Abstract
We share our experience of 2000 spinal tuberculosis (TB) cases, including both typical and atypical presentations. The aim of the study is to estimate the incidence and types of spinal TB referred to our department for diagnosis. 2000 patients were selected by convenience sampling from January 2006 to September 2010. Study design was descriptive and among 2000 mostly unknown cases without evidence of symptoms of systemic TB (1080 males and 920 females). MRI without and with IV contrast, CECT with MPR, and in some cases not fit for MRI, CT myelography, were performed. Out of 2000 cases of tuberculous spine, 1080 (54%) were male and 920 (46%) were female. Their age ranged from 8–60 years. About 90% of patients were below the age of 40 years. Peak age among the males and females was 20–29 years and 14–35 years, respectively. The most common site of involvement was dorsal spine (45%) followed by lumbo-sacral spine (33%), cervical spine (10%) and at multiple levels (12%). Biopsies were done in 240 (12%) cases. Spinal TB should always be suspected when radiographs demonstrate a destructive spinal process. Awareness and prompt management of TB spine will help in reducing the continuing morbidity of this disease.
Keywords: Spine, TB
Introduction
Infection of the spine is a major category of spinal disease that is difficult to differentiate clinically from degenerative disease, non-inflammatory lesions, and spinal neoplasm.1 Granulomatous infections are still a frequent cause of infective spondylitis in many parts of the world. Tuberculosis (TB) is a very common disease in developing countries and has been found to affect almost all parts of the body. Spinal TB is most common in Pakistan. Spinal TB is a destructive form of tuberculosis. It accounts for approximately half of all cases of musculoskeletal TB. Spinal TB is more common in children and young adults. The incidence of spinal TB is increasing in developed nations. Early diagnosis and prompt treatment are essential to prevent permanent neurological deficit and/or spinal deformity.
In the diagnosis of spinal infections and their sequelae, magnetic resonance imaging (MRI) has become an established imaging technique.2-5 In high-contrast resolution, direct multiplanar imaging, the usefulness in detecting marrow infiltration and the ease by which intra-dural disease can be assessed are definite advantages.
Neuroimaging thus becomes one of the most important initial investigations, offering prompt and conclusive diagnosis and allowing treatment to be given at an earlier stage. The majority of the previous studies have evaluated neuroimaging features in a case study or case series. The aim of this study is to portray the different features and presentation of spinal TB.
Subject and methods
A total of 2000 patients were selected by convenience sampling from January 2006 to September 2010. Study design was descriptive and among 2000 mostly unknown cases without evidence of symptoms of systemic TB (1080 males and 920 females). MRI without and with IV contrast, contrast-enhanced computed tomography (CT) with multiplanar reconstruction, and in some cases not fit for MRI, CT myelography, were performed. In a few selected patients’ cases, MR spectrograms were also obtained to rule out the possibility of metastasis. MRI remained the choice of imaging and was performed on 1.5 Tesla Phillips MRI at Lahore General Hospital, PGMI, Lahore, Pakistan. Histopathological biopsies were available in 240 cases.
Results
Out of 2000 cases of tuberculous spine, 1080 (54%) were male and 920 (46%) were female. Their age ranged from 8–60 years. About 90% of patients were below the age of 40 years. Peak age among the males and females was 20–29 years and 14–35 years, respectively. The most common site of involvement was dorsal spine (45%), followed by lumbo-sacral spine (33%), cervical spine (10%) and at multiple levels (12%). Biopsies were done in 240 (12%) cases. On MRI, vertebral end plate destruction and reduced disc space were the commonest sequelae in all the biopsied cases (100%). Among those at multiple levels, all 240 cases (100%) showed the involvement of dorsal spine. Some 88% of typical cases were further classified according to type of presentation: central (22%), marginal (71%) and peripheral (7%). The incidence of atypical cases was 12%.
Discussion
TB remains endemic in most developing countries. However, a resurgence of TB, with a rising incidence of spinal TB, has also been reported in recent decades in developed countries, mainly in immigrants from countries with a high prevalence and in patients with immunodeficiency (HIV and AIDS) or other underlying chronic diseases. In addition, numerous sociological factors such as poor nutritional status, poor living facilities, and alcohol and drug abuse have contributed the re-emergence of TB. TB involves both pulmonary and extra-pulmonary sites. Skeletal involvement has been reported to occur in 1–5% of all patients with TB.6 The exact incidence and prevalence of spinal TB in most parts of the world are not known. Approximately 10% of patients with extra-pulmonary TB have skeletal involvement. The vertebral column is the most common site of osseous involvement, comprising in most series about 50% cases of skeletal TB.7
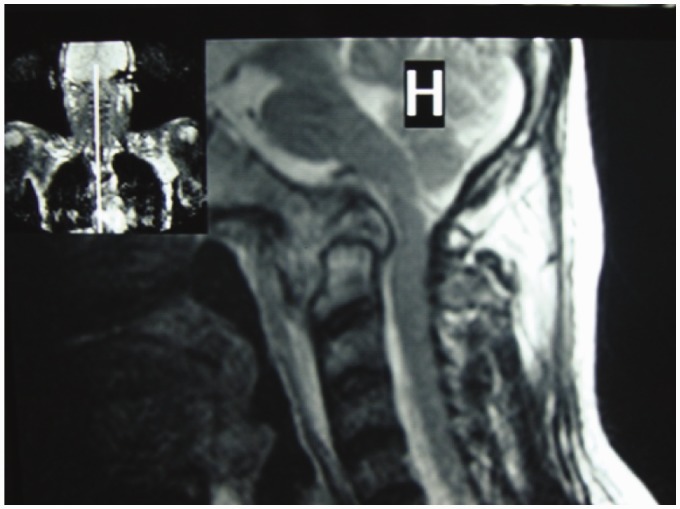
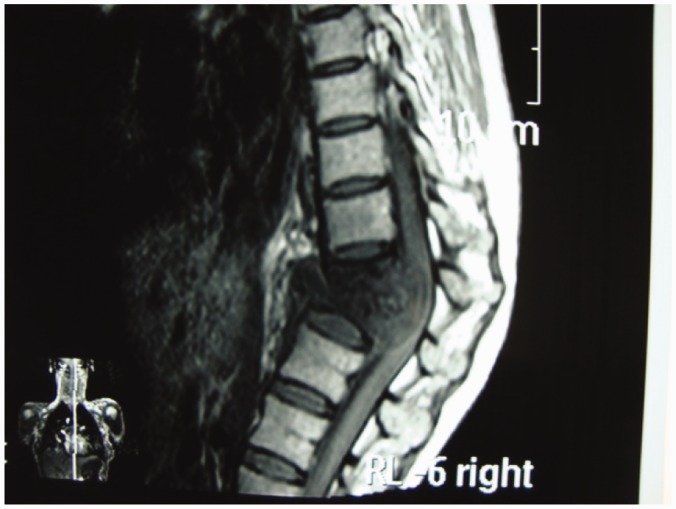
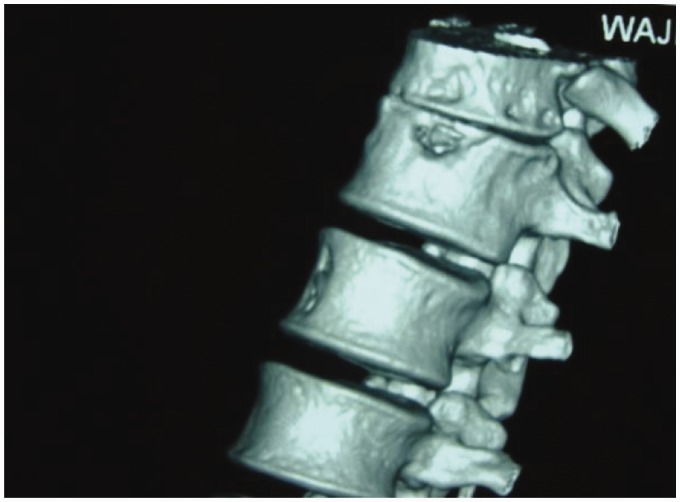
The male-to-female ratio is 1:1, with no gender predilection. Vertebral TB is most often found in the lower thoracic and upper lumbar regions. Cervical and sacral involvement is not uncommon (Figure 1). Two distinct patterns of vertebral osteomyelitis have been reported: typical classic spondylodiscitis and atypical spondylitis without disc involvement.8
Figure 1.
Cranio-cervical junction TB.
Common clinical manifestations include constitutional symptoms, back pain, spinal tenderness, paraplegia and spinal deformities. In our study most of the patients presented with chronic back pain with focal tenderness. Some of the young patients also presented with kyphosis/gibbus formation, reflecting gradual, insidious onset of the disease.
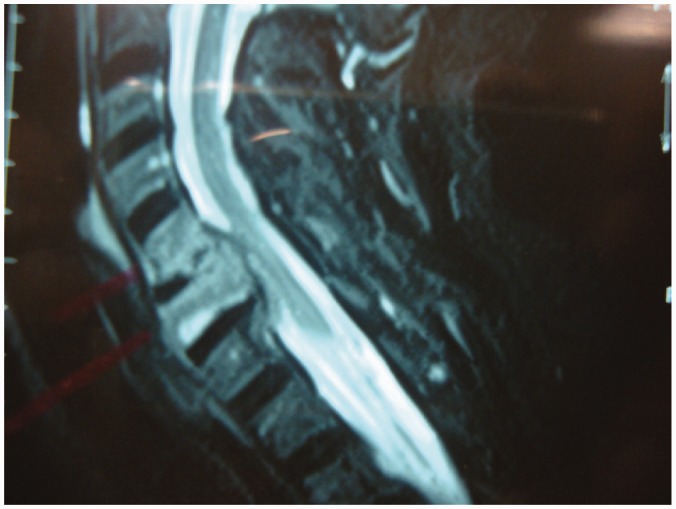
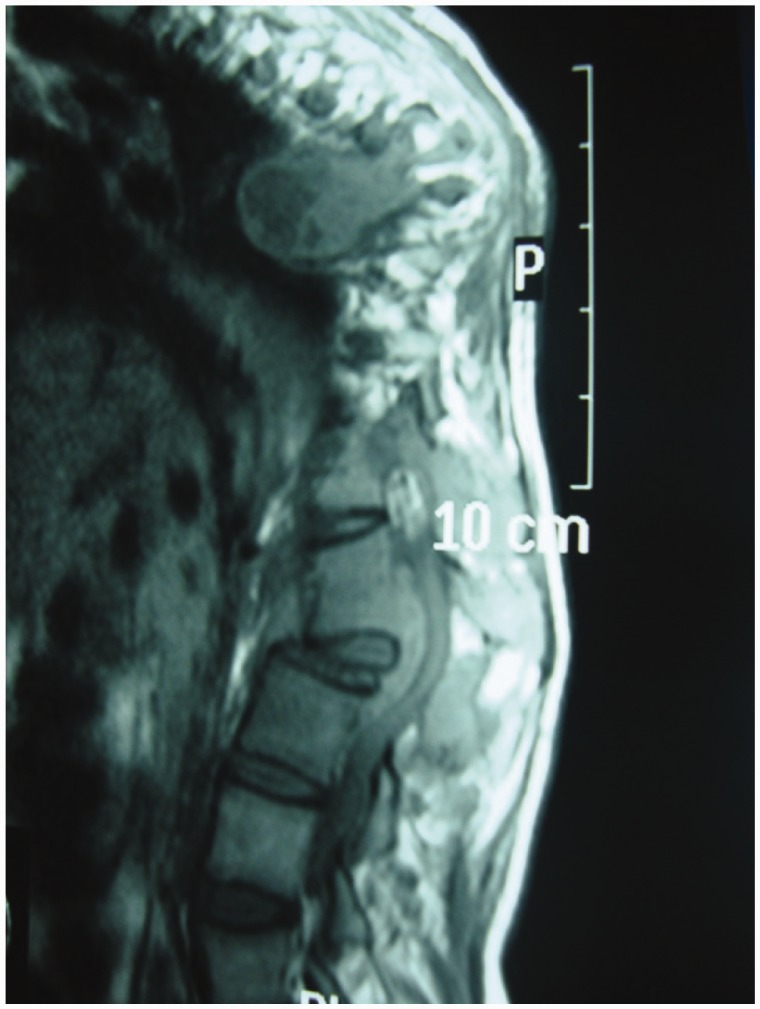
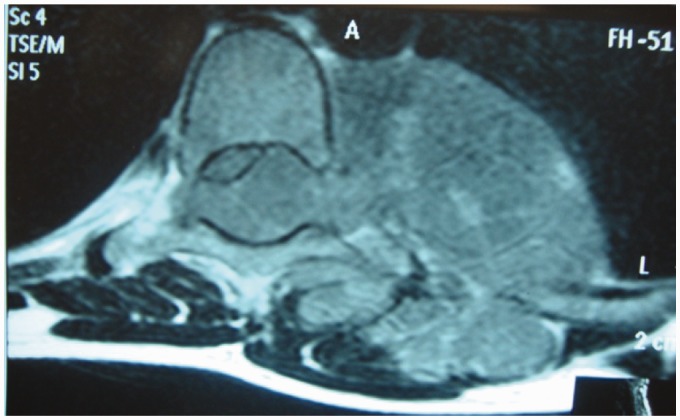
Spinal TB is initially apparent in the anterior inferior portion of the vertebral body (Figure 2). Later on it spreads into the central part of the body or disc. Paradiscal, anterior, and central lesions are the common types of vertebral involvement.9
Figure 2.
Posterior end plates TB with cord compression.
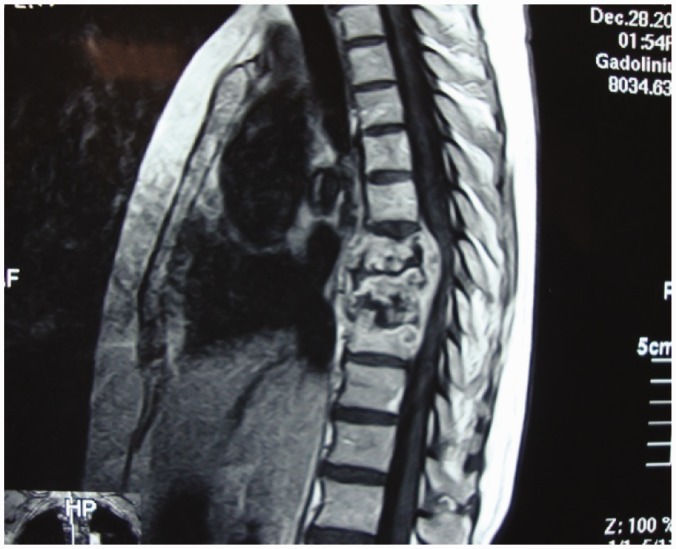
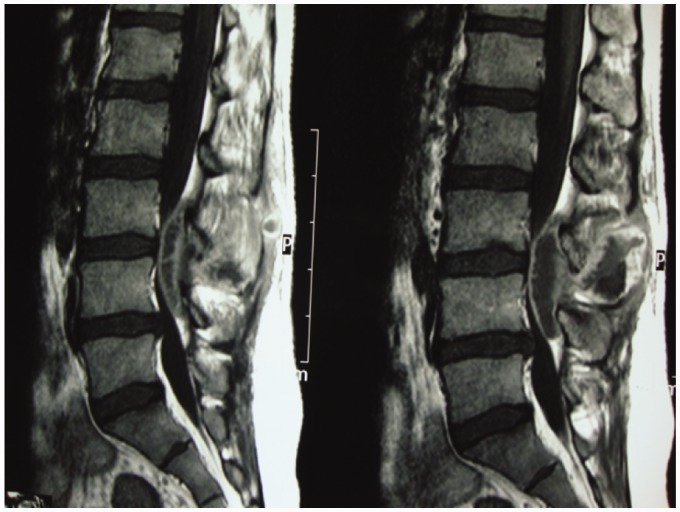
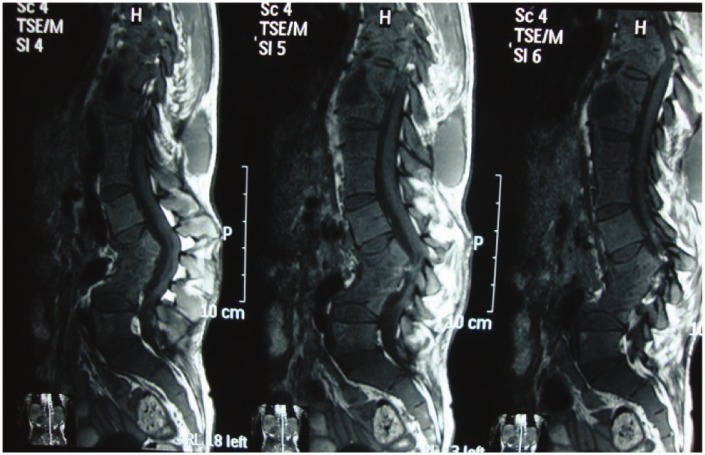
Advances in diagnostic imaging have led to the successful treatment of spinal TB. Multiplanar capability and superior tissue contrast make MR imaging the modality of first choice in the evaluation and follow-up of spondylodiscitis (Figure 3).
Figure 3.
Follow-up case with IV contrast: subligamentous healed TB.
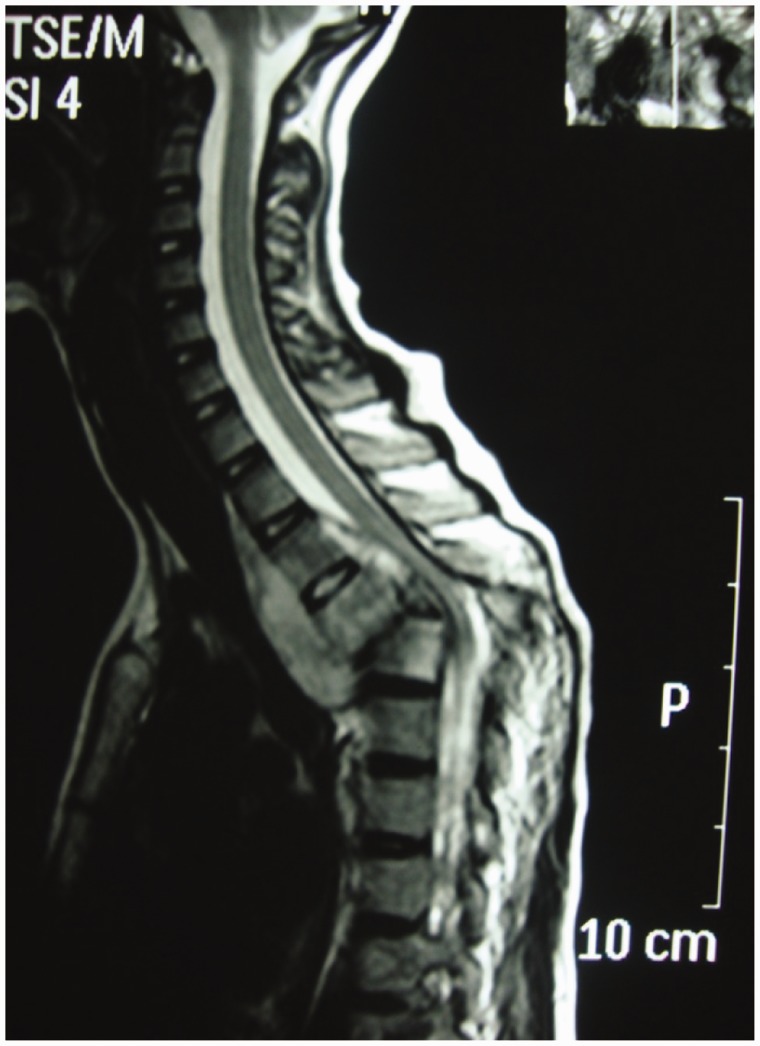
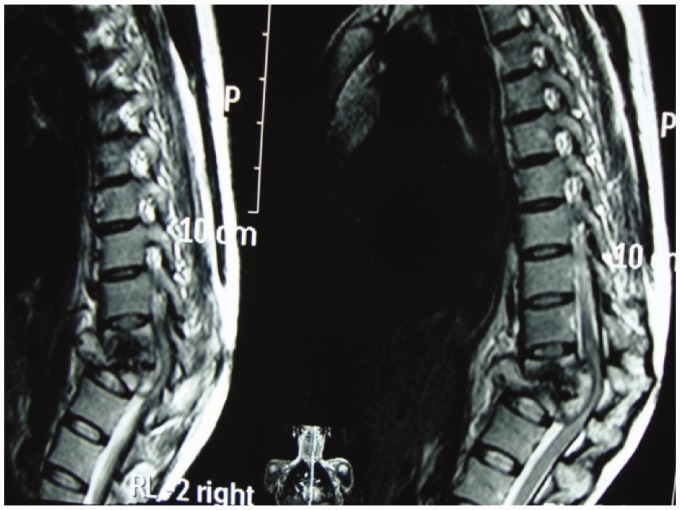
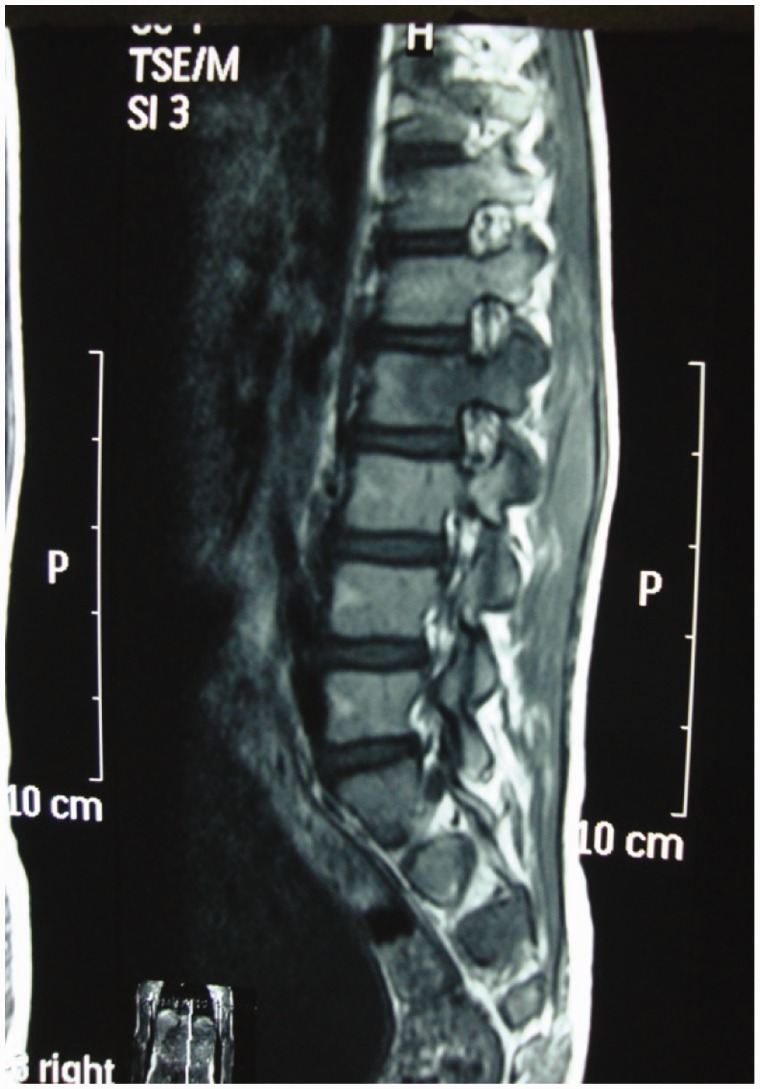
A major advantage of MR imaging, compared with CT scan and plain radiography, is the higher sensitivity for the detection of the early inflammatory bone marrow changes and infiltrative end plate changes in the vertebra.8 MRI frequently demonstrates involvement of the vertebral bodies on either side of the disc, disc destruction, cold abscess, vertebral collapse and presence of vertebral column deformities (Figure 4). T1W images usually show decreased signal from the affected vertebral marrow, reduced disc height, morphologic alteration of paraspinal soft tissues, and epidural extension (Figure 5).
Figure 4.
Gibbus formation.
Figure 5.
Sagittal T1W MR image shows decreased signal from the affected vertebral marrow and epidural extension.
On T2W images, an indiscriminate increase in signal is noted from the vertebrae, discs, and soft tissues (Figure 3). Enhanced MR studies are particularly useful for characterizing tuberculous spondylitis. Rim enhancement around intra-osseous and para spinal soft tissue abscess had not been demonstrated in other spinal infection (Figures 6 and 7).
Figure 6.
Paravertebral and epidural extension.
Figure 7.
Sagittal T1W enhanced MR image shows dorsal epidural and intraspinous tuberculous abscess.
Epidural extension and meningeal involvement are seen to better advantage on enhanced examination. MRI is also useful in detecting intramedullary or extramedullary tuberculoma, spinal cord cavitation, spinal cord edema, and possibly unsuspected non-contiguous lesions of the spine.2,3,10,11 The subligamentous spread of a paraspinal mass and the involvement of multiple contiguous bones and intramedullary spinal changes can be very well demonstrated by MRI.4
Neuroimaging-guided needle biopsy from the affected site in the center of the vertebral body is the gold standard technique for early histopathological diagnosis.5 With early diagnosis and early treatment, prognosis is generally good.9
Typical spinal TB can be diagnosed easily and treated successfully (Figure 8). However, spinal TB with atypical clinical and radiographic findings can result in a delayed diagnosis.12 Several different features of atypical radiological presentation of TB have been described and reported.13,14 Atypical features described are (1) involvement of spinal posterior element with sparing of anterior column; (2) skipped lesions; and (3) neural component compression as a result of tuberculosis granuloma.15
Figure 8.
Typical TB.
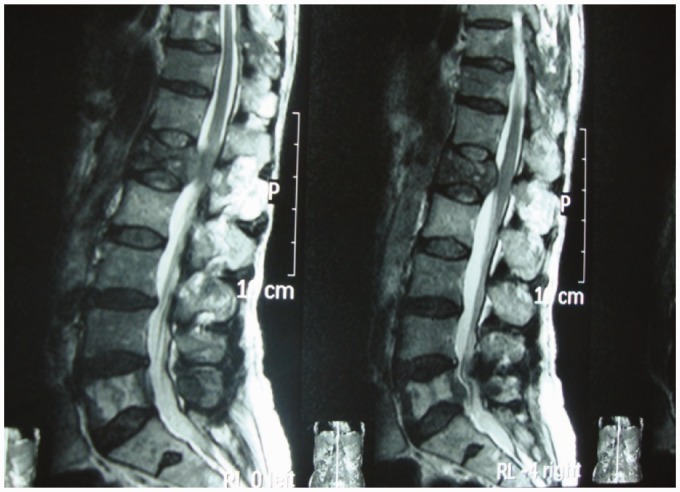
The most common site of involvement of the posterior element of the spine is the pedicle.16 These posterior lesions need to be distinguished from the metastatic lesions (Figures 9 and 10). The posterior spinal element, specifically pedicle involvement, is generally not a characteristic feature of spinal TB. In one study, pedicle involvement was noted in an unusually high (65%) number of patients. In this study the highest involvement was at the thoracic level. The mean vertebral body, disc collapse, prevertebral abscess, and kyphosis were more severe in the group with pedicle involvement.17
Figure 9.
Pedicle involvement.
Figure 10.
Paravertebral tuberculous soft tissue mass eroding adjacent rib.
Multi-level non-contiguous spinal TB is an atypical form of spinal TB that affects two non-contiguous vertebrae without destruction of the adjacent vertebral bodies and intervertebral discs. Current research indicates that the incidence of multiple-level non-contiguous vertebral TB is 1.1–16%.18 In our study, in 240 cases (12%), the involved lesions were multiple and skipped, which led to spinal cord compression as a result of an epidural mass and co-existing spondylosis (Figure 11). Our incidence of non-contiguous spinal TB is 12%, less than that reported in the literature (Pandit et al.19 (15%) and Polley and Dunn2 (16.3%)).
Figure 11.
Multi-level lesions.
However, Kalia et al.18 had reported that with whole-spinal MRI the incidence of multi-level non-contiguous spinal tuberculosis reaches as high as 71.4%, and a large proportion of patients with affected non-contiguous vertebral sites were asymptomatic. In this retrospective analysis, patients were included if spinal infection was identified by whole-spine MRI and confirmed as TB by a combination of histology and microbiology.18 In another study, 16 cases of non-contiguous spinal TB were identified from a single surgeon series of 98 patients.2
CT is of great importance in demonstrating small, early foci of bone infection and extension of the bone and soft tissue involvement. End plate destruction, fragmentation of the vertebrae, and paravertebral calcification are adequately demonstrated.8 CT-guided fine needle aspiration has become widely accepted for both culture and histological diagnosis.8 The pattern of bone destruction is reported as fragmentary in 47% of cases, osteolytic in 34%, localized and sclerotic in 10%, and subperiosteal in 30% of cases. Other findings include soft tissue involvement and paraspinal tissue abscess.20 In most of our cases, the pattern of bone destruction was fragmentary in 48%, osteolytic in 34%, localized and sclerotic in 10%, and subperiosteal in 27% cases, similar to these findings (Figure 12).
Figure 12.
Marginal TB.
In the central lesion, the disc is not involved, and collapse of the vertebral body produces vertebra plana. Vertebra plana indicates complete compression of the vertebral body mimicking compression fracture and in elderly/middle-aged patients as metastatic collapse.9
In our study, we encountered two cases of vertebral collapse without involvement of discs simulating mitotic compression fracture (Figure 13).
Figure 13.
TB simulating metastases.
Magnetic resonance spectroscopy (MRS) was carried out in addition to MRI to evaluate its role. MRS findings were of granulomatous infection as increased lipid/lactate peaks and the diagnosis of spinal TB was made.
Spinal TB should be considered in the differential diagnosis of chronic back pain (with or without constitutional, neurological, or musculoskeletal manifestations) and in young persons. Several spinal diseases need be differentiated from spinal TB.9 Common differential diagnosis includes granulomatous diseases, traumatic and osteoporotic fractures, and primary and metastatic neoplasms which may have features comparable with those of vertebral TB.
Conclusion
Young males belonging to the age group 20–29 years are more affected, and the most common site is dorsal spine (45%). MRI is an excellent tool to investigate the diseases of spine, particularly tuberculous spine. However, an atypical radiological presentation of spinal TB presents a challenge for an appropriate diagnosis and early treatment, due to atypical clinical and radiological features. Spinal TB should always be suspected when radiographs demonstrate a destructive spinal process. Awareness and prompt management of TB spine will help in reducing the continuing morbidity of this disease.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Sharif HS. Role of MR imaging in the management of spinal infection. AJR 1992; 158: 1333–1341. [DOI] [PubMed] [Google Scholar]
- 2.Polley P, Dunn R. Noncontiguous spinal tuberculosis: Incidence and management. Eur Spine J 2009; 18: 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung NY, Jee WH, Ha KY, et al. Discrimination of tuberculous spondylitis from pyogenic spondylitis on MRI. Am J Roentgenol 2004; 182: 1405–1410. [DOI] [PubMed] [Google Scholar]
- 4.Oguz E, Sehirlioglu A, Altinmakas M, et al. A new classification and guide for surgical treatment of spinal tuberculosis. Int Orthop 2008; 32: 127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jain R, Sawhney S, Berry M. Computed tomography of tuberculosis: Patterns of bone destruction. Clin Radiol 1993; 47: 196–199. [DOI] [PubMed] [Google Scholar]
- 6.Pertuiset E, Beaudreuil J, Horusitzky A, et al. Epidemiological aspects of osteoarticular tuberculosis in adults – Retrospective study of 206 cases diagnosed in Paris area from 1980 to 1994. Press Med 1997; 26: 311–315. [PubMed] [Google Scholar]
- 7.Moore SL, Rafii M. Imaging of musculoskeletal and spinal tuberculosis. Radiol Clin North Am 2001; 39: 329–342. [DOI] [PubMed] [Google Scholar]
- 8.De Becker AI, Mortele KJ, Vanschoubroeck IJ, et al. Tuberculosis of the spine: CT and MR imaging features. JBR - BTR 2005; 88: 92–77. [PubMed] [Google Scholar]
- 9.Garg RK, Somvanshi DS. Spinal tuberculosis: A review. J Spinal Cord Med 2011; 34: 440–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shanley DJ. Tuberculosis of the spine: Imaging features. Am J Roentgenol 1995; 164: 659–664. [DOI] [PubMed] [Google Scholar]
- 11.Moorthy S, Prabhu NK. Spectrum of MR imaging findings in spinal tuberculosis. Am J Roentgenol 2002; 179: 979–983. [DOI] [PubMed] [Google Scholar]
- 12.Jahng J, Kim YH, Lee KS. Tuberculosis of the lower lumbar spine with atypical radiological presentation – a case mimicking a malignancy. Asian Spine J 2007; 1: 102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmadi J, Bajaj A, Destian S, et al. Spinal tuberculosis: Atypical observations at MR imaging. Radiology 1993; 189: 489–493. [DOI] [PubMed] [Google Scholar]
- 14.Postacchini F, Montanaro A. Tuberculous epidural granuloma simulating a herniated lumbar disk: A report of a case. Clin Orthop Relat Res 1980; 148: 182–185. [PubMed] [Google Scholar]
- 15.Pande KC, Pande SK, Babhulkar SS. An atypical presentation of tuberculosis of the spine. Spinal Cord 1996; 34: 716–719. [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa K, Murata H, Naitoh K, et al. Spinal tuberculosis: Report of an atypical presentation. Clin Orthop Relat Res 2002; 403: 100–103. [PubMed] [Google Scholar]
- 17.Yusof MI, Hassan E, Rahmat N, et al. Spinal tuberculosis: The association between pedicle involvement and anterior column damage and kyphotic deformity. Spine (Phila PA 1976) 2009; 34: 713–717. [DOI] [PubMed] [Google Scholar]
- 18.Kaila R, Malhi AM, Mahmood B, et al. The incidence of multiple level noncontiguous vertebral tuberculosis detected using whole spine MRI. J Spinal Disord Tech 2007; 20: 78–81. [DOI] [PubMed] [Google Scholar]
- 19.Pandit HG, Sonsale PD, Shikare SS, et al. Bone scintigraphy in tuberculous spondylodiscitis. Eur Spine J 1999; 8: 205–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perronne C, Saba J, Behloul Z. Pyogenic and tuberculosis spondylodiskitis (vertebral osteomyelitis) in 80 adult patients. Clin Infect Dis 1994; 19: 746–750. [DOI] [PubMed] [Google Scholar]