Abstract
The strong negative correlation between grain protein concentration (GPC) and grain yield (GY) in bread wheat complicates the simultaneous improvement of these traits. However, earlier studies have concluded that the deviation from this relationship (grain protein deviation or GPD) has strong genetic basis. Genotypes with positive GPD have an increased ability to uptake nitrogen (N) during the post-flowering period independently of the amount of N taken up before flowering, suggesting that genetic variability for N satiety could enable the breakage of the negative relationship. This study is based on two genotypes markedly contrasted for GPD grown under semi-hydroponic conditions differentiated for nitrate availability both before and after flowering. This allows exploration of the genetic determinants of post-flowering N uptake (PANU) by combining whole plant sampling and targeted gene expression approaches. The results highlights the correlation (r² = 0.81) with GPC of PANU occurring early during grain development (flowering–flowering + 250 degree-days) independently of GY. Early PANU was in turn correlated (r² = 0.80) to the stem-biomass increment after flowering through its effect on N sink activity. Differences in early PANU between genotypes, despite comparable N statuses at flowering, suggest that genetic differences in N satiety could be involved in the establishment of the GPC. Through its strong negative correlation with genes implied in N assimilation, root nitrate concentration appears to be a good marker for evaluating instantaneous plant N demand, and may provide valuable information on the genotypic N satiety level. This trait may help breeders to identify genotypes having high GPC independently of their GY.
Introduction
Grain yield (GY) and grain protein concentration (GPC) are two major breeding objectives in wheat, as these traits are the dominant determinants of the economic value of the harvested product. GPC influences price, in particular because of its impact on the rheological qualities of the flour [1,2]. It is also a necessary quality criterion for wheat to be eligible for export. However, there is a strong negative relationship between GY and GPC [3–7] and this presents a major obstacle to the simultaneous improvement of these two traits in breeding programmes. In most developed nations, including in Europe, GY increased greatly during the second half of the 20th century [6,8]. This gain was the combined result of improved genotypes obtained through breeding and improved management practices. Unfortunately, the global rise in GY has been associated with a concomitant decrease in GPC [9,10]. Nevertheless, as stated by Simmonds [3], breeding programs focusing on GPC would have been counter-productive because the economic cost of the associated GY penalty would have far exceeded the economic benefit of the protein gain. The classical agronomic strategy for achieving high GY coupled with a good level of GPC is to grow varieties having high GY potential and then to boost their GPC through a protocol in which the final fertiliser application is delayed to just before heading. However, as the global valorisation of fertilizer inputs is estimated between 50% and 60% in high potential conditions [11], this approach is now being questioned in the context of reduced-input agriculture, due to the large economic and ecologic cost of excessive mineral fertiliser usage [12].
A new strategy to counteract the negative relationship between GY and GPC, without compromising either of these two traits was proposed by Monaghan et al. [5]. This originates in the observation that some genotypes deviate from the linear regression of GPC on GY, either positively or negatively. This deviation, called the Grain Protein Deviation (GPD), has a strong genetic basis [7,10] and may thus provide an alternative selection criterion for simultaneously improving GY and GPC.
Grain N may originate from N taken up either before or after flowering. N taken up before flowering is stored in the vegetative organs but later, during their senescence, is remobilised into the grain. Genotypic differences have been detected for the fraction of grain N originating from remobilisation, both through variation in the amount of N already present in the plant at flowering [13] and through variation in the N remobilisation efficiency [14,15]. N taken up by the plant during the post-flowering period can account for between 5 and 40% of total grain N under field conditions [14,16,17]. The relative contribution of this later N source to grain N is strongly influenced by the environment–especially the availabilities of soil N and water, but also to a lesser extent by genotype [18]. Under controlled conditions where environmental constraints are minimised, wheat has the ability to take up N until near grain maturity [19–22]. However, under field conditions, pre-flowering and post-flowering N uptakes are negatively correlated [5,17,18,23]. This negative relationship may be explained by the presence in the soil of a finite amount of available N that can be absorbed either before or after flowering.
It has been shown that post-flowering N uptake (PANU) has a strong impact on GPC [5,14,24–26]. In a growth chamber, under post-flowering conditions where N is non-limiting, PANU may completely meet grain need for N [27]. Although the physiological basis of the GPD remains unclear, Bogard et al. [23] demonstrated that it was highly correlated with genotypic capacity to absorb N during the post-flowering period independently of the amount of N taken up before flowering. This leads to the hypothesis that genetic variability for GPD could be associated with variations in satiety for N. The positive GPD could thus be associated with genotypes having an increased ability to take up N during the post-flowering period despite a high N uptake before flowering. PANU is hypothesised to be both linked to plant N status at flowering and to the strength of the demand for N exerted by the growing grain [27]. Such internal regulations of N uptake could represent a potential node of genetic variability that might explain the increased ability of some genotypes to capture N after flowering in a way that was independent of the level of N uptake realised before flowering [23].
Under aerobic soil conditions (classically the case for wheat), N is principally taken up as nitrate (NO3-) [28]. Two main families of root transporters are involved in plants, and these are differentiated by their affinity for NO3-. The first is a high-affinity transport system (HATS), coded by NRT2 family genes, and the second is a low-affinity transport system (LATS) coded by NPF genes [29], formerly known as NRT1 family genes [30–33]. In Arabidopsis thaliana, it has been shown that the involvement of the two transport systems in NO3- uptake is dependent on the NO3- concentration in the external medium. LATS predominates when the NO3- concentration in the medium is high (>1 mM), while HATS predominates when NO3- concentration is low (<1 mM) [31]. Unlike the model species, information on wheat NRT genes is limited. Until recently, only one gene belonging to the NRT1 family had been studied [34]. This gene exhibit a high degree of homology to OsNRT1.1 from Oriza sativa, whose nitrate transport function has been confirmed [35]. A more recent study has however proposed 16 genes as putative homologs to the A. thaliana NRT1 family genes [36]. Similarly, TaNRT2.1 is the only gene belonging to the NRT2 family which has so far been characterised in wheat [37]. This gene is hypothesised to play a central role in wheat post-flowering N uptake [27]. The major implication of HATS in wheat NO3- uptake independently of NO3- concentration in the external medium has also been suggested in recent studies [38,39], although Pang et al. [40] observed a significant involvement of LATS at the tillering stage.
Progress in the understanding of the mechanisms underlying N uptake and N assimilation opens the possibility of understanding the genetic variability for complex mechanisms linked to N uptake, such as GPD, at the molecular scale. To an applied perspective, such knowledge could provide the ability to select varieties with increased capacity to valorise late fertiliser inputs into grain proteins. The objective of this study was to characterise the physiological and molecular markers of GPD, using two genotypes (cvs. Récital and Renan) which contrast markedly with respect of this trait. Employing a semi-hydroponic culture method and closely controlled conditions, the experimental approach allowed fine control of NO3- availability and testing for the effects of post-flowering NO3- availability on plants having contrasting N statuses at flowering. The study includes physiological measurements, gene expression quantification, and NO3- assays at four developmental stages distributed between flowering and maturity.
Material and Methods
Plant material
Two winter wheat (Triticum aestivum L.) genotypes were used in this study, the cvs. Récital and Renan. These were chosen for their strongly contrasting GPD performance found in multi-environment trials [10,23] associated with closely comparable grain yields and similar earliness. Both are semi dwarf Rht-B1b [15]. At comparable levels of GY, Récital has a GPC which is about 1.5% lower than Renan [10,23,41,42]. In this genotype pairing, Récital represents the GPD negative partner (GPD-) and Renan the positive one (GPD+).
Calibrated grains (55 mg ± 5 for Récital and 65 mg ± 5 for Renan, according to their respective mean thousand kernel weight values) were sown in germination trays filled with compost and placed in a heated greenhouse (20°C) for two weeks. Seedlings were subsequently vernalised for six weeks in a growth chamber (6°C, 8 h photoperiod, light intensity 350 μmol PAR m-2s-1). After vernalisation, they were transplanted to PVC tubes (7 cm diameter, 60 cm high) filled with a perlite:sand substrate (1:1, v:v) for semi-hydroponic culture, with two plants per tube. A total of 256 tubes (512 plants) was set up for each genotype. Tubes were placed vertically in eight containers (four containers per genotype, container area 0.49 m²) at a density of 64 tubes per container, each container containing eight rows of eight tubes. This configuration represents a cover density of 260 plants m-², which is comparable to that under field conditions under local agronomic practice. To avoid any “edge effect” on plants located on the outer edges of containers, appropriate shading nets were positioned around each container. These shading nets, positioned at the canopy height, were regularly raised during plant development.
Plants were then placed in a growth chamber under a long-day photoperiod (16 h light at 20°C, 8 h dark at 18°C, light intensity 650 μmol PAR m-2s-1). Each tube was fitted with its own automated micro-irrigation system which provided nutrient solution at a rate of 66 ml per 3 h. Nutrient solution composition was adapted from Castle and Randall [43] (S1 Table). Before flowering, the nutrient solution contained either 4 or 10 mM of NO3- to create two pre-flowering N treatments which we will refer to as N4 and N10, respectively. During the pre-flowering period, two containers of each genotype were exposed to the N4 pretreatment, and two to N10.
From flowering and for the remainder of the post-flowering period, the nutrient solutions were reassigned among the containers so that one container from the N4 pretreatment and one from N10, were now exposed to low NO3- availability (4 mM of NO3-). Meanwhile, the other two containers (one from the N4 pretreatment and one from N10) were now exposed to high NO3- availability (10 mM of NO3-). This created the two post-flowering N treatments, LN and HN, respectively. The factorial design allowed independent observation of the effects of low and high NO3- availability in both the pre- and post-flowering periods. For each genotype, it created the four treatment combinations: N4-LN, N4-HN, N10-LN and N10-HN. The homogeneity of environmental parameters within the growth chamber (temperature, humidity, light intensity) was beforehand validated, and particular care was taken concerning preparation of substrate and precision of each individual micro-irrigation system.
Sampling protocol
Four destructive samplings were carried out during the post-flowering period. The first took place one day after flowering (GS65, [44]), and the other three at GS65+250 degree-day (DD), GS65+450 DD and maturity (GS92). Each sampling was carried out between one and two hours after lighting. At each sampling date, plants were harvested by rows from the outside to the inside of the container.
Physiological measurements
Physiological measurements were carried out on five biological replicates at the four sampling dates, the two pooled plants of each tube being considered as a single replicate. Plants were each divided into six fractions: root, stem, green laminae, senescent laminae, grain and chaff. Only at GS65, spikes were not divided into grain and chaff. After fractioning, areas of green laminae were measured (LI-3100 area meter, LI-COR, Lincoln NE, USA) and spike numbers were counted. The different fractions were then oven-dried at 80°C for 48 h before dry weight (DW) measurements. Samples were subsequently ground using a ball mill, and total N concentrations were measured by the Dumas combustion method using a Flash EA 1112 Series CNS analyser (ThermoFisher Scientific, Waltham MA, USA). GPC was estimated as grain N x 5.7.
Nitrogen Nutrition Index (NNI) was calculated as the above-ground N concentration divided by a critical plant N concentration, defined as the minimum N concentration needed for maximum growth rate [45]. The critical plant N concentration was calculated using the equation described for winter wheat in Justes et al. [46]. PANU was calculated as the difference in total plant N between GS92 and GS65, and early PANU as the difference in total plant N between GS65+250 DD and GS65. Remobilisation of N was calculated as the difference between total vegetative N (total plant N, minus grain N) between GS92 and GS65.
Preparation of root samples for NO3- concentration and gene expression analysis
Nitrate concentration and gene expression analyses were carried out for the first three sampling dates only, samples collected at maturity exhibited an advanced state of senescence. The same samples were used to assay NO3- concentration and gene expression. Analyses were carried out on three biological replicates, the two pooled plants from each tube were considered a single replicate. Roots were separated from the aerial parts, washed free of substrate residue with water, and frozen in liquid nitrogen. Root samples were ground while still frozen using a ball mill, and stored at -80°C pending analysis.
Metabolite measurements
Nitrate concentration measurements were carried out at the Bordeaux INRA Metabolome Platform (https://www.bordeaux.inra.fr/umr619/RMN_index.htm; Bordeaux, France) on sub-samples of root frozen powder (20 mg), using the spectrophotometric method described in Cross et al. [47].
qRT-PCR analyses
Total RNA was extracted from sub-samples of frozen root powder (100 mg) using the Nucleomag 96 RNA kit (Macherey-Nagel, Düren, Germany) on the Biosprint 96 (Qiagen, Hilden, Germany). Total RNA was subsequently purified with the NucleoSpin 96 RNA kit (Macherey-Nagel). This purification step allowed a complete elimination of both protein residues and residual genomic DNA contamination, with another DNase digestion step. Reverse transcription was carried out on 1 μg of RNA with the iScript Select cDNA synthesis kit (Bio-Rad, Richmond, CA, USA). Steps of extraction, purification and reverse transcription were carried out according to the manufacturer’s instructions.
The primer pairs used to quantify expression levels of genes coding for two root nitrate transporters TaNRT1 (GenBank AY587264) and TaNRT2.1 (GenBank AF332214.1), the nitrate reductase TaNR (whose partial sequence comes from Boisson et al. [48]) and the glutamine synthetase 2 TaGS2 (GenBank DQ124212.1) have been described in Taulemesse et al. [27]. The specificities and the efficiencies of the primer pairs had been validated previously.
Quantitative real-time experiments were carried on the Light Cycler 380 (Roche, Indianapolis, IN, USA) with the LightCycler 480 SYBR Green 1 Master Kit. Reactions were made with amounts of 12.5 ng cDNA for TaNR and TaGS2, or 31.25 ng cDNA for TaNRT1 and TaNRT2.1 because of lower expression levels observed for the two latter genes. PCR reactions were cycled for 10 min at 95°C followed by 45 cycles of 95°C for 10 s, 60°C for 15 s and 72°C for 15s. A melting curve was analysed at the end of each assay to ensure that single products were amplified. Relative expression was determined using the ∆CT method corrected for primers efficiency [49], and results were normalised to the expression of two housekeeping genes, Ta54280 and Ta54948, selected from Paolacci et al. [50], whose expression stability had already been validated under our experimental conditions.
Statistical analyses
Statistical analyses were carried out using R v2.15.1 [51] after conversion to a per-square-meter basis based on tube surface area. Graphics were drawn using SigmaPlot v8.0.
Results
Plant N status at flowering
Two contrasting N treatments were imposed on each of the two cvs. Renan and Récital before flowering (N4 and N10), to obtain plants having distinct N statuses at the flowering stage.
For both Renan and Récital, spike number per square meter, green laminae area, whole plant dry weight, whole plant N concentration, whole plant N and NNI were all strongly impacted by the pre-flowering N treatments (p<0.001) (Table 1). However, the N response of the two genotypes differed for some traits. Both Renan and Récital showed similar spike numbers under N10 condition, and again under N4 conditions. The mean decreases in spike number in response to reduced N were about 24% and 33%, for Renan and Récital respectively. Green laminae area was similar for the two genotypes across the two pre-flowering N treatments (p = 0.27), but a significant interaction term between genotype and N treatment (p = 0.02) revealed that Récital was more affected by the N4 treatment than Renan. The decrease in green laminae area between N10 and N4 was about 21% for Renan, and 57% for Récital. Whole plant dry weight responded to N deficiency in both genotypes, with an average decrease between N10 and N4 of 32% for Renan and 38% for Récital, but biomass was significantly higher in Renan than Récital across N treatments (p<0.001). The opposite result was observed for whole plant N concentration, with significantly lower levels for Renan than for Récital (p<0.001), despite similar responses to N deficiency with decreases of plant N concentration of 30% for Renan, and 29% for Récital. Total plant N showed a low significant difference between the two genotypes (p<0.05). The average decrease in total plant N in response to N deficiency was 53% for Renan, and 58% for Récital. Lastly, NNI levels were similar in the two genotypes under both N4 and N10 conditions (Table 1). Initially, the NNI index was developed to assess plant N status under field conditions [45], where a value less than ‘1’ indicates N deficiency [46]. Here, our NNI values were always higher than ‘1’, which suggests this index is not well-suited to the semi-hydroponic conditions of this study. Nevertheless, the results do confirm that the pre-flowering N treatments led to contrasting N statuses at flowering with average NNI values for the N10 and N4 treatments of 1.82 and 1.12, respectively.
Table 1. Spike number, green laminae area, plant dry weight, plant N concentration, total plant N and Nitrogen Nutrition Index (NNI) at flowering for the two genotypes at two contrasting pre-flowering N treatments.
Values are the means of sixteen biological repetitions ± 1 standard error (SE) for Plant dry weight, plant N concentration and total plant N, and the means of ten biological repetitions ± 1 standard error (SE) for spike number, green laminae area and NNI. Statistical groups are given by post-ANOVA Tukey HSD test for α = 0.05.
| Genotype | N treatment | Spike number (per m-2) ±SE | Green laminae area (m² m-2) ±SE | Plant dry weight (g m-2) ±SE | Plant N concentration (%DW) ±SE | Total plant N (g m-2) ±SE | NNI ±SE |
| Récital | N4 | 653 ±34 c | 4.8 ±0.4 c | 1152 ±83 d | 1.93 ±0.11 b | 21.1 ±0.8 d | 1.10 ±0.04 b |
| N10 | 971 ±66 a | 11.3 ±0.6 a | 1868 ±64 b | 2.73 ±0.05 a | 50.5 ±1.1 b | 1.82 ±0.03 a | |
| Renan | N4 | 735 ±53 bc | 8.3 ±0.5 b | 1555 ±67 c | 1.77 ±0.06 b | 27.0 ±0.6 c | 1.14 ±0.02 b |
| N10 | 970 ±94 ab | 13.0 ±1.6 a | 2292 ±88 a | 2.52 ±0.09 a | 57.6 ±2.4 a | 1.83 ±0.12 a | |
| Genotype | N treatment | Spike number (per m-2) ±SE | Green laminae area (m² m-2) ±SE | Plant dry weight (g m-2) ±SE | Plant N concentration (%DW) ±SE | Total plant N (g m-2) ±SE | NNI ±SE |
| Récital | N4 | 653 ±34 c | 4.8 ±0.4 c | 1152 ±83 d | 1.93 ±0.11 b | 21.1 ±0.8 d | 1.10 ±0.04 b |
| N10 | 971 ±66 a | 11.3 ±0.6 a | 1868 ±64 b | 2.73 ±0.05 a | 50.5 ±1.1 b | 1.82 ±0.03 a | |
| Renan | N4 | 735 ±53 bc | 8.3 ±0.5 b | 1555 ±67 c | 1.77 ±0.06 b | 27.0 ±0.6 c | 1.14 ±0.02 b |
| N10 | 970 ±94 ab | 13.0 ±1.6 a | 2292 ±88 a | 2.52 ±0.09 a | 57.6 ±2.4 a | 1.83 ±0.12 a |
Impact of N treatments on agronomic traits at maturity
At flowering, plants from the N4 and N10 pre-flowering treatments were divided into two identical groups. One group was exposed to a low-N post-flowering treatment (LN), and the other to a high-N treatment (HN). This factorial design allowed us to record the behaviours of initially high- and low-N status plants under high- and low-N conditions during the post-flowering period. At maturity, four key agronomic traits were recorded to characterise the performances of the two genotypes. The traits were: GY, grain N concentration, grain N yield, and N harvest index (Table 2).
Table 2. Grain yield, grain N concentration, total grain N and N harvest index at maturity for the two genotypes studied under four N treatments.
Values are the means of five biological repetitions ± 1 standard error (SE). Statistical groups are given by post-ANOVA Tukey HSD test for α = 0.05.
| Genotype | N treatment | Grain yield (g m-2) ±SE | Grain N concentration (%DW) ±SE | Total grain N (g m-2) ±SE | N Harvest Index ±SE |
|---|---|---|---|---|---|
| Récital | N 4-LN | 527.4 ±62.3 d | 2.62 ±0.19 ab | 13.47 ±1.33 e | 0.41 ±0.02 b |
| N4-HN | 778.6 ±133.7 cd | 2.65 ±0.11ab | 20.06 ±2.74 de | 0.46 ±0.04 b | |
| N10-LN | 1531.6 ±116.5 a | 2.31 ±0.06 b | 35.14 ±1.95 abc | 0.49 ±0.04 b | |
| N10-HN | 1384.0 ±153.4 ab | 2.33 ±0.04 b | 32.25 ±3.72 c | 0.43 ±0.03 b | |
| Renan | N 4-LN | 1281.4 ±80.9 ab | 2.68 ±0.03 ab | 34.29 ±2.01 bc | 0.66 ±0.05 a |
| N4-HN | 1016.6 ±77.4 bc | 2.64 ±0.11 ab | 26.51 ±1.35 cd | 0.50 ±0.05 b | |
| N10-LN | 1576.3 ±72.5 a | 2.81 ±0.08 a | 44.28 ±2.52 ab | 0.62 ±0.03 a | |
| N10-HN | 1502.8 ±81.4 a | 3.04 ±0.10 a | 45.41 ±1.62 a | 0.48 ±0.03 b |
The semi-hydroponic growth conditions led to GY values of between 527 and 1576 g DW m-2 (Table 2). As expected, GY was positively affected by pre-flowering N treatments in both Récital and Renan (p = <0.001) but no significant effect of the post-flowering N treatments was detected (p = 0.42). Combining the results across all treatments, Renan exhibited a higher GY than Récital (p<0.001). A significant interaction between genotype and pre-flowering N treatment (p<0.001) showed that the GY for Récital was more affected by the low-N pre-flowering treatment (N4) than that for Renan.
Grain N concentrations varied between 2.3% and 3.0% (Table 2), these corresponding to high levels of GPC (13.2% and 17.3% respectively). Interestingly, the Anova revealed no significant effect of post-flowering N treatment on grain N concentration, as previously observed for grain yield. However, there was a strong genotype effect (p<0.001) and a significant interaction between genotype and pre-flowering N treatment (p<0.001). Hence, Renan had a higher grain-N concentration than Récital, which resulted principally from strong differences observed between the two genotypes when submitted to N10 pre-flowering treatment. An interesting observation was the opposite-going response of the two genotypes to the pre-flowering N treatments, revealed in the significant interaction term. Here, an increase in pre-flowering N availability led to a decrease in grain N concentration in Récital, but to an increase in Renan.
Total grain N was influenced mostly by GY as the latter trait varied more in response to N availability. Analysis of total grain N showed a significant genotype effect (p<0.001) and pre-flowering N treatment effect (p<0.001). The two genotypes both responded positively to high pre-flowering N availability, although when all treatments were combined, Renan had a higher grain N yield, than Récital.
The N harvest index (NHI) provides information on the distribution of N between the vegetative organs and the grain. Interestingly NHI was significantly influenced by genotype (p<0.001), by post-flowering N treatment (p<0.001) and by the interaction between genotype and post-flowering N treatment (p<0.001). Renan had a higher NHI than Récital, due to the high NHI levels recorded in the low-N post-flowering treatment (LN). The significant interaction term between genotype and post-flowering N treatment reveals that the increase in NHI observed for the post-flowering LN treatment in Renan was not apparent in Récital.
Relations between grain yield and grain N concentration
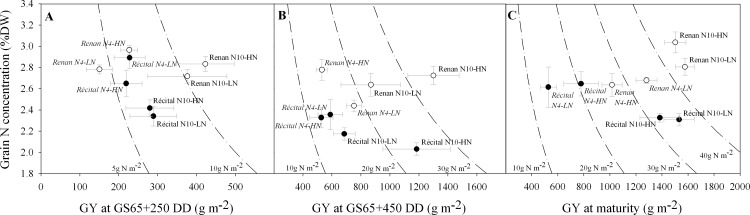
The relationship between grain yield and grain N concentration is at the basis of the calculation of GPD. This study does not allow GPD to be calculated stricto sensu because it considers only two genotypes. Nevertheless the graphical representation of the relationship between GY and grain N concentration at maturity (Fig 1C) does offer a good visualisation of both genotypes and N treatment effects. In particular, the causes for Renan’s higher total grain N appears clearly. While this was caused by a higher GY than Récital in the case of the low-N pre-flowering treatment (N4), this trait was caused by a higher grain N concentration in the high-N pre-flowering treatment (N10).
Fig 1. Relations between grain yield and grain N concentration at three post-flowering developmental stages.
Data were collected at GS65+250 DD (A), GS65+250 DD (B) and at maturity (C) for Récital (black circles) and Renan (white circles) exposed to two contrasting pre-flowering nitrate treatments N4 (italic labels) and N10 (regular labels) combined with two contrasting post-flowering nitrate treatments LN and HN. Dotted lines are iso-grain N yield. Values are the means of five biological replicates ± 1 standard error.
The relation between GY and grain N concentration was also observed at two intermediate stages of grain development. At GS65+250 DD (Fig 1A) and at GS65+450 DD (Fig 1B), there was no significant genotype effect on GY, whereas grain N concentration was already significantly higher in Renan across all treatments (p<0.001). These results show that differences between the two genotypes in grain N concentration observed under N10 are likely to have been established early in grain development. Oppositely, the differences in grain yield observed under N4 occurred at a later stage. Pre-flowering N treatment effects on grain N concentration and GY were also already effective (p<0.001 and p = 0.003, respectively), revealing that for Récital, the N4 pre-flowering treatment led to higher grain N concentration than N10 early during grain filling.
Post-flowering N uptake and N remobilisation
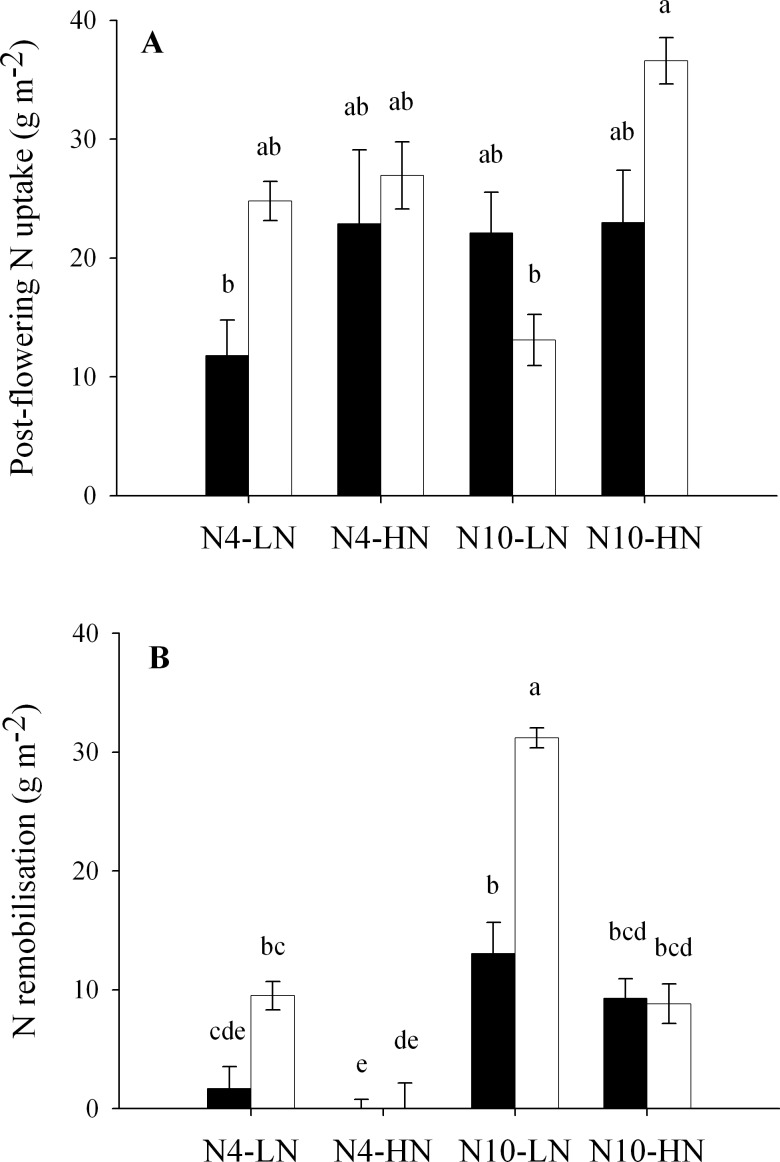
Post-flowering N uptake (PANU) ranged between 11 and 36 g m-² (Fig 2A), representing from 30 to 100% of total grain N at maturity. In the case of the HN post-flowering treatment, PANU represents the equivalent of 71 to 100% of total grain N. The Anova reveals a significant genotypic effect, with higher PANU levels in Renan (p = 0.04), and a strong post-flowering N treatment effect (p<0.001) showing that the HN treatment led to overall increases in PANU. However, a third-order interaction, genotype × post-flowering N treatment × pre-flowering N treatment (p = 0.003) emphasises that this trait is responsive to complex interaction effects, so the main factor effects should be interpreted cautiously. The main point to highlight for PANU is the marked difference observed between LN (12 g m-²) and HN (36 g m-²) post-flowering treatments in Renan plants subjected to the N10 pre-flowering treatment. This was clearly highly influenced by N availability during the post-flowering period.
Fig 2.
Post-flowering N uptake (A) and N remobilisation (B) for Récital (black circles) and Renan (white circles) exposed to two contrasting pre-flowering nitrate treatments N4 and N10 combined with two contrasting post-flowering nitrate treatments LN and HN. Values are the means of five biological replicates ± 1 standard error. Statistical groups are given by post-ANOVA Tukey HSD test for α = 0.05.
In this study, it was found that PANU was not significantly correlated with N remobilisation (p = 0.458), which contrasts with significant and negative correlations reported from experiments carried out under field conditions that focused on genotypic effects [5,16,23]. Remobilisation of N from vegetative parts to the grain varied between 0 and 31 g m-2 (Fig 2B), representing from 0 to 70% of total grain N. In the case of HN post-flowering treatment, remobilisation always represented less than 29% of total grain N. Anova revealed that N remobilisation is a complex trait, impacted by all three main factors (genotype, p<0.001; pre-flowering N treatment, p<0.001; post-flowering N treatment, p<0.001) and interaction terms between genotype and post-flowering N treatment (p<0.001), together with a third-order interaction (p = 0.038). Overall, Renan remobilised more than Récital, which is mostly explained by the high values observed for Renan plants exposed to N10-LN and N4-LN treatments. Remobilisation was also generally favoured in the N10 pre-flowering treatment and LN post-flowering treatment, leading to high N stocks in vegetative parts at flowering and low N availability during the post-flowering period, respectively. Lastly, the interaction between genotype and post-flowering N treatment effects reflects the higher capacity of Renan to increase its remobilisation when exposed to the LN post-flowering N treatment.
Relation between grain N concentration and early PANU
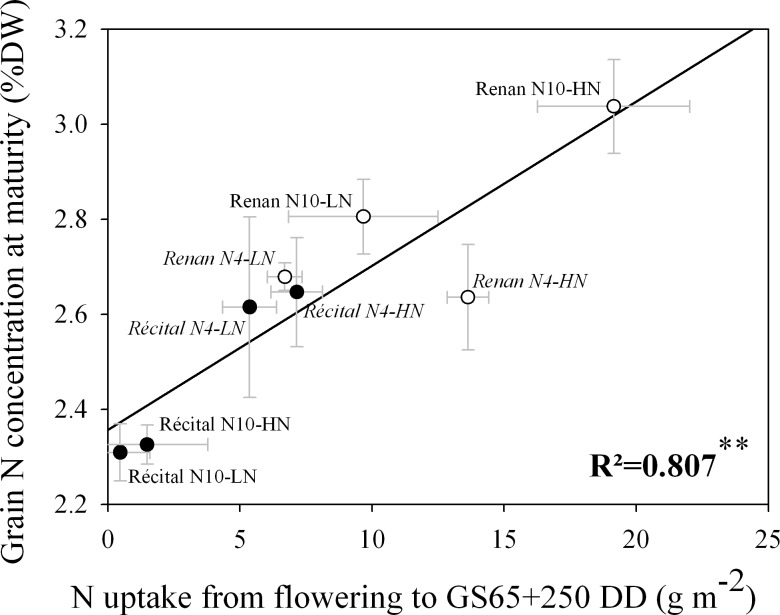
In this study, early PANU occurring from GS65 to GS65+250 DD represents a highly variable part of total PANU, ranging between 2% and 74% depending on genotype and N treatment. This early PANU was positively correlated with grain N concentration at maturity (p = 0.002) (Fig 3), as well as with grain N concentration measured at GS65+250 DD (p = 0.040), and at GS65+450 DD (p = 0.002) (S1 Fig). Among all measured traits in this study, early PANU was the only one which displayed a significant correlation with grain N concentration at maturity. Oppositely, total PANU measured at maturity was not significantly correlated with grain N concentration (p = 0.414).
Fig 3. Relation between N uptake from flowering to GS65+250 DD and grain N concentration at maturity for Récital (black circles) and Renan (white circles) exposed to two contrasting pre-flowering nitrate treatments N4 (italic labels) and N10 (regular labels) combined with two contrasting post-flowering nitrate treatments LN and HN.
Values are the means of five biological replicates ± 1 standard error.
Physiological and molecular determination of early PANU
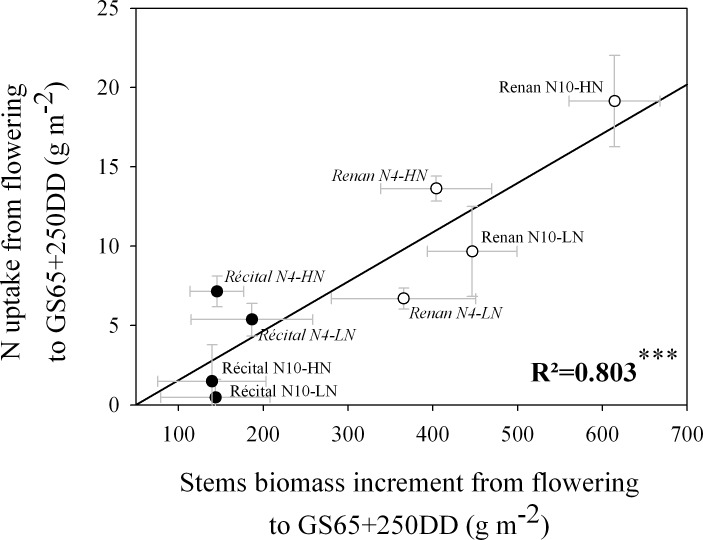
At a physiological level, early PANU at GS65+250 DD was positively correlated with the stem biomass increment between GS65 and GS65+250 DD (r² = 0.80; p = 0.003) (Fig 4); the stem biomass increment from GS65 to GS65+250 DD being itself under the influence of a genotypic effect (p<0.001) with higher levels observed for Renan.
Fig 4. Relation between stem biomass increment and N uptake from flowering to GS65+250 DD for Récital (black circles) and Renan (white circles) exposed to two contrasting pre-flowering nitrate treatments N4 (italic labels) and N10 (regular labels) combined with two contrasting post-flowering nitrate treatments LN and HN.
Values are the means of five biological replicates ± 1 standard error.
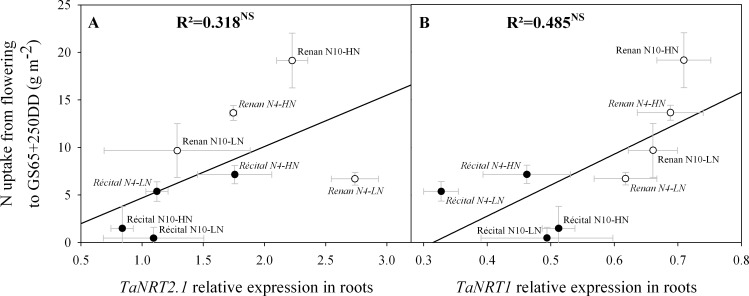
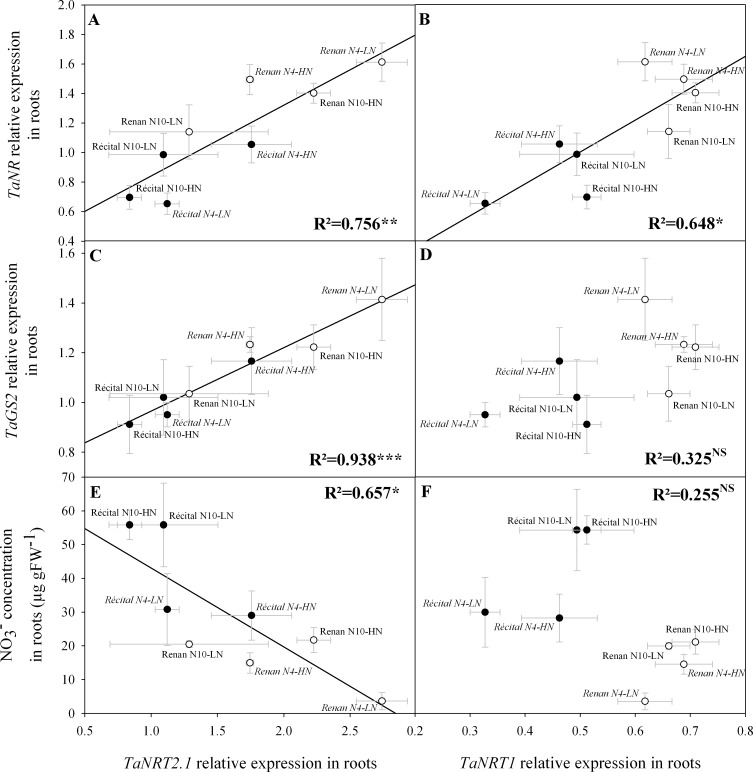
At a molecular scale, although not statistically significant, PANU at GS65+250 DD was positively correlated with both the expression of the NO3- high-affinity transporter TaNRT2.1 (p = 0.145) (Fig 5A) and that of the low-affinity transporter TaNRT1 (p = 0.055) (Fig 5B). At GS65+250 DD, TaNRT2.1 expression levels were significantly correlated with those of TaNR (Fig 6A) and TaGS2 (Fig 6C). At the same developmental stage, TaNRT1 expression levels correlated positively with those of TaNR (Fig 6B), but were not correlated with those of TaGS2 (Fig 6D). Genes coding for root NO3- transporters as well as genes coding for NR and GS2 exhibited higher expression levels in Renan than in Récital at GS65+250DD (p = 0.001, p<0.001, p<0.001, and p = 0.02, respectively for TaNRT2.1, TaNRT1, TaNR, and TaGS2).
Fig 5. Relations between expression levels of genes coding for root nitrate transporters and N uptake from flowering to GS65+250 DD.
Relations are for TaNRT2.1 relative expression in roots at GS65+250 DD (A) and TaNRT1 relative expression in roots at GS65+250 DD (B). Values are for Récital (black circles) and Renan (white circles) exposed to two contrasting pre-flowering nitrate treatments N4 (italic labels) and N10 (regular labels) combined with two contrasting post-flowering nitrate treatments LN and HN. Values are the means of five biological replicates ± 1 standard error.
Fig 6. Relations between relative expression levels of genes coding for root nitrate transporters and relative expression levels of genes involved in nitrate assimilation, or nitrate concentration in roots at GS65+250 DD.
Relations are for TaNRT2.1 and TaNR (A), TaNRT2.1 and TaGS2 (C), TaNRT2.1 and root NO3- concentration (E), TaNRT1 and TaNR (B), TaNRT1 and TaGS2 (D), TaNRT1 and root NO3- concentration (F). Values are for Récital (black circles) and Renan (white circles) exposed to two contrasting pre-flowering nitrate treatments N4 (italic labels) and N10 (regular labels) combined with two contrasting post-flowering nitrate treatments LN and HN. Values are the means of five biological replicates ± 1 standard error.
Finally, TaNRT2.1 expression and root NO3- concentrations were negatively correlated at GS65+250 DD (p = 0.015) (Fig 6E), whereas no correlation was observed between TaNRT1 expression and root NO3- concentration (p = 0.202) (Fig 6F). Root NO3- concentrations were significantly higher in Récital than in Renan (p<0.001). An effect of pre-flowering NO3- treatment was also revealed by the Anova test, showing that the N10 treatment led to higher root NO3- concentrations than the N4 treatment (p = 0.001).
Discussion
Growing conditions and choice of genotypes
The literature reveals that most studies of wheat root processes at a fine scale used plants grown under hydroponic conditions. The semi-hydroponic approach used here allows a fine control of N availability and it also allows to perform rapid changes to the effective N availability because of the low N-retention capacity of the substrate. Compared to field trials, these features are particularly useful in allowing independent control of the available N before and after flowering, without neglecting any genetic differences in terms of plant phenology.
In this study, we used an approach based on two contrasting NO3- concentrations (4 and 10 mM) before flowering. The 10 mM NO3- concentration is generally considered non-limiting for hydroponic wheat [27,34,52], while 4 mM NO3- generates a significant N stress but without having drastic effects on canopy structure in terms of tiller density and dynamics [27]. Clearly, our objective of generating contrasting N statuses at flowering was met as shown by the significant differences between N4 and N10 for all the physiological traits measured at flowering (Table 1). After flowering, plants from each pre-flowering treatment were subjected to either a high-N (10 mM; HN) or a low-N (4 mM; LN) post-flowering N treatment in order to observe the independent effects of N availability before and after flowering. Although N status at flowering had large effects on GY, post-flowering N availability effects were lower than expected on GPC. This shows that even if the N4 treatment was clearly limiting for N during the pre-flowering period, the LN post-flowering treatment was not highly N deficient. However, these contrasting treatments allow significant differences in total grain N at maturity to be created, a circumstance particularly favourable for research into GPD determinism. To this aim, our strategy was to use two significantly contrasting genotypes for GPD based on an earlier robust characterisation of this trait in field studies [10,23]. The experimental design used in this study, comprising only two genotypes, did not allow calculating GPD values for Récital and Renan. However, genotypic ranking for GPD can be reasonably estimated by a ranking for protein yield [7,53]. Under our conditions, Renan clearly exhibited larger grain N values than Récital, showing that the genetic component implied in protein yield was expressed under our controlled conditions in a way similar to in the field [23,42,54]. These results suggest that GPD may be a sufficiently robust genetic character to be expressed even under such different growing conditions.
GPC at maturity is correlated with early PANU at GS65+250 DD
Under field conditions, numerous studies have highlighted that PANU has a larger effect on grain N than on yield [5,14,24–26]. In the present study, although no correlation was observed between total PANU and GPC (r² = 0.11; p = 0.414), a strong correlation was found between GPC and early PANU (e.g. PANU calculated only between flowering and GS65 + 250 DD). This result, although only correlative, suggests that this trait could be a strong driver of final GPC. Quantitatively, early PANU represented on average 36% of total PANU. In a study based on four spring wheat genotypes grown hydroponically under condition of gradual decrease of N availability during post-flowering period mimicking field conditions, Oscarson et al. [19] observed an even higher value, with about 50% of total PANU occurring before the end of grain cell division. Hence, early PANU may represent a large part of total PANU under field conditions because of the various environmental limits to PANU occurring later during grain filling, water and N stresses in particular. This could be the reason for the stronger effect of total PANU on GPC observed under field conditions than in the present study. Taken together, these results plead for strong effect of early PANU on GPC, accounting both for existing genetic and environmental variabilities.
Datasets that allow us to temporally dissect the relationship established over the entire post-flowering period are scarce. Under non-limiting conditions for N, in hydroponic culture, Taulemesse et al. [27] highlighted that PANU exhibits a marked dynamic during the post-flowering period. Further, these authors showed that PANU occurring during the early post-flowering phase (grain cell division) was strongly impacted by plant N status at flowering, whereas that occurring during the later post-flowering phase (active phase of grain N filling) was hypothesised to be mainly controlled by N demand exerted by grain growth. Clearly, our results suggest that the N taken up after flowering has a differential effect on GPC depending of the timing at which it occurs during the post-flowering period. Supporting this idea, in a study based on a high GPC genotype grown hydroponically, Oscarson et al. [20] have shown that the timing of short extra applications of N during the post-flowering period impacts GPC for N-limited plants. However, it is difficult to know whether the results of Oscarson et al. can be transposed to non-deficient plants where GY is not impacted by N starvation during the post-flowering period as in our study. With a different approach based on a multi-local study under field conditions on mapping populations, Bogard et al. [23] reported that GPD measured at GS65+250 DD is well correlated with GPD measured at maturity (r² = 0.50), possibly indicating an early determinism of this trait. As GPD was strongly correlated with genotype capacity to take up N after flowering independently of the level of N uptake before flowering, these authors hypothesised that early PANU could play a strong role in GPD. This hypothesis is in accordance with our results which show that a high early PANU is associated with a high GPC under our controlled conditions, independently of GY level.
Genetic variability for early PANU regulation
Quantitatively, early PANU represents a highly variable component of total grain N at maturity, ranging from 1% to 51% depending on genotype and N treatment. On average, early PANU was higher for Renan than for Récital (12.28 g m-2 and 3.6 g m-2, respectively). In addition to these quantitative variations, the two genotypes displayed contrasting controls of early PANU (S2 Fig). On the one hand, in Récital, the ratio of early PANU over total grain N at maturity was influenced mainly by plant N status at flowering (i.e. showing a strong effect of pre-flowering N treatment) with values of about 41% for plants exposed to the N4 pre-flowering treatment, and less than 7% for plants exposed to the N10 one, with no effect of the post-flowering N treatment. Comparable results have already been described for Récital, with higher early PANU observed for plants having low N status at flowering [27]. On the other hand, in Renan, this ratio was impacted mainly by post-flowering N availability, with values around 20% for plants exposed to the LN post-flowering treatment, compared with about 45% for plants exposed to the HN one, while the effect of pre-flowering N treatment was negligible. This argues strongly for genotypic differences in the control of early PANU.
The regulation of N uptake is under the control of complex mechanisms. Earlier studies have shown that N uptake is driven not only by soil N availability but also by plant N demand [4,55–57]. Under hydroponic conditions, plant N uptake occurs until late in plant development [19–22,27,58], and roots have the ability to take up N even when the concentration in the medium is extremely low [19]. Under such conditions, plant N demand could play a central role in N uptake variability. Accordingly, the early PANU observed in Récital was not significantly affected by post-flowering N availability, while the effect of plant N status at flowering was dominant. However, the observations in Renan suggest plant N status at flowering have less impact on early PANU for this genotype because early PANU was clearly limited by N availability for both N statuses at flowering under LN post-flowering condition. Despite the fact that Renan and Récital had roughly comparable NNI at flowering, their N uptakes differed during the days following flowering, particularly for plants exposed to the N10 pre-flowering treatment. This suggests that NNI, which is a valuable integrative indicator of limitation of carbon acquisition by plant N concentration during vegetative growth [46], does not allow the assessment of post-flowering N uptake as genetic variability exists at particular NNI values. These findings argue for the existence of a genetic difference in N satiety at similar NNI, which could be defined as the maximum level of plant N accumulation corresponding to a state of plant N saturation. Genotypic variability for N satiety may thus be related to genotypic deviations from the “maximum N dilution curve” described by Justes et al. [46]. Hence, N satiety may influence the genotypic capacity to take up luxury levels of N, i.e. amounts not strictly necessary for growth but possibly beneficial to GPC. Genetic differences for N satiety have previously been hypothesised to be involved in the determination of GPD [23], and could also explain the reasons for the higher sensibility to N starvation of Récital observed previously [59].
Genetic variability for early PANU is linked to vegetative growth after flowering
As noted above, internal regulatory mechanisms of N uptake are driven mainly by physiological parameters such as organ growth and the N concentration of already-formed organs. Early PANU occurs during a period when growing organs represent only a moderate biomass accumulation, because grains have not yet reached a rapid growth phase and the photosynthetic apparatus is already fully developed. The grain biomass increment from GS65 to GS65+250 DD does not explain early PANU, as these two traits were not significantly correlated in the present study (p = 0.261). This finding is in accordance with previous results obtained by Taulemesse et al. [27] in Récital which rather suggest a major role for plant N status in N uptake at this developmental stage. In the framework of model development for grain protein concentration in wheat, Martre et al. [60] suggested that the rate of N uptake after flowering could be limited by the N storage capacity of the stem. In this sense, some studies have shown that stem biomass may continue to increase during about one week after flowering, especially through peduncle elongation [61,62]. Potentially, the growth of this structural organ could create a sink demand for N, thus influencing N uptake, especially as stem biomass increments from GS65 to GS65+250 DD exceeded grain biomass accumulation for some genotype x N treatment combinations in the present study. As a result, early PANU displayed a positive correlation with the stem biomass increment from GS65 to GS65+250DD (Fig 4) but not with stem N concentration variation during the same phase (p = 0.946). Clearly, the correlative approach used in the present study does not provide any causal demonstration of the link between the two traits. However, the high levels of NNI measured at flowering do seem to indicate that plant growth during the early post-flowering phase was not limited by plant N under our conditions, with values always higher than 1. This suggests that observed genetic differences for stem growth after flowering were probably not a consequence of N uptake, but were more likely partly causal. Regulation of stem growth after flowering could thus be involved in grain N concentration determination.
Molecular determination of early PANU
Genes implicated in plant N uptake and N assimilation network have been described largely in model species such as A. thaliana [30–33]. Not all homologs of these genes have yet however been characterised in wheat, leading to an incomplete characterisation of N uptake through gene expression studies. Nevertheless, expression quantification of some key genes involved at different levels of N metabolism allows the establishment of a good overview of the N assimilation process. In this study, we observed no significant correlation between early PANU and root N transporter expression, either for TaNRT2.1 or TaNRT1. This result differs from that of a previous study [27] showing a significant correlation between PANU and TaNRT2.1 expression level in wheat. Similarly, in maize, Garnett et al. [63] showed that ZmNRT2.1 and ZmNRT2.2 expression patterns correlated well with the N uptake pattern. The methodology used in the present study to calculate early PANU may partly explain this lack of significant correlation, as PANU was calculated based on total plant N differences between two sampling dates (and therefore integrating plant functioning over the whole period) while expression results were based on instantaneous measurements. We believe this may have reduced the accuracy of the relationship which nevertheless shows a positive trend (Fig 5A).
In addition, the relation between TaNRT2.1 expression at GS65+250 DD and early PANU was significantly positive (p = 0.007) after exclusion of Renan N4-LN. This treatment x Genotype combination exhibited the highest TaNRT2.1 expression level but a very low N uptake. In this case, a consequent N demand that cannot be fulfilled under LN conditions can be strongly hypothesised. The positive correlation between early PANU and TaNRT2.1 expression levels at GS65+250 DD without Renan N4-LN suggests that TaNRT2.1 could have played an important role in early PANU, although its expression levels did not explain N uptake when N demand was not satisfied. This assumption is in accordance with recent studies suggesting a preponderant involvement of HATS on wheat nitrogen uptake independently of NO3- concentration in the external medium [38,39].
This assumption is also strengthened by the observation that TaNRT2.1 expression was in turn significantly correlated with TaNR and TaGS2 expression levels at GS65+250 DD, suggesting a consistency between N uptake, N reduction and N assimilation genes (Fig 6). A synchronous regulation of genes coding for N transport, reduction and assimilation against NO3- environment has been substantially described in a range of species such as A. thaliana [64], Oriza sativa [65], and Zea mays [66–69]. However, the regulation of N uptake and N assimilation systems at the molecular level has not clearly been established, as at least two putative regulatory signals have been proposed for N uptake regulation. The first is circulating amino-acids such as glutamine [70–72] and the second is NO3- itself [73–75]. In Taulemesse et al. [27] and in the present study, TaNRT2.1 expression levels at GS65+250 DD were negatively correlated with NO3- concentration in roots, suggesting that NO3- concentration could be an internal marker of plant N demand with a putative direct or indirect regulatory role on genes coding for N metabolism. Similarly, TaNR and TaGS2 expression levels were negatively correlated with root NO3- concentration (S3 Fig). The hypothesis identifying NO3- concentration in roots as a marker of plant N demand is also supported by physiological measurements, as NO3- levels in roots were significantly lower in N4 than in N10 at flowering independently of genotype. The rapid increase in NO3- level in roots after the switch from N4 to HN tends to corroborate this assumption. The putative role of NO3- concentration in roots as a marker of plant N demand is also implied by genotypic differences. Indeed, the higher levels of early PANU observed in Renan were accompanied by lower root NO3- concentrations in this genotype than in Récital, regardless of the pre-flowering N treatment (S4 Fig).
Excluding Renan N4-LN, the relation between NO3- concentration and early PANU became significant (p = 0.018; S5 Fig). The outlier nature of Renan N4-LN is probably a result of the same factors previously suggested for the relation between TaNRT2.1 expression level and early PANU. Genetic effects on early PANU could thus be based on differences in NO3- concentration in roots, despite comparable NNI, revealing different N satiety levels.
Putative influence of satiety level on the balance between PANU and N remobilisation
In the present study, PANU and N remobilisation were not significantly correlated (p = 0.458) when considering the two genotypes and the four N conditions. The absence of correlation was probably influenced by the contrasting N environments used in both the pre-flowering and the post-flowering periods, but was also a result of a genotypic effect. Renan showed both higher PANU and N remobilisation than Récital with all treatments combined (Fig 2), showing that Récital did not compensate for its lower N uptake by N remobilisation. The N remobilisation under LN treatment in Renan was generally higher, leading to a higher NHI of this genotype than Récital when exposed to low N availability during the post-flowering period (Table 2). Under field conditions, the low capacity of Récital to achieve high remobilisation levels has already been observed [14]. These results seem to indicate that Renan has both a higher capacity to take up N when N is available and a lower threshold for N remobilisation than Récital. Based on the fact that remobilisation is involved when N uptake cannot meet N demand, it is plausible that the specific satiety level of each genotype influences the triggering of this phenomenon. According to this, despite comparable NNI levels, a variety having a high luxury N demand must have more difficulty taking up enough N to be satiated than a variety having a low luxury N demand, which may precipitate the onset of senescence. Thus, as root NO3- concentration seems to be a reliable indicator of plant N demand, cultivars presenting low NO3- concentrations in their roots even at high NNI, could be both more effective in N uptake when N is available, and better able to use N stored in vegetative parts when the availability of N is insufficient during grain filling.
Conclusions
This study aimed to identify genetic differences in the establishment of GPC in order to better understand the genetic bases of GPD. Based on the behavior of two genotypes having strongly contrasting GPC grown under controlled conditions with varying NO3- availabilities, we show that GPC is positively correlated with early PANU from GS65 to GS65+250 DD independently of GY level. At a physiological level, the study suggests that early PANU could be impacted by stem biomass increment during early growth stages following flowering, although the regulatory mechanisms of this are unknown. At a molecular scale, the negative correlation between root NO3- concentration and N network genes expression levels suggests that root NO3- concentration is a good candidate for evaluating instantaneous plant N demand, and may provide valuable information on genotype satiety level when measured at high NNI. Although these findings have still to be validated with a larger number of genotypes, this study attempts to open new research routes to develop a better understanding of the physiological and molecular bases of genetic determination of grain N concentration involved in GPD. On the basis of our results, genotypes having a low NO3- concentration in the roots, even at high NNI levels, may be interesting subjects for breeding as they have a tendency to accumulate more N both at flowering and during the early post-flowering growth stages, but they also seem more able to remobilise N, when its availability is reduced during grain filling.
Supporting Information
(XLSX)
Relation between N uptake from flowering to GS65+250 DD and grain N concentration at GS65+250 DD (A) or at GS65+450 DD (B).
(TIF)
(TIF)
Relations between nitrate concentration and TaNR relative expression (A) or between nitrate concentration and TaGS2 relative expression (B) in roots at GS65+250 DD.
(TIF)
(TIF)
(TIF)
(PDF)
Acknowledgments
The authors thank Joelle Messaoud, Antoine Bordes and Julien Mazuel (INRA Clermont-Ferrand) for their help with experiments. The authors are also grateful to Patricia Ballias and Duyen Prodhomme (INRA Villenave d’Ornon) for their help in nitrate assays. Sandy Lang (rescript.co.nz) proofread and provided language assistance.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the French “Fonds de Soutien à l’Obtention Végétale” (FSOV) 2010F project (2011–2013). The authors are also grateful to the ANRT (Association Nationale de la Recherche et de la Technologie) which supports the PhD thesis (CIFRE 878/2011) during which the analyses were conducted.
References
- 1.Branlard G, Dardevet M, Saccomano R, Lagoutte F, Gourdon J. Genetic diversity of wheat storage proteins and bread wheat quality. Euphytica. 2001;119: 59–67. [Google Scholar]
- 2.Shewry PR. Improving the protein content and composition of cereal grain. J Cereal Sci. 2007;46: 239–250. [Google Scholar]
- 3.Simmonds NW. The relation between yield and protein in cereal grain. J Sci Food Agric. 1995;67: 309–315. [Google Scholar]
- 4.Feil B. The inverse yield-protein relationship in cereals: possibilities and limitations for genetically improving the grain protein yield. Trends Agron. 1997;1: 103–119. [Google Scholar]
- 5.Monaghan JM, Snape JW, Chojecki AJS, Kettlewell PS. The use of grain protein deviation for identifying wheat cultivars with high grain protein concentration and yield. Euphytica. 2001;122: 309–317. [Google Scholar]
- 6.Triboi E, Martre P, Girousse C, Ravel C, Triboi-Blondel A-M. Unravelling environmental and genetic relationships between grain yield and nitrogen concentration for wheat. Eur J Agron. 2006;25: 108–118. [Google Scholar]
- 7.Oury F-X, Godin C. Yield and grain protein concentration in bread wheat: how to use the negative relationship between the two characters to identify favourable genotypes? Euphytica. 2007;157: 45–57. [Google Scholar]
- 8.Calderini DF, Slafer GA. Changes in yield and yield stability in wheat during the 20th century. Field Crops Res. 1998;57: 335–347. [Google Scholar]
- 9.Calderini DF, Torres-León S, Slafer GA. Consequences of wheat breeding on nitrogen and phosphorus yield, grain nitrogen and phosphorus concentration and associated traits. Ann Bot. 1995;76: 315–322. [Google Scholar]
- 10.Oury FX, Berard P, Brancourt-Hulmel M, Heumez E, Pluchard P, Rousset M, et al. Yield and grain protein concentration in bread wheat: a review and a study of multi-annual data from a French breeding program [Triticum aestivum L.]. J Genet Breed. 2003;57. [Google Scholar]
- 11.Foulkes MJ, Hawkesford MJ, Barraclough PB, Holdsworth MJ, Kerr S, Kightley S, et al. Identifying traits to improve the nitrogen economy of wheat: Recent advances and future prospects. Field Crops Res. 2009;114: 329–342. [Google Scholar]
- 12.Rothstein SJ. Returning to our roots: making plant biology research relevant to future challenges in agriculture. Plant Cell. 2007;19: 2695–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbottin A, Lecomte C, Bouchard C, Jeuffroy M- H. Nitrogen Remobilization during Grain Filling in Wheat. Crop Sci. 2005;45: 1141. [Google Scholar]
- 14.Kichey T, Hirel B, Heumez E, Dubois F, Le Gouis J. In winter wheat (Triticum aestivum L.), post-anthesis nitrogen uptake and remobilisation to the grain correlates with agronomic traits and nitrogen physiological markers. Field Crops Res. 2007;102: 22–32. [Google Scholar]
- 15.Gaju O, Allard V, Martre P, Snape JW, Heumez E, LeGouis J, et al. Identification of traits to improve the nitrogen-use efficiency of wheat genotypes. Field Crops Res. 2011;123: 139–152. [Google Scholar]
- 16.Cox MC, Qualset CO, Rains DW. Genetic variation for nitrogen assimilation and translocation in wheat. III. Nitrogen translocation in relation to grain yield and protein. Crop Sci. 1986;26: 737–740. [Google Scholar]
- 17.Palta JA, Fillery IRP. N application enhances remobilization and reduces losses of pre-anthesis N in wheat grown on a duplex soil. Aust J Agric Res. 1995;46: 519–531. [Google Scholar]
- 18.Cox MC, Qualset CO, Rains DW. Genetic variation for nitrogen assimilation and translocation in wheat. II. Nitrogen assimilation in relation to grain yield and protein. Crop Sci. 1985;25: 435–440. [Google Scholar]
- 19.Oscarson P, Lundborg T, Larsson CM. Genotypic differences in nitrate uptake and nitrogen utilization for spring wheat grown hydroponically. Crop Sci. 1995;35: 1056–1062. [Google Scholar]
- 20.Oscarson P, Lundborg T, Larsson M, Larsson C-M. Fate and effects on yield components of extra applications of nitrogen on spring wheat (Triticum aestivum L.) grown in solution culture. Plant Soil. 1995;175: 179–188. [Google Scholar]
- 21.Oscarson P. Transport of recently assimilated 15N nitrogen to individual spikelets in spring wheat grown in culture solution. Ann Bot. 1996;78: 479–488. [Google Scholar]
- 22.Oscarson P. The strategy of the wheat plant in acclimating growth and grain production to nitrogen availability. J Exp Bot. 2000;51: 1921–1929. [DOI] [PubMed] [Google Scholar]
- 23.Bogard M, Allard V, Brancourt-Hulmel M, Heumez E, Machet J-M, Jeuffroy M-H, et al. Deviation from the grain protein concentration-grain yield negative relationship is highly correlated to post-anthesis N uptake in winter wheat. J Exp Bot. 2010;61: 4303–4312. 10.1093/jxb/erq238 [DOI] [PubMed] [Google Scholar]
- 24.Van Sanford DA, MacKown CT. Cultivar differences in nitrogen remobilization during grain fill in soft red winter wheat. Crop Sci. 1987;27: 295–300. [Google Scholar]
- 25.Gooding MJ, Davies WP. Foliar urea fertilization of cereals: a review. Fertil Res. 1992;32: 209–222. [Google Scholar]
- 26.Gooding MJ, Gregory PJ, Ford KE, Ruske RE. Recovery of nitrogen from different sources following applications to winter wheat at and after anthesis. Field Crops Res. 2007;100: 143–154. [Google Scholar]
- 27.Taulemesse F, Le Gouis J, Gouache D, Gibon Y, Allard V. Post-flowering nitrate uptake in wheat is controlled by N status at flowering, with a putative major role of root nitrate transporter NRT2.1. Yang H, editor. PLOS ONE. 2015;10: e0120291 10.1371/journal.pone.0120291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maathuis FJ. Physiological functions of mineral macronutrients. Curr Opin Plant Biol. 2009;12: 250–258. 10.1016/j.pbi.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 29.Léran S, Varala K, Boyer J-C, Chiurazzi M, Crawford N, Daniel-Vedele F, et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014;19: 5–9. 10.1016/j.tplants.2013.08.008 [DOI] [PubMed] [Google Scholar]
- 30.Miller AJ, Fan X, Orsel M, Smith SJ, Wells DM. Nitrate transport and signalling. J Exp Bot. 2007;58: 2297–2306. [DOI] [PubMed] [Google Scholar]
- 31.Tsay Y-F, Chiu C-C, Tsai C-B, Ho C-H, Hsu P-K. Nitrate transporters and peptide transporters. FEBS Lett. 2007;581: 2290–2300. [DOI] [PubMed] [Google Scholar]
- 32.Glass ADM. Nitrate uptake by plant roots. Botany. 2009;87: 659–667. [Google Scholar]
- 33.Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. Nitrogen uptake, assimilation and remobilization in plants: challenges for sustainable and productive agriculture. Ann Bot. 2010;105: 1141–1157. 10.1093/aob/mcq028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang P, Wang Z, Cai R, Li Y, Chen X, Yin Y. Physiological and molecular response of wheat roots to nitrate supply in seedling stage. Agric Sci China. 2011;10: 695–704. [Google Scholar]
- 35.Lin C-M, Koh S, Stacey G, Yu S-M, Lin T-Y, Tsay Y-F. Cloning and functional characterization of a constitutively expressed nitrate transporter gene, OsNRT1, from rice. Plant Physiol. 2000;122: 379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buchner P, Hawkesford MJ. Complex phylogeny and gene expression patterns of members of the NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family (NPF) in wheat. J Exp Bot. 2014;65: 5697–5710. 10.1093/jxb/eru231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin L-P, Li P, Wen B, Taylor D, Berry JO. Characterization and expression of a high-affinity nitrate system transporter gene (TaNRT2.1) from wheat roots, and its evolutionary relationship to other NTR2 genes. Plant Sci. 2007;172: 621–631. [Google Scholar]
- 38.Liu J, Fu J, Tian H, Gao Y. In-season expression of nitrate and ammonium transporter genes in roots of winter wheat (Triticum aestivum L.) genotypes with different nitrogen-uptake efficiencies. Crop Pasture Sci. 2015;66: 671. [Google Scholar]
- 39.Melino VJ, Fiene G, Enju A, Cai J, Buchner P, Heuer S. Genetic diversity for root plasticity and nitrogen uptake in wheat seedlings. Funct Plant Biol. 2015;42: 942. [DOI] [PubMed] [Google Scholar]
- 40.Pang J, Milroy SP, Rebetzke GJ, Palta JA. The influence of shoot and root size on nitrogen uptake in wheat is affected by nitrate affinity in the roots during early growth. Funct Plant Biol. 2015;42: 1179–1189. [DOI] [PubMed] [Google Scholar]
- 41.Martre P. Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant Physiol. 2003;133: 1959–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Le Bail M, Jeuffroy M-H, Bouchard C, Barbottin A. Is it possible to forecast the grain quality and yield of different varieties of winter wheat from Minolta SPAD meter measurements? Eur J Agron. 2005;23: 379–391. [Google Scholar]
- 43.Castle SL, Randall PJ. Effects of sulfur deficiency on the synthesis and accumulation of proteins in the developing wheat seed. Funct Plant Biol. 1987;14: 503–516. [Google Scholar]
- 44.Tottman DR, Makepeace RJ, Broad H. An explanation of the decimal code for the growth stages of cereals, with illustrations. Ann Appl Biol. 1979;93: 221–234. [Google Scholar]
- 45.Lemaire G, Gastal F. N uptake and distribution in plant canopies Diagnosis of the Nitrogen Status in Crops. Berlin, Heidelberg: Springer Berlin Heidelberg; 1997. pp. 3–43. [Google Scholar]
- 46.Justes E, Mary B, Meynard JM, Machet JM, Thelier-Huche L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann Bot. 1994;74: 397–407. [Google Scholar]
- 47.Cross JM, von Korff M, Altmann T, Bartzetko L, Sulpice R, Gibon Y, et al. Variation of enzyme activities and metabolite levels in 24 Arabidopsis accessions growing in carbon-limited conditions. Plant Physiol. 2006;142: 1574–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boisson M, Mondon K, Torney V, Nicot N, Laine A-L, Bahrman N, et al. Partial sequences of nitrogen metabolism genes in hexaploid wheat. Theor Appl Genet. 2005;110: 932–940. [DOI] [PubMed] [Google Scholar]
- 49.Pfaffl MW. Quantification strategies in real-time PCR. AZ Quant PCR. 2004;1: 89–113. [Google Scholar]
- 50.Paolacci AR, Tanzarella OA, Porceddu E, Ciaffi M. Identification and validation of reference genes for quantitative RT-PCR normalization in wheat. BMC Mol Biol. 2009;10: 11 10.1186/1471-2199-10-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.R Core Team. R: A language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: Available: http://www.R-project.org/. 2012. [Google Scholar]
- 52.Carillo P, Mastrolonardo G, Nacca F, Fuggi A. Nitrate reductase in durum wheat seedlings as affected by nitrate nutrition and salinity. Funct Plant Biol. 2005;32: 209–219. [DOI] [PubMed] [Google Scholar]
- 53.Koekemoer FP, Labuschagne MT, Van Deventer CS. A selection strategy for combining high grain yield and high protein content in South African wheat cultivars. Cereal Res Commun. 1999; 107–114. [Google Scholar]
- 54.Groos C, Robert N, Bervas E, Charmet G. Genetic analysis of grain protein-content, grain yield and thousand-kernel weight in bread wheat. Theor Appl Genet. 2003;106: 1032–1040. [DOI] [PubMed] [Google Scholar]
- 55.Imsande J, Touraine B. N demand and the regulation of nitrate uptake. Plant Physiol. 1994;105: 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olesen JE, Berntsen J, Hansen EM, Petersen BM, Petersen J. Crop nitrogen demand and canopy area expansion in winter wheat during vegetative growth. Eur J Agron. 2002;16: 279–294. [Google Scholar]
- 57.Sadras VO, Rodriguez D. Modelling the nitrogen-driven trade-off between nitrogen utilisation efficiency and water use efficiency of wheat in eastern Australia. Field Crops Res. 2010;118: 297–305. [Google Scholar]
- 58.Mattsson M, Lundborg T, Larsson M, Larsson C-M. Nitrogen utilization in N-limited barley during vegetative and generative growth III. Post-anthesis kinetics of net nitrate uptake and the role of the relative root size in determining the capacity for nitrate acquisition. J Exp Bot. 1992;43: 25–30. [Google Scholar]
- 59.Le Gouis J, Béghin D, Heumez E, Pluchard P. Genetic differences for nitrogen uptake and nitrogen utilisation efficiencies in winter wheat. Eur J Agron. 2000;12: 163–173. [Google Scholar]
- 60.Martre P, Jamieson PD, Semenov MA, Zyskowski RF, Porter JR, Triboi E. Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. Eur J Agron. 2006;25: 138–154. [Google Scholar]
- 61.Waldren RP, Flowerday AD. Growth stages and distribution of dry matter, N, P, and K in winter wheat. Agron J. 1979;71: 391–397. [Google Scholar]
- 62.Bertheloot J, Andrieu B, Martre P. Light–nitrogen relationships within reproductive wheat canopy are modulated by plant modular organization. Eur J Agron. 2012;42: 11–21. [Google Scholar]
- 63.Garnett T, Conn V, Plett D, Conn S, Zanghellini J, Mackenzie N, et al. The response of the maize nitrate transport system to nitrogen demand and supply across the lifecycle. New Phytol. 2013;198: 82–94. 10.1111/nph.12166 [DOI] [PubMed] [Google Scholar]
- 64.Wang R, Guegler K, LaBrie ST, Crawford NM. Genomic analysis of a nutrient response in Arabidopsis reveals diverse expression patterns and novel metabolic and potential regulatory genes induced by nitrate. Plant Cell Online. 2000;12: 1491–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cao Y, Fan XR, Sun SB, Xu GH, Hu J, Shen QR. Effect of nitrate on activities and transcript levels of Nitrate Reductase and Glutamine Synthetase in Rice. Pedosphere. 2008;18: 664–673. [Google Scholar]
- 66.Gowri G, Kenis JD, Ingemarsson B, Redinbaugh MG, Campbell WH. Nitrate reductase transcript is expressed in the primary response of maize to environmental nitrate. Plant Mol Biol. 1992;18: 55–64. [DOI] [PubMed] [Google Scholar]
- 67.Redinbaugh MG, Campbell WH. Glutamine synthetase and ferredoxin-dependent glutamate synthase expression in the maize (Zea mays) root primary response to nitrate (evidence for an organ-specific response). Plant Physiol. 1993;101: 1249–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stitt M. Nitrate regulation of metabolism and growth. Curr Opin Plant Biol. 1999;2: 178–186. [DOI] [PubMed] [Google Scholar]
- 69.Forde BG. Nitrate transporters in plants : structure, function and regulation. Biochim Biophys Acta BBA-Biomembr. 2000;1465: 219–235. [DOI] [PubMed] [Google Scholar]
- 70.Vidmar JJ, Zhuo D, Siddiqi MY, Schjoerring JK, Touraine B, Glass AD. Regulation of high-affinity nitrate transporter genes and high-affinity nitrate influx by nitrogen pools in roots of barley. Plant Physiol. 2000;123: 307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nazoa P, Vidmar JJ, Tranbarger TJ, Mouline K, Damiani I, Tillard P, et al. Regulation of the nitrate transporter gene AtNRT2. 1 in Arabidopsis thaliana: responses to nitrate, amino acids and developmental stage. Plant Mol Biol. 2003;52: 689–703. [DOI] [PubMed] [Google Scholar]
- 72.Barneix AJ. Physiology and biochemistry of source-regulated protein accumulation in the wheat grain. J Plant Physiol. 2007;164: 581–590. [DOI] [PubMed] [Google Scholar]
- 73.Siddiqi MY, Glass AD, Ruth TJ, Rufty TW. Studies of the uptake of nitrate in barley I. kinetics of 13NO3- influx. Plant Physiol. 1990;93: 1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King BJ, Siddiqi MY, Ruth TJ, Warner RL, Glass AD. Feedback regulation of nitrate influx in barley roots by nitrate, nitrite, and ammonium. Plant Physiol. 1993;102: 1279–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang R. Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol. 2004;136: 2512–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Relation between N uptake from flowering to GS65+250 DD and grain N concentration at GS65+250 DD (A) or at GS65+450 DD (B).
(TIF)
(TIF)
Relations between nitrate concentration and TaNR relative expression (A) or between nitrate concentration and TaGS2 relative expression (B) in roots at GS65+250 DD.
(TIF)
(TIF)
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.