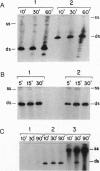
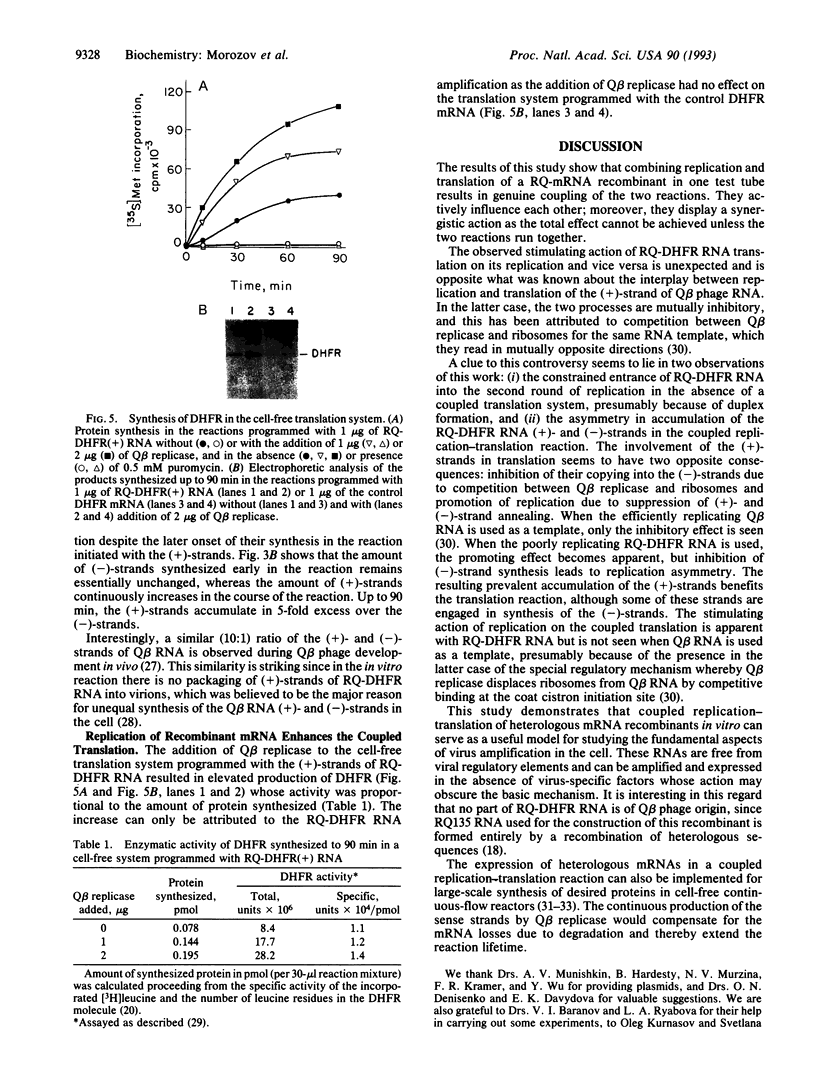
Abstract
Combination of the Q beta replicase reaction with the Escherichia coli cell-free translation system markedly enhances replication of a recombinant RQ-DHFR RNA consisting of the dihydrofolate reductase (DHFR) mRNA sequence inserted into RQ135(-1) RNA, an efficient naturally occurring Q beta replicase template. The enhancement is associated with a replication asymmetry previously described for the replication of Q beta phage RNA in vivo; the sense (+)-strands are produced in large excess over the antisense (-)-strands. This, in turn, results in increased synthesis of the functionally active DHFR. These effects are not observed when DHFR mRNAs or RQ135(-1) RNAs are used as templates, if the translation system is not complete, or if it is inhibited by puromycin. The coupled replication-translation of nonviral mRNA recombinants can serve as a useful model for studying the fundamental aspects of virus amplification and can be implemented for large-scale protein synthesis in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod V. D., Brown E., Priano C., Mills D. R. Coliphage Q beta RNA replication: RNA catalytic for single-strand release. Virology. 1991 Oct;184(2):595–608. doi: 10.1016/0042-6822(91)90430-j. [DOI] [PubMed] [Google Scholar]
- Banerjee A. K., Rensing U., August J. T. Replication of RNA viruses. X. Replication of a natural 6 s RNA by the Q-beta RNA polymerase. J Mol Biol. 1969 Oct 28;45(2):181–193. doi: 10.1016/0022-2836(69)90098-9. [DOI] [PubMed] [Google Scholar]
- Baranov V. I., Morozov IYu, Ortlepp S. A., Spirin A. S. Gene expression in a cell-free system on the preparative scale. Gene. 1989 Dec 14;84(2):463–466. doi: 10.1016/0378-1119(89)90521-0. [DOI] [PubMed] [Google Scholar]
- Biebricher C. K., Diekmann S., Luce R. Structural analysis of self-replicating RNA synthesized by Qbeta replicase. J Mol Biol. 1982 Feb 5;154(4):629–648. doi: 10.1016/s0022-2836(82)80019-3. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Libonati M., Viñuela E., Weissmann C. Replication of viral ribonucleic acid. X. Turnover of virus-specific double-stranded ribonucleic acid during replication of phage MS2 in Escherichia coli. J Biol Chem. 1966 Oct 25;241(20):4750–4757. [PubMed] [Google Scholar]
- Chetverin A. B., Chetverina H. V., Munishkin A. V. On the nature of spontaneous RNA synthesis by Q beta replicase. J Mol Biol. 1991 Nov 5;222(1):3–9. doi: 10.1016/0022-2836(91)90729-p. [DOI] [PubMed] [Google Scholar]
- Feix G., Hake H. Primer directed initiation of RNA synthesis catalysed by Qbeta replicase. Biochem Biophys Res Commun. 1975 Jul 22;65(2):503–509. doi: 10.1016/s0006-291x(75)80176-8. [DOI] [PubMed] [Google Scholar]
- Feix G. Primer-dependent copying of rabbit globin mRNA with Qbeta replicase. Nature. 1976 Feb 19;259(5544):593–594. doi: 10.1038/259593a0. [DOI] [PubMed] [Google Scholar]
- Gavrilova L. P., Kostiashkina O. E., Koteliansky V. E., Rutkevitch N. M., Spirin A. S. Factor-free ("non-enzymic") and factor-dependent systems of translation of polyuridylic acid by Escherichia coli ribosomes. J Mol Biol. 1976 Mar 15;101(4):537–552. doi: 10.1016/0022-2836(76)90243-6. [DOI] [PubMed] [Google Scholar]
- Gurevich V. V., Pokrovskaya I. D., Obukhova T. A., Zozulya S. A. Preparative in vitro mRNA synthesis using SP6 and T7 RNA polymerases. Anal Biochem. 1991 Jun;195(2):207–213. doi: 10.1016/0003-2697(91)90318-n. [DOI] [PubMed] [Google Scholar]
- Haruna I., Spiegelman S. Autocatalytic synthesis of a viral RNA in vitro. Science. 1965 Nov 12;150(3698):884–886. doi: 10.1126/science.150.3698.884. [DOI] [PubMed] [Google Scholar]
- Kolakofsky D., Weissmann C. Possible mechanism for transition of viral RNA from polysome to replication complex. Nat New Biol. 1971 May 12;231(19):42–46. doi: 10.1038/newbio231042a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miele E. A., Mills D. R., Kramer F. R. Autocatalytic replication of a recombinant RNA. J Mol Biol. 1983 Dec 15;171(3):281–295. doi: 10.1016/0022-2836(83)90094-3. [DOI] [PubMed] [Google Scholar]
- Mills D. R. Engineered recombinant messenger RNA can be replicated and expressed inside bacterial cells by an RNA bacteriophage replicase. J Mol Biol. 1988 Apr 5;200(3):489–500. doi: 10.1016/0022-2836(88)90538-4. [DOI] [PubMed] [Google Scholar]
- Munishkin A. V., Voronin L. A., Ugarov V. I., Bondareva L. A., Chetverina H. V., Chetverin A. B. Efficient templates for Q beta replicase are formed by recombination from heterologous sequences. J Mol Biol. 1991 Sep 20;221(2):463–472. doi: 10.1016/0022-2836(91)80067-5. [DOI] [PubMed] [Google Scholar]
- Obinata M., Nasser D. S., McCarthy B. J. Synthesis of probes for RNA using Qbeta-replicase. Biochem Biophys Res Commun. 1975 May 19;64(2):640–647. doi: 10.1016/0006-291x(75)90369-1. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Diener T. O. Synthesis of RNA complementary to potato spindle tuber viroid using Q beta replicase. Virology. 1977 Jun 1;79(1):109–120. doi: 10.1016/0042-6822(77)90338-5. [DOI] [PubMed] [Google Scholar]
- Palmenberg A., Kaesberg P. Synthesis of complementary strands of heterologous RNAs with Qbeta replicase. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1371–1375. doi: 10.1073/pnas.71.4.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poe M., Greenfield N. J., Hirshfield J. M., Williams M. N., Hoogsteen K. Dihydrofolate reductase. Purification and characterization of the enzyme from an amethopterin-resistant mutant of Escherichia coli. Biochemistry. 1972 Mar 14;11(6):1023–1030. doi: 10.1021/bi00756a012. [DOI] [PubMed] [Google Scholar]
- Smith D. R., Calvo J. M. Nucleotide sequence of the E coli gene coding for dihydrofolate reductase. Nucleic Acids Res. 1980 May 24;8(10):2255–2274. doi: 10.1093/nar/8.10.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman S., Pace N. R., Mills D. R., Levisohn R., Eikhom T. S., Taylor M. M., Peterson R. L., Bishop D. H. The mechanism of RNA replication. Cold Spring Harb Symp Quant Biol. 1968;33:101–124. doi: 10.1101/sqb.1968.033.01.015. [DOI] [PubMed] [Google Scholar]
- Spirin A. S., Baranov V. I., Ryabova L. A., Ovodov S. Y., Alakhov Y. B. A continuous cell-free translation system capable of producing polypeptides in high yield. Science. 1988 Nov 25;242(4882):1162–1164. doi: 10.1126/science.3055301. [DOI] [PubMed] [Google Scholar]
- Vournakis J. N., Carmichael G. G., Efstratiadis A. Synthesis of RNA complementary of rabbit globin mRNA by Qbeta replicase. Biochem Biophys Res Commun. 1976 Jun 7;70(3):774–782. doi: 10.1016/0006-291x(76)90659-8. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Colthart L., Libonati M. Determination of viral plus and minus ribonucleic acid strands by an isotope dilution assay. Biochemistry. 1968 Feb;7(2):865–874. doi: 10.1021/bi00842a045. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Feix G., Slor H. In vitro synthesis of phage RNA: the nature of the intermediates. Cold Spring Harb Symp Quant Biol. 1968;33:83–100. doi: 10.1101/sqb.1968.033.01.014. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Feix G., Slor H., Pollet R. Replication of viral RNA. XIV. Single-stranded minus strands as template for the synthesis of viral plus strands in vitro. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1870–1877. doi: 10.1073/pnas.57.6.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhang D. Y., Kramer F. R. Amplifiable messenger RNA. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11769–11773. doi: 10.1073/pnas.89.24.11769. [DOI] [PMC free article] [PubMed] [Google Scholar]