Abstract
Nucleic acid amplification-based diagnostics offer rapid, sensitive, and specific means for detecting and monitoring the progression of infectious diseases. However, this method typically requires extensive sample preparation, expensive instruments, and trained personnel. All of which hinder its use in resource-limited settings, where many infectious diseases are endemic. Here, we report on a simple, inexpensive, minimally-instrumented, smart cup platform for rapid, quantitative molecular diagnostics of pathogens at the point of care. Our smart cup takes advantage of water-triggered, exothermic chemical reaction to supply heat for the nucleic acid-based, isothermal amplification. The amplification temperature is regulated with a phase-change material (PCM). The PCM maintains the amplification reactor at a constant temperature, typically, 60-65°C, when ambient temperatures range from 12 to 35°C. To eliminate the need for an optical detector and minimize cost, we use the smartphone’s flashlight to excite the fluorescent dye and the phone camera to record real-time fluorescence emission during the amplification process. The smartphone can concurrently monitor multiple amplification reactors and analyze the recorded data. Our smart cup’s utility was demonstrated by amplifying and quantifying herpes simplex virus type 2 (HSV-2) with LAMP assay in our custom-made microfluidic diagnostic chip. We have consistently detected as few as 100 copies of HSV-2 viral DNA per sample. Our system does not require any lab facilities and is suitable for use at home, in the field, and in the clinic, as well as in resource-poor settings, where access to sophisticated laboratories is impractical, unaffordable, or nonexistent.
Keywords: Chemical heating, Loop mediated isothermal amplification, HSV-2 virus detection, Smartphone, microfluidics
1. Introduction
In the past decade, there has been a growing interest in developing simple, inexpensive, point of care (POC), molecular diagnostic devices for home care, clinic and dental office use, individualized medicine and therapeutics, and field testing [1,2]. In particular, there is an urgent need for such a technology in resource-limited settings, where laboratory facilities and skilled personnel are in short supply [3-5]. Lateral flow strip (immunochromatographic) devices have been widely applied to detect various infections such as the human immunodeficiency virus (HIV) [6] and tuberculosis [7] as well as to identify drugs of abuse [8]. Although lateral flow strips are simple to use, low-cost, and do not require instrumentation, they lack the sensitivity and specificity of nucleic acid-based tests and are inappropriate in certain cases [9].
Nucleic acid amplification-based assays have a critical advantage over immunoassay-based methods because pathogen-associated DNA or RNA can be enzymatically amplified at least a million-fold with great specificity, allowing the detection of very few target molecules. Moreover, when the target is known, the design of the nucleic acid-based assay is relatively simple, inexpensive, and rapid. Currently, polymerase chain reaction (PCR) is the most frequently used nucleic acid amplification method that serves as the gold standard and confirmatory test for the diagnosis of many infectious diseases [10, 11]. Conventional PCR amplification requires, however, elaborate sample preparation and complex thermal cycling, which is not amenable to low-cost POC diagnostic applications.
Over the last decade, microfluidics (“lab on chip”) technology has emerged as a viable means for on-site, molecular diagnostics because the technology offers low cost, short test times, small sample and reagent volumes, reduced contamination, better containment and disposal of infectious material and, most importantly, integration of most, if not all, processes from sample preparation to detection in an inexpensive portable device [12-14]. In particular, the integration of affordable, microfluidic-based diagnostic devices with the ubiquitous mobile phone to perform biomedical tests that would normally require expensive laboratory-based instruments and well-trained personnel creates a new paradigm shift for health monitoring in resource-limited settings, may foster a revolution in the delivery of home care, and may improve the quality of care in clinics by enabling real time, informed decisions. Nowadays, mobile phone technology has a growing and pervasive influence on our society, with nearly seven billion mobile phone subscribers worldwide and a mobile phone penetration rate of 95.5% globally [18]. As a result, when designing new diagnostic systems, one can take the availability of cell phones for granted, and exclude the cost of the phone when pricing the system. So far, cell phones have been applied for cell analysis [15], lateral flow strip’s signal reading [16] and colorimetric detection [17].
Although early microfluidic implementations incorporated PCR, the need to cycle the temperature complicates instrumentation, reduces reliability, and increases cost. As an attractive alternative to PCR, several isothermal nucleic-acid amplification methods such as loop-mediated isothermal amplification (LAMP) [19], recombinase polymerase amplification (RPA) [20], nucleic acid sequence-based amplification (NASBA) [21], and rolling circle amplification (RCA) [22] have been developed. These isothermal nucleic-acid amplification processes are more suitable for low-cost, POC diagnostics as they require merely a constant temperature incubation, which simplifies thermal control and supporting electronics. They consume less power than PCR, without compromising sensitivity [23].
Most isothermal amplification schemes require the heating of the reactants to an elevated temperature. Usually, an electrically (grid or battery) powered resistive heater or Peltier (thermoelectric) module is used to control the reaction chamber’s temperature, along with temperature sensors (thermocouples or RTDs) and electronic temperature control circuitry. To eliminate the need for electrical power and electronic circuits, several research groups have recently explored the use of exothermic chemical reaction-based heating for isothermal amplification. For example, Weigl et al. [24] proposed non-instrumented and/or minimally instrumented diagnostic devices heated with an exothermic reaction. Curtis et al. [25] report a non-instrumented nucleic acid amplification (NINA) device for HIV-1 detection in whole blood. In their device, vials are immersed in a calcium-oxide heat source that is thermally coupled to phase change material for temperature regulation. Kubota et al. [26] have successfully carried out LAMP amplification of Salmonella enterica DNA in a modified teacup by adding a small amount (<150 g) of boiling water. In our earlier work [27], we first reported on the use of commercially available Mg-Fe alloy, typically used for heating food rations, as the fuel for heating a microfluidic device for E. coli DNA amplification and endpoint detection with a camera. In our previous work, the exothermic reaction chamber was part of the disposable chip. Recently, Singleton et al. [28] have demonstrated an electricity-free heater for bi-plexed LAMP assay of HIV-1 and a ß-actin internal control with lateral flow (NALF) strip for visual readout. However, all the above-mentioned devices lack sample processing capabilities. They either require purified nucleic acids [26-28] that were isolated from clinical specimens with other instrumentation, typically of the type used in a laboratory or operate with unprocessed specimen [25]. In the latter case, a very small sample volume, typically a few μl is added to the reaction volume. Naturally, the use of very small sample volume severely impairs test sensitivity. Additionally, all the aforementioned devices report only endpoint qualitative (positive or negative) test results and do not afford quantification. In many cases, however, quantification is desired to monitor disease progression and the effectiveness of therapy.
In this article, we describe a simple, inexpensive, easy-to-use, minimally-instrumented, smart cup equipped with a smartphone for quantitative molecular detection of pathogens at the point of care. The smart cup is powered by an exothermic chemical reaction (‘thermal battery’) without any need for external instruments and electrical power to operate and control the heater. The heat is generated by reacting magnesium with water in the presence of iron. The device temperature is regulated and maintained independent of the ambient temperature with the aid of phase change material (PCM). To eliminate the need for an expensive detector and minimize detection cost, a mobile phone’s flashlight is used for dye excitation and a mobile phone’s camera is adapted for real time fluorescence monitoring of the amplification process. The utility of this smart cup is demonstrated by using LAMP to amplify HSV-2 virus. This simple and inexpensive smart cup apparatus is particularly suitable for use in the field, in resource-poor regions of the world (where funds and trained personnel are in short supply), in remote areas without electricity, at home, in clinics, and in doctor and dentist offices.
2. Material and methods
2.1. Smart cup design and chemical-heating
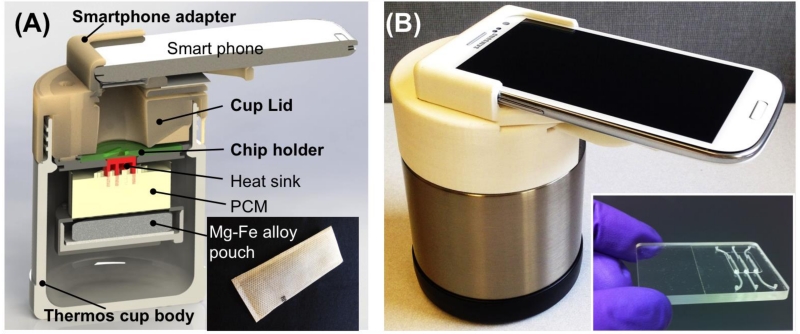
An exploded view of the minimally-instrumented, chemically-heated smart cup for rapid, quantitative molecular diagnostics is shown in Fig. 1A. The cup consists of four main components: a thermos cup body with vacuum insulation (which can be replaced with an inexpensive Styrofoam cup), a microfluidic chip holder, a cup lid, and a smartphone adapter. For expediency and due to its excellent insulating properties, we used a commercially-available, vacuum-insulated thermos cup body (Thermos, USA) to house our chip holder. The smartphone adapter, chip holder and cup lid were fabricated with 3D-printing (Dimension Elite 3D printer, Minnesota). These can also be casted from Styrofoam.
Fig. 1.
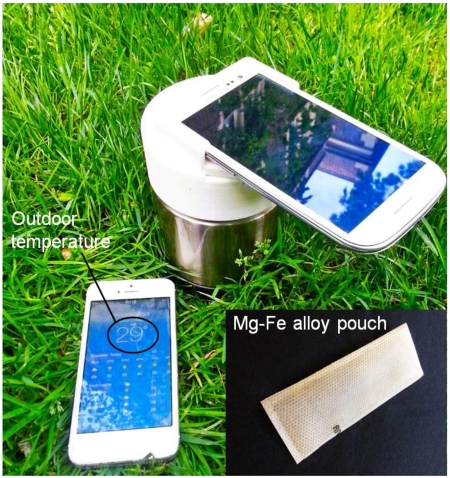
(A) Exploded view of smart cup for minimally-instrumented, point-of-care molecular diagnostics. The smart cup consists of a thermos cup body, a 3D-printed cup lid, a chip holder, and a smartphone adapter. Inset is a photograph of Mg-Fe alloy pouch ($ 0.15 per pouch) used as a heating source. (B) A photograph of a fully assembled, smart cup equipped with a Samsung Galaxy S3 smartphone. Inset is a photograph of an integrated microfluidic chip containing three independent, multifunctional, isothermal amplification reactors for nucleic acid extraction and amplification.
PCM with a melting temperature of 68°C (PureTemp 68, Entropy Solutions Inc., Plymouth, MN, USA) [29] was used to regulate the chip’s temperature. To improve heat transfer, a high thermal conductivity aluminum heat sink was embedded in the PCM. A custom chip holder was designed to accommodate our microfluidic chip (inset of Fig. 1B) and interface the chip with the heat sink.
To facilitate real time fluorescence emission monitoring, a detection window was formed in the center of the cup lid. The smartphone adapter equipped with excitation optical filter (MF475-35, Thorlabs Inc.) and emission optical filter (MF530-43, Thorlabs Inc.) was mounted on top of the cup lid. The filters were selected to minimize the overlap between the emission and excitation spectra. Fig. 1B is a photograph of our fully assembled chemically heated, smart cup equipped with a Samsung Galaxy S3 phone.
In our experiments, a commercially available Mg-Fe alloy pouch (~$0.15 per pouch) (inset of Fig. 1A) of the type employed for self-heating meals (Innotech Products Ltd., USA) was used in each run. First, the hermetically, heat-sealed pouch is placed on a tray in the chip holder (Fig. 1A). Subsequently, the tray is filled with water through the water inlet in the cup lid. The water oxidizes the magnesium alloy and induces galvanic corrosion, which is an exothermic process. The generated heat is transferred through the heat sink and PCM to the chip. Byproducts of the exothermic reaction, such as water vapor and hydrogen gas, are vented through small holes in the cup’s lid. Within nearly two hours, part, but not all, the PCM melts, maintaining nearly fixed temperature. This amount of time is more than sufficient for the LAMP reaction, which typically lasts less than an hour.
2.2. Temperature measurement and calibration
To evaluate the thermal performance of the smart cup, we constructed a calibration chip as previously described [30]. With the exception of hosting a thermocouple, the calibration chip is identical in all respects to the diagnostic chip. A K-type thermocouple (Omega Engineering) was inserted in the amplification reactor through a 1.0 mm diameter hole, which was then sealed. The thermocouple wires were connected to a terminal block (SCC-68) that interfaced with a National Instruments data acquisition card (PXI-6281). Once the chemical, exothermic reaction was initiated by adding water into the tray, the temperature was monitored and displayed using Labview™ software (National Instruments, Austin, TX, USA).
To examine the effect of the ambient temperature on the smart cup’s temperature, experiments were carried out in an environmental oven (Isotemp Vacuum Oven Model 280A, Fisher Scientific Inc., Pittsburgh, PA) at various oven temperatures. An infrared image of the heat sink’s surface heated by our chemical heater (Mg-Fe alloy pouch) was taken with an infrared thermography camera T360 (FLIR Systems, Wilsonville, USA) to assess temperature uniformity.
2.3. Detection of herpes simplex virus type 2
To demonstrate the applicability of the smart cup for molecular diagnosis of infectious disease, we carried out real time quantitative LAMP assay of HSV-2 virus using our custom-made, microfluidic, diagnostic chip. The diagnostic chip contains three independent, multifunctional, isothermal amplification reactors (inset of Fig. 1B) [31]. Each of these reactors is equipped with a flow-through Qiagen™ silica membrane (QIAamp DNA Blood Mini Kit) at its entry port.
HSV-2 virus samples were prepared by spiking 200 μL of virus transport media (Dulbecco’s modified Eagle’s medium (DMEM) containing 5% fetal bovine serum supplemented with 25 μg/ml vancomycin) with 0, 10, 100 and 1000 plaque forming unit (PFU) HSV-2 as determined by plaque assay on Vero cells [32]. Virus lysis buffer (QIAamp DNA Blood Mini Kit) was mixed with the HSV-2 containing virus transport media in 1:1 ratio. Then, 200 μL of ethanol was added.
We used our custom-made microfluidic chips [30, 31]. These chips contain an array of multifunctional reaction chambers, each 22 μL in volume. Each chamber is equipped with one nucleic acid isolation (Qiagen™ silica) membrane at its inlet. For each test, 250 μL lysate was filtered through the isolation membrane of one of the amplification reactors. The nucleic acids bound to the membrane. Subsequent to the sample introduction, 150 μL of Qiagen wash buffer 1 (AW1) was injected into the chip to remove any remaining amplification inhibitors. Then, the silica membrane was washed with 150 μL of Qiagen™ wash buffer 2 (AW2), followed by air-drying for 30 seconds. Witness that our chip performs the function of nucleic acid isolation and concentration and does not require a separate elution step, all of which are typically carried out with benchtop spin columns. Another advantage of the chip is that the sample volume is decoupled from the reaction volume. Relatively large sample volumes can be filtered through the membrane enabling one to obtain high sensitivity.
Next, 22 μL of LAMP master mixture which contains all the reagents necessary for the LAMP, and 0.5 × EvaGreen@ fluorescence dye (Biotium, Hayward, CA), was injected into each reactor through its inlet port. The inlet and outlet ports were then sealed using transparent tape to minimize evaporation and contamination during the amplification process. The primers and their respective concentrations for HSV-2 DNA amplification were the same as previously described [33].
2.4. Smart cup operation and real time fluorescent quantitative detection
To activate the chemical heater in our smart cup, 7.5 mL of tap water was introduced into smart cup through the water inlet in the cup lid. The Mg-Fe immediately reacted with the water and produced heat. After approximately 10 minutes, once the heat sink’s temperature exceeded 60°C, the microfluidic chip was inserted into the chip holder. Then, the smartphone was initiated to record in real time the fluorescence emission from the reactors. The phone camera acquired an image once every min over a time interval of 60 min. After the test run, the exothermal reaction products contained in the pouch and the microfluidic chip were discarded, while the other components, including the thermos cup body, cup lid, PCM, heat sink, adapter and holder were retained and reused. The images obtained with smartphone camera were analyzed with MATLAB™ and the normalized and the average fluorescence intensity signal for each reactor were extracted from each image.
3. Results and discussion
3.1. Chemical heating and amplification reaction
Although there are various exothermic chemical reactions that can generate heat [24, 27, 34], we selected the Mg-Fe alloy because of its high efficiency, safety, low cost, and availability. The Mg-Fe alloy is commercially available in a pouch, known as flameless ration heater (FRH) and is designed to heat ready to eat meals (MREs). We used the commercially available FRH without any modifications. When water is added to the FRH (inset of Fig. 1A) in the absence of PCM, the exothermic chemical reaction proceeds rapidly and the pouch surface temperature may exceed 90°C within several seconds. This is, however, not desired in our application. We need the smart cup to maintain the LAMP assay mixture in the microfluidic chip at a temperature ranging from 60 to 65°C for the duration of the amplification process [19]. To regulate the temperature of the amplification reactors, we take advantage of a PCM with a melting temperature of 68°C. The exothermic reaction heats the PCM to its melting temperature. As long as the PCM is only partially melted, its temperature remains fixed at its melting temperature. Due to thermal resistance between the PCM and the chip, the LAMP reactor temperature was slightly lower, and maintained within the desired range.
Our experiments indicate a potential risk of false-positive when the microfluidic chip was slowly heated, possibly due to non-specific priming at low temperatures. To avoid a non-specific amplification, we insert the chip into the cup after about 10-minute pre-warming, after the cup has reached its desired temperature. In future implementations, we will pre-store the amplification reagents, encapsulated in paraffin, in the amplification reactors as we have previously described [35]. The encapsulating paraffin will melt and the reagents will get hydrated only when the reactor has reached its operating temperature, assuring a hot-start and eliminating the possibility of premature priming. With this dry storage scheme, the chip can be inserted into the cup without waiting for the cup to warm up.
Since the LAMP process is highly efficient, it can amplify low abundance targets to detectable quantities in less than an hour. LAMP products have been detected directly by visual observation of end-point turbidity or fluorescence emission [36, 37]. Here, we use the fluorescence emission of the intercalating dye Eva® Green to monitor the LAMP process in real time. To eliminate the need for an extra excitation light source, we use the flashlight of the smartphone with an excitation optical filter. The fluorescence emissions from the reactors were recorded in real time with the smartphone camera. To block any background emission from the heat sink, black 3M Scotch electrical tape was used to cover its surface.
In our experiments, the cellphone acquired images once every minute for an hour, consuming less than 5% (~1400 J) of the cellphone battery’s energy per test (Samsung Galaxy S3 SIII Li-ion Battery energy capacity: 7.98Wh). This energy consumption can be further reduced by reducing the frequency and duration of image collection. Since the cell phone energy consumption is insignificant, we did not attempt to optimize its energy consumption.
How does the cellphone energy consumption compare to the energy required to heat the reactors? The heating needs depend on the ambient temperature, the insulation, and device design and are difficult to estimate with precision. In our experiments, the heating value of the flameless ration heater (one Mg-Fe alloy pouch) is estimated in excess of 43kJ, which far exceeds the energy consumed by the cellphone-based detection. Given that we rely on dry storage [35], there is no energy consumption associated with a cold chain.
Here, we used Samsung Galaxy S3 smartphone. Clearly, the smart cup, with minor modifications, can accommodate other cellphones. Although the cost of the phone is not trivial, since cellphones are ubiquitous in the third world and their cost is continuously declining, it would be reasonable to assume that the cellphone is available to the testers and that its cost can be excluded from the cost of the test.
3.2. Evaluation of the effect of the ambient temperature
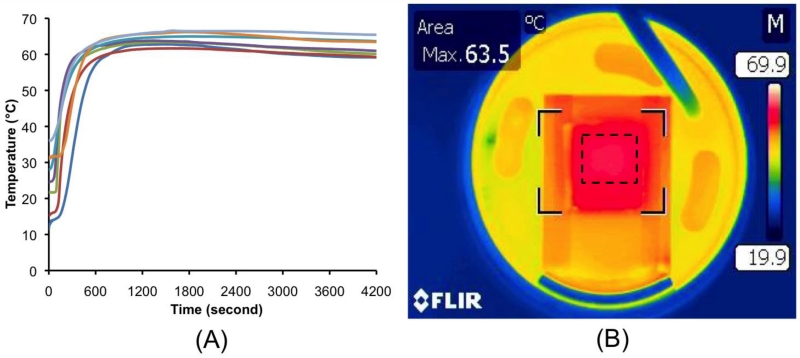
To operate at various ambient temperatures, it is necessary to isolate the microfluidic chip’s temperature from ambient conditions. To evaluate the performance of our PCM thermal control, we monitored the reactor’s temperature at different ambient temperatures. To this end, we carried out our experiment in an environmental oven with both cooling and heating functions. Fig. 2A depicts the amplification reactor’s temperature as a function of time at different oven (ambient) temperatures, ranging from 12 to 35 °C. In Fig. 2A, time zero coincides with the instant when water was added into the smart cup. Once water has been introduced into the smart cup, it takes less than 10 minutes for the reactor’s temperature to reach ~60 °C. The higher the ambient temperature is, the shorter the ramp time is. When the ambient temperature increased from 12 to 35 °C, the amplification reactor temperature increased from 60 to 65 °C and was maintained at a nearly uniform level for well over an hour, which is longer than necessary to amplify target molecules to detectable levels. Since the LAMP reaction operates efficiently over a relatively broad temperature range (i.e., 60-65°C) [19], the temperature range maintained by the smart cup is adequate.
Fig. 2.
(A) The amplification reactor’s temperature as a function of time when the ambient temperature varied between 12 °C to 35 °C. (B) A thermograph of the heated surface of the microfluidic chip holder taken with an infrared camera (T360). The three reactors of the microfluidic chip are located within the dashed square.
To evaluate temperature uniformity of our heating system, a thermograph of the chip holder’s surface was taken with an infrared camera. The three reactors of the microfluidic chip, located within the dashed square, showed excellent temperature uniformity (Fig. 2B).
3.3. Real time quantitative detection of HSV-2 virus
Herpes simplex virus (HSV) is a ubiquitous and contagious human pathogen that causes a variety of diseases [38]. World wide, approximately 500 million people are infected with HSV-2 with 23 million new infections annually [39]. Of those infected with HSV-2, 81.1% are unaware of their infection [40]. Primary HSV-1 or HSV-2 genital infection in the mother at the time of delivery can cause life threatening, disseminated disease in the newborn [41].
To test the suitability of our minimally-instrumented, smart cup for point-of-care, nucleic acid-based amplification tests, we chose HSV-2 virus as a model analyte and carried out viral DNA extraction and amplification process in our custom-made, microfluidic chip (inset of Fig. 1B). To evaluate the sensitivity of our device, we compared performance of chip-based LAMP assay in our smart cup and in a benchtop thermal cycler, amplifying known concentrations of HSV-2 viral DNA. The results obtained with our smart cup were similar to the ones obtained with a Peltier Thermal Cycler PTC-200 (Bio-Rad DNA Engine, Hercules, CA, USA). In each case, we obtained a sensitivity of less than 100 copies/reaction of HSV-2 viral DNA (Table 1).
Table 1.
HSV-2 LAMP assay in our minimally-instrumented, smart cup and in a benchtop thermal cycler. The table documents the number of positive results normalized with the number of tests.
| Samples | Benchtop thermal cycler | Smart cup |
|---|---|---|
| 1,000 copies/reaction | 3/3 | 3/3 |
| 100 copies/reaction | 3/3 | 3/3 |
| 50 copies/reaction | 2/3 | 2/3 |
| 0 (negative control) | 0/3 | 0/3 |
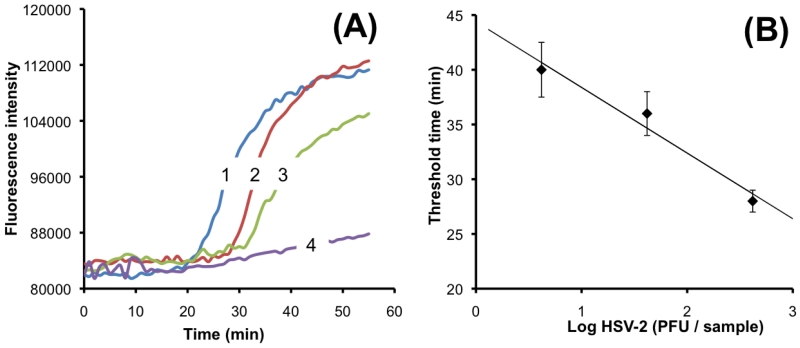
The Video in Supporting Information shows real time fluorescence monitoring of three multifunctional reactors with 420, 42 and 4.2 PFU HSV-2 virus. The higher the target concentration, the earlier the amplification reactor lights up. Fig. 3A depicts the fluorescence emission intensity as a function of time when the sample contains 420 (n=3), 42 (n=3), 4.2 (n=3) and 0 (negative control, n=3) HSV-2 copies. The fluorescence intensity of the negative control (no target) remained nearly constant throughout the entire detection period, indicating negligible primer-dimer production. We define the threshold time (Tt) as the time that elapses from the start of the enzymatic reaction until the fluorescent intensity increases above a preselected baseline level. Fig. 3B depicts the threshold time Tt (min) as a function of the viral DNA concentration (C) on a semi-log plot. In the range 4.2 < C < 420 PFU per sample, the threshold time Tt decreases nearly linearly as a function of log (C). The data correlates well with the formula Tt =44.4-6 log (C), where Tt is expressed in minutes. R2=0.96. The experiment indicates that the smart cup is suitable for nucleic acid amplification, and that the use of a simple threshold time measurement provides reasonable target quantification.
Fig. 3.
(A) Real-time monitoring of LAMP amplification of transport media samples spiked with 420 PFU per sample (curve 1), 42 PFU per sample (curve 2), 4.2 PFU per sample (curve 3), and 0 PFU per sample (curve 4) (negative control). (B) The threshold time Tt (in minutes) is depicted as a function of the HSV-2 concentration (expressed in terms of PFU per sample) (n = 3).
4. Conclusions and Outlook
We designed, constructed, and tested a simple, inexpensive, water-activated, chemically heated, minimally-instrumented smart cup for nucleic acid amplification and detection. The device utilizes the exothermic reaction between Mg-Fe alloy and water as the heat source without any need for electrical power to operate and control the heater. The amplification reactors’ temperature is regulated with a phase change material and maintained at the desired level (60-65°C), suitable for LAMP-based amplification, independent of the ambient temperature, over ambient temperatures, ranging from 12°C to 35 °C. LAMP is just one example of an isothermal amplification process. With proper modifications, our smart cup can be adapted to accommodate other isothermal amplification schemes such as Nucleic Acid Sequence-based Amplification (NASBA) [21, 42], Strand Displacement Amplification (SDA) [43], Self-Sustained Sequence Reaction (3SR) [44], and Rolling Circle Amplification (RCA) [45].
To demonstrate the utility of our device for molecular diagnostics, we amplified HSV-2 viral DNA and consistently detected down to 100 copies per sample. The sensitivity of our device is comparable to that of state of the art, benchtop thermal cyclers. Furthermore, we quantitatively detected the HSV-2 virus with our smart cup in combination with our custom-made, microfluidic, diagnostic chip.
To maintain low system cost, we monitored the amplification results with the ubiquitous smartphone. We used the smartphone flashlight to excite the fluorescence dye, eliminating the need for a separate light source. The dye emission was detected with the smartphone camera without a need for optical instrumentation. We needed, however, to include optical filters to prevent a significant overlap between the excitation and emission spectra. By real time monitoring of the emission with the smartphone camera and transmitting the data to a laptop computer for processing, we were able to obtain amplification curves and threshold times for quantifying the number of target molecules in the sample. In the future, we will program the smart phone for image processing, data analysis and communications to eliminate the need for a separate computer.
Although a low cost lateral flow immunochromatographic, antibody-based point of care test, utilizing serum and blood samples, for HSV-2 infection is available [46], such immunoassay approach provides only limited information about the state of the disease. The lateral flow test is not designed to detect presence of herpes simplex virus; it detects anti-HSV antibody that develops after HSV infection, therefore the lateral flow test is not useful for detecting primary infection as it takes 2-3 weeks for anti-herpes antibodies to develop. The antibody test also remains positive for many years after infection; therefore, it is not useful to identify subjects undergoing a flare in their infection, which is a common manifestation of herpes infection. The advantages of a nucleic acid amplification-based test are that it detects the presence of the virus itself, which is a better indicator of active state of disease than antibodies.
Our smart cup technology can be used in delivery rooms to screen mothers suspected of primary genital herpes infection even in developed nations. The smart cup allows testing for the presence of herpes simplex virus in the vaginal canal by rapid nucleic acid amplification. In contrast, the conventional PCR test for HSV is expensive and may take 24-48 hours in hospitals equipped with PCR machine for HSV-1/2 diagnosis in developed nations and may take weeks in resource limited settings. Rapid smart cup test can also be useful when encephalitis is suspected. A quick diagnostics, followed by intervention with antiviral drugs, may control the infection faster and reduce health care cost.
For future field application, further modifications and improvements of the device will include on-chip pre-storage of liquid reagents (lysis buffer, wash buffer, and water) in a pouch format as we have previously described [12, 13]. The pouch can be actuated by an electricity-free, spring-driven, timer-actuator [5] or by a finger [13]. Another improvement includes dry storage of LAMP/RT-LAMP reagents in the isothermal amplification reactors [35]. The dry stored reagents will be encapsulated with paraffin to protect them from the various solutions transmitted through the reactor. The paraffin will melt and move out of the away when the amplification reactor is heated to the isothermal amplification temperature (e.g., 60°C), allowing the hydration of the reagents. This storage scheme eliminates the need for a cold chain.
The integrated, minimally-instrumented, smart cup can be operated at the point of care with little training or technical skill while performing all the necessary steps from sample introduction to detection. With appropriate modifications of the reagents, the system can be used to detect various infectious diseases, monitor the health of individuals, provide a trigger for the administration of expensive or dangerous medications, and facilitate monitoring water and food quality. The device is suitable for use in the field, in resource-poor regions, especially in areas without reliable electric power, in remote areas, or at home.
Supplementary Material
Highlights.
An inexpensive, mininally-instrumented, smart cup is developed for molecular diagnostics.
Smart cup takes advantage of exothermic chemical heat for nucleic acid amplification.
Smartphone is used for real-time fluorescence quantitative detection.
A limit of detection of 100 copies of HSV-2 DNA is achieved.
Smart cup is very suitable for point of care diagnostics.
Acknowledgments
The work was supported by NIH Grants K25AI099160, R41AI104418 and Penn CFAR Pilot Grant (AI045008).
Biographies
Shih-Chaun Liao received a Master’s degree from the Department of Biomechatronics Engineering, National Pingtung University of Science and Technology, Taiwan in 2015. He was a visiting student at Department of Mechanical Engineering and Applied Mechanics, University of Pennsylvania from 2014 to 2015. His research interest is microfluidic chip technololgy.
Jing Peng received her M.S. degree in Analytical Chemistry from Hebei University, in 2011. She is currently a Research Assistant at the Department of Mechanical Engineering and Applied Mechanics, University of Pennsylvania. Her research interest focuses on microfluidic chips fabrication and smartphone detection for point of care diagnostics.
Michael G. Mauk has a PhD in Electrical Engineering from the University of Delaware (USA). He is a Researcher at the University of Pennsylvania with an interest in developing microfluidic point of care diagnostics devices for infectious diseases.
Sita Awasthi received her M.Sc. and Ph.D. in Biochemistry from Devi Ahilya University in India. After receiving her post-doctoral training at Rocky Mountain Laboratory, NIH, Baylor College of Medicine, Houston, TX, and University of Pennsylvania, in 2007 she joined as a Research Assistant Professor in Infectious Disease Division, Department of Medicine, Perelman School of Medicine at University of Pennsylvania, Philadelphia. Her research interests include pathogenesis, virus-host interactions, and evasion of host immunity by human herpes viruses. In addition she is intensely involved in development of a subunit based prophylactic and therapeutic vaccine candidate against genital herpes disease.
Jinzhao Song received his PhD degree in Chemistry from the Institute of Chemistry, Chinese Academy of Sciences (ICCAS), Beijing, China in 2013. He is currently a Post Doctoral Researcher at the Department of Mechanical Engineering and Applied Mechanics, University of Pennsylvania. His research interest focuses on isothermal nucleic acid amplification technologies, nanotechnologies, and microfluidic chips for point of care diagnostics.
Harvey Friedman received his medical degree from McGill University and did his residency in Internal Medicine at the Jewish General Hospital in Montreal, Quebec. He is a professor of Medicine and the Director of the Botswana-UPenn Partnership (BUP) program at the University of Pennsylvania. His research involves studies of the immune evasion properties of herpes simplex virus. In recent years, his lab is evaluating strategies to develop a herpes vaccine that involves blocking the virus’ ability to evade host immunity.
Haim H. Bau received his PhD in Mechanical Engineering from Cornell University, Ithaca, NY, in 1980. He is currently a Professor of Mechanical Engineering and Applied Mechanics at the University of Pennsylvania. His research interests are micro and nanofluidics and point of care diagnostics.
Changchun Liu received his PhD degree in Electronics Engineering from the Institute of Electronics, Chinese Academy of Sciences (IECAS), Beijing, China in 2005. Currently, he is a Research Assistant Professor at the Department of Mechanical Engineering and Applied Mechanics, University of Pennsylvania. His research interest focuses on the microfluidic chips, biosensors and BioMEMS devices for point-of-care diagnostics.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hart RW, Mauk MG, Liu C, Qiu X, Thompson JA, Chen D, Malamud D, Abrams WR, Bau HH. Point-of-care oral-based diagnostics. Oral diseases. 2011;17:745–752. doi: 10.1111/j.1601-0825.2011.01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Niemz A, Ferguson TM, Boyle DS. Point-of-care nucleic acid testing for infectious diseases. Trends in Biotechnology. 2011;29:240–250. doi: 10.1016/j.tibtech.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Yager P, Edwards T, Fu E, Helton K, Nelson K, Tam MR, Weigl BH. Microfluidic diagnostic technologies for global public health. Nature. 2006;442:412–418. doi: 10.1038/nature05064. [DOI] [PubMed] [Google Scholar]
- [4].Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012;9:e1001306. doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu C, Qiu X, Ongagna S, Chen D, Chen Z, Abrams WR, Malamud D, Corstjens PLAM, Bau HH. A timer-actuated immunoassay cassette for detecting molecular markers in oral fluids. Lab on a chip. 2009;9:768–776. doi: 10.1039/b814322f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].O’Connell RJ, Merritt TM, Malia JA, VanCott TC, Dolan MJ, Zahwa H, Bradley WP, Branson BM, Michael NL, De Witt CC. Performance of the oraquick rapid antibody test for diagnosis of human immunodeficiency virus type 1 infection in patients with various levels of exposure to highly active antiretroviral therapy. Journal of clinical microbiology. 2003;41:2153–2155. doi: 10.1128/JCM.41.5.2153-2155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lawn SD, Dheda K, Kerkhoff AD, Peter JG, Dorman S, Boehme CC, Nicol MP. Determine TB-LAM lateral flow urine antigen assay for HIV-associated tuberculosis: recommendations on the design and reporting of clinical studies. BMC infectious diseases. 2013;13:407. doi: 10.1186/1471-2334-13-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wong R. The effect of adulterants on urine screen for drugs of abuse: detection by an on-site dipstick device. American Clinical Laboratory. 2002;21:37–39. [PubMed] [Google Scholar]
- [9].Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey, Analytical and Bioanalytical Chemistry. 2009;393:569–582. doi: 10.1007/s00216-008-2287-2. [DOI] [PubMed] [Google Scholar]
- [10].Fiscus SA, Cheng B, Crowe SM, Demeter L, Jennings C, Miller V, Respess R, Stevens W. Forum for Collaborative HIV Research Alternative Viral Load Assay Working Group, HIV-1 viral load assays for resource-limited settings. PLoS Med. 2006;3:e417. doi: 10.1371/journal.pmed.0030417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang J, Chen Z, Corstjens PL, Mauk MG, Bau HH. A disposable microfluidic cassette for DNA amplification and detection. Lab on a Chip. 2006;6:46–53. doi: 10.1039/b511494b. [DOI] [PubMed] [Google Scholar]
- [12].Chen D, Mauk M, Qiu X, Liu C, Kim J, Ramprasad S, Ongagna S, Abrams WR, Malamud D, Corstjens PLAM, Bau HH. An integrated, self-contained microfluidic cassette for isolation, amplification, and detection of nucleic acids. Biomedical Microdevices. 2010;12:705–719. doi: 10.1007/s10544-010-9423-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Qiu X, Thompson JA, Chen Z, Liu C, Chen D, Ramprasad S, Mauk MG, Ongagna S, Barber C, Abrams WR, Malamud D, Corstjens PLAM, Bau HH. Finger-actuated, self-contained immunoassay cassettes. Biomedical Microdevices. 2009;11:1175–1186. doi: 10.1007/s10544-009-9334-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Easley CJ, Karlinsey JM, Bienvenue JM, Legendre LA, Roper MG, Feldman SH, Hughes MA, Hewlett EL, Merkel TJ, Ferrance JP, Landers JP. A fully integrated microfluidic genetic analysis system with sample-in-answer-out capability. Proceedings of the National Academy of Sciences. 2006;103:19272–19277. doi: 10.1073/pnas.0604663103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Optofluidic fluorescent imaging cytometry on a cell phone. Anal Chem. 2011;83:6641–6647. doi: 10.1021/ac201587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mudanyali O, Dimitrov S, Sikora U, Padmanabhan S, Navruz I, Ozcan A. Integrated rapid-diagnostic-test reader platform on a cellphone. Lab Chip. 2012;12:2678–2686. doi: 10.1039/c2lc40235a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shen L, Hagen JA, Papautsky I. Point-of-care colorimetric detection with a smartphone. Lab Chip. 2012;12:4240–4243. doi: 10.1039/c2lc40741h. [DOI] [PubMed] [Google Scholar]
- [18].Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: A systematic review of reviews. Annual review of public health. 2015;36:393. doi: 10.1146/annurev-publhealth-031914-122855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic acids research. 2000;28:e63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lutz S, Weber P, Focke M, Faltin B, Hoffmann J, Müller C, Mark D, Roth G, Munday P, Armes N, Piepenburg O, Zengerleabe R, Stetten F. Microfluidic lab-on-a-foil for nucleic acid analysis based on isothermal recombinase polymerase amplification (RPA) Lab on a Chip. 2010;10:887–893. doi: 10.1039/b921140c. [DOI] [PubMed] [Google Scholar]
- [21].Dimov IK, Garcia-Cordero JL, O’Grady J, Poulsen CR, Viguier C, Kent L, Daly P, Lincoln B, Maher M, O’Kennedy R, Smith TJ, Ricco AJ, Lee LP. Integrated microfluidic tmRNA purification and real-time NASBA device for molecular diagnostics. Lab on a Chip. 2008;8:2071–2078. doi: 10.1039/b812515e. [DOI] [PubMed] [Google Scholar]
- [22].Lizardi PM, Huang X, Zhu Z, Bray-Ward P, Thomas DC, Ward DC. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nature Genetics. 1998;19:225–232. doi: 10.1038/898. [DOI] [PubMed] [Google Scholar]
- [23].Asiello PJ, Baeumner AJ. Miniaturized isothermal nucleic acid amplification, a review. Lab on a Chip. 2011;11:1420–1430. doi: 10.1039/c0lc00666a. [DOI] [PubMed] [Google Scholar]
- [24].Weigl B, Domingo G, LaBarre P, Gerlach J. Towards non-and minimally instrumented, microfluidics-based diagnostic devices. Lab on a Chip. 2008;8:1999–2014. doi: 10.1039/b811314a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Curtis KA, Rudolph DL, Nejad I, Singleton J, Beddoe A, Weigl B, LaBarre P, Owen SM. Isothermal amplification using a chemical heating device for point-of-care detection of HIV-1. PLoS One. 2012;7:e31432. doi: 10.1371/journal.pone.0031432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kubota R, Labarre P, Weigl BH, Li Y, Haydock P, Jenkins DM. Molecular diagnostics in a teacup: non-instrumented nucleic acid amplification (NINA) for rapid, low cost detection of salmonella enterica. Chinese Science Bulletin. 2013;58:1162–1168. doi: 10.1007/s11434-012-5634-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Liu C, Mauk MG, Hart R, Qiu X, Bau HH. A self-heating cartridge for molecular diagnostics. Lab on a Chip. 2011;11:2686–2692. doi: 10.1039/c1lc20345b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Singleton J, Osborn JL, Lillis L, Hawkins K, Guelig D, Price W, Johns R, Ebels K, Boyle D, Weigl B, LaBarre P. Electricity-free amplification and detection for molecular point-of-care diagnosis of HIV-1. PLoS ONE. 2014;9:e113693. doi: 10.1371/journal.pone.0113693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Singleton J, Zentner C, Buser J, Yager P, LaBarre P, Weigl BH. Instrument-free exothermic heating with phase change temperature control for paper microfluidic devices. Proc, SPIE. 2013;8615:86150R-1–14. doi: 10.1117/12.2005928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Liu C, Geva E, Mauk M, Qiu X, Abrams WR, Malamud D, Curtis K, Owen SM, Bau HH. An isothermal amplification reactor with an integrated isolation membrane for point-of-care detection of infectious diseases. Analyst. 2011;136:2069–2076. doi: 10.1039/c1an00007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Liu C, Mauk M, Gross R, Bushman FD, Edelstein PH, Collman RG, Bau HH. Membrane-based, sedimentation-assisted plasma separator for point-of-care applications. Analytical chemistry. 2013;85:10463–10470. doi: 10.1021/ac402459h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Awasthi S, Balliet JW, Flynn JA, Lubinski JM, Shaw CE, DiStefano DJ, Caia M, Brown M, Smith JF, Kowalski R, Swoyer R, Galli J, Copeland V, Rios S, Davidson RC, Salnikova M, Kingsley S, Bryan J, Casimiro DR, Friedman HM. Protection provided by a herpes simplex virus 2 (HSV-2) glycoprotein C and D subunit antigen vaccine against genital HSV-2 infection in HSV-1-seropositive guinea pigs. Journal of Virology. 2014;88:2000–2010. doi: 10.1128/JVI.03163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kaneko H, Iida T, Aoki K, Ohno S, Suzutani T. Sensitive and rapid detection of herpes simplex virus and varicella-zoster virus DNA by loop-mediated isothermal amplification. Journal of clinical microbiology. 2005;43:3290–3296. doi: 10.1128/JCM.43.7.3290-3296.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hatano B, Maki T, Obara T, Fukumoto H, Hagisawa K, Matsushita Y, Okutani A, Bazartseren B, Inoue S, Sata T, Katano H. LAMP using a disposable pocket warmer for anthrax detection, a highly mobile and reliable method for anti-bioterrorism. Jpn J Infect Dis. 2010;63:36–40. [PubMed] [Google Scholar]
- [35].Kim J, Byun D, Mauk MG, Bau HH. A disposable, self-contained PCR chip. Lab on a Chip. 2009;9:606–612. doi: 10.1039/b807915c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liu C, Mauk MG, Hart R, Bonizzoni M, Yan G, Bau HH. A low-cost microfluidic chip for rapid genotyping of malaria-transmitting mosquitoes. PloS ONE. 2012;7:e42222. doi: 10.1371/journal.pone.0042222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang X, Liao M, Jiao P, Luo K, Zhang H, Ren T, Zhang G, Xu C, Xin C, Cao W. Development of a loop-mediated isothermal amplification assay for rapid detection of subgroup J avian leukosis virus. Journal of clinical microbiology. 2010;48:2116–2121. doi: 10.1128/JCM.02530-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Awasthi S, Friedman HM. Status of prophylactic and therapeutic genital herpes vaccines. Current opinion in virology. 2014;6:6–12. doi: 10.1016/j.coviro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- [39].Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bulletin of the World Health Organization. 2008;86:805–812A. doi: 10.2471/BLT.07.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–973. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]
- [41].Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. New England Journal of Medicine. 2009;361:1376–1385. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Compton J. Nucleic acid sequence-based amplification. Nature. 1991;350:91–92. doi: 10.1038/350091a0. [DOI] [PubMed] [Google Scholar]
- [43].Walker GT, Fraiser MS, Schram JL, Little MC, Nadeau JG, Malinowski DP. Strand displacement amplification--an isothermal, in vitro DNA amplification technique. Nucleic Acids Res. 1992;20:1691–1696. doi: 10.1093/nar/20.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Guatelli JC, Whitfield KM, Kwoh DY, Barringer KJ, Richman DD, Gingeras TR. Isothermal, in vitro amplification of nucleic acids by a multienzyme reaction modeled after retroviral replication. Proc Natl Acad Sci U S A. 1990;87:1874–1878. doi: 10.1073/pnas.87.5.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Fire A, Xu SQ. Rolling replication of short DNA circles. Proc Natl Acad Sci U S A. 1995;92:4641–4645. doi: 10.1073/pnas.92.10.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Laderman EI, Whitworth E, Dumaual E, Jones M, Hudak A, Hogrefe W, Carney J, Groen J. Rapid, sensitive, and specific lateral-flow immunochromatographic point-of-care device for detection of herpes simplex virus type 2-specific immunoglobulin G antibodies in serum and whole blood. Clinical and Vaccine Immunology. 2008;15:159–163. doi: 10.1128/CVI.00218-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.