Abstract
Once tumor cells metastasize to the bone, the prognosis for prostate cancer patients is generally very poor. The mechanisms involved in bone metastasis, however, remain elusive, because of lack of relevant animal models. In this manuscript, we describe step-by-step protocols for the xenograft mouse models that are currently used for studying prostate cancer bone metastasis. The different routes of tumor inoculation (intraosseous, intracardiac, intravenous and orthotopic) presented are useful for exploring the biology of bone metastasis.
Introduction
Prostate cancer is the second leading cause of cancer death in men in the US. Because of the recent improvement of treatment strategies for localized disease, a 5-year overall survival rate of over 90% has been achieved in prostate cancer patients (http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-survival-rates). However, once cells spread to specific secondary organs such as bone, the 5-year survival rate of prostate cancer patients significantly decreases to 28% (http://www.cancer.org/cancer/prostatecancer/detailedguide/prostate-cancer-survival-rates). Aside from the poor prognosis, bone metastasis significantly impairs the quality of life of prostate cancer patients, as it is associated with myelophthisis, hypercalcemia, bone pain, fracture and/or nerve compression. Therefore, there is an urgent need to understand the biology of bone metastasis in order to fight against this complex disease and its painful consequences.
Prostate cancer is known as a heterogeneous tumor.1 For instance, some prostate cancers, when they disseminate to the bone, present both osteolytic and osteoblastic bone lesions, although bone metastasis from prostate cancer is classically classified as osteoblastic. In addition to tumor heterogeneity, bone marrow also contains heterogeneous populations, including hematopoietic lineage cells and cells regulating bone remodeling. The interaction between these bone marrow cells and cancer cells is thought to be important for cancer progression.2 This heterogeneous and complex organization makes revealing the mysterious bone metastatic properties of prostate cancer much more difficult.
Metastasis involves multiple steps. First, tumor cells induce neovascularization (angiogenesis), acquire an invasive phenotype (epithelial mesenchymal transition), invade surrounding tissues (invasion), move into the circulation through the basal membrane (intravasation), travel throughout the body (circulation), and then adhere to a specific endothelium and disseminate into a secondary organ (extravasation). Once there, these disseminated tumor cells adapt to this new environment (colonization), survive for a long time without growing (dormancy), and eventually regrow (metastatic growth or recurrence). As this extremely complicated metastatic process is impossible to recreate with an in vitro or ex vivo approach, and experiments with humans are not reasonable, we need to rely on a pre-clinical approach with animal models to study bone metastasis. However, to date, there has been no ideal animal model that allows us to follow all these steps simultaneously.
Recently, in vivo xenograft mouse models have been developed that involve intraosseous, intracardiac, intravenous and orthotopic injections, which provide a means to better study prostate cancer bone metastasis. In this manuscript, we will provide details of the methods used in these animal models.
Xenograft mouse models for studying prostate cancer bone metastasis
Murine xenograft models are commonly used for experimental bone metastasis. Tumor growth in marrow is achieved through injection of human cancer cell lines via different routes.
Preparation before prostate cancer cell inoculation
Preparation of prostate cancer cell suspension
Materials
Prostate cancer cell lines.
Tissue culture flasks or dishes.
Appropriate antibiotics (for example, penicillin–streptomycin).
Culture medium supplemented with 10% fetal bovine serum (FBS) and antibiotics.
0.05% trypsin-EDTA.
Sterile 1 × phosphate-buffered saline (PBS, pH=7.2-7.4).
70 μm cell strainer.
15 ml and 50 ml sterile conical tubes.
0.4% trypan blue.
Hemacytometer or automated cell counter.
Ca2+-free and Mg2+-free Hanks' Balanced Salt Solution with Phenol Red Indicator (HBSS).
Procedures
One day prior to inoculation, passage prostate cancer cells so they will be 70–80% confluent on the day of the experiment. This ensures that cells are in the exponential growth phase, as recommended for optimal tumor onset *NOTE: prepare approximately 2–3 times more cells than you anticipate needing.*NOTE: slow growing cells (for example, LNCaP, LNCaP C4-2B, VCaP) may need to be passaged 3–4 days earlier.
Trypsinize the cells, quench the trypsin activity by adding a sufficient volume of media containing FBS and wash one or two times with 1 × PBS.
Filter cells through a 70 μm cell strainer to exclude large cell aggregates.
Count the total live and dead cell number and determine the viability of the cells with trypan blue by using hemacytometer or automated cell counter.*NOTE: the viability of cells should be ⩾90% prior to injection.
Centrifuge the cells in a swinging bucket centrifuge at 100–400 g for 3 min.
Carefully remove the media without disturbing the cell pellet.
Re-suspend cells in an appropriate volume of HBSS.
Transport cells to surgical room on ice.
Draw the prepared cell suspensions into the syringe and push out any air bubbles that may exist.
*NOTE: the number of cells for injection depends on the metastatic ability of the cell line and the experimental model. Usually, osteolytic prostate cancer cells require fewer cells to grow within the marrow (for example, 2500–50 000 cells), whereas osteoinductive prostate cancer cells need more cells (for example, 500 000 cells). Recommended cell number and injection volume for different inoculation models are as follows:
Intraosseous inoculation: 2500 cells—5 × 105 cells per 5–10 μl.
Intracardiac inoculation: 1–5 × 105 cells per 100 μl.
Intravenous inoculation: 1–5 × 106 cells per 100 μl.
Orthotopic inoculation: 1–5 × 104 cells per 10 μl.
*NOTE: set up the experiment keeping time from harvest to injection at a minimum, to assure that the cells stay alive throughout the injection process. The viability of cells will decline with time. At the end of an injection experiment, the cell viability should be above 80%.
Preparation for surgery
Materials
Animals: depending on the prostate cancer cell line to be inoculated, immunodeficient mice (BALB/c nu/nu, CB17 SCID) are used. Animals should be between 4 and 8 weeks of age.
Isoflurane
Ketamine
Xylazine
Eye ointment (vitamin A)
Shaver or chemical hair remover
Warming pad
Procedures
Set up the laminar flow with all necessary equipment and materials (Figures 1a and b)*NOTE: surgery should be conducted under aseptic conditions and use sterile instruments, supplies and wound closure materials within a designated surgical room, and following procedures approved by the facility department of animal care oversight.
- Anesthetize animals if necessary.
- This anesthesia should be performed only if equipped with a proper ventilated area. Two percent isoflurane mixed with 98% oxygen in the induction chamber. Mice should be kept under anesthesia for the duration of the surgery by being placed on their back and their head (nose) inserted into the breathing tube.
- Alternatively, xylazine–ketamine cocktail (Ketamine (100 mg ml−1): Xylazine (20 mg ml−1): 1× PBS=3:2:5) can be administered 300 μl per 1 kg body weight intraperitoneally (i.p.)
Monitor animals until fully anesthetized (loss of reflexes) and place them on a warming pad.*NOTE: prior to surgery, depth of anesthesia should be ensured by gently pinching the hind paw or tail tip. Mice should be unresponsive. If there is a response (movement) wait longer or (in the case of ketamine injection) administer another small dose. Please consult with your local authority, as the regulation may vary.
Apply ophthalmic ointment and remove the hair if necessary.
Place the animal in an appropriate position (for example, supine or prone) on a heated operation table within the laminar flow.
Disinfect the surgical area with 70% ethanol.
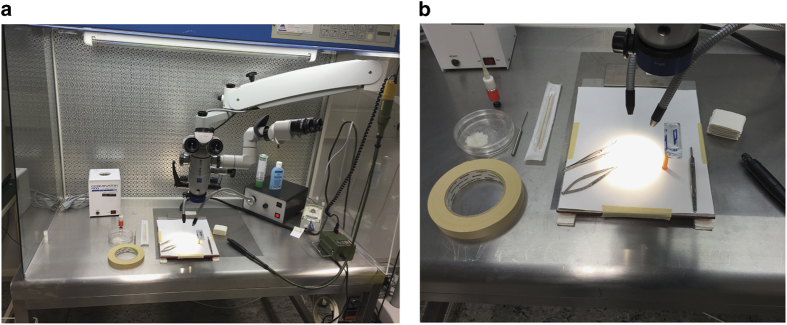
Figure 1.
An example images for sterile surgical suite. (a, b) laminar flow with mounted microscope, heated operation table and necessary equipment.
Prostate cancer cell inoculation routes
Intraosseous inoculation (Written by JH)
Materials
Sterile 1 × PBS.
Dental drill (power supply and drill, www.proxxon.com) and dental needles (Hedstroem files 28 mm size 30).
Surgical scissors, tweezers, scalpel and blade.
Cotton swabs.
70% ethanol gauze.
1 ml syringes with a 27G needle.
0.5 or 1 ml insulin syringes (30G, ½ inch).
Surgical bone wax (Ethicon, Inc., Somerville, NJ, USA), capillary pistons (CP25, Gilson Microman, Gilson, Inc., Middleton, WI, USA) and spatula (Figure 2a).
Suturing thread.
Betadine, Purdue Pharma, Stamford, CT, USA.
Faxitron radiography system (MX-20, Faxitron, Tucson, AZ, USA).
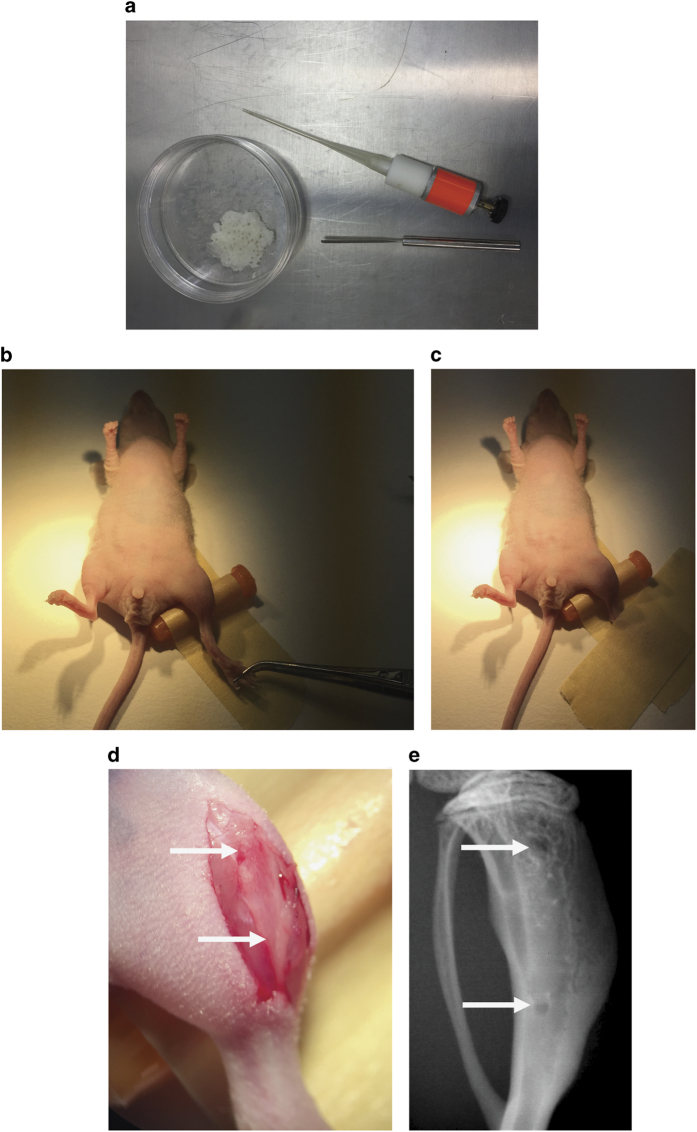
Figure 2.
Intraosseous inoculation of prostate cancer cells. (a) Capillary piston, bone wax and spatula. (b, c) Fixation of hind limb. Gently grab the hind limb of the animal with a tweezer to avoid damaging to a mouse foot and tape it to the operation table. (d) Skin incision (white arrows point to the position of holes to be drilled). (e) Radiograph of tibia after intraosseous inoculation (white arrows point to the drilled holes).
Procedures
Intratibial injection (Method 1)
Place the animal in supine position on a heated operation table within the laminar flow (administer anesthesia as indicated above).
Disinfect the hind limb with 70% ethanol.
Flex the knee at about 90 angle.
Insert a 27G needle into the joint surface through the patellar tendon and tibial plateau in order to enter the intramedullary canal of the tibia.*NOTE: the needle should be inserted with a drilling movement. Alternatively, an incision over the patellar tendon, followed by a longitudinal arthrotomy along the medial border of the patellar tendon, can be performed to expose the tibial plateau.
Inject the cancer cell suspension with a 30G needle via the proximal hole (10 μl maximal volume, but less volume would be ideal).
Inject an appropriate analgesic and monitor mice until they fully recover.
*NOTE: alternatively to this method, a needle can be used to drill a hole proximal to the tibial tuberosity. After penetration of the cortical bone, the cancer cell suspension can be injected.
*NOTE: bone marrow ablation creates space for the injection, and drilled holes can be sealed with bone wax preventing cancer cells to migrate out of the medullary cavity. (please see Method 2).
Intraosseous inoculation (Method 2)
Place the animal in supine position on a heated operation table within the laminar flow (administer anesthesia as indicated above).*NOTE: tape the animal's hind limb to the operation table (if the leg is tape-fixed over a needle cap with a rotation to the inner side, drilling of the holes is facilitated) (Figures 2b and c).
Disinfect the hind limb with 70% ethanol.
Make a skin incision with a scalpel starting slightly below the knee joint to the end of the tibial bone marrow cavity (Figure 2d).*NOTE: take care to not damage the tibial vein or artery.
Carefully resect the caput mediale of the gastrocnemius along the anterior tibial margin, and then continue to resect its insertion on the medial surface of the tibia to uncover the underlying bone.
Constantly apply 1 × PBS to prevent drying of the wound.
Drill two holes, one in close proximity to the metaphyseal growth plate and one at the distal end of the tibial bone marrow cavity (Figure 2d: arrows indicate the position of holes to be drilled; Figure 2e: arrows indicate position of drilled holes as seen on radiographs).*NOTE: the dental drill bit should be held perpendicular to the tibia while drilling.
Flush the bone marrow with 0.5 ml 1 × PBS using a 30G needle.
Insert bone wax with a capillary piston into the distal hole and press with a spatula.
Inoculate the cancer cell suspension intramedullarily with a 30G needle via the proximal hole (10 μl maximal volume, but less volume would be ideal).
Seal the proximal hole with bone wax and suture the wound with 2–3 stitches.
Disinfect the wound with betadine.
Inject an appropriate analgesic and monitor mice until they fully recover.
*NOTE: both Method 1 and Method 2 are invasive. Either the injection channel using Method 1 or the drilled holes using Method 2 will be identified in histological sections. The injection volume has to be kept minimal for both methods because of the space limitation in the medullary cavity.
*NOTE: mice should be monitored regularly, according to an approved mouse protocol, and killed if clinical signs match the experimental end point. Signs include, but are not limited to, rapid weight loss and any condition interfering with daily activities (for example, eating or drinking, ambulation or elimination).
*NOTE: bone lesions can be monitored by radiography (Faxitron, 25 kV, 6 s) (Figures 3a and b: representative radiography pictures of osteolytic and osteoblastic bone metastases), and cancer cell growth can be monitored using bioluminescence imaging (only for luciferase-labeled cells).
Figure 3.
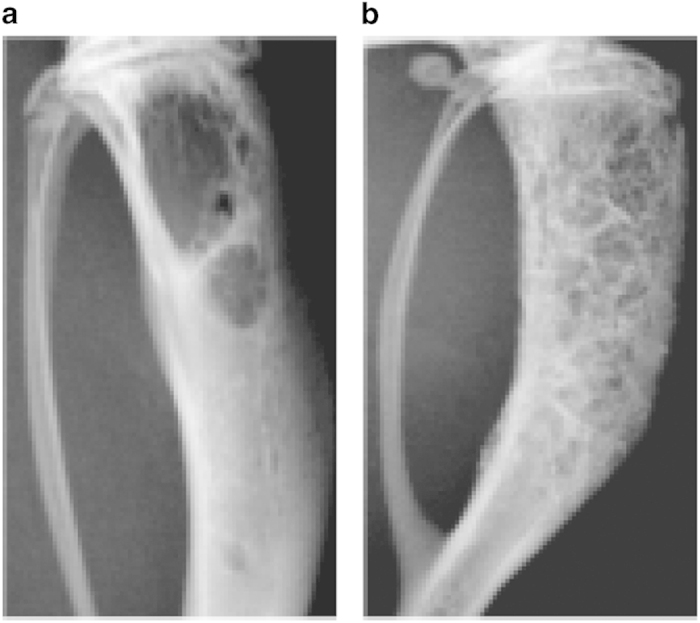
Representative radiographs after intraosseous inoculation. Representative images of radiographs after the intraosseous injection of (a) osteolytic prostate cancers cells (PC-3) into the bone marrow cavity of CB17 SCID mice (Day 32, 2 500 cells delivered) and (b) osteoinductive prostate cancer cells (VCaP) into the bone marrow cavity of CB17 SCID mice (day 91, 500 000 cells delivered).
Intracardiac inoculation (Written by NW)
Materials
70% ethanol gauze
0.5 or 1 ml Insulin syringes (30G, ½ inch)
Procedures
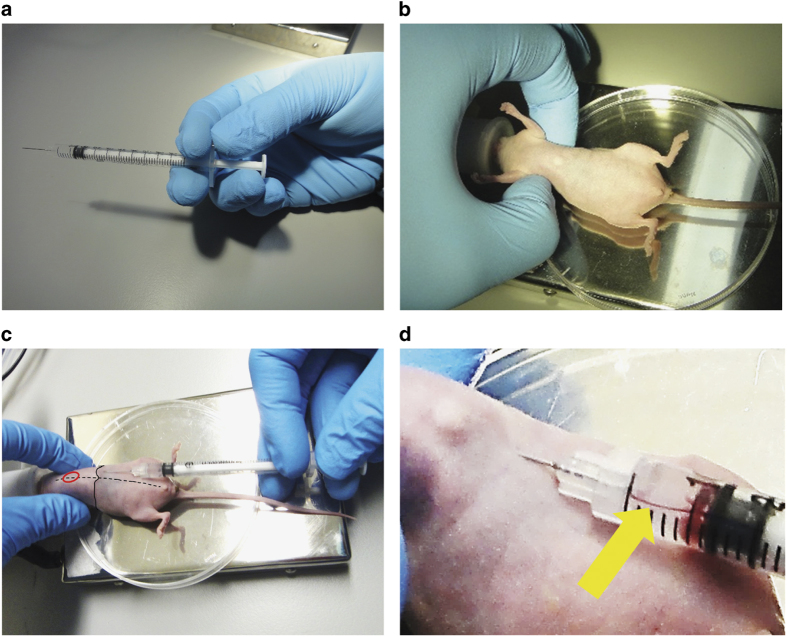
Hold the syringe with thumb, index finger and middle finger. Ring finger and little finger are used to withdraw and push the plunger, respectively (Figure 4a).
Use thumb and index finger of the other hand to slightly squeeze the rib cage of mouse from dorsal side upward to raise the position of the heart (Figure 4b) (administer anesthesia as indicated above).
Disinfect the chest area with 70% ethanol.
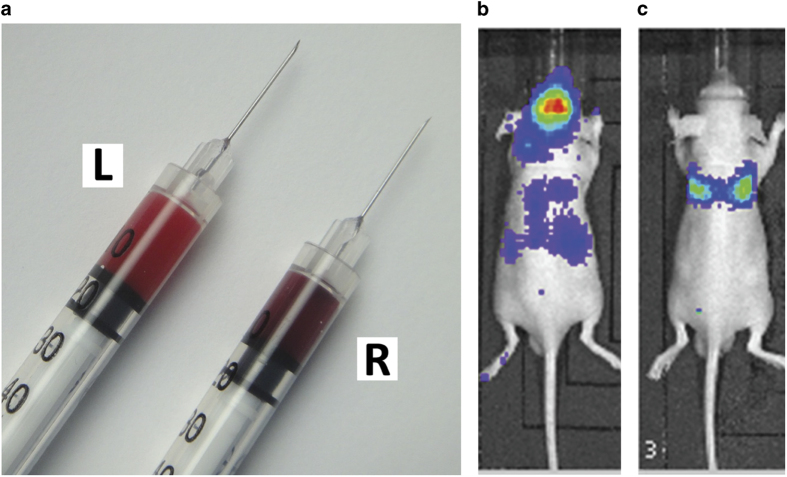
Insert needle horizontally into the injection point and slightly withdraw the plunger after the needle has been inserted 7–8 mm*NOTE: push and withdraw the plunger of syringe several time to reduce friction force before loading the syringe with 120 μl cell suspensions (20 μl in excess).*NOTE: alternatively, needle can be inserted vertically.*NOTE: injection point is 1–2 mm left of the midline and 1–2 mm beneath the edge of rib cage of the mouse (Figure 4c).*NOTE: if the needle is properly inserted in the left ventricle, bright red blood will be pumped back into the cell suspension and pulsation should be visible from the air bubble remaining in the syringe (Figure 4d). If there is no blood pumping back or dark red blood observed, retract and re-insert the needle, but no more than three attempts should be carried out within 24 h.
Inject the cell suspension at a slow pace (∼30 s for total 100 μl).*NOTE: avoid positional change of injecting hand during injection.*NOTE: correct injection can be verified during the process of injection by the color of blood pumped back to syringe. Should the needle be correctly injected into the left ventricle, bright red, oxygenated arterial blood will be seen, which is distinctive from the darker venous blood of the right ventricle (Figure 5a).*NOTE: tumor cells in the systemic circulation can also be verified within 24 h after injection by bioluminescence imaging, if you are using luciferase-labeled cells. If tumor cells are correctly injected into the left heart ventricle, bioluminescence signals can be observed in the whole body (Figure 5b). Otherwise, signals will be concentrated in the chest area, and resemble the shape of lung, or will be completely absent (Figure 5c).
Slightly withdraw the plunger during the inoculation, and observe whether blood is still pumping back into the syringe to confirm that the needle is still placed in the left ventricle.
Complete the injection of 100 μl of cell suspension with the 20 μl excess remaining in the syringe. Wait for 5 s before the needle is taken out.
Monitor mice until they fully recover.
Figure 4.
Intracardiac inoculation of prostate cancer cells. (a) Proper holding of the syringe. Thumb, index finger and middle finger are used to hold the syringe, whereas ring finger and little finger are responsible for withdrawing and pushing the plunger. (b) Mouse is placed in a supine position and the rib cage is squeezed up to raise the position of the heart. (c) Insert the needle horizontally into the injection point 1–2 mm left of the midline (the dotted line) and 1–2 mm beneath the edge of rib cage (the solid line). The approximate position of the heart is marked in red. (d) When the needle is successfully inserted into the left ventricle, slightly withdrawing the plunger will pump bright red blood back into the cell suspension in the syringe (yellow arrow).
Figure 5.
Verification after intracardiac inoculation. (a) Bright red, oxygenated arterial blood from correct injection into the left ventricle (L) and darker venous blood of the right ventricle from incorrect injection (R). Representative images of in vivo bioluminescence imaging within 24 h after injection (b) in the correctly injected mouse (whole body bioluminescence signals) and (c) in the incorrectly injected mouse (lung-shape signals over thorax).
*NOTE: after the successful injection, the mouse is placed back into a clean cage on a heating pad, until fully recovered from the anesthesia. Closely monitor the mouse for the first 24 h after injection.
Intravenous inoculation (Written by JD)
Materials
Mouse restrainers.
70% ethanol gauze.
1 ml syringes with 26–28G needle, ½ inch
*NOTE: various mouse restrainers are available, but the mouse tail illuminator restrainer from Braintree Scientific, Inc. (Braintree, MA, USA) is recommended, because it efficiently illuminates and warms a mouse tail for easy injection.
Procedures
Gently grab the tail of a mouse and pull it into the commercial or custom mouse restrainer, with its back against the slit and its tail sticking out of the small opening in the back of the restrainer. Slowly close doors and keep mouse gently restrained.
Find the lateral tail vein (Figure 6) and promote vasodilation of the vein for easy injection by warming the mouse using a heat lamp or a warming device or by immersion of the tail in warm water for about 5 min*NOTE: the time will be dependent on the distance from the heat source, and the animals must be monitored carefully to prevent being burned or overheated by the heat source.
Disinfect the tail with 70% ethanol.
Pull the tail straight.
Hold the tip of tail with thumb, and support the point for injection with the index finger.
With the bevel facing up, insert a needle into one of the lateral tail veins in the proximal 1/3 of the tail, and gently inject 100 μl of the cell suspension.*NOTE: a successful injection is based on lack of resistance while pushing the plunger.*NOTE: start from the distal end of the tail; thus, if the first trial fails, a more proximal region of the tail can be used if another attempt is needed.*NOTE: the maximum volume that may be injected depends on numerous factors including the mouse's size, background strain and properties of the cells. Most users try to limit the injection to a maximum of 0.25 ml. Keep in mind that overconcentration of cells may lead to embolism in lung capillaries and death of the mice.
Withdraw the needle after injection and press a clean piece of paper towel or cotton swab on the injection site to facilitate clotting.
Release the mouse from the restrainer and return it to the cage.
Figure 6.
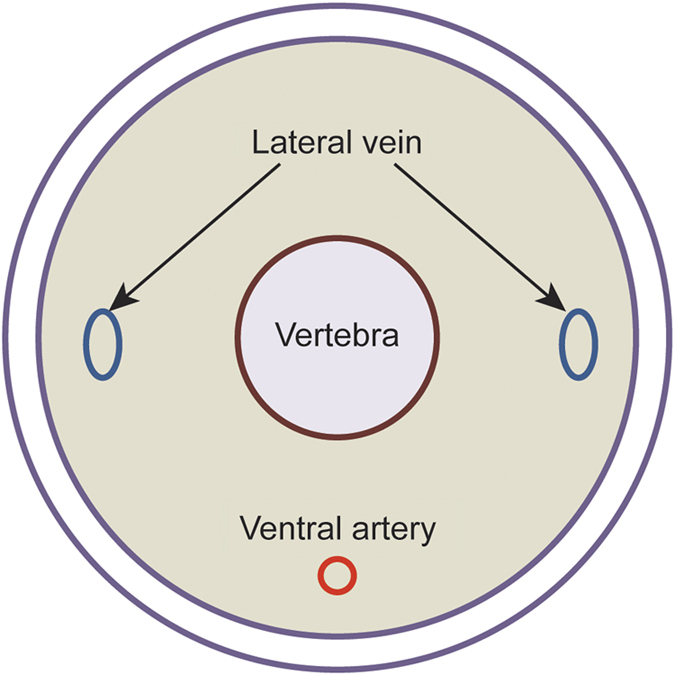
Anatomy of murine tail. Murine tail veins are found in both sides of murine tail (just under the skin). Intravenous injections should be performed via the lateral tail vein.
*NOTE: most mice show only reduced activity levels. The mice should be observed for the first hour following injection. If no problems are observed other than reduced activity, the mice may be returned to standard housing.
Orthotopic inoculation (Written by MK-dJ)
Materials
Sterile 1 × PBS.
Surgical scissors and tweezers.
Cotton swabs.
70% ethanol gauze.
0.5 or 1 ml Insulin syringes (30G, ½ inch).
Suturing thread (wound clip is not ideal for imaging).
Betadine.
Procedure
Place a mouse in supine position, immobilization is not necessary (administer anesthesia as indicated above).
Disinfect the lower abdomen with 70% ethanol.
Make a small (1 cm) incision in the lower midline of the peritoneum.
Exteriorize and gently stabilize the prostate dorsal lobes with a wet (1 × PBS) cotton swab (Figure 7).
Insert the needle into the right dorsal lobe of the prostate at a 45 angle.
Slowly inject 10 μl of the cell suspension.*NOTE: a well-localized bleb indicates a successful injection.
Retract the needle gently and place a cotton swab over the injection site for about 1 min to prevent bleeding and spillage of material.
Return the prostate to the peritoneum and suture the abdominal wall first and the skin layer after with a 4–0 silk suture.
Disinfect the wound with betadine.
Inject an appropriate analgesic and monitor mice until they fully recover.
Figure 7.
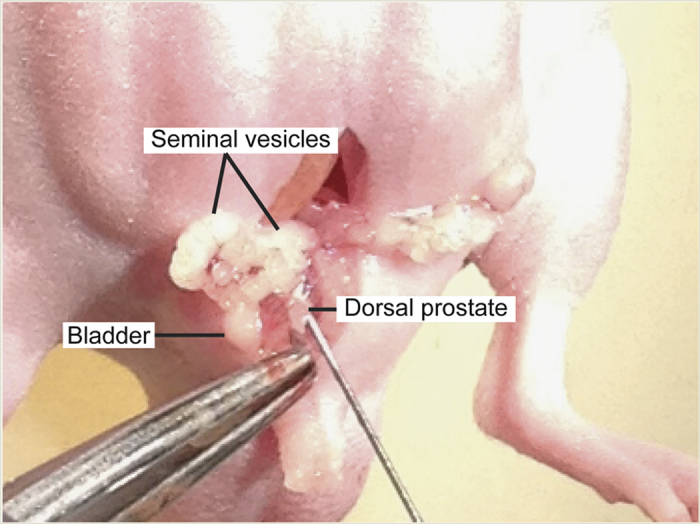
Orthotopic inoculation of prostate cancer cells. The prostate dorsal lobes are gently exteriorized, and prostate cancer cells will be inoculated.
*NOTE: mice should be monitored regulary according to an approved mouse protocol and killed if clinical signs match experimental end point. Signs include, but are not limited to, rapid weight loss and any condition interfering with daily activities (for example, eating or drinking, ambulation or elimination).
In vivo bioluminescence imaging
Materials
Luciferase-labeled prostate cancer cell line (PCa-luc cells)*NOTE: luciferase-labeled prostate cancer cells are greatly useful to follow metastasis in vivo. Lentiviral transfer of luciferase gene and subsequent selection and verification of stable clones should be performed in advance. Luciferase-labeled PC-3 cells can be purchased from Caliper Life Sciences or generated individually by transducing cells with lentiviral vectors and luciferase reporter vectors (pGL4 Luciferase Reporter Vectors from Promega, Madison, WI, USA). Verify luciferase activity in cells before injection.
Sterile 1 × PBS.
1 ml syringes with a 25G needle.
D-Luciferin (from Regis Technologies, Morton Grove, IL, USA, or Caliper Life Sciences, Waltham, MA, USA or Promega).
Isoflurane.
Oxygen tank.
Isoflurane/oxygen-based anesthesia system fitted with an induction chamber and inhalation masks for mice.*NOTE: xylazine–ketamine cocktail can be used if no Isoflurane/oxygen-based anesthesia system available.
In vivo imaging system (Xenogen IVIS, Perkin-Elmer, Waltham, MA, USA).
Procedure
Prepare a sterile stock solution of D-luciferin in 1 × PBS (40 mg ml−1).
Inject 150 μg g−1 D-luciferin into the intraperitoneal cavity of mice, using a syringe with a 25G needle.
Initialize the ‘Living Image software (Perkin-Elmer)' provided by the manufacturer on a computer attached to the IVIS machine.
Set exposure time and imaging parameters.
Anesthetize the mice in 2% isoflurane mixed with 98% oxygen in induction chamber.
Place mice in the IVIS platform while keeping mice under anesthesia through a nose cone, and take the image 12 min after D-luciferin injection.*NOTE: it is recommended to perform an initial kinetic experiment for each animal model taking images during different time points. This will allow you to determine the D-luciferin distribution for your experiment.
Analyze and quantify the photons emitted from luciferase-labeled cells within the animal according to the manufacturer's protocol.
*NOTE: for the intravenous inoculation model of prostate cancer, a highly metastatic cancer cell line (for example, PC-3 and DU145) typically gives rise to many metastases, especially at hind limb with bone metastatic lesions (Figure 8a), whereas very few metastases will be detected from a poorly metastatic cancer cell line (for example, LNCaP and LNCaP C4-2B).
Figure 8.
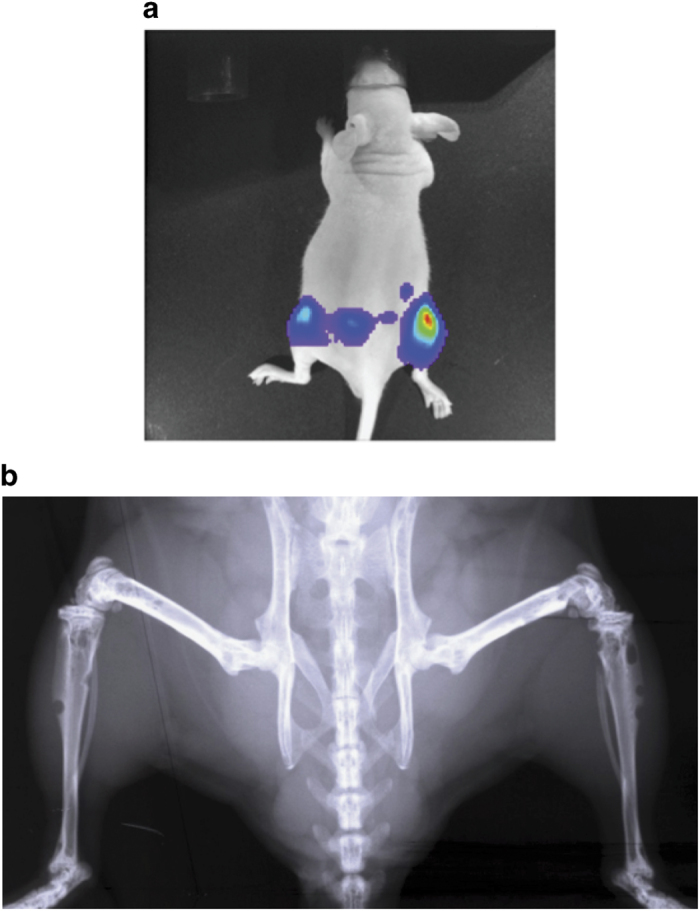
Representative images after intravenous injection. Representative images of (a) in vivo bioluminescence imaging and (b) radiograph after the intravenous injection of luciferase-labeled PC-3 cells into a nude mouse. Multiple bone metastatic lesions are detected at mouse hind limbs.
*NOTE: bioluminescence is a well-established technique commonly utilized to track tumor growth and to locate and monitor the presence of metastases within living animals. In vivo bioluminescence imaging system is a highly sensitive imaging technology to quantify bioluminescence via a digital camera and advanced computer software. This system detects photons emitted from luciferase-expressing cells within the living animals.
*NOTE: in certain cases, it may be important to get both dorsal and ventral images.
*NOTE: many imaging techniques can also be used to detect the prostate cancer metastatic lesions in mice, such as molecular imaging techniques or small animal diagnostic imaging (microcomputed tomography or magnetic resonance imaging or Faxitron cabinet x-ray systems) (Figure 8b). If both bioluminescence and other imaging techniques are not available, histopathological examination of metastatic nodules subsequent to necropsy is the best approach.
Discussions/Conclusion
In this manuscript, we discussed the mouse xenograft models that are commonly used to study prostate cancer bone metastasis. Using these models with human prostate cancer cell lines (Table 1),3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28 we have gained a greater understanding of the process of bone metastasis. However, there are limitations for using these xenograft models, as the immune systems of animals used for developing these models are weak or compromised to avoid an immune response to the human tissue. There are currently some transgenic spontaneous prostate cancer models that may overcome this limitation; however, the transgenic mice currently available rarely result in bone metastases, unlike those seen in human prostate cancer.29 Although humanized animal models30,31 or ex vivo tissue engineered models32,33 have been recently proposed to potentially mimic bone metastasis, models that perfectly recapitulate the human prostate cancer bone metastatic process are still missing. To unveil the mystery of bone metastasis, further improvement of animal models currently available and/or development of new animal models to overcome the limitations that we are facing are clearly warranted.
Table 1. Prostate cancer cell line used in bone metastasis research.
| Cell line | Origin | PSA | AR | Bone lesion | Model | References |
|---|---|---|---|---|---|---|
| DU145 | Central nerve system metastasis (Human) | Negative | Negative | Osteolytic | Intratibial injection, Intracardiac injection, Intravenous injection into human bone implanted animals | Conley-LaComb et al.,3 Stone et al.,4 Nemeth et al.,5 Yin et al.6 |
| PC-3 | Vertebral bone metastasis (Human) | Negative | Negative | Osteolytic | Intratibial injection, Intrafemoral injection, intracardiac injection, Intravenous injection into human bone implanted animals | Kaighn et al.,7 Nemeth et al.,5 Chu et al.,8 Wu et al.,9 Zhang et al.10 |
| LNCaP | Lymph node metastasis (Human) | Positive | Positive | Osteoblastic/Osteolytic | Intrafemoral injection, Intravenous injection into human bone implanted animals | Nemeth et al.,5 Horoszewicz et al.,11 Wu et al.9 |
| LNCaP C4-2 | LNCaP subline C4 xenograft line | Positive | Positive | Osteoblastic | Intratibial injection, Intrafemoral injection, intracardiac injection | Thalmann et al.,12 Wu et al.9 |
| LNCaP C4-2B | LNCaP subline C4-2 xenograft line | Positive | Positive | Osteoblastic | Intrafemoral injection | Thalmann et al.,12 Wu et al.9 |
| MDA PCa 2b | Bone metastasis (Human) | Positive | Positive | Osteoblastic | Intrafemoral injection | Navone et al.,13 Cheng et al.,14 Yang et al.15 |
| VCaP | Vertebral bone metastasis (Human) | Positive | Positive | Osteoblastic | Intratibial injection | Korenchuk et al.,16 Kirschenbaum et al.17 |
| 22Rv1 | CWR22R xenograft line | Positive | Positive | Osteoblastic/Osteolytic | Intratibial injection | Sramkoski et al.,18 Henry et al.19 |
| LAPC-9 | Femoral bone metastasis (Human) | Positive | Positive | Osteoblastic | Intratibial injection | Craft et al.,20 Lee et al.,21 Lee et al.22 |
| ARCaP | Ascites of a patient with advanced metastatic disease (Human) | Positive | Positive | Osteoblastic/Osteolytic | Intracardiac injection | Zhau et al.,23 Odero-Marah et al.,24 Xu et al.25 |
| LuCaP23.1 | Lymph node metastasis (Human) | Positive | Positive | Osteoblastic | Intratibial injection | Ellis et al.,26 Morrissey et al.,27 Brubaker et al.28 |
Abbreviations: AR, androgen receptor; PSA, prostate-specific antigen.
Acknowledgments
We thank Ms Janice E Berry for critical reading of this manuscript and Dr Antoinette Wetterwald for insightful scientific discussion. This work is directly supported by the National Cancer Institute (CA163124, YS; P01Ca093900, JD; and R01CA190554, JD), the Department of Defense (W81XWH-14-1-0403, YS), the Prostate Cancer Foundation (YS), Cancer Research UK (NW), Yorkshire Cancer Research (NW), and Wellcome Trust (NW). Supported by the IBMS-ECTS Young Investigators.
Footnotes
The authors declare no conflict of interest.
References
- Boyd LK, Mao X, Lu YJ. The complexity of prostate cancer: genomic alterations and heterogeneity. Nat Rev Urol 2012; 9: 652–664. [DOI] [PubMed] [Google Scholar]
- Shiozawa Y, Eber MR, Berry JE, Taichman RS. Bone marrow as a metastatic niche for disseminated tumor cells from solid tumors. BoneKey Rep 2015; 4: 689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley-LaComb MK, Saliganan A, Kandagatla P, Chen YQ, Cher ML, Chinni SR. PTEN loss mediated Akt activation promotes prostate tumor growth and metastasis via CXCL12/CXCR4 signaling. Mol Cancer 2013; 12: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU 145). Int J Cancer 1978; 21: 274–281. [DOI] [PubMed] [Google Scholar]
- Nemeth JA, Harb JF, Barroso U Jr, He Z, Grignon DJ, Cher ML. Severe combined immunodeficient-hu model of human prostate cancer metastasis to human bone. Cancer Res 1999; 59: 1987–1993. [PubMed] [Google Scholar]
- Yin J, Pollock C, Tracy K, Chock M, Martin P, Oberst M et al. Activation of the RalGEF/Ral pathway promotes prostate cancer metastasis to bone. Mol Cell Biol 2007; 27: 7538–7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest Urol 1979; 17: 16–23. [PubMed] [Google Scholar]
- Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ, Chen DT et al. Cadherin-11 promotes the metastasis of prostate cancer cells to bone. Mol Cancer Res 2008; 6: 1259–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF et al. Establishing human prostate cancer cell xenografts in bone: induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer 1998; 77: 887–894. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li K, Zhang X, Fang Z, Xiong W, Chen Q et al. Effect of Id1 knockdown on formation of osteolytic bone lesions by prostate cancer PC3 cells in vivo. J Huazhong Univ Sci Technolog Med Sci 2012; 32: 364–369. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Chu TM, Wajsman ZL, Friedman M, Papsidero L et al. The LNCaP cell line–a new model for studies on human prostatic carcinoma. Prog Clin Biol Res 1980; 37: 115–132. [PubMed] [Google Scholar]
- Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res 1994; 54: 2577–2581. [PubMed] [Google Scholar]
- Navone NM, Olive M, Ozen M, Davis R, Troncoso P, Tu SM et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin Cancer Res 1997; 3: (12 Pt 1): 2493–2500. [PubMed] [Google Scholar]
- Cheng CJ, Ye XC, Vakar-Lopez F, Kim J, Tu SM, Chen DT et al. Bone microenvironment and androgen status modulate subcellular localization of ErbB3 in prostate cancer cells. Mol Cancer Res 2007; 5: 675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Fizazi K, Peleg S, Sikes CR, Raymond AK, Jamal N et al. Prostate cancer cells induce osteoblast differentiation through a Cbfa1-dependent pathway. Cancer Res 2001; 61: 5652–5659. [PubMed] [Google Scholar]
- Korenchuk S, Lehr JE, L MC, Lee YG, Whitney S, Vessella R et al. VCaP, a cell-based model system of human prostate cancer. In Vivo 2001; 15: 163–168. [PubMed] [Google Scholar]
- Kirschenbaum A, Liu XH, Yao S, Leiter A, Levine AC. Prostatic acid phosphatase is expressed in human prostate cancer bone metastases and promotes osteoblast differentiation. Ann NY Acad Sci 2011; 1237: 64–70. [DOI] [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow TG 2nd, Giaconia JM, Pretlow TP, Schwartz S, Sy MS et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol Anim 1999; 35: 403–409. [DOI] [PubMed] [Google Scholar]
- Henry MD, Silva MD, Wen S, Siebert E, Solin E, Chandra S et al. Spiculated periosteal response induced by intraosseous injection of 22Rv1 prostate cancer cells resembles subset of bone metastases in prostate cancer patients. Prostate 2005; 65: 347–354. [DOI] [PubMed] [Google Scholar]
- Craft N, Chhor C, Tran C, Belldegrun A, DeKernion J, Witte ON et al. Evidence for clonal outgrowth of androgen-independent prostate cancer cells from androgen-dependent tumors through a two-step process. Cancer Res 1999; 59: 5030–5036. [PubMed] [Google Scholar]
- Lee Y, Schwarz E, Davies M, Jo M, Gates J, Wu J et al. Differences in the cytokine profiles associated with prostate cancer cell induced osteoblastic and osteolytic lesions in bone. J Orthop Res 2003; 21: 62–72. [DOI] [PubMed] [Google Scholar]
- Lee YP, Schwarz EM, Davies M, Jo M, Gates J, Zhang X et al. Use of zoledronate to treat osteoblastic versus osteolytic lesions in a severe-combined-immunodeficient mouse model. Cancer Res 2002; 62: 5564–5570. [PubMed] [Google Scholar]
- Zhau HY, Chang SM, Chen BQ, Wang Y, Zhang H, Kao C et al. Androgen-repressed phenotype in human prostate cancer. Proc Natl Acad Sci USA 1996; 93: 15152–15157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odero-Marah VA, Wang R, Chu G, Zayzafoon M, Xu J, Shi C et al. Receptor activator of NF-kappaB Ligand (RANKL) expression is associated with epithelial to mesenchymal transition in human prostate cancer cells. Cell Res 2008; 18: 858–870. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang R, Xie ZH, Odero-Marah V, Pathak S, Multani A et al. Prostate cancer metastasis: role of the host microenvironment in promoting epithelial to mesenchymal transition and increased bone and adrenal gland metastasis. Prostate 2006; 66: 1664–1673. [DOI] [PubMed] [Google Scholar]
- Ellis WJ, Vessella RL, Buhler KR, Bladou F, True LD, Bigler SA et al. Characterization of a novel androgen-sensitive, prostate-specific antigen-producing prostatic carcinoma xenograft: LuCaP 23. Clin Cancer Res 1996; 2: 1039–1048. [PubMed] [Google Scholar]
- Morrissey C, Dowell A, Koreckij TD, Nguyen H, Lakely B, Fanslow WC et al. Inhibition of angiopoietin-2 in LuCaP 23.1 prostate cancer tumors decreases tumor growth and viability. Prostate 2010; 70: 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker KD, Brown LG, Vessella RL, Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer 2006; 6: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter SF, Cooper AB, Greenberg NM. Models of metastatic prostate cancer: a transgenic perspective. Prostate Cancer Prostatic Dis 2003; 6: 204–211. [DOI] [PubMed] [Google Scholar]
- Hesami P, Holzapfel BM, Taubenberger A, Roudier M, Fazli L, Sieh S et al. A humanized tissue-engineered in vivo model to dissect interactions between human prostate cancer cells and human bone. Clin Exp Metastasis 2014; 31: 435–446. [DOI] [PubMed] [Google Scholar]
- Holzapfel BM, Wagner F, Loessner D, Holzapfel NP, Thibaudeau L, Crawford R et al. Species-specific homing mechanisms of human prostate cancer metastasis in tissue engineered bone. Biomaterials 2014; 35: 4108–4115. [DOI] [PubMed] [Google Scholar]
- Thibaudeau L, Taubenberger AV, Holzapfel BM, Quent VM, Fuehrmann T, Hesami P et al. A tissue-engineered humanized xenograft model of human breast cancer metastasis to bone. Dis Model Mech 2014; 7: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin P, Youm H, Salih E. Three-dimensional cancer-bone metastasis model using ex-vivo co-cultures of live calvarial bones and cancer cells. Biomaterials 2012; 33: 1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]