Abstract
Objectives
To assess the feasibility of a vitamin D intervention delivered through a Meals-on-Wheels (MOW) program to improve 25-hydroxyvitamin D (25(OH)D) concentrations and reduce falls in homebound older adults.
Design
Single-blind, cluster randomized trial.
Setting
MOW, Forsyth County, North Carolina.
Participants
Community-dwelling homebound adults aged 65 to 102 (N=68).
Intervention
MOW clients were randomized to vitamin D3 (100,000 IU/month; n=38) or active placebo (400 IU vitamin E/month; n=30) according to MOW delivery route.
Measurements
Serum 25(OH)D was assessed at baseline and 5-month follow-up; proportions of participants in 25(OH)D categories were compared using the Fisher exact test. Falls were assessed using monthly fall calendars, and rate of falls was estimated using negative binomial generalized estimating equation models.
Results
Mean±standard deviation 25(OH)D concentrations were 20.9±11.5 ng/mL at baseline, with 57% having 25(OH)D concentrations less than 20 ng/mL. Retention and adherence were high (>90%). After the 5-month intervention, only one of 34 participants randomized to vitamin D3 had 25(OH)D concentrations less than 20 ng/mL, compared with 18 of 25 participants randomized to placebo (P<.001). In unadjusted analyses, the rate of falls over 5 months was not significantly different according to intervention group (risk ratio (RR)=0.48, 95% confidence interval (CI)=0.19–1.19), but after adjustment for sex, race, season of year, baseline 25(OH)D status, and history of falls, participants randomized to vitamin D3 had a lower rate of falling than those randomized to placebo (RR=0.42, 95% CI=0.21–0.87).
Conclusion
A vitamin D intervention delivered through MOW was feasible, resulting in improvements in 25(OH)D levels and a lower rate of falls in adjusted analyses. Further research is needed to validate the reduction in falls from this type of intervention.
Keywords: homebound elderly adults, Meals-on-Wheels, 25-hydroxyvitamin D, falls
More than one-third of community-dwelling adults aged 65 and older fall annually, with approximately one in 10 falls resulting in serious injury.1 Fall-related injuries in older adults are a common cause of pain, functional limitation, disability, placement in long- term care, and death.2,3 Although most falls do not result in significant physical injury, the psychological effects of a fall often result in fear of falling and restriction of activities, leading to a loss of independence.4 Evidence suggests that sufficient 25-hydroxyvitamin D (25(OH)D) concentrations may be important for maintaining muscle integrity and physical function and, consequently, preventing falls.
Recent data from the National Health and Nutrition Examination Surveys (NHANES) 2001 to 2006 show that insufficient 25(OH)D concentrations (<20 ng/mL) are common in community-dwelling older adults (≥70), with approximately 31% of older men and 38% of older women having low 25(OH)D concentrations.5 Homebound older adults are at greater risk of vitamin D insufficiency because they are exposed to sunlight less and often have inadequate dietary intake.6,7 Serum 25(OH)D concentrations have been shown to be low in older adults who fall.8,9 Although low 25(OH)D concentrations have been associated with greater risk of falls in older adults in assisted living or nursing homes,10,11 studies examining the association between low 25(OH)D concentrations and risk of falls in community-dwelling older adults have been inconsistent.12–14 Results of intervention trials of vitamin D with regard to falls have also been mixed,15–18 although to the knowledge of the authors of the current study, none have been conducted in community-dwelling homebound elderly adults.
The goal of this pilot study was to assess the feasibility of a vitamin D intervention in homebound older adults delivered through a Meals-on-Wheels (MOW) program to improve 25(OH)D concentrations and reduce falls in homebound older adults. Specifically, the study sought to determine adherence to and acceptability of a vitamin D3 supplementation regimen to home-delivered meal recipients, rate of participant drop-out due to leaving the program or death, 25(OH)D response to a monthly vitamin D dose, and effect of the intervention on falls.
METHODS
Subjects
Older adults were recruited from the Forsyth County Senior Services MOW program to participate in a 5-month, single-blind, cluster randomized, active placebo-controlled trial of vitamin D supplementation and falls. Participants were enrolled in the study based on the following criteria: aged 65 and older, not taking prescription vitamin D2 or more than 1,000 IU/d of vitamin D3, no primary hyperparathyroidism, no history of hypercalcemia or kidney stones (within the past 2 years), not on dialysis, not confined to a wheelchair while inside their home, and willing to be randomized to vitamin D3 or active placebo control. All participants provided written informed consent to participate in the study according to the guidelines set forth by the Wake Forest School of Medicine institutional review board for human research.
Recruitment and baseline assessments
Forsyth County Senior Services MOW staff described the study to potential participants as clients were assessed (or reassessed) for MOW eligibility. Interested MOW clients were initially contacted for a telephone screen during which their willingness to have study staff come to their home for study visits, provide a blood sample, take a monthly vitamin supplement, and complete a monthly fall calendar was ascertained. At the baseline home visit, eligibility was confirmed and written informed consent obtained. Participants were asked about their medical history, and medications and dietary supplements were reviewed. Fall history (Have you fallen in the past six months?) and fear of falling (Are you afraid of falling?) was assessed using a questionnaire. Self-reported physical function was assessed using the Pepper Assessment Tool for Disability (PAT-D), a 19-item questionnaire containing subscales assessing mobility, activities of daily living (ADLs), and instrumental ADLs (IADLs).19 Participants answered questions on a Likert scale from 1 (usually do with no difficulty) to 5 (unable to do), and a summary score was calculated for each subscale (lower score indicating better functioning). A trained phlebotomist obtained nonfasting blood samples to assess liver and kidney function, vitamin D status (serum 25(OH)D; LIAISON, DiaSorin, Saluggia, Italy), and serum calcium (colorimetric assay) at an independent clinical laboratory (LabCorp, Greensboro, NC). Participants were provided instructions on how to fill out a monthly fall calendar, in which they were to indicate each day whether they had fallen.
Participants were randomized according to MOW delivery route. Once the baseline visit was completed for all participants on a route, the route was randomized using a computer-generated randomization table with mixed block sizes to one of two conditions: vitamin D supplementation (17 routes, n=38) or active placebo control (vitamin E; 16 routes, n=30). Vitamin E was chosen as the active placebo because it has not been shown to increase 25(OH)D concentrations or reduce falls. Thirty-three routes were randomized in four waves starting approximately 1 month apart from December 2010 through March 2011 (Wave 1, 9 routes; Wave 2, 9 routes; Wave 3, 8 routes; Wave 4, 7 routes). The vitamin D3 supplement (two 50,000-IU capsules/month; vitamin D3–50, Bio-Tech Pharmacal, Inc., Fayetteville, AR) or vitamin E supplement (400 IU capsule/month; GNC Natural E 400, General Nutrition Corp., Pittsburgh, PA) were individually packaged, and MOW volunteers delivered them monthly with the home-delivered meal. Up to three attempts during the same week were made on a monthly basis to deliver the supplement to the MOW participant’s home. The study coordinator called the participant on the day supplements were delivered to confirm that they had received and taken the supplement and were completing their monthly fall calendars.
Follow-up assessments
The 5-month follow-up home visits were scheduled approximately 2 weeks after participants received the final supplement dose (range 1.5–2.5 weeks). At the follow-up visit, medications and dietary supplements were reviewed; fall history, fear of falling, and self-reported physical function (PAT-D) were assessed according to questionnaire; and the monthly fall calendars were collected. Nonfasting blood samples were obtained to assess serum 25(OH)D concentrations and serum calcium.
Statistics
Statistical analyses were performed using SAS version 9.3 (SAS Institute, Inc., Cary, NC). Descriptive statistics were calculated for the study population at baseline; values are reported as means ± standard deviations or frequencies. Differences in baseline participant characteristics were compared according to intervention group using chi-square or Fisher exact tests for categorical variables and t-tests for continuous variables. Mean change in 25(OH)D concentrations at follow-up adjusted for age, sex, race, and season was estimated in a mixed model with a random intercept and an intervention group–by-time interaction; interactions between group, time, and race were also fit to assess differences in 25(OH)D change according to race. It was attempted to include a random route effect in the modeling, but there were not enough participants per route to allow the models to be fit. Proportions of participants in 25(OH)D categories at follow-up were compared according to intervention group using the Fisher exact test. Proportions of participants in each intervention group who reported any fall during the follow-up period were compared using a chi-square test. Negative binomial generalized estimating equation models (to account for the five monthly assessments of falls) were used to estimate rate ratios and 95% confidence intervals of the number of falls per month (as a count variable) according to intervention group. Models were adjusted for sex, race, history of falls in the previous 6 months, baseline 25(OH)D status (<20 vs ≥20 ng/mL), and season of year (time varying). A two-sided alpha level of .05 was considered significant.
RESULTS
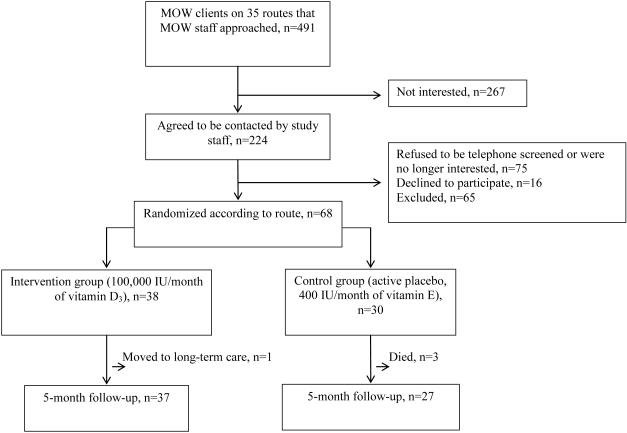
MOW staff approached MOW clients on 35 routes (n=491) regarding their interest in participating in the study; 224 (46%) expressed interest and were contacted by study staff to determine eligibility. Seventy-five refused participation upon being contacted by study staff, 65 were ineligible, and 16 were eligible but declined further participation. Figure 1 shows a consort diagram of selection of the participant sample. Characteristics of those randomized (n=68) are shown in Table 1. Mean age was 77.9±8.7, 72% were female, and 75% were black. Approximately one-third lived alone and had less than an 8th grade education. More than one-third reported their health as fair or poor. Most had hypertension, and almost half had diabetes mellitus. Sixty-three percent were taking five or more prescription medications, and approximately one-fifth were taking a vitamin D–containing supplement. More than half reported having fallen in the previous year and that they were afraid of falling. Mean PAT-D mobility scale score was 3.3±1.2, indicating some difficulty with mobility-related tasks. Overall baseline 25(OH)D concentrations were low (20.9 ± 11.5) and did not differ significantly between those randomized to vitamin D3 and those randomized to active placebo (P=.22). Baseline 25(OH)D concentrations were slightly higher in whites (24.5 ± 10.1 ng/mL) than in blacks (19.6 ± 11.8 ng/mL) (P=.13).
Figure 1.
Consort diagram of participant selection: Meals-on-Wheels vitamin D and falls feasibility study.
Table 1.
Participant Baseline Characteristics: Meals-on-Wheels Vitamin D and Falls Feasibility Study
| Characteristic | Total Sample, n=68 |
Intervention Group, n=38 |
Control Group, n=30 |
P- Valuea |
|---|---|---|---|---|
| Age, mean ± SD | 77.9±8.7 | 77.6±9.0 | 78.2±8.4 | .75 |
| Female, % | 72.1 | 79.0 | 63.3 | .15 |
| Black, % | 75.0 | 71.0 | 80.0 | .40 |
| Winter, % | 55.9 | 50.0 | 63.3 | .27 |
| Living alone, % | 30.9 | 29.0 | 33.3 | .70 |
| Education, % | .54 | |||
| Elementary | 30.9 | 31.6 | 30.0 | |
| High school | 54.4 | 57.9 | 50.0 | |
| College | 14.7 | 10.5 | 20.0 | |
| Assistive device, % | .81 | |||
| None | 30.9 | 34.2 | 26.7 | |
| Cane | 23.5 | 23.7 | 23.3 | |
| Walker | 14.7 | 15.8 | 13.3 | |
| Wheelchair | 30.9 | 26.3 | 36.7 | |
| Self-rated health, % | .006 | |||
| Excellent or very good | 11.8 | 2.6 | 23.3 | |
| Good | 48.5 | 44.7 | 53.3 | |
| Fair | 29.4 | 34.2 | 23.3 | |
| Poor | 10.3 | 18.4 | 0 | |
| Chronic conditions, % | ||||
| Hypertension | 88.2 | 97.4 | 76.7 | .008 |
| Diabetes mellitus | 45.6 | 52.6 | 36.7 | .19 |
| Heart attack | 20.6 | 21.1 | 20.0 | .92 |
| Heart failure | 20.6 | 23.7 | 16.7 | .48 |
| Stroke | 22.1 | 29.0 | 13.3 | .12 |
| Lung disease | 11.8 | 13.2 | 10.3 | .99 |
| Cancer in past year | 8.8 | 2.6 | 16.7 | .08 |
| Medications, % | ||||
| Benzodiazepines | 5.9 | 5.3 | 6.7 | .81 |
| Narcotics | 14.7 | 13.2 | 16.7 | .69 |
| Antidepressants | 19.1 | 15.8 | 23.3 | .43 |
| Diuretics | 47.1 | 50.0 | 43.3 | .58 |
| Osteoporosis medications | 2.9 | 2.6 | 3.3 | .87 |
| Total number of prescription medications, mean ± SD |
6.1±4.2 | 6.4±3.4 | 5.8±5.1 | .59 |
| Dietary supplements, % | ||||
| Multivitamin | 20.6 | 26.3 | 13.3 | .21 |
| Calcium | 17.6 | 18.4 | 16.7 | .90 |
| Vitamin D-containing | 22.1 | 23.7 | 20.0 | .77 |
| Falls in past year, % | ||||
| 0 | 38.8 | 36.8 | 41.4 | .51 |
| 1 | 19.4 | 15.8 | 24.1 | |
| ≥2 | 41.8 | 47.4 | 34.5 | |
| Fall requiring medical attention in past year, %b |
19.5 | 16.7 | 23.5 | .58 |
| Afraid of falling, % | 57.4 | 63.2 | 50.0 | .28 |
| Pepper Assessment Tool for Disability score, mean ± SD |
||||
| Mobility score | 3.3±1.2 | 3.4±1.2 | 3.1±1.1 | .22 |
| Activity of daily living score | 1.6±0.7 | 1.7±0.8 | 1.5±0.5 | .20 |
| Instrumental activity of daily living score | 1.6±0.7 | 1.7±0.8 | 1.6±0.5 | .60 |
| 25-hydroxyvitamin D, ng/mL, mean ± SD | 20.9±11.5 | 22.5±12.2 | 18.9±10.6 | .22 |
Based on chi-square or Fisher exact test for categorical variables and t-test for continuous variables.
Among those who reported falling in past year.
SD=standard deviation.
The 5-month follow-up home visit was completed in 64 (94%) of the 68 individuals randomized; one individual randomized to vitamin D3 was moved to long-term care, and three randomized to active placebo died before the follow-up visit. Of those with a follow-up home visit, all 37 randomized to vitamin D3 and 24 of 27 (89%) randomized to active placebo received at least four of the five monthly supplement doses. Four individuals randomized to vitamin D3 and three individuals randomized to active placebo were missing baseline (n=3) or follow-up (n=5) blood samples (unable to obtain blood sample = 6; refused = 2).
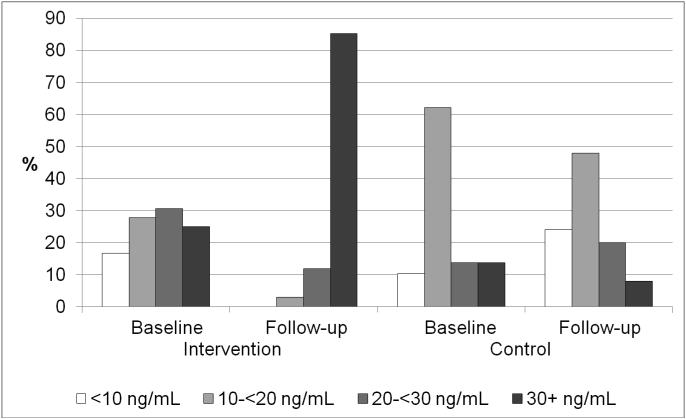
Overall, 57% had 25(OH)D concentrations less than 20 ng/mL, and 80% had 25(OH)D concentrations less than 30 ng/mL at baseline. Blacks (31/48, 64%) were more likely than whites (6/17, 35%) to have 25(OH)D concentrations less than 20 ng/mL at baseline (P=.04), but the proportion with 25(OH)D concentrations less than 30 ng/mL was similar (39/48 blacks, 81%; 13/17 whites, 76%, P=.67). Figure 2 shows the distribution of 25(OH)D at baseline and 5-month follow-up in individuals randomized to vitamin D3 or active placebo. Although the distribution of 25(OH)D did not differ according to randomization group at baseline (Table 1), fewer individuals randomized to vitamin D3 (16/36, 44%) had 25(OH)D concentrations less than 20 ng/mL at baseline than of those randomized to active placebo (21/29, 72%) (P=.02). The prevalence of 25(OH)D concentrations less than 30 ng/mL at baseline was similar in those randomized to vitamin D3 (27/36, 75%) and active placebo (25/29, 86%) (P=.26). The mean (standard error) change in 25(OH)D concentrations after the 5-month intervention was 21.7 (3.1) ng/mL in those randomized to vitamin D3 and 0.0 (4.0) ng/mL in those randomized to active placebo after adjusting for age, sex, race, and season (P<.001 for group difference). The interaction between race and change in 25(OH)D concentrations was not significant (P=.82); in those randomized to vitamin D3, the mean 25(OH)D change was 22.6 (3.5) ng/mL in blacks and 20.8 (3.6) ng/mL in whites. At 5-month follow-up, only one of 34 (3%) participants randomized to vitamin D3 had 25(OH)D concentrations less than 20 ng/mL, versus 18 of 25 (72%) randomized to active placebo (P<.001); and five of 34 (15%) participants randomized to vitamin D3 had 25(OH)D concentrations less than 30 ng/mL, versus 23 of 25 (92%) randomized to active placebo (P<.001). Of those randomized to vitamin D3, one of 23 (4%) blacks and 0 of 11 (0%) whites had 25(OH)D less than 20 ng/mL at follow-up (p>.99); three (13%) blacks and two (18%) whites had 25(OH)D less than 30 ng/mL at follow-up (p>.99). There were no cases of hypercalcemia (serum calcium >10.6 mg/dL) at follow-up.
Figure 2.
Serum 25(OH)D status at baseline and 5-month follow-up: Meals-on-Wheels vitamin D and falls feasibility study.
Monthly fall calendars were complete for 59 (92%) of the 64 participants with a follow-up visit; four participants randomized to vitamin D3 and one randomized to active placebo did not complete the monthly fall calendars for all 5 months. Eleven of 33 (33%) participants randomized to vitamin D3 and 12 of 26 (46%) randomized to active placebo reported falling during the 5-month follow-up period; the rate of falling did not differ between the two groups (P=.32). The mean number of reported falls over the 5-month follow-up period was 0.5 (range 0–4) in participants randomized to vitamin D3 and 1.1 (range 0–8) in participants randomized to active placebo. In unadjusted analyses, there was not a significant association between the vitamin D3 intervention and number of falls over the 5-month follow-up period (Table 2), but after adjusting for sex and race, the association between vitamin D3 intervention and falls was significant, with participants randomized to vitamin D3 having fewer falls over the 5-month follow-up period than those randomized to active placebo. The association remained significant after further adjustment for season, baseline 25(OH)D status (<20 vs ≥20 ng/mL), and history of falls in the previous 6 months. Of the covariates included in the multivariate analyses, sex and fall history had the largest effect on the model.
Table 2.
Randomization to Vitamin D3 and Rate of Falls: Meals-on-Wheels Vitamin D and Falls Feasibility Study
| Model | Rate Ratio (95% Confidence Interval) |
|---|---|
| Unadjusted | 0.48 (0.19–1.19) |
| Adjusted | |
| + sex and race | 0.36 (0.16–0.84) |
| + season and baseline 25-hydroxyvitamin D status | 0.41 (0.18–0.90) |
| + fall history | 0.42 (0.21–0.87) |
DISCUSSION
The goal of this single-blind, cluster-randomized, active placebo–controlled pilot study was to assess the feasibility of delivering a vitamin D intervention through a MOW program to improve 25(OH)D concentrations and reduce falls in homebound older adults. There was a high prevalence of vitamin D insufficiency among homebound older adults receiving home-delivered meals, with more than half having 25(OH)D concentrations less than 20 ng/mL and four-fifths having 25(OH)D concentrations less than 30 ng/mL. Delivery of a vitamin D supplement once a month by MOW volunteer drivers was feasible; more than 90% participants received at least four of the five monthly doses. The monthly dose of 100,000 IU of vitamin D3 was effective in increasing 25(OH)D concentrations to 20 ng/mL or greater at follow-up in all but one participant and increasing 25(OH)D concentrations to 30 ng/mL or greater in all but five of the 34 participants randomized to vitamin D supplementation. Furthermore, randomization to vitamin D supplementation resulted in a rate of falls in the intervention group that was approximately half that of the control group over the 5-month period.
Homebound older adults are a subgroup of older adults particularly vulnerable to poor dietary intake and nutritional health, nutrition-related health conditions, and functional decline and disability. It wa reported that the Dietary Reference Intakes (DRIs) for energy and several micronutrients including vitamin D were not met in home-delivered meal recipients in North Carolina.20 Furthermore, a summary measure of musculoskeletal nutrient intake (calcium, vitamin D, magnesium, phosphorus) was associated with lower-extremity physical performance such that those with a low musculoskeletal nutrient intake had worse performance,21 possibly putting them at greater risk of falls. Of older adults in northeast Georgia receiving home-delivered meals, 38% had insufficient 25(OH)D concentrations (<20 ng/mL).22 More than half of homebound older adults receiving home-delivered meals in the current pilot study had insufficient 25(OH)D concentrations.
The Older Americans Act (OAA) mandates that home-delivered meals provide a minimum of one-third of the DRIs established by the Food and Nutrition Board of the Institute of Medicine (IOM) and National Academy of Sciences. Although studies in general have found home-delivered meal programs to improve diet quality and nutrient intake,23 the current pilot study and a previous study22 suggest that a high prevalence of vitamin D insufficiency remains in elderly adults receiving home-delivered meals. In the 2006 OAA Amendment, Congress recognized that a “single, daily multivitamin-mineral supplement can be a safe and inexpensive strategy to help ensure the nutritional health of older adults” to help prevent nutrition deficiencies common in many older adults when diet does not fully meet the nutritional needs of older adults.24 Although OAA nutrition programs can provide education and counseling to encourage the use of vitamin–mineral supplements, the provision of vitamin–mineral supplements is currently not a fundable OAA service. In older community-dwelling adults in Georgia participating in the OAA congregate meal program, a 4-month falls prevention education program that included information on dietary and supplemental calcium and vitamin D resulted in a significant increase in calcium- and vitamin D–rich foods and calcium- and vitamin D–containing supplements,25 although falls were not measured.
Previous fall intervention trials of vitamin D have been mixed,15–18 although individuals with low 25(OH)D concentrations at baseline appeared to have the greatest reduction in falls after vitamin D supplementation.17,18 Furthermore, results from a vitamin D and falls metaanalysis suggest that 25(OH)D concentrations of 24 ng/mL or greater are required for fall prevention.15 In contrast to the IOM’s report on DRIs for calcium and vitamin D, which concluded that 25(OH)D concentrations of 20 ng/mL or greater were sufficient for bone and overall health,26 a consensus statement on vitamin D for prevention of falls from the American Geriatrics Society concluded that a “25(OH)D concentration of 30 ng/mL should be a minimum goal for older adults, particular for frail adults, who are at higher risk for falls, injuries, and fractures.” 27 In the current pilot study, the majority of participants randomized to vitamin D3 achieved 25(OH)D concentrations of 30 ng/mL or greater, and those randomized to the vitamin D3 group had a lower rate of falls than the control group.
To maximize adherence to the vitamin D supplement, a monthly dose of 100,000 IU of vitamin D3 was used. This monthly dose is equivalent to a daily dose of 3,300 IU/d, which is above the IOM’s recommended dietary allowance of 800 IU/d for individuals aged 70 and older but below the tolerable upper intake of 4,000 IU/d.26 The American Geriatrics Society consensus statement on vitamin D for prevention of falls recommended 4,000 IU/d from all sources and that a supplementation schedule that would achieve the best adherence should be used because daily, weekly, and monthly doses of vitamin D3 are equally effective at achieving target 25(OH)D concentrations of 30 ng/mL.27 A recent randomized controlled trial using an annual high-dose 500,000 IU vitamin D3 showed greater risk of falls in those randomized to 500,000 IU vitamin D3 annually, with the greater risk of falls occurring in the 3-month period after the annual dose.28 In contrast, the current study showed that monthly vitamin D dosing at 100,000 IU, for a total dose of 500,000 IU over the 5 months, was effective in increasing 25(OH)D concentrations to 30 ng/mL or greater and reducing the rate of falls over 5 months. The annual 500,000 IU vitamin D3 dose28 may have upregulated 25-hydroxyvitamin D-24-hydroxylase, resulting in a decrease in blood and tissue levels of the active form of vitamin D (1,25-dihydroxyvitamin D).29 Thus, a vitamin D3 dosing regimen of at least monthly may be best to avoid adverse effects.
Important to the success of this vitamin D pilot intervention was gaining the support of the local Forsyth County Senior Services and the staff who initially approached MOW clients regarding the pilot study. MOW clients who were seen in person to be assessed or reassessed for MOW eligibility were more likely to agree to be contacted for the pilot study than those who were contacted by telephone. Barriers included client relocation (to a different MOW route or to a location not serviced by Forsyth County Senior Services) and disconnected phone lines when staff attempted to contact clients. Some clients were unwilling (e.g., distrust of academic medical centers/clinical research) or unable (e.g., impaired cognition) to sign the informed consent, and legally authorized representatives were unavailable to consent on their behalf. Some physicians discouraged clients from participating, and some clients or their caregivers thought participation would be too much of a burden. MOW meals were not delivered when clients had doctor or other appointments or short-term hospital stays or were out of town, but because staff were able to make up to three attempts to deliver the supplement within the same week, most clients received at least four of the five monthly doses.
This study has notable strengths and limitations. The study sample of homebound older adults was a high-risk population for vitamin D insufficiency and falls, although the response rate was low and thus may not be a true reflection of the average MOW client. As a pilot study, its small sample size was a limitation. Randomization was clustered according to MOW route so that all participants on a route received the same supplement to avoid contamination of groups, but due to small numbers of participants per route, it was not possible to include a random route effect in the statistical models. Open-label supplements were used, but the supplements were individually repackaged to allow for delivery of the monthly supplement dose and to keep participants blinded to randomization assignment. Delivery of a once-monthly vitamin D3 supplement was feasible, and the monthly dose of 100,000 IU was sufficient to increase 25(OH)D concentrations to 30 ng/mL or greater in a majority of participants randomized to vitamin D. Although a high monthly vitamin D dosing regimen was used, there were no cases of hypercalcemia at 5-month follow-up. There are seasonal variations in 25(OH)D concentrations, and thus, some of the observed increase in 25(OH)D concentrations may have been due to the follow-up visits occurring in the spring and early summer, but given the homebound status of these individuals, the seasonal effect would be expected to be minimal. Monthly fall calendars were used to ascertain falls, with more than 90% of individuals completing calendars for all months. Finally, participant adherence and retention were excellent.
In conclusion, this pilot study showed that delivering vitamin D supplements through the MOW program was feasible and improved 25(OH)D concentrations in homebound older adults. The rate of falls over 5 months of follow-up was lower in those randomized to vitamin D. Vitamin D supplementation delivered by a MOW program may help older homebound adults remain independent in the community by improving 25(OH)D concentrations and reducing falls and their consequences.
ACKNOWLEDGMENTS
We would like to thank the MOW staff at Senior Services, Inc., and the Forsyth County, North Carolina, MOW clients who participated in this research project.
Financial Disclosure: Supported by the Wake Forest Translational Science Institute and Center for Integrative Medicine and the Wake Forest University Pepper Center (NIH, NIA, P30-AG21332).
Sponsor’s role: None.
Footnotes
ClinicalTrials.gov Identifier: NCT01410084
Portions of this work were presented in abstract form at the American Society for Nutrition Annual Meeting/Experimental Biology, San Diego, California, April 23, 2012, and the American Geriatrics Society Annual Meeting, Seattle, Washington, May 4, 2012.
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Author Contributions: Houston, Tooze, Kritchevsky, Williamson: study concept and design. Houston, Tooze, Kritchevsky, Williamson: obtained funding. Demons, Davis: guidance on how to deliver the intervention. Demons: medical oversight for the intervention. Shertzer-Skinner: data collection, project management. Kearsley: oversight of MOW staff involved in project. Houston, Tooze: data analysis. Houston: drafting the manuscript. All authors: critical review for intellectual content.
REFERENCES
- 1.Tinetti ME. Clinical practice. Preventing falls in elderly persons. N Engl J Med. 2003;348:42–49. doi: 10.1056/NEJMcp020719. [DOI] [PubMed] [Google Scholar]
- 2.Kannus P, Sievanen H, Palvanen M, et al. Prevention of falls and consequent injuries in elderly people. Lancet. 2005;366:1885–1893. doi: 10.1016/S0140-6736(05)67604-0. [DOI] [PubMed] [Google Scholar]
- 3.Tinetti ME, Williams CS. Falls, injuries due to falls, and the risk of admission to a nursing home. N Engl J Med. 1997;337:1279–1284. doi: 10.1056/NEJM199710303371806. [DOI] [PubMed] [Google Scholar]
- 4.Li F, Fisher KJ, Harmer P, et al. Fear of falling in elderly persons: Association with falls, functional ability, and quality of life. J Gerontol B Psychol Sci Soc Sci. 2003;58B:283–290. doi: 10.1093/geronb/58.5.p283. [DOI] [PubMed] [Google Scholar]
- 5.Looker AC, Johnson CL, Lacher DA, et al. Vitamin D status: United States, 2001–2006. NCHS Data Brief. 2011:1–8. [PubMed] [Google Scholar]
- 6.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 7.Bailey RL, Dodd KW, Goldman JA, et al. Estimation of total usual calcium and vitamin D intakes in the United States. J Nutr. 2010;140:817–822. doi: 10.3945/jn.109.118539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein MS, Wark JD, Scherer SC, et al. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47:1195–1201. doi: 10.1111/j.1532-5415.1999.tb05199.x. [DOI] [PubMed] [Google Scholar]
- 9.Dhesi JK, Bearne LM, Moniz C, et al. Neuromuscular and psychomotor function in elderly subjects who fall and the relationship with vitamin D status. J Bone Miner Res. 2002;17:891–897. doi: 10.1359/jbmr.2002.17.5.891. [DOI] [PubMed] [Google Scholar]
- 10.Flicker L, Mead K, Macinnis RJ, et al. Serum vitamin D and falls in older women in residential care in Australia. J Am Geriatr Soc. 2003;51:1533–1538. doi: 10.1046/j.1532-5415.2003.51510.x. [DOI] [PubMed] [Google Scholar]
- 11.Sambrook PN, Chen JS, March LM, et al. Serum parathyroid hormone predicts time to fall independent of vitamin D status in a frail elderly population. J Clin Endocrinol Metab. 2004;89:1572–1576. doi: 10.1210/jc.2003-031782. [DOI] [PubMed] [Google Scholar]
- 12.Snijder MB, van Schoor NM, Pluijm SM, et al. Vitamin D status in relation to one-year risk of recurrent falling in older men and women. J Clin Endocrinol Metab. 2006;91:2980–2985. doi: 10.1210/jc.2006-0510. [DOI] [PubMed] [Google Scholar]
- 13.Faulkner KA, Cauley JA, Zmuda JM, et al. Higher 1,25-dihydroxyvitamin D3 concentrations associated with lower fall rates in older community-dwelling women. Osteoporos Int. 2006;17:1318–1328. doi: 10.1007/s00198-006-0071-8. [DOI] [PubMed] [Google Scholar]
- 14.Pramyothin P, Techasurungkul S, Lin J, et al. Vitamin D status and falls, frailty, and fractures among postmenopausal Japanese women living in Hawaii. Osteoporos Int. 2009;20:1955–1962. doi: 10.1007/s00198-009-0910-5. [DOI] [PubMed] [Google Scholar]
- 15.Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: A meta-analysis of randomised controlled trials. BMJ. 2009;339:b3692. doi: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalyani RR, Stein B, Valiyil R, et al. Vitamin D treatment for the prevention of falls in older adults: Systematic review and meta-analysis. J Am Geriatr Soc. 2010;58:1299–1310. doi: 10.1111/j.1532-5415.2010.02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;9:CD007146. doi: 10.1002/14651858.CD007146.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murad MH, Elamin KB, Abu Elnour NO, et al. Clinical review: The effect of vitamin D on falls: A systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:2997–3006. doi: 10.1210/jc.2011-1193. [DOI] [PubMed] [Google Scholar]
- 19.Rejeski WJ, Ip EH, Marsh AP, et al. Measuring disability in older adults: The International Classification System of Functioning, Disability and Health (ICF) framework. Geriatr Gerontol Int. 2008;8:48–54. doi: 10.1111/j.1447-0594.2008.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharkey JR, Branch LG, Zohoori N, et al. Inadequate nutrient intakes among homebound elderly and their correlation with individual characteristics and health-related factors. Am J Clin Nutr. 2002;76:1435–1445. doi: 10.1093/ajcn/76.6.1435. [DOI] [PubMed] [Google Scholar]
- 21.Sharkey JR, Giuliani C, Haines PS, et al. Summary measure of dietary musculoskeletal nutrient (calcium, vitamin D, magnesium, and phosphorus) intakes is associated with lower-extremity physical performance in homebound elderly men and women. Am J Clin Nutr. 2003;77:847–856. doi: 10.1093/ajcn/77.4.847. [DOI] [PubMed] [Google Scholar]
- 22.Johnson MA, Fischer JG, Park S. Vitamin D deficiency and insufficiency in the Georgia Older Americans Nutrition Program. J Nutr Elder. 2008;27:29–46. doi: 10.1080/01639360802059704. [DOI] [PubMed] [Google Scholar]
- 23.Zhu H, An R. Impact of home-delivered meal programs on diet and nutrition among older adults: A review. Nutr Health. 2013;22:89–103. doi: 10.1177/0260106014537146. [DOI] [PubMed] [Google Scholar]
- 24. Older Americans Act reauthorization amendment. H.R. 6197. 2006. 2014. 8–4-0014 [on-line]. Available at: http://www.i4ainfo.org/OAA_2006_reauth_HR6197.pdf Accessed August 4, 2014.
- 25.Teems J, Hausman DB, Fischer JG, et al. Older adults attending Georgia senior centers increase preventive behaviors for falls and fractures following a community-based intervention. J Nutr Gerontol Geriatr. 2011;30:72–85. doi: 10.1080/01639366.2011.545042. [DOI] [PubMed] [Google Scholar]
- 26.Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board . Dietary Reference Intakes for Calcium and Vitamin D. National Academies Press; Washington, DC: 2011. [Google Scholar]
- 27.Recommendations abstracted from the American Geriatrics Society consensus statement on vitamin D for prevention of falls and their consequences. J Am Geriatr Soc. 2014;62:147–152. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 28.Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA. 2010;303:1815–1822. doi: 10.1001/jama.2010.594. [DOI] [PubMed] [Google Scholar]
- 29.Dawson-Hughes B, Harris SS. High-dose vitamin D supplementation: Too much of a good thing? JAMA. 2010;303:1861–1862. doi: 10.1001/jama.2010.598. [DOI] [PubMed] [Google Scholar]