Abstract
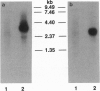
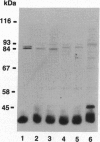
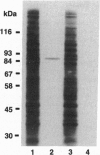
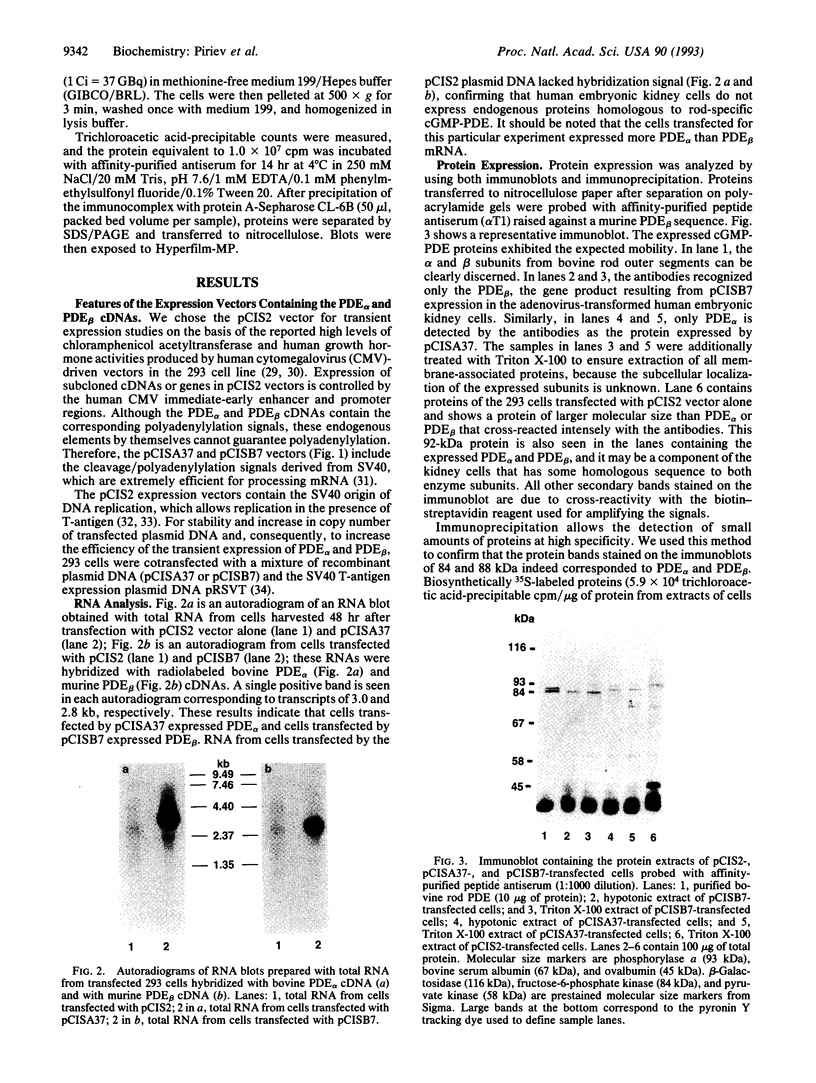
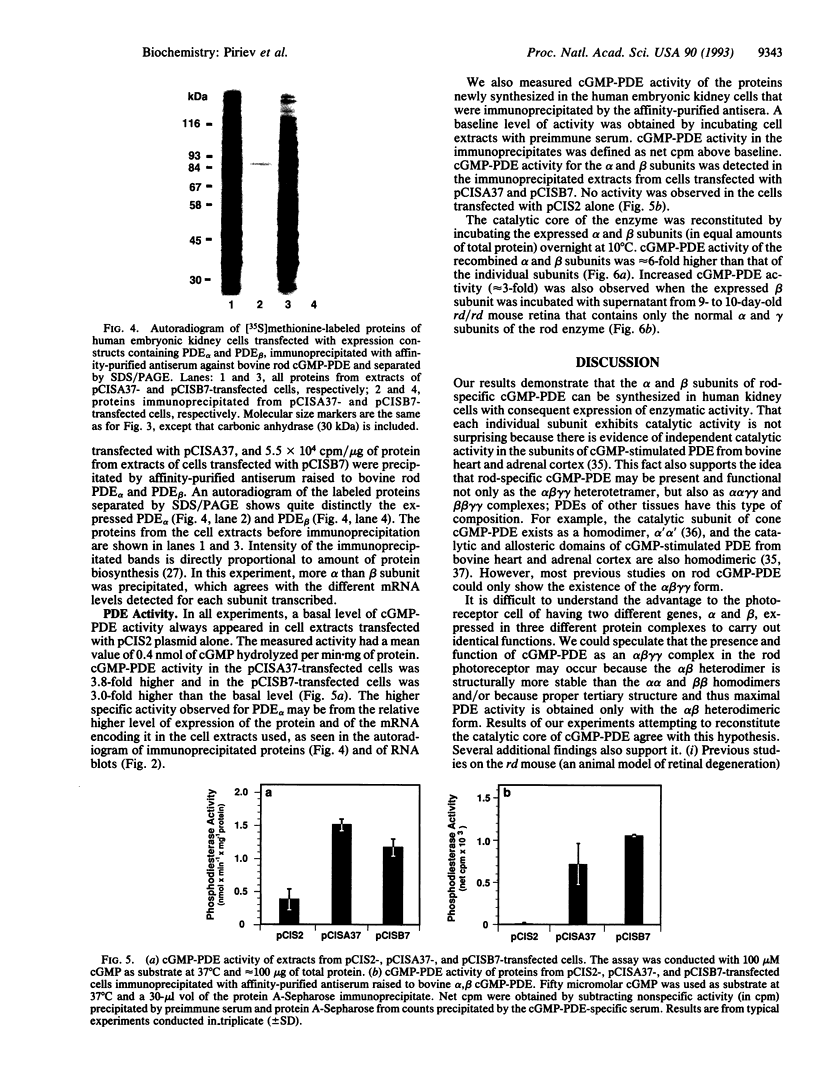
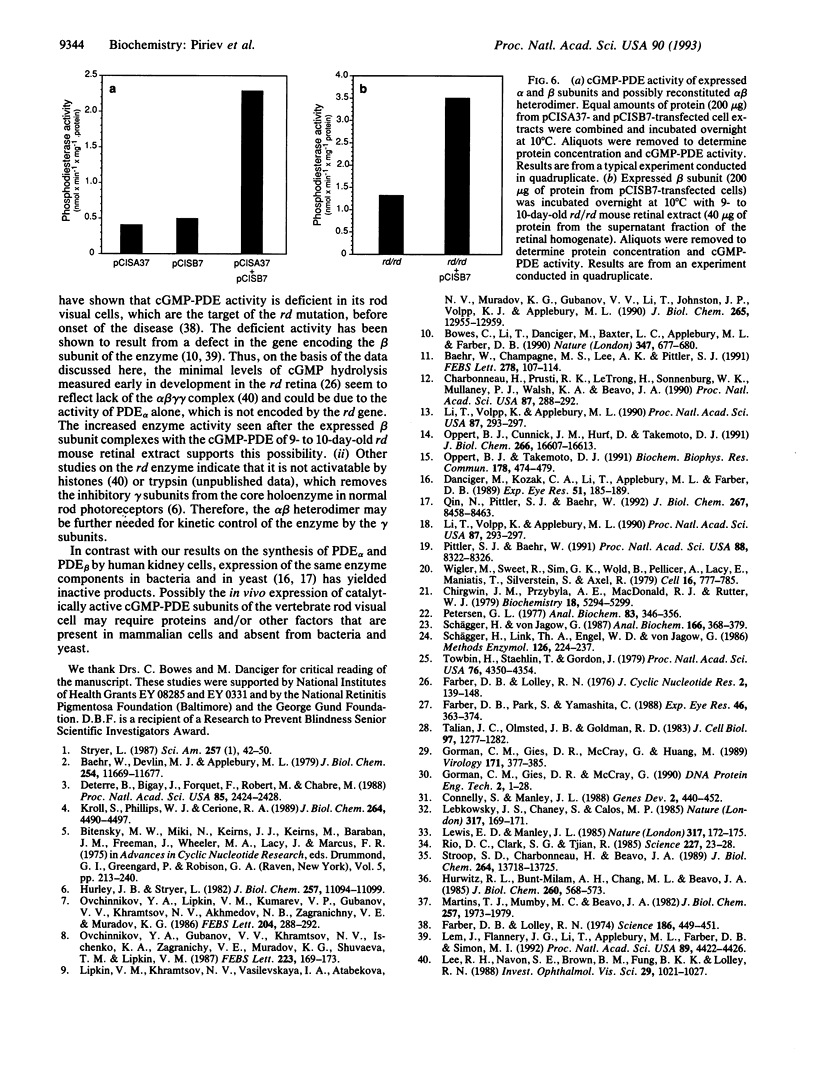
The bovine alpha and murine beta subunits of rod-photoreceptor cGMP-phosphodiesterase (PDE alpha and PDE beta) were expressed in adenovirus-transformed 293 human embryonic kidney cells. RNA blots from transfected cells showed transcripts of 3.0 and 2.8 kb corresponding to PDE alpha and PDE beta, respectively. Protein expression was analyzed by using affinity-purified antibodies against cGMP-PDE on immunoblots and by immunoprecipitation. PDE alpha and PDE beta exhibited the expected mobility (and thus apparent molecular size) and had cGMP hydrolytic activity. Reconstitution of the PDE alpha beta heterodimer with the expressed proteins increased by approximately 6-fold the activity of the individual alpha and beta subunits. Addition of expressed beta subunit to retinal extracts from 9- to 10-day-old rd/rd mice (which have only normal alpha and gamma subunits of rod cGMP-PDE and thus minimal activity) increased enzyme activity by approximately 3-fold. Our results therefore demonstrate that photoreceptor-specific cGMP-PDE can be synthesized in human kidney cells with consequent expression of enzymatic activity.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bitensky M. W., Miki N., Keirns J. J., Keirns M., Baraban J. M., Freeman J., Wheeler M. A., Lacy J., Marcus F. R. Activation of photoreceptor disk membrane phosphodiesterase by light and ATP. Adv Cyclic Nucleotide Res. 1975;5:213–240. [PubMed] [Google Scholar]
- Deterre P., Bigay J., Forquet F., Robert M., Chabre M. cGMP phosphodiesterase of retinal rods is regulated by two inhibitory subunits. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2424–2428. doi: 10.1073/pnas.85.8.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Stryer L. Purification and characterization of the gamma regulatory subunit of the cyclic GMP phosphodiesterase from retinal rod outer segments. J Biol Chem. 1982 Sep 25;257(18):11094–11099. [PubMed] [Google Scholar]
- Kroll S., Phillips W. J., Cerione R. A. The regulation of the cyclic GMP phosphodiesterase by the GDP-bound form of the alpha subunit of transducin. J Biol Chem. 1989 Mar 15;264(8):4490–4497. [PubMed] [Google Scholar]
- Ovchinnikov YuA, Gubanov V. V., Khramtsov N. V., Ischenko K. A., Zagranichny V. E., Muradov K. G., Shuvaeva T. M., Lipkin V. M. Cyclic GMP phosphodiesterase from bovine retina. Amino acid sequence of the alpha-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1987 Oct 19;223(1):169–173. doi: 10.1016/0014-5793(87)80530-6. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Lipkin V. M., Kumarev V. P., Gubanov V. V., Khramtsov N. V., Akhmedov N. B., Zagranichny V. E., Muradov K. G. Cyclic GMP phosphodiesterase from cattle retina. Amino acid sequence of the gamma-subunit and nucleotide sequence of the corresponding cDNA. FEBS Lett. 1986 Aug 18;204(2):288–292. doi: 10.1016/0014-5793(86)80830-4. [DOI] [PubMed] [Google Scholar]
- Stryer L. The molecules of visual excitation. Sci Am. 1987 Jul;257(1):42–50. doi: 10.1038/scientificamerican0787-42. [DOI] [PubMed] [Google Scholar]