Abstract
Heterotopic ossification (HO) is a pathological process where bone forms in connective tissues such as skeletal muscle. Previous studies have suggested that muscle-resident non-myogenic mesenchymal progenitors are the likely source of osteoblasts and chondrocytes in HO. However, the previously identified markers of muscle-resident osteoprogenitors label up to half the osteoblasts within heterotopic lesions, suggesting other cell populations are involved. We have identified alpha smooth muscle actin (αSMA) as a marker of osteoprogenitor cells in bone and periodontium, and of osteo-chondro progenitors in the periosteum during fracture healing. We therefore utilized a lineage tracing approach to evaluate whether αSMACreERT2 identifies osteoprogenitors in the muscle. We show that in the muscle, αSMACreERT2 labels both perivascular cells, and satellite cells. αSMACre-labeled cells undergo osteogenic differentiation in vitro and form osteoblasts and chondrocytes in BMP2-induced HO in vivo. In contrast, Pax7CreERT2-labeled muscle satellite cells were restricted to myogenic differentiation in vitro, and rarely contributed to HO in vivo. Our data indicate that αSMACreERT2 labels a large proportion of osteoprogenitors in skeletal muscle, and therefore represents another marker of muscle-resident cells with osteogenic potential under HO-inducing stimulus. In contrast, muscle satellite cells make minimal contribution to bone formation in vivo.
Keywords: heterotopic ossification, mesenchymal progenitor, alpha smooth muscle actin, satellite cell, osteogenesis
Introduction
Heterotopic ossification (HO) refers to formation of skeletal tissue in soft tissues such as muscle and subcutaneous tissues. It is a feature of the rare genetic diseases fibrodysplasia ossificans progressiva (FOP) and progressive osseous heteroplasia[1]. FOP is caused by mutations that result in abnormal activation of ACVR1, a bone morphogenetic protein (BMP) receptor, in response to Activin A, a ligand that is normally inhibitory, thereby implicating dysregulation of BMP signaling as an important player in formation of HO[2, 3]. HO is also a complication associated with high impact orthopedic injuries, such as those sustained in combat, and neurological damage, in particular spinal cord injury[4]. Most HO lesions undergo a process similar to endochondral ossification, and analogous with fracture healing. HO lesions are initiated in areas of tissue damage, and begin with inflammation and infiltration of cells of the immune system. Formation of fibrocartilage occurs, followed by ossification, and infiltration of bone marrow[5]. Once formed, lesions generally persist unless removed surgically, and there is currently no proven pharmacological treatment for prevention or removal of HO lesions.
Muscle contains multiple populations of progenitor cells: satellite cells, non-satellite mesenchymal progenitors present within the interstitium, as well as perivascular cells. Satellite cells are characterized by their location below the muscle fiber basal lamina, and by expression of Pax7, and are critical for muscle fiber regeneration. Most studies suggest that in vivo, satellite cells are lineage-restricted self-renewing muscle stem cells[6–11]. Interstitial cells characterized by expression of PDGFRα, or Sca1 and CD34, act as fibro/adipogenic progenitors, and their in vivo differentiation potential is dictated by the muscle microenvironment[7, 8, 12]. Perivascular cells constitute a third muscle-resident population and may have multiple potential fates. Perivascular cells can contribute to the satellite cell pool in rare circumstances such as during early postnatal growth, or upon transplantation into diseased muscle[13, 14]. In addition, perivascular cells derived from many tissues including muscle are capable of osteogenic differentiation under appropriate conditions[15].
In order to better understand the pathophysiology of HO, numerous studies have investigated the source of cells within muscle that differentiate into chondrocytes and osteoblasts. Studies from FOP patients have suggested that both circulating cells and endothelial cells contribute to osteogenesis[16, 17]. However, studies using Cre-directed lineage tracing in murine models have indicated that hematopoietic, endothelial, and smooth muscle lineages do not contribute to bony elements within lesions formed in BMP-induced HO[18–21]. In addition, myogenic lineages make little or no contribution to osteoblasts or chondrocytes in HO based on studies using Myf5-Cre and MyoD-Cre[18, 19]. Studies with Tie2-Cre, which labels both endothelial, hematopoietic, and, in some Tie2-Cre lines, mesenchymal lineages, indicated that only the CD45−CD31−Sca1+PDGFRα+ population significantly contributed to bone formation[20, 21]. However, Tie2-Cre only labeled 40–50% of osteoblasts and chondrocytes in BMP-induced HO suggesting that other cell populations may be involved[19, 20]. Another recent study indicated that Glast-CreERT2, which predominantly labels a Tie2 negative perivascular population also contributed to ossification in HO, particularly in more mature lesions[22]. Together, these data imply that tissue resident mesenchymal subpopulations, under appropriate stimulation, can form bone tissue in HO. This is consistent with data from muscle tissue collected after blast injury that shows expansion of tissue adherent mesenchymal cells that also had increased osteogenic differentiation capacity[23].
We have previously identified alpha smooth muscle actin (αSMA) as a marker of osteogenic progenitor cells in bone and periodontium, of osteo-chondro progenitors in the periosteum during fracture healing, and of tendon progenitors[24–28]. We therefore hypothesized that αSMA expression may also label progenitors in the muscle. In this study we show that in the muscle, αSMACreERT2 labels both perivascular and satellite cells. αSMACre-labeled cells are capable of osteogenic differentiation in vitro and in vivo, while Pax7-labeled satellite cells show very limited osteogenic potential. αSMACreERT2 activity therefore represents a useful marker to track progenitor cell activity in muscle during HO, and in response to potential treatments.
Materials and Methods
Mouse strains and procedures
All animal procedures were approved by an institutional animal care and use committee. The αSMACreERT2[24] and Col2.3GFP mice[29] were previously described. Ai9 reporter mice (B6;129S6-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, stock # 007905) and Pax7CreERT2 mice (B6.129S-Pax7tm1(cre/ERT2)Gaka/J, stock # 017763) were obtained from Jackson Labs (Bar Harbor, ME). In order to label cells, tamoxifen (Sigma Aldrich, St Louis, MO) dissolved in corn oil was administered by intraperitoneal injection at a dose of 75μg/g of bodyweight, either once, or twice on consecutive days. Heterotopic ossification was induced in 2-month-old mice. Animals were anaesthetized with isoflurane, and 2.5μg BMP2 (Infuse, Medtronic, Minneapolis, MN) in 50μL matrigel (Corning, Tewksbury, MA) was injected into the tibialis anterior muscle using a 28G insulin syringe[20]. Mice were sacrificed 11 days after BMP2 injection and ossicles harvested. Lineage tracing in growing animals was initiated at 4-5 weeks of age, and mice were sacrificed at the indicated time points. Lineage tracing in mature animals was initiated at 4-5 months of age.
Histology
Tissue was fixed in 10% formalin for at least 1 hour, then incubated overnight in 30% sucrose/PBS, and embedded in Cryomatrix (Thermo Fisher Scientific, Waltham, MA). Sections (7μm) were obtained on a cryostat using a tape transfer system as previously described[24]. Sections were coverslipped in 50% glycerol containing DAPI. Imaging was performed using an Observer.Z1 microscope or Axioscan (Carl Zeiss, Thornwood, NY) using appropriate filter cubes. Consistent exposure times were maintained to allow for image analysis. After fluorescent imaging, coverslips were removed and the tissue was stained with 0.025% toluidine blue.
Immunostaining
Immunostaining was performed for CD31 (1:20, AF3628, R&D Systems, Minneapolis, MN), PDGFRα (1:80, AF1062, R&D Systems), PDGFRβ (1:100, MA5-15143, Thermo Scientific) and laminin (1:25, L9393, Sigma Aldrich). Sections for immunostaining were rehydrated in PBS, permeabilized in 0.03% Triton X for 10 minutes, and blocked with Powerblock (Biogenex, Fremont, CA) for 10 minutes. Primary antibody diluted in PBS 0.1% BSA was applied overnight at 4°C. After washing, secondary antibodies diluted 1:500 were applied for 1 hour at room temperature (donkey anti-goat Alexa Fluor 647, followed by washing then goat anti-rabbit Alexa Fluor 488; or for PDGFRβ staining, goat anti-rabbit Alexa Fluor 647, Life Technologies, Carlsbad, CA). A modified protocol was used for PDGFRβ: sections were incubated in citrate-based Antigen Unmasking Solution at 60°C (Vector Laboratories, Burlingame, CA) for 10 hours prior to blocking and the permeabilization step was omitted. Confocal imaging was performed for PDGFRα and CD31 immunostaining using a Zeiss LSM 780.
Flow cytometry
Muscle cells were prepared for analysis or sorting two days after tamoxifen injections. Hindlimb muscles were dissected free of fat, tendon and major vasculature, and the perimysium was removed. Tissue was then shredded, and incubated in DMEM/0.2% collagenase A at 37°C with agitation. After 30 min, tissue was homogenized by passing through an 18G needle, followed by a further 15 min incubation. Cells were washed and resuspended before staining proceeded in 1× Hank's balanced salt solution, 10mM HEPES, 2% fetal bovine serum (FBS). FACS reagents were purchased from eBioscience (San Diego, CA) unless otherwise stated. Cells were stained with combinations of the following antibodies: CD45 (clone 30-F11), CD31 (clone 390), Sca1 (D7), PDGFRα (clone APA5), PDGFRβ (clone APB5), CD34 (clone RAM34), αSMA (clone 1A4, Abcam, Cambridge, MA), SM/C-2.6 (provided by S. Fukada[30]). Avidin-APC eFluor 780 was used as a secondary. Dead cells were excluded using SYTOX green (Life Technologies). Cell sorting and analysis was performed using a FACSAria II (BD Biosciences, San Jose, CA). Voltages and gates were set based on unstained cells from Cre negative and Cre positive animals. Cell surface marker analysis was performed on cell preparations from both single animals and up to 4 animals pooled, aged 6–10 weeks, αSMA: n=17, Pax7: n=6. Both sexes were used, and no difference between sexes was evident. For intracellular staining, cells were stained with Live-Dead UV Blue, then surface marker antibodies, followed by fixation with 3% paraformaldehyde and permeablization with Permeablization Buffer. The samples were split up and stained with either αSMA-FITC antibody, or isotype control (FITC-conjugated mouse IgG2a kappa), which was used for determining gates.
Tissue culture
Muscle cell suspensions were prepared from 3–8 mice as described above. Cells were sorted into tdTomato+ and tdTomato− fractions, and unsorted cells were also cultured. Cells were seeded at a density of 1.5 × 104 cells/cm2. For myogenesis, cells were seeded on matrigel coated plates in muscle growth medium (HAMS F-10, 20% FBS, 5ng/ml FGF2). Once cells reached approximately 70% confluence (7–10 days), medium was switched to DMEM 2% horse serum for 3 days, and formation of myotubes was determined visually and by immunofluorescence for Myosin Heavy Chain (MyHC). Following fixation with 4% paraformaldehyde for 15 minutes, cells were permeabilized and blocked as described for tissue immunostaining. MyHC antibody conjugated with Alexa Fluor 488 (1:500, eBioscience) was applied for 2h at room temperature, then mounted in 50% Glycerol/PBS/Dapi. For osteogenesis and adipogenesis, cells were cultured in basal medium (αMEM 10% FBS) until confluence (7–9 days). Osteogenesis was induced by addition of osteogenic medium (basal medium + 50μg/mL ascorbic acid + 4mM β-glycerophosphate) containing 100ng/ml BMP2. After 14 days of differentiation, cells were harvested in Trizol (Life Technologies) for RNA extraction followed by gene expression analysis. Adipogenesis was induced by addition of adipogenic differentiation medium (basal medium + 1μM insulin + 0.5μM rosiglitazone). After 3–5 days of differentiation, cells were imaged, then harvested in Trizol for RNA extraction or fixed for perilipin immunostaining (anti-Perilipin A antibody, 1:500 (Abcam) for 2h at room temperature followed by goat anti-rabbit Alexa Fluor 488). Medium was changed three times weekly. Cultures of unsorted cells were harvested for RNA extraction at confluence as undifferentiated controls. Reverse transcription and real time PCR were performed using Taqman assays (Life Technologies) as previously described[25].
Image analysis
Cell contribution to HO lesions was quantified in histological sections using ImageJ (NIH). Individual cells within the lesion were defined using the DAPI channel, and separated using the watershed algorithm. Appropriate standardized thresholds for the red and green channels were determined, then signal levels in both channels were determined for each nuclear region, and used to count the number of red, green, and dual-labeled cells. The proportion of labeled osteoblasts was determined by calculating the proportion of green cells that were also red. Chondrocytes were quantified by drawing ROIs around areas of chondrocytes, as determined by toluidine blue staining, then calculating the proportion of red nuclei in these ROIs. To ensure values were representative of the entire ossicle, 2–5 sections at least 50μm intervals apart were analyzed per sample.
Statistics
To determine if the differences in contribution to osteoblasts and chondrocytes in HO lesions were different, data from different sections within a lesion were averaged. Differences between groups were determined using one-way ANOVA with Tukey's post test, or, where appropriate, Student's t test.
Results
αSMA labels satellite and perivascular cell populations in muscle
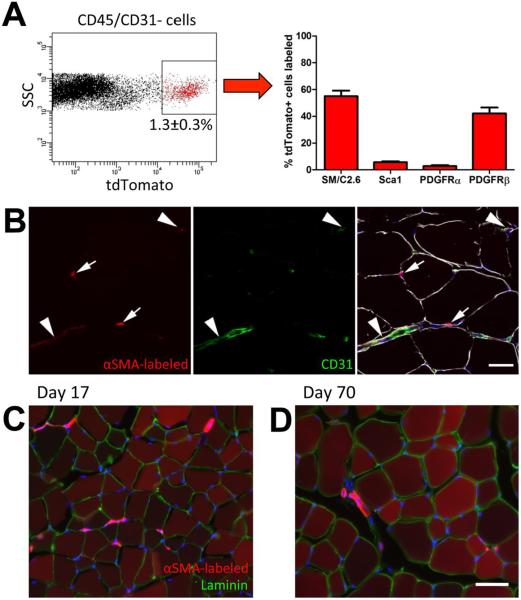
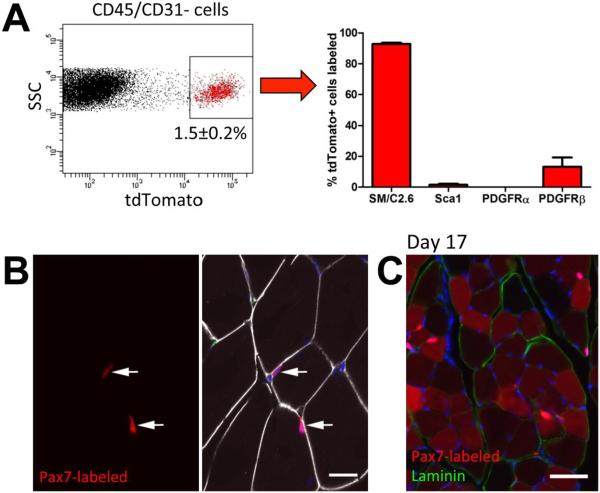
Our previous studies have indicated that Cre driven by the αSMA promoter labels mesenchymal progenitor populations in a number of tissues including periosteum, periodontium and tendon[24–28]. In order to investigate the identity of cells labeled by αSMA in the muscle, we crossed αSMACreERT2 animals with the Ai9 reporter mouse. Cells were labeled by tamoxifen injection and we performed FACS analysis on digested hind-limb muscle of adult αSMACre/Ai9 mice two days later. αSMA-labeled cells were negative for hematopoietic (CD45) and endothelial (CD31) markers, and made up around 1.3% of the CD45/CD31− population (Figure 1A). Approximately half the αSMA-labeled population expressed muscle satellite cell marker SM/C2.6. 3–6% of the αSMACre/Ai9+ cells expressed mesenchymal progenitor markers Scal or PDGFRα (Figure 1A), and the majority of these cells were within the SM/C2.6− population (Supplemental Figure S1B). Around 40% of αSMA-labeled cells expressed perivascular marker PDGFRβ. Histology of labeled muscle indicated that, like in other tissues, perivascular cells adjacent to CD31+ blood vessels were labeled as well as cells resident under the basal lamina, consistent with a satellite cell phenotype (Figure 1B). Labeled cells very rarely colocalized with PDGFRα immunostaining, although they were often closely associated with these cells (Supplemental Figure S2A). However, labeled cells with perivascular morphology were consistently PDGFRβ+ (Supplemental Figure S2B). In order to determine the fate of αSMA-labeled cells, we performed lineage tracing experiments in growing mice starting at 4-5 weeks of age. After 17 or 70 days, the majority of muscle fibers were labeled, and perivascular labeling remained (Figure 1C–D). This extensive labeling of muscle fibers was specific to the growth period, as lineage tracing in mature mice (4-5 months of age) showed some labeling of muscle fibers over time, but to a much lesser extent than during growth (Supplemental Figure S3). Together these results indicate that αSMA labels a mixed cell population in the muscle, which includes both satellite and perivascular cells. To help distinguish between the contribution of the perivascular and satellite cell fractions, we used an established satellite cell marker, Pax7CreERT2[11]. Using the same reporter strain (Ai9) and tamoxifen dosing, we found that Pax7-labeled cells comprised a similar proportion of CD45/CD31− muscle cells to αSMA, but over 90% were SM/C2.6+Sca1−PDGFRα−, consistent with a satellite cell phenotype (Figure 2A, Supplemental Figure S1). We did detect PDGFRβ expression in 13% of Pax7-labeled cells suggesting that expression of this receptor is not exclusive to perivascular cells. Pax7CreERT2 labeled 75.9±3.5% (mean±SEM) of CD45−CD31−SM/C2.6+Sca1− satellite cells, while αSMACreERT2 labeled a smaller proportion of satellite cells (26.3±5.1%). Using CD34 as an alternative marker of satellite cells[8], we confirmed that the majority of Pax7-labeled cells were CD34+Sca1− (Supplemental Figure 1C). αSMA-labeled cells also showed a large CD34+Sca1− satellite cell population using this method, in addition to CD34+Sca1+ and CD34−Sca1− populations. Both Pax7-labeled and αSMA-labeled CD34+Sca1− satellite cell populations showed PDGFRβ expression on a large proportion of the cells (34–45%), and over 70% stained for αSMA. Histology indicated that the Pax7-labeled cells are sublaminar, and not obviously associated with vasculature (Figure 2B), or PDGFRα staining (Supplemental Figure S2C). Consistent with the FACS data, while most Pax7-labeled cells were PDGFRβ−, we were able to identify some PDGFRβ+ Pax7-labeled cells by immunostaining (Supplemental Figure S2D). Following lineage tracing, labeling was evident in the majority of myofibers after 17 days in a growing animal, similar to observations with αSMA (Figure 2C). We also saw evidence of some contribution to myofibers in adult animals, consistent with a recent study (Supplemental Figure S3)[31]. These data are consistent with the established findings that Pax7CreERT2 specifically labels satellite cells in the muscle[11].
Figure 1. Cell surface marker expression and localization of αSMA labeled cells.
(A) αSMACre/Ai9 mice were injected with tamoxifen to label cells, and two days later, hindlimb muscles were processed for FACS analysis. The labeled population represented around 1.3% of the non-hematopoietic, non-endothelial cell fraction (CD45/CD31−). Expression of cell surface markers of muscle satellite cells (SM/C2.6) and mesenchymal progenitor markers in the tdTomato+ population is indicated. (B) αSMACre/Ai9 muscle two days post labeling was immunostained for CD31 (green) and laminin (white). Some labeled cells are perivascular (arrowheads), while others reside below the basal lamina (indicated by laminin staining) consistent with a muscle satellite cell phenotype (arrows). Lineage tracing initiated in 4-5 week old mice indicates that after (C) 17 days, and (D) 70 days, the majority of muscle fibers are labeled. Images representative of tracing in at least 6 animals are shown, and similar results were seen in male and female mice. Scale bars indicate 20μm (B) or 50μm (C,D).
Figure 2. Cell surface marker expression and localization of Pax7 labeled cells.
(A) Pax7Cre/Ai9 mice were injected with tamoxifen to label cells, and two days later, hindlimb muscles were processed for FACS analysis. The labeled population represented around 1.5% of the CD45/CD31− cell fraction, and the presence of cell surface markers of muscle satellite cells (SM/C2.6) and mesenchymal progenitor markers in the tdTomato+ population is indicated. (B) Pax7Cre/Ai9 muscle two days post labeling was immunostained for CD31 (green) and laminin (white). All labeled cells resided below the basal lamina consistent with a muscle satellite cell phenotype (arrows). (C) Lineage tracing initiated in 4-5 week old mice indicates that after 17 days the majority of muscle fibers are labeled. Images representative tracing in 4 animals are shown. Scale bars indicate 20μm (B) or 50μm (C).
In vitro differentiation potential of isolated muscle derived progenitor cell populations
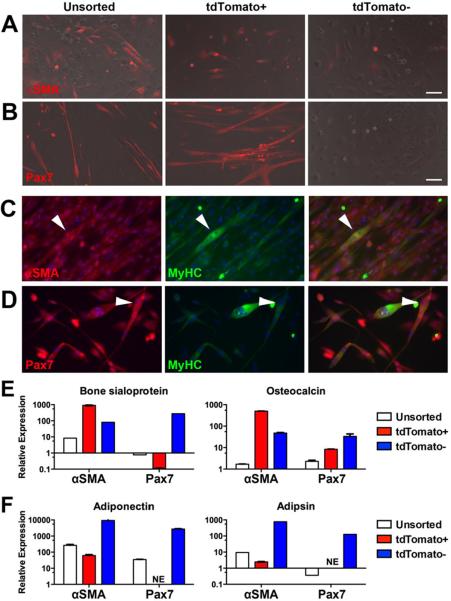
We evaluated the ability of the labeled cell populations to undergo differentiation into several lineages. Cells were isolated from muscle and sorted two days after labeling with tamoxifen (Supplemental Figure S4A–B). Under myogenic conditions, myofibers that formed in unsorted cultures from both αSMACre/Ai9 and Pax7Cre/Ai9 mice were labeled, and the tdTomato+ cell fractions were capable of MyHC+ myofiber formation (Figure 3A–D). Myofibers did not form in the negative cell fractions. Osteogenic differentiation was induced in the same cell populations using BMP2 and evaluated by expression of osteoblast marker genes including bone sialoprotein and osteocalcin. Unsorted cultures showed minimal induction of these markers after two weeks under osteogenic conditions (Figure 3E, Supplemental Table S1). αSMA-labeled cells showed the highest expression of osteoblast marker genes, suggesting enrichment of osteoprogenitors, while Pax7-labeled cells did not undergo osteogenic differentiation in vitro at this BMP2 concentration, suggesting that the osteoprogenitors reside within the αSMA-labeled perivascular population. However, we did note significant induction of osteogenic markers in Pax7-labeled satellite cell cultures treated with a higher concentration of BMP2 (500ng/ml) suggesting that these cells can be directed into the osteoblast lineage with sufficient stimulation (data not shown). Negative cells, depleted of muscle satellite cells, showed upregulation of osteogenic markers, albeit to a lesser extent that αSMA+ cells, suggesting that αSMA does not label all osteoprogenitors. Adipogenesis, as assessed by adiponectin and adipsin expression did not occur efficiently in the unsorted cultures (Figure 3F, Supplemental Figure S4C–F), or the αSMA-labeled cells, and was totally absent in the Pax7-labeled cells, which instead showed evidence of myofiber formation (Supplemental Figure S4D, F). Abundant adipogenesis did, however, occur in both negative fractions (Figure 3D, Supplemental Figure S4C–D). Adipocyte formation was confirmed by perilipin staining, shown in αSMA-labeled cells (Supplemental Figure S4E).
Figure 3. In vitro differentiation potential of αSMA and Pax7 labeled muscle cells.
αSMACre or Pax7Cre-expressing cells were labeled in vivo, then muscle cells harvested. tdTomato+ and tdTomato− cell fractions were sorted and unsorted cells were also cultured. Myogenic differentiation was evaluated visually in (A) αSMA-labeled cultures and (B) Pax7-labeled cultures. Muscle phenotype was confirmed by immunostaining of αSMA+ cells (C) Pax7+ cells (D) for myosin heavy chain (E) Osteogenic differentiation in the presence of BMP2 was evaluated by expression of bone sialoprotein and osteocalcin. (F) Adipogenic differentiation was evaluated by expression of adiponectin and adipsin. Scale bars indicate 100μm. Real time PCR data was normalized to GAPDH expression, then to expression levels of the genes in unsorted cultures harvested at confluence before the addition of differentiation medium. Results from a culture representative of 3–4 biological replicates are shown. NE, not expressed.
In vivo contribution to ectopic bone formation
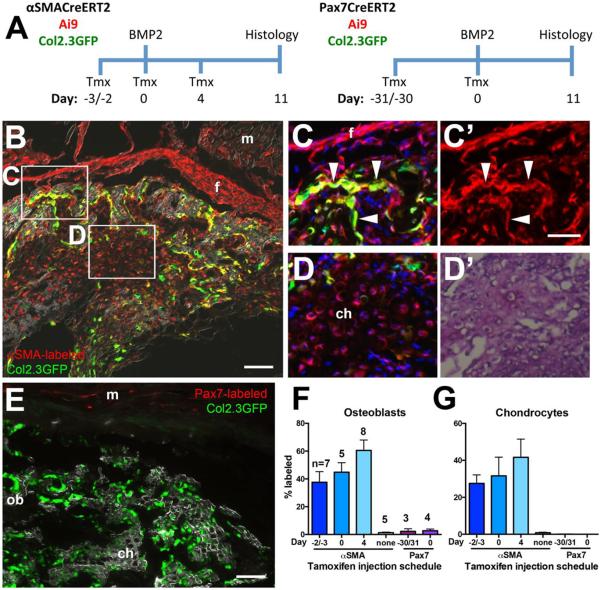
In order to evaluate the ability of αSMA and Pax7 labeled cells to contribute to ectopic bone formation in the muscle, we performed lineage tracing experiments using a model of HO induced by intramuscular BMP2 injection (Figure 4A). BMP2 consistently formed macroscopically evident ossicles as previously reported[20]. To detect the transition of αSMA labeled cells into osteoblasts we utilized Col2.3GFP reporter mice in which mature osteoblasts are GFP+. Since expression of αSMA is known to be induced or upregulated in fibroblasts following injury[32], we performed tamoxifen labeling both before and after BMP2 administration. In all cases, αSMA labeled cells contributed to a significant proportion of osteoblasts and chondrocytes within the lesions, as well as labeling fibrous tissue, and often surrounding muscle (Figure 4B–D). This labeling was not due to tamoxifen-independent activation of the reporter, as there were very few labeled cells under these conditions (Figure 4F–G, Supplemental Figure S5A–C). Our results leave open the possibility that some osteoprogenitors activate or up-regulate αSMA expression after exposure to BMP2, as there is a trend towards increased labeling when tamoxifen is administered after BMP2 administration, however this difference did not reach statistical significance. The αSMA lineage tracing experiments were performed in male mice, but similar experiments in female mice with tamoxifen labeling at the time of BMP2 injection (Day 0) indicated that 58.9±11.1% of osteoblasts and 57.5±6.8% chondrocytes (n=3) were labeled, which is not significantly different from the males. In contrast to αSMA, Pax7-labeled cells showed little or no contribution to heterotopic bone formation (Figure 4E–G). There was no evidence of Pax7Cre-labeled chondrocytes, but in most cases we observed low numbers of labeled osteoblasts (0–6% of osteoblasts derived from Pax7+ precursors), and very few tdTomato+ cells within the borders of the lesion. We observed no significant difference in contribution to the osteoblast lineage both when satellite cells were labeled on the day of BMP2 administration, and when the muscle lineage was labeled more extensively by administering tamoxifen one month prior to BMP2 while mice were still growing (Figure 4F). Damaging the muscle with cardiotoxin prior to BMP2 administration did not significantly increase the contribution of the muscle lineage to osteoblasts (Supplemental Figure S5D–E), consistent with previous studies using MyoD-Cre[19, 20]. These results indicate the αSMACre/Ai9+ perivascular cells are a significant source of osteo- and chondroprogenitors during HO, while muscle lineage cells make minimal contribution.
Figure 4. Contribution of endogenous αSMA and Pax7 labeled muscle cells to heterotopic ossification.
(A) Heterotopic ossification was induced by intramuscular injection of BMP2, with cell labeling initiated at different time points by tamoxifen (Tmx) injection(s). Cell contribution was evaluated histologically after 11 days. (B) αSMA-labeled cells contribute to multiple lineages within the ossicle. tdTomato expression is evident in osteoblasts (C), indicated by co-expression of Col2.3GFP (arrowheads), and chondrocytes (D), indicated by toluidine blue staining and morphology (D'). Fibrous tissue is also labeled. (E) Pax7 labeled cells contribute to muscle, but not heterotopic bone or cartilage tissue. Both images are from animals that received tamoxifen on the day of BMP2 injection. Quantification of the contribution of labeled cells to (F) osteoblasts and (G) chondrocytes in heterotopic bone indicates that αSMACreERT2 labels a significant proportion of bone lineage cells, while Pax7 labels very few. The days on the x-axis indicate the timing of tamoxifen administration in relation to BMP2 administration (Day 0). The n values on (F) represent the number of ossicles analyzed (1 ossicle/mouse) in each group, and also apply to (G). Scale bars represent 100μm (B, E) or 50μm (C, D). Abbreviations: ch, chondrocytes; f, fibrous tissue; m, muscle; ob, osteoblasts.
Discussion
HO is a debilitating complication of around half of severe musculoskeletal injuries sustained in combat zones, and a serious complication of other orthopedic injuries and procedures, for which no effective prevention exists[33]. Identification of the progenitor cells that differentiate into cartilage and bone within the lesions will enable targeted evaluation of potential treatments. In agreement with previous studies in mice, our data indicates that non-hematopoietic, non-endothelial, non-myogenic muscle-resident mesenchymal cells are the main contributor to bone and cartilage in heterotopic lesions[18, 20, 22]. αSMACreERT2 labels a mixed population of cells in the muscle, composed of satellite cells in addition to perivascular cells, some of which show a phenotype characteristic of fibro/adipogenic progenitors[8]. Pax7CreERT2 was used in parallel to rule out the satellite cell fraction as the major contributors to the HO tissue mass. Pax7CreERT2 targets satellite cells very efficiently[11, 31], and over 70% of Pax7-labeled cells were αSMA+, meaning that the αSMA-labeled satellite cells are most likely also labeled by Pax7Cre. In contrast to the Tie2-Cre-labeled population that contribute to HO demonstrated by Wosczyna et al[20], the αSMA-labeled, non-satellite cell fraction are primarily Sca1− and PDGFRα−, and mainly reside in a perivascular, rather than interstitial, location. It is unclear whether αSMA+ cells overlap with the Glast-CreERT2 labeled cells that also contribute to HO, but were shown to be αSMA−, and Glast expression generally labels a different population of perivascular cells than αSMA[22, 34]. Dysregulation of BMP2 signaling is thought to be important in most, if not all forms of HO, and a recent study showed inhibition of burn-induced HO by administration of a BMP receptor inhibitor[35]. For our studies we used intramuscular injection of BMP2 to model the HO process. While this is not directly clinically relevant, it provides a reliable means to monitor cell contribution during HO lesion formation, and is similar to methods of inducing HO used in many other studies[19−22].
In vivo and in vitro, αSMA-labeled cells are capable of osteogenesis, while Pax7-labeled cells generally are not, suggesting the perivascular αSMA+ fraction is enriched for osteoprogenitors. On average, less than half the osteoblasts and chondrocytes in the lesions were labeled by αSMACreERT2, and the αSMA− fraction also showed some evidence of osteogenesis in vitro, suggesting that there is also an αSMA− population contributing to osteogenesis. Our previous data has suggested that αSMA expression is activated in cells that undergo osteo-chondrogenic differentiation in the early stages after injury, so we therefore evaluated labeling when tamoxifen was administered both before and after BMP2. Labeling after BMP2 administration tended to result in a higher contribution of αSMA-labeled cells in HO lesions, in some instances up to 85% of osteoblasts and 75% of chondrocytes, suggesting that the majority of cells that contribute to bone formation express αSMA at some point during the osteogenic commitment phase. The variable labeling suggests that the Cre recombination efficiency is less than 100%. αSMACreERT2 labeling, particularly if combined with methods to exclude satellite cells, may therefore represent a powerful tool to selectively identify osteochondroprogenitors and fibroblasts in early lesions.
In vivo lineage tracing studies using both αSMA and Pax7 as markers of satellite cells suggested incorporation of satellite cells into the majority of muscle fibers within 17 days in growing animals (4-5 weeks of age). Many authors have suggested that satellite cells are a major contributor to postnatal muscle growth, however there is little direct evidence for this phenomenon[36, 37]. Extensive labeling of muscle was specific to the growing animals, consistent with the gradual conversion of most satellite cells to quiescence during the postnatal period[36]. Interestingly, we observed labeling of a selection of muscle fibers within a relatively short timeframe in 4-month-old animals. This suggests that satellite cells continue to incorporate into muscle fibers in adult animals under normal homeostatic conditions, even though their function at this stage appears to be dispensable for tissue homeostasis unless there is major tissue damage, as evidenced by a number of recent studies using models of satellite cell ablation[38–40]. This agrees with the results of Fry et al. who showed a small contribution of BrdU-labeled nuclei to myotubes after a two-week pulse in adult mice, which was abrogated with satellite cell ablation[39]. It also replicates the recent findings of Keefe et al. that demonstrated contribution of satellite cells to muscle fibers of 6-month-old animals over the following 6 months or more[31]. Our results and others suggest that satellite cells, when present, contribute to muscle growth and homeostasis throughout postnatal life.
It is clear from many studies that the fate of mesenchymal progenitor cells in the muscle microenvironment is tightly regulated, as bone, fat or fibrous tissue generally only invade muscle in disease states, such as Duchenne muscular dystrophy, chronic inflammation, or the conditions leading to HO described[41]. In our in vitro studies, the unsorted cultures showed little evidence of osteogenic differentiation, even in the presence of BMP2, and low levels of glitazone-induced adipogenesis, however αSMA− and Pax7− fractions underwent both differentiation programs much more readily suggesting the satellite cells and their progeny were producing signals that inhibited differentiation. A recent study showed similar effects of myotube conditioned media in vitro[42]. Despite this, HO in FOP and other settings is confined to a limited range of tissues including the muscle. Many other tissues and organs have resident MSC-like cell populations capable of osteogenic differentiation in vitro suggesting that specific changes induced by injury and other factors in the muscle microenvironment are required for heterotopic ossification to occur, however further studies are required to identify these mechanisms[15].
In conclusion, we have shown that αSMA labels progenitor cell populations within the muscle that include both satellite cells, and perivascular cells. The perivascular αSMA-labeled cell population is capable of osteogenic differentiation both in vitro and in vivo, and makes a significant contribution to heterotopic bone lesions. In contrast, Pax7-labeled satellite cells appear to be restricted to the myogenic lineage under most conditions.
Supplementary Material
Highlights.
αSMACreERT2 labels both satellite cells and perivascular cells in murine muscle.
αSMA-labeled muscle cells are capable of BMP2-induced osteogenic differentiation.
Pax7-labeled satellite cells rarely form osteoblasts.
Perivascular αSMA+ cells form about half the osteoblasts and chondrocytes in BMP2-induced lesions.
Satellite cells are rapidly incorporated into muscle fibers during adolescent growth and adulthood.
Acknowledgements
Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number AR055607 to I.K. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Connecticut Stem Cell Research Initiative grant 14-SCA-UCHC-02 supported B.G.M. We thank Dr. S Fukada, Osaka University for providing the SM/C-2.6 antibody.
Supported by: This work has been supported by NIH/NIAMS AR055607 grant to I.K and by Connecticut Stem Cell Research Initiative grant 14-SCA-UCHC-02 to B.G.M.
Abbreviations
- αSMA
alpha smooth muscle actin
- BMP
bone morphogenetic protein
- FOP
fibrodysplasia ossificans progressiva
- HO
heterotopic ossification
- MyHC
myosin heavy chain
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: BGM: Conception and design, acquisition and analysis of the data and interpretation, drafting and revising the manuscript, approved the final version and agree to be accountable for all aspects of the work.
ET: Collection and assembly of data, data analysis and interpretation, final approval of manuscript.
ER, IM: Collection and assembly of data, final approval of manuscript.
DG: Collection and assembly of data, data analysis and interpretation, final approval of manuscript.
IK: Conception and design, collection and assembly of data, data analysis and interpretation, financial support, revision and final approval of manuscript and agree to be accountable for all aspects of the work.
Competing financial interests The authors have no conflicts of interest to disclose.
References
- [1].Kaplan FS, Chakkalakal SA, Shore EM. Fibrodysplasia ossificans progressiva: mechanisms and models of skeletal metamorphosis. Disease Models & Mechanisms. 2012;5:756–762. doi: 10.1242/dmm.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho TJ, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–7. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- [3].Hatsell SJ, Idone V, Wolken DM, Huang L, Kim HJ, Wang L, Wen X, Nannuru KC, Jimenez J, Xie L, Das N, Makhoul G, Chernomorsky R, D'Ambrosio D, Corpina RA, Schoenherr CJ, Feeley K, Yu PB, Yancopoulos GD, Murphy AJ, Economides AN. ACVR1R206H receptor mutation causes fibrodysplasia ossificans progressiva by imparting responsiveness to activin A. Sci Transl Med. 2015;7:303ra137. doi: 10.1126/scitranslmed.aac4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Forsberg JA, Pepek JM, Wagner S, Wilson K, Flint J, Andersen RC, Tadaki D, Gage FA, Stojadinovic A, Elster EA. Heterotopic ossification in high-energy wartime extremity injuries: prevalence and risk factors. J Bone Joint Surg Am. 2009;91:1084–91. doi: 10.2106/JBJS.H.00792. [DOI] [PubMed] [Google Scholar]
- [5].Kan L, Hu M, Gomes WA, Kessler JA. Transgenic mice overexpressing BMP4 develop a fibrodysplasia ossificans progressiva (FOP)-like phenotype. American Journal of Pathology. 2004;165:1107–15. doi: 10.1016/S0002-9440(10)63372-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pannerec A, Formicola L, Besson V, Marazzi G, Sassoon DA. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 2013;140:2879–2891. doi: 10.1242/dev.089326. [DOI] [PubMed] [Google Scholar]
- [7].Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nature Cell Biology. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- [8].Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nature Cell Biology. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- [10].Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, Yamaguchi M, Ogawa R, Matev MM, Miyagoe-Suzuki Y, Takeda S, Tsujikawa K, Tsuchida K, Yamamoto H, Fukada S. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011;124:3654–64. doi: 10.1242/jcs.086629. [DOI] [PubMed] [Google Scholar]
- [13].Dellavalle A, Maroli G, Covarello D, Azzoni E, Innocenzi A, Perani L, Antonini S, Sambasivan R, Brunelli S, Tajbakhsh S, Cossu G. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nature Communications. 2011;2:499. doi: 10.1038/ncomms1508. [DOI] [PubMed] [Google Scholar]
- [14].Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nature Cell Biology. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- [15].Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- [16].Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR. Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med. 2010;16:1400–6. doi: 10.1038/nm.2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Suda RK, Billings PC, Egan KP, Kim JH, McCarrick-Walmsley R, Glaser DL, Porter DL, Shore EM, Pignolo RJ. Circulating osteogenic precursor cells in heterotopic bone formation. Stem Cells. 2009;27:2209–19. doi: 10.1002/stem.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Kan L, Liu Y, McGuire TL, Berger DM, Awatramani RB, Dymecki SM, Kessler JA. Dysregulation of local stem/progenitor cells as a common cellular mechanism for heterotopic ossification. Stem Cells. 2009;27:150–6. doi: 10.1634/stemcells.2008-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lounev VY, Ramachandran R, Wosczyna MN, Yamamoto M, Maidment ADA, Shore EM, Glaser DL, Goldhamer DJ, Kaplan FS. Identification of Progenitor Cells That Contribute to Heterotopic Skeletogenesis. Journal of Bone and Joint Surgery. 2009;91A:652–663. doi: 10.2106/JBJS.H.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wosczyna MN, Biswas AA, Cogswell CA, Goldhamer DJ. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification. Journal of Bone and Mineral Research. 2012;27:1004–1017. doi: 10.1002/jbmr.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kolind M, Bobyn JD, Matthews BG, Mikulec K, Aiken A, Little DG, Kalajzic I, Schindeler A. Lineage tracking of mesenchymal and endothelial progenitors in BMP-induced bone formation. Bone. 2015;81:53–59. doi: 10.1016/j.bone.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kan LX, Peng CY, McGuire TL, Kessler JA. Glast-expressing progenitor cells contribute to heterotopic ossification. Bone. 2013;53:194–203. doi: 10.1016/j.bone.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Davis TA, O'Brien FP, Anam K, Grijalva S, Potter BK, Elster EA. Heterotopic ossification in complex orthopaedic combat wounds: quantification and characterization of osteogenic precursor cell activity in traumatized muscle. J Bone Joint Surg Am. 2011;93:1122–31. doi: 10.2106/JBJS.J.01417. [DOI] [PubMed] [Google Scholar]
- [24].Grcevic D, Pejda S, Matthews BG, Repic D, Wang LP, Li HT, Kronenberg MS, Jiang X, Maye P, Adams DJ, Rowe DW, Aguila HL, Kalajzic I. In vivo fate mapping identifies mesenchymal progenitor cells. Stem Cells. 2012;30:187–196. doi: 10.1002/stem.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, Shin DG, Adams DJ, Kalajzic I. Analysis of alphaSMA-labeled progenitor cell commitment identifies Notch signaling as an important pathway in fracture healing. Journal of Bone and Mineral Research. 2014;29:1283–1294. doi: 10.1002/jbmr.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Roguljic H, Matthews BG, Yang W, Cvija H, Mina M, Kalajzic I. In vivo Identification of periodontal progenitor cells. Journal of Dental Research. 2013;92:709–15. doi: 10.1177/0022034513493434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dyment NA, Hagiwara Y, Matthews BG, Li Y, Kalajzic I, Rowe DW. Lineage Tracing of Resident Tendon Progenitor Cells during Growth and Natural Healing. PLoS ONE. 2014;9:e96113. doi: 10.1371/journal.pone.0096113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kalajzic Z, Li H, Wang LP, Jiang X, Lamothe K, Adams DJ, Aguila HL, Rowe DW, Kalajzic I. Use of an alpha-smooth muscle actin GFP reporter to identify an osteoprogenitor population. Bone. 2008;43:501–510. doi: 10.1016/j.bone.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kalajzic I, Kalajzic Z, Kaliterna M, Gronowicz G, Clark SH, Lichtler AC, Rowe D. Use of type I collagen green fluorescent protein transgenes to identify subpopulations of cells at different stages of the osteoblast lineage. Journal of Bone and Mineral Research. 2002;17:15–25. doi: 10.1359/jbmr.2002.17.1.15. [DOI] [PubMed] [Google Scholar]
- [30].Fukada S, Higuchi S, Segawa M, Koda K, Yamamoto Y, Tsujikawa K, Kohama Y, Uezumi A, Imamura M, Miyagoe-Suzuki Y, Takeda S, Yamamoto H. Purification and cell-surface marker characterization of quiescent satellite cells from murine skeletal muscle by a novel monoclonal antibody. Experimental Cell Research. 2004;296:245–55. doi: 10.1016/j.yexcr.2004.02.018. [DOI] [PubMed] [Google Scholar]
- [31].Keefe AC, Lawson JA, Flygare SD, Fox ZD, Colasanto MP, Mathew SJ, Yandell M, Kardon G. Muscle stem cells contribute to myofibres in sedentary adult mice. Nat Commun. 2015;6:7087. doi: 10.1038/ncomms8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Barisic-Dujmovic T, Boban I, Clark SH. Fibroblasts/myofibroblasts that participate in cutaneous wound healing are not derived from circulating progenitor cells. Journal of Cellular Physiology. 2010;222:703–712. doi: 10.1002/jcp.21997. [DOI] [PubMed] [Google Scholar]
- [33].McCarthy EF, Sundaram M. Heterotopic ossification: A review. Skeletal Radiology. 2005;34:609–619. doi: 10.1007/s00256-005-0958-z. [DOI] [PubMed] [Google Scholar]
- [34].Goritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisen J. A pericyte origin of spinal cord scar tissue. Science. 2011;333:238–42. doi: 10.1126/science.1203165. [DOI] [PubMed] [Google Scholar]
- [35].Peterson JR, De La Rosa S, Eboda O, Cilwa KE, Agarwal S, Buchman SR, Cederna PS, Xi C, Morris MD, Herndon DN, Xiao W, Tompkins RG, Krebsbach PH, Wang SC, Levi B. Treatment of heterotopic ossification through remote ATP hydrolysis. Science Translational Medicine. 2014;6 doi: 10.1126/scitranslmed.3008810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chakkalakal JV, Christensen J, Xiang W, Tierney MT, Boscolo FS, Sacco A, Brack AS. Early forming label-retaining muscle stem cells require p27kip1 for maintenance of the primitive state. Development. 2014;141:1649–1659. doi: 10.1242/dev.100842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Montarras D, L'Honore A, Buckingham M. Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 2013;280:4036–50. doi: 10.1111/febs.12372. [DOI] [PubMed] [Google Scholar]
- [38].McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont-Versteegden EE, Peterson CA. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011;138:3657–66. doi: 10.1242/dev.068858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fry CS, Lee JD, Mula J, Kirby TJ, Jackson JR, Liu F, Yang L, Mendias CL, Dupont-Versteegden EE, McCarthy JJ, Peterson CA. Inducible depletion of satellite cells in adult, sedentary mice impairs muscle regenerative capacity without affecting sarcopenia. Nat Med. 2015;21:76–80. doi: 10.1038/nm.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Jackson JR, Mula J, Kirby TJ, Fry CS, Lee JD, Ubele MF, Campbell KS, McCarthy JJ, Peterson CA, Dupont-Versteegden EE. Satellite cell depletion does not inhibit adult skeletal muscle regrowth following unloading-induced atrophy. American Journal of Physiology-Cell Physiology. 2012;303:C854–C861. doi: 10.1152/ajpcell.00207.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Uezumi A, Ikemoto-Uezumi M, Tsuchida K. Roles of nonmyogenic mesenchymal progenitors in pathogenesis and regeneration of skeletal muscle. Frontiers in Physiology. 2014;5:68. doi: 10.3389/fphys.2014.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Johnson RW, White JD, Walker EC, Martin TJ, Sims NA. Myokines (muscle-derived cytokines and chemokines) including ciliary neurotrophic factor (CNTF) inhibit osteoblast differentiation. Bone. 2014;64:47–56. doi: 10.1016/j.bone.2014.03.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.