SYNOPSIS
Using a life span model, this article presents new scientific findings regarding risk factors for pelvic floor disorders (PFDs), with a focus on the role of childbirth in the development of single or multiple co-existing PFDs. Phase I of the life span model includes predisposing factors such as genetic predisposition and race. Phase II of the model includes inciting factors such as obstetric events. Prolapse, urinary incontinence (UI) and fecal incontinence (FI) are more common among vaginally parous women, although the impact of vaginal delivery on risk of FI is less dramatic than for prolapse and UI. Finally, Phase III includes intervening factors such as age and obesity. Both age and obesity are associated with prevalence of PFDs. The prevention and treatment of obesity is an important component to PFD prevention.
Keywords: Pelvic floor disorders, childbirth, vaginal delivery, cesarean section, urinary incontinence, pelvic organ prolapse, fecal incontinence
The study of the epidemiology of any disease starts with case definition. Pelvic floor disorders (PFDs) include stress urinary incontinence (SUI), urgency urinary incontinence (UUI), overactive bladder (OAB), pelvic organ prolapse (POP), and fecal or anal incontinence (FI, AI). Numerous definitions and classification schemes have been proposed for these individual PFDs. Variations in definitions, both in clinical practice and in the literature, create variability in estimates of prevalence and incidence.1 For instance, mild POP, defined as any degree of prolapse on examination, is practically universal in older women,2 but women may not have symptoms unless prolapse is more severe.3 Thus, estimates of the prevalence of POP will be impacted by the threshold used to define the condition.
Additionally, definitions proposed for clinical practice may not be sufficiently precise for epidemiologic research. In 2009, the International Continence Society (ICS) and International Urogynecologic Association provided updates on defining PFDs.4 Urinary incontinence (UI) is defined as involuntary loss of urine. However, the definition does not specify a measurement standard for this condition. Similar limitations are noted with the ICS definitions for other PFDs. OAB is defined as urinary urgency (usually accompanied by frequency and nocturia), with or without UUI, in the absence of urinary tract infection or other obvious pathology. POP is defined as the “descent of one or more of the anterior vaginal wall, posterior vaginal wall, the uterus (cervix) or the apex of the vagina (vaginal vault or cuff scar after hysterectomy)”, correlated with symptoms, assisted by any relevant imaging. FI is defined as involuntary loss of feces, while AI is involuntary loss of feces or flatus.
As noted by Sung and Hampton in 2009,1 “the generalized lack of agreement on an epidemiologic definition of incontinence has limited the ability to obtain precise and consistent estimates of prevalence, incidence, and remission rates. In addition, differences in target populations, study and survey methodology, and questionnaire design increase the variability of estimates between studies.” Prior publications have addressed various validated standards for the measurement and classification of PFDs.1,5–7
Prevalence and public health burden of pelvic floor disorders
Despite the challenges of measuring and classifying PFDs in epidemiologic research, recent studies have provided valuable estimates of the prevalence of these conditions. Pelvic floor disorders are common. Based on a cross-sectional study of a nationally representative population of women in the United States (US), the prevalence of at least one PFD was 23.7%. The prevalence was more than doubled in women 80 years or older.8 The probability that a woman will undergo surgical correction of POP by age 80 is estimated to be one in five.9,10
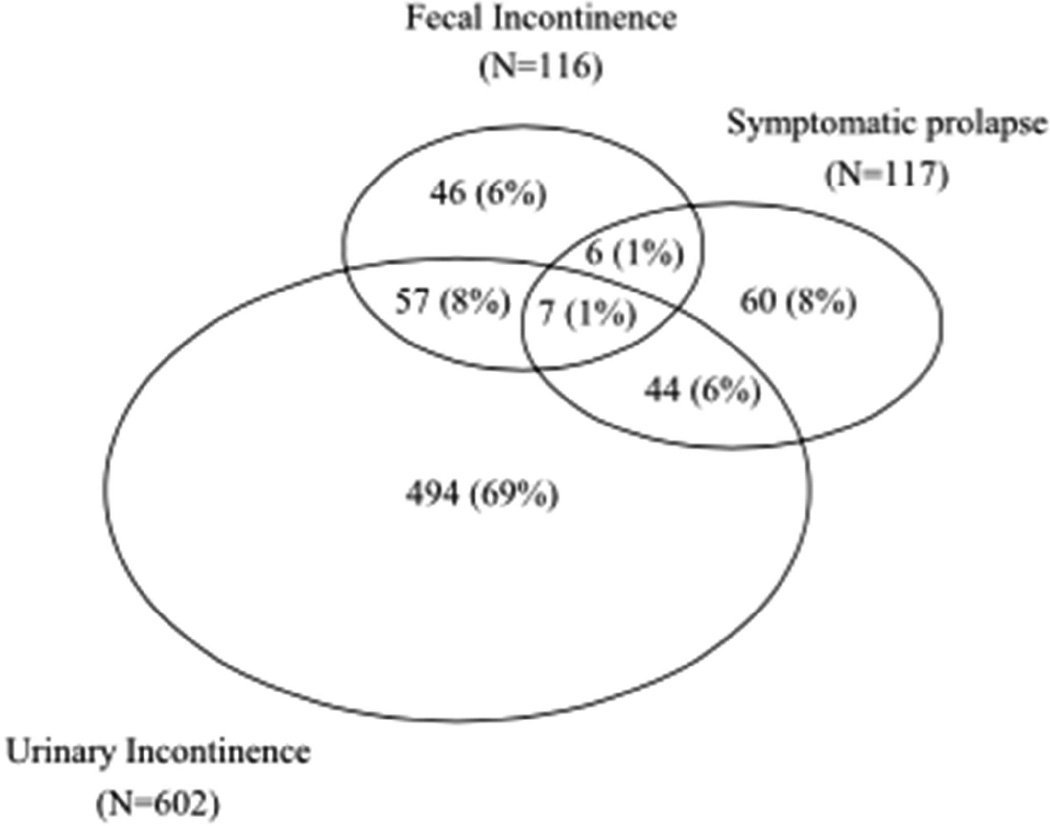
Research also suggests that PFDs often coexist. For example, in a study of more than 5000 parous Swedish women,11 46% had at least one disorder and almost a third of these symptomatic women had two or more disorders. Similarly, in a Kaiser study, at least one PFD was reported by 34% of women over 40 years of age, and 16% of symptomatic women had more than one disorder; specifically, 9% of the symptomatic women had both UI and FI and 7% had both UI and POP (Figure 1).12
Figure 1.
Overlap of the prevalence of urinary incontinence (weekly or more), symptomatic pelvic organ prolapse, and fecal incontinence (leaking monthly or more) in 714 symptomatic women.
From Rortveit G, Subak LL, Thom DH, et al. Urinary Incontinence, Fecal Incontinence and Pelvic Organ Prolapse in a Population-Based, Racially Diverse Cohort. Female Pelvic Medicine & Reconstructive Surgery. 2010;16(5):278–283, with permission.
Due to their high prevalence, PFDs have a large economic burden. In 2006, the estimated direct annual costs of ambulatory care for PFDs in the US was $412 million.13 As the population ages, health care utilization for PFDs is predicted to grow. Wu et al used US Census Bureau population projections to estimate the total number of women who will undergo surgery for POP from 2010 to 2050, and determined that this number is expected to increase by 48.2% over these four decades.14
The limitation of studies on health care utilization is that some women with PFDs do not seek care. In a population-based sample of women 40 years or older, the prevalence of UI was 41%, but only 25% of symptomatic women sought care, 23% received some care, and 12% received subspecialty care.15 In a community-based Internet survey of women older than 45 years, 19% reported accidental bowel leakage but only 29% of those had sought care.16 Thus, the incidence of care seeking provides an underestimate of the public health burden of PFDs among US women.
Pelvic floor disorders as chronic disease
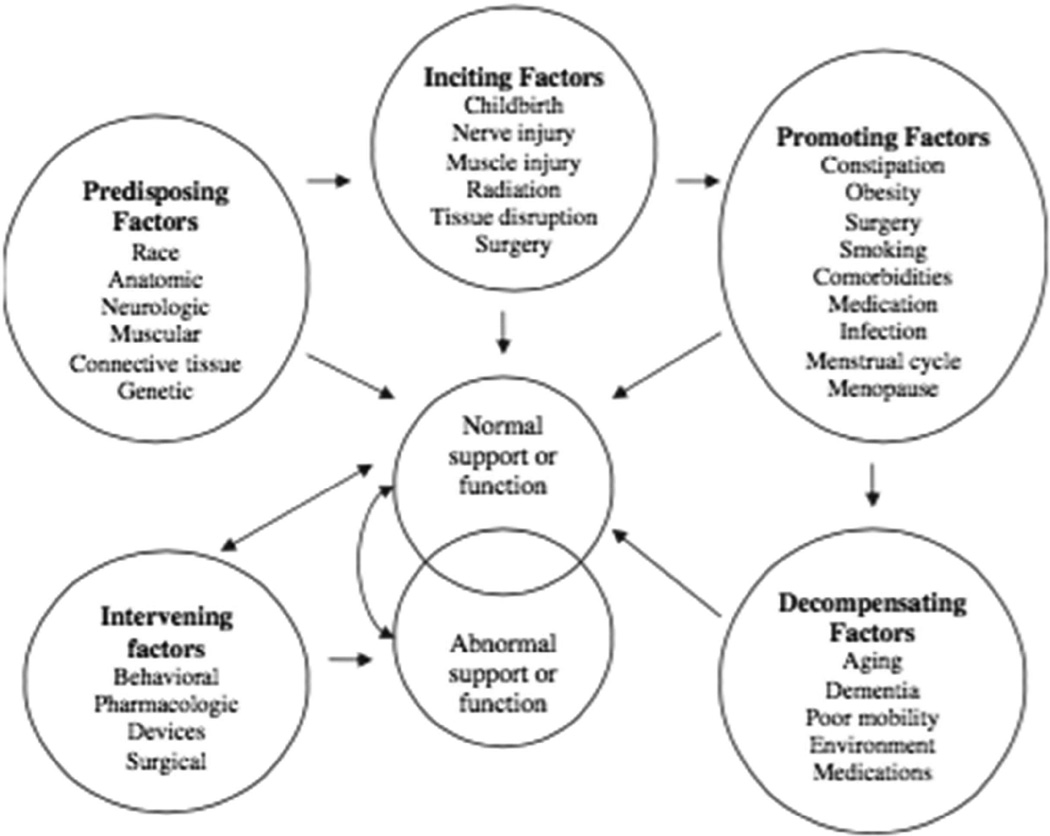
One model for conceptualizing the development of PFDs in women was published in 1998 by Bump and Norton17 (Figure 2). This model provides a framework for discussion of the progression from normal to abnormal pelvic support and pelvic organ function, organizing risk factors into categories: intervening, predisposing, inciting, promoting, and decompensating. As others have observed,1 this model may not explain an individual’s progression of disease, nor does it provide an indication of the burden of one risk factor compared to others.
Figure 2.
Model of the development of pelvic floor dysfunction in women.
From Bump RC, Norton P. Epidemiology and Natural History of Pelvic Floor Dysfunction. Obstetrics and Gynecology Clinics of NA. 1998;25(4):723–746; with permission.
Another way of conceptualizing the epidemiology of PFDs is a model that takes into account the chronic nature of the disorders. The life course or life span approach is a theory used for chronic disease epidemiology.18 The key concepts include the study of the disease process during gestation, childhood, adolescence, young adulthood and later adult life, within the individual’s life course and across generations. The model emphasizes the temporal relationship between exposure variables and their inter-relationships.
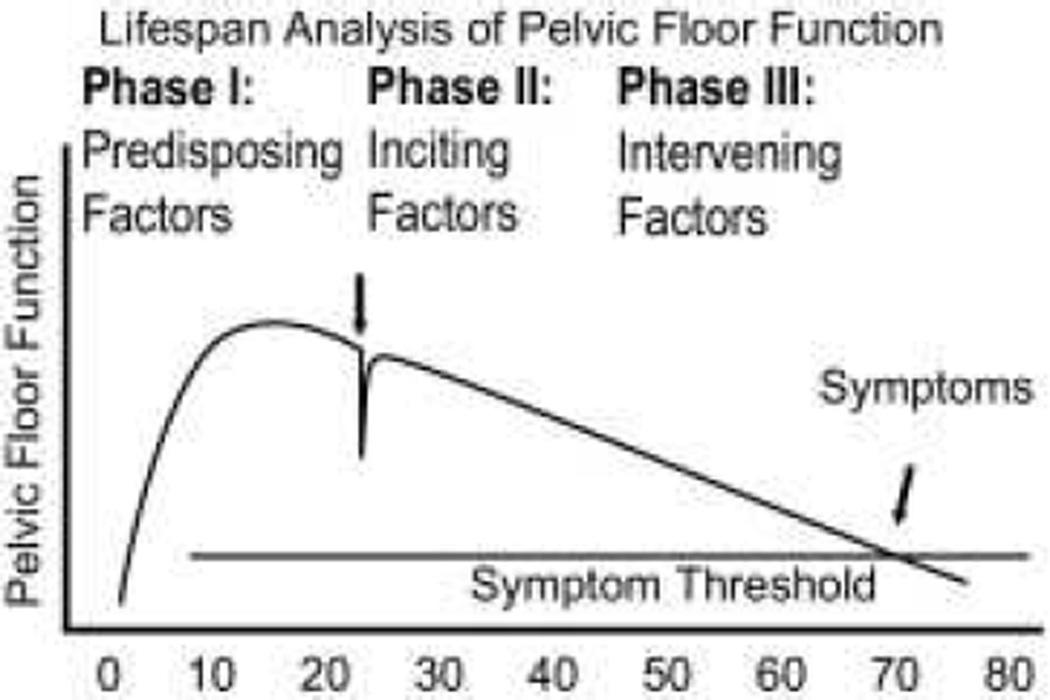
The life span approach can be used for reproductive health.19 In 2008, DeLancey et al proposed the Lifespan Model for Pelvic Floor Disorders (Figure 3).20 Three phases were explored: phase I for predisposing factors such as growth and development, phase II for inciting factors such as birth-induced changes, and finally phase III for intervening factors such as age-related changes or lifestyle factors. It seems reasonable that the life span approach can be used to further characterize known and suspected risk factors for PFDs.
Figure 3.
Graphical display of the life span model of pelvic floor function. This graph shows the development of pelvic floor disorders across three phases of a woman’s life.
From DeLancey JOL, Low LK, Miller JM, Patel DA, Tumbarello JA. Graphic integration of causal factors of pelvic floor disorders: an integrated life span model. American Journal of Obstetrics and Gynecology. 2008;199:610.e1-.e5; with permission.
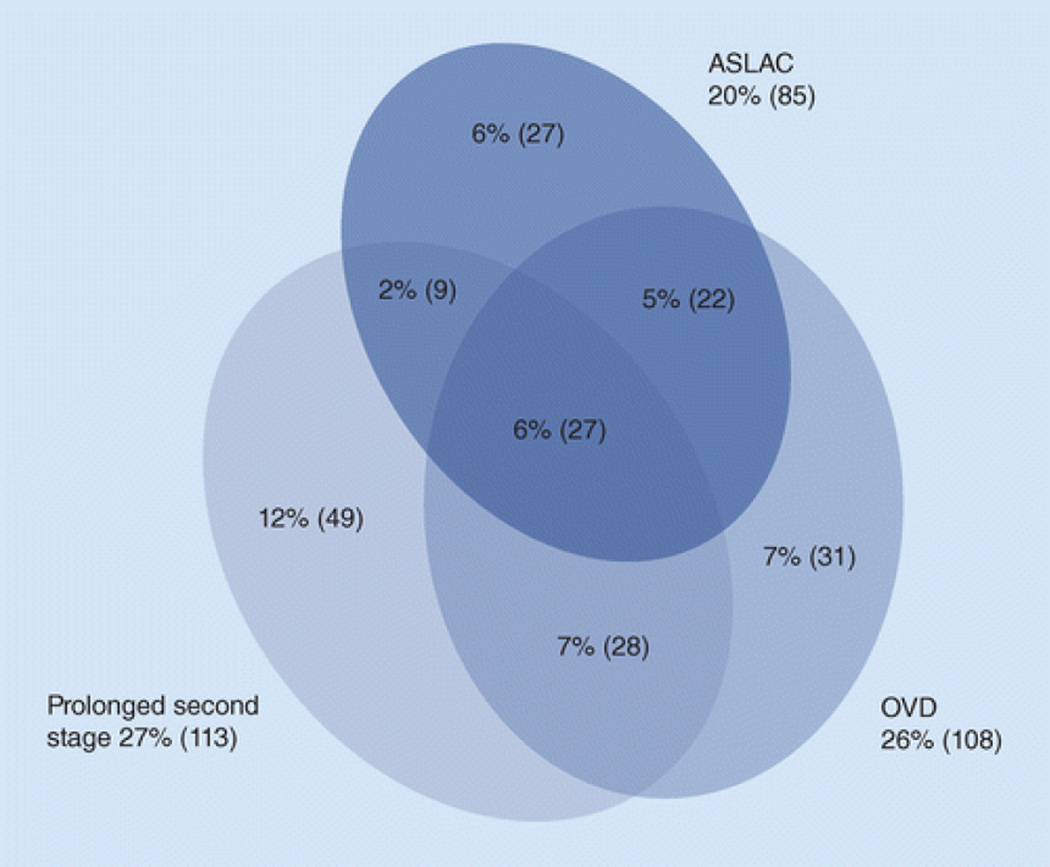
Two methods of using the life span theory are the Critical Period method and the Accumulation of Risk method. The latter can be further categorized based on whether individual risk factors (a) act separately, (b) cluster together, (c) accumulate in an additive fashion (known as a ‘chain of risk’), or (d) have a final ‘trigger effect’ risk factor. Thus, Accumulation of Risk may be most appropriate for modeling PFD epidemiology. For instance, Memon & Handa provided a graphical display of obstetric exposures in a population of primiparous women who delivered vaginally (Figure 4).21 As the figure shows, these obstetric exposures are clustered and have considerable overlap. Using a life span model with an Accumulation of Risk framework, the sections that follow present a perspective on new scientific findings regarding risk factors for PFDs.
Figure 4.
Clustering of obstetrical exposures in a population of 418 primiparous women who delivered vaginally. ASLAC: anal sphincter laceration. OVD: operative vaginal delivery.
From Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Women's Health. 2013;9(3):265–277; with permission.
Phase I: Predisposing factors for pelvic floor disorders
Genetic predisposition
A genetic cause for PFDs has not been identified, although epidemiologic evidence for a genetic predisposition accumulates. For example, a meta-analysis of clinical studies on family history of POP calculated that the relative odds for POP among women with a genetic predisposition was of 2.58.22 A meta-analysis provided moderate evidence of the genetic association for urinary symptoms in women, with certain genes having an odds ratio (OR) of 2.5 for OAB and 2.1 for SUI.23 Although specific genetic predisposition has not been identified, a systematic review of genetic studies found that collagen type 3 alpha 1 was associated with POP (OR 4.79).24
Race
Some studies suggest that the prevalence of prolapse and incontinence are associated with race. For example, Kaiser investigators found that Latina and white women had a higher risk of symptomatic POP than African American women (prevalence ratio 4.89 and 5.35, respectively).25 In a related Kaiser study, the age-adjusted prevalence of weekly UI was significantly different among Hispanic (36%), white (30%), African American (25%), and Asian-American (19%) women (p>0.001).26 However, other studies have not found significant racial differences in UI.8,28 Also, FI does not appear to be associated with race.27 It is worth noting that studies based on symptoms reported by women of different race may be biased by culture-based differences in perceptions of symptoms.29
Phase II: Inciting factors for pelvic floor disorders
The second phase of the Lifespan Model focuses on the impact of acquired risk factors, such as obstetric events.
Severe and symptomatic POP is much more common in vaginally parous versus nulliparous women.30–32 Among multiparous women, the increase is most dramatic with the first birth.30,31 In a cross-sectional study by Quiroz et al of women aged 40 years or older, vaginal delivery (VD) increased the odds of prolapse to or beyond the hymen (OR 9.73), but an additional VD was not associated with an increased odds.30 In contrast, delivery by cesarean delivery (CD) was not associated with prolapse. Two additional epidemiologic studies of parous women suggest a strong association between vaginal (versus cesarean) delivery on the odds of prolapse later in life. In a study of over 1000 parous women 5–10 years from a first delivery, a history of one or more vaginal births was strongly associated with pelvic organ prolapse to or beyond the hymen on physical examination (OR 5.6, 95% confidence interval (CI) 2.2–14.7).31 An association between vaginal delivery and prolapse symptoms was also observed among primiparous Swedish women (20 years after one VD or CD).32
Urinary incontinence, most notably SUI, is also strongly associated with vaginal childbirth.31,33,34 In a Swedish population of women 20 years from a single delivery, vaginal delivery was associated with higher UI severity (adjusted OR 1.68, 95% CI 1.40–2.03) and bothersomeness of UI (adjusted OR 1.85, 95% CI 1.42–2.39).34 In a cohort of women 5–10 years from first delivery,31 history of one or more vaginal births was associated with significantly greater odds of SUI (OR 2.9, 95% CI 1.5–5.5) but not OAB (OR 1.7, 95% CI 0.8–3.5). The impact of vaginal birth on UI is most notable in the immediate postpartum period. For example, in this same cohort study, severe SUI and OAB symptoms were more common after VD than CD, but differences between these groups decreased as time since childbirth increased.33
FI also appears to be more common among vaginally parous women, although the impact of vaginal delivery on risk of FI is less dramatic than for other PFDs. Using data from the Nurses’ Health Study, Townsend et al reported that the odds of liquid FI increased with each pregnancy beginning with the first (OR 1.29, 1.66, 1.75, 1.74, and 1.84 for 1, 2, 3, 4, and 5 or more pregnancies, respectively).35 The authors hypothesized a cumulative effect of multiple births on global pelvic floor dysfunction. However, other studies have suggested that vaginal delivery (in the absence of a recognized sphincter laceration) does not appear to increase risk of anal incontinence compared to cesarean delivery.36
Labor, in the absence of vaginal delivery, does not appear to modify the later development of PFDs. Among women who have delivered exclusively by cesarean (i.e., across all their births), the risk of PFD does not appear to be increased by a history of active labor or complete cervical dilation prior to CD.31 In contrast, operative vaginal birth appears to be a powerful risk factor for the development of PFDs. Compared with un-instrumented vaginal delivery, operative delivery (by forceps or vacuum) significantly increases the odds for all PFDs, with the highest increase for POP (OR 7.5, 95% CI 2.7–20.9).31
Unfortunately, in some studies the impact of childbirth on the later incidence of PFDs may be influenced by recall bias. For instance, one study of parous women suggested that women with symptoms of PFDs were more likely to report a history of anal sphincter laceration, even if the medical record did not confirm a laceration (P=0.025).37 Thus, studies that rely on participant recall may not accurately reflect the association between obstetric events and development of PFDs.
Given the strong accumulating evidence of an association between vaginal birth and PFDs, it is important to identify pathophysiologic mechanisms for the observed association. One potential mechanism may be damage to the levator ani muscle (LAM). The work of Delancey38–40 and Dietz41–43 has done much to draw attention to the role of levator tears in the development of PFDs. The LAM can be injured by stretch (overdistension, or microtrauma) or avulsion (disruption of the muscle, or macrotrauma).44 In a review by Schwertner-Tiepelmann et al, the prevalence of LAM injuries was 13–36% in vaginally parous women.45
However, the prognosis after levator injury is uncertain. Imaging suggests that levator injury may resolve over time.46,47 Van Delft48 found that the majority of levator avulsions diagnosed at 3 months postpartum were not evident at 12 months (62%, 95% CI 41–79%). Of thirty levator avulsions, 9 were persistent. Women with persistent evidence of avulsion were more likely to have symptoms of PFD and weakened pelvic floor muscle strength. While both levator avulsion and overdistension have been associated with reduced contractile function,44 the fact that women with levator ani defects are still able to contract the pelvic floor muscles may provide evidence that perhaps non-injured muscles are compensating for injured muscles.49
Even in the absence of levator injury, levator muscle dynamics may be impacted by childbirth. On ultrasound, number of vaginal births has been associated with increased hiatal area (p<0.001); the effect is most pronounced after the first vaginal birth.50 Changes in the levator hiatus are also more likely to persist after VD than after pre-labor CD.51 However, regardless of delivery route, the levator hiatus remains more distensible after delivery compared with measurements at 12 weeks gestation.51 Additionally, changes in the appearance of vaginal fornices on MR imaging has been observed after delivery;52 this suggests a possible role of paravaginal injury during delivery on subsequent development of anterior vaginal wall prolapse.
As more information emerges about the incidence of levator injury, a critical question is whether this obstetrical complication leads to PFDs. In a study of women delivered by operative vaginal birth, LAM avulsion observed on 3D transperineal ultrasound was associated with symptoms of prolapse (p=0.036) but not objective evidence of prolapse (p=0.20).53 Others have found that objective evidence of prolapse is more common among those with levator ani muscle injury.43 For example, among primiparous women 12 months after delivery,54 levator avulsion was associated with objective and subjective measures of POP (OR 4.8, 95% CI 1.99–11.34).
Levator injury has been associated with persistent enlargement of the levator hiatus.48 This may plausibly contribute to the development of prolapse. For example, the size of the genital hiatus on physical examination (a proxy for the size of the levator hiatus55,56) is a predictor of worsening pelvic organ support over time.57 Specifically, the odds for worsening support over 12–18 months appears to be doubled among women with a genital hiatus greater than or equal to 2 compared to those with a smaller genital hiatus (OR 2.36; 95% CI 1.03–5.43). In another study, women with de novo SUI two days postpartum had higher levator hiatal transverse diameter and area (p<0.05).58 This suggest that the size of the levator hiatus may be a risk factor for the development of PFDs.
Mechanism of injury
In a 3D computer model created from digitized cadaveric anatomy, investigators determined that the nerves to the anal sphincter stretched during VD, beyond the 15% threshold known to cause permanent damage in appendicular peripheral nerves.59 This model showed that greater nerve strain occurred if the nerve fixation point was more proximal. The inferior rectal branch was the most strained (35%), followed by the perineal nerve branch innervating the anal sphincter (33%), then the branches to the posterior labia (15%) and urethral sphincter (13%). Thus, neuropraxia is likely after vaginal birth, but the impact on the later development of PFDs is not currently known.
Among women who deliver vaginally, maternal and fetal factors may influence the probability of levator trauma and other obstetrical sequelae. One obvious fetal factor is birth weight. Birth weight appears to be a risk factor for symptomatic POP.32 In one study of Swedish women (assessed by mailed symptom questionnaire), symptomatic POP increased by 3% (OR 1.03; 95% CI 1.02–1.05) for each 100 g increase of infant weight. Short mothers who were 160 cm or less and who delivered a child weighting ≥4000 g had a doubled prevalence of symptomatic POP compared with short mothers with smaller babies (OR 2.06; 95% CI 1.19–3.55).
The probability of obstetrical neuromuscular injuries may also be influenced by antepartum accommodation to childbirth. In a recent editorial, Nygaard proposed a “bendy person toy” analogy for vaginal childbirth.60 She proposed that the pelvic floor is a dynamic structure that changes to accommodate the fetal head. For instance, it has been shown that the levator hiatus enlarges during pregnancy.51 Recent studies using rat models found that pre-delivery, there was a 21–37% increase in pelvic floor muscle fiber length,61 as additional sarcomeres were added and collagen synthesis increased in intramuscular extracellular matrix.62 Women who experience less levator enlargement may possibly be predisposed to injury; smaller levator hiatal dimensions with voluntary contraction have been associated with subsequent operative vaginal delivery or cesarean delivery.63 Additionally, greater first-trimester elastase activity has been associated with uncomplicated spontaneous vaginal delivery.61 Differences in adaptation during late pregnancy might contribute to differences among women with respect to progress during labor and the occurrence of soft tissue trauma with vaginal delivery.
Phase III: Intervening factors for pelvic floor disorders
Age
Age is known to be associated with the prevalence and severity for all PFDs. For example, in a population-based cross-sectional study, Miedel et al asked 5489 women aged 30 to 79 years old with an intact uterus and no prior pelvic surgery to complete a survey using validated questionnaires for symptomatic POP.64 They found age had an independent significant association with symptomatic prolapse. Age was also an independent risk factor for FI among participants in the U.S. National Health and Nutrition Examination Survey 2005–2010.27 Prevalence increased from 2.91% among 20 to 29 year old participants, to 16.16% among participants 70 years and older. Similarly, in the Nurses’ Health Study, the reported prevalence of liquid or solid stool incontinence at least monthly increased from 9% in women aged 62 to 64 years, to 17% in women aged 85 to 87 years.65
Obesity
Obesity appears to be a risk factor for symptoms of prolapse,32,66 although an association with objective measures of prolapse is less clear. For example, in the SWEPOP study,32 symptomatic POP increased 3% with each unit increase of current body mass index (OR 1.03; 95% CI 1.01–1.05). Unfortunately, there was no objective measure of prolapse severity in this study. In a clinical trial of a weight loss program,66 symptoms of POP were recorded for 338 women at baseline and 6 months after a weight loss or educational program. At baseline, a higher proportion of obese women reported feeling vaginal bulging compared with overweight women (13% vs 0%, P<0.01), and of the obese women 9% reported bothersome sensation of vaginal bulge. Unfortunately, at 6 months, there were no significant differences in improvement of self-reported bothersome prolapse symptoms in women in the weight loss or the control group.
There is increasing evidence that obesity is a strong risk factor for the incidence and progression of urinary and anal incontinence.33 In a mailed questionnaire to women contemplating bariatric surgery, Chen et al determined that obese women had a fourfold risk of UI and twofold risk of AI compared with non-obese controls.67 The prevalence of at least one PFD was 75% in the obese group, compared with 44% of the non-obese (p<0.0001). In a longitudinal cohort study of parous women, obese women experienced a significantly greater increase in UI bother over time, compared to non-obese women.33 Thus, obesity is not only a risk factor for incontinence but also appears to accelerate incontinence progression over time.
Fortunately, either diet/exercise or bariatric surgery can reduce UI. The investigators of the Longitudinal Assessment of Bariatric Surgery 2 recently published their 3 year follow-up data.68 Prevalent UI, defined as at least weekly UI episodes, was 49.3% at baseline in the women, 18.3% at 1 year (mean weight loss 29.5%), and 24.8% at 3 years. Similar optimistic findings were reported by the investigators of the Program to Reduce Incontinence by Diet and Exercise (PRIDE), even with modest weight loss defined as 5–10% of body weight.69 Compared with a reference of participants who gained weight, the women who lost 5–10% of their body weight were significantly more likely to experience reduction in UI episodes and were more likely to achieve at least a 70% reduction in the frequency of total and urge UI episodes. Similarly, women with liquid stool FI who lost at least 5 kg and/or increased their dietary fiber intake had reduced FI frequency (p = 0.001 and p=0.05, respectively) at up to 18 months follow-up.70
Conclusion
The lifespan approach provides a conceptual framework for the interaction of causal factors for PFDs over time.20 This is particularly true for understanding the role of childbirth, and vaginal delivery in particular, in the development of single or multiple co-existing PFDs. The complex interactions between pregnancy physiology, childbirth mechanics, obstetric interventions, and predisposing factors (such as genetics) are still unclear. For instance, while cesarean birth is associated with a lower incidence of PFD later in life, cesarean section without labor does not completely protect a woman from developing PFDs31; moreover, while PFDs are highly prevalent among vaginally parous women, severe PFDs are not universal among such women.
Recent studies have shown that the pelvic floor is a dynamic structure that adapts during pregnancy and delivery by expanding the levator hiatus, increasing elastase activity, and lengthening pelvic floor muscle fibers. Future studies with animal or imaging models will provide even more insight into these mechanics. While the impact of childbirth on the development of PFDs is clear, other inciting and intervening factors play a critical role. Fortunately, not all women with obstetric risk factors develop symptomatic PFDs. Some remain asymptomatic, others experience transient symptoms, and yet others develop significant symptoms much later in life. Antenatal prediction of pelvic floor injury is not currently feasible.71,72 At the current time, the prevention and treatment of obesity is an important component to PFD prevention. Other proposed primary and secondary prevention strategies (such as elective cesarean section73,74) may be based on insufficient evidence; recommendations for intervention based on perceived risk should be balanced against risks and costs.
KEY POINTS.
Pelvic floor disorders are highly prevalent among adult women.
Vaginal childbirth is strongly associated with the incidence of pelvic floor disorders later in life.
Injury to the levator ani muscle, as well as functional changes in the muscle, may result from vaginal birth and may contribute to the development of incontinence and prolapse.
Prevention and treatment of obesity may reduce the burden of symptomatic pelvic floor disorders.
Acknowledgments
DISCLOSURE STATEMENT
This work was supported by R01HD056275 and R01HD082070
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jennifer L. Hallock, Female Pelvic Medicine & Reconstructive Surgery, Johns Hopkins School of Medicine, Baltimore, MD.
Victoria L. Handa, Gynecology & Obstetrics, Johns Hopkins School of Medicine, Baltimore, MD.
References
- 1.Sung VW, Hampton BS. Epidemiology of Pelvic Floor Dysfunction. Obstetrics and Gynecology Clinics of NA. 2009;36(3):421–443. doi: 10.1016/j.ogc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Nygaard I, Bradley C, Brandt D. Pelvic Organ Prolapse in Older Women: Prevalence and Risk Factors. Obstetrics & Gynecology. 2004;104(3):489–497. doi: 10.1097/01.AOG.0000136100.10818.d8. [DOI] [PubMed] [Google Scholar]
- 3.Gutman RE, Ford DE, Quiroz LH, Shippey SH, Handa VL. Is there a pelvic organ prolapse threshold that predicts pelvic floor symptoms? American Journal of Obstetrics and Gynecology. 2008;199(6):683.e1–683.e7. doi: 10.1016/j.ajog.2008.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haylen B, de Ridder D, Freeman RM, et al. An International Urogyncological Association (IUGA) / International Continence Society (ICS) Joint Report on the Terminology for Female Pelvic Floor Dysfunction. Neurourol Urodyn. 2010;29:4–20. doi: 10.1002/nau.20798. [DOI] [PubMed] [Google Scholar]
- 5.Madoff RD, Parker SC, Varma MG, Lowry AC. Faecal incontinence in adults. The Lancet. 2004;364(9434):621–632. doi: 10.1016/S0140-6736(04)16856-6. [DOI] [PubMed] [Google Scholar]
- 6.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. The Lancet. 2007;369(9566):1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 7.Barber MD. Questionnaires for women with pelvic floor disorders. Int Urogynecol J. 2006;18(4):461–465. doi: 10.1007/s00192-006-0252-1. [DOI] [PubMed] [Google Scholar]
- 8.Nygaard I, Barber MD. Prevalence of Symptomatic Pelvic Floor Disorders in US Women. JAMA. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu JM, Matthews CA, Conover MM, Pate V, Jonsson Funk M. Lifetime Risk of Stress Urinary Incontinence or Pelvic Organ Prolapse Surgery. Obstetrics & Gynecology. 2014;123(6):1201–1206. doi: 10.1097/AOG.0000000000000286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith FJ, Holman CDJ, Moorin RE, Tsokos N. Lifetime risk of undergoing surgery for pelvic organ prolapse. Obstetrics & Gynecology. 2010;116(5):1096–1100. doi: 10.1097/AOG.0b013e3181f73729. d. [DOI] [PubMed] [Google Scholar]
- 11.Gyhagen M, Åkervall S, Milsom I. Clustering of pelvic floor disorders 20 years after one vaginal or one cesarean birth. Int Urogynecol J. 2015;26(8):1115–1121. doi: 10.1007/s00192-015-2663-3. [DOI] [PubMed] [Google Scholar]
- 12.Rortveit G, Subak LL, Thom DH, et al. Urinary Incontinence, Fecal Incontinence and Pelvic Organ Prolapse in a Population-Based, Racially Diverse Cohort. Female Pelvic Medicine & Reconstructive Surgery. 2010;16(5):278–283. doi: 10.1097/SPV.0b013e3181ed3e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sung VW, Washington B, Raker CA. Costs of ambulatory care related to female pelvic floor disorders in the United States. American Journal of Obstetrics and Gynecology. 2010;202(5):483.e1–483.e4. doi: 10.1016/j.ajog.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu JM, Kawasaki A, Hundley AF, Dieter AA, Myers ER, Sung VW. Predicting the number of women who will undergo incontinence and prolapse surgery, 2010 to 2050. American Journal of Obstetrics and Gynecology. 2011;205(3):230.e1–230.e5. doi: 10.1016/j.ajog.2011.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Minassian VA, Yan XS, Lichtenfeld MJ, Sun H, Stewart WF. The iceberg of health care utilization in women with urinary incontinence. Int Urogynecol J. 2012;23(8):1087–1093. doi: 10.1007/s00192-012-1743-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown HW, Wexner SD, Lukacz ES. Factors Associated With Care Seeking Among Women With Accidental Bowel Leakage. Female Pelvic Medicine & Reconstructive Surgery. 2013;19(2):66–71. doi: 10.1097/SPV.0b013e31828016d3. [DOI] [PubMed] [Google Scholar]
- 17.Bump RC, Norton P. Epidemiology and Natural History of Pelvic Floor Dysfunction. Obstetrics and Gynecology Clinics of NA. 1998;25(4):723–746. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Epidemiological Association. 2002;31:285–293. [PubMed] [Google Scholar]
- 19.Mishra GD, Cooper R, Kuh D. A life course approach to reproductive health: Theory and methods. Maturitas. 2010;65(2):92–97. doi: 10.1016/j.maturitas.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeLancey JOL, Low LK, Miller JM, Patel DA, Tumbarello JA. Graphic integration of causal factors of pelvic floor disorders: an integrated life span model. American Journal of Obstetrics and Gynecology. 2008;199:610.e1–610.e5. doi: 10.1016/j.ajog.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Women's Health. 2013;9(3):265–277. doi: 10.2217/whe.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lince SL, van Kempen LC, Vierhout ME, Kluivers KB. A systematic review of clinical studies on hereditary factors in pelvic organ prolapse. Int Urogynecol J. 2012;23(10):1327–1336. doi: 10.1007/s00192-012-1704-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cartwright R, Mangera A, Tikkinen KAO, et al. Systematic Review and Meta-analysis of Candidate Gene Association Studies of Lower Urinary Tract Symptoms in Men. European Urology. 2014;66(4):752–768. doi: 10.1016/j.eururo.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ward RM, Velez Edwards DR, Edwards T, Giri A, Jerome RN, Wu JM. Genetic epidemiology of pelvic organ prolapse: a systematic review. American Journal of Obstetrics and Gynecology. 2014;211(4):326–335. doi: 10.1016/j.ajog.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitcomb EL, Rortveit G, Brown JS, et al. Racial Differences in Pelvic Organ Prolapse. Obstetrics & Gynecology. 2009;114(6):1271–1277. doi: 10.1097/AOG.0b013e3181bf9cc8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thom DH, Van Den Eeden SK, Ragins AI, et al. Differences in prevalence of urinary incontinence by race/ ethnicity. Journal of Urology. 2006;175(1):259–264. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ditah I, Devaki P, Luma HN, et al. Prevalence, Trends, and Risk Factors for Fecal Incontinence in United States Adults, 2005–2010. Clinical Gastroenterology and Hepatology. 2014;12(4):636.e2–643.e2. doi: 10.1016/j.cgh.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 28.Waetjen LE, Xing G, Johnson WO, Melnikow J, Gold EB. Factors Associated With Seeking Treatment for Urinary Incontinence During the Menopausal Transition. Obstetrics & Gynecology. 2015;125(5):1071–1079. doi: 10.1097/AOG.0000000000000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maserejian NN, Chen S, Chiu GR, et al. Treatment Status and Progression or Regression of Lower Urinary Tract Symptoms in a General Adult Population Sample. The Journal of Urology. 2014;191(1):107–113. doi: 10.1016/j.juro.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quiroz L, Muñoz A, Shippey SH, Gutman RE, Handa VL. Vaginal Parity and Pelvic Organ Prolapse. J Reprod Med. 2011;55(3–4):93–98. [PMC free article] [PubMed] [Google Scholar]
- 31.Handa VL, Blomquist JL, Knoepp LR, Hoskey KA, McDermott KC, Muñoz A. Pelvic Floor Disorders 5–10 Years After Vaginal or Cesarean Childbirth. Obstetrics & Gynecology. 2011 Sep;:1. doi: 10.1097/AOG.0b013e3182267f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. Prevalence and risk factors for pelvic organ prolapse 20 years after childbirth: a national cohort study in singleton primiparae after vaginal or caesarean delivery. BJOG: Int J O&G. 2012;120(2):152–160. doi: 10.1111/1471-0528.12020. [DOI] [PubMed] [Google Scholar]
- 33.Handa VL, Pierce CB, Muñoz A, Blomquist JL. Longitudinal changes in overactive bladder and stress incontinence among parous women. Neurourol Urodyn. 2014;34(4):356–361. doi: 10.1002/nau.22583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gyhagen M, Bullarbo M, Nielsen TF, Milsom I. A comparison of the long-term consequences of vaginal delivery versus caesarean section on the prevalence, severity and bothersomeness of urinary incontinence subtypes: a national cohort study in primiparous women. BJOG: Int J O&G. 2013;120:1548–1555. doi: 10.1111/1471-0528.12367. [DOI] [PubMed] [Google Scholar]
- 35.Matthews CA, Whitehead WE, Townsend MK, Grodstein F. Risk Factors for Urinary, Fecal, or Dual Incontinence in the Nurses’ Health Study. Obstetrics & Gynecology. 2013;122(3):539–545. doi: 10.1097/AOG.0b013e31829efbff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evers EC, Blomquist JL, McDermott KC, Handa VL. Obstetrical anal sphincter laceration and anal incontinence 5–10 years after childbirth. American Journal of Obstetrics and Gynecology. 2012;207(5):425.e1–425.e6. doi: 10.1016/j.ajog.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Smith LJ, Pierce CB, Blomquist JL, Handa VL. Do Symptoms of Pelvic Floor Disorders Bias Maternal Recall of Obstetrical Events Up to 10 Years After Delivery? Female Pelvic Medicine & Reconstructive Surgery. 2015;21(3):129–134. doi: 10.1097/SPV.0000000000000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strohbehn K, Ellis JH, Strohbehn JA, DeLancey JOL. Magnetic Resonance Imaging of the Levator Ani With Anatomic Correlation. Obstetrics & Gynecology. 1996;87:277–285. doi: 10.1016/0029-7844(95)00410-6. [DOI] [PubMed] [Google Scholar]
- 39.DeLancey JOL, Kearney R, Chou Q, Speights S, Binno S. The Appearance of Levator Ani Muscle Abnormalities in Magnetic Resonance Images After Vaginal Delivery. Obstetrics & Gynecology. 2003;101:46–53. doi: 10.1016/s0029-7844(02)02465-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashton-Miller JA, DeLancey JOL. On the Biomechanics of Vaginal Birth and Common Sequelae. Annu Rev Biomed Eng. 2009;11(1):163–176. doi: 10.1146/annurev-bioeng-061008-124823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dietz HP, Wilson PD. Childbirth and pelvic floor trauma. Best Practice & Research Clinical Obstetrics & Gynaecology. 2005;19(6):913–924. doi: 10.1016/j.bpobgyn.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Dietz HP, Lanzarone V. Levator Trauma After Vaginal Delivery. Obstetrics & Gynecology. 2005;106:707–712. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 43.Dietz HP, Simpson JM. Levator trauma is associated with pelvic organ prolapse. BJOG: Int J O&G. 2008;115(8):979–984. doi: 10.1111/j.1471-0528.2008.01751.x. [DOI] [PubMed] [Google Scholar]
- 44.Guzmán Rojas R, Wong V, Shek KL, Dietz HP. Impact of levator trauma on pelvic floor muscle function. Int Urogynecol J. 2013;25(3):375–380. doi: 10.1007/s00192-013-2226-4. [DOI] [PubMed] [Google Scholar]
- 45.Schwertner-Tiepelmann N, Thakar R, Sultan AH, Tunn R. Obstetric levator ani muscle injuries: current status. Ultrasound in Obstetrics & Gynecology. 2012;39(4):372–383. doi: 10.1002/uog.11080. [DOI] [PubMed] [Google Scholar]
- 46.Star-Jensen J, Siafarikas F, Hilde G, Benth JŠ, Bø K, Engh ME. Postpartum Recovery of Levator Hiatus and Bladder Neck Mobility in Relation to Pregnancy. Obstetrics & Gynecology. 2015;125(3):531–539. doi: 10.1097/AOG.0000000000000645. [DOI] [PubMed] [Google Scholar]
- 47.Miller JM, Low LK, Zielinski R, Smith AR, DeLancey JOL, Brandon C. Evaluating maternal recovery from labor and delivery: bone and levator ani injuries. American Journal of Obstetrics and Gynecology. 2015;213(2):188.e1–188.e11. doi: 10.1016/j.ajog.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Delft K, Thakar R, Sultan AH, IntHout J, Kluivers KB. The natural history of levator avulsion one year following childbirth: a prospective study. BJOG: Int J O&G. 2014 Dec;:1–8. doi: 10.1111/1471-0528.13223. [DOI] [PubMed] [Google Scholar]
- 49.Hilde G, Staer-Jensen J, Siafarikas F, Gjestland K, Ellström Engh M, Bo K. How well can pelvic floor muscles with major defects contract? A cross-sectional comparative study 6 weeks after delivery using transperineal 3D/4D ultrasound and manometer. BJOG: Int J O&G. 2013;120(11):1423–1429. doi: 10.1111/1471-0528.12321. [DOI] [PubMed] [Google Scholar]
- 50.Atan IK, Gerges B, Shek KL, Dietz HP. The association between vaginal parity and hiatal dimensions: a retrospective observational study in a tertiary urogynaecological centre. BJOG: Int J O&G. 2014;122(6):867–872. doi: 10.1111/1471-0528.12920. [DOI] [PubMed] [Google Scholar]
- 51.van Veelen GA, Schweitzer KJ, van der Vaart CH. Ultrasound imaging of the pelvic floor: changes in anatomy during and after first pregnancy. Ultrasound in Obstetrics & Gynecology. 2014;44(4):476–480. doi: 10.1002/uog.13301. [DOI] [PubMed] [Google Scholar]
- 52.Cassadó-Garriga J, Wong V, Shek K, Dietz HP. Can we identify changes in fascial paravaginal supports after childbirth? Aust N Z J Obstet Gynaecol. 2014;55(1):70–75. doi: 10.1111/ajo.12261. [DOI] [PubMed] [Google Scholar]
- 53.Memon HU, Blomquist JL, Dietz HP, Pierce CB, Weinstein MM, Handa VL. Comparison of Levator Ani Muscle Avulsion Injury After Forceps-Assisted and Vacuum-Assisted Vaginal Childbirth. Obstetrics & Gynecology. 2015;125(5):1080–1087. doi: 10.1097/AOG.0000000000000825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durnea CM, Khashan AS, Kenny LC, Durnea UA, Smyth MM, O’Reilly BA. Prevalence, etiology and risk factors of pelvic organ prolapse in premenopausal primiparous women. Int Urogynecol J. 2014;25(11):1463–1470. doi: 10.1007/s00192-014-2382-1. [DOI] [PubMed] [Google Scholar]
- 55.Dietz HP, Shek C, De Leon J, Steensma AB. Ballooning of the levator hiatus. Ultrasound in Obstetrics & Gynecology. 2008;31(6):676–680. doi: 10.1002/uog.5355. [DOI] [PubMed] [Google Scholar]
- 56.Khunda A, Shek KL, Dietz HP. Can ballooning of the levator hiatus be determined clinically? American Journal of Obstetrics and Gynecology. 2012;206(3):246.e1–246.e4. doi: 10.1016/j.ajog.2011.10.876. [DOI] [PubMed] [Google Scholar]
- 57.Pierce CB, Hallock JL, Blomquist JL, Handa VL. Longitudinal Changes in Pelvic Organ Support Among Parous Women. Female Pelvic Medicine & Reconstructive Surgery. 2012;18(4):227–232. doi: 10.1097/SPV.0b013e3182626294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Falkert A, Endress E, Weigl M, Seelbach-Göbel B. Three-dimensional ultrasound of the pelvic floor 2 days after first delivery: influence of constitutional and obstetric factors. Ultrasound in Obstetrics & Gynecology. 2010;35(5):583–588. doi: 10.1002/uog.7563. [DOI] [PubMed] [Google Scholar]
- 59.Lien K-C, Morgan DM, DeLancey JOL, Ashton-Miller JA. Pudendal nerve stretch during vaginal birth: A 3D computer simulation. American Journal of Obstetrics and Gynecology. 2005;192(5):1669–1676. doi: 10.1016/j.ajog.2005.01.032. [DOI] [PubMed] [Google Scholar]
- 60.Nygaard I. New directions in understanding how the pelvic floor prepares for and recovers from vaginal delivery. American Journal of Obstetrics and Gynecology. 2015;213(2):121–122. doi: 10.1016/j.ajog.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 61.Oliphant SS, Nygaard IE, Zong W, Canavan TP, Moalli PA. Maternal adaptations in preparation for parturition predict uncomplicated spontaneous delivery outcome. American Journal of Obstetrics and Gynecology. 2014;211(6):630.e1–630.e7. doi: 10.1016/j.ajog.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 62.Alperin M, Lawley DM, Esparza MC, Lieber RL. Pregnancy-induced adaptations in the intrinsic structure of rat pelvic floor muscles. American Journal of Obstetrics and Gynecology. 2015;213(2):191.e1–191.e7. doi: 10.1016/j.ajog.2015.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Veelen GA, Schweitzer KJ, van Hoogenhuijze NE, van der Vaart CH. Association between levator hiatal dimensions on ultrasound during first pregnancy and mode of delivery. Ultrasound in Obstetrics & Gynecology. 2015;45(3):333–338. doi: 10.1002/uog.14649. [DOI] [PubMed] [Google Scholar]
- 64.Miedel A, Tegerstedt G, Moehle-Schmidt M, Nyrén O, Hammarström M. Nonobstetric Risk Factors for Symptomatic Pelvic Organ Prolapse. Obstetrics & Gynecology. 2009;113:1089–1097. doi: 10.1097/AOG.0b013e3181a11a85. [DOI] [PubMed] [Google Scholar]
- 65.Townsend MK, Matthews CA, Whitehead WE, Grodstein F. Risk Factors for Fecal Incontinence in Older Women. The American Journal of Gastroenterology. 2012;108(1):113–119. doi: 10.1038/ajg.2012.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myers DL, Sung VW, Richter HE, Creasman JM, Subak LL. Prolapse symptoms in overweight and obese women before and after weight loss. Female Pelvic Medicine & Reconstructive Surgery. 2012;18(1):55–59. doi: 10.1097/SPV.0b013e31824171f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen CCG, Gatmaitan P, Koepp S, et al. Obesity is associated with increased prevalence and severity of pelvic floor disorders in women considering bariatric surgery. SOARD. 2015;5(4):411–415. doi: 10.1016/j.soard.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 68.Subak LL, King WC, Belle SH, et al. Urinary Incontinence Before and After Bariatric Surgery. JAMA Intern Med. 2015;175(8):1378–1310. doi: 10.1001/jamainternmed.2015.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wing RR, Creasman JM, West DS, et al. Improving Urinary Incontinence in Overweight and Obese Women Through Modest Weight Loss. Obstetrics & Gynecology. 2010;116(2, Part 1):284–292. doi: 10.1097/AOG.0b013e3181e8fb60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Markland AD, Richter HE, Burgio KL, Myers DL, Hernandez AL, Subak LL. Weight loss improves fecal incontinence severity in overweight and obese women with urinary incontinence. Int Urogynecol J. 2011;22(9):1151–1157. doi: 10.1007/s00192-011-1444-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.van Delft K, Thakar R, Sultan AH, Schwertner-Tiepelmann N, Kluivers K. Levator ani muscle avulsion during childbirth: a risk prediction model. BJOG: Int J O&G. 2014;121(9):1155–1163. doi: 10.1111/1471-0528.12676. [DOI] [PubMed] [Google Scholar]
- 72.Lavy Y, Sand PK, Kaniel CI, Hochner-Celnikier D. Can pelvic floor injury secondary to delivery be prevented? Int Urogynecol J. 2011;23(2):165–173. doi: 10.1007/s00192-011-1530-0. [DOI] [PubMed] [Google Scholar]
- 73.Koc O, Duran B. Role of elective cesarean section in prevention of pelvic floor disorders. Current Opinion in Obstetrics and Gynecology. 2012;24(5):318–323. doi: 10.1097/GCO.0b013e3283573fcb. [DOI] [PubMed] [Google Scholar]
- 74.Wilson D, Dornan J, Milsom I, Freeman R. UR-CHOICE: can we provide mothers-to-be with information about the risk of future pelvic floor dysfunction? Int Urogynecol J. 2014;25(11):1449–1452. doi: 10.1007/s00192-014-2376-z. [DOI] [PubMed] [Google Scholar]