Abstract
Background
Cancer is responsible for elevated HIV-related morbidity and mortality. Research on HIV-infected patients with concurrent cancer is rare in China. The purpose of our study was to investigate the prevalence and risk factors associated with cancer among HIV-infected inpatients in Beijing, and to investigate the mortality and risk factors among HIV-infected inpatients with cancer.
Methods
Hospital records from a total of 1946 HIV-infected patients were collected from the Beijing Ditan Hospital. The data, from 2008 to 2013, were collected retrospectively. The cancer diagnoses included AIDS-defining cancers (ADC) and non-AIDS defining cancers (NADC). Logistic regression was used to identify risk factors predicting the concurrence of cancer with HIV. Mortality was examined using Kaplan-Meier estimates and Cox proportional hazards models.
Results
7.7 % (149 cases) of all HIV-infected inpatients had concurrent cancer at their first hospital admission; of those, 33.6 % (50 cases) had ADCs, and 66.4 % (99 cases) had NADCs. The most prevalent NADCs were Hodgkin’s lymphoma, gastrointestinal cancer, liver cancer, and lung cancer. Patients who did not accept antiretroviral therapy (ART) were more likely to suffer from cancer [AOR = 2.07 (1.42–3.01), p = 0.001]. Kaplan-Meier curves indicated that the survival probability of HIV-positive cancer patients was significantly lower than that of HIV-positive cancer-free patients (log-rank test, p < 0.001). For patients diagnosed with cancer, the mortality was also higher among those who did not receive ART [AHR = 2.19 (1.84–2.61), p < 0.001].
Conclusion
The prevalence of cancer concurrence among hospitalized HIV-infected patients was 7.7 %. Concurrent cancer also increased mortality among HIV-infected patients. ART was protective against concurrent cancer as well as mortality among HIV-infected cancer patients. These results highlight the importance of promoting cancer screening and early ART initiation among HIV-infected patients.
Keywords: HIV, Cancer, Concurrence, Prevalence, Mortality, Antiretroviral therapy, China
Background
People living with HIV/AIDS (PLWHA) are at higher risk of developing concurrent cancer due to the impairment of the immune system [1]. Cancer is in turn responsible for increased HIV-related morbidity and mortality [2, 3]. Cancers can be categorized by their associations with HIV/AIDS as AIDS-defining cancers (ADCs) and non-AIDS-defining cancers (NADCs) [4]. Generally, risk factors for developing cancer among PLWHA include low CD4 cell count or late stage of AIDS, co-infection with other viruses, cigarette smoking, alcohol consumption, and advanced age [5].
Globally, the wide application of antiretroviral therapy (ART), which has changed HIV/AIDS from a fatal disease to a chronic one, has contributed to a shift in cancer patterns among HIV-infected patients [6]. Evidence reveals that risk of ADCs has been dropping among HIV/AIDS patients since 1996 [6]. However, NADCs burden among HIV/AIDS patients are still on the rise [5–7] for reasons that remain unclear. In addition, although ART has been reported to be effective in lowering the risk of opportunistic infection and treating cancer-related diseases [8–10], evidence for the efficacy of ART in preventing the development of concurrent cancer among HIV-infected patients is lacking.
The mortality rate of ADCs has decreased while the mortality rate of NADCs has increased in the ART era [7, 11, 12]. While the former was always attributed to the wider application of ART [13, 14], deaths due to NADCs have been on the rise. Some studies attributed the increasing mortality of NADCs to lifestyle factors such as alcohol consumption and drug abuse [15], while others considered older age and lower CD4 counts to be the responsible factors [16].
In 2004, China started its free national ART program and began to provide free ART medications as well as related services to HIV-infected patients. The mortality of Chinese AIDS patients subsequently experienced a significant decline in the following decade [17]. However, very limited information is available on concurrent cancer among HIV/AIDS patients in China. Some HIV-infected patients were not identified until their CD4 counts decreased to less than 200 cells/mm3, after the onset of AIDS [17–19]. Late identification of HIV infection and under-resourced healthcare settings, especially in rural China, made concurrent cancer identification difficult. As a result, the prevalence of cancer concurrence among PLWHA was underestimated.
This study, based on a 5-year retrospective cohort at Beijing Ditan Hospital, focuses on the epidemiology of cancer among PLWHA. Our hypothesis was that ART was protective against concurrent cancer as well as cancer-related mortality among HIV-infected patients. We aimed to identify the risk factors associated with concurrent cancer among PLWHA and risk factors associated with mortality among PLWHA with cancer.
Methods
Study population
Beijing Ditan Hospital is one of largest specialized hospitals providing healthcare services to HIV-infected patients in China. As Beijing Ditan Hospital is a comprehensive, well-staffed, and well-equipped hospital with a professional reputation, HIV-infected patients from across China travel to the hospital to seek medical services. Therefore, the sample from Beijing Ditan Hospital is composed of a mix of HIV patients from all over the country.
Data collection
Information on each patient was collected from his/her hospital records. Permission was obtained from Beijing Ditan Hospital for accessing the patient medical records. The follow-up data within the observational period were extracted from the national AIDS information system, which compiles compulsory reports of HIV cases as well as follow-up data every 6 months. Information collected in this system included demography, updated health status, CD4 counts, ART status, and survival status. The national AIDS information system is not publicly available. For the study, we applied the permission of data use for this study from National Center for AIDS/STD Control and Prevention, which took responsibility of managing the information system.
Sampling criteria
We included all HIV-infected inpatients older than 20 who were admitted to Beijing Ditan Hospital between January 1, 2008 and December 31, 2013. Patients were considered to be enrolled in the study from the time of their first admission to Beijing Ditan Hospital until death or the end of the study period on December 31, 2013. Outpatient records were not included as the records did not include all of the necessary information. The retrospective data collection was performed from May 1 to June 30, 2014. The sampling framework for this study is shown in Fig. 1. Data from the national AIDS information system was linked to hospital data to determine their time of HIV diagnosis, first CD4 count after HIV diagnosis, subsequent CD4 record, the time of ART initiation and the death time.
Fig. 1.
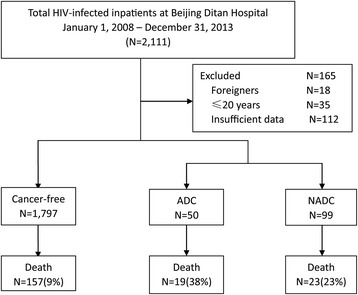
Framework of the study
Definition of the variables
HIV infection was defined as being laboratory-confirmed using Western blot. As is widely accepted [20, 21], cancers were categorized as ADCs (Kaposi’s sarcoma, KS; non-Hodgkin’s lymphoma, NHL; and cervical cancer) or NADCs (cancers of the lung, liver, kidney, anus, head and neck, skin, Hodgkin’s lymphoma, etc.). Cancer was diagnosed by image examination and biomarker analysis and further verified by biopsy. The time of first cancer diagnosis was not collected in this study.
Statistical analysis
Patients with no record of death were censored at the end of the study period on December 31, 2013. Continuous variables were summarized using median and interquartile range (IQR), and categorical variables were summarized using frequencies and percentages. Cross-tabulations with χ2 or Fisher’s exact tests were performed to relate variables of interest. A multivariate logistic regression model was used to identify factors of concurrent cancer at the first hospitalization. Kaplan-Meier curves were used to estimate the survival function during the study period. A multivariate Cox proportional hazards model was used to identify the prognostic risk of death among patients with cancer. The start time of Cox analysis was first HIV diagnosis. Updated CD4 counts and ART status were modelled as time-dependent covariates. CD4 count was kept as continuous variable. A p-value of less than 0.05 was considered significant in the final reduced multivariate model. Patients with missing data were kept in the table and classified in the ‘unknown’ group. Data was managed and analysed using the SAS 9.2 statistical software.
Ethical considerations
This study was reviewed and approved by the Institutional Review Board of the National Center for AIDS/STD Control and Prevention of the Chinese Center for Disease Control and Prevention. All participants signed the routine informed consent form when they were admitted to the hospital and no further informed consent was required. Patient information was de-identified for data analysis.
Results
From January 1, 2008 to December 31, 2013, a total of 2111 hospitalized HIV-infected patients’ records were incorporated into the study from their admissions to the hospital. 165 cases (18 foreigners, 35 below the age requirement, 112 without sufficient information) were excluded based on our inclusion criteria. Ultimately, 1946 patients were enrolled in this study; their records were analysed with a total of 59,016 patient-months of follow-up.
Characteristics of the patients
The baseline characteristics of ADC-concurrent, NADC-concurrent, and cancer-free HIV-infected inpatients are summarized in Table 1. 149 out of 1946 HIV-infected inpatients were diagnosed with concurrent cancer, accounting for 7.7 % of the study population. Among them, 33.6 % (50 patients) were diagnosed with ADCs, and 66.4 % (99 patients) were diagnosed with NADCs. The distribution of ADCs, as shown in Fig. 2, was 46 % NHL, 38 % KS, and 16 % cervical cancer. The most prevalent NADCs were Hodgkin’s lymphoma (27 %), gastrointestinal cancer (16 %), liver cancer (14 %), and lung cancer (13 %).
Table 1.
Baseline clinical characteristics classified by cancer status among HIV-infected inpatients at Beijing Ditan Hospital, January 1, 2008 – December 31, 2013
| Characteristics | Cancer-Free | Cancer | p-value* | ||
|---|---|---|---|---|---|
| N (%) | N (%) | ||||
| All | All | ADCs | NADCs | ||
| Total | 1797 | 149 | 50 | 99 | |
| Gender | 0.045 | ||||
| Female | 359 (20.0) | 40 (26.8) | 15 (30.0) | 25 (25.3) | |
| Male | 1438 (80.0) | 109 (73.2) | 35 (70.0) | 74 (74.7) | |
| Age (years) | |||||
| Median (IQR) | 37 (30–45) | 44 (37–53) | 50 (39–57) | 42 (36–49) | 0.026 |
| Age group | 0.002 | ||||
| 21–30 | 466 (25.9) | 14 (9.4) | 1 (2.0) | 13 (15.4) | |
| 31–40 | 621 (34.6) | 48 (32.2) | 14 (28.0) | 34 (34.3) | |
| 41–50 | 455 (25.3) | 37 (24.8) | 8 (16.0) | 29 (29.3) | |
| 51–60 | 171 (9.5) | 32 (21.5) | 16 (32.0) | 16 (16.2) | |
| ≥ 61 | 84 (4.7) | 18 (12.1) | 11 (22.0) | 7 (7.1) | |
| HIV transmission mode | 0.085 | ||||
| Men who have sex with men | 635 (35.3) | 35 (23.5) | 6 (12.0) | 29 (29.3) | |
| Injection drug use | 81 (4.5) | 2 (1.3) | 0 (0.0) | 2 (2.0) | |
| Heterosexual contact | 800 (44.5) | 83 (55.7) | 31 (62.0) | 52 (52.5) | |
| Blood transmission | 224 (12.5) | 24 (16.1) | 12 (24.0) | 12 (12.1) | |
| Unknown | 57 (3.2) | 5 (3.4) | 1 (2.0) | 4 (4.0) | |
| Median days from HIV diagnosis to the first hospital admission (IQR) | 111 (27–663) | 215 (49–760) | 350 (58–847) | 140 (36–402) | 0.015 |
| Median first CD4 cell count after HIV diagnosis (IQR) (cells/μl) | 131 (36–265) | 127 (51–369) | 146 (85–402) | 91 (20–255) | 0.213 |
| 0–200 | 997 (55.5) | 79 (53.0) | 26 (56.0) | 53 (51.5) | |
| 201–350 | 422 (23.5) | 29 (19.5) | 10 (20.0) | 19 (19.2) | |
| 351–500 | 245 (13.6) | 27 (18.1) | 8 (16.0) | 19 (19.2) | |
| > 500 | 133 (7.4) | 14 (9.4) | 6 (12.0) | 8 (8.1) | |
| Median CD4 cell count at the first hospital admission (IQR) (cells/μl) | 289 (135–441) | 265 (115–374) | 297 (201–396) | 240 (95–351) | 0.381 |
| ART initiation before the first hospital admission | 1206 (67.1) | 78 (52.3) | 29 (58.0) | 49 (49.5) | 0.004 |
| The CD4 cell count at ART initiation | 147 (60–273) | 137 (66–265) | 160 (90–297) | 102 (36–228) | 0.067 |
*p-value for comparing of cancer-free versus cancer group
Fig. 2.
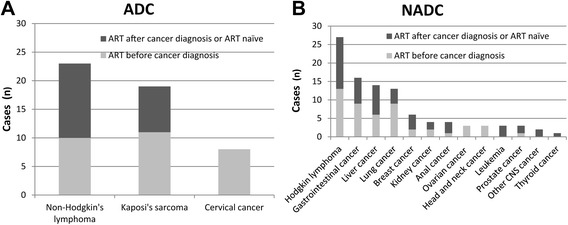
Distribution of cancers among HIV-infected inpatients with ADCs (A) and NADCs (B) at Beijing Ditan Hospital, January 1, 2008 – December 31, 2013
There were nearly four times as many male patients (1547) as female patients (399), but 10 % of female HIV patients were diagnosed with cancer compared to 7 % of male HIV patients in the study (p < 0.05). The median age of HIV-infected patients without cancer was 37 years old, which was significantly younger than that of HIV-infected patients with cancer, at 44 years old (p < 0.001). 52.3 % of HIV-infected cancer patients initiated ART before admission at Beijing Ditan Hospital compared to 67.1 % of cancer-free patients (p = 0.004).
From the logistic regression model (Table 2), we identified ART status as a strong independent factor of cancer status among HIV-infected patients. Patients not on ART were more likely to have cancer than patients who were on ART [AOR = 2.07 (1.42–3.01), p = 0.001]. Advanced age is also associated with a slightly elevated risk of developing cancer [AOR = 1.05 (1.03–1.06), p = 0.001], while gender, route of HIV contraction, and CD4 cell count at HIV diagnosis were not found to be predictive of cancer status.
Table 2.
Risk factors associated with concurrent cancer as calculated using logistic regression modeling among 1946 HIV-infected inpatients at Beijing Ditan Hospital, January 1, 2008 – December 31, 2013
| Characteristic | OR (95 % CI) | p-value | Adjusted OR (95 % CI) | p-value |
|---|---|---|---|---|
| Total | ||||
| Gender | ||||
| Female | 1.00 | 1.00 | ||
| Male | 0.68 (0.47–0.99) | 0.042 | 0.68 (0.45–1.05) | 0.422 |
| Age group every 10-year increase | 1.05 (1.03–1.06) | 0.011 | 1.05 (1.03–1.06) | 0.001 |
| HIV transmission mode | ||||
| Men who have sex with men | 1.00 | 1.00 | ||
| Injection drug use | 0.45 (0.11–1.90) | 0.156 | 0.37 (0.09–1.61) | 0.085 |
| Heterosexual contact | 1.88 (1.25–2.83) | 0.037 | 1.42 (0.84–1.82) | 0.524 |
| Blood transmission | 1.94 (1.13–3.34) | 0.041 | 1.07 (0.86–1.32) | 0.554 |
| Unknown | 1.59 (0.60–4.22) | 0.263 | 0.89 (0.32–2.54) | 0.734 |
| First CD4 cell count after HIV diagnosis (cells/μl) | ||||
| 0–200 | 1.00 | 1.00 | ||
| 201–350 | 0.87 (0.56–1.35) | 0.533 | 1.08 (0.67–1.75) | 0.745 |
| 351–500 | 0.86 (0.55–1.35) | 0.514 | 1.02 (0.63–1.64) | 0.950 |
| > 500 | 0.57 (0.32–1.02) | 0.057 | 0.71 (0.39–1.30) | 0.263 |
| ART initiation before the first hospital admission | ||||
| Yes | 1.00 | 1.00 | ||
| No | 1.86 (1.33–2.60) | 0.01 | 2.07 (1.42–3.01) | 0.001 |
OR odds ratio, CI confidence interval
Patients without cancer had significantly higher survival rates over the follow-up period than patients with ADCs or NADCs (log-rank test p < 0.001) (Fig. 3). A multivariate Cox proportional hazards analysis (Table 3) showed that patients with cancer who were not on ART were at higher risk for death [AHR = 2.19 (1.84–2.61), p < 0.001]. Time updating CD4 cell counts (from HIV diagnosis to the endpoint of this study) and advanced age did not affect mortality.
Fig. 3.
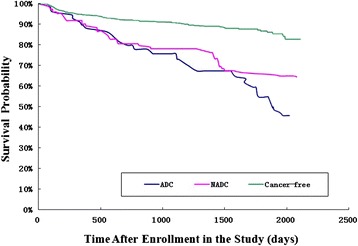
Kaplan-Meier survival curves for HIV-infected inpatients stratified by cancer type at Beijing Ditan Hospital, January 1, 2008 – December 31, 2013
Table 3.
Risk factors associated with mortality as calculated using Cox proportional hazards models among 149 inpatients with cancer at Beijing Ditan Hospital, January 1, 2008 – December 31, 2013
| Characteristic | Number of Deaths (%) | Observed person-months | Mortality rate (deaths per 100 person-months) | HR(95 % CI) | p-value | Adjusted HR (95 % CI) | p-value |
|---|---|---|---|---|---|---|---|
| Total | 42 (28.2) | 5355 | 0.78 | – | – | – | – |
| Gender | |||||||
| Female | 13 (32.5) | 2012 | 0.65 | 1.00 | 1.00 | ||
| Male | 29 (26.6) | 3343 | 0.87 | 1.30 (0.67–2.53) | 0.071 | 0.94 (0.41–2.13) | 0.826 |
| Age (every 10-year increase) | 42 (28.2) | 5355 | 0.78 | 1.02 (1.00–1.05) | 0.923 | 1.02 (0.99–1.05) | 0.912 |
| HIV transmission mode | |||||||
| Men who have sex with men | 8 (22.9) | 1029 | 0.78 | 1.00 | 1.00 | ||
| Injection drug use | 0 (0.0) | 110 | 0.00 | – | – | – | – |
| Heterosexual contact | 23 (27.7) | 2468 | 0.93 | 1.17 (0.52–2.63) | 0.227 | 1.27 (0.44–3.66) | 0.363 |
| Blood transmission | 9 (37.5) | 1601 | 0.56 | 0.58 (0.21–1.62) | 0.189 | 0.78 (0.23–2.67) | 0.314 |
| Unknown | 2 (40.0) | 146 | 1.37 | 1.85 (0.39–8.76) | 0.456 | 6.71 (0.67–66.89) | 0.532 |
| CD4 cell counta (per cell/μl increase) | 42 (28.2) | 5355 | 0.78 | 0.99 (0.99–0.99) | <0.001 | 1.01 (0.99–1.02) | 0.343 |
| No ART initiationa | 42 (28.2) | 5355 | 0.78 | 2.52 (2.22–2.86) | <0.001 | 2.19 (1.84–2.61) | <0.001 |
| Classification of cancer | |||||||
| NADC | 23 (23.2) | 3151 | 0.73 | 1.00 | 1.00 | ||
| ADC | 19 (38.0) | 2204 | 0.86 | 1.12 (0.61–2.08) | 0.121 | 0.86 (0.38–1.93) | 0.082 |
HR hazard ratio, CI confidence interval
aVariables were modelled as time-dependent covariates
Discussion
Research on HIV-infected patients with concurrent cancer is lacking in China [22]. The purpose of this study was to investigate the prevalence of cancer in HIV-infected inpatients and to identify the risk factors associated with cancer among PLWHA and with mortality among PLWHA with cancer.
Related factors for developing cancer among the HIV-infected population include low CD4 cell count, route of HIV contraction, co-infection with other viruses, late stage of AIDS, cigarette smoking, alcohol consumption, and advanced age [5]. The finding of advanced age as an independent predictor of developing cancer is consistent with previous studies [7]. In our study, patients with cancer were on average 7 years older than those without cancer (44 years versus 37 years, p = 0.026), suggesting that it may be meaningful to advocate regular cancer screening and care among older HIV patients in China.
The capacity for cancer screening and diagnosis largely depends on techniques such as biomarker analysis, CT and MRI scanning, endoscopy, and biopsy [23]. These methods are available at major hospitals, but many HIV patients in rural China may miss the opportunity to be screened for cancer due to the high cost burden or the inaccessibility of medical facilities or service providers.
The prevalence, of cancer among patients enrolled in our study was 7.7 % (149/1946). Data from the national registry indicated that the crude incidence rate of cancer in 2011 was 235/100,000 and that cancers of the lung, female breast, gastrointestinal tract, liver, and cervix were the most common malignancies in China [24]. In our HIV-positive population, the most prevalent NADCs were Hodgkin’s lymphoma, gastrointestinal cancer, liver cancer, and lung cancer, different from the national pattern.
Previous research in developed countries showed the decrease of the incidences of ADCs and NADCs in the post-ART era [25]. As China’s national ART program began later than those of most developed countries and some HIV-infected patients were first identified at a late stage with low CD4 counts [19], it is reasonable that China still experiences HIV-related cancer epidemics in the ART era [26]. It is therefore important to initiate surveillance to track changes in the incidences of ADCs and NADCs.
Effective ART was found to reduce some of the excess risk of developing cancer among HIV patients [27]. The ART rate among HIV patients diagnosed with cancer was lower than among cancer-free patients (52.3 % versus 67.1 %, p = 0.004). Patients without ART were more likely to get cancer than patients who accepted ART [2.07 (1.42–3.01), p = 0.001]. This result indicated that treatment could be beneficial in reducing the risk of cancer among HIV-infected patients. Additionally, reasons why patients who were not on ART even they were in the serious progression should be considered. Being very sick and near death reduced the likelihood that ART was prescribed by their physician.
Mortality rates in both the cancer-concurrent group as well as the cancer-free group were relatively high, in part because our study population was drawn from hospital admissions who were more likely to have advanced disease progression. Our finding that HIV-infected patients with cancer who received ART were less likely to experience premature death is encouraging. A large-scale and long-term cohort study in the U.S. found that overall cancer-related mortality among PLWHA experienced a major drop (from 302 to 29 per 1000 patients) between 1980 and 2006 [28]. Another U.S. cohort study reported that mortality rates among HIV patients with cancer who received ART were much lower than among HIV patients who did not, while the time of ART initiation, before or after cancer diagnosis, did not make a significant difference [3]. The Johns Hopkins AIDS Clinic cohort discovered no difference in mortality rates in ADCs and NADCs among patients who received ART [29].
Limitations
Our study has a few limitations. The sample from Beijing Ditan Hospital could be biased because it is a referral center for infection disease treatment. HIV-infected patients admitted by Beijing Ditan Hospital came from all over China, but the study population cannot be considered a random sample as only those who had relatively severe complications and who could afford the medical expenses would come to this hospital. As a result, selection bias may have occurred, leading to the overestimation of the prevalence of concurrent cancer among HIV-infected patients.
There was another major weakness of this study. The time of first cancer diagnosis was not collected in this study. Patients were assessed concurrent cancer when they were firstly hospitalized in our study facility, Beijing Ditan hospital. We could not accurately describe the occurrence of cancer.
Due to the nature of the retrospective study, it was not possible for us to collect data on as many clinical variables as may have been needed for the study, such as viral load. Therefore, there exists the possibility of residual confounding due to data insufficiency. For example, the regression models did not include covariates such as alcohol dependence and co-infection with other viruses as in other studies, as they were not available in all of our routine clinical data.
Conclusion
The concurrence of cancers among HIV-infected patients was 7.7 % in inpatients in our study hospital. Cancers increased mortality rates among HIV-infected patients, but ART was effective both in reducing the risk of cancer development among HIV-infected patients and in reducing the risk of cancer-related mortality. It is therefore important to increase cancer screening and to promote early initiation of ART among HIV-infected patients.
Acknowledgments
We thank Dr. Zunyou Wu for his contribution of study design, interpretation of results, and review for this manuscript. The China Office of the U.S. CDC Global AIDS Program provided partial financial support for this study. The authors are solely responsible for the contents of the paper. The funding organizations had no role in the design of the study, data collection, analysis, and interpretation, or the final decision to submit the manuscript for publication.
Footnotes
Jun Yang and Shu Su were the co-first authors of this article.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JY: contributed to conception and design of study, interpretation of results, and drafting of the manuscript; SS: contributed to cleaning of data, analysis of data, and drafting of the manuscript; HZ: contributed to conception and design of study, acquisition of data, interpretation of data, and critical review of manuscript; DW: contributed to interpretation of data, and revision of manuscript; JW: contributed to interpretation of data, and revision of manuscript; FZ: contributed to interpretation of results, and critical review of the manuscript; YZ: contributed to conception and design of study, interpretation of results, and critical review of the manuscript. All authors read and approved the final manuscript.
Contributor Information
Hongxin Zhao, Email: 13911022130@163.com.
Yan Zhao, Email: zhaoyan1973@sina.cn.
References
- 1.Biggar RJ, Chaturvedi AK, Goedert JJ, Engels EA. AIDS-related cancer and severity of immunosuppression in persons with AIDS. J Natl Cancer Inst. 2007;99(12):962–72. doi: 10.1093/jnci/djm010. [DOI] [PubMed] [Google Scholar]
- 2.Coghill AE, Shiels MS, Suneja G, Engels EA. Elevated Cancer-Specific Mortality Among HIV-Infected Patients in the United States. J Clin Oncol. 2015;33(21):2376–2383. [DOI] [PMC free article] [PubMed]
- 3.Riedel DJ, Mwangi EI, Fantry LE, Alexander C, Hossain MB, Pauza CD, et al. High cancer-related mortality in an urban, predominantly African-American, HIV-infected population. AIDS (London, England). 2013;27(7):1109–17. [DOI] [PMC free article] [PubMed]
- 4.Yanik EL, Napravnik S, Cole SR, Achenbach CJ, Gopal S, Olshan A, et al. Incidence and timing of cancer in HIV-infected individuals following initiation of combination antiretroviral therapy. Clin Infect Dis. 2013;57(5):756–64. [DOI] [PMC free article] [PubMed]
- 5.Pinzone MR, Fiorica F, Di Rosa M, Malaguarnera G, Malaguarnera L, Cacopardo B, et al. Non-AIDS-defining cancers among HIV-infected people. Eur Rev Med Pharmacol Sci. 2012;16(10):1377–88. [PubMed]
- 6.Engels EA, Biggar RJ, Hall HI, Cross H, Crutchfield A, Finch JL, et al. Cancer risk in people infected with human immunodeficiency virus in the United States. Int J Cancer J Int Du Cancer. 2008;123(1):187–94. [DOI] [PubMed]
- 7.Deeken JF, Tjen ALA, Rudek MA, Okuliar C, Young M, Little RF, et al. The rising challenge of non-AIDS-defining cancers in HIV-infected patients. Clin Infect Dis. 2012;55(9):1228–35. [DOI] [PMC free article] [PubMed]
- 8.International Collaboration on HIV and Cancer Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92(22):1823–30. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 9.Clifford GM, Polesel J, Rickenbach M, Dal Maso L, Keiser O, Kofler A, et al. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425–32. [DOI] [PubMed]
- 10.Jones JL, Hanson DL, Dworkin MS, Ward JW, Jaffe HW. Effect of antiretroviral therapy on recent trends in selected cancers among HIV-infected persons. Adult/Adolescent Spectrum of HIV Disease Project Group. J Acquir Immune Defic Syndr. 1999;21 Suppl 1:S11–7. [PubMed] [Google Scholar]
- 11.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 12.Spagnuolo V, Galli L, Salpietro S, Gianotti N, Guffanti M, Cossarini F, et al. Ten-year survival among HIV-1-infected subjects with AIDS or non-AIDS-defining malignancies. Int J Cancer J Int Du Cancer. 2012;130(12):2990–6. [DOI] [PubMed]
- 13.Hleyhel M, Belot A, Bouvier AM, Tattevin P, Pacanowski J, Genet P, De Castro N, Berger JL, Dupont C, Lavole A et al. Trends in survival after cancer diagnosis among HIV-infected individuals between 1992 and 2009. Results from the FHDH-ANRS CO4 cohort. Int J Cancer J Int Du Cancer. 2015;137(10):2443–2453. [DOI] [PubMed]
- 14.Gotti D, Raffetti E, Albini L, Sighinolfi L, Maggiolo F, Di Filippo E, et al. Survival in HIV-infected patients after a cancer diagnosis in the cART Era: results of an italian multicenter study. PLoS One. 2014;9(4):e94768. [DOI] [PMC free article] [PubMed]
- 15.Aberg JA. The changing face of HIV care: common things really are common. Ann Intern Med. 2006;145(6):463–5. doi: 10.7326/0003-4819-145-6-200609190-00011. [DOI] [PubMed] [Google Scholar]
- 16.Falster K, Choi JY, Donovan B, Duncombe C, Mulhall B, Sowden D, et al. AIDS-related and non-AIDS-related mortality in the Asia-Pacific region in the era of combination antiretroviral treatment. AIDS (London, England). 2009;23(17):2323–36. [DOI] [PMC free article] [PubMed]
- 17.Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–24. [DOI] [PubMed]
- 18.Fisher M. Late diagnosis of HIV infection: major consequences and missed opportunities. Curr Opin Infect Dis. 2008;21(1):1–3. doi: 10.1097/QCO.0b013e3282f2d8fb. [DOI] [PubMed] [Google Scholar]
- 19.Tang H, Mao Y, Shi CX, Han J, Wang L, Xu J, et al. Baseline CD4 cell counts of newly diagnosed HIV cases in China: 2006–2012. PLoS One. 2014;9(6):e96098. [DOI] [PMC free article] [PubMed]
- 20.Grulich AE, Wan X, Law MG, Coates M, Kaldor JM. Risk of cancer in people with AIDS. AIDS (London, England) 1999;13(7):839–43. doi: 10.1097/00002030-199905070-00014. [DOI] [PubMed] [Google Scholar]
- 21.Layton JL, Castillo JJ. Non-AIDS defining cancers. Med Health Rhode Island. 2010;93(10):296–8. [PubMed] [Google Scholar]
- 22.Zhang YX, Gui XE, Zhong YH, Rong YP, Yan YJ. Cancer in cohort of HIV-infected population: prevalence and clinical characteristics. J Cancer Res Clin Oncol. 2011;137(4):609–14. doi: 10.1007/s00432-010-0911-y. [DOI] [PubMed] [Google Scholar]
- 23.Sigel K, Dubrow R, Silverberg M, Crothers K, Braithwaite S, Justice A. Cancer screening in patients infected with HIV. Curr HIV/AIDS Rep. 2011;8(3):142–52. doi: 10.1007/s11904-011-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen W, Zheng R, Zhang S, Zhao P, Zeng H, Zou X, et al. Annual report on status of cancer in China. Chin J Cancer. 2010;26(1):48–58. [DOI] [PMC free article] [PubMed]
- 25.Shiels MS, Pfeiffer RM, Gail MH, Hall HI, Li J, Chaturvedi AK, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011;103(9):753–62. [DOI] [PMC free article] [PubMed]
- 26.Mutyaba I, Phipps W, Krantz EM, Goldman JD, Nambooze S, Orem J, et al. A population-level evaluation of the effect of antiretroviral therapy on cancer incidence in Kyadondo County, Uganda, 1999–2008. J Acquir Immune Defic Syndr. 2015;69(4):481–6. [DOI] [PMC free article] [PubMed]
- 27.Grulich AE. Cancer: the effects of HIV and antiretroviral therapy, and implications for early antiretroviral therapy initiation. Curr Opin HIV AIDS. 2009;4(3):183–7. doi: 10.1097/COH.0b013e328329c5b2. [DOI] [PubMed] [Google Scholar]
- 28.Simard EP, Engels EA. Cancer as a cause of death among people with AIDS in the United States. Clin Infect Dis. 2010;51(8):957–62. doi: 10.1086/656416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long JL, Engels EA, Moore RD, Gebo KA. Incidence and outcomes of malignancy in the HAART era in an urban cohort of HIV-infected individuals. AIDS (London, England) 2008;22(4):489–96. doi: 10.1097/QAD.0b013e3282f47082. [DOI] [PMC free article] [PubMed] [Google Scholar]


