Abstract
This review examines the effect of β-glucan, the viscous soluble fiber in oats, on satiety. A literature search for studies that examined delivery of the fiber in whole foods or as an extract was conducted. Viscosity interferes with the peristaltic mixing process in the small intestine to impede digestion and absorption of nutrients, which precipitates satiety signals. From measurements of the physicochemical and rheological properties of β-glucan, it appears that viscosity plays a key role in modulating satiety. However, the lack of standardized methods to measure viscosity and the inherent nature of appetite make it difficult to pinpoint the reasons for inconsistent results of the effects of oats on satiety. Nevertheless, the majority of the evidence suggests that oat β-glucan has a positive effect on perceptions of satiety.
Keywords: appetite, β-glucan, dietary fiber, oats, satiety
INTRODUCTION
The prevalence of overweight and obesity has been increasing globally over the past several years. Between 1980 and 2013, the prevalence of overweight and obesity worldwide rose by 27.5% among adults and 41.5% among children. No country has come close to reversing this trend, although some regions have achieved a stabilization of the average body mass index.1 Obesity does not discriminate. It is evident in countries with high as well as low income levels and across all strata of society.2 Progress in the reduction of obesity can only be described as abysmal.
The chronic nature of obesity and its related diseases makes a case for comprehensive management approaches to achieve and maintain weight loss.3 There is some debate as to who should be responsible for taking action. Advocates of the “hard approach” envision a strong role for society, involving government regulatory and fiscal interventions. A “soft approach” involves education and voluntary action undertaken by industry.4 The multidimensional nature of obesity2 complicates the solutions to prevent obesity. Undoubtedly, the food environment interacts with personal vulnerabilities to foster a situation that promotes overconsumption. Little is achieved by laying the blame or the responsibility on either the individual or the environment. Individuals may be susceptible to the allures of the environment, but they still have to make their own food choices. Hence, there will always be an element of personal responsibility.5
There are important physiologic barriers to losing excess weight, once gained. Weight loss induces neuroendocrine changes that synchronize appetite perception, food intake behavior, and energy homeostasis. These changes make both weight loss and weight maintenance incredibly difficult.6 The strong biological resistance to weight loss and the predisposition to weight regain prompts a vicious cycle of failed attempts and personal misgivings.5 Counter-regulatory adaptations that occur in response to energy deprivation include an increase in the drive to eat.7 Controlling appetite in order to adhere to dietary recommendations is a daunting task for most individuals, especially in an environment rife with enticing food choices.8
Human appetite is controlled by central and peripheral mechanisms that interact with the environment. The nutrient composition of foods is especially important. Foods varying in their nutrient content engage differently with the mediating processes to exert different physiologic effects. Some of these effects are signals that induce satiety, which is the inhibition of hunger after a meal is eaten.9 Foods that increase satiety, such as fiber-rich foods, have been an area of active investigation.10–15 The results have been promising and offer an avenue by which the scientific community and industry can together work toward reversing the obesity trends and countering the impending adverse health effects.
The 2010 Dietary Guidelines for Americans16 recommend that whole grains comprise at least half of the 6–11 daily servings (1-oz equivalent per serving) of grains to reduce the risk of chronic diseases such as obesity, type 2 diabetes, and cardiovascular disease. Whole grains contain a host of nutrients, most notably, n-3 fatty acids, dietary fiber, minerals (magnesium, iron, zinc, manganese, copper, selenium, phosphorus, calcium, sodium, and potassium), vitamins (vitamin E, thiamin, niacin, pantothenic acid, biotin, pyridoxine, and folate), and phytochemicals.17 The precise nature of the physiologic effects of dietary fiber is not well understood, mostly because whole grains are abundant in many bioactive components.18 Nevertheless, there is evidence to suggest that the dietary fiber component of whole grains may mediate the effects of whole grains on chronic diseases such as obesity, type 2 diabetes, and cardiovascular disease.19–21
Dietary fiber – which, for the most part, consists of carbohydrate polymers that are undigested by human enzymes – has never been formally proposed as an essential component of the diet.22 However, the scientific report of the 2015 Dietary Guidelines Advisory Committee23 recognizes the potential role of dietary fiber in preventing coronary heart disease, colorectal and other cancers, type 2 diabetes, and obesity. Biomarkers of fiber intake are singularly lacking; hence, on the basis of very low consumption across all sectors of the population in the United States, dietary fiber is designated as a nutrient of public health concern.
Advocates of whole foods argue that the relationship between diet and disease cannot be clearly identified from the effects of individual nutrients.24 Thus, isolating dietary fiber from the overall field of nutrition derived from foods of plant origins is suggestive of assigning preeminence to one component.22 The scientific report of the 2015 Dietary Guidelines Advisory Committee proposes consumption of high-fiber cereals, whole grains, fruits, and vegetables to meet the recommendations for dietary fiber intake.23 However, there is evidence that dietary fiber delivered in supplements or added to food has favorable effects on weight loss as well as on the risk and progression of cardiovascular disease.20,25 Thus, whole foods and fiber-enriched foods both have a place in the diet. It is important to recognize that, while there may be synergy between the bioactive components of a whole grain, there is no value in ignoring the potential contribution of foods that contain added fiber.
Oats are usually processed as a whole grain and are particularly high in a type of dietary fiber called β-glucan.26 There is evidence to suggest that oat β-glucan reduces low-density lipoprotein cholesterol.27–29 The US Food and Drug Administration allows a health claim for an association between consumption of rolled oats, oat bran, whole oat flour, and oatrim and a reduced risk of coronary heart disease.30 There is growing evidence to suggest that oat products, when compared with similar wheat foods or a glucose control, reduce the human glycemic response.31 This review summarizes the effects of dietary fiber on the regulation of energy balance and explores the effects of oats and oat β-glucan on appetite control.
DIETARY FIBER
In human nutrition, the term dietary fiber was first described by Hipsley32 in the 1950s as the nondigestible components of the plant cell wall. The properties of dietary fiber, such as its chemical composition, physiologic functions, and the food matrix in which it is delivered, can be very diverse, with different types of fibers sharing some, all, or none of these characteristics.33 Varied definitions of dietary fiber have been proposed in an attempt to capture its multifaceted nature.
The American Association of Cereal Chemists International defines dietary fiber as “the edible part of plants and analogous carbohydrates that is resistant to digestion and absorption in the human small intestine with complete or partial fermentation in the large intestine. Dietary fiber includes polysaccharides, oligosaccharides, lignin, and associated plant substances.” The definition also acknowledges that dietary fiber promotes beneficial physiologic effects.34 The Institute of Medicine (IOM) defines dietary fiber as nondigestible carbohydrates, including lignin, that are intrinsic and intact in plants. Dietary fiber is distinguished from functional fiber, which consists of isolated, nondigestible carbohydrates with beneficial physiologic effects in humans. The sum of dietary fiber and functional fiber is total fiber.35 Thus, the IOM reserves the term dietary fiber solely for materials that are intrinsic and intact or inherent within food, as opposed to extracted, modified, or synthesized fiber, which is termed functional fiber.33
The term used in labeling and nutrient databases is dietary fiber, which includes fiber from foods as well as fiber added to foods, which cannot be distinguished analytically when a food inherently contains the fiber that has been added, thereby introducing an element of ambiguity. However, the proposed amendments to nutrition labeling regulations recognize these analytical limitations. A single definition for dietary fiber, which is equivalent to the IOM’s definition for total fiber and includes carbohydrates of 3 or more monomeric units rather than a separation of the definition into dietary fiber and functional fiber, has been proposed. The isolated and synthetic nondigestible carbohydrates would qualify as dietary fiber only pursuant to the US Food and Drug Administration’s approval of a citizen’s petition or health claim petition providing evidence of a physiologic effect beneficial to human health. Under the proposed provisions, β-glucan soluble fiber added to foods meets the definition of added fiber.36
Nonstarch polysaccharides are complex polysaccharides – other than starch – that comprise several thousand monosaccharide units joined through glycosidic linkages.37 The definition of nonstarch polysaccharides essentially includes plant cell wall components and excludes synthetic resistant carbohydrate polymers or those extracted from foods by physical, enzymatic, or chemical means.33 This definition excludes resistant starch. which is starch or starch degradation products that are indigestible by enzymes in the small intestine.38 Moreover, the definition of nonstarch polysaccharides excludes the term dietary fiber altogether.33
The Codex Alimentarius definition includes dietary fibers that are intrinsic and intact, extracted from food, and synthesized or modified.33 The Codex definition captures the essence of the American Association of Cereal Chemists International and IOM definitions and further stipulates that isolated or synthetic fibers must show a proven physiologic benefit to health. This definition attempts to harmonize the definition of dietary fiber among countries. However, the Codex Alimentarius places the decision of whether to include polymers of 3–9 monomeric units in the definition of dietary fiber on national authorities.33,39 Thus, the efforts to arrive at a definition that has international unanimity may miss the mark when countries do not accept short-chain oligomers as dietary fiber.
Whether defined using the term dietary fiber or nonstarch polysaccharides, all of the definitions characterize dietary fiber as carbohydrate polymers or oligomers that escape digestion in the small intestine and are partially or fully fermented upon reaching the large intestine.33 The origin of the fiber as inherent in a food or added to the food does not change the way it is metabolized in the body, although it has been argued that fiber in its original matrix (such as the nonstarch polysaccharide) has other nutrients attached to it that may influence its effects.38 Nevertheless, most definitions of dietary fiber include nondigested carbohydrate components that are extracted from foods, synthesized, or modified, if such components exhibit a beneficial physiologic effect. The Codex definition, which includes carbohydrate polymers of 3–9 monomeric units, appears to encompass the nuances of accepted definitions and will define dietary fiber in this review.
Physicochemical properties
The physical and chemical properties of dietary fiber, such as hydration, solubility, viscosity, and adsorption to organic molecules, determine its physiologic effects. Polysaccharide networks are formed by an ordered packing of chain segments, as in insoluble fibers. However, hydration and swelling are promoted by interconnecting sequences that are disordered, as found in solution. The noncovalent bonds stabilizing these ordered junctions are individually weak, and therefore the junctions are stable only above a certain critical minimum length. The length requirement for ordered packing makes the network properties of specific polysaccharides highly dependent on the spacing of minor structural irregularities. The formation and disruption of the junctions can occur in response to relatively small changes in factors such as temperature, pH, ionic environment, or Maillard reaction products formed during processing or cooking.40 Processes such as grinding, drying, heating, or extrusion cooking that modify the physical properties of the fiber affect the hydration properties. The physicochemical properties of the matrix in which the fiber is delivered, as well as the gut environment, play a role in determining the hydration or swelling and water-retention capacity of the fiber.41
The relative stability of the ordered and disordered forms of the polysaccharide networks determines the solubility of a polysaccharide. If the structure is such that the molecules fit together in a crystalline array, as occurs in a linear structure such as cellulose, the polymer is more energetically stable in a solid state than in solution. Polysaccharides with structural irregularities, such as β-glucan, tend to be soluble. Some fibers that are insoluble in cold water will dissolve readily in hot water, which promotes conversion to the disordered form.41
Viscosity of a fluid is described as resistance to flow.41 Although the terms viscosity and gelling are often used interchangeably, their properties differ. A gel does not flow, but it stretches elastically or breaks under a force.42 When soluble polysaccharides are present in the digesta as disordered coils, they confer viscosity by interpenetration of individual polymer chains to form an entangled network. The viscosity generated depends upon the number and the size of the coils present. Viscosity will only occur at or above a critical polymer concentration.43 Therefore, increasing the concentration or molecular weight will increase the viscosity. However, structure and solubility also influence viscosity.37 Lowering the moisture content and increasing the particle size have been shown to increase viscosity.44 Both the food matrix in which the fiber is delivered45 and the processing conditions to which the food has been subjected influence the viscosity generated by the fiber.46
Coil volume and, hence, viscosity may also be altered by other constituents in the digesta and by the secretion or absorption of aqueous fluids along the gastrointestinal (GI) tract. For instance, the hydrodynamic volume of charged polysaccharides is reduced by salts, which allows the coils to contract to a more compact form through reduced electrostatic repulsions. Further, the concentration of soluble dietary fiber in the lumen may be different from that ingested, as a result of the gut adapting to ingestion of a viscous solution. The polysaccharides can also undergo depolymerization during transit in the GI tract.41 Thus, measuring viscosity in vitro may not be fully indicative of true physiologic effects of viscous soluble dietary fiber.40 However, measuring viscosity generated by dietary fiber in vivo also has some limitations, mostly due to the practical difficulties in obtaining access to the GI tract in humans and taking accurate and reproducible measurements of viscosity.43
Physiologic effects
Dietary fiber in the small intestine has two primary physiologic effects: (1) reducing the rate or extent of absorption of nutrients, which is mediated by dietary fiber in part by physically trapping nutrients, and (2) increasing the viscosity of luminal contents to deter the transport of enzymes to their substrates, bile salts to fat for emulsification, and nutrients to the gut wall.40 Nutrients in the intestinal contents are brought into contact with the intestinal mucosa by contractions, creating turbulence that allows digesta from the center of the lumen to be transported to the vicinity of the epithelium. Diffusion across the thin unstirred layer of fluid close to the epithelium is then necessary for absorption to occur. This peristaltic mixing process is hampered with increased viscosity.40
At high concentrations of polymers, dissolved polysaccharides present a physical obstacle to the diffusion of small molecules across the unstirred water layer.40,47 As the particle size of the grain is reduced (through cracking or milling), the rate of digestion increases because the surface-to-volume ratio of the grain increases, allowing greater access to enzymes. However, while this occurs with wheat and corn, which contain larger proportions of insoluble fiber, an increased rate of digestion with a reduced particle size of oats occurs in vitro, but not in vivo, because of the increased viscosity generated by the soluble fiber content of oats, which restricts digestive enzymes from coming into contact with their substrates.48
A vast and diverse microbial community inhabits the human GI tract, with the greatest number of organisms found in the distal gut. The constituency of the gut microbiota is determined by the host phylogeny and diet.49 The genome of this indigenous microbial community, termed the microbiome, encodes myriad gene products, providing a diverse range of biochemical and metabolic functions that humans have not had to evolve fully on their own,49 including the processing of otherwise indigestible components of the diet, such as plant polysaccharides.50
In response to changes in the diet, there are dramatic and rapid alterations in the cellular composition as well as the gene transcription network of microbiota.51 Oat β-glucan supplementation for 5 weeks has been shown to promote the proliferation of bacteria such as Bifidobacterium species in healthy humans.52 These bacteria are associated with a beneficial effect on the host through their potential involvement in diabetes-related inflammation and the development of obesity.53 In the colon, dietary fiber may be fermented by gut microbes to short-chain fatty acids, namely butyrate, propionate, and acetate, which activate the enteroendocrine cells of the gut to secrete a host of metabolically active peptides involved in food intake, lipid storage, and energy homeostasis.54 However, as previously reviewed, lactulose, galactooligosaccharides, and fructan-type oligosaccharides dominate prebiotic research in humans. In vitro studies provide the majority of the data to support the prebiotic potential of oat β-glucan, but more evidence is needed before a prebiotic effect may be attributed to oat β-glucan.55
Dietary fiber influences bowel function by increasing fecal volume and weight, which improves stool consistency and frequency, thereby preventing constipation. This bulking effect is largely due to the nonfermentable fiber, but fermentable fiber can also contribute by increasing bacterial mass, thereby increasing stool weight to promote laxation.56 Soluble fibers are, for the most, part completely fermented by colonic bacteria and have a higher viscosity than insoluble fibers. However, not all soluble fibers are viscous, and some insoluble fibers may be fermented.
Effects on appetite regulation
Appetite reflects a complex interaction between the external environment, the behavioral profile, and subjective states as well as the storage and metabolism of energy.9 The entire field of food intake, including food selection, motivation, and food preference, is encompassed within the broad definition of appetite.57 When food intake reduces hunger and inhibits further intake, two processes are involved, namely satiation and satiety. Satiation develops during the course of eating and eventually causes meal termination, whereas satiety is the state in which further eating is inhibited and is preceded by an eating episode. Thus, satiety is not an instantaneous process but occurs over a period of time. Satiation and satiety are mediated by sensory, cognitive, postingestive, and postabsorptive processes.9
The mastication of foods high in dietary fiber requires time and effort, which prolongs oral exposure and allows time for signals that mediate satiety sensations.58 The duration of oral exposure has an important role in reducing energy intake and may be comparable with signals of gastric filling, which have also been shown to promote a feeling of fullness.59,60 Approximately 20 g of nonstarch polysaccharides and other carbohydrates are fermented in the human colon each day, producing approximately 200 mmol of short-chain fatty acids. Only 7–20 mmol of these fatty acids are excreted in feces. Therefore, it is estimated that fermentable fibers provide approximately 1–2 kcal/g, which lowers the energy density, or the amount of energy per unit weight of a food or beverage.40 Thus, dietary fiber increases the volume of foods while lowering the metabolizable energy.61 Increasing the energy density has a positive effect on the rate of gastric emptying in humans, and diets high in fiber appear to consistently slow gastric emptying.62
Using magnetic resonance imaging, it has been shown that appetite decreases with increased viscosity of locust bean gum solutions, which may be related to the increase in gastric volumes and the decrease in gastric emptying. There was substantial dilution of viscosity, possibly due to salivary and gastric secretions. Although this minimized differences in gastric emptying between meals of varying doses of locust bean gum, the initial meal viscosity influenced satiety significantly.63 The addition of nutrients to the locust bean gum solution resulted in an additive effect in delaying gastric emptying and increasing satiety sensations.64 Magnetic resonance imaging also showed that guar gum added to a milk-based beverage increased viscosity compared with a similar beverage without the fiber, producing greater satiety.65
The increased viscosity of intestinal contents prolongs transit time and the absorption rate of nutrients. The prolonged presence of nutrients in the GI tract raises the possibility of interaction between nutrients and the intestinal mucosa to stimulate the release of peptides involved in appetite regulation66 (Table 1).67 Additionally, short-chain fatty acids produced from colonic fermentation of nondigestible carbohydrates activate G-protein–coupled receptors that are present in the colon.68 It is postulated that short-chain fatty acids may mediate satiety through activation of these receptors to modulate the release of peptides involved in appetite regulation.69,70 In mice, acetate has been shown to cross the blood–brain barrier and suppress appetite through hypothalmic mechanisms.71
Table 1.
Major gut hormones: sites of synthesis and mechanism of action relating to appetitea
| Hormone | Primary sites of synthesis | Major effects on appetite |
|---|---|---|
| CCK | I-cells of duodenum and jejunum; widespread CNS expression | Slows gastric emptying and reduces food intake |
| Ghrelin | A-cells of gastric fundus; small and large intestines; hypothalmic nuclei | Promotes gastric motility and increases food intake |
| GLP-1 | L-cells of distal small and large intestines; hypothalamus, dorsovagal complex, pituitary gland | Inhibits gastric emptying and reduces food intake |
| PYY3-36 | L-cells of distal small and large intestines; hypothalamus, medulla, pons | Reduces gut motility and reduces food intake |
Abbreviations: CCK, cholecystokinin; CNS, central nervous system; GLP-1, glucagon-like peptide-1; PYY, peptide YY.
aAdapted from Chaudhri et al.67
The intake of whole grains and dietary fiber in the diets of Americans falls dismally short of the recommendation. Based on the National Health and Nutrition Examination Survey data from 2001 to 2010, the average whole-grain intake among adults in the United States is approximately 0.61–0.86 ounce equivalents per day, which is not even close to the recommended 3–6 ounce equivalents.72 The average dietary fiber intake is approximately 16.1 g/d, far short of the 25–38 g/d recommended by the IOM.16,72 Consumption of whole grains and foods rich in cereal fiber such as bran is a good way to increase the intake of dietary fiber.73
OATS
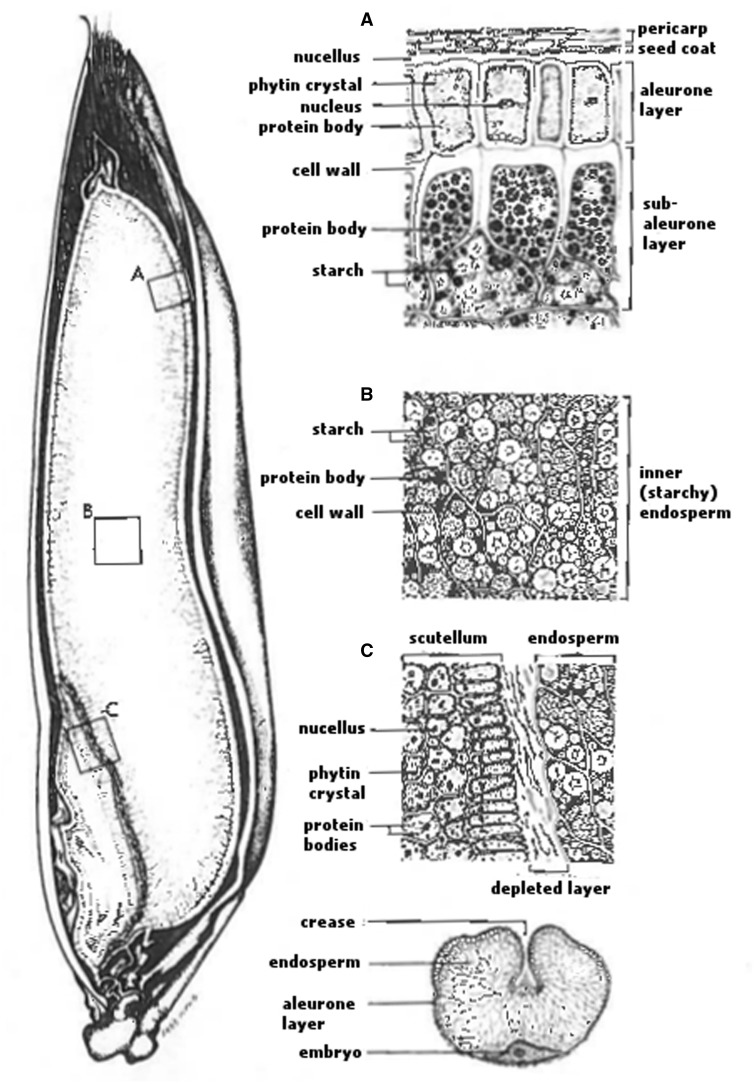
Whole oats have a hard outer hull. The hulls of cereal grains are designed to protect the seed from harsh environments and can pass through the digestive system with little or no digestion.26 The hull must be removed to obtain maximum nutritional benefits. Hulled oats, known as oat groats, have 3 fractions: the bran, the starchy endosperm, and the germ. The outer layers of the groats form the bran and typically include the pericarp, the testa or seed coat, the nucellus, the aleurone layer, and a large portion of the subaleurone layer of the starchy endosperm74 (Figure 1).
Figure 1.
Diagram of the oat caryopsis (with the hull) that has been split longitudinally to display the major fractions of the groats: (A) bran that includes the pericarp, seed coat, nucellus, aleurone layer, and a large portion of the subaleurone layer of the starchy endosperm; (B) starchy endosperm; (C) germ-endosperm interface. Below (C) is a cross-section of the groat diagrammed in (C). Reproduced with permission from Miller and Fulcher.74
The aleurone cell wall contains some β-glucan, but the amount is small compared with that in the underlying starchy endosperm, which is the primary storage site of starch, protein, lipid, and β-glucan. As with most cereals, starch is the main component of the groats. The germ contains high levels of proteins and lipids, but very little starch.74 Oat is usually processed as a whole grain because its groat is softer than that of other grains like wheat and thus cannot be easily separated into the germ, endosperm, and bran fractions. Milling is designed to remove foreign materials and to isolate and stabilize the groats and convert them into a form suitable for cooking.26 This involves cleaning, dehulling, and kilning (heat denaturing of lipase and lipoxygenase released during milling). After milling, the oats may be cut, flaked, or ground to produce steel-cut oats, oat flakes, oat flour, and oat bran.26
Steel-cut or pinhead oats are made by passing the groats through steel cutters that cut each groat 2–4 times. Rolled oats are made by steaming the groats and then flattening them into oat flakes using rollers. Flake thickness can be controlled and, in general, quick-cooking oats are rolled thinner than whole oat flakes.26 Instant oats are prepared in a similar way to quick-cooking oats; however, they are steamed for a longer period and rolled more thinly.75 Oat bran, the coarse fraction of oat flour, consists of the outer aleurone and subaleurone layers of the groats and is higher in fiber than the fine fraction of oat flour.26 The hull contains insoluble fiber, which is commonly called oat fiber in some countries, including the United States, as opposed to oat hull fiber in other countries; this difference may cause some confusion.76 The fiber from the hulls, if finely ground, has applications in animal feed, in some food ingredients for humans, and as biomass for power plants.26
OAT β-GLUCAN
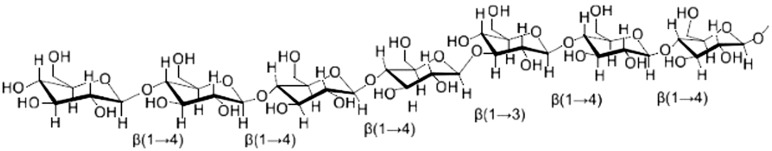
Oat β-glucans are linear polysaccharides that can be viewed as a cellulose chain. Approximately 70% are 4-O-linked units interrupted by 3-O-linked β-d glucopyranosyl units. The (1→3) linkages occur singly, leading to a structure of predominantly β (1→3)-linked cellotriosyl and cellotetraosyl units42 (Figure 2). Structure affects the water solubility of β-glucan. The soluble form of β-glucans has a greater ratio of (1→4) linkages and cellotriosyl units than the insoluble form.77
Figure 2.
Oat β-glucan, a linear polysaccharide consisting of 4-O-linked units interrupted by 3-O-linked β-D glucopyranosyl units.
Oats typically contain 3%–5% (dry-weight basis) β-glucan.78 Oat β-glucan has a native chain length of approximately 20 000 glucosidyl units and has a molecular weight of up to 3 million Daltons.46,79 A considerable range in the average or peak molecular weight of cereal β-glucans has been reported in the literature. The average molecular weight of oat β-glucan is above 106 g/mol and is probably in the range of 2 × 106 g/mol.42 However, the chain of glucopyranosyl units is easily disrupted by enzymatic or chemical hydrolysis, by mechanical shear, or by heat treatments.46 Thus, the molecular weights in commercial food products range from 0.4 to 2 × 106 Daltons.79
The solubility of β-glucan is influenced by the structure of the polymer and by the properties of the solute. The amount of β-glucan dissolved depends on the temperature, the ionic strength, and the pH of the solvent.80 Impediments to the penetration of water and the diffusion of dissolved substances also affect the solubility.46 Viscosity is a property of fluids; therefore, it is the amount of β-glucan solubilized in food and not just the total β-glucan content that is pertinent. Thus, the manner in which a fiber will modify solution properties depends upon the amount, the solubility, or extractability of the fiber under physiologic conditions, as well as the molecular weight and structure of the fiber. Changes in these properties of β-glucan in a food product can greatly influence the physiologic response.42
Food processing operations can influence the degree of polymerization, the molecular interactions within the structure, and the physicochemical properties of the fiber, depending upon the processing methods employed.81 For instance, hydrothermal treatments such as extrusion, which prevents fragmentation of β-glucan arising from enzymatic hydrolysis, can substantially improve the molecular weight and, thus, the viscosity, whereas physical disruption of the cell wall material can increase the pool of soluble β-glucan.82 However, the molecular size of the fiber may also be altered by conditions of high shear arising from mechanical processing, which results in reduced viscosity.83 Adverse structural changes such as depolymerization of the linear polysaccharides can also occur during commercial purification processes.84 Nevertheless, there is a range in which the physiologic response is sensitive to viscosity and, at higher levels, little change in response will be observed despite large changes in viscosity.78,85
OATS, β-GLUCAN, AND SATIETY
In animal studies, β-glucan has been shown to increase satiety-related hormones as well as reduce energy intake and body weight.86,87 In one study using mice with diet-induced obesity, the satiety effects of a 6-week supplementation of the diet with different concentrations (0.7%, 3.5%, and 7%) of β-glucan from oat bran were investigated. Energy intake and body weight decreased, while plasma peptide YY (PYY) concentrations increased in a dose-dependent manner; moreover, the expression of neuropeptide Y mRNA in the arcuate nucleus of the hypothalamus decreased with the highest concentration of β-glucan. The neurons that coexpress neuropeptide Y and agouti-related peptide increase appetite.88 The molecular weight of the β-glucan was approximately 1.7 × 106 g/mol. with solubility of approximately 13% (dry-weight basis). However, the viscosity was not measured.86 In another study using mice fed a high-fat diet, similar results were obtained using β-glucan doses of 0.5, 1, and 1.5 g/kg of body weight. At the end of 3 weeks of supplementing the high-fat diet with β-glucan, energy intake and body weight were reduced in a dose-dependent manner when compared with findings in mice on the high-fat diet without β-glucan. Moreover, the expression of neuropeptide Y mRNA in the hypothalamic arcuate nucleus was lower in the groups receiving the β-glucan supplementation than in the mice on the high-fat diet without β-glucan. However, no details on the physicochemical properties of the fiber were provided in this study.87
Human studies evaluating the effects of oat β-glucan on satiety have, for the most part, evaluated acute effects and have tested whole foods as well as β-glucan extracts added to food products. In a crossover study, breakfast meals containing a control (0 g), low (2.16 g), medium (3.82 g), and high (5.45 g) doses of oat β-glucan in extruded cereals, and a cereal with an ethanolic extract of β-glucan (added in equivalence to the highest dose among the extruded cereals), were compared for their effects on satiety.13 Consistent with previous reports of the effects of extrusion on β-glucan,82,89 a decrease in molecular weight was offset by an increase in solubility to increase viscosity as the concentration of β-glucan in the cereals increased. There was no significant effect on suppression of ghrelin. However, regression analysis identified a significant relationship between the dose of β-glucan and the cholecystokinin response (R2 ≥ 0.97, P = 0.002), but there was no difference in the cholecystokinin response between the various doses and the control condition. Although the cholecystokinin response between the control condition and the low-, medium-, and high-fiber conditions was statistically significant in females (P = 0.036, 0.032, and 0.006, respectively), the sample size of females was small.
A reduction in energy intake was significant between the cereal containing the ethanolic extract of β-glucan and the control, although the repeated-measures analysis of variance for the overall effect on energy intake at lunch was not significant. However, subjective satiety increased at all doses compared with the control, although there was no dose–response relationship. Despite the crossover design of this study, the differences between the overall effects and the effects between the conditions suggest that larger sample sizes may have been required to detect differences at all levels of the analysis. In an extension of this study, PYY was measured. Regression analysis showed a significant correlation between PYY concentrations and the fiber dose (P = 0.003; R2 = 0.994). The total levels of plasma PYY increased linearly with increasing doses of β-glucan from 2.2 g to 5.45 g 4 hours following the meal.12
In studies evaluating the effects of oat-based breakfast cereals on satiety, a 250-kcal serving of “old fashioned” oatmeal containing 2.6 g of β-glucan increased perceptions of satiety compared with an isocaloric oat-based ready-to-eat cereal containing 1.7 g of β-glucan. However, when a single serving (150 kcal) of old fashioned oatmeal was compared with an isocaloric serving of the ready-to-eat cereal, the effect on satiety was far less potent than that of the 250-kcal serving.90,91 Nevertheless, both serving sizes of instant oatmeal increased subjective satiety, while the 250-kcal serving of instant oatmeal also reduced energy intake. Unlike old fashioned oatmeal, instant oatmeal displayed a higher initial meal viscosity (after oral and initial gastric digestion) than the ready-to-eat cereal.91,92 It is likely that initial meal viscosity mediates the induction of signaling through orosensory stimuli to influence the overall satiety response. Thus, these studies91,92 corroborate the results of other studies that used magnetic resonance imaging and found that initial meal viscosity influenced satiety, possibly through the oral, gastric, and intestinal signals working in concert.63,65 The sugar content of the ready-to-eat cereal in each of the studies90–92 was higher than that of the oatmeal. Although the kinetics of starch digestion and glucose release measured using in vitro mechanisms were not different between the breakfast cereals used in these studies, the possibility that differences in the nutrient composition influenced the results cannot be completely ruled out.
In another study using a crossover design, the satiety effect of isocaloric breakfast meals (352 kcal, including milk) consisting of oatmeal (4 g β-glucan and 4 g insoluble fiber), frosted cornflakes (<1 g of fiber per 110 kcal, per Kellogg’s Nutrition Facts panel), and water were compared. Perceptions of satiety increased, and energy intake was lower, at an ad libitum lunch meal consisting of a liquid formulation after consumption of the oatmeal breakfast compared with consumption of the cornflakes breakfast meal or water. These effects were more pronounced among overweight individuals. Gastric emptying was slower after oatmeal was consumed than after the cornflakes meal or water was consumed, which may have contributed to the increase in satiety. Although the sugar content of the frosted cornflakes (35.5 g) in this study was higher than that of oatmeal (8.9 g), the effects on satiety were independent of the glycemic area under the curve, which did not differ between the oatmeal and frosted cornflakes conditions. However, the physicochemical properties of the fiber were not measured.93 Further, when the meals were eaten every day for 4 weeks, the group not eating breakfast lost more weight than the other groups, but there was no difference in body weight between the oatmeal and cornflakes groups, despite increased satiety reported by participants in the oatmeal group.94 This study conducted in 1998–1999 used the Likert-type rating scale to measure subjective satiety.94 Unlike the visual analog scale, which is continuous rather than interrupted by nonequivalent scale point ratings, the Likert-type scale has unknown magnitudes of satiety at equally spaced intervals along the scale.95
Other studies evaluated the effects of the viscosity generated by oat β-glucan on satiety by delivering the preload meal containing β-glucan in a beverage.96–98 In a comparison between beverages (each approximately 167 kcal) containing 0 g, 5 g (2.5 g of β-glucan), or 10 g of (5 g of β-glucan) of fiber from oats, satiety over a 3-hour period after consumption of the beverages was measured. The fiber-containing beverages increased satiety, but the effect was not dose dependent. Other conditions included in the study were a high-viscosity beverage (167 kcal) containing 10 g of fiber from oats (5 g of β-glucan), the 10 -g fiber-containing beverage treated with β-glucanase enzyme to reduce the viscosity, and a fiber-free beverage. The viscosity of the beverages was measured to ensure a difference in viscosity; however, neither the molecular weight nor the solubility of β-glucan was measured. The enzymatically treated beverage and the high-viscosity beverage increased perceptions of satiety and reduced hunger compared with the 0-g fiber beverage, but there was no difference in hunger ratings between the fiber-containing beverages.98 This study also evaluated the effects of energy levels of 167 kcal and 334 kcal at β-glucan doses of 0 g and 10 g and found that, at both levels of energy, the fiber-containing beverages increased satiety, with no significant differences between the energy levels. The fiber was added to the beverages just prior to serving to prevent viscosity levels that might make it unpalatable to ingest, which raises questions as to practical significance of delivering β-glucan in a liquid formulation.
Contrasting results were obtained when 2 isocaloric beverages (300 kcal) equal in volume but differing in measured viscosity were compared.96 Each beverage contained 5 g of soluble and 5 g of insoluble fiber from oat bran concentrate; however, the viscosity of 1 test beverage was reduced enzymatically using β-glucanase. The study had a crossover design with 20 subjects, but no details of a power analysis were provided. The low-viscosity beverage produced significantly greater postprandial cholecystokinin, glucagon-like peptide-1, and PYY responses compared with the high-viscosity beverage. There was no difference in energy intake at the ad libitum test meal, although the low-viscosity beverage did produce an increase in one of the subjective measures, which was the response to the question “How satiated are you?” The high-viscosity beverage, however, delayed gastric emptying. It is likely that, consistent with previous research, the physiologic response may not change in proportion to viscosity,85 or that, above a certain viscosity level, the response is insensitive.78
In another study, subjects were served 4 different breakfast meals consisting of biscuits and a juice drink to investigate the influence of the food matrix on the effects of β-glucan. Four grams of β-glucan was either added or not added to the biscuits and juice drink (55% orange juice and 45% water). Each type of biscuit was combined with each type of juice drink. The viscosity increased as the β-glucan content of the meal increased, regardless of the food form. While the addition of β-glucan increased perceptions of satiety compared with the control, the fortified juice drink and fortified biscuits combination produced the strongest effects on satiety. Moreover, the addition of oat bran was more effective in increasing satiety when added to the juice drink than when added to biscuits.97 However, when a comparison was made between solid forms, arabinoxylan, oat β-glucan, and rye kernels in bread all increased subjective satiety compared with refined wheat bread, although there was no effect on energy intake.99 Thus, the food matrix may play a role in mediating the effects of viscosity on appetite, as demonstrated by the lack of effect in studies using a semisolid form.100–102
When delivered in a semisolid pudding, isocaloric servings (300 kcal) containing 1.5 g of dietary fiber, 10.3 g of insoluble fiber from wheat bran, 10.2 g of fiber from oat bran (5 g β-glucan), and a combination of wheat and oat brans providing 10.1 g of fiber (2.5 g β-glucan), there were no significant differences in the postprandial ghrelin or PYY responses. Appetite ratings and energy intake at a subsequent meal were also not significantly different between the conditions. When rated by subjects before eating, the meal with no added fiber was expected to be more filling. Cognitive factors, such as an estimation of the satiating effect of foods, contribute to making eating largely a learned behavior57 and could have influenced the results, which only underscores the complex nature of appetite and the responses to dietary manipulations. In this study, the physicochemical properties of the fiber were not evaluated.100
Similarly, when delivered in semisolid form, such as yogurt, no differences in satiety or gastric emptying were observed in a comparison between oat bran containing 4 g of β-glucan and cornflakes.101 Satiety was assessed at 15 and 90 minutes following the meal, using a single numerical scale ranging from extreme hunger to extreme satiety and punctuated with phrases describing various degrees of hunger and satiety. Using a similar design, the effects of breakfast cereals consisting of wheat bran flakes (7.5 g fiber), oat flakes (4 g total fiber, 0.5 g β-glucan), and cornflakes (1.5 g fiber) were compared, with results similar to those of the previous study; however, in this study, the β-glucan content of oat flakes was almost negligible.102 Subjective ratings usually measure perceptions of hunger, fullness, desire to eat, and the prospect of future consumption, terms relating to differing aspects of the motivation to eat.57,103,104 These measures include an element of introspection, which may not always be amenable to capture. Therefore, measuring several states repeatedly provides a better measure of satiety than the single scale used in this study. However, even when delivered in a breakfast cereal bar, fiber from oat bran had no effect on subjective satiety or energy intake compared with a control product.105 In these studies,101,102,105 the physicochemical properties of the fiber were not evaluated.
A 3-month intervention evaluated the effect of an energy-restricted meal plan supplemented with β-glucan from oat bran in ready-to-eat cereals and snacks. The control group consumed oat glucan at 0.2 g/d, while the intervention groups consumed similar products containing β-glucan at a moderate (5–6 g/d) or high (8–9 g/d) dose. The molecular weight and solubility of β-glucan were altered, as expected, with food processing; however, the viscosity increased with β-glucan content. The average total dietary fiber consumption at the end of 3 months, based on self-reported food intakes in the control, moderate-fiber, and high-fiber groups, was 21.6 g, 27.4 g, and 33 g, respectively. There were no differences in body weight or satiety hormones between the 3 groups.106 However, self-reported food intakes tend to be imprecise and prone to underreporting.107,108 Further, compliance with the diet was likely compromised by imposing a diet that included the same foods for a period of 3 months. A review of the human trials is presented in Table 2.
Table 2.
Summary of the human studies reviewed that investigated the effects of oat β-glucan on satiety
| Source | Study overview | Summary of results | Conclusions |
|---|---|---|---|
| Beck et al. (2009)12,13 |
|
|
Conclusions: β-glucan increases satiety, possibly acting through CCK and PYY. Optimal dose of β-glucan: 4–6 g |
| Rebello et al. (2013)90 |
|
|
Conclusions: oatmeal increases satiety, which may be related to viscosity generated by β-glucan |
| Rebello et al. (2014)91 |
|
|
Conclusions: oatmeal increases satiety compared with RTEC. Initial viscosity may be important for inducing satiety |
| Rebello et al. (2015)92 |
|
|
Conclusions: oatmeal increases satiety and reduces energy intake compared with RTEC. Initial viscosity may be important for inducing satiety |
| Geliebter et al. (2015)93 |
|
|
Conclusions: oatmeal increases satiety compared with cornflakes, which may be related to delayed gastric emptying |
| Geliebter et al. (2014)94 |
|
|
Conclusions: oatmeal increases satiety, but breakfast skippers lost more weight over 4 wk |
| Lyly et al. (2010)98 |
|
|
Conclusions: β-glucan increases viscosity and enhances satiety, but the effect may not be related to the amount of fiber |
| Pentikainen et al. (2014)97 |
|
|
Conclusions: 4 g or 8 g β- glucan increases satiety. Food matrix is an important factor influencing satiety |
| Juvonen et al. (2009)96 |
|
|
Conclusions: differences in viscosity may not clearly reflect differences in appetite sensations or energy intake |
| Juvonen et al. (2011)100 |
|
|
Conclusions: β-glucan in a semisolid pudding has no effect on appetite sensations or gut hormone release. Food matrix may influence satiety response |
| Korczak et al. (2014)105 |
|
|
Conclusions: oat bran does not induce greater satiety than barley bran or a low-fiber control |
| Hlebowicz et al. (2007)102 |
|
|
Conclusions: presence of fiber in semisolid meal has no effect on satiety, despite reduction in gastric emptying rate |
| Hlebowicz et al. (2008)101 |
|
|
Conclusions: 4 g oat β-glucan in a semisolid meal has no effect on satiety or gastric emptying |
| Beck et al. (2010)106 |
|
|
Conclusions: addition of oat β-glucan does not enhance the effect of energy restriction over 3 mo |
| Hartvigsen et al. (2014)99 |
|
|
Conclusions: breads with added fiber increase satiety, with no effect on ghrelin concentrations or energy intake |
Abbreviations: CCK, cholecystokinin; GLP-1, glucagon-like peptide-1; mPa.s, millipascal-second; MW, molecular weight; cP, centipoise; PYY, peptide YY; RTEC, ready-to-eat cereal; VAS, visual analog scale.
While oats are the focus of this review, other sources of β-glucan, especially barley, are worth mentioning. Barley contains 3%–7% β-glucan and is considered a good source of this fiber.109 Oat and barley β-glucan are very similar in structure and properties, although some differences exist. The molar ratio of (1→3)-linked cellotriosyl units to (1→3)-linked cellotetraosyl is lower in oats than in barley. Oat and barley β-glucans of the same molecular weight at the same concentration exhibit the same viscosity behavior but have different gelation characteristics, largely due to the higher proportions of (1→3)-linked cellotriosyl in barley. which induces more rapid gelation, especially, at low molecular weights of the fiber.42 Several studies investigating the effects of β-glucan on satiety used extracts of the fiber from barley. While a number of studies found a positive effect of β-glucan on satiety and energy intake,10,15,110–113 some studies produced inconsistent results.114–116 β-Glucan is also found in cereals such as sorghum, rye, maize, triticale, wheat, and rice, as well as in certain seaweed and mushroom species, but the β-glucan content is much lower than that of oats or barley.117 Except for rye, which has arabinoxylan as the dominant fiber,118 the other sources have not been actively investigated for their effects on satiety.
Some studies investigating the effects of oat β-glucan supplementation on satiety have not been able to show a positive effect on satiety.100,101,105,106 Despite the inconsistencies, a majority of the human studies have demonstrated that oat β-glucan increases perceptions of satiety.13,90–94,96–98 However, this may not always translate into a reduction in energy intake or body weight.105,106 A number of studies did not provide details on the physicochemical properties of the fiber; nevertheless, it is clear that viscosity is an important factor in stimulating the effects on satiety. Varying ranges of viscosity have been able to deliver the desired result. In some studies, the increase in satiety was congruent with the increase in viscosity,90,91,97 whereas in others, a beverage with a low viscosity produced greater effects on satiety than a beverage with higher viscosity.96 In a study that demonstrated changes in gut hormones in the desired directions with a low-viscosity beverage compared with a high-viscosity beverage, 85% of the β-glucan in the low-viscosity beverage had a molecular weight <100 000 g/mol.96 At sufficiently high concentrations (above 3.5%–4%), solutions of low-molecular-weight β-glucan (35 000–110 000 g/mol) tend to abandon the random coil flow behavior over time and form gels.74 Thus, the contrary results of the rheological effects of β-glucan in beverages warrant further investigation in future trials.
Some of the inconsistencies in the results may be explained by differences in the terms used to describe viscosity. For instance, apparent viscosity is defined as the viscosity of a non-Newtonian fluid expressed as if it were a Newtonian fluid. Fluids such as tea, coffee, edible oils, or milk display a true viscous flow and are termed Newtonian fluids. A number of fluid foods as well as biological fluids have non-Newtonian flow behavior. Unlike Newtonian fluids, these fluids increase in viscosity when the shear rate increases and are not disposed to being measured at a single shear rate. The literature on dietary fiber appears to favor the use of the apparent viscosity. Moreover, the use of different instrumentation could provide different results.85 Thus, in addition to reporting physicochemical data, there is clearly a need for standardization of procedures used to measure viscosity.
Satiety has been shown to increase at doses of β-glucan ranging from 2.2 g to 5.5 g; however, the effects of dose on satiety are inconsistent.90,91,98,101 Viscosity depends upon the solubility or extractability as well as the molecular weight of the fiber and is an important determinant of the physiologic response.109 Thus, it is of importance to ensure that the food product provides not only a sufficient dose but also good extractability. Further, the preload meal must be of sufficient caloric value to sustain satiety during the period of evaluation. Since viscosity is important for bioactivity, any processing, cooking, or storage treatments that affect solubility and molecular weight of β-glucan must be considered.42
The sensation of hunger is an important factor that determines what and how much is eaten.9 However, the control of appetite is not merely a question of satisfying biological needs. Individuals eat for various reasons, including customary eating patterns, the social context, or even boredom. The interaction of social and physiologic factors lends complexity to human appetite control. Eating patterns are maintained by habits, attitudes, opinions about the value or suitability of the food, liking for the food, and a motivational drive to actually engage in eating.9 Individuals experience a decline in the pleasure derived from a consuming a particular food in comparison with foods not consumed.119 This phenomenon, known as sensory-specific satiety, is what prompts consumers to search for variety when making food choices and may explain why it is difficult for subjects to comply with a study protocol that requires consumption of 2 or 3 test products for a length of time.
Psychological and biological factors exercise a control over appetite that is anything but tenuous. Therefore, the development of foods that promote satiety requires a certain amount of ingenuity and highlights the need to expand research of components demonstrated to play role in promoting satiety, such as β-glucan. Ideally, further studies of the effects of processing and cooking are required. Understanding the relationships between viscosity of isolates, viscosity of in vitro extracts from foods, and physiologic responses would help clarify the mechanisms by which β-glucan affects satiety and the processing techniques that could facilitate development of satiety-enhancing products.
CONCLUSION
The mechanisms by which soluble dietary fiber exerts its physiologic effects on satiety are biologically plausible. Increased viscosity delays gastric emptying and reduces the absorption of nutrients. The increased interaction with the cells that release satiety hormones stimulates the release of peptides involved in appetite regulation. Whether delivered in a whole food or an extract from the food, oat β-glucan appears to have a positive effect on perceptions of satiety. Whether the effects are enduring with repeated exposure remains to be established.
Eating behavior, which arises from metabolic and sensory factors as well as the reward value of foods, is largely learned.57 The sensory factors drive food choice, but preferences are influenced by various exposures, availability, cultures, and social norms surrounding the food. Repeated exposure leads to the development of habits. Although preferences are resistant to change, they are amenable to modification.120 Consumption of whole foods or extracts from foods shown to promote satiety offers a means of helping individuals adhere to diet regimens by controlling hunger and the desire to eat.
Acknowledgments
Funding/support. This work is based in part on work that was supported by the National Institutes of Health under an award (T32 A T004094) from the National Center for Complementary and Integrative Health, and in part on work that was supported by the National Institute of Food and Agriculture, US Department of Agriculture, under an award from the USDA Hatch Project LAB 94209. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the US Department of Agriculture.
Declaration of interest. The authors have no relevant interests to declare.
References
- 1.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gortmaker SL, Swinburn BA, Levy D, et al. Changing the future of obesity: science, policy, and action. Lancet. 2011;378:838–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dietz WH, Baur LA, Hall K, et al. Management of obesity: improvement of health-care training and systems for prevention and care. Lancet. 2015;385:2521–2533. [DOI] [PubMed] [Google Scholar]
- 4.Swinburn B, Kraak V, Rutter H, et al. Strengthening of accountability systems to create healthy food environments and reduce global obesity. Lancet. 2015;385:2534–2545. [DOI] [PubMed] [Google Scholar]
- 5.Roberto CA, Swinburn B, Hawkes C, et al. Patchy progress on obesity prevention: emerging examples, entrenched barriers, and new thinking. Lancet. 2015;385:2400–2409. [DOI] [PubMed] [Google Scholar]
- 6.Rosenbaum M, Leibel RL. Adaptive thermogenesis in humans. Int J Obes (London). 2010;34(suppl 1):S47–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doucet E, Cameron J. Appetite control after weight loss: what is the role of bloodborne peptides? Appl Physiol Nutr Metab. 2007;32:523–532. [DOI] [PubMed] [Google Scholar]
- 8.Cohen DA. Neurophysiological pathways to obesity: below awareness and beyond individual control. Diabetes. 2008;57:1768–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blundell JE, Lawton CL, Cotton JR, et al. Control of human appetite: implications for the intake of dietary fat. Annu Rev Nutr. 1996;16:285–319. [DOI] [PubMed] [Google Scholar]
- 10.Vitaglione P, Lumaga RB, Stanzione A, et al. β-glucan-enriched bread reduces energy intake and modifies plasma ghrelin and peptide YY concentrations in the short term. Appetite. 2009;53:338–344. [DOI] [PubMed] [Google Scholar]
- 11.Lyly M, Liukkonen KH, Salmenkallio-Marttila M, et al. Fibre in beverages can enhance perceived satiety. Eur J Nutr. 2009;48:251–258. [DOI] [PubMed] [Google Scholar]
- 12.Beck EJ, Tapsell LC, Batterham MJ, et al. Increases in peptide Y-Y levels following oat β-glucan ingestion are dose-dependent in overweight adults. Nutr Res. 2009;29:705–709. [DOI] [PubMed] [Google Scholar]
- 13.Beck EJ, Tosh SM, Batterham MJ, et al. Oat β-glucan increases postprandial cholecystokinin levels, decreases insulin response and extends subjective satiety in overweight subjects. Mol Nutr Food Res. 2009;53:1343–1351. [DOI] [PubMed] [Google Scholar]
- 14.Schroeder N, Gallaher DD, Arndt EA, et al. Influence of whole grain barley, whole grain wheat, and refined rice-based foods on short-term satiety and energy intake. Appetite. 2009;53:363–369. [DOI] [PubMed] [Google Scholar]
- 15.Vitaglione P, Lumaga RB, Montagnese C, et al. Satiating effect of a barley β-glucan-enriched snack. J Am Coll Nutr. 2010;29:113–121. [DOI] [PubMed] [Google Scholar]
- 16.US Department of Agriculture, Center for Nutrition Policy and Promotion. Dietary guidelines for Americans 2010. http://www.cnpp.usda.gov/dietary-guidelines-2010. Published December 2010. Accessed July 27, 2015.
- 17.Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre? Nutr Res Rev. 2010;23:65–134. [DOI] [PubMed] [Google Scholar]
- 18.Anderson JW. Whole grains protect against atherosclerotic cardiovascular disease. Proc Nutr Soc. 2003;62:135–142. [DOI] [PubMed] [Google Scholar]
- 19.Jonnalagadda SS, Harnack L, Liu RH, et al. Putting the whole grain puzzle together: health benefits associated with whole grains – summary of American Society for Nutrition 2010 Satellite Symposium. J Nutr. 2011;141:1011S–1022S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Moura FF, Lewis KD, Falk MC. Applying the FDA definition of whole grains to the evidence for cardiovascular disease health claims. J Nutr. 2009;139:2220S–2226S. [DOI] [PubMed] [Google Scholar]
- 21.Newby PK, Maras J, Bakun P, et al. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr. 2007;86:1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eastwood M, Kritchevsky D. Dietary fiber: how did we get where we are? Annu Rev Nutr. 2005;25:1–8. [DOI] [PubMed] [Google Scholar]
- 23.2015 Dietary Guidelines Advisory Committee. Scientific Report of the Dietary Guidelines Advisory Committee. Rockville, MD: Office of Disease Prevention and Health Promotion; 2015. [Google Scholar]
- 24.Willett WC, Ludwig DS. The 2010 Dietary Guidelines – the best recipe for health? N Engl J Med. 2011;365:1563–1565. [DOI] [PubMed] [Google Scholar]
- 25.Anderson JW, Baird P, Davis RH, Jr, et al. Health benefits of dietary fiber. Nutr Rev. 2009;67:188–205. [DOI] [PubMed] [Google Scholar]
- 26.Decker EA, Rose DJ, Stewart D. Processing of oats and the impact of processing operations on nutrition and health benefits. Br J Nutr. 2014;112(suppl 2):S58–S64. [DOI] [PubMed] [Google Scholar]
- 27.Othman RA, Moghadasian MH, Jones PJ. Cholesterol-lowering effects of oat β-glucan. Nutr Rev. 2011;69:299–309. [DOI] [PubMed] [Google Scholar]
- 28.Rebello CJ, Greenway FL, Finley JW. Whole grains and pulses: a comparison of the nutritional and health benefits. J Agric Food Chem. 2014;62:7029–7049. [DOI] [PubMed] [Google Scholar]
- 29.Whitehead A, Beck EJ, Tosh S, et al. Cholesterol-lowering effects of oat β-glucan: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2014;100:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.US Food and Drug Administration. Health claims meeting significant scientific agreement (SSA). http://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm2006876.htm. Updated October 5, 2015. Accessed November 17, 2015. [Google Scholar]
- 31.Tosh SM. Review of human studies investigating the post-prandial blood-glucose lowering ability of oat and barley food products. Eur J Clin Nutr. 2013;67:310–317. [DOI] [PubMed] [Google Scholar]
- 32.Hipsley EH. Dietary “fibre” and pregnancy toxaemia. Br Med J. 1953;2:420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones JM. CODEX-aligned dietary fiber definitions help to bridge the ‘fiber gap'. Nutr J. 2014;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AACC International. Dietary fiber. http://www.aaccnet.org/initiatives/definitions/Pages/DietaryFiber.aspx. Published June 1, 2000. Accessed July 27, 2015. [Google Scholar]
- 35.Institute of Medicine. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids (macronutrients) (2005). http://books.nap.edu/openbook.php?record_id=10490&page=339. Published 2005. Accessed July 27, 2015. [Google Scholar]
- 36.Food labeling; revision of the nutrition and supplements facts labels. Unified agenda 0910-AF22. Fed Regis. Spring 2014. [PubMed]
- 37.Kumar V, Sinha AK, Makkar HP, et al. Dietary roles of non-starch polysaccharides in human nutrition: a review. Crit Rev Food Sci Nutr. 2012;52:899–935. [DOI] [PubMed] [Google Scholar]
- 38.Englyst KN, Liu S, Englyst HN. Nutritional characterization and measurement of dietary carbohydrates. Eur J Clin Nutr. 2007;61(suppl 1):S19–S39. [DOI] [PubMed] [Google Scholar]
- 39.Food and Agricultural Organization of the United Nations/World Health Organization, Joint FAO/WHO Food Standards Programme. CODEX Alimentarius (CODEX) Guidelines on Nutrition Labelling CAC/GL-2-1985 as last amended 2010. Rome: FAO; 2010.
- 40.Eastwood MA, Morris ER. Physical properties of dietary fiber that influence physiological-function: a model for polymers along the gastrointestinal tract. Am J Clin Nutr. 1992;55:436–442. [DOI] [PubMed] [Google Scholar]
- 41.Guillon F, Champ M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res Int. 2000;33:233–245. [Google Scholar]
- 42.Wood PJ. Cereal β-glucans in diet and health. J Cereal Sci. 2007;46:230–238. [Google Scholar]
- 43.Ellis PR, Rayment P, Wang Q. A physico-chemical perspective of plant polysaccharides in relation to glucose absorption, insulin secretion and the entero-insular axis. Proc Nutr Soc. 1996;55:881–898. [DOI] [PubMed] [Google Scholar]
- 44.Lam CD, Flores RA. Effect of particle size and moisture content on viscosity of fish feed. Cereal Chem. 2003;80:20–24. [Google Scholar]
- 45.Gallaher DD, Wood KJ, Gallaher CM, et al. Intestinal contents supernatant viscosity of rats fed oat-based muffins and cereal products. Cereal Chem. 1999;76:21–24. [Google Scholar]
- 46.Malkki Y, Virtanen E. Gastrointestinal effects of oat bran and oat gum: a review. Lebensm Wiss Technol. 2001;34:337–347. [Google Scholar]
- 47.Edwards CA, Johnson IT, Read NW. Do viscous polysaccharides slow absorption by inhibiting diffusion or convection? Eur J Clin Nutr. 1988;42:307–312. [PubMed] [Google Scholar]
- 48.Heaton KW, Marcus SN, Emmett PM, et al. Particle size of wheat, maize, and oat test meals: effects on plasma glucose and insulin responses and on the rate of starch digestion in vitro. Am J Clin Nutr. 1988;47:675–682. [DOI] [PubMed] [Google Scholar]
- 49.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backhed F, Ley RE, Sonnenburg JL, et al. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. [DOI] [PubMed] [Google Scholar]
- 51.Greiner T, Backhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22:117–123. [DOI] [PubMed] [Google Scholar]
- 52.Martensson O, Biorklund M, Lambo AM, et al. Fermented, ropy, oat-based products reduce cholesterol levels and stimulate the bifidobacteria flora in humans. Nutr Res. 2005;25:429–442. [Google Scholar]
- 53.Roberfroid M, Gibson GR, Hoyles L, et al. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104(suppl 2):S1–S63. [DOI] [PubMed] [Google Scholar]
- 54.Nicholson JK, Holmes E, Kinross J, et al. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. [DOI] [PubMed] [Google Scholar]
- 55.Cloetens L, Ulmius M, Johansson-Persson A, et al. Role of dietary β-glucans in the prevention of the metabolic syndrome. Nutr Rev. 2012;70:444–458. [DOI] [PubMed] [Google Scholar]
- 56.Raninen K, Lappi J, Mykkanen H, et al. Dietary fiber type reflects physiological functionality: comparison of grain fiber, inulin, and polydextrose. Nutr Rev. 2011;69:9–21. [DOI] [PubMed] [Google Scholar]
- 57.Blundell J, de Graaf C, Hulshof T, et al. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11:251–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sakata T. A very-low-calorie conventional Japanese diet: its implications for prevention of obesity. Obes Res. 1995;3(suppl 2):233S–239S. [DOI] [PubMed] [Google Scholar]
- 59.Wijlens AG, Erkner A, Alexander E, et al. Effects of oral and gastric stimulation on appetite and energy intake. Obesity. 2012;20:2226–2232. [DOI] [PubMed] [Google Scholar]
- 60.Deutsch JA, Young WG, Kalogeris TJ. The stomach signals satiety. Science. 1978;201:165–167. [DOI] [PubMed] [Google Scholar]
- 61.Baer DJ, Rumpler WV, Miles CW, et al. Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J Nutr. 1997;127:579–586. [DOI] [PubMed] [Google Scholar]
- 62.Yao M, Roberts SB. Dietary energy density and weight regulation. Nutr Rev. 2001;59:247–258. [DOI] [PubMed] [Google Scholar]
- 63.Marciani L, Gowland PA, Spiller RC, et al. Gastric response to increased meal viscosity assessed by echo-planar magnetic resonance imaging in humans. J Nutr. 2000;130:122–127. [DOI] [PubMed] [Google Scholar]
- 64.Marciani L, Gowland PA, Spiller RC, et al. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1227–G1233. [DOI] [PubMed] [Google Scholar]
- 65.Hoad CL, Rayment P, Spiller RC, et al. In vivo imaging of intragastric gelation and its effect on satiety in humans. J Nutr. 2004;134:2293–2300. [DOI] [PubMed] [Google Scholar]
- 66.Kristensen M, Jensen MG. Dietary fibres in the regulation of appetite and food intake. Importance of viscosity. Appetite. 2011;56:65–70. [DOI] [PubMed] [Google Scholar]
- 67.Chaudhri O, Small C, Bloom S. Gastrointestinal hormones regulating appetite. Philos Trans R Soc Lond B Biol Sci. 2006;361:1187–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brown AJ, Goldsworthy SM, Barnes AA, et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. [DOI] [PubMed] [Google Scholar]
- 69.Cherbut C, Ferrier L, Roze C, et al. Short-chain fatty acids modify colonic motility through nerves and polypeptide YY release in the rat. Am J Physiol. 1998;275:G1415–G1422. [DOI] [PubMed] [Google Scholar]
- 70.Samuel BS, Shaito A, Motoike T, et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci U S A. 2008;105:16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611 doi:10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGill CR, Iii VL, Devareddy L. Ten-year trends in fiber and whole grain intakes and food sources for the United States population: National Health and Nutrition Examination Survey 2001–2010. Nutrients. 2015;7:1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cho SS, Qi L, Fahey GC, Jr, et al. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. Am J Clin Nutr. 2013;98:594–619. [DOI] [PubMed] [Google Scholar]
- 74.Miller SS, Fulcher RG. Microstructure and chemistry of the oat kernel. In: Webster FH, Wood PJ, eds. Oats: Chemistry and Technology. 2nd ed. St. Paul, MN: AACC International; 2011:77–94. [Google Scholar]
- 75.Singh R, De S, Belkheir A. Avena sativa (oat), a potential neutraceutical and therapeutic agent: an overview. Crit Rev Food Sci Nutr. 2013;53:126–144. [DOI] [PubMed] [Google Scholar]
- 76.Virtanen JK, Nurmi T, Voutilainen S, et al. Association of serum 25-hydroxyvitamin D with the risk of death in a general older population in Finland. Eur J Nutr. 2011;50:305–312. [DOI] [PubMed] [Google Scholar]
- 77.Gaidosova A, Petruldkova Z, Havrilentova M, et al. The content of water-soluble and water-insoluble beta-D-glucans in selected oats and barley varieties. Carbohyd Polym. 2007;70:46–52. [Google Scholar]
- 78.Wood PJ. Relationships between solution properties of cereal β-glucans and physiological effects – a review. Trends Food Sci Tech. 2004;15:313–320. [Google Scholar]
- 79.Wood PJ, Weisz J, Mahn W. Molecular characterization of cereal β-glucans II. Size-exclusion chromatography for comparison of molecular weight. Cereal Chem. 1991;68:530–536. [Google Scholar]
- 80.Wood PJ, Paton D, Siddiqui IR. Determination of β-glucan in oats and barley. Cereal Chem. 1977;54:524–533. [Google Scholar]
- 81.Tiwari U, Cummins E. Factors influencing β-glucan levels and molecular weight in cereal-based products. Cereal Chem. 2009;86:290–301. [Google Scholar]
- 82.Izydorczyk MS, Storsley J, Labossiere D, et al. Variation in total and soluble β-glucan content in hulless barley: effects of thermal, physical, and enzymic treatments. J Agr Food Chem. 2000;48:982–989. [DOI] [PubMed] [Google Scholar]
- 83.Wood PJ, Weisz J, Fedec P, et al. Large-scale preparation and properties of oat fractions enriched in (1→3)(1→4)-β-D-glucan. Cereal Chem. 1989;66:97–103. [Google Scholar]
- 84.Wursch P, Pi-Sunyer FX. The role of viscous soluble fiber in the metabolic control of diabetes. A review with special emphasis on cereals rich in β-glucan. Diabetes Care. 1997;20:1774–1780. [DOI] [PubMed] [Google Scholar]
- 85.Dikeman CL, Fahey GC. Viscosity as related to dietary fiber: a review. Crit Rev Food Sci Nutr. 2006;46:649–663. [DOI] [PubMed] [Google Scholar]
- 86.Huang XF, Yu Y, Beck EJ, et al. Diet high in oat β-glucan activates the gut-hypothalamic (PYY3–36-NPY) axis and increases satiety in diet-induced obesity in mice. Mol Nutr Food Res. 2011;55:1118–1121. [DOI] [PubMed] [Google Scholar]
- 87.Lin N, Li Y, Tang L, et al. In vivo effect of oat cereal β-glucan on metabolic indexes and satiety-related hormones in diet-induced obesity C57-Bl mice. Mol Nutr Food Res. 2013;57:1291–1294. [DOI] [PubMed] [Google Scholar]
- 88.Buhmann H, le Roux CW, Bueter M. The gut–brain axis in obesity. Best Pract Clin Gastroenerol. 2014;28:559–571. [DOI] [PubMed] [Google Scholar]
- 89.Zhang M, Bai X, Zhang ZS. Extrusion process improves the functionality of soluble dietary fiber in oat bran. J Cereal Sci. 2011;54:98–103. [Google Scholar]
- 90.Rebello CJ, Johnson WD, Martin CK, et al. Acute effect of oatmeal on subjective measures of appetite and satiety compared to a ready-to-eat breakfast cereal: a randomized crossover trial. J Am Coll Nutr. 2013;32:272–279. [DOI] [PubMed] [Google Scholar]
- 91.Rebello CJ, Chu YF, Johnson WD, et al. The role of meal viscosity and oat β-glucan characteristics in human appetite control: a randomized crossover trial. Nutr J. 2014;13:49 doi:10.1186/1475-2891-13-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rebello CJ, Johnson WD, Martin CK, et al. Instant oatmeal increases satiety and reduces energy intake compared to a ready-to-eat oat based breakfast cereal: a randomized crossover trial [published online August 14, 2015]. J Am Coll Nutr. doi:10.1080/07315724.2015.1032442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Geliebter A, Grillot CL, Aviram-Friedman R, et al. Effects of oatmeal and corn flakes cereal breakfasts on satiety, gastric emptying, glucose, and appetite-related hormones. Ann Nutr Metab. 2015;66:93–103. [DOI] [PubMed] [Google Scholar]
- 94.Geliebter A, Nerys MA, Aviram-Friedman R, et al. Skipping breakfast leads to weight loss but also elevated cholesterol compared with consuming daily breakfasts of oat porridge or frosted cornflakes in overweight individuals: a randomised controlled trial. J Nutr Sci. 2014;3:e56–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cardello AV, Schutz HG, Lesher LL, et al. Development and testing of a labeled magnitude scale of perceived satiety. Appetite. 2005;44:1–13. [DOI] [PubMed] [Google Scholar]
- 96.Juvonen KR, Purhonen AK, Salmenkallio-Marttila M, et al. Viscosity of oat bran-enriched beverages influences gastrointestinal hormonal responses in healthy humans. J Nutr. 2009;139:461–466. [DOI] [PubMed] [Google Scholar]
- 97.Pentikainen S, Karhunen L, Flander L, et al. Enrichment of biscuits and juice with oat β-glucan enhances postprandial satiety. Appetite. 2014;75:150–156. [DOI] [PubMed] [Google Scholar]
- 98.Lyly M, Ohls N, Lahteenmaki L, et al. The effect of fibre amount, energy level and viscosity of beverages containing oat fibre supplement on perceived satiety. Food Nutr Res. 2010;54 doi:10.3402/fnr.v54i0.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hartvigsen ML, Gregersen S, Laerke HN, et al. Effects of concentrated arabinoxylan and β-glucan compared with refined wheat and whole grain rye on glucose and appetite in subjects with the metabolic syndrome: a randomized study. Eur J Clin Nutr. 2014;68:84–90. [DOI] [PubMed] [Google Scholar]
- 100.Juvonen KR, Salmenkallio-Marttila M, Lyly M, et al. Semisolid meal enriched in oat bran decreases plasma glucose and insulin levels, but does not change gastrointestinal peptide responses or short-term appetite in healthy subjects. Nutr Metab Cardiovasc Dis. 2011;21:748–756. [DOI] [PubMed] [Google Scholar]
- 101.Hlebowicz J, Darwiche G, Bjorgell O, et al. Effect of muesli with 4 g oat β-glucan on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized crossover trial. J Am Coll Nutr. 2008;27:470–475. [DOI] [PubMed] [Google Scholar]
- 102.Hlebowicz J, Wickenberg J, Fahlstrom R, et al. Effect of commercial breakfast fibre cereals compared with corn flakes on postprandial blood glucose, gastric emptying and satiety in healthy subjects: a randomized blinded crossover trial. Nutr J. 2007;6:22 doi:10.1186/1475-2891-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flint A, Raben A, Blundell JE, et al. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24:38–48. [DOI] [PubMed] [Google Scholar]
- 104.Rogers PJ, Blundell JE. Effect of anorexic drugs on food intake and the micro-structure of eating in human subjects. Psychopharmacology. 1979;66:159–165. [DOI] [PubMed] [Google Scholar]
- 105.Korczak R, Lindeman K, Thomas W, et al. Bran fibers and satiety in women who do not exhibit restrained eating. Appetite. 2014;80:257–263. [DOI] [PubMed] [Google Scholar]
- 106.Beck EJ, Tapsell LC, Batterham MJ, et al. Oat β-glucan supplementation does not enhance the effectiveness of an energy-restricted diet in overweight women. Br J Nutr. 2010;103:1212–1222. [DOI] [PubMed] [Google Scholar]
- 107.de Vries JH, Zock PL, Mensink RP, et al. Underestimation of energy intake by 3-d records compared with energy intake to maintain body weight in 269 nonobese adults. Am J Clin Nutr. 1994;60:855–860. [DOI] [PubMed] [Google Scholar]
- 108.Goris AH, Westerterp-Plantenga MS, Westerterp KR. Undereating and underrecording of habitual food intake in obese men: selective underreporting of fat intake. Am J Clin Nutr. 2000;71:130–134. [DOI] [PubMed] [Google Scholar]
- 109.Skendi A BC, Lazaridou A, Izydorczyk MS. Structure and rheological properties of water soluble β-glucans from oat cultivars of Avena sativa and Avena bysantina. J Cereal Sci. 2002;38:15–31. [Google Scholar]
- 110.Barone Lumaga R, Azzali D, Fogliano V, et al. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage. Food Funct. 2012;3:67–75. [DOI] [PubMed] [Google Scholar]
- 111.Schroeder N, Gallaher DD, Arndt EA, et al. Influence of whole grain barley, whole grain wheat, and refined rice-based foods on short-term satiety and energy intake. Appetite. 2009;53:363–369. [DOI] [PubMed] [Google Scholar]
- 112.Aoe S, Ikenaga T, Noguchi H, et al. Effect of cooked white rice with high β-glucan barley on appetite and energy intake in healthy Japanese subjects: a randomized controlled trial. Plant Foods Hum Nutr. 2014;69:325–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Granfeldt Y, Liljeberg H, Drews A, et al. Glucose and insulin responses to barley products: influence of food structure and amylose-amylopectin ratio. Am J Clin Nutr. 1994;59:1075–1082. [DOI] [PubMed] [Google Scholar]
- 114.Kim H, Behall KM, Vinyard B, et al. Short-term satiety and glycemic response after consumption of whole grains with various amounts of β-glucan. Cereal Food World. 2006;51:29–33. [Google Scholar]
- 115.Paquin J, Bedard A, Lemieux S, et al. Effects of juices enriched with xanthan and β-glucan on the glycemic response and satiety of healthy men. Appl Physiol Nutr Metab. 2013;38:410–414. [DOI] [PubMed] [Google Scholar]
- 116.Peters HP, Boers HM, Haddeman E, et al. No effect of added β-glucan or of fructooligosaccharide on appetite or energy intake. Am J Clin Nutr. 2009;89:58–63. [DOI] [PubMed] [Google Scholar]
- 117.El Khoury D, Cuda C, Luhovyy BL, et al. Beta glucan: health benefits in obesity and metabolic syndrome. J Nutr Metab. 2012;2012:851362 doi:10.1155/2012/851362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ragaee SM, Campbell GL, Scoles GJ, et al. Studies on rye (Secale cereale L.) lines exhibiting a range of extract viscosities. 2. Rheological and baking characteristics of rye and rye/wheat blends and feeding value for chicks of wholemeals and breads. J Agric Food Chem. 2001;49:2446–2453. [DOI] [PubMed] [Google Scholar]
- 119.Rolls BJ, Rolls ET, Rowe EA, et al. Sensory specific satiety in man. Physiol Behav. 1981;27:137–142. [DOI] [PubMed] [Google Scholar]
- 120.Hawkes C, Smith TG, Jewell J, et al. Smart food policies for obesity prevention. Lancet. 2015;385:2410–2421. [DOI] [PubMed] [Google Scholar]