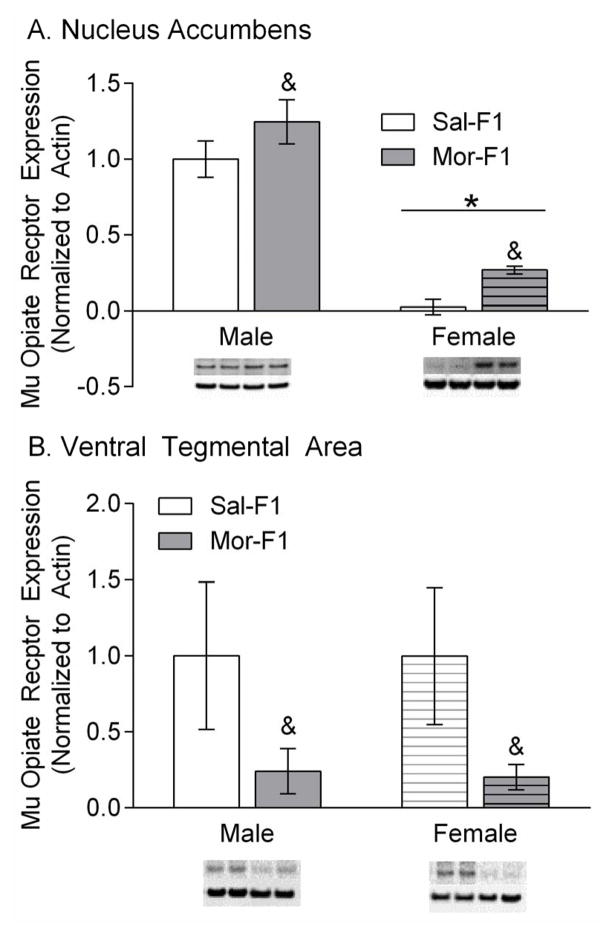
There is increased mu opioid receptor protein expression in the NAc of Mor-F1 females and decreased mu opioid receptor protein expression in the VTA of all Mor-F1 animals
Protein expression of mu opioid receptors in the NAc and the VTA was measured in adult (PND 65–70) naïve F1 animals. The data are expressed as mu opioid receptor expression as a percent control (male Sal-F1), normalized to beta actin (mean +/− SEM). Analysis of the NAc revealed a significant main effect of maternal treatment [F(1, 18)=5.174; p=0.035] a significant effect of sex [F(1, 18)=82.23; p<0.0001] but no interaction [F(1, 18)=0.00018; p=0.9892)(Figure 5a). Analysis of the VTA showed a significant maternal treatment effect [F(1,20)=5.1901; p=0.033] but no effect of sex [F(1,20)=0.003679; p=0.9522] and no interaction [F(1,20)=0.00312; p=0.9560] (Figure 5b).
Figure 5.
Mu Opioid Receptor (MOR) expression in the Nac (Panel A) and VTA (Panel B). Data are expressed as % of control and normalized to beta actin. All samples were run in duplicate or triplicate. Representative images from the blots are placed below the bar graph. & refers to p<0.05 main effect of maternal treatment and * refers to p<0.05 main effect of sex n=5–6/group
Discussion
The data presented here show that preconception exposure of morphine to female adolescent animals affects their offspring’s response to morphine in a sex-specific manner, which may be mediated in part by differential expression of the mu opioid receptor in the NAc and VTA.
In the CPP experiments, there was a significant preference for the morphine paired side at the 2.5, 5, and 10 mg/kg doses in all male animals, which is similar to previous studies using a comparable conditioning regimen (Bardo et al., 1995). There were no significant effects of maternal adolescent drug history on CPP behavior in the male offspring. The data from the females did not show a main effect of dose, although there was a trend in that direction (p=0.055). There is a paucity of literature examining CPP in females but the range of doses that were utilized covered previously published reports (Bardo et al., 1995; Olmstead and Burns, 2005). One reason for the lack of a main effect in females may be the use of a 3-chamber apparatus as opposed to a 2-chamber apparatus. With the 2-chamber method, the animal is forced to make a choice between the two compartments which may artificially inflate the reward association. In a 3-chamber design, the animal may spend time in the small center compartment. The current data indicate that females, on average, spent over 1/3 of their time in the center compartment, which was significantly more time than males. However, despite the large amount of time spent in the center compartment, Mor-F1 females did show a significant increase in preference compared to the Sal-F1 females at both of the lower doses (1 and 2.5 mg/kg). All females, regardless of maternal opioid history showed increased locomotor activity compared with males in response to a novel environment. This has been shown repeatedly and recapitulates what is known in the literature (Hensleigh et al., 2011; Spivey et al., 2009; Wooters et al., 2006). CPP is posited to measure cue-elicited conditioning that can motivate drug-taking behavior (Bardo et al., 1995; Robbins and Ehrman, 1992). Therefore, this suggests either an increased sensitivity to the rewarding properties of morphine in the Mor-F1 females and/or the Mor-F1 female animals may have an increased capacity to form associations between external cues and internal states.
While there were clear effects on the sensitivity to the reward/associative effects of morphine in the female offspring, there were no differences in the levels of sensitization observed as a function of maternal treatment in either sex. Thus, the sensitization data show that both male and female animals are capable of demonstrating a sensitized locomotor response when administered a low dose of morphine in a new context. Sensitization is a phenomenon whereby following repeated exposure to a stimulus that same stimulus elicits an enhanced response (first characterized in psychostimulants but also well established in opioids) (Curran et al., 1996; Steketee and Kalivas, 2011; Trujillo, 2000). While there were no differences between the Sal-F1 and Mor-F1 animals using a context-independent paradigm, previous findings using a similar (although more prolonged) adolescent morphine exposure paradigm revealed increased levels of morphine-induced locomotor sensitization in Mor-F1 males but not females (Byrnes, 2005). The two key differences in the current study are the use of 10 day as opposed to a 20 day adolescent exposure regimen and the use of the more traditional model of context-dependent locomotor sensitization paradigm (i.e. locomotor activity was measured in the same environment throughout the experimental paradigm). In the current study, our goal was to determine whether similar differences in locomotor sensitization could be discerned in the absence of contextual cues. Thus, we assessed morphine-induced locomotor sensitization in a novel environment (i.e. an apparatus not previously associated with drug or saline). The current findings indicate that maternal treatment has no effect on context-independent sensitization. Whether similar results using this more abbreviated adolescent exposure paradigm would be observed in a context-dependent model is unknown.
Sensitization is thought to model critical neural modifications associated with repeated exposure to drugs of abuse (O’Brien and Gardner, 2005; Steketee and Kalivas, 2011). Interestingly, sensitization occurs following repeated administration of a wide variety of drugs of abuse and great cross-sensitization exists between various drugs of abuse, lending evidence to the idea that behavioral sensitization is caused by common neural adaptations by most, if not all abused substances (Steketee and Kalivas, 2011). Sensitization has been shown to be dependent on contextual conditioning in that a greater sensitized response is observed if the environment is a conditioned stimulus for the drug (Pierce and Kalivas, 1997). Therefore, the choice to evaluate context-independent sensitization was aimed at teasing apart the contextual conditioning aspect of addiction from the physical neural adaptation. In that context the Mor-F1 female animals show increased levels of contextual association to morphine without demonstrating alterations in locomotor response to morphine compared with Sal-F1 females. This could be due to increased reward of the opioid itself, providing a greater appetitive stimulus, or enhanced contextual learning in the Mor-F1 females.
Due to the differences observed within these two divergent models of addiction-like behavior, we sought to determine molecular mechanisms that might underlie these changes. Therefore, we utilized naïve littermates to examine the level of protein expression of the mu opioid receptor prior to drug exposure. The data show differences in baseline levels of mu opioid receptor protein expression in both the VTA and the NAc, although the patterns are quite distinct. In the VTA there was a main effect of maternal treatment showing decreased basal mu opioid receptor protein levels in the Mor-F1 animals. In the NAc there was a main effect of both sex and maternal treatment which showed an increase in basal mu opioid receptor levels in the Mor-F1 animals and lower levels of mu opioid receptor in females. The mu opioid receptor subtype is one of three main classes of opioid receptors in the central nervous system (mu, delta, and kappa). The mu subtype is thought to be the primary mediator of reward related processes associated with endogenous and exogenous opioids, although delta opioid receptors also play a role (Bals-Kubik et al., 1990). The mu opioid receptor subtype is expressed in high levels in both the NAc and the VTA (Mansour et al., 1995). While stimulation of mu receptors in both of these areas produce reward in rats, these effects are mediated by different mechanisms of action (Bozarth and Wise, 1981; Hakan and Henriksen, 1989; Olds, 1982). Thus, mu opioid receptor stimulation in the VTA produces reward by increasing dopamine in the NAc, while mu opioid receptor simulation in the NAc likely works through inhibition of medium spiny neuron output (David et al., 2002; Hakan and Henriksen, 1989). While these hypotheses have a significant body of supporting evidence, there are likely mechanisms yet to be discovered that represent additional means by which mu opioid receptor agonists provide reward (Nutt et al., 2015).
Nevertheless, for opioid drugs accumbal mu opioid receptors play a primary role in mediating reinforcement (Hakan and Henriksen, 1989). These receptors have been shown to play a role in morphine-induced CPP, with microinjections of morphine directly into the NAc capable of inducing CPP (Liang et al., 2006; van der Kooy et al., 1982). In the current study, the effect of maternal treatment on CPP was only significant in Mor-F1 females, which demonstrated a robust increase in mu opioid receptors in the NAc. This increase in mu receptors may partially underlie the increased sensitivity to lower doses of morphine-induced CPP in the MOR-F1 females. In fact, the slightly smaller non-significant effect observed in the Mor-F1 males at the 1 mg/kg dose mirrors the small increase in accumbal mu opioid receptor. Previously, it has been shown that prenatal opioid exposure has sex-specific effects on the binding characteristics and expression of opioid receptors (Rimanoczy et al., 2001; Vathy et al., 2000). These data, combined with our own preconception model, implicates a relationship to the vulnerability of preconception and/or prenatal opioid effects which may manifest in a sex-specific manner. Likely, hormones such as estrogen play a role in the sex-specific development of opioidergic differences in morphine exposure (Vathy, 2002).
Additionally, within the accumbens there were lower levels of mu opioid receptors in female animals compared with males. There is a growing literature indicating sex-specific expression patterns of opioid receptors as well as endogenous opioid expression, particularly in relation to the differential antinociception response between males and females (Gerald et al., 2008; Kren et al., 2008; Meyer et al., 2000; Wang et al., 2012). For example, it has been shown that there are sex-specific increases in mu opioid receptor expression within the spinal cord in male but not female rats (Verzillo et al., 2014) and the periaqueductal gray region (Loyd et al., 2008). In terms of changes observed in the accumbens, it has been shown that endogenous opioid gene expression is higher in females than males in the nucleus accumbens (Gugusheff et al., 2014). Moreover, prenatal morphine exposure is associated with higher levels of mu opioid receptor in the nucleus accumbens of males but not females (Vathy et al., 2003). Additionally, within the accumbens, hypermethylation was observed on the oprm1 gene of male rats, which may indicate altered expression of the mu opioid receptor (Hao et al., 2011). The data presented here adds to the existing literature, which indicates that there are numerous aspects of the endogenous opioid system that are divergent based on sex.
The VTA is also known to be involved in mediating the rewarding effects of opioids and thus contributing to CPP and sensitization. For example, intra VTA infusions of mu opioid receptor agonists, such as morphine, induce CPP in rats (Bozarth, 1987; Jaeger and van der Kooy, 1996). One mechanism by which the activity of mu opioid receptors in the VTA is thought contribute to reward is by increasing dopamine in the NAc (Pierce and Kumaresan, 2006). Opioids bind mu opioid receptors located on GABAergic interneurons, decreasing tonic inhibition of VTA dopaminergic neurons and increasing release of dopamine in the NAc. Here, we report that Mor-F1 animals have significantly decreased levels of mu opioid receptors in the VTA at baseline. This is apparently at odds with their CPP behavior where the Mor-F1 animals, particularly the females, showed CPP at lower doses than the Sal-F1 females. However, protein expression does not measure the functional activity of the receptors. Additionally, the animals we measured were drug naïve. Following drug exposure the regulation of the receptor may change dramatically. For example, previous work with Mor-F1 animals in this model indicates dramatic changes in dopamine receptor mRNA expression following repeated injections of quinpirole (Byrnes et al., 2013). Or, perhaps the effect is driven by opioid receptors in different brain regions, such as the accumbens which correlated more closely with the observed behaviors. In terms of sensitization, the VTA mu opioid receptors have been associated with the initiation of sensitization rather than the expression(Steketee and Kalivas, 2011). We did not measure locomotor activity during initiation which may have revealed differences between Mor-F1 and Sal-F1 animals. Indeed, in our previous studies using the more prolonged adolescent morphine exposure model, both male and female Mor F1 animals demonstrated more rapid development of sensitization (Byrnes, 2005).
It is important to emphasize that there was no gestational exposure to opioids in the F1 animals. The females were exposed to morphine only during adolescence and remained drug free for at least 3 weeks prior to mating. The mechanism of transmission is currently unknown but may include direct oocyte exposure to opioids. It is known that opioid receptors are expressed on oocytes and that stimulation delays oocyte maturation (Agirregoitia et al., 2012; Iorga et al., 2009), which can be reversed using the opioid antagonist naloxone (Dell’Aquila et al., 2002; Iorga et al., 2009). In fact, multiple studies have shown that endogenous opioids and their receptors play a substantial role in the development and function of the reproductive axis (Vuong et al., 2010). Thus, opioid administration can have both acute and chronic effects on the endocrine system including increased growth hormone and prolactin, altered ACTH, decreased luteinizing hormone, and decreased testosterone, just to name a few (Vuong et al., 2010). These represent direct effects of morphine on the maturation of the reproductive axis as well as oocyte development, which may contribute to the observed phenotype in the subsequent generation. Additionally, alterations in maternal care, or other epigenetic influences may also play a role (Vassoler et al., 2013). However, previously, we examined the maternal care in these animals and found only very subtle differences (Johnson et al., 2011). Yet this cannot be discounted as a potential mechanism and future work is necessary to tease this apart.
Both research and clinical experience indicate that drug use disorders tend to run in families. However, the ability to distinguish between genetic and environmental factors is extraordinarily difficult. However, there is a literature that shows that children of addicts are more likely to become addicts themselves and have many other behavioral problems including conduct disorder, attention-deficit/hyperactivity disorder, psychiatric disorders, and major depression (Johnson and Leff, 1999; Nunes et al., 2000; Nunes et al., 1998; Tsuang et al., 1996; van den Bree et al., 1998; Weissman et al., 1999). Additionally, the role of the environment appears to be particularly important, especially for females. Thus, in one study, it was found that for female opiate users, environmental influences actually played a much more important role in the development of abuse/dependence with genetic influences carrying no weight at all (van den Bree et al., 1998). Here, we are introducing yet another variable that should be taken into consideration, preconception drug use in the absence of continued intake. Unfortunately, there are no human studies to date that utilize longitudinal methods to examine preconception drug use on outcomes in children. Additionally, the transmission of this effect is unknown and likely encompasses environmental, biological, genetic, and psychological factors. Because preconception drug exposure is so difficult to tease apart in humans, preclinical models are necessary to determine what, if any influence this drug exposure has on future offspring.
Taken together our results indicate that female adolescent exposure to opioids has the potential to induce increased sensitivity to the rewarding effects of opioids, particularly in females, in the subsequent generation and this effect may be mediated by mu opioid receptor expression in the NAc and VTA.
Adult offspring of females exposed to morphine during adolescence are studied
Offspring demonstrate enhanced sensitivity to the rewarding properties of morphine
Mu opioid receptors of offspring are altered in the nucleus accumbens
Mu opioid receptors of offspring are altered in the ventral tegmental area
Acknowledgments
Funding
This work was funded by NIH: NIDA 5R01DA025674-06 (EMB), NIDA 5R03DA034886-02 (EMB), and T35RR029724 (SJW).
Footnotes
Disclosures
The authors have no disclosures or conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agirregoitia E, Peralta L, Mendoza R, Exposito A, Ereno ED, Matorras R, Agirregoitia N. Expression and localization of opioid receptors during the maturation of human oocytes. Reprod Biomed Online. 2012;24:550–557. doi: 10.1016/j.rbmo.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Bals-Kubik R, Shippenberg TS, Herz A. Involvement of central mu and delta opioid receptors in mediating the reinforcing effects of beta-endorphin in the rat. Eur J Pharmacol. 1990;175:63–69. doi: 10.1016/0014-2999(90)90153-w. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bozarth MA. Neuroanatomical boundaries of the reward-relevant opiate-receptor field in the ventral tegmental area as mapped by the conditioned place preference method in rats. Brain Res. 1987;414:77–84. doi: 10.1016/0006-8993(87)91327-8. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Britt MD, Wise RA. Ventral tegmental site of opiate reward: antagonism by a hydrophilic opiate receptor blocker. Brain Res. 1983;258:105–108. doi: 10.1016/0006-8993(83)91232-5. [DOI] [PubMed] [Google Scholar]
- Byrnes EM. Transgenerational consequences of adolescent morphine exposure in female rats: effects on anxiety-like behaviors and morphine sensitization in adult offspring. Psychopharmacology (Berl) 2005;182:537–544. doi: 10.1007/s00213-005-0122-4. [DOI] [PubMed] [Google Scholar]
- Byrnes JJ, Babb JA, Scanlan VF, Byrnes EM. Adolescent opioid exposure in female rats: transgenerational effects on morphine analgesia and anxiety-like behavior in adult offspring. Behav Brain Res. 2011;218:200–205. doi: 10.1016/j.bbr.2010.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Carini LM, Byrnes EM. Multigenerational effects of adolescent morphine exposure on dopamine D2 receptor function. Psychopharmacology (Berl) 2013;227:263–272. doi: 10.1007/s00213-012-2960-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JJ, Johnson NL, Schenk ME, Byrnes EM. Cannabinoid exposure in adolescent female rats induces transgenerational effects on morphine conditioned place preference in male offspring. J Psychopharmacol. 2012;26:1348–1354. doi: 10.1177/0269881112443745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadoret RJ, Troughton E, O’Gorman TW, Heywood E. An adoption study of genetic and environmental factors in drug abuse. Arch Gen Psychiatry. 1986;43:1131–1136. doi: 10.1001/archpsyc.1986.01800120017004. [DOI] [PubMed] [Google Scholar]
- Calcaterra S, Glanz J, Binswanger IA. National trends in pharmaceutical opioid related overdose deaths compared to other substance related overdose deaths: 1999–2009. Drug Alcohol Depend. 2013;131:263–270. doi: 10.1016/j.drugalcdep.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo G, Lovric J, Chen CC, Pytte CL, Bodnar RJ. Ventromedial and medial preoptic hypothalamic ibotenic acid lesions potentiate systemic morphine analgesia in female, but not male rats. Behav Brain Res. 2010;214:301–316. doi: 10.1016/j.bbr.2010.05.046. [DOI] [PubMed] [Google Scholar]
- Curran EJ, Akil H, Watson SJ. Psychomotor stimulant- and opiate-induced c-fos mRNA expression patterns in the rat forebrain: comparisons between acute drug treatment and a drug challenge in sensitized animals. Neurochem Res. 1996;21:1425–1435. doi: 10.1007/BF02532384. [DOI] [PubMed] [Google Scholar]
- David V, Durkin TP, Cazala P. Differential effects of the dopamine D2/D3 receptor antagonist sulpiride on self-administration of morphine into the ventral tegmental area or the nucleus accumbens. Psychopharmacology (Berl) 2002;160:307–317. doi: 10.1007/s00213-001-0981-2. [DOI] [PubMed] [Google Scholar]
- Dell’Aquila ME, Casavola V, Reshkin SJ, Albrizio M, Guerra L, Maritato F, Minoia P. Effects of beta-endorphin and Naloxone on in vitro maturation of bovine oocytes. Mol Reprod Dev. 2002;63:210–222. doi: 10.1002/mrd.10163. [DOI] [PubMed] [Google Scholar]
- Djurendic-Brenesel M, Mimica-Dukic N, Pilija V, Tasic M. Gender-related differences in the pharmacokinetics of opiates. Forensic Sci Int. 2010;194:28–33. doi: 10.1016/j.forsciint.2009.10.003. [DOI] [PubMed] [Google Scholar]
- DuRant RH, Smith JA, Kreiter SR, Krowchuk DP. The relationship between early age of onset of initial substance use and engaging in multiple health risk behaviors among young adolescents. Arch Pediatr Adolesc Med. 1999;153:286–291. doi: 10.1001/archpedi.153.3.286. [DOI] [PubMed] [Google Scholar]
- Fiellin DA. Treatment of adolescent opioid dependence: no quick fix. JAMA. 2008;300:2057–2059. doi: 10.1001/jama.2008.567. [DOI] [PubMed] [Google Scholar]
- Gerald TM, Howlett AC, Ward GR, Ho C, Franklin SO. Gene expression of opioid and dopamine systems in mouse striatum: effects of CB1 receptors, age and sex. Psychopharmacology (Berl) 2008;198:497–508. doi: 10.1007/s00213-008-1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gugusheff JR, Ong ZY, Muhlhausler BS. Naloxone treatment alters gene expression in the mesolimbic reward system in ‘junk food’ exposed offspring in a sex-specific manner but does not affect food preferences in adulthood. Physiol Behav. 2014;133:14–21. doi: 10.1016/j.physbeh.2014.04.007. [DOI] [PubMed] [Google Scholar]
- Hakan RL, Henriksen SJ. Opiate influences on nucleus accumbens neuronal electrophysiology: dopamine and non-dopamine mechanisms. J Neurosci. 1989;9:3538–3546. doi: 10.1523/JNEUROSCI.09-10-03538.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Huang W, Nielsen DA, Kosten TA. Litter gender composition and sex affect maternal behavior and DNA methylation levels of the oprm1 gene in rat offspring. Front Psychiatry. 2011;2:21. doi: 10.3389/fpsyt.2011.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensleigh E, Smedley L, Pritchard LM. Sex, but not repeated maternal separation during the first postnatal week, influences novel object exploration and amphetamine sensitivity. Dev Psychobiol. 2011;53:132–140. doi: 10.1002/dev.20499. [DOI] [PubMed] [Google Scholar]
- Heyman RB, Adger H., Jr Office approach to drug abuse prevention. Pediatr Clin North Am. 1997;44:1447–1455. doi: 10.1016/s0031-3955(05)70568-x. [DOI] [PubMed] [Google Scholar]
- Holman JE, Stoddard GJ, Higgins TF. Rates of prescription opiate use before and after injury in patients with orthopaedic trauma and the risk factors for prolonged opiate use. J Bone Joint Surg Am. 2013;95:1075–1080. doi: 10.2106/JBJS.L.00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman JE, Stoddard GJ, Horwitz DS, Higgins TF. The effect of preoperative counseling on duration of postoperative opiate use in orthopaedic trauma surgery: a surgeon-based comparative cohort study. J Orthop Trauma. 2014;28:502–506. doi: 10.1097/BOT.0000000000000085. [DOI] [PubMed] [Google Scholar]
- Hwang J, Kim JE, Kaufman MJ, Renshaw PF, Yoon S, Yurgelun-Todd DA, Choi Y, Jun C, Lyoo IK. Enlarged cavum septum pellucidum as a neurodevelopmental marker in adolescent-onset opiate dependence. PLoS One. 2013;8:e78590. doi: 10.1371/journal.pone.0078590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iorga AI, Valentini L, De Santis T, Ambruosi B, Albrizio M, Guaricci AC, Caira M, Dell’Aquila M. Expression of the mu opioid receptor and effects of the opioid antagonist naloxone on in vitro maturation of oocytes recovered from anoestrous bitches. Reprod Domest Anim. 2009;44(Suppl 2):263–268. doi: 10.1111/j.1439-0531.2009.01423.x. [DOI] [PubMed] [Google Scholar]
- Jaeger TV, van der Kooy D. Separate neural substrates mediate the motivating and discriminative properties of morphine. Behav Neurosci. 1996;110:181–201. doi: 10.1037//0735-7044.110.1.181. [DOI] [PubMed] [Google Scholar]
- Johnson JL, Leff M. Children of substance abusers: overview of research findings. Pediatrics. 1999;103:1085–1099. [PubMed] [Google Scholar]
- Johnson NL, Carini L, Schenk ME, Stewart M, Byrnes EM. Adolescent opiate exposure in the female rat induces subtle alterations in maternal care and transgenerational effects on play behavior. Front Psychiatry. 2011;2:29. doi: 10.3389/fpsyt.2011.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future national survey results on drug use: 1975–2014: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research The University of Michigan; 2015. p. 90. [Google Scholar]
- Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kren MC, Haller VL, Welch SP. The role of gonadal hormones on opioid receptor protein density in arthritic rats. Eur J Pharmacol. 2008;578:177–184. doi: 10.1016/j.ejphar.2007.08.036. [DOI] [PubMed] [Google Scholar]
- Li CQ, Luo YW, Bi FF, Cui TT, Song L, Cao WY, Zhang JY, Li F, Xu JM, Hao W, Xing XW, Zhou FH, Zhou XF, Dai RP. Development of anxiety-like behavior via hippocampal IGF-2 signaling in the offspring of parental morphine exposure: effect of enriched environment. Neuropsychopharmacology. 2014;39:2777–2787. doi: 10.1038/npp.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Li Y, Ping X, Yu P, Zuo Y, Wu L, Han JS, Cui C. The possible involvement of endogenous ligands for mu-, delta- and kappa-opioid receptors in modulating morphine-induced CPP expression in rats. Peptides. 2006;27:3307–3314. doi: 10.1016/j.peptides.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18:22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, Stolar M, Stevens DE, Goulet J, Preisig MA, Fenton B, Zhang H, O’Malley SS, Rounsaville BJ. Familial transmission of substance use disorders. Arch Gen Psychiatry. 1998;55:973–979. doi: 10.1001/archpsyc.55.11.973. [DOI] [PubMed] [Google Scholar]
- Meyer JS, Shani I, Rice D. Effects of neonatal cocaine treatment and gender on opioid agonist-stimulated [(35)S]GTP gamma S binding in the striatum and nucleus accumbens. Brain Res Bull. 2000;53:147–152. doi: 10.1016/s0361-9230(00)00323-3. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Weissman MM, Goldstein R, McAvay G, Beckford C, Seracini A, Verdeli H, Wickramaratne P. Psychiatric disorders and impairment in the children of opiate addicts: prevalances and distribution by ethnicity. Am J Addict. 2000;9:232–241. doi: 10.1080/10550490050148062. [DOI] [PubMed] [Google Scholar]
- Nunes EV, Weissman MM, Goldstein RB, McAvay G, Seracini AM, Verdeli H, Wickramaratne PJ. Psychopathology in children of parents with opiate dependence and/or major depression. J Am Acad Child Adolesc Psychiatry. 1998;37:1142–1151. doi: 10.1097/00004583-199811000-00013. [DOI] [PubMed] [Google Scholar]
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nat Rev Neurosci. 2015;16:305–312. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- O’Brien CP, Gardner EL. Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther. 2005;108:18–58. doi: 10.1016/j.pharmthera.2005.06.018. [DOI] [PubMed] [Google Scholar]
- Olds ME. Reinforcing effects of morphine in the nucleus accumbens. Brain Res. 1982;237:429–440. doi: 10.1016/0006-8993(82)90454-1. [DOI] [PubMed] [Google Scholar]
- Olmstead MC, Burns LH. Ultra-low-dose naltrexone suppresses rewarding effects of opiates and aversive effects of opiate withdrawal in rats. Psychopharmacology (Berl) 2005;181:576–581. doi: 10.1007/s00213-005-0022-7. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Rimanoczy A, Slamberova R, Vathy I. Prenatal morphine exposure alters estrogen regulation of kappa receptors in the cortex and POA of adult female rats but has no effects on these receptors in adult male rats. Brain Res. 2001;894:154–156. doi: 10.1016/s0006-8993(00)03326-6. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN. Designing studies of drug conditioning in humans. Psychopharmacology (Berl) 1992;106:143–153. doi: 10.1007/BF02801965. [DOI] [PubMed] [Google Scholar]
- SAMHSA. Substance Abuse and Mental Health Services Administration, Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2011. NSDUH Series H-41, HHS Publication No. (SMA) 11–4658. [Google Scholar]
- Schulteis G, Koob GF. Reinforcement processes in opiate addiction: a homeostatic model. Neurochem Res. 1996;21:1437–1454. doi: 10.1007/BF02532385. [DOI] [PubMed] [Google Scholar]
- Schwartz RH. Adolescent heroin use: a review. Pediatrics. 1998;102:1461–1466. doi: 10.1542/peds.102.6.1461. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spivey JM, Shumake J, Colorado RA, Conejo-Jimenez N, Gonzalez-Pardo H, Gonzalez-Lima F. Adolescent female rats are more resistant than males to the effects of early stress on prefrontal cortex and impulsive behavior. Dev Psychobiol. 2009;51:277–288. doi: 10.1002/dev.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drug-seeking behavior. Pharmacol Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szutorisz H, DiNieri JA, Sweet E, Egervari G, Michaelides M, Carter JM, Ren Y, Miller ML, Blitzer RD, Hurd YL. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology. 2014;39:1315–1323. doi: 10.1038/npp.2013.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo KA. Are NMDA receptors involved in opiate-induced neural and behavioral plasticity? A review of preclinical studies. Psychopharmacology (Berl) 2000;151:121–141. doi: 10.1007/s002130000416. [DOI] [PubMed] [Google Scholar]
- Tsuang MT, Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Eaves L. Genetic influences on DSM-III-R drug abuse and dependence: a study of 3,372 twin pairs. Am J Med Genet. 1996;67:473–477. doi: 10.1002/(SICI)1096-8628(19960920)67:5<473::AID-AJMG6>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Vaccarino FJ, Bloom FE, Koob GF. Blockade of nucleus accumbens opiate receptors attenuates intravenous heroin reward in the rat. Psychopharmacology (Berl) 1985;86:37–42. doi: 10.1007/BF00431681. [DOI] [PubMed] [Google Scholar]
- van den Bree MB, Johnson EO, Neale MC, Pickens RW. Genetic and environmental influences on drug use and abuse/dependence in male and female twins. Drug Alcohol Depend. 1998;52:231–241. doi: 10.1016/s0376-8716(98)00101-x. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Mucha RF, O’Shaughnessy M, Bucenieks P. Reinforcing effects of brain microinjections of morphine revealed by conditioned place preference. Brain Res. 1982;243:107–117. doi: 10.1016/0006-8993(82)91124-6. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Byrnes EM, Pierce RC. The impact of exposure to addictive drugs on future generations: Physiological and behavioral effects. Neuropharmacology. 2013 doi: 10.1016/j.neuropharm.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassoler FM, Johnson-Collins NL, Carini LM, Byrnes EM. Next generation effects of female adolescent morphine exposure: sex-specific alterations in response to acute morphine emerge before puberty. Behav Pharmacol. 2014;25:173–181. doi: 10.1097/FBP.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vathy I. Prenatal opiate exposure: long-term CNS consequences in the stress system of the offspring. Psychoneuroendocrinology. 2002;27:273–283. doi: 10.1016/s0306-4530(01)00049-x. [DOI] [PubMed] [Google Scholar]
- Vathy I, Rimanoczy A, Slamberova R. Prenatal exposure to morphine differentially alters gonadal hormone regulation of delta-opioid receptor binding in male and female rats. Brain Res Bull. 2000;53:793–800. doi: 10.1016/s0361-9230(00)00409-3. [DOI] [PubMed] [Google Scholar]
- Vathy I, Slamberova R, Rimanoczy A, Riley MA, Bar N. Autoradiographic evidence that prenatal morphine exposure sex-dependently alters mu-opioid receptor densities in brain regions that are involved in the control of drug abuse and other motivated behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:381–393. doi: 10.1016/S0278-5846(02)00355-X. [DOI] [PubMed] [Google Scholar]
- Verzillo V, Madia PA, Liu NJ, Chakrabarti S, Gintzler AR. Mu-opioid receptor splice variants: sex-dependent regulation by chronic morphine. J Neurochem. 2014;130:790–796. doi: 10.1111/jnc.12768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev. 2010;31:98–132. doi: 10.1210/er.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–306. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Huang P, Ung A, Blendy JA, Liu-Chen LY. Reduced expression of the mu opioid receptor in some, but not all, brain regions in mice with OPRM1 A112G. Neuroscience. 2012;205:178–184. doi: 10.1016/j.neuroscience.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, McAvay G, Goldstein RB, Nunes EV, Verdeli H, Wickramaratne PJ. Risk/protective factors among addicted mothers’ offspring: a replication study. Am J Drug Alcohol Abuse. 1999;25:661–679. doi: 10.1081/ada-100101885. [DOI] [PubMed] [Google Scholar]
- Woolf AD, Greco C. Why can’t we retire codeine? Pediatrics. 2014;133:e1354–1355. doi: 10.1542/peds.2013-4057. [DOI] [PubMed] [Google Scholar]
- Wooters TE, Dwoskin LP, Bardo MT. Age and sex differences in the locomotor effect of repeated methylphenidate in rats classified as high or low novelty responders. Psychopharmacology (Berl) 2006;188:18–27. doi: 10.1007/s00213-006-0445-9. [DOI] [PubMed] [Google Scholar]
- Yohn NL, Bartolomei MS, Blendy JA. Multigenerational and transgenerational inheritance of drug exposure: The effects of alcohol, opiates, cocaine, marijuana, and nicotine. Prog Biophys Mol Biol. 2015;118:21–33. doi: 10.1016/j.pbiomolbio.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]